Abstract
Unlike most other endogenous messengers that are deposited in vesicles, processed on demand and/or secreted in a regulated fashion, NO (nitric oxide) is a highly active molecule that readily diffuses through cell membranes and thus cannot be stored inside the producing cell. Rather, its signalling capacity must be controlled at the levels of biosynthesis and local availability. The importance of temporal and spatial control of NO production is highlighted by the finding that differential localization of NO synthases in cardiomyocytes translates into distinct effects of NO in the heart. Thus NO synthases belong to the most tightly controlled enzymes, being regulated at transcriptional and translational levels, through co- and post-translational modifications, by substrate availability and not least via specific sorting to subcellular compartments, where they are in close proximity to their target proteins. Considerable efforts have been made to elucidate the molecular mechanisms that underlie the intracellular targeting and trafficking of NO synthases, to ultimately understand the cellular pathways controlling the formation and function of this powerful signalling molecule. In the present review, we discuss the mechanisms and triggers for subcellular routing and dynamic redistribution of NO synthases and the ensuing consequences for NO production and action.
Keywords: acylation cycle, differential activation, intracellular trafficking, nitric oxide synthase, protein–protein interaction, subcellular targeting
Abbreviations: [Ca2+]i, intracellular Ca2+ concentration; CaM, calmodulin; EBP50, ezrin/radixin/moesin-binding phosphoprotein-50; EC, endothelial cell; Hsp90, heat-shock protein 90; LDL, low-density lipoprotein; NMDA, N-methyl-D-aspartate; NOS, nitric oxide synthase; eNOS, endothelial NOS; iNOS, inducible NOS; nNOS, neuronal NOS; NOSIP, NOS-interacting protein; NOSTRIN, NOS trafficking inducer; PECAM, platelet/endothelial cell-adhesion molecule; PI3K, phosphoinositide 3-kinase; PM, plasma membrane; PSD, post-synaptic density; sGC, soluble NO-sensitive guanylate cyclase; VEGF, vascular endothelial growth factor
INTRODUCTION
Small and swift, but certainly not soft: NO (nitric oxide) is a hyper-reactive radical that is nearly ubiquitous, truly pleiotropic and often ambivalent in its actions. Such multi-talented molecules need close surveillance and tightly controlled synthesis, and NO is no exception to this rule. In mammalian species, three types of NOSs (NO synthases) orchestrate the production of NO from L-arginine, i.e. nNOS (neuronal NOS), iNOS (inducible NOS) and eNOS (endothelial NOS). This NOS trio shares similar structures and catalytic modes, yet the mechanisms that control their activity in time and space are quite diverse. Above all, expression of iNOS is induced by inflammatory stimuli, while eNOS and nNOS are more or less constitutively expressed [1]. A number of variables collectively dictate the amount of NO that is produced by cells, including the availability of substrates such as L-arginine, NADPH and tetrahydrobiopterin, cofactors such as FAD and FMN, and protein–protein interactions such as NOS dimerization and association with PSD-95 (post-synaptic density 95), caveolins and CaM (calmodulin) [2,3]. All three isoenzymes bind to CaM, which is required for maximum activity. However, only nNOS and eNOS do so in a strictly Ca2+-dependent manner, whereas iNOS forms an active complex with CaM even when intracellular Ca2+ levels are low [1]. In addition, the activity of all three isoforms is regulated by complex phosphorylation and dephosphorylation events that differ substantially among the various NOS types [4].
Finally, another layer of regulation is provided by the subcellular distribution of the NOS isoenzymes. The notion that the intracellular location of NOS is critical for the coupling of extracellular signals to efficient NO production is based on the initial observations that NOS molecules are not dispersed randomly throughout the cytosol, but are in fact located within distinct subcellular structures and that misrouted NOS mutants display reduced activity [5,6]. These findings have prompted important questions as to how subcellular targeting has an impact on NOS activity. At present, the mechanisms that underlie targeting, sequestration and trafficking of NOS are not fully understood, and the (patho)physiological consequences of their distinct subcellular distributions are still largely hypothetical. In this review, we summarize our present knowledge of the intracellular targeting of the different NOS isoforms, present the mechanisms involved in NOS translocation and critically discuss the potential implications of differential NOS distribution for NO production.
MECHANISMS FOR NOS TARGETING
All three NOS isoforms are targeted to distinct subcellular locations, albeit through different mechanisms. Lipid modification and protein–protein interactions are proven strategies to establish and maintain the spatial organization of NOS within a cell.
Membrane anchorage through lipid modification
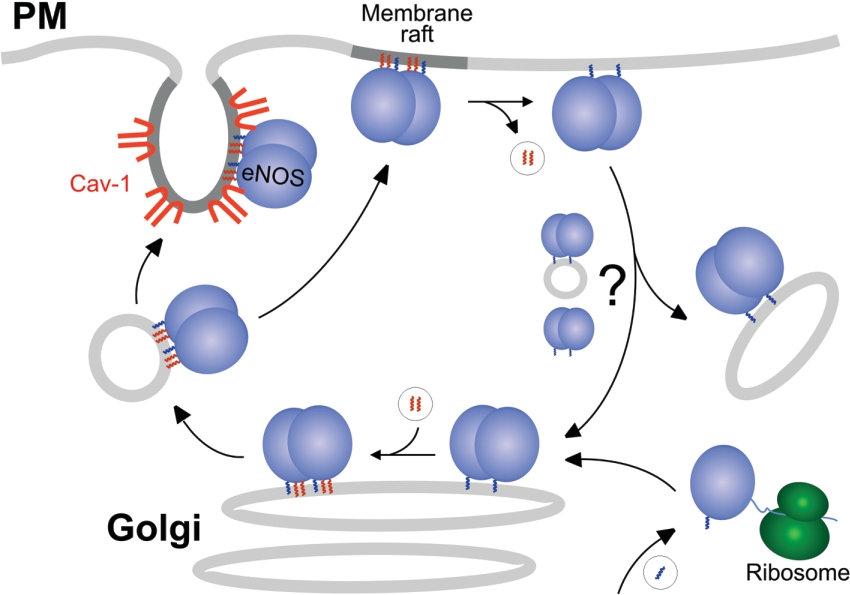
Prominent compartments for eNOS are the PM (plasma membrane) and the Golgi apparatus. A two-step process targets eNOS to these membranes (Figure 1): myristoylation of a glycine residue (Gly-2) at the very N-terminus of eNOS provides general membrane association, whereas dual palmitoylation of N-terminally located cysteine residues (Cys-15 and Cys-26) targets eNOS to the PM, where it resides partially in caveolae (see below). Myristoylation of eNOS occurs co-translationally, is essentially irreversible and is a prerequisite for its subsequent palmitoylation. Accordingly, myristoylation-deficient eNOS mutants are distributed diffusely in the cytosol [7–10]. In general, myristoylation is catalysed by two types of N-myristoyltransferases that can be targeted to distinct cellular locations [11]. For eNOS, this process has not been investigated in detail. Palmitoylation of eNOS is a reversible process and is likely to be carried out post-translationally at the Golgi complex [7,8]. However, from the trafficking behaviour of an eNOS–CD8 membrane-spanning chimaera, it has been concluded that palmitoylation might also take place at the PM [12]. Typically, palmitoylation can occur in non-enzymatic fashion in vitro [13], but the recent identification of palmitoyl acyltransferases in yeast and mammals [14] suggests that enzyme-mediated palmitoylation is important in vivo.
Figure 1. Putative acylation cycle of eNOS trafficking between plasma and Golgi membranes.
The anterograde transport (left-hand side) is thought to be vesicular, while the nature of the retrograde transport (right-hand side) is still unknown. Myristoyl residues are shown in blue, and palmitoyl residues are in red. Cav-1, caveolin-1.
Recently, it has been shown that (at least a fraction of) iNOS is palmitoylated, but not myristoylated, and targeted to the PM [15]. iNOS palmitoylation occurs both in lipopolysaccharide-challenged myocytes and after iNOS overexpression, and appears to be necessary for proper passage of the enzyme through the Golgi network as well as for PM targeting and NO production, although it does not mediate specific localization to caveolae [15]. In addition, association of iNOS with caveolin lowers the stability of the enzyme, thereby controlling NO release [16]. Accordingly, induction of iNOS can be accompanied by down-regulation of caveolin protein levels [17].
At present, the mechanisms governing anterograde transport of acylated NOS from the Golgi to the PM are incompletely understood. Given that other dually acylated proteins such as myristoylated/palmitoylated Src kinases and heterotrimeric Gαi [18], as well as farnesylated/palmitoylated Ras [14], undergo vectorial transport from the Golgi to the PM, it is tempting to speculate that NOS molecules use similar routes to reach their final destination at the cytosolic leaflet of the PM (Figure 1, left-hand side). In the case of eNOS, the role of caveolin in anterograde transport and caveolar targeting is still debated. Both eNOS and caveolin reside to a considerable extent at the Golgi [19], and some studies have indicated that palmitoylation may facilitate the interaction of eNOS with caveolin [20,21]. This could suggest a caveolin-assisted anterograde transport. However, it has been challenged that eNOS and caveolin meet at the same Golgi subcompartment [22] and that palmitoylation increases the eNOS–caveolin interaction [12]. Moreover, at the PM, a considerable fraction of eNOS is localized outside caveolae, for example at lamellipodia [23,24] and possibly non-invaginated lipid rafts [25]. Finally, depalmitoylation may initiate the retrograde transport of NOS back from the PM to the Golgi (Figure 1, right-hand side), closing the putative acylation–deacylation cycle (see below).
Subcellular targeting by protein–protein interactions
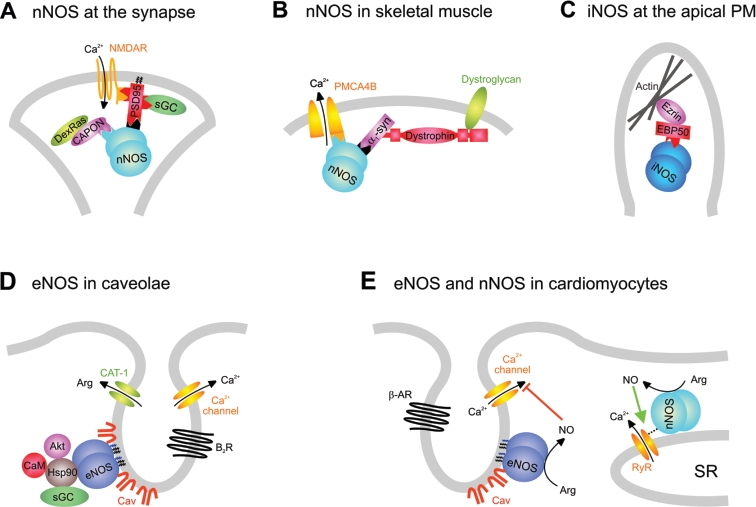
Unlike the other two isoforms, nNOS appears to lack acylation and therefore uses other targeting mechanisms [26]. Rather, nNOS is recruited to membranes such as the PSD or the sarcolemma via protein–protein interactions involving its unique N-terminal PDZ domain which is absent from the other NOS isoforms (Figures 2A and 2B) [4]. This PDZ domain carries two distinct motifs, i.e. a consensus and a near-consensus (‘pseudo-peptide’) sequence [27] that can bind to the PDZ domain of adaptor molecules such as PSD-95 in neurons or α1-syntrophin in myocytes [28,29]. PSD-95 itself is targeted to the post-synaptic membrane by palmitoylation [30] and serves as a multivalent scaffolding protein important for protein clustering, e.g. with the NMDA (N-methyl-D-aspartate) receptor [31]. Through its PDZ domain, nNOS can also bind to the adaptor protein CAPON (C-terminal PDZ ligand of nNOS) [32], which in turn mediates interaction with members of the synapsin family that are associated with synaptic vesicles and involved in their exocytosis. Synapsin I- and II-knockout mice display an altered cellular nNOS distribution, suggesting an important role of synapsin in nNOS localization [32]. In skeletal muscle, nNOS utilizes the PDZ-containing protein α1-syntrophin for membrane association (Figure 2B). This protein recruits nNOS as well as other proteins to the dystrophin–dystroglycan complex at the sarcolemma [33], and deficiency of α1-syntrophin or dystrophin, as in Duchenne muscular dystrophy, results in redistribution of nNOS from the sarcolemma to the cytosol [34,35]. Similarly, nNOS interacts through its PDZ domain with the Ca2+-efflux pump PMCA4b (PM Ca2+-ATPase 4b) at caveolae of different cell types [36]. The targeting mechanism for nNOS at the sarcoplasmic reticulum of cardiomyocytes (cf. Figure 2E) is still unknown, but might involve the interaction with ryanodine receptor-1 [37].
Figure 2. Association of NOS isoforms with various biological membranes.
(A, B) nNOS at the synapse (A) and at the sarcolemma (B) of skeletal muscle. (C) iNOS at the epithelial membrane. (D) eNOS at the endothelial membrane. (E) Differential distribution of eNOS and nNOS at the cardiomyocyte membranes. α1-Syn, α1-syntrophin; Akt, protein kinase B/Akt; β-AR, β-adrenergic receptor; B2R, bradykinin B2 receptor; CaM, Ca2+/calmodulin; CAT, cationic amino acid transporter; Cav, caveolin-1/-3; NMDAR, NMDA receptor; RyR, ryanodine receptor-1; SR, sarcoplasmic reticulum.
A similar picture evolves for the compartmentalization of iNOS and eNOS (Figures 2C–2E). In epithelial cells, iNOS localizes to the apical domain in a submembranous protein complex linked tightly to cortical actin [38]. iNOS is recruited to this complex by binding to the PDZ-containing protein EBP50 (ezrin/radixin/moesin-binding phosphoprotein-50), which in turn is linked to cortical actin via ezrin [39]. Like PSD-95, EBP50 harbours multiple PDZ domains, implicating it in protein clustering (Figure 2C).
eNOS resides in caveolae, which represent sites of clustered signal-transduction networks (see below), reminiscent of the situation of nNOS at the PSD. eNOS directly binds to caveolin-1 in endothelial cells (ECs) (Figure 2D) [20] and, additionally, to caveolin-3 at the sarcolemma of cardiomyocytes (Figure 2E) [40]. Finally, eNOS has been found to be associated with the cytoskeleton and cell–cell contacts [41–45]. A direct interaction with actin has been reported [44], but the precise targeting mechanism is unclear. A protein–protein interaction with PECAM (platelet/endothelial cell-adhesion molecule) resident at cell–cell contacts might contribute to this particular localization of eNOS, since peripheral membrane staining of eNOS is lost in aortic endothelium of PECAM-knockout mice [41]. eNOS has also been found in association with mitochondria [46], and even the nucleus [4], although the targeting mechanisms remain elusive.
FUNCTIONAL CONSEQUENCES OF SUBCELLULAR TARGETING
A hypothesis has been put forward that, within the cell, there are compartments that allow for full activation of NOS and other locales where NOS cannot be efficiently activated. Such ‘active’ compartments may be defined by free access to substrates and cofactors, as well as the presence of upstream activators. Targeting of NOS into these active compartments can be considered a prerequisite for efficient NO production, e.g. at cell boundaries.
NO production in different subcellular compartments
Previous studies have shown that eNOS activity in ECs is higher in confluent than in subconfluent cell cultures and that this higher activity is correlated with a greater fraction of total cellular eNOS residing at the PM [42]. Other studies have shown that palmitoylation-deficient mutants of eNOS (C15S, C26S and C15S/C26S) which fail to reach the PM [7,10,47] produce less NO in the intact cell, although they fully retain their enzymatic activity in vitro [47], and that the highest eNOS activity is found at the PM [48]. Collectively, these findings suggest that the ‘correct’ subcellular localization of eNOS is critical for NO production and that its close proximity to the PM is optimal for the efficient synthesis of NO (Table 1).
Table 1. Relative NO-producing activities of NOS in various cellular compartments.
| NOS isoform | Subcellular location | Activity | Reference(s) |
|---|---|---|---|
| eNOS | PM | High | [42,49,90] |
| eNOS | Cytosol | Low | [5,115] |
| eNOS | Golgi (cis) | Moderate | [49] |
| eNOS | Golgi (trans) | Low | [49] |
| eNOS | Nucleus | Very low | [50] |
| eNOS | Mitochondrion | Low | [50] |
| eNOS | Cytoskeleton (actin) | Low | [43,44] |
| eNOS | Cytoskeleton (tubulin) | High | [51] |
| iNOS | Peroxisome | Unknown | [61] |
| iNOS | PM | High | [50] |
| iNOS | Nucleus | High | [50] |
| iNOS | Mitochondrion | High | [50] |
In addition to the PM, eNOS is present in multiple other locations where the regulation of its activity is not well understood and experimentally difficult to tackle. To overcome these inherent problems, the targeting of the non-myristoylated G2A mutant of eNOS to various intracellular compartments has offered a unique window into how eNOS activity is regulated within different intracellular locations. Indeed, the specific targeting of eNOS to the PM has confirmed the pre-eminence of this location as a place to produce NO [49]. These studies also showed that targeting of eNOS to intracellular organelles such as the Golgi results in an enzyme that is actually functional and therefore contributes to the total cellular pool of NO, albeit at a lower level compared with the PM (Table 1). The targeting of eNOS to other locations such as the nucleus or within the mitochondrial matrix, however, resulted in a greatly reduced capacity to produce NO [50]. Cytoskeletal proteins such as actin filaments or microtubules may also play a role in the regulation of eNOS activity [44,51]. However, it remains to be shown whether interaction of eNOS with the cytoskeleton has direct consequences for its activity in intact cells.
Molecular constraints affecting NOS activity
While it is clear that subcellular location has a profound effect on eNOS activity, the molecular constraints affecting NO production in the different compartments are largely unknown. The targeting of Ca2+-independent iNOS to the cellular compartments that display either high or low eNOS activity has provided new insights into these mechanisms [50]. Whether present at the PM, mitochondria, Golgi or cytosol, the activity of iNOS and production of NO is invariant. As the substrate and cofactor requirements for eNOS and iNOS are virtually identical, these results largely exclude the spatial restriction of substrate and cofactors as variables that limit eNOS activity (although this is disputed, cf. the ‘arginine paradox’ [52]). By exclusion, this positions Ca2+/CaM as the prime candidate. A number of studies have demonstrated the presence of Ca2+ gradients within cells. The highest intracellular Ca2+ concentrations ([Ca2+]i) are detected in the submembranous space, which is also consistent with the area of highest eNOS activity [53]. However, most of these measurements have only recorded the brief capacitive Ca2+ influx (seconds) and have not looked at whether this difference is maintained during the time frame of increased NOS activity (5–10 min) [54]. Interestingly, Ca2+-dependent activation of eNOS has been reported to trigger its association with Hsp90 (heat-shock protein 90) and activated Akt, leading to eNOS phosphorylation and sustained activation, independently of maintained high [Ca2+]i [55].
According to these studies, the presence of Ca2+ and the action of Ca2+/CaM are important features of an ‘active’ NOS compartment. However, differences in [Ca2+]i cannot solely account for the varied activity of eNOS throughout the cell, but changes in Ca2+ sensitivity can. Notably, the Ca2+-sensitivity of eNOS is controlled to a large part by changes in the phosphorylation state of key serine residues. The specificity of protein kinases is achieved by both temporal and spatial constraints and therefore it is not surprising that the phosphorylation state of eNOS is heavily dependent on its intracellular location [49,56,57]. The cytosolic non-acylated eNOS mutant is hypophosphorylated on serine residues (positions 617, 635 and 1179 of the bovine isoform) when compared with eNOS at the Golgi or the PM. The degree of phosphorylation correlates directly with the ability of these constructs to produce NO, with the greatest activity seen in the extensively phosphorylated constructs. Mutation of the key serine to non-phosphorylatable alanine residues equalizes activity and nullifies the importance of location [58]. Phosphorylation of Ser-617, Ser-635 and Ser-1179 enhances the sensitivity of eNOS to Ca2+/CaM and may therefore account for the differences in eNOS activity in the different parts of the cell [59,60].
Little is known about how subcellular location influences the activity of other NOS isoforms. A number of the important eNOS phosphorylation sites are conserved on nNOS, and nNOS is also targeted to distinct subcellular structures (see above). The influence of these locations on the ability of nNOS to produce NO is not known. Although intracellular location is considered to have little influence on the activity of iNOS itself (see above), in some cells types targeting of iNOS to the PM appears to be necessary for efficient NO production [15,61,62].
Functional consequences of spatial NOS organization
What is the biological goal of producing a freely diffusible gas within specific intracellular locations? Spatial constriction in the cell periphery might be essential for delivering NO to targets outside the NO-generating cell. For example, NO produced by nNOS at the PSD diffuses to the pre-synaptic cell (retrograde signalling) and eNOS-derived NO in ECs affects platelets and adjacent smooth-muscle cells [26,63]. In addition, eNOS present at cell–cell contacts may be essential for local regulation of endothelial permeability [64]. Likewise, NO produced by nNOS within the dystrophin complex at the skeletal-muscle sarcolemma dilates adjacent blood vessels, and mutation of dystrophin in muscular dystrophy results in loss of peripheral nNOS and impairment of blood flow [65]. In all of these examples, the biological effect is mediated by the activation of sGC (soluble NO-sensitive guanylate cyclase) and the consequent production of cGMP. The binding of NO to sGC is an extremely efficient and specific reaction [66] that requires only a small amount of NO, explaining why the NO signal is transmitted so efficiently to adjacent cells. Also, NOS localization at the cell periphery might help to protect the host cell from damage induced by NO. This is most relevant for epithelial cells, where iNOS targeted to the EBP50 complex produces excessive amounts of NO specifically at the apical cell membrane (whereas virtually no NO is released at the basolateral side), thereby building a first line of defence against pathogens [67].
Another important consequence of spatial NOS organization is the efficient coupling of NO production to upstream signals. Tethering of nNOS to the NMDA receptor via PSD-95 (see above) underlies the ‘source specificity hypothesis’, which states that Ca2+ influx through the NMDA receptor is especially neurotoxic, most likely through excessive NO production via Ca2+ influx [28,68,69]. Accordingly, depletion of PSD-95 [70] or inhibition of PSD-95–nNOS complex formation attenuates excitotoxic cell death [71].
In caveolae, association of eNOS with caveolin-1 leads to inhibition of the enzyme, and mice lacking caveolin-1 have increased eNOS activity [72]. Proteins of the NO signalling pathway clustering in caveolae include arginine succinate synthase and lyase, arginine and Ca2+ channels as well as bradykinin and acetylcholine receptors [4], providing access to upstream signals and substrates (see Figure 2D). Hsp90, also present in caveolae, is thought to facilitate signal transduction by bringing eNOS into close proximity to its upstream activators, Ca2+/CaM and protein kinase B/Akt [73–75], as well as to its downstream target sGC [76]. At the sarcolemma of cardiomyocytes, caveolae contain muscarinic acetylcholine and β-adrenergic receptors [77–79] signalling through Ca2+ mobilization, as well as L-type Ca2+ channels that are inhibited by NO via a negative-feedback mechanism (Figure 2E). Of note, NO generated by nNOS at the sarcoplasmic reticulum of cardiomyocytes evokes opposing effects on contractility, highlighting the fundamental importance of subcellular localization for NO signalling specificity [37].
Numerous biological functions of NO are independent of cGMP and rely on higher concentrations of NO, making the controlled production of a reactive and diffusible gas within a confined space a viable strategy. One such paradigm is the reversible nitrosylation of proteins (Table 2). Interestingly, eNOS nitrosylates itself, thereby down-regulating its own enzymatic activity [80,81]. The degree of nitrosylation varies with its subcellular location: membrane-targeted eNOS exhibits much greater nitrosylation than cytosolic eNOS, and the transient translocation of eNOS from membranes to the cytosol may facilitate denitrosylation [82]. Also eNOS-associated proteins become nitrosylated, such as Hsp90, resulting in a reduction of its positive effect on eNOS activity [83]. In the case of dynamin, which interacts with both eNOS and NOSTRIN (NOS trafficking inducer) (see below), nitrosylation enhances dynamin-mediated internalization processes [84]. NMDA receptors that bind indirectly to nNOS are nitrosylated as well, resulting in reduced Ca2+ influx [85]. Another example for a functional signal cluster is the complex of nNOS and DexRas via the adaptor CAPON in neurons, which is necessary for the NO-dependent activation of DexRas and subsequent modulation of transcription [26,86]. Thus spatial proximity of NOS signalling complexes is crucial to facilitate nitrosylation and to provide important feedback mechanisms, supporting the concept that NOS has to go where NO is needed.
Table 2. Subcellular location of confirmed targets for NOS-dependent nitrosylation.
DYNAMIC REDISTRIBUTION OF NOS: TRIGGERS, MECHANISMS AND CONTROL
Targeting of NO synthases is not unidirectional. For instance, upon stimulation of ECs, eNOS is sequestered from the PM and translocated to interior compartments of the cell. At present, the mechanisms and driving forces that underlie intracellular redistribution of NOS have best been studied for eNOS, so we will focus on this isoform.
Mechanisms of eNOS redistribution: the acylation cycle
The distribution of eNOS appears to vary among different cell types and even within a given cell. The former is illustrated by blood vessels of various calibres, where ECs of larger arteries keep their eNOS strictly at the Golgi, whereas those of the endocardium display a substantial amount of eNOS at the PM [87].
The observation that the turnover of palmitate residues attached to eNOS is more rapid than that of the protein itself has pointed to the possibility that eNOS may undergo a cycle of depalmitoylation and repalmitoylation, translating into a constant shuttling of the enzyme between Golgi and PM [7]. Such an acylation cycle has recently been described for the Ras oncogene [88]. For both eNOS and Ras, this cycle comprises two types of protein modifications, i.e. irreversible acylation (myristoylation of eNOS and farnesylation of Ras) as well as reversible palmitoylation (Figure 1). While the former provides general membrane association, the latter directs proteins specifically to the PM. According to this acylation cycle model, proteins become depalmitoylated at the PM and are consequently displaced from this locale and redistributed indiscriminately to other intracellular membranes. Because (re)palmitoylation occurs specifically at the Golgi, this compartment provides a kinetic trap from which the proteins are targeted again to the PM [88,89]. Thus the acylation cycle provides both the transfer of active proteins from the PM to the Golgi and the continuous access to the sorting machinery of the Golgi, allowing redistribution to distinct membrane microdomains.
At present, the nature of depalmitoylation and the possible involvement of specific thioesterases are still unclear. Palmitoylation and depalmitoylation rates of eNOS in ECs were shown to be independent of stimuli such as bradykinin or ionomycin [90], suggesting a constitutive process as seen for Ras [88]. However, agonist-induced depalmitoylation has also been reported [91]. Palmitoyl-protein thioesterase and acyl-protein thioesterase-1 have been implicated in eNOS depalmitoylation, although the consequences for its subcellular localization remain to be demonstrated [92,93]. To date, it is unclear whether the retrograde traffic of eNOS is brought about by molecular or vesicular transport. Protein–protein interactions might contribute to the intracellular redistribution of eNOS and might involve the recently identified proteins NOSIP (NOS-interacting protein) and NOSTRIN (see below), as well as the co-chaperone CHIP (C-terminus of heat-shock cognate 70-interacting protein) [94]. In the case of Ras, the retrograde transport from the PM to the Golgi is probably non-vesicular and may require carrier proteins such as the δ subunit of phosphodiesterase, accommodating the lipid anchor of its acylated cargo protein [95,96].
In addition to constant shuttling, eNOS localization may be regulated dynamically. For ECs, an important factor that determines eNOS location appears to be the plasma concentration of LDL (low-density lipoprotein)/cholesterol. Short-term incubation of porcine arterial ECs with oxidized LDL results in decreased cholesterol content of the PM and in the loss of eNOS from the PM, possibly due to caveolar disintegration [97]. The ensuing eNOS redistribution reduces acetylcholine-stimulated activity, presumably due to absence of the enzyme from the ‘active’ compartment. Prolonged exposure of human umbilical-vein ECs to oxidized LDL leads to redistribution of eNOS without concomitant changes in the cholesterol content of the PM and to an attenuated thrombin-stimulated activity of the enzyme [98]. In either case of treatment with oxidized LDL, myristoylation and palmitoylation of eNOS are unaffected, suggesting that the enzyme remains associated with membranes. Umbilical-vein ECs treated with native LDL display a higher proportion of caveolin-1 and eNOS at the PM and stronger association of the two proteins, resulting in a decreased basal activity and a strongly reduced activity after Ca2+ ionophore stimulation [99]. Thus a well-balanced cholesterol supply appears to influence the localization of eNOS within ECs.
Triggers for eNOS redistribution
Several stimuli can induce a transient displacement of eNOS from the PM. When subconfluent bovine arterial ECs are exposed to stimulating agents such as bradykinin, oestradiol or ceramide, the majority of the eNOS molecules leave the PM for a short while, and, after 1 h, the original situation is more or less restored [100–102]. In case of bradykinin, this could be due to the decrease in the eNOS–bradykinin B2 receptor interaction [103]. None of these studies has, however, monitored the state (e.g. acylation or phosphorylation) of eNOS during translocation, and the question of the whereabouts of eNOS during its absence from the PM has remained unanswered. Similar observations were made using confluent layers of umbilical-vein ECs treated with bradykinin or VEGF (vascular endothelial growth factor), demonstrating that eNOS and caveolin-1 leave the PM shortly after stimulation [104]. This was accompanied by an increase of the eNOS signal in the perinuclear region as well as in vesicular structures throughout the cytoplasm after both bradykinin and VEGF stimulation. Interestingly, the time course of translocation differs for the two stimuli, with bradykinin creating a transient, and VEGF creating a sustained, translocation.
The location of eNOS following translocation was investigated in more detail by an in vivo study of the hamster cheek-pouch microcirculation [105]. Shortly after stimulation with acetylcholine, eNOS distribution had clearly shifted from the microsomal (PM) into the heavy membrane fraction, representing Golgi and possibly cytoskeletal components. The cytosolic fraction was only marginally enriched in eNOS after stimulation, and, shortly after acetylcholine application, eNOS started to return to the microsomal fraction. Another vivid example of eNOS translocation comes from renal epithelium [106,107]. Here, luminal flow induces redistribution of eNOS and Hsp90 from all over the cells towards the apical PM. This shift is accompanied by an increased NO production. Translocation and activation of eNOS are blocked by the Hsp90 inhibitor geldanamycin, the actin polymerization inhibitor cytochalasin D and the PI3K (phosphoinositide 3-kinase) inhibitor wortmannin. The suggested vesicular transport for eNOS is well in line with a role of PI3K in basolateral-to-apical trafficking of other proteins [108].
Mediators of eNOS redistribution
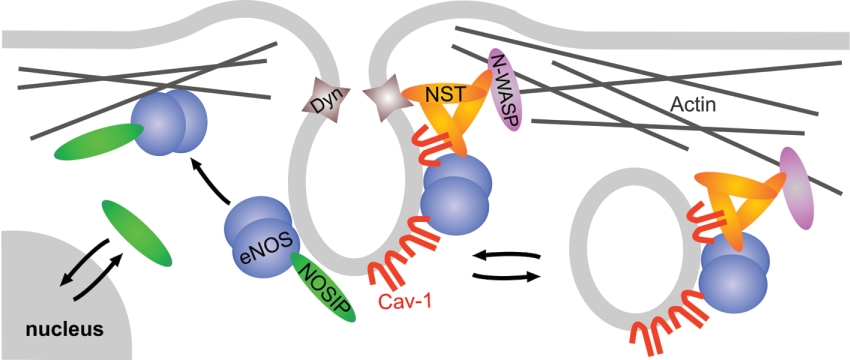
Recent studies involving the eNOS-interacting protein NOSTRIN have indicated that translocation of eNOS can be at least partially achieved by vesicular trafficking (Figure 3). Upon overexpression, NOSTRIN induces trafficking of eNOS away from the PM to intracellular vesicular structures, concomitant with a decrease in eNOS activity [109]. Further investigation showed that NOSTRIN serves as an oligomeric adaptor protein for the large GTPase dynamin and the Arp2/3 (actin-related protein 2/3 complex) activating protein N-WASP (neuronal Wiskott–Aldrich syndrome protein), suggesting a role in co-ordinating vesicle fission and transport [110,111]. NOSTRIN binds directly to caveolin-1 without affecting the ability of either of the two proteins to interact with eNOS, and thus allows for the formation of a ternary complex of eNOS, caveolin-1 and NOSTRIN (A. Icking, unpublished work). NOSTRIN is enriched at caveolar membranes and may be critical for caveolar transport of eNOS both in internalization and to the PM.
Figure 3. Potential roles of the eNOS-associated proteins NOSIP and NOSTRIN in the redistribution of eNOS from the PM.
NOSIP may target eNOS from its caveolar localization towards the actin cytoskeleton. In contrast, NOSTRIN may have a role in vesicular internalization of eNOS, probably involving a caveolar mechanism. Cav, caveolin; Dyn, dynamin; NST, NOSTRIN; N-WASP, neuronal Wiskott–Aldrich syndrome protein.
Unlike NOSTRIN, the eNOS-interacting protein NOSIP competes with the caveolin scaffolding domain for binding of eNOS. Accordingly, overexpression of NOSIP results in the dislocation of eNOS from the PM and inhibition of NO release [112]. NOSIP is a nucleocytoplasmic shuttling protein with predominantly nuclear localization in proliferating cells, but its localization is regulated dynamically, and NOSIP accumulates in the cytoplasm specifically during the G2 phase of the cell cycle. Detailed analysis has shown that this cytoplasmic accumulation, corresponding to NOSIP overexpression, mediates cytoskeletal targeting and inhibition of eNOS in a cell-cycle-dependent manner [43]. This highlights the notion that the actin cytoskeleton represents an ‘inactive’ compartment for eNOS and indicates that targeting into such inactive locales serves to regulate the overall activity of the enzyme. NOSIP also has inhibitory potential towards nNOS [113]. Although the exact mechanism of its action remains to be determined, it is likely to involve NOSIP-induced translocation of nNOS from distal dendrites towards the perikaryon. Interestingly, subcellular localization of NOSIP in hippocampal neurons varies with neuronal activity: it changes in favour of the cytoplasm after NMDA stimulation and towards the nuclear compartment when neuronal activity is silenced. The finding that nNOS activity may be regulated by subcellular targeting is supported by the observation that NMDA induces the translocation of nNOS from intracellular locales towards the PM in differentiated PC12 (pheocytochroma) cells [114].
CONCLUSIONS AND FUTURE PROSPECTS
Accumulating evidence indicates that NOSs are subject to specific targeting to subcellular compartments and that this translocation is crucial for specific nitrosylation of target proteins and proper functional performance. Yet our knowledge of the precise molecular mechanisms governing the intracellular redistribution processes is still rather limited. We anticipate that emerging techniques such as live-cell imaging and single-molecule tracking will be instrumental in shedding new light on the molecular details of NOS trafficking and reshuffling within the cell. It appears that NOS, very much like small GTPases such as Ras, beautifully exemplifies Nature's efforts to develop sophisticated machineries to keep the activity of its key enzymes under tight control. Future studies will unravel whether NOS trafficking uses cargo-specific vehicles or whether it utilizes ‘public’ transportation systems that are shared by other proteins cycling in the cell.
Acknowledgments
We apologize to all those colleagues whose important work could not be included because of space limitations. Our work is supported by grants from the Deutsche Forschungsgemeinschaft (S.O., A.I., W.M.-E.), the Netherlands Heart Foundation (grant 99.041 to R.G.), and the Netherlands Organization for Scientific Research (NWO grant 902-26-224 to R.G.).
References
- 1.Alderton W. K., Cooper C. E., Knowles R. G. Nitric oxide synthases: structure, function and inhibition. Biochem. J. 2001;357:593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fulton D., Gratton J. P., Sessa W. C. Post-translational control of endothelial nitric oxide synthase: why isn't calcium/calmodulin enough? J. Pharmacol. Exp. Ther. 2001;299:818–824. [PubMed] [Google Scholar]
- 3.Kone B. C. Protein–protein interactions controlling nitric oxide synthases. Acta Physiol. Scand. 2000;168:27–31. doi: 10.1046/j.1365-201x.2000.00629.x. [DOI] [PubMed] [Google Scholar]
- 4.Govers R., Oess S. To NO or not to NO: ‘where?’ is the question. Histol. Histopathol. 2004;19:585–605. doi: 10.14670/HH-19.585. [DOI] [PubMed] [Google Scholar]
- 5.Sakoda T., Hirata K., Kuroda R., Miki N., Suematsu M., Kawashima S., Yokoyama M. Myristoylation of endothelial cell nitric oxide synthase is important for extracellular release of nitric oxide. Mol. Cell. Biochem. 1995;152:143–148. doi: 10.1007/BF01076076. [DOI] [PubMed] [Google Scholar]
- 6.Sessa W. C., Barber C. M., Lynch K. R. Mutation of N-myristoylation site converts endothelial cell nitric oxide synthase from a membrane to a cytosolic protein. Circ. Res. 1993;72:921–924. doi: 10.1161/01.res.72.4.921. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Cardena G., Oh P., Liu J., Schnitzer J. E., Sessa W. C. Targeting of nitric oxide synthase to endothelial cell caveolae via palmitoylation: implications for nitric oxide signaling. Proc. Natl. Acad. Sci. U.S.A. 1996;93:6448–6453. doi: 10.1073/pnas.93.13.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu J., Hughes T. E., Sessa W. C. The first 35 amino acids and fatty acylation sites determine the molecular targeting of endothelial nitric oxide synthase into the Golgi region of cells: a green fluorescent protein study. J. Cell Biol. 1997;137:1525–1535. doi: 10.1083/jcb.137.7.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu J., Sessa W. C. Identification of covalently bound amino-terminal myristic acid in endothelial nitric oxide synthase. J. Biol. Chem. 1994;269:11691–11694. [PubMed] [Google Scholar]
- 10.Shaul P. W., Smart E. J., Robinson L. J., German Z., Yuhanna I. S., Ying Y., Anderson R. G., Michel T. Acylation targets endothelial nitric-oxide synthase to plasmalemmal caveolae. J. Biol. Chem. 1996;271:6518–6522. doi: 10.1074/jbc.271.11.6518. [DOI] [PubMed] [Google Scholar]
- 11.Farazi T. A., Waksman G., Gordon J. I. The biology and enzymology of protein N-myristoylation. J. Biol. Chem. 2001;276:39501–39504. doi: 10.1074/jbc.R100042200. [DOI] [PubMed] [Google Scholar]
- 12.Prabhakar P., Cheng V., Michel T. A chimeric transmembrane domain directs endothelial nitric-oxide synthase palmitoylation and targeting to plasmalemmal caveolae. J. Biol. Chem. 2000;275:19416–19421. doi: 10.1074/jbc.M001952200. [DOI] [PubMed] [Google Scholar]
- 13.Smotrys J. E., Linder M. E. Palmitoylation of intracellular signaling proteins: regulation and function. Annu. Rev. Biochem. 2004;73:559–587. doi: 10.1146/annurev.biochem.73.011303.073954. [DOI] [PubMed] [Google Scholar]
- 14.Magee T., Seabra M. C. Fatty acylation and prenylation of proteins: what's hot in fat. Curr. Opin. Cell Biol. 2005;17:190–196. doi: 10.1016/j.ceb.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Navarro-Lerida I., Corvi M. M., Barrientos A. A., Gavilanes F., Berthiaume L. G., Rodriguez-Crespo I. Palmitoylation of inducible nitric-oxide synthase at Cys-3 is required for proper intracellular traffic and nitric oxide synthesis. J. Biol. Chem. 2004;279:55682–55689. doi: 10.1074/jbc.M406621200. [DOI] [PubMed] [Google Scholar]
- 16.Felley-Bosco E., Bender F. C., Courjault-Gautier F., Bron C., Quest A. F. Caveolin-1 down-regulates inducible nitric oxide synthase via the proteasome pathway in human colon carcinoma cells. Proc. Natl. Acad. Sci. U.S.A. 2000;97:14334–14339. doi: 10.1073/pnas.250406797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Navarro-Lerida I., Portoles M. T., Barrientos A. A., Gavilanes F., Bosca L., Rodriguez-Crespo I. Induction of nitric oxide synthase-2 proceeds with the concomitant downregulation of the endogenous caveolin levels. J. Cell Sci. 2004;117:1687–1697. doi: 10.1242/jcs.01002. [DOI] [PubMed] [Google Scholar]
- 18.Resh M. D. Fatty acylation of proteins: new insights into membrane targeting of myristoylated and palmitoylated proteins. Biochim. Biophys. Acta. 1999;1451:1–16. doi: 10.1016/s0167-4889(99)00075-0. [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Cardena G., Fan R., Stern D. F., Liu J., Sessa W. C. Endothelial nitric oxide synthase is regulated by tyrosine phosphorylation and interacts with caveolin-1. J. Biol. Chem. 1996;271:27237–27240. doi: 10.1074/jbc.271.44.27237. [DOI] [PubMed] [Google Scholar]
- 20.Feron O., Belhassen L., Kobzik L., Smith T. W., Kelly R. A., Michel T. Endothelial nitric oxide synthase targeting to caveolae: specific interactions with caveolin isoforms in cardiac myocytes and endothelial cells. J. Biol. Chem. 1996;271:22810–22814. doi: 10.1074/jbc.271.37.22810. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Cardena G., Martasek P., Masters B. S., Skidd P. M., Couet J., Li S., Lisanti M. P., Sessa W. C. Dissecting the interaction between nitric oxide synthase (NOS) and caveolin: functional significance of the NOS caveolin binding domain in vivo. J. Biol. Chem. 1997;272:25437–25440. doi: 10.1074/jbc.272.41.25437. [DOI] [PubMed] [Google Scholar]
- 22.Govers R., van der Sluijs P., van Donselaar E., Slot J. W., Rabelink T. J. Endothelial nitric oxide synthase and its negative regulator caveolin-1 localize to distinct perinuclear organelles. J. Histochem. Cytochem. 2002;50:779–788. doi: 10.1177/002215540205000604. [DOI] [PubMed] [Google Scholar]
- 23.Bulotta S., Cerullo A., Barsacchi R., Palma C. D., Rotiroti D., Clementi E., Borgese N. Endothelial nitric oxide synthase is segregated from caveolin-1 and localizes to the leading edge of migrating cells. Exp. Cell Res. 2006;312:877–889. doi: 10.1016/j.yexcr.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 24.Heijnen H. F., Waaijenborg S., Crapo J. D., Bowler R. P., Akkerman J. W., Slot J. W. Colocalization of eNOS and the catalytic subunit of PKA in endothelial cell junctions: a clue for regulated NO production. J. Histochem. Cytochem. 2004;52:1277–1285. doi: 10.1177/002215540405201004. [DOI] [PubMed] [Google Scholar]
- 25.Sowa G., Pypaert M., Sessa W. C. Distinction between signaling mechanisms in lipid rafts vs. caveolae. Proc. Natl. Acad. Sci. U.S.A. 2001;98:14072–14077. doi: 10.1073/pnas.241409998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mungrue I. N., Bredt D. S. nNOS at a glance: implications for brain and brawn. J. Cell Sci. 2004;117:2627–2629. doi: 10.1242/jcs.01187. [DOI] [PubMed] [Google Scholar]
- 27.Wang P., Zhang Q., Tochio H., Fan J. S., Zhang M. Formation of a native-like β-hairpin finger structure of a peptide from the extended PDZ domain of neuronal nitric oxide synthase in aqueous solution. Eur. J. Biochem. 2000;267:3116–3122. doi: 10.1046/j.1432-1327.2000.01318.x. [DOI] [PubMed] [Google Scholar]
- 28.Christopherson K. S., Hillier B. J., Lim W. A., Bredt D. S. PSD-95 assembles a ternary complex with the N-methyl-D-aspartic acid receptor and a bivalent neuronal NO synthase PDZ domain. J. Biol. Chem. 1999;274:27467–27473. doi: 10.1074/jbc.274.39.27467. [DOI] [PubMed] [Google Scholar]
- 29.Hillier B. J., Christopherson K. S., Prehoda K. E., Bredt D. S., Lim W. A. Unexpected modes of PDZ domain scaffolding revealed by structure of nNOS–syntrophin complex. Science. 1999;284:812–815. [PubMed] [Google Scholar]
- 30.Imamura F., Maeda S., Doi T., Fujiyoshi Y. Ligand binding of the second PDZ domain regulates clustering of PSD-95 with the Kv1.4 potassium channel. J. Biol. Chem. 2002;277:3640–3646. doi: 10.1074/jbc.M106940200. [DOI] [PubMed] [Google Scholar]
- 31.Kim E., Sheng M. PDZ domain proteins of synapses. Nat. Rev. Neurosci. 2004;5:771–781. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- 32.Jaffrey S. R., Benfenati F., Snowman A. M., Czernik A. J., Snyder S. H. Neuronal nitric-oxide synthase localization mediated by a ternary complex with synapsin and CAPON. Proc. Natl. Acad. Sci. U.S.A. 2002;99:3199–3204. doi: 10.1073/pnas.261705799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Z. P., Stephens T. J., Murthy S., Canny B. J., Hargreaves M., Witters L. A., Kemp B. E., McConell G. K. Effect of exercise intensity on skeletal muscle AMPK signaling in humans. Diabetes. 2003;52:2205–2212. doi: 10.2337/diabetes.52.9.2205. [DOI] [PubMed] [Google Scholar]
- 34.Brenman J. E., Chao D. S., Xia H., Aldape K., Bredt D. S. Nitric oxide synthase complexed with dystrophin and absent from skeletal muscle sarcolemma in Duchenne muscular dystrophy. Cell. 1995;82:743–752. doi: 10.1016/0092-8674(95)90471-9. [DOI] [PubMed] [Google Scholar]
- 35.Kameya S., Miyagoe Y., Nonaka I., Ikemoto T., Endo M., Hanaoka K., Nabeshima Y., Takeda S. α1-Syntrophin gene disruption results in the absence of neuronal-type nitric-oxide synthase at the sarcolemma but does not induce muscle degeneration. J. Biol. Chem. 1999;274:2193–2200. doi: 10.1074/jbc.274.4.2193. [DOI] [PubMed] [Google Scholar]
- 36.Schuh K., Uldrijan S., Telkamp M., Rothlein N., Neyses L. The plasmamembrane calmodulin-dependent calcium pump: a major regulator of nitric oxide synthase I. J. Cell Biol. 2001;155:201–205. doi: 10.1083/jcb.200104131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barouch L. A., Harrison R. W., Skaf M. W., Rosas G. O., Cappola T. P., Kobeissi Z. A., Hobai I. A., Lemmon C. A., Burnett A. L., O'Rourke B., et al. Nitric oxide regulates the heart by spatial confinement of nitric oxide synthase isoforms. Nature (London) 2002;416:337–339. doi: 10.1038/416337a. [DOI] [PubMed] [Google Scholar]
- 38.Glynne P. A., Darling K. E., Picot J., Evans T. J. Epithelial inducible nitric-oxide synthase is an apical EBP50-binding protein that directs vectorial nitric oxide output. J. Biol. Chem. 2002;277:33132–33138. doi: 10.1074/jbc.M205764200. [DOI] [PubMed] [Google Scholar]
- 39.Reczek D., Berryman M., Bretscher A. Identification of EBP50: a PDZ-containing phosphoprotein that associates with members of the ezrin–radixin–moesin family. J. Cell Biol. 1997;139:169–179. doi: 10.1083/jcb.139.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feron O., Dessy C., Opel D. J., Arstall M. A., Kelly R. A., Michel T. Modulation of the endothelial nitric-oxide synthase–caveolin interaction in cardiac myocytes: implications for the autonomic regulation of heart rate. J. Biol. Chem. 1998;273:30249–30254. doi: 10.1074/jbc.273.46.30249. [DOI] [PubMed] [Google Scholar]
- 41.Dusserre N., L'Heureux N., Bell K. S., Stevens H. Y., Yeh J., Otte L. A., Loufrani L., Frangos J. A. PECAM-1 interacts with nitric oxide synthase in human endothelial cells: implication for flow-induced nitric oxide synthase activation. Arterioscler. Thromb. Vasc. Biol. 2004;24:1796–1802. doi: 10.1161/01.ATV.0000141133.32496.41. [DOI] [PubMed] [Google Scholar]
- 42.Govers R., Bevers L., de Bree P., Rabelink T. J. Endothelial nitric oxide synthase activity is linked to its presence at cell–cell contacts. Biochem. J. 2002;361:193–201. doi: 10.1042/0264-6021:3610193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schleicher M., Brundin F., Gross S., Müller-Esterl W., Oess S. Cell cycle-regulated inactivation of endothelial NO synthase through NOSIP-dependent targeting to the cytoskeleton. Mol. Cell. Biol. 2005;25:8251–8258. doi: 10.1128/MCB.25.18.8251-8258.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Su Y., Edwards-Bennett S., Bubb M. R., Block E. R. Regulation of endothelial nitric oxide synthase by the actin cytoskeleton. Am. J. Physiol. Cell Physiol. 2003;284:C1542–C1549. doi: 10.1152/ajpcell.00248.2002. [DOI] [PubMed] [Google Scholar]
- 45.Zharikov S. I., Sigova A. A., Chen S., Bubb M. R., Block E. R. Cytoskeletal regulation of the L-arginine/NO pathway in pulmonary artery endothelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001;280:L465–L473. doi: 10.1152/ajplung.2001.280.3.L465. [DOI] [PubMed] [Google Scholar]
- 46.Gao S., Chen J., Brodsky S. V., Huang H., Adler S., Lee J. H., Dhadwal N., Cohen-Gould L., Gross S. S., Goligorsky M. S. Docking of endothelial nitric oxide synthase (eNOS) to the mitochondrial outer membrane: a pentabasic amino acid sequence in the autoinhibitory domain of eNOS targets a proteinase K-cleavable peptide on the cytoplasmic face of mitochondria. J. Biol. Chem. 2004;279:15968–15974. doi: 10.1074/jbc.M308504200. [DOI] [PubMed] [Google Scholar]
- 47.Liu J., Garcia-Cardena G., Sessa W. C. Palmitoylation of endothelial nitric oxide synthase is necessary for optimal stimulated release of nitric oxide: implications for caveolae localization. Biochemistry. 1996;35:13277–13281. doi: 10.1021/bi961720e. [DOI] [PubMed] [Google Scholar]
- 48.Shaul P. W. Regulation of endothelial nitric oxide synthase: location, location, location. Annu. Rev. Physiol. 2002;64:749–774. doi: 10.1146/annurev.physiol.64.081501.155952. [DOI] [PubMed] [Google Scholar]
- 49.Fulton D., Babbitt R., Zoellner S., Fontana J., Acevedo L., McCabe T. J., Iwakiri Y., Sessa W. C. Targeting of endothelial nitric-oxide synthase to the cytoplasmic face of the Golgi complex or plasma membrane regulates Akt- versus calcium-dependent mechanisms for nitric oxide release. J. Biol. Chem. 2004;279:30349–30357. doi: 10.1074/jbc.M402155200. [DOI] [PubMed] [Google Scholar]
- 50.Jagnandan D., Sessa W. C., Fulton D. Intracellular location regulates calcium-calmodulin-dependent activation of organelle-restricted eNOS. Am. J. Physiol. Cell Physiol. 2005;289:C1024–C1033. doi: 10.1152/ajpcell.00162.2005. [DOI] [PubMed] [Google Scholar]
- 51.Su Y., Zharikov S. I., Block E. R. Microtubule-active agents modify nitric oxide production in pulmonary artery endothelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002;282:L1183–L1189. doi: 10.1152/ajplung.00388.2001. [DOI] [PubMed] [Google Scholar]
- 52.Wyat A. W., Steinert J. R., Mann G. E. Modulation of the L-arginine/nitric oxide signalling pathway in vascular endothelial cells. Biochem. Soc. Symp. 2004;71:143–156. doi: 10.1042/bss0710143. [DOI] [PubMed] [Google Scholar]
- 53.Marsault R., Murgia M., Pozzan T., Rizzuto R. Domains of high Ca2+ beneath the plasma membrane of living A7r5 cells. EMBO J. 1997;16:1575–1581. doi: 10.1093/emboj/16.7.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin S., Fagan K. A., Li K. X., Shaul P. W., Cooper D. M., Rodman D. M. Sustained endothelial nitric-oxide synthase activation requires capacitative Ca2+ entry. J. Biol. Chem. 2000;275:17979–17985. doi: 10.1074/jbc.275.24.17979. [DOI] [PubMed] [Google Scholar]
- 55.Brouet A., Sonveaux P., Dessy C., Balligand J. L., Feron O. Hsp90 ensures the transition from the early Ca2+-dependent to the late phosphorylation-dependent activation of the endothelial nitric-oxide synthase in vascular endothelial growth factor-exposed endothelial cells. J. Biol. Chem. 2001;276:32663–32669. doi: 10.1074/jbc.M101371200. [DOI] [PubMed] [Google Scholar]
- 56.Fulton D., Fontana J., Sowa G., Gratton J. P., Lin M., Li K. X., Michell B., Kemp B. E., Rodman D., Sessa W. C. Localization of endothelial nitric-oxide synthase phosphorylated on serine 1179 and nitric oxide in Golgi and plasma membrane defines the existence of two pools of active enzyme. J. Biol. Chem. 2002;277:4277–4284. doi: 10.1074/jbc.M106302200. [DOI] [PubMed] [Google Scholar]
- 57.Gonzalez E., Kou R., Lin A. J., Golan D. E., Michel T. Subcellular targeting and agonist-induced site-specific phosphorylation of endothelial nitric-oxide synthase. J. Biol. Chem. 2002;277:39554–39560. doi: 10.1074/jbc.M207299200. [DOI] [PubMed] [Google Scholar]
- 58.Church J. E., Fulton D. Differences in eNOS activity because of subcellular localization are dictated by phosphorylation state rather than the local calcium environment. J. Biol. Chem. 2006;281:1477–1488. doi: 10.1074/jbc.M505968200. [DOI] [PubMed] [Google Scholar]
- 59.McCabe T. J., Fulton D., Roman L. J., Sessa W. C. Enhanced electron flux and reduced calmodulin dissociation may explain “calcium-independent” eNOS activation by phosphorylation. J. Biol. Chem. 2000;275:6123–6128. doi: 10.1074/jbc.275.9.6123. [DOI] [PubMed] [Google Scholar]
- 60.Michell B. J., Harris M. B., Chen Z. P., Ju H., Venema V. J., Blackstone M. A., Huang W., Venema R. C., Kemp B. E. Identification of regulatory sites of phosphorylation of the bovine endothelial nitric-oxide synthase at serine 617 and serine 635. J. Biol. Chem. 2002;277:42344–42351. doi: 10.1074/jbc.M205144200. [DOI] [PubMed] [Google Scholar]
- 61.Collins J. L., Vodovotz Y., Hierholzer C., Villavicencio R. T., Liu S., Alber S., Gallo D., Stolz D. B., Watkins S. C., Godfrey A., et al. Characterization of the expression of inducible nitric oxide synthase in rat and human liver during hemorrhagic shock. Shock. 2003;19:117–122. doi: 10.1097/00024382-200302000-00005. [DOI] [PubMed] [Google Scholar]
- 62.Vodovotz Y., Russell D., Xie Q. W., Bogdan C., Nathan C. Vesicle membrane association of nitric oxide synthase in primary mouse macrophages. J. Immunol. 1995;154:2914–2925. [PubMed] [Google Scholar]
- 63.Sessa W. C. eNOS at a glance. J. Cell Sci. 2004;117:2427–2429. doi: 10.1242/jcs.01165. [DOI] [PubMed] [Google Scholar]
- 64.Predescu D., Predescu S., Shimizu J., Miyawaki-Shimizu K., Malik A. B. Constitutive eNOS-derived nitric oxide is a determinant of endothelial junctional integrity. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005;289:L371–L381. doi: 10.1152/ajplung.00175.2004. [DOI] [PubMed] [Google Scholar]
- 65.Grange R. W., Isotani E., Lau K. S., Kamm K. E., Huang P. L., Stull J. T. Nitric oxide contributes to vascular smooth muscle relaxation in contracting fast-twitch muscles. Physiol. Genomics. 2001;5:35–44. doi: 10.1152/physiolgenomics.2001.5.1.35. [DOI] [PubMed] [Google Scholar]
- 66.Zhao Y., Brandish P. E., Ballou D. P., Marletta M. A. A molecular basis for nitric oxide sensing by soluble guanylate cyclase. Proc. Natl. Acad. Sci. U.S.A. 1999;96:14753–14758. doi: 10.1073/pnas.96.26.14753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang W., Kuncewicz T., Yu Z. Y., Zou L., Xu X., Kone B. C. Protein–protein interactions involving inducible nitric oxide synthase. Acta Physiol. Scand. 2003;179:137–142. doi: 10.1046/j.1365-201X.2003.01119.x. [DOI] [PubMed] [Google Scholar]
- 68.Aarts M. M., Tymianski M. Novel treatment of excitotoxicity: targeted disruption of intracellular signalling from glutamate receptors. Biochem. Pharmacol. 2003;66:877–886. doi: 10.1016/s0006-2952(03)00297-1. [DOI] [PubMed] [Google Scholar]
- 69.Kornau H. C., Schenker L. T., Kennedy M. B., Seeburg P. H. Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science. 1995;269:1737–1740. doi: 10.1126/science.7569905. [DOI] [PubMed] [Google Scholar]
- 70.Sattler R., Xiong Z., Lu W. Y., Hafner M., MacDonald J. F., Tymianski M. Specific coupling of NMDA receptor activation to nitric oxide neurotoxicity by PSD-95 protein. Science. 1999;284:1845–1848. doi: 10.1126/science.284.5421.1845. [DOI] [PubMed] [Google Scholar]
- 71.Cao J., Viholainen J. I., Dart C., Warwick H. K., Leyland M. L., Courtney M. J. The PSD95–nNOS interface: a target for inhibition of excitotoxic p38 stress-activated protein kinase activation and cell death. J. Cell Biol. 2005;168:117–126. doi: 10.1083/jcb.200407024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Drab M., Verkade P., Elger M., Kasper M., Lohn M., Lauterbach B., Menne J., Lindschau C., Mende F., Luft F. C., et al. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293:2449–2452. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- 73.Fontana J., Fulton D., Chen Y., Fairchild T. A., McCabe T. J., Fujita N., Tsuruo T., Sessa W. C. Domain mapping studies reveal that the M domain of hsp90 serves as a molecular scaffold to regulate Akt-dependent phosphorylation of endothelial nitric oxide synthase and NO release. Circ. Res. 2002;90:866–873. doi: 10.1161/01.res.0000016837.26733.be. [DOI] [PubMed] [Google Scholar]
- 74.Song Y., Zweier J. L., Xia Y. Heat-shock protein 90 augments neuronal nitric oxide synthase activity by enhancing Ca2+/calmodulin binding. Biochem. J. 2001;355:357–360. doi: 10.1042/0264-6021:3550357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Takahashi S., Mendelsohn M. E. Synergistic activation of endothelial nitric-oxide synthase (eNOS) by HSP90 and Akt: calcium-independent eNOS activation involves formation of an HSP90–Akt–CaM-bound eNOS complex. J. Biol. Chem. 2003;278:30821–30827. doi: 10.1074/jbc.M304471200. [DOI] [PubMed] [Google Scholar]
- 76.Venema R. C., Venema V. J., Ju H., Harris M. B., Snead C., Jilling T., Dimitropoulou C., Maragoudakis M. E., Catravas J. D. Novel complexes of guanylate cyclase with heat shock protein 90 and nitric oxide synthase. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H669–H678. doi: 10.1152/ajpheart.01025.2002. [DOI] [PubMed] [Google Scholar]
- 77.Darby P. J., Kwan C. Y., Daniel E. E. Caveolae from canine airway smooth muscle contain the necessary components for a role in Ca2+ handling. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000;279:L1226–L1235. doi: 10.1152/ajplung.2000.279.6.L1226. [DOI] [PubMed] [Google Scholar]
- 78.Feron O., Smith T. W., Michel T., Kelly R. A. Dynamic targeting of the agonist-stimulated m2 muscarinic acetylcholine receptor to caveolae in cardiac myocytes. J. Biol. Chem. 1997;272:17744–17748. doi: 10.1074/jbc.272.28.17744. [DOI] [PubMed] [Google Scholar]
- 79.Rybin V. O., Xu X., Lisanti M. P., Steinberg S. F. Differential targeting of β-adrenergic receptor subtypes and adenylyl cyclase to cardiomyocyte caveolae: a mechanism to functionally regulate the cAMP signaling pathway. J. Biol. Chem. 2000;275:41447–41457. doi: 10.1074/jbc.M006951200. [DOI] [PubMed] [Google Scholar]
- 80.Erwin P. A., Lin A. J., Golan D. E., Michel T. Receptor-regulated dynamic S-nitrosylation of endothelial nitric-oxide synthase in vascular endothelial cells. J. Biol. Chem. 2005;280:19888–19894. doi: 10.1074/jbc.M413058200. [DOI] [PubMed] [Google Scholar]
- 81.Hurshman A. R., Marletta M. A. Nitric oxide complexes of inducible nitric oxide synthase: spectral characterization and effect on catalytic activity. Biochemistry. 1995;34:5627–5634. doi: 10.1021/bi00016a038. [DOI] [PubMed] [Google Scholar]
- 82.Erwin P. A., Mitchell D. A., Sartoretto J., Marletta M. A., Michel T. Subcellular targeting and differential S-nitrosylation of endothelial nitric-oxide synthase. J. Biol. Chem. 2006;281:151–157. doi: 10.1074/jbc.M510421200. [DOI] [PubMed] [Google Scholar]
- 83.Martinez-Ruiz A., Villanueva L., Gonzalez de Orduna C., Lopez-Ferrer D., Higueras M. A., Tarin C., Rodriguez-Crespo I., Vazquez J., Lamas S. S-nitrosylation of Hsp90 promotes the inhibition of its ATPase and endothelial nitric oxide synthase regulatory activities. Proc. Natl. Acad. Sci. U.S.A. 2005;102:8525–8530. doi: 10.1073/pnas.0407294102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang G., Moniri N. H., Ozawa K., Stamler J. S., Daaka Y. Nitric oxide regulates endocytosis by S-nitrosylation of dynamin. Proc. Natl. Acad. Sci. U.S.A. 2006;103:1295–1300. doi: 10.1073/pnas.0508354103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim W. K., Choi Y. B., Rayudu P. V., Das P., Asaad W., Arnelle D. R., Stamler J. S., Lipton S. A. Attenuation of NMDA receptor activity and neurotoxicity by nitroxyl anion, NO. Neuron. 1999;24:461–469. doi: 10.1016/s0896-6273(00)80859-4. [DOI] [PubMed] [Google Scholar]
- 86.Fang M., Jaffrey S. R., Sawa A., Ye K., Luo X., Snyder S. H. Dexras1: a G protein specifically coupled to neuronal nitric oxide synthase via CAPON. Neuron. 2000;28:183–193. doi: 10.1016/s0896-6273(00)00095-7. [DOI] [PubMed] [Google Scholar]
- 87.Andries L. J., Brutsaert D. L., Sys S. U. Nonuniformity of endothelial constitutive nitric oxide synthase distribution in cardiac endothelium. Circ. Res. 1998;82:195–203. doi: 10.1161/01.res.82.2.195. [DOI] [PubMed] [Google Scholar]
- 88.Rocks O., Peyker A., Kahms M., Verveer P. J., Koerner C., Lumbierres M., Kuhlmann J., Waldmann H., Wittinghofer A., Bastiaens P. I. An acylation cycle regulates localization and activity of palmitoylated Ras isoforms. Science. 2005;307:1746–1752. doi: 10.1126/science.1105654. [DOI] [PubMed] [Google Scholar]
- 89.Sowa G., Liu J., Papapetropoulos A., Rex-Haffner M., Hughes T. E., Sessa W. C. Trafficking of endothelial nitric-oxide synthase in living cells: quantitative evidence supporting the role of palmitoylation as a kinetic trapping mechanism limiting membrane diffusion. J. Biol. Chem. 1999;274:22524–22531. doi: 10.1074/jbc.274.32.22524. [DOI] [PubMed] [Google Scholar]
- 90.Liu J., Garcia-Cardena G., Sessa W. C. Biosynthesis and palmitoylation of endothelial nitric oxide synthase: mutagenesis of palmitoylation sites, cysteines-15 and/or -26, argues against depalmitoylation-induced translocation of the enzyme. Biochemistry. 1995;34:12333–12340. doi: 10.1021/bi00038a029. [DOI] [PubMed] [Google Scholar]
- 91.Robinson L. J., Michel T. Mutagenesis of palmitoylation sites in endothelial nitric oxide synthase identifies a novel motif for dual acylation and subcellular targeting. Proc. Natl. Acad. Sci. U.S.A. 1995;92:11776–11780. doi: 10.1073/pnas.92.25.11776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Michel J. B., Michel T. The role of palmitoyl-protein thioesterase in the palmitoylation of endothelial nitric oxide synthase. FEBS Lett. 1997;405:356–362. doi: 10.1016/s0014-5793(97)00222-6. [DOI] [PubMed] [Google Scholar]
- 93.Yeh D. C., Duncan J. A., Yamashita S., Michel T. Depalmitoylation of endothelial nitric-oxide synthase by acyl-protein thioesterase 1 is potentiated by Ca2+-calmodulin. J. Biol. Chem. 1999;274:33148–33154. doi: 10.1074/jbc.274.46.33148. [DOI] [PubMed] [Google Scholar]
- 94.Jiang J., Cyr D., Babbitt R. W., Sessa W. C., Patterson C. Chaperone-dependent regulation of endothelial nitric-oxide synthase intracellular trafficking by the co-chaperone/ubiquitin ligase CHIP. J. Biol. Chem. 2003;278:49332–49341. doi: 10.1074/jbc.M304738200. [DOI] [PubMed] [Google Scholar]
- 95.Hanzal-Bayer M., Renault L., Roversi P., Wittinghofer A., Hillig R. C. The complex of Arl2-GTP and PDEδ: from structure to function. EMBO J. 2002;21:2095–2106. doi: 10.1093/emboj/21.9.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Meder D., Simons K. Cell biology: Ras on the roundabout. Science. 2005;307:1731–1733. doi: 10.1126/science.1110551. [DOI] [PubMed] [Google Scholar]
- 97.Blair A., Shaul P. W., Yuhanna I. S., Conrad P. A., Smart E. J. Oxidized low density lipoprotein displaces endothelial nitric-oxide synthase (eNOS) from plasmalemmal caveolae and impairs eNOS activation. J. Biol. Chem. 1999;274:32512–32519. doi: 10.1074/jbc.274.45.32512. [DOI] [PubMed] [Google Scholar]
- 98.Nuszkowski A., Grabner R., Marsche G., Unbehaun A., Malle E., Heller R. Hypochlorite-modified low density lipoprotein inhibits nitric oxide synthesis in endothelial cells via an intracellular dislocalization of endothelial nitric-oxide synthase. J. Biol. Chem. 2001;276:14212–14221. doi: 10.1074/jbc.M007659200. [DOI] [PubMed] [Google Scholar]
- 99.Zhu Y., Liao H. L., Niu X. L., Yuan Y., Lin T., Verna L., Stemerman M. B. Low density lipoprotein induces eNOS translocation to membrane caveolae: the role of RhoA activation and stress fiber formation. Biochim. Biophys. Acta. 2003;1635:117–126. doi: 10.1016/j.bbalip.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 100.Goetz R. M., Thatte H. S., Prabhakar P., Cho M. R., Michel T., Golan D. E. Estradiol induces the calcium-dependent translocation of endothelial nitric oxide synthase. Proc. Natl. Acad. Sci. U.S.A. 1999;96:2788–2793. doi: 10.1073/pnas.96.6.2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Igarashi J., Thatte H. S., Prabhakar P., Golan D. E., Michel T. Calcium-independent activation of endothelial nitric oxide synthase by ceramide. Proc. Natl. Acad. Sci. U.S.A. 1999;96:12583–12588. doi: 10.1073/pnas.96.22.12583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Prabhakar P., Thatte H. S., Goetz R. M., Cho M. R., Golan D. E., Michel T. Receptor-regulated translocation of endothelial nitric-oxide synthase. J. Biol. Chem. 1998;273:27383–27388. doi: 10.1074/jbc.273.42.27383. [DOI] [PubMed] [Google Scholar]
- 103.Marrero M. B., Venema V. J., Ju H., He H., Liang H., Caldwell R. B., Venema R. C. Endothelial nitric oxide synthase interactions with G-protein-coupled receptors. Biochem. J. 1999;343:335–340. [PMC free article] [PubMed] [Google Scholar]
- 104.Thuringer D., Maulon L., Frelin C. Rapid transactivation of the vascular endothelial growth factor receptor KDR/Flk-1 by the bradykinin B2 receptor contributes to endothelial nitric-oxide synthase activation in cardiac capillary endothelial cells. J. Biol. Chem. 2002;277:2028–2032. doi: 10.1074/jbc.M109493200. [DOI] [PubMed] [Google Scholar]
- 105.Figueroa X. F., Gonzalez D. R., Martinez A. D., Duran W. N., Boric M. P. ACh-induced endothelial NO synthase translocation, NO release and vasodilatation in the hamster microcirculation in vivo. J. Physiol. 2002;544:883–896. doi: 10.1113/jphysiol.2002.021972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ortiz P. A., Hong N. J., Garvin J. L. Luminal flow induces eNOS activation and translocation in the rat thick ascending limb. II. Role of PI3-kinase and Hsp90. Am. J. Physiol. Renal Physiol. 2004;287:F281–F288. doi: 10.1152/ajprenal.00383.2003. [DOI] [PubMed] [Google Scholar]
- 107.Ortiz P. A., Hong N. J., Garvin J. L. Luminal flow induces eNOS activation and translocation in the rat thick ascending limb. Am. J. Physiol. Renal Physiol. 2004;287:F274–F280. doi: 10.1152/ajprenal.00382.2003. [DOI] [PubMed] [Google Scholar]
- 108.Hansen S. H., Olsson A., Casanova J. E. Wortmannin, an inhibitor of phosphoinositide 3-kinase, inhibits transcytosis in polarized epithelial cells. J. Biol. Chem. 1995;270:28425–28432. doi: 10.1074/jbc.270.47.28425. [DOI] [PubMed] [Google Scholar]
- 109.Zimmermann K., Opitz N., Dedio J., Renné C., Müller-Esterl W., Oess S. NOSTRIN: a protein modulating nitric oxide release and subcellular distribution of endothelial nitric oxide synthase. Proc. Natl. Acad. Sci. U.S.A. 2002;99:17167–17172. doi: 10.1073/pnas.252345399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Icking A., Matt S., Opitz N., Wiesenthal A., Müller-Esterl W., Schilling K. NOSTRIN functions as a homotrimeric adaptor protein facilitating internalization of eNOS. J. Cell Sci. 2005;118:5059–5069. doi: 10.1242/jcs.02620. [DOI] [PubMed] [Google Scholar]
- 111.Icking A., Schilling K., Wiesenthal A., Opitz N., Müller-Esterl W. FCH/Cdc15 domain determines distinct subcellular localization of NOSTRIN. FEBS Lett. 2006;580:223–228. doi: 10.1016/j.febslet.2005.11.078. [DOI] [PubMed] [Google Scholar]
- 112.Dedio J., König P., Wohlfart P., Schroeder C., Kummer W., Müller-Esterl W. NOSIP, a novel modulator of endothelial nitric oxide synthase activity. FASEB J. 2001;15:79–89. doi: 10.1096/fj.00-0078com. [DOI] [PubMed] [Google Scholar]
- 113.Dreyer J., Schleicher M., Tappe A., Schilling K., Kuner T., Kusumawidijaja G., Müller-Esterl W., Oess S., Kuner R. Nitric oxide synthase (NOS)-interacting protein interacts with neuronal NOS and regulates its distribution and activity. J. Neurosci. 2004;24:10454–10465. doi: 10.1523/JNEUROSCI.2265-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Arundine M., Sanelli T., Ping He B., Strong M. J. NMDA induces NOS 1 translocation to the cell membrane in NGF-differentiated PC 12 cells. Brain Res. 2003;976:149–158. doi: 10.1016/s0006-8993(03)02568-x. [DOI] [PubMed] [Google Scholar]
- 115.Sessa W. C., Garcia-Cardena G., Liu J., Keh A., Pollock J. S., Bradley J., Thiru S., Braverman I. M., Desai K. M. The Golgi association of endothelial nitric oxide synthase is necessary for the efficient synthesis of nitric oxide. J. Biol. Chem. 1995;270:17641–17644. doi: 10.1074/jbc.270.30.17641. [DOI] [PubMed] [Google Scholar]
- 116.Martinez-Moreno M., Alvarez-Barrientos A., Roncal F., Albar J. P., Gavilanes F., Lamas S., Rodriguez-Crespo I. Direct interaction between the reductase domain of endothelial nitric oxide synthase and the ryanodine receptor. FEBS Lett. 2005;579:3159–3163. doi: 10.1016/j.febslet.2005.04.078. [DOI] [PubMed] [Google Scholar]
- 117.Sun J., Xin C., Eu J. P., Stamler J. S., Meissner G. Cysteine-3635 is responsible for skeletal muscle ryanodine receptor modulation by NO. Proc. Natl. Acad. Sci. U.S.A. 2001;98:11158–11162. doi: 10.1073/pnas.201289098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang J., Jin B., Li L., Block E. R., Patel J. M. Nitric oxide-induced persistent inhibition and nitrosylation of active site cysteine residues of mitochondrial cytochrome-c oxidase in lung endothelial cells. Am. J. Physiol. Cell Physiol. 2005;288:C840–C849. doi: 10.1152/ajpcell.00325.2004. [DOI] [PubMed] [Google Scholar]
- 119.Choi Y. B., Tenneti L., Le D. A., Ortiz J., Bai G., Chen H. S., Lipton S. A. Molecular basis of NMDA receptor-coupled ion channel modulation by S-nitrosylation. Nat. Neurosci. 2000;3:15–21. doi: 10.1038/71090. [DOI] [PubMed] [Google Scholar]
- 120.Eu J. P., Hare J. M., Hess D. T., Skaf M., Sun J., Cardenas-Navina I., Sun Q. A., Dewhirst M., Meissner G., Stamler J. S. Concerted regulation of skeletal muscle contractility by oxygen tension and endogenous nitric oxide. Proc. Natl. Acad. Sci. U.S.A. 2003;100:15229–15234. doi: 10.1073/pnas.2433468100. [DOI] [PMC free article] [PubMed] [Google Scholar]