Abstract
Male cotton-top tamarins have been shown to be responsive to female scent cues of ovulation, and are known to actively participate in infant care during the time when their mates are fertile. We measured urinary androgen levels and glucocorticoids in seven father tamarins for the first month following the birth of infants to determine 1) whether male tamarins showed an androgen response to their mate’s postpartum ovulation, 2) when androgens rise relative to ovulation, 3) whether there is a glucocorticoid response, and 4) whether males alter their parenting behavior during their mate’s receptive period. All of the males showed a significant increase in urinary androgens prior to the female’s postpartum LH peak, which indicated ovulation. The hormonal increase, which included estradiol, occurred 3–7 days prior to the female’s LH peak at a time that coincided with the female’s follicular period. Corticosterone levels also peaked during that time, but did not correlate with androgen changes. Fathers did not alter their daily infant-carrying patterns relative to the androgen increase or at the time of the mate’s LH peak. We conclude that male cotton-top tamarins experience an increase in androgens that coincides with their mate’s postpartum ovulation, which ensures optimal fertility. However, this sexual communication does not alter father–infant interactions, which already occur at a high rate in this species.
Keywords: cotton-top tamarin, androgens, glucocorticoids, chemical signaling, ovulation, parenting behavior
INTRODUCTION
Many female primates signal their fertile period with changes in sexual characteristics such as sex skin swellings, genital coloration, or attractiveness [Dixson, 1998]. Depending on the social structure, these cues may allow multiple males to compete to fertilize a female, or allow one male to monopolize a female at the time of conception. Under the social conditions of marmosets and tamarins (family Callitrichidae) there is usually one breeding pair per group, and little or no competition among males to mate with a female [French, 1997]. Marmosets and tamarins do not show obvious external signs of ovulation, such as sex skin swellings or genital coloration, but they do appear to produce olfactory or chemical signals indicating their reproductive state [Converse et al., 1995; Smith & Abbott, 1998; Ziegler et al., 1993].
While male marmosets are known to respond to other cues of female attractiveness (e.g., auditory and visual cues), chemical cues appear to be an especially important sensory system for mediating male sexual behavior [Dixson, 1998]. We have found that male cotton-top tamarins (Saguinus oedipus) are receptive to the chemical signals of ovulation. In a previous study, male cotton-top tamarins that were presented with sequential scent marks from a novel female throughout her ovulatory cycle for 10 min per day showed a significant increase in mounting their own mate and in the frequency of erections only while the scent originated during the periovulatory period [Ziegler et al., 1993c]. Other callitrichids also show responses to chemical signals. Studies have shown that male pygmy marmosets (Cebuella pygmaea) respond to the periovulatory period with increased anogenital investigation of their mates [Converse et al., 1995]. Male common marmosets (Callithrix) monitored during the postpartum period showed sexual behavior in response to nonbehavioral cues [Dixson & Lunn, 1987], and preferred periovulatory phase odors over luteal phase odors [Smith & Abbott, 1998]. These studies indicate that males respond behaviorally to female attractiveness. However, only one study in callitricids has examined a hormonal response to the female’s receptive state [Ziegler et al., in press]. Isolated periovulatory scent secretions presented to single and mated males are followed by an increase in testosterone, but not cortisol.
In a previous study we found that male cotton-top tamarins show higher levels of androgens postpartum when their mates ovulate early (≤15 days) compared to males whose mates ovulate late [Ziegler et al., 2000b]. However, males were monitored only during the first 2 weeks postpartum, and therefore the hormonal data were incomplete for males whose females ovulated late (>15 days). In captivity, cotton-top tamarins are highly fertile. Females give birth to multiple infants and experience postpartum ovulation a mean of 19 days following birth [Ziegler et al., 1990]. Females are nursing while ovulation and conception occur (over 80% of postpartum ovulations end in conception) [Ziegler et al., 1987]. Therefore, males should be optimally responsive at the time females ovulate. Additionally, perception of the ovulatory signal may produce a glucocorticoid response in the male, as suggested by observations of experienced cotton-top tamarin fathers responding to the pregnancy of their mates [Ziegler et al., 2004].
Male callitrichids respond behaviorally and perhaps hormonally to the female’s receptive period. However, in biparental species this occurs at the same time the fathers are providing the optimal amount of parental care. If fathers are increasing their parental investment during the mate’s fertile period, the males might benefit by ensuring exclusive mating with the female. One study of cotton-top tamarins showed that males carried infants more during the female’s receptive period [Sanchez et al., 1999]; however, that study lacked hormonal measurements to document the timing of ovulation. Another study found that cotton-top tamarin males mounted their mates more often when they were carrying infants than when they were not [Price, 1990], but this study also had no direct measures of ovulation. However, Tardif and Bales [1997] found no indication that either male cotton-top tamarin or common marmoset fathers exhibited higher rates of copulation while carrying infants, and they found no relationship between the frequency of copulations and the amount of time spent carrying infants during the suspected ovulatory period. Alternatively, high parental investment may have a negative effect on males and their reproductive hormones. In a study of Weids marmosets (Callithrix kuhli), fathers increased infant-carrying after the second week, at a time when females were likely to be ovulating, but their testosterone levels decreased with increasing infant-carrying rates [Nunes et al., 2001]. Male cotton-top tamarins may lose up to 11% of their body weight following the birth of their infants, while they are spending a great amount of time carrying infants [Achenbach & Snowdon, 2002; Sanchez et al., 1999].
We designed the current study to examine male hormone levels and infant-carrying rates for fathers, and determine the timing of ovulations in the mother. With these data we sought to determine 1) whether male tamarins show an androgen response to their mate’s postpartum ovulation, 2) when androgens rise relative to ovulation, 3) whether there is a glucocorticoid response, and 4) whether males alter their parenting behavior during their mate’s receptive period.
MATERIALS AND METHODS
Subjects
Seven male–female tamarin reproductive adult pairs were used in this study. These breeding pairs lived under family conditions. The animal husbandry and diet employed for this colony have been described elsewhere [Ginther et al., 2001]. Each pair entered the study at the time of parturition, and all births occurred between March 2002 and October 2003. Table I lists the study subjects, their ages, and their family conditions at the time of birth. All parents had similar experiences with sibling births while they lived in their natal families. All parents but one were experienced, having had previous births.
TABLE I.
Tamarin Mated Pairs, Age, Previous Births, Their Offspring, and Infant Survivor-ship During the Month Following Birth
| Tamarin pairs Fe-Ma | Age(years) at this birth | No. previous births | Other family members (offspring) | No. infants born | No. surviving infants |
|---|---|---|---|---|---|
| Ant-Sue | 5–3 | 1 | None | 2 | 2 |
| Fre-Odi | 12–10 | 4 | None | 2 | 1 |
| Phl-Nep | 6–8 | 5 | FF | 2 | 2 |
| Sun-Lok | 7–11 | 2 | MM | 2 | 2 |
| Jaz-Axl | 4–4 | 0 | MM | 2 | 0 |
| Val-Jun | 7–8 | 6 | MF, FF | 2 | 2 |
| Net-Wol | 5–8 | 2 | None | 2 | 1 |
Starting the day after the birth of an infant, urine was collected daily (or attempted daily) from the first morning void of both the mother and father, for 28 days. The urine was mixed, centrifuged, and frozen at −20°C as previously described [Ziegler et al., 1987]. The timing of the female’s urinary LH peak was determined for each female, and each male’s urinary steroids were normalized to the day of the female’s LH peak.
Observational Data
Family participation in carrying infants was recorded in instantaneous scan samples at five different times per day for each family, for a total of 6 weeks. The method correlated well with focal animal observations, and has been described in detail by Ziegler et al. [2000b]. We recorded which animal carried the infant(s) at each time point. The amount of time each father was seen carrying an infant was divided by the number of daily scans. A percent carrying time was calculated daily for each father during the first month postpartum. Father carrying percentages were also averaged by blocks of 5 days for each male, for comparison with averaged hormonal levels.
Hormonal Analysis
To determine the timing of ovulation in the females, we analyzed daily urine samples for LH using a previously described and validated method [Ziegler et al., 1993a]. This method uses 100 μl of urine in duplicate. The intra- and interassay coefficients for 34 assays were 5.92 and 11.24, respectively.
Male urine samples were processed so that testosterone, DHT, estradiol, and corticosterone levels could be measured. Since quantification of multiple steroids was desired, we processed each 1-ml sample of urine by solvolysis to break the conjugates [Ziegler et al., 1996], purified and concentrated the sample by solid phase extraction (60 mg/3 ml polymeric sorbent; Strata; Phenomenex, Torrance, CA), and separated the steroids by HPLC as previously described [Ziegler et al., 2004]. Basically, a 1-ml sample was acidified and extracted in ethyl acetate after it was incubated at 40°C for 2 hr. After the samples were dried, they were reconstituted in 1 ml of 30% methanol, and applied to the solid phase extraction columns. Eluted fractions were dried and resuspended in 20 μl acetonitrile/H2O 1:1. Samples were then ready for injection on the Beckman (Schaumburg, IL) autosampler (508) by using μl pickup to inject the entire sample. The samples were run isocratically at 1 ml/min in mobile phase 40/60% acetonitrile/H2O for 30 min through a precolumn (5 μm, 4.6 × 4.5 cm; Beckman) and a C18 column (5 μm, 4.6 × 25 cm; Ultrasphere; Beckman). From each sample we collected fractions representing the elution of testosterone and DHT for subsequent analyses by EIA. We measured the other steroids (estradiol and corticosterone) by UV absorption using the online diode array detector and standard curves generated for each steroid. The interassay coefficients of variation (CVs) for the UV absorption assays were 11.54 for corticosterone, and 18.9 for estradiol (n = 11). For the testosterone and DHT EIAs, the intra- and interassay CVs were T = 2.7 and 10.1, respectively (n = 6); and DHT = 2.5 and 15.3, respectively (n = 11).
All of the hormone concentrations were indexed by creatinine. The creatinine was determined by the Jaffe reaction with the use of methods described by Ziegler et al. [1995]. The creatinine levels were divided into all hormone values for each day.
Statistical Analyses
The days over which the urine samples were collected were normalized to the day of the female’s urinary LH peak, with the LH peak day being day 0. Since male cotton-top tamarins in our colony excrete different basal levels of androgens [Ziegler et al., 2000a], we calculated the data as a percent change from the values on the day of the female’s postpartum LH peak for comparisons between males. Data from each hormone were averaged as the percent change across 5-day blocks for each subject, with 10 days before and after the LH peak day yielding the following blocks: −10/−6, −5/−1, 1/5, and 6/10. Since some females ovulated early, and others ovulated later in the postpartum month of collection, we were unable to obtain urine samples from all of the males for more than three time periods. However, data were available for five of seven males for the 6/10 block. We used a nonparametric analysis to measure percent changes in hormone values, and Friedman’s two-way ANOVA to test hormone levels across time blocks. A Wilcoxon signed-ranks test was used as a posthoc analysis to determine significant differences between time blocks. To determine the onset of changes in androgen levels more precisely, we examined the first day when the concentration of each hormone (estradiol, testosterone, DHT, and corticosterone) had increased by ≥2 standard deviations (SDs) from the mean hormone level of the previous days. Correlations between the timing of the LH peak and the reproductive parameters were analyzed by means of a Pearson correlation. Significance was assessed by P-values ≤0.05.
RESULTS
The timing of the female’s LH peak (indicating ovulation) ranged between 13 to 25 days following parturition (18.7± 5.5 days (mean± SD), median = 18 days). The results for each female are presented in Table II. Five of the ovulations resulted in conceptions, but we found no relationship between the timing of the LH peak and conception. The number of infants born, number of infants that survived, number of previous births, family size, or resulting conceptions did not correlate with the timing of the LH peak. However, the number of infants that survived from the present birth correlated with conception from the following postpartum ovulation (r = 0.81, P = 0.03). All but one female that conceived during the postpartum ovulation also had two surviving infants in the subsequent parturition.
TABLE II.
Timing of the LH Peak Postpartum and Resulting Conceptions in Female Cotton-Top Tamarins
| Tamarin pairs Fe-Ma | Ov from birth in days | Resulting conceptions |
|---|---|---|
| Ant-Sue | 24 | + |
| Fre-Odi | 18 | − |
| Phl-Nep | 13 | + |
| Sun-Lok | 25 | + |
| Jaz-Axl | 13 | − |
| Val-Jun | 24 | + |
| Net-Wol | 14 | + |
Steroid Changes Around Ovulation
Figure 1 depicts a representative profile of steroid production from one male cotton-top tamarin whose female ovulated the earliest, and one male whose female ovulated late. Levels of testosterone, DHT, estradiol, and corticosterone were elevated for 5 days before and after the female’s LH peak. Testosterone and DHT profiles were similar for both males, except that in male A, DHT levels were basal following birth until 5 days before ovulation, and testosterone levels were not basal. Estradiol levels were elevated longer compared to the other steroids, and did not closely follow the androgens. Corticosterone levels peaked on the fourth day prior to the LH peak for the male whose female ovulated early, and remained high from 5 days prior to the LH peak through the remainder of the sample-collecting period.
Fig. 1.

Steroid profiles for two male cotton-top tamarins from birth through the following 28 days. A: Testosterone, DHT, corticosterone, and estradiol levels for a male tamarin whose female ovulated early (day 13). B: Steroid profiles for a male whose female ovulated late (day 24) following birth.
Significant changes in male androgens occurred around the time of ovulation (Fig. 2). Testosterone percent levels changed significantly across the four time blocks for all males (F = 7.8, P = 0.05), and the mean percent levels from −5 to −1 days and 1–5 days were significantly higher than those from 6–10 days (Zs = 2.0, P = 0.04). DHT percent levels changed significantly across the four time blocks for all males (F = 6.0, P = 0.05), and the mean change from −10 to −6 days was significantly lower than that during −5 to −1 days (Z = 2.2, P = 0.03). Estadiol percent levels changed significantly across the four time blocks for six of the seven males (F = 9, P = 0.01), but significance was marginal with all seven males in the analysis (F = 5.4, P = 0.07). The mean percent levels were significantly lower during the −10 to −6 days than the −5 to −1 days (Z = 2.2, P = 0.03) or the 1–5 days after the LH peak (Z = 2.2, P = 0.03). Mean corticosterone percent levels did not change significantly over the time blocks (F = 2.3, P = 0.31).
Fig. 2.

Mean percent change in androgen levels over four time blocks (5 days each) from the female’s LH peak. Day 0 is the day of the LH peak, and is normalized to 100%. The top graph indicates significant changes in testosterone across the time blocks: −10/−6 is significantly lower than −5/−1 and 1/5 (Wilcoxon signed-ranks test, P < 0.05). The second graph indicates significant changes in DHT across the time blocks: −10/−6 is significantly lower than −5/−1 (P < 0.03). The third graph indicates significant changes in estradiol across the time blocks: −10/−6 is significantly lower than −5/−1 and 1/5 (P < 0.03). The fourth graph indicates changes in corticosterone across the 5-day time blocks, but these were not significant (F = 2.33, P = 0.31).
Preovulation Stimulation of Androgens
The day on which androgen levels rose and sustained an elevation two times greater than the SD of the mean of the previous days is shown for each male in Table III. Testosterone was significantly elevated for all but one male 4–5 days prior to the LH peak. The significant elevation in DHT occurred 3–6 days prior to the LH peak for six of the males. The significant elevation in estradiol occurred 3–6 days prior to the LH peak, except for two males in which no predictable change occurred. The average duration of the follicular phase in cotton-top tamarins is 5 ± 0.45 (mean ± SEM), with a low variance (8.94%) [Ziegler et al., 1993b].
TABLE III.
The Onset of Sustained Hormonal Elevaton for Individual Male Cotton-Top Tamarins Relative to the Female’s Postpartum Urinary LH Peak
| Male | Testosterone | DHT | Estradiol | Corta |
|---|---|---|---|---|
| Jun | −7 | −4 | −5 | −5 |
| Sue | −5 | −6 | −6 | −6 |
| Lok | −5 | −5 | −5 | −5 |
| Nep | −4 | −3 | −3 | −4 |
| Odi | −5 | No change | No change | −1 |
| Axl | −5 | −5 | −5 | −4 |
| Wol | −4 | −4 | No change | −3 |
| Mean±SD | −5±1 | −4.5±1.05 | −4.8±1.1 | −4.9±1.2 |
Indicates timing of corticosterone concentration.
Each male showed an increase in corticosterone prior to the female’s ovulation. The days prior to the LH peak are shown in Table III. Peak levels occurred 1–6 days prior to the LH peak for the males, but there was no predictable relationship between the timing of the corticosterone peak and that of the androgen increase.
Parental Care Changes Around the Time of Ovulation
The amount of time fathers carried one or both infants varied among males, and across the first month postpartum. Since both infants were lost to one father, there were six fathers remaining in which we could examine carrying time. There were no significant correlations between daily androgen (estradiol, testosterone, and DHT) levels and daily percent carrying time for any male. When male androgen levels were averaged by 5-day blocks and compared with averaged percent carrying time by blocks, there were no significant correlations. There were no consistent changes in percent carrying around the LH peak for either males or females. Figure 3 shows the mean percent carrying across time blocks for males and females. All fathers, except one, carried more on the first day of birth and throughout the first month than did mothers, but there was no significant change around the time of the female’s LH peak.
Fig. 3.
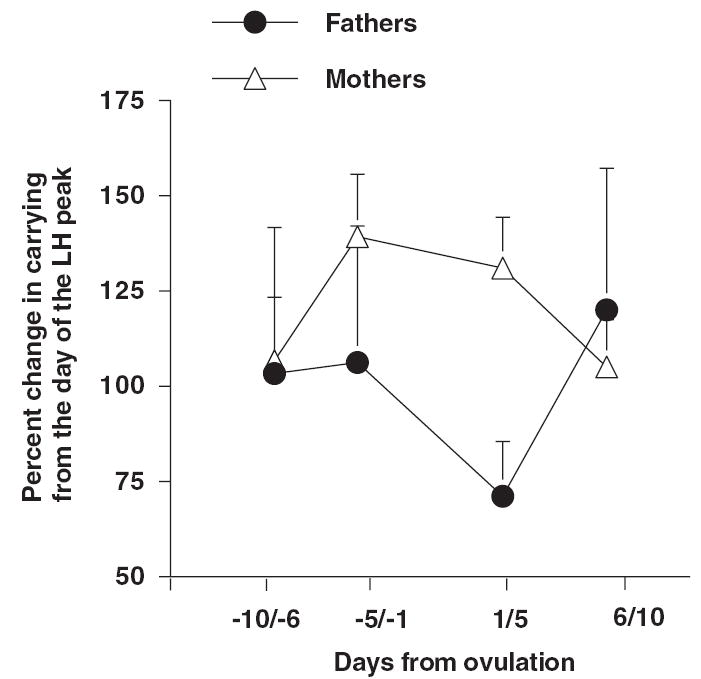
Mean percent time males and females carried infants relative to the timing of the female’s postpartum ovulation. Day 0 represents the day of a female’s urinary LH peak, and percent carrying was normalized to 100%.
DISCUSSION
The cotton-top tamarin fathers in this study consistently anticipated their mate’s postpartum ovulation with an increase in urinary androgens (testosterone, DHT, and estradiol). The fact that androgens increased during the female’s follicular phase may indicate that the males increased their reproductive hormones to prepare for the female’s fertile phase. While males were presumably responsive to ovulatory signals from their mate [Ziegler et al., 1993c], there was no evidence that these hormone elevations influenced their infant-carrying patterns.
It seems that the male’s hormonal response to the female’s ovulation may work to increase male sexual responsiveness at the optimum time for mating. The tamarin female’s ability to nurse and ovulate simultaneously is due to the infrequency of nursing across the day [Ziegler et al., 1990], and to the great amount of time fathers and other helpers spend on carrying infants in the first few days postpartum [Ziegler et al., 2000b]. Cotton-top tamarin females cannot raise infants without help. Since fathers are in such close association with mothers and infants during this time, it is not surprising that males receive and respond to fertility signals with increased androgen production. Increased testosterone production in males stimulates spermatid maturation and may influence fertility in males [Williams-Ashman, 1988]. Additionally, male mating behavior is responsive to testosterone in male marmosets [Dixson, 1993], squirrel monkeys [Mendoza and Mason, 1989], and rhesus monkeys [Wallen et al., 1991], and increased testosterone promotes a preference for female estrus odors in rodents [Stern, 1970]. Therefore, the increase in androgens in male cotton-top tamarins may work to increase both a male’s fertility and his interest in mating at the appropriate time during the period of a female’s optimum fertility.
Urinary testosterone levels increased prior to or simultaneously with DHT and estradiol except in one male. Both urinary DHT and estradiol have been shown to be derived from testosterone in male tamarins, and are gonadal in origin [Ziegler et al., 2000a]. In all males except one, the relative change in estradiol was higher than that in testosterone or DHT. Estrogen levels (both circulating and urinary) are high relative to androgens in cotton-top tamarins and other New World monkeys compared to Old World monkeys and apes [Ziegler et al., 2000a]. High levels of estrogen may also help to stimulate male parental care; however, in the present study there was no daily or 5-day relationship between estradiol and infant-carrying in fathers. In the California mouse, estrogen has been shown to promote maternal behavior in females, and paternal behavior in males [Trainor & Marler, 2002]. Blocking the conversion of testosterone to estradiol decreased the amount of pup-directed behaviors in California male mice. Therefore, testosterone may be important in these biparental males for both its impact on fertility and its influence on parenting behaviors via estrogen excretion.
Changes in the urinary androgens of males occurred simultaneously with the timing of the female’s follicular phase. This is a time when estrogens are elevated and female scent-marking increases [Savage et al., 1988; Ziegler et al., 1993b]. Male primates are sexually attracted to estrogen-primed females [Estep et al., 1984; Michael et al., 1967], and copulation attempts are most frequent during the follicular and periovulatory phase in the common marmoset [Kendrick & Dixson, 1983]. Chemical signaling of the female’s optimum fertility would likely occur during this phase of the cycle.
Glucocorticoids may mediate and initiate hormonal responses to chemical signals. Studies in mice have shown that both the release of signal and the response of the recipient are associated with elevated glucocorticoids (i.e., corticosterone) [Drickamer & McIntosh, 1980; Marchlewska-Koj & Zacharczuk-Kakietek, 1990; Weidong et al., 1998]. Experienced male tamarins also show a glucocorticoid response to their mate’s pregnancy exactly 1 week after glucocorticoid levels rise in the female’s urine due to fetal adrenal production [Ziegler et al., 2004]. A higher increase in corticosterone occurred than for cortisol in the male’s response to the female’s pregnancy. In this study corticosterone increased in response to the female’s follicular phase. Evidence from both studies indicating that the pathway of conversion from progesterone to deoxycorticosterone may be important factors in chemical signaling.
It has been hypothesized that males use infant-carrying as a courtship strategy. Male care of young may represent a means by which males increase their mating success and therefore incur a benefit in exchange for infant care [Smuts & Gubernick, 1992]. Indeed, Price [1990] reported that cotton-top tamarin fathers mount their mate significantly more often when they are carrying infants than when they are not. Those observations were made during the 2–4 weeks following birth, when females were likely to be ovulating. However, a different study of the postpartum period in cotton-top tamarins and common marmosets did not find higher rates of copulations by males carrying infants, or any other indicators of special attention paid to infants during the females’ fertile period [Tardif & Bales, 1997].
Weid’s marmoset fathers appear to carry infants more frequently during the second week postpartum, when females are likely to be ovulating; however, in this species of marmoset, males do not begin to carry infants until the second week postpartum [Nunes et al., 2001]. None of these studies [Prince, 1990; Tardif & Bales, 1997; Nunes et al., 2001] used endocrine data to identify the time of the female’s ovulation, but their behavioral data did reflect the general period in which ovulation should have occurred. Our data on male infant-carrying did not show any increase in carrying around the time of postpartum ovulation. Male infant-carrying did not significantly correlate, positively or negatively, with any of the hormone changes that occurred in these males. We did not examine all aspects of male parenting during the postpartum period, and did not look at sexual behavior in the pairs. Therefore, we do not know whether the males carried infants most often during the mounting of females. However, the daily frequency of carrying infants did not increase. Since cotton-top tamarin fathers exhibit a high rate of carrying during the first month, they may not need to prove their worth as infant caretakers to their mate only at the time of her ovulation. Additionally, in captive family groups the male has exclusive mating rights to the female.
Sexual communication between paired male and female cotton-top tamarins ensures mating at the time of optimum fertility. Cotton-top tamarins have a high fertility rate, with multiple births from each pregnancy, and a high rate of postpartum conception when food availability is adequate. If a high reproductive rate is important for this species due to high mortality of offspring, then a clear signaling process to indicate fertility is essential. Since breeding males participate in infant care at a high rate during the time that females are ovulating again, the males will be in close enough proximity to respond to such signals. Elevated corticosterone may indicate reception of the signal. It has been shown that males respond to ovulatory signals with signs of behavioral arousal, indicating reception of the signal [Ziegler et al., 1993c], and now we know that they respond hormonally as well. We hope future work will help us to understand the mechanisms that underlie reproductive signaling in this species.
Acknowledgments
We thank Kate Washabaugh, Aimee Kurian, Carla Boe, and Jillian Scott for processing the tamarin samples; the tamarin laboratory as a whole for urine collection and storage; and Fritz Wegner and Dan Wittwer for consultation on the hormonal analyses. This work was supported by grants from the National Institutes of Health (MH 35,215 to C.T.S. and T.E.Z., and RR000167 to the National Primate Research Center, U.W.–Madison).
Footnotes
Presented in part at the 26th meeting of the American Society of Primatologists, 2003, Calgary, Canada.
Contract grant sponsor: National Institutes of Health; Contract grant numbers: MH 35,215; RR000167.
References
- Achenbach GG, Snowdon CT. Costs of caregiving: weight loss in captive adult male cotton-top tamarins (Saguinus oedipus) following the birth of infants. Int J Primatol. 2002;23:179–189. doi: 10.1023/A:1013210226793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Converse LJ, Carlson AA, Ziegler TE, Snowdon CT. Communication of ovulatory state to mates by female pygmy marmosets, Cebuella pygmaea. Anim Behav. 1995;49:615–621. [Google Scholar]
- Dixson AF, Lunn SF. Postpartum changes in hormones and sexual behavior in captive groups of marmosets (Callithrix jacchus) Physiol Behav. 1987;41:577–583. doi: 10.1016/0031-9384(87)90314-3. [DOI] [PubMed] [Google Scholar]
- Dixson AF. Effects of testosterone propionate upon the sexual and aggressive behavior of adult male marmosets (Callithrix jacchus) castrated as neonates. Horm Behav. 1993;27:216–230. doi: 10.1006/hbeh.1993.1016. [DOI] [PubMed] [Google Scholar]
- Dixson AF. 1998. Primate sexuality: comparative studies of the prosimians, monkeys, apes, and human beings. Oxford, UK: Oxford University Press. XIV + 546 p.
- Drickamer LC, McIntosh TK. Effects of adrenalectomy on the presence of a maturation delaying pheromone in the urine of female mice. Horm Behav. 1980;14:146–152. doi: 10.1016/0018-506x(80)90006-9. [DOI] [PubMed] [Google Scholar]
- Estep DQ, Bruce KEM, Johnston ME, Gordon TP. Sexual behavior of group-housed stumptail macaques (Macaca arctoides): temporal, demographic and sociosexual relationships. Folia Primatol. 1984;42:115–126. doi: 10.1159/000156154. [DOI] [PubMed] [Google Scholar]
- French JA. 1997. Proximate regulation of singular breeding in callithricid primates. In: Solomon NG, French JA, editors. Cooperative breeding in mammals. Cambridge: Cambridge University Press. p. 34–75.
- Ginther AJ, Ziegler TE, Snowdon CT. Reproductive biology of captive male cotton-top tamarin monkeys as a function of social environment. Anim Behav. 2001;61:65–78. doi: 10.1006/anbe.2000.1587. [DOI] [PubMed] [Google Scholar]
- Kendrick KM, Dixson AF. The effect of the ovarian cycle on the sexual behaviour of the common marmoset (Callithrix jacchus) Physiol Behav. 1983;30:735–742. doi: 10.1016/0031-9384(83)90171-3. [DOI] [PubMed] [Google Scholar]
- Marchlewska-Koj A, Zacharczuk-Kakietek M. Acute increase in plasma corticosterone level in female mice evoked by pheromones. Physiol Behav. 1990;48:577–580. doi: 10.1016/0031-9384(90)90194-9. [DOI] [PubMed] [Google Scholar]
- Mendoza SP, Mason WA. Behavioral and endocrine consequences of heterosexual pair formation in squirrel monkeys. Physiol Behav. 1989;46:597–603. doi: 10.1016/0031-9384(89)90338-7. [DOI] [PubMed] [Google Scholar]
- Michael RP, Herbert J, Wellegalla J. Ovarian hormones and the sexual behaviour of the male rhesus monkey (Macaca mulatta) under laboratory conditions. J Endocrinol. 1967;39:81–98. doi: 10.1677/joe.0.0390081. [DOI] [PubMed] [Google Scholar]
- Nunes S, Fite JE, Patera KJ, French JA. Interactions among paternal behavior, steroid hormones, and parental experience in male marmosets (Callithrix kuhlii) Horm Behav. 2001;39:70–82. doi: 10.1006/hbeh.2000.1631. [DOI] [PubMed] [Google Scholar]
- Price E. Infant carrying as a courtship strategy of breeding male cotton-top tamarins. Anim Behav. 1990;40:784–786. [Google Scholar]
- Sanchez S, Pelaez F, Gil-Burmann C, Kaumanns W. Costs of infant-carrying in the cotton-top tamarin (Saguinus oedipus) Am J Primatol. 1999;48:99–111. doi: 10.1002/(SICI)1098-2345(1999)48:2<99::AID-AJP2>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Savage A, Ziegler TE, Snowdon CT. Sociosexual development, pair bond formation, and mechanisms of fertility suppression in female cotton-top tamarins (Saguinus oedipus oedipus) Am J Primatol. 1988;14:345–359. doi: 10.1002/ajp.1350140404. [DOI] [PubMed] [Google Scholar]
- Smith TE, Abbott DH. Behavioral discrimination between circumgenital odor from periovulatory dominant and anovulatory female common marmosets (Callithrix jacchus) Am J Primatol. 1998;46:265–284. doi: 10.1002/(SICI)1098-2345(1998)46:4<265::AID-AJP1>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Smuts BB, Gubernick DJ. 1992. Male-infant relationships in non-human primates: paternal investment or mating effort? In Heulett BS, editor. Father-child relations: cultural and biosocial contexts. New York. Aldin de Gruyter. p 1–30.
- Stern J. Responses of male rats to sex odors. Physiol Behav. 1970;5:519–524. doi: 10.1016/0031-9384(70)90260-x. [DOI] [PubMed] [Google Scholar]
- Tardif SD, Bales K. Is infant-carrying a courtship strategy in callitrichid primates? Anim Behav. 1997;53:1001–1007. [Google Scholar]
- Trainor BC, Marler CA. Testosterone promotes paternal behaviour in a monogamous mammal via conversion to oestrogen. Proc R Soc Lond B. 2002;269:823–829. doi: 10.1098/rspb.2001.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallen K, Eisler JA, Tannenbaum PL, Nagell KM, Mann DR. Antide (Nal-Lys GnRH antagonist) suppression of pituitarytesticular function and sexual behavior in group-living rhesus monkeys. Physiol Behav. 1991;50:429–435. doi: 10.1016/0031-9384(91)90090-b. [DOI] [PubMed] [Google Scholar]
- Weidong M, Zhongshan M, Novotny MV. Role of the adrenal gland and adrenal-mediated chemosignals in suppression of estrus in the house mouse: the Lee-Boot effect revisited. Biol Reprod. 1998;59:1317–1320. doi: 10.1095/biolreprod59.6.1317. [DOI] [PubMed] [Google Scholar]
- Williams-Ashman HG. 1988. Perspectives on the male sexual physiology of eutherian mammals. In: Knobil E, Neill JD, editors. The physiology of reproduction. New York: Raven Press. p 727–751.
- Ziegler TE, Bridson WE, Snowdon CT, Eman S. Urinary gonadotropin and estrogen excretion during the postpartum estrus, conception and pregnancy in the cotton-top tamarin (Saguinus oedipus) Am J Primatol. 1987;12:127–140. doi: 10.1002/ajp.1350120202. [DOI] [PubMed] [Google Scholar]
- Ziegler TE, Widowski TM, Larson ML, Snowdon CT. Nursing does affect the duration of the postpartum to ovulation interval in cotton-top tamarins (Saguinus oedipus) J Reprod Fertil. 1990;90:563–570. doi: 10.1530/jrf.0.0900563. [DOI] [PubMed] [Google Scholar]
- Ziegler TE, Matteri RL, Wegner FH. Detection of urinary gonadotropins in callitrichid monkeys with a sensitive immunoassay based upon a unique monoclonal antibody. Am J Primatol. 1993a;31:181–188. doi: 10.1002/ajp.1350310303. [DOI] [PubMed] [Google Scholar]
- Ziegler TE, Wittwer TE, Snowdon CT. Circulating and excreted hormones during the ovarian cycle in the cotton-top tamarin, Saguinus oedipus. Am J Primatol. 1993b;31:55–65. doi: 10.1002/ajp.1350310106. [DOI] [PubMed] [Google Scholar]
- Ziegler TE, Epple G, Snowdon CT, Porter TA, Belcher AM, Kuderling I. Detection of the chemical signals of ovulation in the cotton-top tamarin, Saguinus oedipus. Anim Behav. 1993c;45:313–322. [Google Scholar]
- Ziegler TE, Scheffler G, Snowdon CT. The relationship of cortisol levels to social environment and reproductive functioning in female cotton-top tamarins, Saguinus oedipus. Horm Behav. 1995;29:407–424. doi: 10.1006/hbeh.1995.1028. [DOI] [PubMed] [Google Scholar]
- Ziegler TE, Scheffler G, Wittwer DJ, Schultz-Darken NJ, Snowdon CT, Abbott DH. Metabolism of reproductive steroids during the ovarian cycle in two species of callitrichids, Saguinus oedipus and Callithrix jacchus, and estimation of the ovulatory period from fecal steroids. Biol Reprod. 1996;54:91–99. doi: 10.1095/biolreprod54.1.91. [DOI] [PubMed] [Google Scholar]
- Ziegler TE, Carlson AA, Ginther AJ, Snowdon CT. Gonadal source of testosterone metabolites in urine of male cotton-top tamarin monkeys (Saguinus oedipus) Gen Comp Endocrinol. 2000a;118:332–343. doi: 10.1006/gcen.2000.7476. [DOI] [PubMed] [Google Scholar]
- Ziegler TE, Wegner FH, Carlson AA, Lazaro-Perea C, Snowdon CT. Prolactin levels during the periparturitional period in the biparental cotton-top tamarin (Saguinus oedipus): interactions with gender, androgen levels, and parenting. Horm Behav. 2000b;38:111–122. doi: 10.1006/hbeh.2000.1606. [DOI] [PubMed] [Google Scholar]
- Ziegler TE, Washabaugh KF, Snowdon CT. Responsiveness of expectant male cotton-top tamarins, Saguinus oedipus, to mate’s pregnancy. Horm Behav. 2004;45:84–92. doi: 10.1016/j.yhbeh.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Ziegler TE, Schultz-Darken NJ, Scott JJ, Snowdon CT, Ferris CF. Neuroendocrine response to female periovulatory odors depends upon social condition in male common marmosets, Callithrix jacchus In review. [DOI] [PubMed]


