Abstract
Membrane fusion mediated by the influenza-virus fusion protein is activated by low pH via a cascade of reactions. Some processes among them are irreversible, such as helix hairpin formation of the ectodomain, whereas others are reversible, such as exposure of the fusion peptide. Using this property, we attempted to dissect, in temporal order, different stages of the fusion reaction involving the fusion peptide by an acidic–neutral–acidic pH cycle. The fluorescence-quenching data indicated that both insertion depth and self-assembly are pH-reversible. In addition, lipid mixing assay was demonstrated to be arrested by neutral pH. By contrast, membrane leakage was shown to be irreversible with respect to pH. Our results, along with those from other studies on the pH-dependence of membrane fusion, are used to build a model for the virus-mediated fusion event from the perspective of pH-reversibility.
Keywords: influenza virus, lipid mixing, membrane fusion, membrane leakage, pH reversibility, pH-arrested process
Abbreviations: bis-ANS, 4,4′-bis-(1-anilinonaphthalene 8-sulphonate); DMPC, 1,2-dimyristoyl-sn-glycero-3-phosphocholine; DMPG, 1,2-dimyristoyl sn-glycero-3-phosphoglycerol; DPA, 2,6-pyridinedicarboxylic acid; Fmoc, fluoren-9-ylmethoxycarbonyl; FP, fusion peptide; gp41, transmembrane glycoprotein 41; HA, haemagglutinin; HA2, haemagglutinin HA2 subunit; HR, heptad repeat; LUV, large unilamellar vesicles; NBD, 4-chloro-7-nitrobenz-2-oxa-1,3-diazole; POPC, 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphatidylcholine; TAMRA, 5(6)-carboxytetramethylrhodamine hydrochloride; RBC, red blood cell; Rho, rhodamine; Rho–PE, Lissamine™ rhodamine B 1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine triethylammonium salt; Tb3+, terbium(III) chloride hexahydrate
INTRODUCTION
The fusion activity of the influenza virus is pH-dependent. Entry of the virus into the target cell is effected by fusion of the viral and cell membranes at the endosomal pH (≈5) in a multi-step mode involving various parts of the fusion protein and co-ordination of neighbouring protein molecules. Examination of the response of different components of the fusion protein to pH change can provide valuable insight into the complex fusion reaction [1–4]. It was demonstrated, by biochemical measurements [5], that the proteolytic fragment of HA2 (haemagglutinin HA2 subunit), TBHA2, consisting of residues 38–175, folds spontaneously into a low-pH conformation, implying that the fusogenic structure is thermodynamically stable and thus is pH-irreversible. A similar proposition has been put forward by Carr and Kim [6] for a synthetic peptide encompassing the coiled-coil domain of HA2. It has been shown that the six-helix bundle of the ectodomain of HIV-1 gp41 (transmembrane glycoprotein 41) is highly thermodynamically stable [7], hence the formation of this core structure is an irreversible process in virus-mediated fusion. The assertion is supported by a recent study on the kinetics of cell–cell fusion induced by the HIV fusion protein [8]. It was found that folding of the helix bundle occurs immediately following formation of the nascent fusion pore, indicating that the helix bundle is probably a post-fusion structure or at least a late product of the fusion reaction. A kinetic study of the fusion process under sub-optimal conditions, for example, lowering the temperature, enabled a dissection of fusion intermediates. Thus Markosyan et al. [9] found that the rate-limiting step of the influenza-HA (haemagglutinin)-induced fusion occurs after the intermediate captured by employing a lower temperature; Leikina et al. [10] examined the irreversible steps of the HA refolding by varying the incubation time at pH 5.0 or 7.4 at 22 °C.
On the other hand, some reversible elements of pH change have been identified. Thus, following a neutral-to-fusogenic pH change, an essential early step of the process is exposure of the N-terminal FP (fusion peptide) of the membrane-anchored subunit HA2, leading to its protrusion towards, and subsequent insertion into, the target membrane. This process has been shown to be pH-reversible by a fluorescence study probed by bis-ANS [4,4′-bis-(1-anilinonaphthalene 8-sulphonate)] binding to the virus [3]. In particular, binding of an N-terminal 20-mer FP to the fluorophore bis-ANS was shown to decrease significantly with increasing pH. In addition, the ability to mediate mixing of phospholipid vesicles by the synthetic 23-mer and 20-mer peptides corresponding to the FP region of HA2 was demonstrated to be reversible in response to pH change [4,11].
It has been shown that the lipid mixing mediated by influenza virus can be arrested by neutralization and resurrected by reacidification [12,13]. Protonation of the highly conserved acidic residues was suggested to be important to the fusion activity. Importantly, Morris et al. [14] observed that the lipid mixing between RBCs (red blood cells) and HA-expressing cells proceeded unaffected by neutralization applied beyond the 15 s incubation time under acidic conditions (pH 5.0, 37 °C). This implies that the reaction leading up to lipid mixing is rapid, with the complete membrane fusion (manifested by content mixing) occurring after the lipid mixing displaying rate-limiting irreversible steps.
Tatulian and Tamm [15] found, in an IR study, that the orientation of influenza HA relative to the supported phospholipid bilayer changed in a pH-reversible manner in the absence, but not in the presence, of target vesicles. They also showed that the reversible components resided in HA2 instead of HA1.
Since the FP is an essential component in the fusion reaction, an important question relevant to the understanding of the fusion mechanism arises as to whether insertion of the FP precedes or follows formation of the helix bundle. In addition, oligomerization of the FP, and hence its pH-dependence in the target membrane, is critical in the fusion event. To address these issues, we took advantage of the variation of HA2-mediated membrane fusion with pH. Using fluorescence-quenching techniques, we showed that the insertion depth and self-assembly of the HA2 FP with membrane bilayer are pH-reversible and that the lipid mixing mediated by the FP can be arrested by neutral pH and the activity resumed with acidification. The latter result is in contrast with the irreversible proceeding of six-helix bundle formation of the HA2 fusion core with respect to pH alteration. A pH-cycle experiment probed by a liposome-encapsulated cation leaking into bulk aqueous medium demonstrated that the process involving disruption of both leaflets of the membrane bilayer is pH-unarrestable and hence irreversible. The dissection of the reversible, arrestable and irreversible processes in the pathway of the fusion reaction illustrated in our proposed scheme may afford a better understanding of this complex event. Taking these data along with the premise that, in general, reversible and arrestable steps are followed by irreversible ones, we constructed a working model for the HA-induced membrane fusion. Our findings can be compared with the scheme advocated recently by Markosyan et al. [8].
EXPERIMENTAL
Materials and peptide synthesis
DMPC (1,2-dimyristoyl-sn-glycero-3-phosphocholine), DMPG (1,2-dimyristoyl sn-glycero-3-phosphoglycerol) and POPC (1-palmitoyl-2-oleoyl-sn-glycero-3-phosphatidylcholine) used in the present study were obtained from Avanti Polar Lipids (Alabaster, AL, U.S.A.). TAMRA [5(6)-carboxytetramethylrhodamine hydrochloride] and Rho–PE (Lissamine™ rhodamine B 1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine triethylammonium salt) were purchased from Molecular Probes (Eugene, OR, U.S.A.) and NBD (4-chloro-7-nitrobenz-2-oxa-1,3-diazole) and Triton X-100 were from Sigma (St. Louis, MO, U.S.A.). Tb3+ [terbium(III) chloride hexahydrate] and DPA (2,6-pyridinedicarboxylic acid) were acquired from Acros Organics (Morris Plains, NJ, U.S.A.), and sodium citrate was from RdH Laborchemikalien GmbH+Co. KG (Seelze, Germany). All reagents were used in the experiments without further purification.
The peptides corresponding to residues 1–25 of HA2, HA2(1–25), namely GLFGAIAGFIENGWEGMIDGWYGFR (influenza virus strain X-31), and its E1 fusion-inactive analogue, E1-HA2(1–25), of influenza virus, were assembled by Fmoc (fluoren-9-ylmethoxycarbonyl)/t-butyl solid-phase peptide synthesis using a peptide synthesizer (model Rainin PS3; Protein Technologies, Tucson, AZ, U.S.A.). The N-terminally free wild-type peptide was labelled with TAMRA following the standard Fmoc–amino acid coupling protocol with a coupling time of 10 h. Labelling with NBD was achieved as described by Rapaport and Shai [16], with the modifications as described in our recent study [17]. Cleavage and purification of the peptide were as described previously [17,18]. The primary sequence of the peptide was ascertained by electrospray MS.
Insertion depth probed by NBD quenching with Co2+
For Co2+ quenching experiments, the NBD-labelled HA2(1–25) was added to DMPC/DMPG vesicles (peptide/DMPC/DMPG, 0.06 μM:150 μM:150 μM) in Tris buffer at pH 4.5 in a total volume of 3 ml. After taking 1 ml of the sample for the first acidic measurement, NaOH was added to the remaining sample solution to adjust the pH to neutral, whereas citric acid was used to change pH to acidic for the third step. An incremental amount of CoCl2 stock solution (0.1 M) was injected into each sample of the three pH stages to give final concentrations in the range of 0.02–0.2 mM. The excitation and emission wavelengths were set at 467 and 530 nm respectively for measurements on a Hitachi F-2500 fluorescence spectrometer at 37 °C using a 1 cm2 semi-micro quartz cuvette with a stirrer. The response time was set at 0.08 s and slit bandwidths for excitation and emission were each 10 nm. Corrections for dilution were made to the observed fluorescence intensities. The data were analysed using the Stern–Volmer equation:
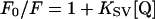 |
(1) |
where F0 and F are the intensities of NBD fluorescence before and after adding a given amount of CoCl2 solution respectively; [Q] is the concentration of the quencher, and the slope KSV, is the Stern–Volmer constant.
Lipid mixing and self-assembly monitored by self-quenching of Rho (rhodamine) fluorescence
For pH cycle experiments using Rho, pure Rho–HA2(1–25) or Rho–HA2(1–25)/HA2(1–25) (1:19, w/w) were added to a DMPC/DMPG (15 μM:15 μM) vesicular solution to give a total concentration of labelled and unlabelled HA2(1–25) of 0.06 μM. After low-pH measurements, NaOH and HCl were used to adjust the pH for the following neutral and acidic stages. To monitor the Rho probe, the excitation and emission wavelengths were set at 530 and 578 nm respectively. A Hitachi F-2500 fluorescence spectrometer with the other parameters the same as those described above, was used to perform the experiments at 37 °C. The fluorescence intensity after the addition of Triton X-100 (0.2%, v/v) was referred to as 100%, and the percentage of lipid mixing (at time t) was estimated from eqn (2):
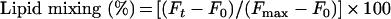 |
(2) |
where F0 is initial residual fluorescence intensity, Ft is the fluorescence intensity at time t and Fmax was obtained by the complete dilution of Rho with Triton X-100.
Using a Jasco FP-777 spectrofluorimeter, Rho-labelled lipid (Rho–PE) was employed for pH variation experiments aimed at detecting inter-trimeric interactions. HA2(1–25) or E1-HA2(1–25) was added to DMPC/Rho–PE vesicles to give peptide/DMPC/Rho–PE final concentrations of 5 μM, 1.0 mM and 3 μM respectively. Also, NaOH and HCl in PBS were used to control pH in the cycle of pH 5.0→7.1→5.0. The excitation wavelength was 556 nm and the emission wavelength was set at 590 nm with a response time of 2 s. The bandwidths for excitation and emission measurements were 5 and 1.5 nm respectively.
Tb3+/DPA leakage assay by enhanced Tb3+ fluorescence
LUVs (large unilamellar vesicles) consisting of POPC were dissolved in chloroform/methanol (4:1, v/v), dried to a thin film under a stream of argon and then further dried under vacuum overnight. LUV lipids were resuspended to a 100 mM concentration in 50 mM Tb3+/100 mM sodium citrate/10 mM Tris/HCl/100 mM NaCl, pH 7.5. Samples were subjected to repeated freezing and thawing for 10 cycles, followed by extrusion through 0.1-μm-pore-size polycarbonate membranes in an Avanti Mini-Extruder. To remove terbium citrate from the liposome external surface, the liposome dispersion was dialysed for 24 h against 10 mM Tris/HCl/100 mM NaCl, pH 7.5 at 4 °C [19,20].
To quantify the extent of leakage observed in the Tb3+/DPA assay, 0.4 μM peptide was added to 40 μM POPC/Tb3+/50 μM DPA in 10 mM Tris/100 mM NaCl, pH 4.5. NaOH and HCl were used to adjust pH in an acidic–neutral–acidic pH cycle. The fluorescence was recorded at room temperature (25 °C) with excitation and emission wavelengths of 270 nm and 490 nm respectively and 10 nm bandwidths for both excitation and emission by using a Hitachi F-2500 fluorescence spectrometer. The percentage leakage of Tb3+ was calculated using eqn (3) as follows:
 |
(3) |
where Fmax is obtained by adding 0.05% (v/v) Triton X-100 and F0 is equivalent to the value for DMSO controls.
RESULTS
Co2+-quenching effect on the NBD-labelled peptide binding to membranes shows the reversibility of insertion depth in the pH change cycle
Figure 1 illustrates the KSV data obtained with quenching of NBD attached to HA2(1–25) embedded in the DMPC/DMPG vesicles in a pH cycle. The KSV values are seen reversible with respect to pH alteration. The pH-dependence of HA2 insertion was previously demonstrated by a photo-sensitizing labelling experiment [21].
Figure 1. Quenching of NBD-labelled HA2(1–25) by Co2+ in a pH cycle.
KSV increases with neutralization, reflecting a decrease in the insertion depth.
Self-quenching of Rho–HA2(1–25) in DMPC/DMPG vesicles in the pH cycle indicates that self-assembly of the FP is a reversible process
In Figure 2(b) the FP molecules used are completely labelled, whereas, in Figure 2(a), only 5% of the FP molecules are labelled. The result indicates that the propensity of the FP to self-assemble is reversible. Results in Figure 2(b) report mainly the intratrimeric interaction, whereas Figure 2(a) reflects primarily intertrimeric interaction, owing to a low proportion of the labelled form. Under both conditions, higher self-quenching of Rho at acidic pH indicates higher propensity for self-association of FP, suggesting that low-pH-enhanced self-assembly of FP is an important part of low-pH-triggered membrane fusion [22,23].
Figure 2. Self-quenching of Rho labelled at the N-terminus of FP to probe self-assembly of FP in the membrane bilayer under pH cycle (pH 4.8→7.3→4.7).
(a), Labelled/unlabelled FP 1:19 (w/w); (b), labelled FP only. The relative intensity is shown in the inset. The former represents inter-trimer association, whereas the latter reflects intra-trimer association. Both sets of results show that FP has higher propensity for self-assembly at acidic pH in a pH-reversible manner.
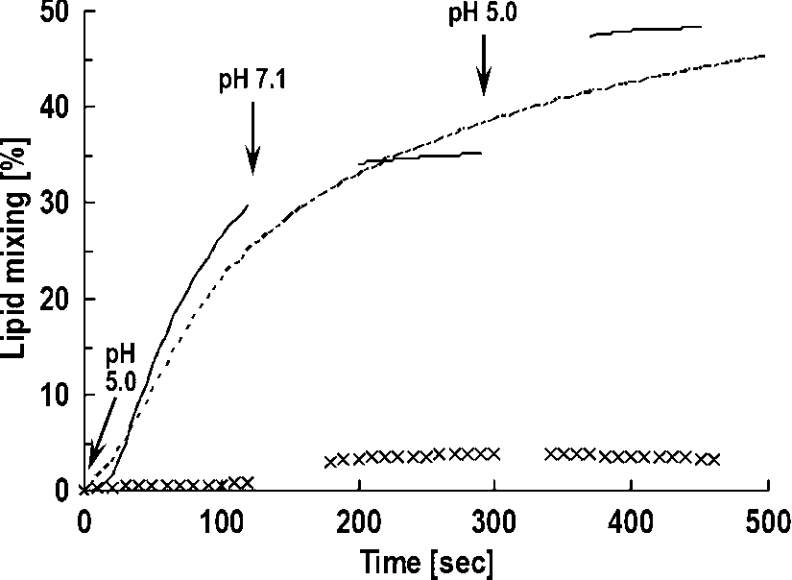
Lipid mixing assayed by dequenching of Rho–PE showed the process is pH-arrestable
Figure 3 displays dequenching of the Rho–PE that is incorporated with the DMPC vesicle measured in a pH cycle (5→7→5). An increase in the fluorescence signifies dilution of the fluorophore due to lipid mixing induced by the FP. The activation of lipid mixing by HA2(1–25) at acidic pH and its arrest at neutral pH indicate that the lipid mixing is not part of the irreversible process. A control experiment in which the pH was kept constant at 5.0 exhibited a gradual decrease in the mixing rate. Note that the extent of mixing is higher for the final low-pH stage in the data for the pH cycle than for the control result. The large increase in lipid mixing at the second stage of re-acidification displayed in Figure 3 is probably due to a better realignment of the trimeric subunits to proceed to the vesicle merger. A similar result has been documented by Stegmann et al. [12], who showed an enhanced fusion rate between influenza virus and RBC ghosts obtained with pre-incubation of the mixture at neutral pH and 0 °C. The gradual levelling off of lipid mixing is likely to be due to a decreased population of the active FPs on the vesicle surface that have free energy available to surmount the barrier to membrane merge. To further check that the observed Rho dequenching arises from organization of active FP molecules, a fusion-inactive analogue, E1-HA2(1–25) [24] was used. A near baseline intensity variation (Figure 3, ×××× trace) is found for the inactive peptide.
Figure 3. Dequenching of Rho conjugated to the membrane-incorporated PE to monitor the FP-induced lipid mixing.
The times at which the pH changes were made are indicated. ——, HA2(1–25); ××××, E1-HA2(1–25). The lack of significant dequenching for the latter inactive peptide throughout the pH variation in contrast with the wild-type FP shows that the lipid coalescence is induced by ordered alignment of FP molecules on the membrane surface, but not membrane deformation caused by non-specific peptide contact. The arrest of lipid mixing, as manifested by the ceasing of the increase in dequenching at pH 7.1 in the pH cycle, suggests that hemifusion is not an irreversible process. - - - -, Lipid mixing triggered by HA2(1–25) under a constant pH of 5.0.
Tb3+/DPA fluorescence assay shows that leakage of the aqueous probe through the membrane, mediated by FP, cannot be arrested by neutral pH
Leakage of Tb3+ from the encapsulated liposome was monitored by an enhanced Tb3+ fluorescence on combining with DPA in the external aqueous medium. As illustrated in Figure 4, Tb3+ leakage continues during the neutral-pH period after a low-pH activation of FP-mediated membrane rupture. This indicates that the leakage is not arrested by neutral pH, in sharp contrast with the data in Figure 3, which indicated an arrest in lipid mixing by neutral pH. Similarly, minimal membrane leakage can be detected for the inactive E1 analogue, demonstrating that the observed activity for HA2(1–25) is mainly due to specific interaction of the active FP molecules with the membrane.
Figure 4. Membrane leakage is pH-irreversible.
Leakage is monitored by Tb3+ fluorescence increase resulting from complexing with DPA. In the continuous curve (——) the continually increased intensity at neutral pH after acidic pH activation of the HA2(1–25)-promoted leakage shows that the process is not pH-arrestable. In the ×××× trace, the E1 analogue is shown to cause a low level of membrane rupture. In particular, the neutral pH stage does not exhibit an abrupt increase in the extent of leakage, in support of the idea that the observed membrane leakage is dominantly induced by the fusion-active FP molecules. The short horizontal traces were obtained with E1-HA2(1–25), used as a negative control for the lipid-mixing assay.
As will be discussed below, the differential results may be explained by a model of membrane fusion in which the opening of both leaflets of bilayer is part of, or subsequent to, the rate-limiting step.
DISCUSSION
To delineate the reversibility of various intermediate steps in the fusion pathway involving in particular the FP, we performed experiments on insertion depth of the FP (Figure 1) and clustering of inserted FPs (Figure 2). The latter result merits more attention, since lipid mixing requires appropriate co-operation of neighbouring FP molecules. It is proposed that the lateral diffusion of the FPs and recruitment of adjacent trimer subunits surrounding the fusing site accounts for the bulk of experimentally observed delay time in initiating membrane fusion, which decreases sharply with increased surface density of the fusion proteins [23,25]. This hypothesis is also supported by a report that the transition from reversible to irreversible forms of HA depends on its surface density [10]. Because self-association of the FP molecules is pH-reversible, Figure 2 indicates that the clustering of FP in the target membrane is not part of a rate-limiting irreversible step of the fusion event. The data presented in Figures 1 and 2 suggest that the pH-reversible insertion depth and self-association may contribute to the arrest of FP-mediated lipid mixing at neutral pH. In a previous study the secondary structure of HA2(1–25) was found be insensitive to pH variation [18]. By contrast, we observed a greater self-quenching of Rho with acidification for the labelled peptide due to longer range of interaction {of the order of 1.5 nm (15 Å) between the fluorophores; see [26]}. This property was therefore used to obtain the data shown in Figure 2. That the insertion of FP precedes lipid mixing or membrane fusion was shown by White and co-workers using the disrupted coiled-coil structure of HA that exhibited membrane insertion devoid of membrane fusion [27].
The decrease in KSV at low pH observed in Figure 1 is likely to be due to an increase in penetration depth of the N-terminal portion of the peptide, but an increase in the propensity for self-assembly (results not shown) may also contribute to KSV change. Evidence that the former change is more important comes from the secondary-structure analysis. Thus the helical structure encompassing residues 1–11 (see the supplementary data at http://www.BiochemJ.org/bj/396/bj3960557add.htm) of the FP incorporated into the membrane mimic is stabilized at pH 4.9 compared with that at pH 7.2, as revealed by coupling-constant analysis of the peptide in SDS micelles. The increase in helical structure has been shown to correlate with insertion of the FPs of influenza virus and HIV-1 into the membrane [18,28], hence the data in Supplementary Figure S1 (http://www.BiochemJ.org/bj/396/bj3960557add.htm) suggest a deeper penetration of the N-terminal half of the peptide at acidic pH. Regardless of whether the former alone or both factors cause(s) the observed pH reversibility in KSV, interaction of the FP with the membrane bilayer is clearly shown in the reversible stage of the fusion event. It is noteworthy that Pak et al. [29] have shown that the FP inserted into the target membrane in a pH-reversible manner.
Stegmann et al. [12] have found that fusion of the influenza virus with an RBC can be arrested by neutral pH and reactivated by re-acidification. We have similarly observed neutral-pH-arrested lipid mixing mediated by the HA2 FP (Figure 3). Strictly speaking, the arrest of lipid transfer by neutral pH does not reflect a reversible process, but indicates that the FPs, along with the fusing membranes, are brought by re-neutralization to a state incapable of progressing to the next stage which, however, is not necessarily the same as that prior to lowering the pH. In other words, the arrested state is not equivalent to a reversible one. Nonetheless, recovery to the fusogenic state can be obtained by re-acidification at this stage of the fusion reaction.
A comparison of Figures 3 and 4 reveals that there is a dramatic difference in the neutral-pH arrestability between the lipid mixing (membrane hemifusion) and complete rupture of the lipid bilayer. The arrest of lipid mixing implies that the step is reversible and hence does not involve a high-energy barrier. The continued increase in Tb3+ fluorescence during the neutral-pH period displayed in Figure 4 means that the opening of the membrane bilayer is irreversible and belongs to, or is subsequent to, the rate-limiting process of the fusion event.
Because the leakage of aqueous ions through the membrane bilayer requires the stable holes created by rupture of both outer and inner leaflets of the membrane (as supported by the minimal leakage caused by the inactive FP analogue), the leakage experiment is equivalent to the complete membrane fusion as far as the fusion reaction pathway is concerned. Thus the irreversibility of the ion leakage deduced from data presented in Figure 4 supports the notion that stable pore opening and growth are subsequent to the rate-limiting step and are irreversible.
The result shown in Figure 3 puts the hemifusion of membrane at the pre-trimer hairpin stage; morevover, it implies that merger of the outer leaflets of the fusing membranes does not constitute the major barrier to the fusion reaction. Rather, the coalescence of inner leaflets to create membrane continuity and generation of robust pores (according to the model proposed by Markosyan et al. [8]) probably imposes a higher energy barrier for the fusing partners to complete the fusion. Furthermore, the required energy is provided, at least in part, by helix-bundle formation. Interestingly, in a kinetic study on lipid mixing, Stegmann et al. [30] found that the lag time necessary for virus/liposome mixing is low-pH-dependent, and the process can be arrested by the neutralization that interrupted the low-pH incubation period at various time points (cf. Figure 4 in [30]), again indicating that the lipid mixing is pH-arrestable. That envelope-induced hemifusion and the nascent fusion pore formation are reversible with respect to pH is implied in a study on the avian sarcoma and leukosis virus with a class I fusion protein [31]. It was concluded that low pH is necessary for the stages leading to hemifusion, but not for pore growth – a result which supports the argument that the pore growth does not require low pH because the process is pH-irreversible and hence would proceed regardless of pH variation (i.e. with or without re-neutralization), whereas low pH is needed for reversible processes to proceed forward. The conclusion that the pore-growth step is irreversible is temporally consistent with the hypothesis that this step is energetically driven primarily by an irreversible formation of the helix hairpin in the late stage of the fusion reaction. Skehel and Wiley [32] deduced from the formation of the N-cap clamping of the N- and C-terminal regions of HA2 – presumably the last step of N- and C-helix refolding – that the free energy of capping is released at a late stage of the fusion events. This energetic consideration will be incorporated into the following fusion model.
As documented by Chernomodik et al. [33], the transition from hemifusion to complete fusion is dependent on the number of HA trimers and their interactions on the membrane surface. This implies that the self-aggregation of HA molecules plays an important role in the membrane fusion (a tight inter-trimer packing), whereas stabilizing the hemifusion diaphragm may hinder fusion-pore expansion. Therefore a non-rigid labile assembly among the adjacent trimers would be conducive to a productive fusion reaction. This contention is in line with the observation that the complete fusion is susceptible to cleavage of HA by proteinase K [33].
On the basis of the foregoing discussion, we summarize our results in terms of reversibility, arrestability and irreversibility with respect to pH to propose a working model of fusion mechanism shown in Figure 5, taking into consideration the concepts inferred from the kinetics studies [9,10]. The first step of a cascade of conformational changes is exposure of the FP and kinked linker regions of the HR1 (heptad repeat 1) and HR2 domains (Figure 5, step 1). This process is pH-reversible [10]. Insertion of the FP into the outer layer of the membrane with a concomitant induction of helical structure [18,34] releases free energy to facilitate subsequent processes; this is arrestable by neutralization, as suggested by Figure 1 (Figure 5, step 2). The membrane diffusion of FP leading to self-association can be arrested by neutral pH (Figure 2), as shown in step 3 (Figure 5). According to Figure 3, stalk formation or hemifusion (step 4, Figure 5) is also arrestable with elevating pH. The time required for the movement and juxtaposition of adjoining FP subunits may be manifested as delay time in the fusion experiments. It is noted that, in accordance with the finding by Morris et al. [14], the steps leading up to hemifusion proceed relatively rapidly. The nascent pore with a narrow passage illustrated at step 5 of Figure 5 flickers (opens and closes rapidly), this arising in part from thermal fluctuation (as revealed by patch-clamp experiments [35,36]), and eventually dilates to a long-lived pore with the required energy provided by interaction between FP and the transmembrane domain, as well as the formation of helix hairpin bundle [8] synchronized by adjacent trimer subunits. That stable pore formation is irreversible is also supported by a Tb3+/DPA leakage experiment (Figure 4). Experimental evidence of a significant free-energy barrier for the fusion intermediates to transit to complete fusion was provided by Markosyan et al. [9] from a kinetics study on HA-mediated fusion. Interestingly, Chernomodik et al. [33] demonstrated that lipid mixing and narrow-pore opening occurred at approximately the same time during the fusion between HA-expressing cells and RBC, implicating a partial overlap of steps 4 and 5.
Figure 5. Proposed working model of HA-mediated membrane fusion from the perspective of pH-reversibility.

Step 1: exposure of FP and the kinked loop (residues 106–112) induced by low pH (pH-reversible). Step 2: insertion of FP into target membrane (insertion depth, pH-reversible). Step 3: self-assembly of the fusion proteins. In this step, several HA2 trimers form a cluster by lateral diffusion and proceed to form fusion stalk and fusion pore. The process may account for the observed delay in the lipid mixing experiments. Step 4: stalk formation and hemifusion of membranes (restricted and unrestricted lipid mixing) by synchronized action of adjacent trimers (pH-arrestable). Step 5: fluctuating (flickering) nascent pore formation. A large-scale movement of a host of fusion protein molecules is required. The step consists of irreversible six-helix bundle formation and co-ordinated release of spring-loaded conformational energy of trimers at the fusion site to sustain and enlarge the initially narrow fusion pore (pH-irreversible). Step 6: a robust fusion pore of the enlarged pore to support the transport of viral nucleocapsid. This step is characterized by concerted formation of the final six-helix bundle structure of adjacent trimers to liberate free energy sufficient to overcome the barrier and realignment of trimers at the fusion site during the pore enlargement. This step does not require low pH to proceed, but is temperature-dependent (pH-irreversible). However, co-operative actions of adjacent trimers effectively lower the kinetic hurdle to the stabilized pore in a kinetically controlled irreversible process. It is stressed that the Tb3+ leakage deduced from Figure 4 is irreversible, which corroborates the concept that the sustained opening of both leaflets of the bilayer is a post-rate limiting step – hence the processes leading up to the hemifusion is rapid, involving low energy barriers.
As shown previously by Leikina et al. [10], exposure of the FP and change in the kinked loop of HA2 (encompassing residues 102–107) are reversible. Similarly, exposure of the FP and the ectodomain of HA has been shown to be pH-reversible [3]. That the insertion of FP into the target membrane precedes the refolding of C-helix to the groove formed by the N-terminal coiled coil is consistent with the finding that binding of the FP domain was not affected, but the membrane fusion was impaired, by a double mutation at positions 63 and 70 of HA2 that disrupted coiled-coil formation [27]. In our model, steps 4 and 5, namely the formation of the lipid stalk and the nascent pore, are at the committed state [8,37] characterized by progress to complete fusion in a highly temperature-dependent fashion. We also note that flickering (rapid opening and closure) of the nascent pore observed in electrophysiological experiments is also reversible [35] at step 5 in our model. It implies that this process precedes the irreversible formation of six-helix hairpin shown in the low-pH crystallographic structure [2,38] and irreversible stable pore formation (Figure 4).
Dissociation of HA1 headgroups is assumed to occur between steps 5 and 6. The pH-irreversibility of the pore enlargement is underscored in part by the thermal stability of the helix bundle and the fact that it exists at both acidic and neutral pH [5]. Moreover, completion of packing of the C-helix domain against the N-terminal coiled coil of the fusion protein should take place during the coalescence of the two inner leaflets to provide free energy to facilitate the close-up of fusing membranes. Therefore the latter part of step 5 would overlap step 6. That the helix-bundle formation is at a post-lipid mixing stage can be inferred from the finding of Muñoz-Barroso et al. [39] on the inhibition of aqueous content mixing induced by HIV-1 fusion protein with a C-helix peptide up to 15 min after co-culture of the effector and target cells. In the final steps of the fusion event, co-operation of adjoining trimer subunits facilitates surmounting of the barrier to the formation of stable, enlarged pores. Because clustering of trimers is likely to be favoured by low pH (Figure 2), the final process is irreversible and kinetically controlled [40]; this means that the last step proceeds under both neutral and acidic conditions, although faster at low pH.
The inactivation of some strains of influenza virus (e.g. X-31) by pre-incubation in the absence of target membrane may be explained by the notion that free energy released upon the helix hairpin formation cannot be used to effect the fusion under this condition. This energy cannot be stored or retrieved as the target membrane approaches at a later time. Nontheless, the energetic aspect of the fusion cascade holds regardless of the molecular mechanism of the low-pH inactivation of influenza virus.
Conclusion
In combination with the results obtained from other pH-dependent changes for HA2 properties, our data suggest some steps in the pathway of the pH-triggered fusion reaction. The insertion depth and angle, the self-association and exposure of the FP domain are reversible in response to pH change and therefore would participate in the pH-induced structural and topological rearrangements of HA2, leading to membrane mixing. By contrast, stable opening of both leaflets of the membrane is irreversible. The overall secondary structure (especially the helix structure) of the FP domain, on the other hand, may not be very sensitive to pH alteration [41].
Online data
Acknowledgments
This work is supported by National Science Council (grant NSC-93-2113-M-001-018 to D.K.C.) and Academia Sinica, Republic of China.
References
- 1.Wilson I. A., Skehel J. J., Wiley D. C. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 Å resolution. Nature (London) 1981;289:366–373. doi: 10.1038/289366a0. [DOI] [PubMed] [Google Scholar]
- 2.Bullough P. A., Hughson F. M., Skehel J. J., Wiley D. C. Structure of influenza haemagglutinin at the pH of membrane fusion. Nature (London) 1994;371:37–43. doi: 10.1038/371037a0. [DOI] [PubMed] [Google Scholar]
- 3.Korte T., Herrmann A. pH-dependent binding of the fluorophore bis-ANS to influenza virus reflects the conformational change of hemagglutinin. Eur. Biophys. J. 1994;23:105–113. doi: 10.1007/BF00208864. [DOI] [PubMed] [Google Scholar]
- 4.Murata M., Sugahara Y., Takahashi S., Ohnishi S. pH-dependent membrane fusion activity of a synthetic twenty amino acid peptide with the same sequence as that of the hydrophobic segment of influenza hemagglutinin. J. Biochem. (Tokyo) 1987;102:957–962. doi: 10.1093/oxfordjournals.jbchem.a122137. [DOI] [PubMed] [Google Scholar]
- 5.Chen J., Wharton S. A., Weissenhorn W., Calder L. J., Hughson F. M., Wiley D. C. A soluble domain of the membrane-anchoring chain of influenza virus hemagglutinin (HA2) folds in Escherichia coli into low-pH-induced conformation. Proc. Natl. Acad. Sci. U.S.A. 1995;92:12205–12209. doi: 10.1073/pnas.92.26.12205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carr C. M., Kim P. S. A spring-loaded mechanism for the conformational change of influenza hemagglutinin. Cell. 1993;73:823–832. doi: 10.1016/0092-8674(93)90260-w. [DOI] [PubMed] [Google Scholar]
- 7.Lu M., Blacklow S. C., Kim P. S. A trimeric structural domain of the HIV-1 transmembrane glycoprotein. Nat. Struct. Biol. 1995;2:1075–1082. doi: 10.1038/nsb1295-1075. [DOI] [PubMed] [Google Scholar]
- 8.Markosyan R. M., Cohen F. S., Melikyan G. B. HIV-1 envelope proteins complete their folding into six-helix bundle immediately after fusion pore formation. Mol. Biol. Cell. 2003;14:926–38. doi: 10.1091/mbc.E02-09-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Markosyan R. M., Melikyan G. B., Cohen F. S. Evolution of influenza virus hemagglutinin-mediated fusion by kinetic measurements of pore formation. Biophys. J. 2001;80:812–821. doi: 10.1016/S0006-3495(01)76060-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leikina E., Ramos C., Markovic I., Zimmerberg J., Chernomodik V. Reversible stages of the low-pH-triggered conformational change in influenza virus hemagglutinin. EMBO J. 2002;21:5701–5710. doi: 10.1093/emboj/cdf559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wharton S. A., Martin S. R., Ruigrok R. W., Skehel J. J., Wiley D. C. Membrane fusion by peptide analogs of influenza virus hemagglutinin. J. Gen. Virol. 1988;69:1847–1857. doi: 10.1099/0022-1317-69-8-1847. [DOI] [PubMed] [Google Scholar]
- 12.Stegmann T., Hoekstra D., Scherphof G., Wilschut J. Fusion activity of influenza virus. J. Biol. Chem. 1986;261:10966–10969. [PubMed] [Google Scholar]
- 13.Ramalho-Santos J., Nir S., Duzgunes N., Carvalho A. P., Lima M. C. P. A common mechanism for influenza virus fusion activity and inactivation. Biochemistry. 1993;32:2771–2779. doi: 10.1021/bi00062a006. [DOI] [PubMed] [Google Scholar]
- 14.Morris S. J., Sarkar D. P., White J. M., Blumenthal R. Kinetics of pH-dependent fusion between 3T3 fibroblasts expressing influenza hemagglutinin and red blood cells. J. Biol. Chem. 1989;264:3972–3978. [PubMed] [Google Scholar]
- 15.Tatulian S. A., Tamm L. K. Reversible pH-dependent conformational change of reconstituted influenza hemagglutinin. J. Mol. Biol. 1996;260:312–316. doi: 10.1006/jmbi.1996.0402. [DOI] [PubMed] [Google Scholar]
- 16.Rapaport D., Shai Y. Aggregation and organization of pardaxin in phospholipid membranes. A fluorescence energy transfer study. J. Biol. Chem. 1992;267:6502–6509. [PubMed] [Google Scholar]
- 17.Kantchev E. A. B., Cheng S. F., Wu C. W., Huang H. J., Chang D. K. Secondary structure, phospholipid membrane interactions, and fusion activity of two glutamate-rich analogs of influenza hemagglutinin FP. Arch. Biochem. Biophys. 2004;425:173–183. doi: 10.1016/j.abb.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 18.Chang D. K., Cheng S. F., Trivedi V. D., Yang S. H. The amino-terminal region of the fusion peptide of influenza virus hemagglutinin HA2 inserts into sodium dodecyl sulfate micelle with residues 16–18 at the aqueous boundary at acidic pH. J. Biol. Chem. 2000;275:19150–19158. doi: 10.1074/jbc.M907148199. [DOI] [PubMed] [Google Scholar]
- 19.Sainz B., Jr, Rausch J. M., Gallaher W. R., Garry R. F. Identification and characterization of the putative fusion peptide of the severe acute respiratory syndrome-associated coronavirus spike protein. J. Virol. 2005;79:7195–7206. doi: 10.1128/JVI.79.11.7195-7206.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bacigalupo M. A., Ius A., Longhi R., Meroni G. Homogeneous immunoassay of atrazine in water by terbium-entrapping liposomes as fluorescent markers. Talanta. 2003;61:539–545. doi: 10.1016/S0039-9140(03)00320-5. [DOI] [PubMed] [Google Scholar]
- 21.Pak C. C., Krumbiegel M., Blumenthal R., Raviv Y. Detection of influenza hemagglutinin with biological membrane by photosensitized activation of [125I]iodonaphthyl azide. J. Biol. Chem. 1994;269:14614–14619. [PubMed] [Google Scholar]
- 22.Danieli T., Pelletiet S. L., Henis Y. I., White J. M. Membrane fusion mediated by the influenza hemagglutinin requires the concerted action of at least three hemagglutinin trimers. J. Cell Biol. 1996;133:559–569. doi: 10.1083/jcb.133.3.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clague M. J., Schoch C., Blumenthal R. Delay time for influenza virus hemagglutinin-induced membrane fusion depends on hemagglutinin surface density. J. Virol. 1991;65:2402–2407. doi: 10.1128/jvi.65.5.2402-2407.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qiao H., Armstrong R. T., Melikyan G. B., Cohen F. S., White J. M. A specific point mutant at position 1 of the influenza hemagglutinin fusion peptide displays a hemifusion phenotype. Mol. Biol. Cell. 1999;10:2759–2769. doi: 10.1091/mbc.10.8.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ellens H., Bentz J., Mason D., Zhang F., White J. M. Fusion of influenza hemagglutinin-expressing fibroblasts with glycophorin-bearing liposomes: role of hemagglutinin surface density. Biochemistry. 1990;29:9697–9707. doi: 10.1021/bi00493a027. [DOI] [PubMed] [Google Scholar]
- 26.Cheng S. F., Kantchev E. A. B., Chang D. K. Fluorescence evidence for a loose self-assembly of the fusion peptide of influenza virus HA2 in the lipid bilayer. Mol. Membr. Biol. 2003;20:345–351. doi: 10.1080/0968708031000138046. [DOI] [PubMed] [Google Scholar]
- 27.Gruenke J. A., Armstrong R. T., Newcomb W. N., Brown J. C., White J. M. New insights into the spring-loaded conformational change of influenza virus hemagglutinin. J. Virol. 2002;76:4456–4466. doi: 10.1128/JVI.76.9.4456-4466.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang D. K., Cheng S. F., Chien W. J. The amino-terminal fusion domain peptide of HIV-1 gp41 inserts into the sodium dodecyl sulfate micelle primarily as a helix with a conserved glycine at the micelle–water interface. J. Virol. 1997;71:6593–6602. doi: 10.1128/jvi.71.9.6593-6602.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pak C. C., Puri A., Blumenthal R. Conformational changes and fusion activity of vesicular stomatitis virus glycoprotein: [125I]iodonaphthyl azide photolabeling studies in biological membranes. Biochemistry. 1997;36:8890–8896. doi: 10.1021/bi9702851. [DOI] [PubMed] [Google Scholar]
- 30.Stegmann T., White J. M., Helenius A. Intermediates in influenza induced membrane fusion. EMBO J. 1990;9:4231–4241. doi: 10.1002/j.1460-2075.1990.tb07871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Melikyan G. B., Barnard R. J. O., Markosyan R. M., Young J. A. T., Cohen J. S. Low pH is required for avian sarcoma and leucosis virus env-induced hemifusion and fusion pore formation but not for pore growth. J. Virol. 2004;78:3753–3762. doi: 10.1128/JVI.78.7.3753-3762.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skehel J. J., Wiley D. C. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu. Rev. Biochem. 2000;69:531–569. doi: 10.1146/annurev.biochem.69.1.531. [DOI] [PubMed] [Google Scholar]
- 33.Chernomodik L. V., Frolov V. A., Leikina E., Bronk P., Zimmerberg J. The pathway of membrane fusion catalyzed by influenza hemagglutinin: restriction of lipids, hemifusion, and lipidic fusion pore formation. J. Cell. Biol. 1998;140:1369–1382. doi: 10.1083/jcb.140.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vaccaro L., Cross K. J., Kleinjung J., Straus S. K., Thomas D. J., Wharton S. A., Skehel J. J., Fraternali F. Plasticity of influenza fusion peptides and their interaction with lipid bilayers. Biophys. J. 2005;88:25–36. doi: 10.1529/biophysj.104.044537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spruce A. E., Iwata A., White J. M., Almers W. Patch clamp studies of single cell-fusion events mediated by a viral fusion protein. Nature (London) 1989;342:355–358. doi: 10.1038/342555a0. [DOI] [PubMed] [Google Scholar]
- 36.Melikyan G. B., Lin S., Roth M. G., Cohen F. S. Amino acid sequence requirements of transmembrane and cytoplasmic domains of influenza virus hemagglutinin for viable membrane fusion. Mol. Biol. Cell. 1999;10:1821–1836. doi: 10.1091/mbc.10.6.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schoch C., Blumenthal R., Clague M. J. A long-lived state for influenza virus–erythrocyte complexes committed to fusion at neutral pH. FEBS Lett. 1992;311:221–225. doi: 10.1016/0014-5793(92)81107-w. [DOI] [PubMed] [Google Scholar]
- 38.Chen J., Skehel J. J., Wiley D. C. N- and C-terminal residues combine in the fusion-pH influenza hemagglutinin HA2 subunit to form an N-cap that terminate triple-stranded coiled coil. Proc. Natl. Acad. Sci. U.S.A. 1999;96:8967–8972. doi: 10.1073/pnas.96.16.8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muñoz-Barroso I., Durell S., Sakaguchi K., Appella E., Blumenthal R. Dilation of the human immunodeficiency virus-1 envelope glycoprotein fusion pore revealed by the inhibitory action of a synthetic peptide from gp41. J. Cell Biol. 1998;140:315–323. doi: 10.1083/jcb.140.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baker D., Agard D. A. Influenza hemagglutinin: kinetic control of protein function. Structure. 1994;2:907–910. doi: 10.1016/s0969-2126(94)00091-3. [DOI] [PubMed] [Google Scholar]
- 41.Gray C., Tamm L. K. pH-induced conformational changes of membrane-bound influenza hemagglutinin and its effect on target lipid bilayers. Protein Sci. 1998;7:2359–2373. doi: 10.1002/pro.5560071113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.