Summary
FSH- or EGF-induced granulosa cell proliferation in intact preantral follicles depends on a novel PKC-mediated MAPK3/1 self-activation loop.
The objective was to reveal whether a PKC-mediated self-sustaining MAPK3/1 activation loop was necessary for FSH- or EGF-induced DNA synthesis in the granulosa cells of intact preantral follicles. For this purpose, hamster preantral follicles were cultured with FSH or EGF in the presence of selective kinase inhibitors. FSH or EGF phosphorylated RAF1, MAP2K1 and MAPK3/1. However, relatively higher dose of EGF was necessary to sustain the MAPK3/1 activity, which was essential for CDK4 activation and DNA synthesis. In intact preantral follicles, FSH or EGF stimulated DNA synthesis only in the granulosa cells. Sustained activation of MAPK3/1 beyond 3h was independent of EGFR kinase activity, but dependent on PKC activity, which appeared to form a self-sustaining MAPK3/1 activation loop by activating RAF1, MAP2K1 and PLA2G4. Inhibition of PKC activity as late as 4h after the administration of FSH or EGF arrested DNA synthesis, which corresponded with attenuated phosphorylation of RAF1 and MAPK3/1, thus suggesting an essential role of PKC in MAPK3/1 activation. Collectively, these data present a novel self-sustaining mechanism comprised of MAPK3/1, PLA2G4, PKC and RAF1 for CDK4 activation leading to DNA synthesis in granulosa cells. Either FSH or EGF can activate the loop to activate CDK4 and initiate DNA synthesis; however, consistent with our previous findings, FSH effect seems to be mediated by EGF, which initiates the event by stimulating EGFR kinase.
Keywords: follicle, MAPK 3/1, PKC, PLA2G4, EGFR, granulosa cells, FSH, cell cycle
Abbreviations used: FSH, follicle-stimulating hormone; EGF, epidermal growth factor; MAPK 3/1extracellular signal regulated kinase 2/1 (also known as ERK2/1); dpMAP3/1, dual-phosphorylated MAP3/1; MAP2K1, MAP3/1 kinase kinase 1 (also known as MEK1); PKC, protein kinase C (all isozymes); PLA2G4, phospholipase A2 (all cytosolic isozymes); CDK4, cyclin dependent kinase 4; RB1, retinoblastoma protein; GFXI, bisindolylmaleimide I; PMA, phorbol myristate acetate
Introduction
Repetitive but controlled division of granulosa cells is essential for follicular growth in the ovary. Previously, we have demonstrated that granulosa cells within hamster preantral follicles contain adequate level of cyclin D2 (CCND2) [1]. Therefore, when these cells are stimulated by FSH, CCND2 transcription or translation is not a prerequisite to activate CDK4 for DNA synthesis [1]. However, CCND2 transcription occurs as a backup mechanism to offset ubiquitination [1].
CDK4 activity is stimulated by EGF, which acts via receptor tyrosine kinase and MAPK3/1 [2]. EGF as well as FSH phosphorylates MAPK3/1 in cultured porcine granulosa cells [3], These factors also phosphorylate MAPK3/1 and CDK4 in the granulosa cells of hamster preantral follicles [1]. Further, EGF-antiserum or antisense EGF deoxyoligonucleotides can block FSH- or cyclic-3′, 5′-AMP stimulated DNA synthesis in the granulosa cells of intact preantral follicles [4, 5]. Evidence indicates that hormones and some growth factors can activate MAPK3/1 via PKC. However, it is evident that besides DNA synthesis, MAPK3/1 activation is required for other cell functions [2, 6, 7]. Further, the expression of EGFR in granulosa cells varies during follicular development [8]; hence, varying degrees of MAPK3/1 signaling are likely during the proliferation and differentiation of granulosa cells. Therefore, a mitogen-induced sustained activation of MAPK3/1 may be necessary for the onset of replicative cell cycle. This hypothesis is supported by an elegant concept developed with computer simulation and limited experimental data [9]. According to the model, a sustained activation of CDK4 is necessary for cells to enter the S phase, and the activation requires a highly stable state of active MAPK3/1. Further, it is conjectured that PKC can elevate MAPK3/1 activity to such an active state by forming a self-sustaining loop [9]. Whether FSH- or EGF-activation of CDK4 and subsequent initiation of DNA synthesis in granulosa cells involve such a loop is not known. The objectives of the present studies were to reveal the signaling steps involved in FSH or EGF stimulation of DNA synthesis in the granulosa cells of hamster preantral follicles, and to test the hypothesis whether a MAPK3/1-PKC self-sustaining activation loop would indeed be necessary for CDK4 activation.
Materials and methods
Golden hamsters (90–100 g) were purchased from SASCO (Madison, WI) and Charles River Laboratories (Wilmington, MA) and maintained in a 14L: 10D cycle in a climate-controlled facility according to the Institutional Animal Care and Use Committee (IACUC) and USDA guidelines. The use of hamsters for this study was approved by the IACUC.
Ovine FSH-20 was purchased from the National Pituitary program, NIH, rabbit polyclonal anti-CDK4 and anti-CCND2 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), recombinant murine EGF was from BD Biosciences (San Diego, CA), antibodies to serine-tyrosine (dual)-phosphorylated MAPK3/1, phospho(Ser259)-RAF1, phospho(Ser217/221)-MAP2K1 were from Cell Signaling (Beverley, MA), polyclonal antibodies to MAPK3/1 and MAP2K1 was from Santa Cruz Biotechnology (Santa Cruz, CA), mouse monoclonal anti-TUBB (also known as beta tubulin) antibody was from Sigma Chemical Company (St. Louis, MO), [3H]-thymidine (specific activity 40 Ci/mmol), 1-acyl-[14C]-arachidonyl phosphoethanolamine (specific activity 56 Ci/mmol) and Advance ECL kit were from GE Healthcare Bio-sciences Corporation (Piscataway, NJ), PD98059, a selective blocker of MAP2K1 activation, U0126, a specific blocker of MAP2K1, AG1478, an EGFR specific tyrosine kinase inhibitor [10], Arachidonyltrifluoromethyl Ketone (AACOCF3), a selective inhibitor of all isoforms of PLA2G4 [9], 2-[1-(3-Dimethylaminopropyl)-1H-indol-3-yl]-3-(1H-indol-3-yl)-maleimide (GFXI), a selective blocker of PKC, and PMA, a selective activator of PKC were purchased from Calbiochem (La Jolla, CA), PKC, PKA and MAPK3/1 assay kits were from Upstate Cell Signaling Solutions (Lake Placid, NY). All analytical grade chemicals were purchased from Fisher Scientific Company or Sigma Chemical Company.
Time course of FSH or EGF effect on follicular CDK4 activity
Ovaries were removed in the morning of proestrous (Day 4) when serum FSH levels were the lowest [11]. Preantral follicles at stages 6 [preantral follicles with 7–8 layers of granulosa cells [12]] and 7 [with incipient antrum, [12]] were dissected and cultured for 2, 4 or 6h without or with 25 ng/ml of ovine FSH-20 or 50 ng/ml recombinant murine EGF in Dulbecco’s modified Eagles’ medium supplemented with ITS+ (insulin, transferrin and selenium) as described previously [1, 13]. Follicles were retrieved, and the activity of CDK4 associated with CCND2 determined as described previously [1].
FSH or EGF activation of follicular signaling intermediaries
Based on the results of experiment 1 and previous study [1], follicles were cultured for 6h with 50 ng/ml EGF or 25 ng/ml FSH. After the culture, follicles were sonicated in a kinase assay buffer (buffer A: 10 mM Tris-HCl, pH 7.4, 100 mM NaCl, 1 mM EGTA, 1 mM EDTA, 1 mM NaF, 20 mM Na4H2PO4, 2 mM sodium orthovanadate, 1% Triton-x100 and 10% glycerol) containing a protease inhibitor cocktail. An equal amount of protein from each sample was immunoblotted to reveal phosphorylated RAF1, MAP2K1 and MAPK3/1, and TUBB (a loading control).
EGF-mediation of FSH effect on follicular DNA synthesis
Based on the results of experiment 1 and previous data [14], FSH and [3H]- thymidine were added 1h after the administration of 100 nM AG1478 and the culture continued for 6h. Incorporation of [3H]-thymidine and the activity of CDK4 were measured as described previously [15].
Follicles were cultured with or without 25 ng/ml FSH or 50 ng/ml EGF and [3H]- thymidine for 6h. Thecal shell was ruptured, granulosa cells squeezed out in ice-cold PBS containing orthovanadate, and each fraction was assayed for [3H]-thymidine incorporation [15].
Next, preantral follicles were cultured for 2h with 50 ng/ml EGF. Granulosa and theca cells were separated as described earlier and analyzed for phospho EGFR, EGFR, phospho MAPK3/1 and MAPK3/1 by immunoblotting.
In the next experiment, follicles were cultured with or without 100 nM AG1478 for 1h before the administration of 25 ng/ml FSH. The culture was continued for another hour. One group of follicles was exposed to 50 ng/ml EGF as a positive control. Granulosa cells were isolated in ice-cold PBS containing orthovanadate, homogenized in ice-cold buffer A without any detergent and a 26,000 g pellet containing crude membrane preparation was used for in vitro EGFR autophosphorylation.
Involvement of PKC in FSH or EGF-induced activation of CDK4 and DNA synthesis
Follicles were exposed to 100 nM GFXI for 1h before the administration of FSH or EGF, and [3H]-thymidine, and the culture continued for 6h. Follicles were cultured with 20 nM PMA as a positive control for PKC activation. Incorporation of [3H]-thymidine, CDK4 activity and phosphorylation of RAF1. MAP2K1 and MAPK3/1 were examined.
In a separate experiment, follicles were exposed to GFXI for 1h before the administration of EGF to examine the effect of PKC inhibition on EGF-induced phosphorylation of MAPK3/1, and the culture continued for 6h. MAPK3/1 phosphorylation was analyzed by immunoblotting. The optimal dosage of GFXI was selected by a dose response analysis based on its efficacy to block PKC activity (data not shown). A similar approach was used for other kinase inhibitors (data not shown).
Because kinases selected for the study were expected to influence each other activity in granulosa cells, a cell-free approach was utilized to check the specificity of the inhibitors used in this study. For this purpose, follicles were cultured without or with FSH for 4h, homogenized in kinase assay buffer and aliquots of a 26,000g supernatant containing 10 μg protein were mixed with GFXI (final concentration, 100 nM), PD98059 (final concentration, 10 μM), U0126 (final concentration, 10 nM) or AACOCF3 (final concentration, 60 μM) for 30 min on ice. Samples with or without the inhibitors were added to respective kinase reaction mixtures containing [32P]-γ-ATP (1.7 μCi/nmole ATP, final concentration) according to the manufacturers’ instructions and the reaction continued at 30°C for 10 min. A 25 μl aliquot of the reaction mixture was spotted on p81 filters, which were washed extensively in 0.75% phosphoric acid and then once with acetone and air dried. Radioactivity incorporated in the substrate was counted in a scintillation counter in the presence of Ecolite Plus cocktail. Enzyme activity was calculated as picomol [32P]- phosphate transferred to substrate per minute per mg protein, and the results were expressed as fold changes relative to untreated control.
Involvement of PLA2G4 in FSH- and EGF-induced DNA synthesis and PKC activation, and the role of MAPK3/1 in PLA2G4 and PKC activation
In the first experiment, follicles were exposed to 60 μM AACOCF3 for 1h followed by a 6h culture with FSH or EGF, and [3H]thymidine. Incorporation of [3H]thymidine determined. In the second experiment, follicles were cultured with 60 μM AACOCF3 or 10 μM PD98059 for 1h before the administration of FSH or EGF, and the culture continued for 6h. The activities of PLA2G4 or PKC in follicular homogenate were determined by thin- layer chromatography or a PKC assay kit, respectively.
Determination of the relationship between EGF-induced follicular MAPK3/1 phosphorylation and the activation of CDK4
Follicles were cultured in the presence of 5, 10, 25 or 50 ng/ml EGF and 1 μCi/ml [3H]thymidine for 6h, and the incorporation of [3H]thymidine measured. Based on the results, follicles were cultured for 6h in the presence of a suboptimal or optimal dose of EGF and the activity of CDK4 determined. Next, follicles were cultured for 10, 15 and 30 minutes with a dose of EGF that was suboptimal to stimulate DNA synthesis, and MAPK3/1 phosphorylation determined.
The effect of EGF in sustaining MAPK3/1 phosphorylation
Follicles were cultured hourly for 6h in the presence of 10 or 50 ng/ml EGF, and phosphorylation of MAPK3/1 examined. To check whether the theca could form a barrier for the suboptimal dose of EGF from reaching the granulosa cell compartment in intact follicles, granulosa cells were separated from the theca and oocytes and exposed to 10 ng/ml EGF for indicated time. Control groups received PBS. One group of culture was exposed to 50 ng/ml EGF to examine whether it would be as effective as it was for intact follicles. The cell lysate was analyzed for phosphorylated and total MAPK3/1, and TUBB.
Identification of a self-sustaining MAPK3/1activation loop
The minimum duration of FSH or EGF exposure needed to initiate CDK4 activation, and DNA synthesis was examined by culturing follicles in the absence or presence of FSH or EGF and [3H]thymidine for 6h and by adding 100 nM AG1478 at 1, 2, 3 or 4h after the commencement of the culture. Incorporation of [3H]thymidine and the activity of CDK4 were measured. Whether PKC activity was needed when DNA synthesis became independent of EGFR action was examined by culturing follicles without or with FSH or EGF for 6h, and adding100 nM GFXI at 4h after the beginning of the culture. Incorporation of [3H]thymidine was examined. The next experiment examined whether MAP2K1 or PKC inhibitor would adversely affect EGFR activation. Follicles were cultured for 6h without or with 50 ng/ml EGF and 10 nM U0126 or 100 nM GFXI, and EGFR phosphorylation examined by immunoblotting. To check whether EGFR kinase was active throughout the culture, follicles were cultured for 6h with or without 50 ng/ml EGF and 100 nM AG1478 was added at 2 or 4h after the beginning of the culture. Phosphorylation of EGFR was examined by immunoblotting.
The minimum duration of EGFR action needed to phosphorylate MAPK3/1 was verified by culturing follicles for 6h with or without 50 ng/ml EGF, and adding 5 μg of a mouse monoclonal anti EGFR IgG at 2h or 4h after the beginning of the culture. Phosphorylation of MAPK3/1 was examined. The monoclonal antibody blocked EGF binding to the receptor, but did not impair EGFR kinase activity. This study also complemented the AG1478 effect indicated earlier.
In the next experiment, follicles were cultured for 6h in the presence of 50 ng/ml EGF and 100 nM AG1478 was added to the culture at 2h or 4h after the beginning of the culture. In a separate experiment, 100 nM GFXI and 100 nM AG1478 were added to the culture 4h after EGF administration. Levels of phosphorylated RAF1 and MAPK3/1 were detected by immunoblotting.
Whether PKC activation depended on MAPK3/1 activity was examined by culturing follicles with 50 ng/ml EGF for 6h, and administering 100 nM AG1478, 10 nM U0126 or 100 nM GFXI at 2h or 4h after the beginning of the culture. The activity of PKC was measured.
Measurement of [3H]thymidine incorporation
This was done essentially as described by Roy and Greenwald [16]. The rate of DNA synthesis was expressed as cpm [3H]thymidine incorporated per ng DNA.
Western blot detection and quantification of cell cycle proteins
The basic protocol was essentially as described by Yang and Roy [1]. After electrophoretic transfer of proteins, the membranes were probed sequentially with antibodies to pRAF1, pMAP2K1, dual-phosphorylated (dp) MAPK3/1, MAPK3/1 and TUBB after completely stripping the ECL signal of the previous detection. The signal was generated using Advanced chemiluminescence Kit (GE Healthcare Biosciences) and the intensity was directly quantified by a UVP (Upland, CA) gel documentation system. Images of representative immunoblots were arranged using Adobe PhotoShop. For the sake of brevity, quantitative values were furnished as bar graphs whenever necessary.
Measurement of CDK4 activity
Because CCND2/CDK4 complex represented active holoenzyme [17], the activity of the complex was determined in CCND2 immunoprecipitate essentially as described previously [1, 18]. The levels of RB1 in each sample were detected by immunoblotting followed by thorough stripping of the membrane. Finally, the membrane was exposed overnight to a phosphor screen and the radioactivity emitted from [32P]RB1 recorded by a Cyclone Phosphorimager (Perkin-Elmer, Shelton, CT).
Autophosphorylation of EGFR
Aliquots of 26,000g pellet of granulosa cells in buffer A without detergent was mixed with an equal volume of an ice-cold buffer containing 50 mM PIPES [Piperazine-1,4- bis(2-ethane sulfonic acid)], pH 7.0, 1 mM MnCl2 and 0.1 mM orthovanadate to a final volume of 40 μl. The mixture was kept on ice and 10 μl of an ATP solution (final concentration: 0.3 μCi of [32P]-γ-ATP in 1 μM ATP) in 0.6% Triton X100 was added to the mixture. After 10 min on ice, the reaction was stopped by 25 μl of a 4X SDS sample buffer, boiled for 5 min, fractionated in a 7.5% polyacrylamide gel, transferred to Optitran membrane and exposed overnight to an X-ray film.
Measurement of PKC activity
The activity of PKC was determined using an assay kit that utilized a PKC-specific substrate peptide and [32P]-γ-ATP essentially as the manufacturer’s instruction. The enzyme activity was expressed as picomol [32P]-phosphate transferred to substrate per minute per mg protein, and the data were expressed as fold changes relative to untreated controls.
Measurement of PKA and MAPK3/1 activities
Activities PKA and MAPK3/1 were measured using assay kits that utilized a PKA- specific substrate peptide and myelin basic protein, respectively, and [32P]-γ-ATP essentially as the manufacturer’s instruction. Each reaction mixture contained inhibitors that allowed the measurement of specific enzymes. Enzyme activities were expressed as picomol [32P]-phosphate transferred to substrate per minute per mg protein, and the data were expressed as fold changes relative to untreated controls.
Measurement of PLA2G4 activity
PLA2G4 assay was done according to Nahas et al [19] with modification to fit small amounts of protein. Briefly, approximately 60 follicles (thecal cells did not contribute to the total enzyme activity, S K Roy, unpublished data) were sonicated in a lysis buffer (50 mM HEPES, pH 7.4 containing 250 mM sucrose, 1 mM EGTA, 1 mM EDTA, 1 mM Na- orthovanadate, 5 mM DTT and a protease inhibitor cocktail) at 20 watt with 2-3, 5-second bursts. Samples were centrifuged at 12,000g for 15 min at 4°C, and the protein content in the supernatant was determined. The assay was done in a final volume of 40 μl containing 40 μg protein and 15 μM [14C]-acryl-arachidonyl phosphoethanolamine (specific activity 74 Ci/mmol) at 37°C for 30 min. The reaction was stopped with 40 μl of a quench solution containing 40 μg of arachidonic acid and 20 μl of glacial acetic acid in 1ml of 100% ethanol, and 50 μl of the mixture was spotted on a LK5PE TLC plates along with pure arachidonic acid as reference. The plates were dried at room temperature for 10 min, developed using a water equilibrated solvent phase containing ethyl acetate: iso-octane: acetic acid (55:75:8), and dried for 5 min at 100°C. Spots corresponding to arachidonic acid were identified by the iodine reaction, cut, mixed with 4 ml of scintillation fluid and counted. The radioactivity migrated with arachidonic acid was converted to picomol arachidonic acid and the enzyme activity was calculated as picomol arachidonic acid produced per min per mg protein. Finally, the data were presented as fold changes relative to untreated control.
Because a short-term culture of intact preantral follicles was used in this study, pharmacological inhibitors were the ideal reagents to address the questions. Nevertheless, we have critically tested the specificity of their effect in the present system for careful data interpretation.
Statistical analysis
All experiments were repeated at least three times to obtain a mean ± SEM. All Immunoblots were quantified directly by the UVP imager and the optical density values were analyzed by 1-way ANOVA. Representative immunoblots were furnished. To avoid redundancies and to maintain clarity of data presentation, bar graphs of immunoblots were furnished whenever it was absolutely necessary. All kinase assays were repeated at least three times. All quantitative data were analyzed using 1-Way ANOVA with Scheffe’s post- hoc test. The level of significance was P< 0.05.
Results
Effect of FSH or EGF on follicular CDK4 activity and signaling intermediaries
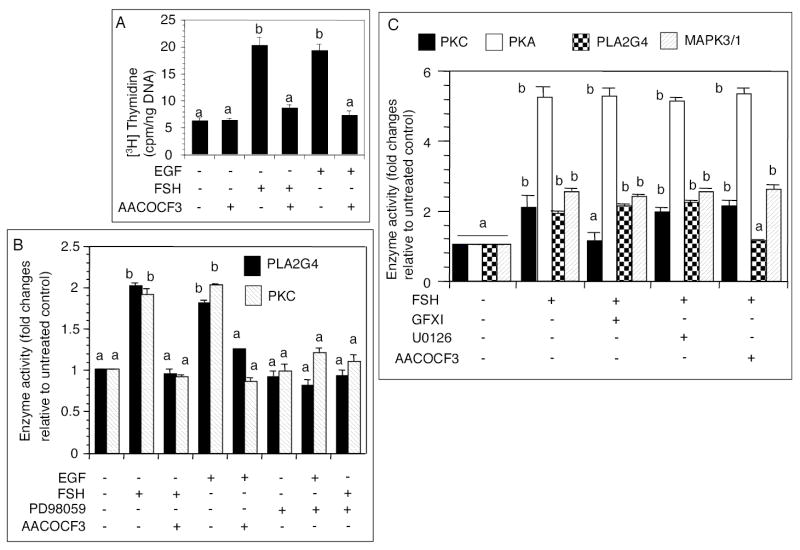
The purpose of the experiment was to determine the latency of FSH-induced stimulation of CDK4 activity, intracellular signaling mechanisms mediating the effects of FSH or EGF, and any thecal contribution to the results obtained using intact follicles. The use of EGF was supported by our previous findings that FSH action on hamster granulosa cells was mediated by EGF [4, 5]. FSH markedly stimulated CDK4 activity by 2h (Fig. 1A). Identical results were obtained when EGF was used instead (data not shown). Either FSH or EGF stimulated RAF1, MAP2K1 and MAPK3/1 phosphorylation in follicular cells (Fig. 1B). Interestingly, FSH stimulated DNA synthesis and CDK4 activation were completely blocked by AG1478 (Fig. 1C).
Fig. 1.
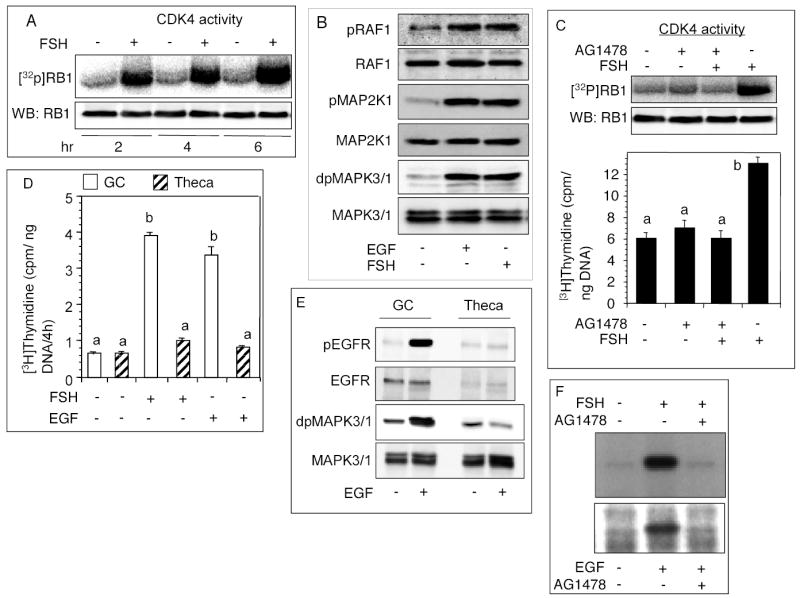
Effect of 25 ng/ml ovine-FSH-20 or 50 ng/ml EGF on kinase and CDK4 activation, and DNA synthesis. (A) Follicles were cultured with FSH for indicated times. CCND2/CDK4 complex was immunoprecipitated from follicular homogenate and used for CDK4 assay. The top panel represents a phosphorimage of [32P]RB1, and the bottom panel represents an immunoblot of RB1 protein added to each sample. (B) Immunoblots of phosphorylated signaling intermediaries in preantral follicles. Follicles were cultured for 6h with or without FSH or EGF. (C) Effect of an EGFR-kinase inhibitor on FSH stimulated follicular CDK4 activity and DNA synthesis. Follicles were cultured for 6h with FSH and [3H]thymidine, both of which were added 1h after the administration of 100 nM AG1478. The top panel represents a phosphorimage of [32P]RB1, middle panel is an immunoblot of RB1 added to each sample and the bottom panel reflects [3H]thymidine incorporation. (D) Effect of FSH or EGF on granulosa cell and thecal DNA synthesis. Follicles were cultured with FSH or EGF for 6h, granulosa cells separated from the theca and the incorporation of [3H]thymidine in both cell types determined. (E) Effect of EGF on EGFR and MAPK3/1 phosphorylation in granulosa and theca cells. Follicles were cultured with FSH or EGF for 2h, granulosa cells separated from the theca and EGFR and MAPK3/1 phosphorylation in both cell types determined by immunoblotting. (F) Autoradiographic analysis of FSH- or EGF-induced EGFR autophosphorylation in granulosa cells. Follicles were cultured with AG1478 1h before the administration of FSH or EGF. Granulosa cells were isolated 1h later, and the crude membrane pellet mixed with [32P]-γ-ATP. After electrophoretic separation of the EGFR, gels were fixed, dried and exposed overnight to an x-ray film. Each bar represents a mean ± SEM of three separate values. P > 0.05: bars with a same letter; P < 0.05: bars with a different letter.
To examine whether theca cells associated with intact preantral follicles contributed to the observed effects, granulosa cells and theca layer were separated after culture and examined. In contrast to granulosa cells, neither FSH nor EGF stimulated thecal DNA synthesis (Fig. 1D). Further, no EGF-induced phosphorylation of thecal EGFR or MAPK3/1 was evident (Fig. 1E). It was noteworthy that on equal protein basis, theca cells had appreciably low levels of EGFR than granulosa cells (Fig. 1E). Based on these results, molecular changes related to cell proliferation in intact follicles would be expected to reflect primarily granulosa cells. Therefore, intact follicles were used to avoid delay in sample preparation unless otherwise indicated.
Phosphorylation of the EGFR in vitro was examined to determine whether FSH action would actually lead to receptor autophosphorylation in granulosa cells. FSH or EGF activated EGFR-kinase leading to phosphorylation of the EGFR, but the effect was completely inhibited by AG1478 (Fig. 1F), suggesting that FSH action on granulosa cells involved activation of EGFR.
PKC mediation of FSH or EGF effect on CDK4 activation
The rationale was to examine whether FSH- or EGF-stimulated DNA synthesis would involve PKC activity. Suppression of PKC activity by GFXI completely blocked FSH or EGF activation of CDK4 and DNA synthesis (Fig. 2A). GFXI also blocked PMA-induced DNA synthesis (Fig. 2A). No attempt was made to determine CDK4 activity following PMA administration because DNA synthesis would not proceed without CDK4 activation. Interestingly, GFXI completely suppressed EGF-induced phosphorylation of MAPK3/1 (Fig. 2B). PMA also stimulated RAF1, MAP2K1 and MAPK3/1 phosphorylation (Fig. 2C). These results, along with those in Fig. 1, suggested that PKC could activate RAF1, MAP2K1 and MAPK3/1 signaling that was necessary for FSH or EGF stimulated granulosa cell DNA synthesis.
Fig. 2.
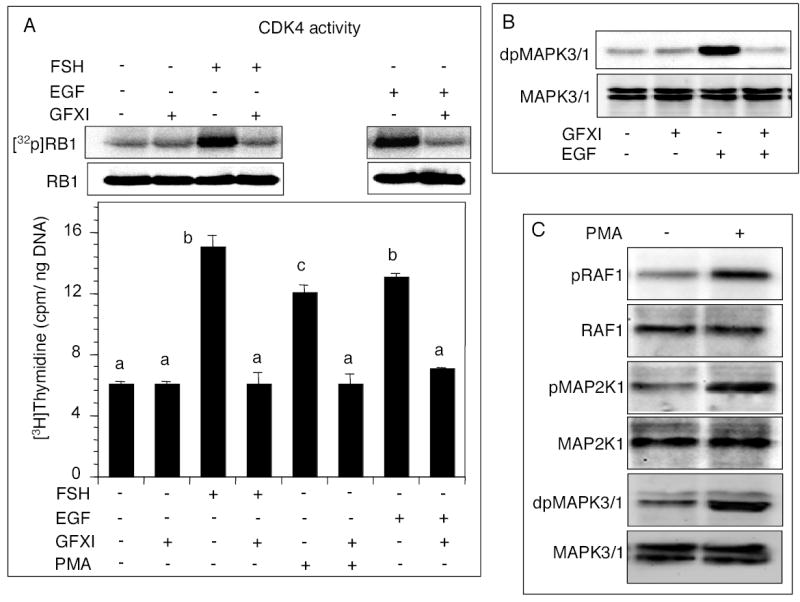
Effects of PKC inhibitor on FSH- or EGF stimulated follicular CDK4 activity, DNA synthesis and MAPK3/1 phosphorylation. (A) Follicles were cultured for 6h without or with 100 nM GFXI, and FSH, EGF or 20 nM PMA, and [3H]-thymidine. The top panel depicts a phosphorimage of [32P]RB1 and immunoblot of RB1 protein. The bottom panel indicates DNA synthesis. Each bar represents a mean ± SEM of three separate values. P > 0.05: bars with a same letter; P < 0.05: bars with a different letter. (B) Immunoblots of dual phosphorylated and total MAPK3/1 in follicles cultured with EGF with or without GFXI. (C) Immunoblots of phosphorylated and total RAF1, MAP2K1 and MAPK3/1 in follicles cultured with 20 nM PMA for 6h.
Activity of PLA2G4 is necessary for FSH or EGF stimulated granulosa cell DNA synthesis, and MAPK3/1 activates PKC by activating PLA2G4
The purpose of these experiments was to determine whether EGF or FSH would activate PKC by activating PLA2G4 as a mechanism to establish the self-sustaining activation loop. AACOCF3 attenuated FSH or EGF stimulated DNA synthesis (Fig. 3A) and PLA2G4 activation (Fig. 3B). AACOCF3 alone did not alter basal [3H]thymidine incorporation (Fig. 3A). Interestingly, stimulation of PLA2G4 by either FSH or EGF was markedly attenuated by PD98059 (Fig. 3B), indicating that MAPK3/1 activity was required for PLA2G4activation. Further, inhibition of either MAP2K1 or PLA2G4 activation resulted in a significant reduction in follicular PKC activity (Fig. 3B), indicating that stimulation of PKC activity by FSH or EGF occurred through a sequential action of MAPK3/1 and PLA2G4. PD98059 alone had no significant effect on basal PLA2G4 or PKC activity (Fig. 3B). The result was similar when AACOCF3 was used, instead (data not shown).
Fig. 3.
Effect of AACOCF3 or PD98059 on FSH- or EGF stimulated follicular DNA synthesis, PLA2G4 and PKC activity. (A) Follicles were cultured with 60 μM AACOCF3 for 1h before the administration of FSH or EGF, and [3H]thymidine. After 6h of culture, DNA synthesis was determined. (B) Follicles were cultured with 60 μM AACOCF3 or 10 μM PD98059 for 1h before the administration of FSH or EGF. After 6h culture, PLA2G4 or PKC activity was measured. AACOCF3 was used as a positive control for PLA2G4 inhibition. (C) Cell-free determination of the specificity of kinase inhibitors at the tested dose level. Follicles were cultured without or with 25 ng/ml FSH, homogenate was mixed with an appropriate concentration of respective inhibitors for 30 min on ice and used in enzyme reaction. Each bar represents a mean ± SEM of three separate values. P > 0.05: bars with a same letter; P < 0.05: bars with a different letter.
The purpose of the cell-free assay of enzyme activities was to determine the specificity of the kinase inhibitors at the tested dose level. FSH stimulated PKC, PLA2G4 and MAPK3/1 activities at least 2-fold, and PKA activity 5-fold (Fig. 3C). Whereas GFXI blocked FSH stimulated PKC activity, it had no effect on the activities of other enzymes (Fig. 3C). Similarly, AACOCF3 blocked the effect of FSH on PLA2G4, but did not show any non-specific inhibition of other enzymes (Fig. 3C). Consistent with the technical information, U0126 did not inhibit any enzyme, including MAPK3/1 (Fig. 3C), which was already activated by MAP2K1, the target of the inhibitor. Similar results were obtained when 10 μM PD98059 was used instead of U0126 (data not shown).
A higher dose of EGF was needed to sustain MAPK3/1 phosphorylation
Results of earlier experiments provided strong evidence that FSH action on granulosa cell DNA synthesis was mediated by EGF via EGFR. Therefore, EGF was used in most of the subsequent experiments to determine whether a self-sustaining MAPK3/1 activation loop was required for granulosa cell DNA synthesis. EGF, up to 25 ng/ml dose level, could not stimulate granulosa cell DNA synthesis, but could do so at 50 ng/ml dose level (Fig. 4A). However, a dose that was suboptimal for CDK4 activation (Fig. 4B), was fully capable of phosphorylating MAPK3/1 within 10 min and maintained the phosphorylation state for 30 min (Fig. 4C). When compared with the effect of an optimal dose for a period of 6h, the suboptimal dose of EGF could not sustain MAPK3/1 phosphorylation beyond 2h (Fig. 4D). To examine whether the inability of the suboptimal dose of EGF to sustain MAPK3/1 phosphorylation was due to the presence of a thecal diffusion barrier, granulosa cell suspension was exposed to suboptimal and optimal doses of EGF, and MAPK3/1 phosphorylation examined. Despite the absence of a theca, the suboptimal dose of EGF failed to maintain MAPK3/1 phosphorylation beyond 3h, whereas MAPK3/1 phosphorylation in response to the optimal dose remained steady after 6h (Fig. 4E). These results suggested that thecal barrier, if any, was not a factor for the effect observed with the suboptimal dose of EGF; rather an optimal dose was indeed necessary to elevate MAPK3/1 activity to a level that could activate CDK4.
Fig. 4.
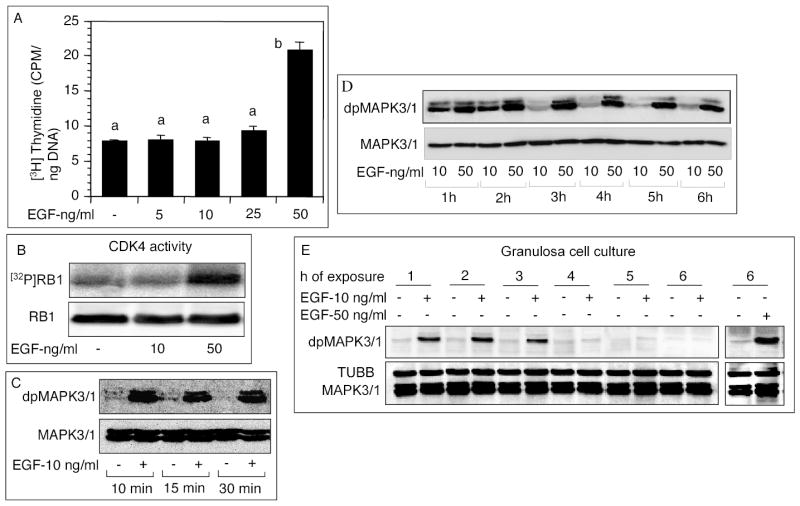
Effects of suboptimal and optimal doses of EGF on follicular DNA synthesis, CDK4 activity or MAPK3/1 phosphorylation. (A-B) Follicles were cultured with indicated doses of EGF and 1 μCI/ml [3H]thymidine. (A) DNA synthesis and (B) CDK4 activity were measured. Each bar represents a mean ± SEM of three separate values. P > 0.05: bars with a same letter; P < 0.05: bars with a different letter. (C) Follicles were cultured with 10 ng/ml EGF for indicated times, and MAPK3/1 phosphorylation was determined by immunoblotting. (D) Follicles were cultured for indicated times with a low (10 ng/ml) or an optimal (50 ng/ml) dose of EGF. Levels of phosphorylated MAPK3/1 were determined by immunoblotting. (E) Granulosa cells were separated from thecal layers and exposed to a suboptimal (10 ng/ml) dose of EGF for indicated times. One group was exposed to 50 ng/ml EGF for 6h to compare the data with those presented in 4D. Phosphorylated MAPK3/1 and TUBB were detected by immunoblotting.
Presence of a self-sustaining MAPK3/1 activation loop
The initiation of a self-sustaining MAPK3/1 activation loop would require signaling to be independent of EGFR activity after a certain period. Therefore, it was important to determine the optimal duration of EGFR-kinase activity needed for activating CDK4 and inducing DNA synthesis. For that purpose, EGFR kinase activity was blocked with AG1478 at different times after EGF or FSH stimulation. Inhibition of EGFR kinase activity beyond 2h of EGF or FSH administration failed to suppress CDK4 activation or DNA synthesis (Fig. 5A). However, a relatively longer FSH exposure seemed necessary to stimulate the full complement of DNA synthesis (Fig. 5A). Similarly, a monoclonal EGFR antibody that would block EGF-induced EGFR activation, could not block EGF-induced MAPK3/1 phosphorylation after 2h (Fig. 5B), suggesting that MAPK3/1 signaling became independent of EGFR action after 2h. In contrast, inhibition of PKC activity by GFXI after 4h of FSH or EGF administration stopped DNA synthesis and the incorporation of thymidine remained at 4h level (Fig. 5C), indicating that PKC was a critical component of the MAPK3/1-activation loop. Neither the MAP2K1 nor the PKC inhibitor could block EGF- induced phosphorylation of the EGFR, whereas AG1478 blocked the phosphorylation even after 4h of EGF administration (Fig. 5D). These results indicated that the observed effect of MAP2K1 or PKC inhibitors was not because of compromised EGFR function.
Fig. 5.
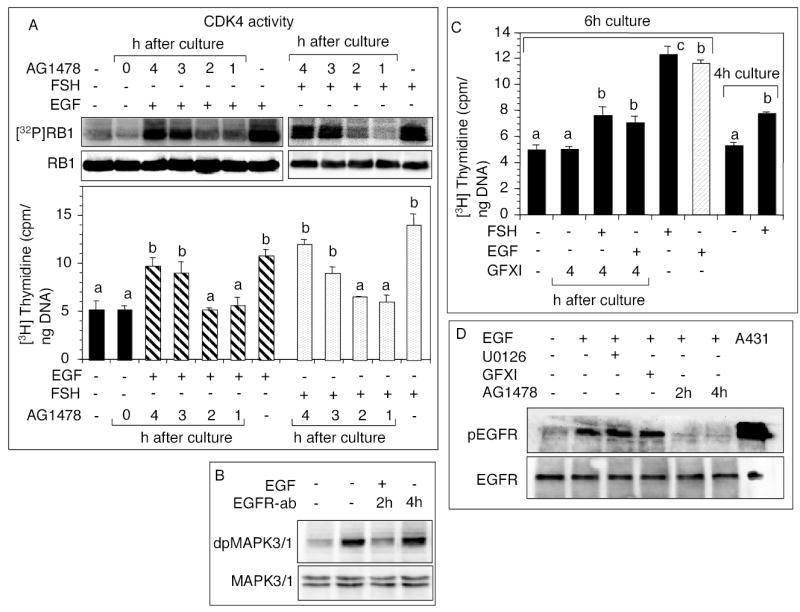
Dependency of FSH or EGF stimulated CDK4 activation and DNA synthesis on EGFR kinase and PKC. (A) Follicles were cultured with 25 ng/ml FSH or 50 ng/ml EGF, and [3H]thymidine for 6h. AG1478 was added at indicated times after the beginning of the culture. CDK4 activity and DNA synthesis were determined. (B) Follicles were cultured with 50 ng/ml EGF for 6h. A mouse monoclonal anti EGFR IgG was added to the culture to a final concentration 5 μg/ml at 2h or 4h after the beginning of the culture, and phosphorylated MAPK3/1 detected by immunoblotting. (C) Follicles were cultured with 25 ng/ml FSH or 50 ng/ml EGF, and [3H]thymidine for 6h. GFXI was added at 4h after the beginning of the culture, and DNA synthesis examined. The data were compared with thymidine incorporation for 4h. (D) Follicles were cultured with 100 nM GFXI or 10 nM U0126 for 1h before the administration of 50 ng/ml EGF. For some groups, 100 nM AG1478 was added at 2h or 4h after beginning of the culture. After 6h, EGFR phosphorylation was examined by immunoblotting. Lysate of activated A431 cells was used as a positive control for phosphorylated EGFR. Each bar represents a mean ± SEM of three separate values. P > 0.05: bars with a same letter; P < 0.05: bars with a different letter.
Because PKC-mediated MAPK3/1 activation loop was expected to involve RAF1, phosphorylation of RAF1and MAPK3/1 was examined after blocking the activation of EGFR kinase by AG1478. Whereas RAF1 and MAPK3/1 phosphorylation was suppressed when AG1478 was added 2h after EGF administration, no inhibition was evident when it was added after 4h (Fig. 6A-B). However, when PKC activity was blocked at 4h after EGF administration, both RAF1 and MAPK3/1 phosphorylation were markedly attenuated (Fig. 6A-B). Measurement of granulosa cell PKC activity following a temporal administration of kinase inhibitors revealed that AG1478 could not block EGF-induced increase in PKC activity after 2h; however, blocking MAP2K1 activity at 2h or 4h completely suppressed PKC activity (Fig. 6C). A direct inhibition of PKC by GFXI had a similar effect (Fig. 6C), thus establishing the specificity of GFXI. These results suggested that MAPK3/1 activated PKC to activate RAF1, which in turn, activated MAPK3/1 to establish the loop.
Fig. 6.
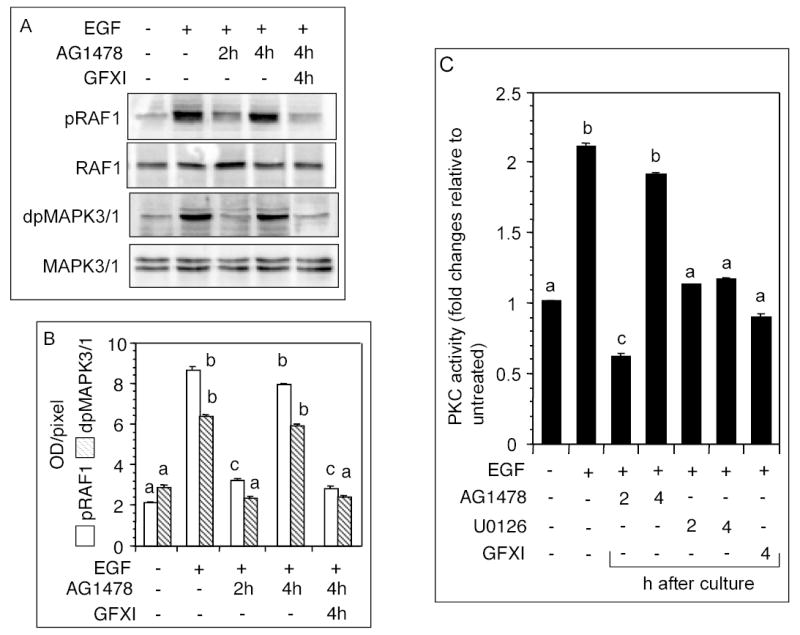
Necessity of PKC to sustain MAPK3/1 phosphorylation. (A) Follicles were cultured with 50 ng/ml EGF for 6h, and 100 nM AG1478 was added at 2h or 4h after beginning of the culture. Another group of follicles received 100 nM GFXI along with AG1478 at 4h. Levels of phosphorylated RAF1 and MAPK3/1 were determined. (B) Quantitative values of the signal intensities presented in A. (C) Follicles were cultured with 50 ng/ml EGF for 6h, and 100 nM AG1478 or 10 nM U0126 was added at 2h or 4h after the beginning of the culture. Another group of follicles received 100 nM GFXI at 4h. PKC activity in follicular homogenate was determined. Each bar represents a mean ± SEM of three separate values. P > 0.05: bars with a same letter; P < 0.05: bars with a different letter.
Discussion
The results of these studies suggest that FSH regulates granulosa cell DNA synthesis in hamster preantral follicles by a novel mechanism that involves an interaction between EGF-EGFR kinase, PLA2G4, PKC and MAPK3/1 signaling. The interaction establishes a self-sustaining MAPK3/1 activation loop that is essential for CDK4 activation. The autophosphorylation of EGFR by FSH suggests that at least part of the FSH action is mediated by EGF. FSH stimulates the synthesis of EGF [5] and EGFR [8] in the granulosa cells of hamster preantral follicles, and EGF seems to mediate the effect of FSH on follicular DNA synthesis [4, 5]. It is apparent that FSH- or EGF stimulated follicular DNA synthesis reflects primarily the activity of granulosa cells and the contribution of theca cells, if any, is minimal. Although the binding of 125I-EGF to rat theca-interstitial cells has been demonstrated [20], the results of the present study corroborate our previous findings that in adult hamsters, EGF in vivo fails to induce protein tyrosine phosphorylation in thecal cells [8]. Alternatively, the results can also highlight a species-specific effect of EGF or the maturation status of preantral follicles. Granulosa and theca cells in preantral follicles are less mature than their counterparts in antral follicles [21].
Sustained MAPK3/1 activity is essential for cells to enter the cell cycle [22]. Bhalla et al [9] have theorized that activated MAPK3/1 can exist either as a monostable or bistable form. Whereas the monostable form of the enzyme represents an all-or-none type transient signaling, the bistable form maintains sustained signaling [9]. This phenomenon seems to exist in granulosa cells because appreciable MAPK3/1 phosphorylation is evident even after 6h of FSH or EGF exposure; however, such a long-term phosphorylation requires a relatively higher dose of EGF that may elevate MAPK3/1 signaling to the bistable level. A similar increase in MAPK3/1 activity by PDGF in NIH 3T3 cells has been reported [9]. In contrast, the suboptimal dose of EGF seems to transiently activates MAPK3/1 that may be necessary for other cell functions [23–28]. Hamster preantral follicles at stage 6 can secrete 36 pg EGF/follicle in response to FSH in vitro [5]. Intrafollicular concentration of EGF is expected to be higher. Although the exact amount of biologically active EGF available to granulosa cells in vitro is difficult to assess, it is evident from the present study that a 50 ng/ml dose provides the amount necessary for DNA synthesis. The impairment of FSH- or EGF-induced MAPK3/1 phosphorylation and DNA synthesis by PKC inhibitor suggests strongly that sustained activation of MAPK3/1 requires PKC action. Bhalla et al [9, 29] proposed that a self-sustaining loop comprising of MAPK3/1, PLA2G4 and PKC functions as a bistable switch wherein an optimal level of extracellular stimulus results in sustained MAPK3/1 activation. Results of the present study lend credence to this hypothesis by providing the first direct evidence for the existence of such a self-sustaining loop in hamster granulosa cells. The attenuation of FSH or EGF stimulated DNA synthesis and PKC activity by PLA2G4 inhibitor provides evidence that PLA2G4 is an integral component of the loop; however, which isozymes participate in the loop is not known at present. FSH has been shown to increase secretory PLA2G4 mRNA levels in the rat ovary [30] and arachidonic acid (AA) production by immature rat Sertoli cells [31]. The results of the present study provide evidence that FSH or EGF activates PLA2G4 in granulosa cells. Similarly, suppression of both PLA2G4 and PKC activities by the MAP2K1 inhibitor suggests strongly that activation of MAPK3/1 is essential to initiate and maintain the self-sustaining loop. On the other hand, FSH- or EGF-induced phosphorylation of RAF1, MAP2K1 and MAPK3/1 suggests that activation of MAPK3/1 occurs via intermediaries that are typical of EGFR signaling [32].
The failure of AG1478 to suppress CDK4 activation and DNA synthesis, and of EGFR-antibody to inhibit MAPK3/1 phosphorylation after 2h of EGF or FSH exposure suggests that the establishment of a self-sustained MAPK3/1 activation loop in granulosa cells requires at least 2h of stimulation by FSH or EGF. However, the active state of MAPK3/1 that is essential for DNA synthesis depends critically on PKC action because the inhibition of PKC activity even after 4h of DNA synthesis can suspend the process in the midstream by suppressing RAF1 and MAPK3/1 phosphorylation. Phosphorylation of RAF1, MAP2K1 and MAPK3/1 by PMA provides additional evidence that PKC can activate the signaling pathway provided the enzyme is activated. Dephosphorylation of RAF1 and MAPK3/1 after 2h of PKC inhibition lends credence to the speculation that one of the functions of PKC in granulosa cells is to suppress the activities of specific phosphatase for maintaining the active states of the kinases. Future studies may address this conjecture.
In summary, the results of the present studies demonstrate that FSH stimulated DNA synthesis in hamster granulosa cells involves a novel mechanism that begins with EGF-EGFR interaction leading to MAPK3/1 phosphorylation. Activated MAPK3/1 stimulates PLA2G4, which activates PKC. Active PKC further stimulates MAPK3/1 via reactivation of RAF1 and MAP2K1. Therefore, at the beginning of FSH or EGF stimulation, MAPK3/1 activation occurs via EGFR signaling as well as PKC signaling, but subsequently, the self-sustaining MAPK3/1 activation loop is established and it elevates MAPK3/1 activity to the bistable mode leading to the activation of CDK4 and DNA synthesis. A model explaining the mechanisms has been proposed (Fig. 7).
Fig. 7.
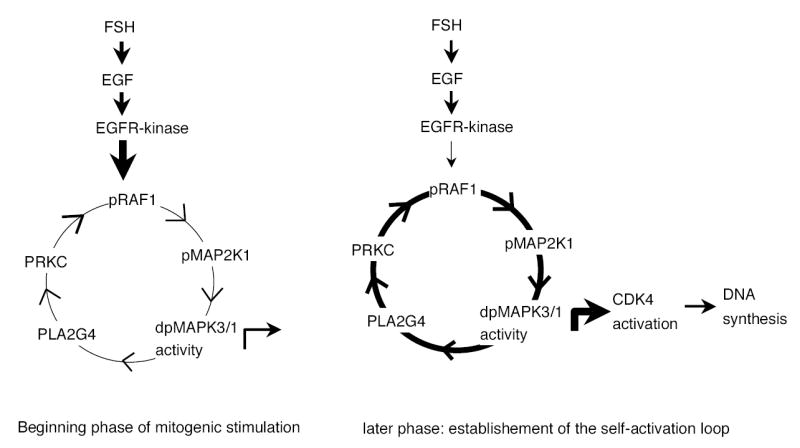
A model depicting the establishment of a self-sustaining MAPK3/1 activation loop for CDK4 activation and DNA synthesis in granulosa cells of hamster preantral follicles. FSH, likely via EGF, activates EGFR kinase that phosphorylates MAPK3/1 by sequential activation of RAF1 and MAP2K1. Active MAPK3/1 stimulates PKC via PLA2G4 and sets the loop in motion. After 2h of exposure to FSH or EGF, the activation loop becomes independent of the receptor kinase and sustains MAPK3/1 activity for at least 6h resulting in CDK4 activation and DNA synthesis.
Acknowledgments
This work was supported by grants HD28165 to SKR from the National Institute of Child Health and Human Development. Peixin Yang was a Lalor Foundation post-doctoral fellow.
References
- 1.Yang P, Roy SK. Follicles stimulating hormone-induced DNA synthesis in the granulosa cells of hamster preantral follicles involves activation of cyclin-dependent kinase-4 rather than cyclin D2 synthesis. Biol Reprod. 2004;70:509–517. doi: 10.1095/biolreprod.103.023457. [DOI] [PubMed] [Google Scholar]
- 2.Blenis J. Signal transduction via the MAP kinases: Proceed at your own RSK. Proc. Natl. Acad. Sci USA. 1993;90:5889–5892. doi: 10.1073/pnas.90.13.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cameron MR, Foster JS, Bukovsky A, Wimalasena J. Activation of mitogen-activated protein kinases by gonadotropins and cyclic adenosine 5′- monophosphates in porcine granulosa cells. Biol Reprod. 1996;55:111–119. doi: 10.1095/biolreprod55.1.111. [DOI] [PubMed] [Google Scholar]
- 4.Roy SK, Greenwald GS. Mediation of follicle-stimulating hormone action on follicular deoxyribonucleic acid synthesis by epidermal growth factor. Endocrinology. 1991;129:1903–1908. doi: 10.1210/endo-129-4-1903. [DOI] [PubMed] [Google Scholar]
- 5.Roy SK, Harris SG. Antisense epidermal growth factor oligodeoxynucleotides inhibit follicle-stimulating hormone-induced in vitro DNA and progesterone synthesis in hamster preantral follicles. Mol Endocrinol. 1994;8:1175–1181. doi: 10.1210/mend.8.9.7838150. [DOI] [PubMed] [Google Scholar]
- 6.Gotoh Y, Nishida E. Signals for mesoderm induction. Roles of fibroblast growth factor (FGF)/mitogen-activated protein kinase (MAPK) pathway. Biochim Biophys Acta Rev Cancer. 1996;1288:F1–F7. doi: 10.1016/0304-419x(96)00009-1. [DOI] [PubMed] [Google Scholar]
- 7.Cowan KJ, Storey KB. Mitogen-activated protein kinases: new signaling pathways functioning in cellular responses to environmental stress. J Exp Biol. 2003;206:1107–1115. doi: 10.1242/jeb.00220. [DOI] [PubMed] [Google Scholar]
- 8.Garnett K, Wang J, Roy SK. Spatiotemporal expression of epidermal growth factor receptor messenger RNA and protein in the hamster ovary: follicle stage-specific differential modulation by follicle-stimulating hormone, luteinizing hormone, estradiol, and progesterone. Biol Reprod. 2002;67:1593–1604. doi: 10.1095/biolreprod.102.005470. [DOI] [PubMed] [Google Scholar]
- 9.Bhalla US, Ram PT, Iyengar R. MAP kinase phosphatase as a locus of flexibility in a mitogen-activated protein kinase signaling network. Science. 2002;297:1018–1023. doi: 10.1126/science.1068873. [DOI] [PubMed] [Google Scholar]
- 10.Seger R, Hanoch T, Rosenberg R, Dantes A, Merz WE, Strauss JF, Amsterdam A. The ERK signaling cascade inhibits gonadotropin-stimulated steroidogenesis. J Biol Chem. 2001;276:13957–13964. doi: 10.1074/jbc.M006852200. [DOI] [PubMed] [Google Scholar]
- 11.Bast JD, Greenwald GS. Serum profiles of follicle-stimulating hormone, luteinizing hormone and prolactin during the estrous cycle of the hamster. Endocrinology. 1974;94:1295–1299. doi: 10.1210/endo-94-5-1295. [DOI] [PubMed] [Google Scholar]
- 12.Roy SK, Greenwald GS. An enzymatic method for dissociation of intact follicles from the hamster ovary: histological and quantitative aspects. Biol Reprod. 1985;32:203– 215. doi: 10.1095/biolreprod32.1.203. [DOI] [PubMed] [Google Scholar]
- 13.Roy SK, Greenwald GS. Hormonal requirements for the growth and differentiation of hamster preantral follicles in long-term culture. J Reprod Fertil. 1989;87:103–114. doi: 10.1530/jrf.0.0870103. [DOI] [PubMed] [Google Scholar]
- 14.Roy SK. Regulation of follicular development: Beyond gonadotropins. In: Jay KP, Krishna A, Halder C (eds.), Comparative Endocrinology and Reproduction. New Delhi: Narosa Publishing House; 1999: 315–330.
- 15.Roy SK, Greenwald GS. In Vitro effects of follicle-stimulating hormone, luteinizing hormone, and prolactin on follicular deoxyribonucleic acid synthesis in the hamster. Biol Reprod. 1988;122:952–958. doi: 10.1210/endo-122-3-952. [DOI] [PubMed] [Google Scholar]
- 16.Roy SK, Greenwald GS. Quantitative analysis of in-vitro incorporation of [3H]thymidine into hamster follicles during the oestrous cycle. J Reprod Fertil. 1986;77:143–152. doi: 10.1530/jrf.0.0770143. [DOI] [PubMed] [Google Scholar]
- 17.Schafer KA. The cell cycle: a review. Vet Pathol. 1998;35:461–478. doi: 10.1177/030098589803500601. [DOI] [PubMed] [Google Scholar]
- 18.Matsushime H, Quelle DE, Shurtleff SA, Shibuya M, Sherr CJ, Kato JY. D-type cyclin-dependent kinase activity in mammalian cells. Mol Cell Biol. 1994;14:2066–2076. doi: 10.1128/mcb.14.3.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nahas N, Waterman WH, Sha'afi RI. Granulocyte-macrophage colony-stimulating factor (GM-CSF) promotes phosphorylation and an increase in the activity of cytosolic phospholipase A2 in human neutrophils. Biochem J. 1996;313 ( Pt 2):503–508. doi: 10.1042/bj3130503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chabot JG, St Arnaud R, Walker P, Pelletier G. Distribution of epidermal growth factor receptors in the rat ovary. Mol Cell Endocrinol. 1986;44:99–108. doi: 10.1016/0303-7207(86)90051-1. [DOI] [PubMed] [Google Scholar]
- 21.Greenwald GS. Follicular activity in the mammalian ovary. In: Jones RE (ed.) The Vertebrate Ovary: Plenum Publishing Corporation; 1978: 639–689.
- 22.Pages G, Lenormand P, L'Allemain G, Chambard JC, Meloche S, Pouyssegur J. Mitogen-activated protein kinases p42mapk and p44mapk are required for fibroblast proliferation. Proc Natl Acad Sci U S A. 1993;90:8319–8323. doi: 10.1073/pnas.90.18.8319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bunone G, Briand PA, Miksicek RJ, Picard D. Activation of the unliganded estrogen receptor by EGF involves the MAP kinase pathway and direct phosphorylation. EMBO J. 1996;15:2174–2183. [PMC free article] [PubMed] [Google Scholar]
- 24.Yang P, Roy SK. Epidermal growth factor modulates transforming growth factor receptor messenger RNA and protein levels in hamster preantral follicles in vitro. Biol Reprod. 2001;65:847–854. doi: 10.1095/biolreprod65.3.847. [DOI] [PubMed] [Google Scholar]
- 25.Ignar-Trowbridge DM, Pimentel M, Teng CT, Korach KS, McLachlan JA. Cross talk between peptide growth factor and estrogen receptor signaling systems. Environ Health Perspect. 1995;103 (Suppl 7):35–38. doi: 10.1289/ehp.95103s735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biswas DK, Cruz AP, Gansberger E, Pardee AB. Epidermal growth factor-induced nuclear factor kB activation: a major pathway of cell-cycle progression in estrogen- receptor negative breast cancer cells. Proc Natl Acad Sci USA. 2000;97:8542–8547. doi: 10.1073/pnas.97.15.8542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leserer M, Gschwind A, Ullrich A. Epidermal growth factor receptor signal transactivation. IUBMB Life. 2000;49:405–409. doi: 10.1080/152165400410254. [DOI] [PubMed] [Google Scholar]
- 28.Klambt C. EGF receptor signalling: the importance of presentation. Curr Biol. 2000;10:R388–R391. doi: 10.1016/s0960-9822(00)00485-1. [DOI] [PubMed] [Google Scholar]
- 29.Bhalla US, Iyengar R. Emergent properties of networks of biological signaling pathways. Science. 1999;283:381–387. doi: 10.1126/science.283.5400.381. [DOI] [PubMed] [Google Scholar]
- 30.Ben-Shlomo I, Kol S, Ando M, Altman KR, Putowski LT, Rohan RM, Adashi EY. Ovarian expression, cellular localization, and hormonal regulation of rat secretory phospholipase A2: increased expression by interleukin-1 and by gonadotropins. Biol Reprod. 1997;57:217–225. doi: 10.1095/biolreprod57.2.217. [DOI] [PubMed] [Google Scholar]
- 31.Jannini EA, Ulisse S, Cecconi S, Cironi L, Colonna R, D'Armiento M, Santoni A, Cifone MG. Follicle-stimulating hormone-induced phospholipase A2 activity and eicosanoid generation in rat Sertoli cells. Biol Reprod. 1994;51:140–145. doi: 10.1095/biolreprod51.1.140. [DOI] [PubMed] [Google Scholar]
- 32.Gschwind A, Zwick E, Prenzel N, Leserer M, Ullrich A. Cell communication networks: epidermal growth factor receptor transactivation as the paradigm for interreceptor signal transmission. Oncogene. 2001;20:1594–1600. doi: 10.1038/sj.onc.1204192. [DOI] [PubMed] [Google Scholar]