Abstract
Objective
To determine how subjects responded to alarms for hypo- and hyperglycemia while they were sleeping.
Research Design and Methods
Twenty subjects with type 1 diabetes (ages 4–17 years) were admitted to a clinical research center for approximately 24 hours. Each subject wore two GlucoWatch® G2TM Biographers (GW2B) and was videotaped using an infrared camera from 9 PM to 7 AM. The videotapes were reviewed to determine if the GW2B alarms were audible on the tape and to document the subject’s response to the alarms. Single or multiple alarms separated by at least ½ hour were considered as discreet alarm “events”.
Results
Downloaded data from the biographers identified 240 individual alarms, 75% of which occurred while the subject was sleeping. Of the 240 alarms 68% were audible on the videotape. Subjects awoke to 29% of individual alarms and to 66% of alarm events. Subjects 4 to 6 years old responded to 17% of alarms; 7 to 11 year olds responded to 20% of alarms, adolescents responded to 53% of alarms, and parents to 37% of alarms. Subjects awoke to 40% of the first alarm during the night, but to only 28% of subsequent alarms. There were 11 events when the glucose was confirmed ≤70 mg/dL, and in each case the subject was awoken. Fifty-five percent of alarm events occurred when there was no hypo- or hyperglycemia confirmed by a reference glucose value.
Conclusions
Subjects awoke to 29% of individual alarms and to 66% of alarm events. Subjects awoke during all alarm events when hypoglycemia was confirmed, but there was a high incidence of false alarms.
Introduction
Nocturnal hypoglycemia is a major concern for patients with type 1 diabetes mellitus (T1DM) (1), and 75% of hypoglycemic seizures occur at night (2). In young children there is concern that severe hypoglycemic events can cause permanent neurologic sequela (3), and in people with type 1 diabetes there is a 6% lifetime risk of “dead-in-bed” (4) which may in part be a result of severe nocturnal hypoglycemia. The GlucoWatch® G2TM Biographer (GW2B, Cygnus, Inc., Redwood City, CA) was the first device approved for home use which provides near real-time glucose measurement (5). After a 2-hour warm up, the GW2B provides glucose readings every 10 minutes for up to 13 hours. The GW2B is equipped with an alarm that sounds when: 1) the measured glucose drops below the level set by the user (hypoglycemia alarm), 2) the trend of consecutive glucose values projects that a glucose value less than the hypoglycemia alarm target will be reached within 20 minutes (down alert alarm), and 3) when the measured glucose is above the level set by the user (hyperglycemic alarm). Once an alarm is initiated, it will continue to beep until either the subject turns off the alarm, or the GW2B glucose reading (determined every 10 minutes) has recovered from the alarm limit and is in a “normal” range. When the biographer is alarming for out of range glucose levels the beep will become more frequent and persistent, but it will not increase in decibels. Because of the lag time between the sampling of the interstitial fluid and the appearance of the glucose value on the device, the hypoglycemia alarm and hyperglycemic alarm reflect the blood glucose level from approximately 18 minutes earlier. The down alert alarm, on the other hand, counterbalances the 18 minute lag time by estimating, based on the blood glucose trend, that the BG may be less than the hypoglycemic target 20 minutes in advance. A previous study described increased detection of night time hypoglycemia when children wore the GlucoWatch compared to children not wearing the device (6).
One of the potential benefits of a “real-time” glucose sensor is that it can obtain frequent glucose values overnight and sound an alarm if glucose values are becoming dangerously high or low while the wearer is sleeping. The accuracy of the GW2B has been previously reported (7–10). As an ancillary project to a Diabetes Research in Children Network (DirecNet) inpatient, clinical research center study that evaluated the accuracy of the GW2B in children with T1DM (9), subjects were videotaped while they were sleeping to determine their response to GW2B alarms. The ancillary study was conducted at two of the DirecNet centers, Stanford University and Barbara Davis Diabetes Center and the goal was to determine if subjects and/or parents heard the alarm, and if they responded to the alarm. As the actual blood glucose increased or decreased to an alarm threshold, there could be single or multiple alarms surrounding this event. We therefore analyzed individual alarms as well as alarm “events” defined as multiple alarms associated with a single hypo or hyperglycemic episode.
Research Design and Methods
The DirecNet Data and Safety Monitoring Board and the Institutional Review Boards at Stanford and the University of Colorado approved the study protocol, consent form and assent form. A parent or guardian gave written consent and subjects 7 years of age or older gave written assent prior to the performance of study procedures.
GlucoWatch 2 biographers were set to alarm for glucose values ≤70 mg/dL (low) and ≥300 mg/dL (high). Both the parents and nurses were instructed to wait at least 5 minutes before responding to alarms overnight to allow the subject an opportunity to awaken and respond. If the GW2B was reading less than or equal to 70 mg/dL, the subject, nurse, or parent was instructed to obtain a One Touch Ultra (Lifescan, Milpitas, CA) glucose value. Reference glucose values were obtained every ½ hour overnight and additional reference levels were obtained whenever the Ultra value at the time of a GW2B alarm was less than or equal to 70 mg/dL. Reference glucose values were obtained from a peripheral intravenous catheter, centrifuged and the serum was shipped frozen on dry ice to the DirecNet Central Biochemistry Laboratory at the University of Minnesota. Reference glucose levels were determined using a hexokinase enzymatic method (11). The results of the reference glucose values were not available the night of the study. The number of true hypoglycemic (≤70 mg/dL) or hyperglycemic (≥300 mg/dL) episodes was based on glucose values measured by the central laboratory. Subjects wore two GW2B devices overnight. There is a 2 hour initialization for each GW2B during which glucose values are not available, therefore start times were staggered so there would not be a time during the night that at least one GW2B was not functioning.
The subjects were videotaped using a Sony TRV 308 video camera equipped with an infrared light source from 9 PM to 7AM. Each videotape was reviewed and scored by two observers, one at each institution (Stanford and Denver). The GW2B alarm times were obtained from a computer download of stored data in the GW2B. Tape reviewers were provided with a list of the alarm times from the download and, unknown the to reviewers, the list also included three to four “pseudo-alarm” times. “Pseudo alarms” are defined as times when there was no GW2B alarm and were included as a measure of inter-reviewer reliability. Pseudo-alarm times were randomly chosen during the night after the subject was recorded to have fallen asleep and before they awoke in the morning. The time the subject fell asleep was recorded and all GW2B alarms after the subject fell asleep were evaluated by viewing the video tape beginning 3 minutes prior to and 3 minutes following each alarm. The tape was reviewed to determine if the alarm was audible to the reviewer, if the subject was awoken by the alarm, and to determine the subject’s response to the alarm. When the subject did not spontaneously awaken to an alarm, the response was coded as “no response.” If the subject was already awake from a previous alarm, when a new alarm was audible, the alarm was not evaluated as an alarm while sleeping. If a parent or nurse awoke the subject, this was also recorded as “no response” since the subject did not spontaneously awaken. Multiple alarms could occur surrounding a decrease or increase in the blood glucose towards an alarm set point. Because many alarms can occur surrounding a change in blood glucose, GW2B alarm “events” are defined as a one or more alarms separated from previous alarms by more than 30 minutes.
Statistics
Agreement between reviewers was assessed by calculating the percent concordance and the unweighted kappa. For all other analyses, data from the two observers were combined. In the case of disagreement between observers, the positive observation dominated. For example, if either observer reported the subject was awakened by the alarm, the subject was considered awakened. Permutation tests were used to account for repeated measures from the same subject in the comparisons of subgroups. Age, HbA1c and time of night were treated as continuous variables in the statistical comparisons. Because multiple comparisons were made in this analysis, p-values > 0.01 were not considered statistically significant. All statistics were calculated using SAS version 8.2 (SAS Institute, Cary, NC).
Results
Twenty-eight subjects with type 1 diabetes consented to be videotaped overnight while wearing one or two GW2B devices. Eight subjects were excluded either because there were no alarms overnight (n=3), all alarms were prior to the subject falling asleep (n=2), the subject had fallen asleep but was awakened prior to the alarm sounding (n=2), or the alarm was not captured on videotape (n=1). Data from the 20 subjects who had GW2B alarms while they were sleeping are analyzed in this report. The ages of the subjects ranged from 4 to 17 years (mean 11 ± 4 years). There were 12 females and 8 males, and 6 were using insulin infusion pumps. The mean A1c was 8.1 ± 1.0. Eighteen of twenty subjects had 2 GW2B devices functioning at the same time for an average of 4.7 hours (because of staggered start of the GW2B devices). Therefore 42% of the time the subject was wearing two functioning GW2B devices. Seven subjects had a parent sleeping in the bed next to them. There were 240 discrete, individual alarms overnight: 100 low blood glucose alarms, 86 down alerts and 54 alarms for a high blood glucose level (Table 1). There were 50 alarm events, 38 for hypoglycemia and 12 for hyperglycemia (Table 1). An example of the GlucoWatch values and alarms, and reference and Ultra blood glucose values and alarm events is given in the Figure. This subject had 24 alarms in 6 events associated with decreases of the blood glucose.
Table 1.
Summary of Alarms
| Subjects with Night Alarms | 20 |
| GWB Alarms | |
| Total Individual Alarms | 240 |
| High Blood Glucose Alarms | 54 |
| Low Blood Glucose Alarms | 100 |
| Down Alerts | 86 |
| Patient Sleep Status | |
| Awake | 60 |
| Sleeping | 180 |
| Alarms Audible on Tape | |
| Either Center | 164 |
| Colorado | 142 |
| Stanford | 147 |
| GWB Alarm Events | |
| Total Alarm Events | 50 |
| Hyperglycemic Alarm Events | 12 |
| Hypoglycemic Alarm Events | 38 |
Figure.
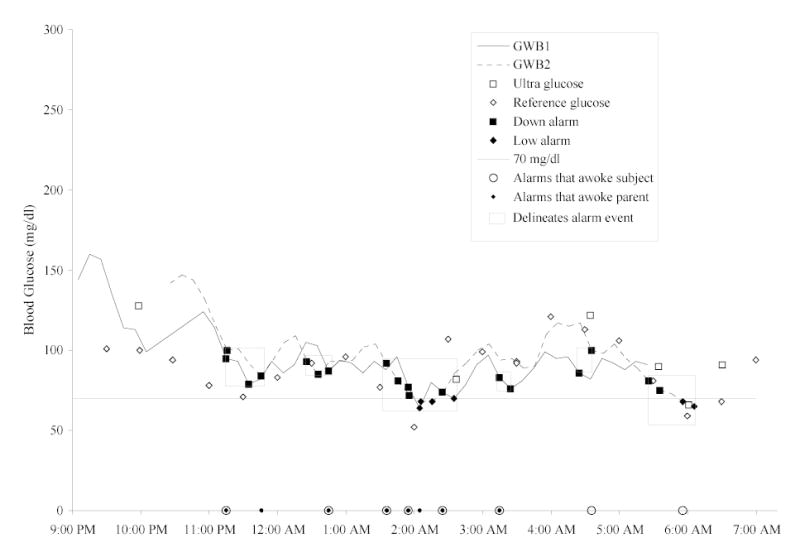
On this night two GlucoWatch biographers were worn (GWB 1 = solid line, and GWB 2 = broken line). Down alarms (▪), and low alarms (♦) are marked on the GlucoWatch plots. Reference (⋄) and Ultra (□) glucose values are plotted as discrete data points. Along the bottom of the graph, alarms that awoke the subject are given as large open circles (○) and alarms that awoke the parent are given as small solid circles (•). Alarm events are denoted by a box.
One-hundred and three “pseudo” alarms (reviewers were told there was an alarm when there actually was no alarm) were introduced in the data, and the reviewers had 95% concordance in their evaluation of these pseudo alarms. For tape reviews where a true alarm occurred the concordance between the two reviewers was 87% (Kappa 0.72). In determining whether a subject had awakened the reviewers concurred 92% of the time (Kappa 0.71). Thirty-two percent of the alarms were not audible on the videotape. It was often difficult to visualize where the arm wearing the watch was located unless it was above the bedcovers. An arm wearing a watch could be under a pillow, under the blankets, or the subject could be lying on the arm wearing the watch. There was 1 instance where the subject responded to an alarm that was not audible to the reviewers on the videotape.
There were 180 individual alarms from 20 children while they were sleeping and 75 alarms occurred when a parent was present in the room. There was a wide range in the number of alarms occurring each night while subjects were sleeping: 1–5 alarms (9 subjects), 7–9 alarms (5 subjects), 14–20 alarms (4 subjects), and 22–24 alarms (2 subjects). Individual alarms woke the subjects 29% of the time and the parent 37% of the time. There was no difference in the response rate between males and females (Table 2). Younger children appeared less likely to respond to alarms, but the trend was only marginally significant due to the small number of subjects (p=.02; Table 2). There was a 40% chance that subjects would awaken to an alarm if it was the first alarm of the night; after the 1st alarm there was a 28% chance of the subject waking to any given alarm (p=.33). When the night was divided into 3 blocks of time (9 PM to 1AM, 1AM to 3 AM, and 3 AM to 7 AM), there was no significant difference in the rate of subjects awakening to the alarms in the first, middle or last time period. Pump users awoke 40% of the time and non-pump users awoke 24% of the time (p=.28).
Table 2.
Response to Alarms (when subject is asleep)
| Individual Alarms | Alarm Events | |||||||
|---|---|---|---|---|---|---|---|---|
| Subjects | Alarms | Awakened | p-valuea | Subjects | Events | Awakened | p-valuea | |
| OVERALL | 20 | 180 | 53 (29%) | 19b | 44 | 29 (66%) | ||
| AGE | .02 | .53 | ||||||
| Age 4–6 | 5 | 30 | 5 (17%) | 5 | 7 | 5 (71%) | ||
| Age 7–11 | 7 | 95 | 19 (20%) | 7 | 20 | 12 (60%) | ||
| Age 12–18 | 8 | 55 | 29 (53%) | 7 | 17 | 12 (71%) | ||
| GENDER | .30 | .50 | ||||||
| Male | 8 | 65 | 25 (38%) | 7 | 20 | 15 (75%) | ||
| Female | 12 | 115 | 28 (24%) | 12 | 24 | 14 (58%) | ||
| INSULIN ROUTE | .28 | .65 | ||||||
| Pump | 6 | 62 | 25 (40%) | 5 | 12 | 9 (75%) | ||
| Multiple Injections | 14 | 118 | 28 (24%) | 14 | 32 | 20 (63%) | ||
| HbA1c | .84 | .51 | ||||||
| < 8.0% | 9 | 87 | 24 (28%) | 8 | 20 | 15 (75%) | ||
| ≥ 8.0% | 11 | 93 | 29 (31%) | 11 | 24 | 14 (58%) | ||
| ALARM TIMEb | .33 | 1.0f | ||||||
| First | 20d | 20 | 8 (40%) | 19d | 19 | 13 (68%) | ||
| Subsequent | 19d | 160 | 45 (28%) | 12d | 25 | 16 (64%) | ||
| NUMBER GWBs ALARMINGc | .61 | .23 | ||||||
| One | 20d | 143 | 44 (31%) | 17d | 34 | 19 (56%) | ||
| Both | 7d | 37 | 9 (24%) | 7d | 10 | 10 (100%) | ||
| TIME OF NIGHT | .86 | .32 | ||||||
| 9pm – 1am | 13d | 53 | 9 (17%) | 13d | 15 | 11 (73%) | ||
| 1am – 3am | 15d | 61 | 23 (38%) | 12d | 14 | 6 (43%) | ||
| 3am – 7am | 11d | 66 | 21 (32%) | 8d | 15 | 12 (80%) | ||
| HYPOGLYCEMIC ALARMSe | .30 | .48 | ||||||
| Glucose confirmed ≤ 70 | 6d | 10 | 3 (30%) | 8d | 11 | 11 (100%) | ||
| Glucose confirmed > 70 | 6d | 16 | 2 (13%) | 7d | 10 | 9 (90%) | ||
| HYPERGLYCEMIC ALARMSe | 1.0f | .67 | ||||||
| Glucose confirmed ≥ 300 | 2d | 3 | 0 | 3d | 3 | 1 (33%) | ||
| Glucose confirmed < 300 | 4d | 14 | 0 | 3d | 7 | 1 (14%) | ||
– Permutation test.
– One subject was awake at the start of their only alarm event, but fell asleep for several alarms during the event.
– Individual alarm classified as “Both” if the other GW2B alarmed within previous 10 minutes. Alarm event classified as “Both” if each GW2B alarmed at least once during the event.
– The same subject can be in more than one group.
– There were 26 individual hypoglycemic alarms and 17 individual hyperglycemic alarms with a reference value available. There were 21 hypoglycemic alarm events and 10 hyperglycemic alarm events with a reference value available.
– All possible permutations resulted in a difference larger than or equal to that actually observed.
There were 44 distinct alarm events defined by GW2B alarms separated by at least 30 minutes while the subject was sleeping (Table 2). The median duration for an alarm event was 25 minutes (25th–75th percentiles = 10 – 70 minutes). The subject awoke during 29 (66%) of these events. Of these 29 events, the subject awoke to the first alarm of the event 18 times (62%) and for the other 11 events, the mean time to awakening was 58 minutes. A reference glucose was available for 21 hypoglycemic alarm events that occurred while the subject was sleeping. In 11 cases the reference glucose was less than or equal to 70 mg/dL and the subject awakened all 11 times. For the 10 alarm events where the reference glucose was > 70 mg/dL, the subject awakened for 9 (90%). There were 10 alarm events for hyperglycemia while the subject was sleeping with a reference glucose value available. In 3 cases the reference glucose was greater than or equal to 300 mg/dL and the subject awoke once. For the 7 alarm events where the reference glucose was < 300 mg/dL, there was 1 awakening (14%).
Discussion
The GW2B is the first glucose sensor to provide alarms in real time and provides important information on how future real-time sensors may work when children are sleeping. Overall subjects awoke to 29% of individual alarms and to 66% of alarm events. One of our concerns was that children would not respond to an alarm when they were truly hypoglycemic. It was therefore encouraging that children awoke to at least one alarm for all 11 alarm events surrounding a confirmed hypoglycemic event while they were sleeping. During 55% (6/11) of alarm events, the subject awoke to the first alarm, but for 45% (5/11) of events it took a mean of 56 minutes to awaken to multiple alarms (in 2 of these 5 cases subjects were wearing 2 functional biographers and both biographers were alarming). We were concerned that subjects may have awoken more frequently when a nurse was entering the room to obtain a reference blood glucose, however this was not a significant factor. Subjects awoke to 71% of alarm events when a reference glucose was obtained, and to 54% of alarm events when a reference glucose was not obtained (p=.11). Since subjects did awaken to alarms while they were hypoglycemic, this makes continued development of real-time hypoglycemic alarms an important consideration for future near-continuous, real-time glucose sensors. In the present study, 48% (10/21) of GW2B alarms for a hypoglycemic event were “false positive” alarms and the subject was not hypoglycemic. This rate of false positive alarms may have an impact on the quality of the users sleep as well as their willingness to use the device. In the previously reported DirecNet study of 90 subjects, the GW2B had a sensitivity (true positive rate) of 23% and false positive rate of 51% (7). Because the GW2B was worn on the forearm, the alarm can be inaudible if the arm is under a pillow, under blankets or under the subject. In reviewing the videotapes, the alarm was not heard by the reviewers 32% of the time. The functionality of a nocturnal alarm could be improved if the alarm signal was also transmitted to a bedside monitor. Such a monitor could have its own audible alarm or it could control another device such as a bedside light. Since parents had a higher response rate to alarms (37%) than did younger children (17%), a remote monitor, perhaps in the parent’s bedroom, could be helpful.
Overall there were 240 individual alarms with 11 episodes of confirmed hypoglycemia and 3 episodes of confirmed hyperglycemia. Eleven of the 20 subjects had more than 5 alarms each night. After the first alarm, there was a trend to have a lower response rate to subsequent alarms (40% vs. 28%). Older children responded to 53% of alarms and younger children responded to 17% of alarms, however because of the correlated data from the same subject (i.e., multiple alarms from the same subject are not statistically independent) this was only marginally significant. In using the GlucoWatch during a diabetes camp (12), it had been our clinical impression that females tended to awaken more frequently to an alarm when compared to males. In the present study this was not confirmed, as both males and females had similar response rates to GW2B alarms. Pump use and HbA1c levels did not significantly effect the frequency of subjects awakening to an alarm. It was difficult for the GW2B alarm to awaken subjects when they were hyperglycemic, whereas all subjects awoke when they were hypoglycemic. We postulate this higher arousal threshold may have been due to catecholamine release in response to the hypoglycemia. Subjects did not have EEG monitoring and we could not determine what stage of sleep they were in when alarms sounded. For each hypoglycemic episode there were often many alarms, both down alert and hypoglycemic alarms, that persisted until addressed. Forty-two percent of the time subjects were wearing two functional biographers so it may have been multiple alarms that awoke them, and with two functional biographers there was a lower probability that an alarm would not be heard due to body positioning muffling an alarm. Since the device obtains frequent blood glucose levels, it is able to develop a trend analysis and predict (down alert) when a glucose may be low. If hypoglycemia treatment was given earlier, at the time of the down alert, without waiting for the glucose to be less than or equal to 70 mg/dL, potentially all episodes of hypoglycemia could have been prevented. With an alarm setting of 70 mg/dL, however, subjects would have been unnecessarily treated 48% of the time. The incidence of false alarms could be significantly improved by setting the hypoglycemic alarm to a lower value (between 50 to 60 mg/dL). Earlier treatment (with a glucose value above 70 mg/dL) could have also substantially decreased the number of subsequent alarms. This is a new treatment paradigm based on trend analysis and prevention of hypoglycemia rather than documentation of hypoglycemia. It remains to be tested in clinical trials to determine if treating predicted hypoglycemia based on glucose trend analysis results in a higher hemoglobin A1c level, although animal models using continuous subcutaneous glucose monitoring to treat predicted hypoglycemia did not result in overshoot hyperglycemia (13).
In summary, subjects awoke to 29% of individual alarms and to 66% of alarm events. There were a high number of individual alarms for a small number of hypoglycemic episodes. The subject was awakened by the GW2B for all hypoglycemic alarms that were confirmed by a reference glucose value.
Acknowledgments
This was an ancillary study to the DirecNet inpatient accuracy study of glucose sensors and was supported by NIH/NICHD grants HD041919 , HD041908, and HD041915 . These studies were also supported by GCRC grants M01 RR00070-41, and M01 RR00069. LifeScan (Milpitas, CA) provided the One Touch Ultra Blood Glucose Monitoring Systems and the glucose test strips. We greatly appreciate the subjects and their families who participated in these studies, the work performed by the CRC Nurses, Elizabeth Bendig for review of videotapes, and the outstanding support of the Jaeb Center for Health Research (Tampa, FL), the coordinating center for DirecNet studies.
References
- 1.Marrero DG, Guare JC, Vandagriff JL, Fineberg NS. Fear of hypoglycemia in the parents of children and adolescents with diabetes: maladaptive or healthy response? Diabetes Educ. 1997;23(3):281–6. doi: 10.1177/014572179702300306. [DOI] [PubMed] [Google Scholar]
- 2.Davis EA, Keating B, Byrne GC, Russell M, Jones TW. Hypoglycemia: incidence and clinical predictors in a large population-based sample of children and adolescents with IDDM. Diabetes Care. 1997;20(1):22–5. doi: 10.2337/diacare.20.1.22. [DOI] [PubMed] [Google Scholar]
- 3.Rovet JF. The effect of hypoglycemic seizures on cognitive function in children with diabetes: a 7-year prospective study. J. Pediatr. 1999;134:503–506. doi: 10.1016/s0022-3476(99)70211-8. [DOI] [PubMed] [Google Scholar]
- 4.Sovik O, Thordarson H. Dead-in-bed syndrome in young diabetic patients. Diabetes Care. 1999;22 (Suppl 2):B40–2. [PubMed] [Google Scholar]
- 5.Potts RO, Tamada JA, Tierney MJ. Glucose monitoring by reverse iontophoresis. Diabetes Metab Res Rev. 2002;18 (Suppl 1):S49–53. doi: 10.1002/dmrr.210. [DOI] [PubMed] [Google Scholar]
- 6.Chase HP, Roberts MD, Wightman C, Klingensmith G, Garg SK, Van Wyhe M, et al. Use of the GlucoWatch biographer in children with type 1 diabetes. Pediatrics. 2003;111(4 Pt 1):790–4. doi: 10.1542/peds.111.4.790. [DOI] [PubMed] [Google Scholar]
- 7.Accuracy of the GlucoWatch G2 Biographer and the continuous glucose monitoring system during hypoglycemia: experience of the Diabetes Research in Children Network. Diabetes Care. 2004;27(3):722–6. doi: 10.2337/diacare.27.3.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.A multicenter study of the accuracy of the One Touch Ultra home glucose meter in children with type 1 diabetes. Diabetes Technol Ther. 2003;5(6):933–41. doi: 10.1089/152091503322640971. [DOI] [PubMed] [Google Scholar]
- 9.The accuracy of the GlucoWatch G2 biographer in children with type 1 diabetes: results of the diabetes research in children network (DirecNet) accuracy study . Diabetes Technol Ther. 2003;5(5):791–800. doi: 10.1089/152091503322526996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The accuracy of the CGMS in children with type 1 diabetes: results of the diabetes research in children network (DirecNet) accuracy study. Diabetes Technol Ther. 2003;5(5):781–9. doi: 10.1089/152091503322526987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Passey RB, Gillum RL, Fuller JB, Urry FM, Giles ML. Evaluation and comparison of 10 glucose methods and the reference method recommended in the proposed product class standard (1974) Clin Chem. 1977;23(1):131–9. [PubMed] [Google Scholar]
- 12.Gandrud LM, Paguntalan HU, Van Wyhe MM, Kunselman BL, Leptien AD, Wilson DM, et al. Use of the Cygnus GlucoWatch biographer at a diabetes camp. Pediatrics. 2004;113(1 Pt 1):108–11. doi: 10.1542/peds.113.1.108. [DOI] [PubMed] [Google Scholar]
- 13.Choleau PD, Klein JC, Ward WK, Wilson GS, Reach G. Prevention of Hpoglycemia Using Risk Assessment With a Continuous Glucose Monitoring System. Diabetes. 2002;51:3263–3273. doi: 10.2337/diabetes.51.11.3263. [DOI] [PubMed] [Google Scholar]


