Abstract
Genes in the S-box family are regulated by binding of S-adenosylmethionine (SAM) to the 5′ region of the mRNA of the regulated gene. SAM binding was previously shown to promote a rearrangement of the RNA structure that results in premature termination of transcription in vitro and repression of expression of the downstream coding sequence. The S-box RNA element therefore acts as a SAM-binding riboswitch in vitro. In an effort to identify factors other than SAM that could be involved in the S-box regulatory mechanism in vivo, we searched for trans-acting mutations in Bacillus subtilis that act to disrupt repression of S-box gene expression during growth under conditions where SAM pools are elevated. We identified a single mutant that proved to have one nucleotide substitution in the metK gene, encoding SAM synthetase. This mutation, designated metK10, resulted in a 15-fold decrease in SAM synthetase activity and a 4-fold decrease in SAM concentration in vivo. The metK10 mutation specifically affected S-box gene expression, and the increase in expression under repressing conditions was dependent on the presence of a functional transcriptional antiterminator element. The observation that the mutation identified in this search affects SAM production supports the model that the S-box RNAs directly monitor SAM in vivo, without a requirement for additional factors.
The S-box regulatory system is used in low-G+C gram-positive organisms, including members of the Bacillus/Clostridium/Staphylococcus group, to regulate expression of genes involved in biosynthesis and transport of methionine and S-adenosylmethionine (SAM) (9-11, 29). Genes in the S-box family exhibit a pattern of conserved sequence and structural elements in the 5′ region of the mRNA, upstream of the start of the regulated coding sequence(s). These conserved elements include an intrinsic terminator and a competing antiterminator that can sequester sequences that otherwise form the 5′ portion of the terminator helix; residues in the 5′ region of the antiterminator can also pair with sequences located further upstream, and this pairing results in formation of a structure (the anti-antiterminator) that sequesters sequences necessary for formation of the antiterminator. The anti-antiterminator helix (helix 1) (Fig. 1) is located at the base of a complex structure comprised of helices 1 to 4. Genetic analyses of S-box leader RNAs supported the model that formation of the anti-antiterminator and transcription termination occur during growth under conditions where methionine is abundant, while starvation for methionine results in destabilization of the anti-antiterminator, allowing antiterminator formation and readthrough of the transcription termination site (9). Mutational analysis also suggested that the helix 1 to 4 region is likely to be the target for binding of a negative regulatory factor or factors (9, 38) and that pairing between residues in the terminal loop of helix 2 and the region between helices 3 and 4 is important for termination during growth in high methionine (21).
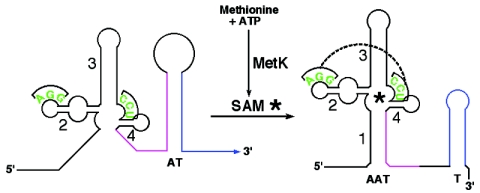
FIG. 1.
The S-box regulatory system. In the absence of SAM, the antiterminator structure forms (AT, red-blue), preventing formation of the competing terminator helix (T, blue) and allowing transcription of the downstream coding sequence. Binding of SAM to the helix 1 to 4 region results in stabilization of helix 1, which serves as an anti-antiterminator (AAT, black-red) by sequestering sequences necessary for formation of the antiterminator, thereby allowing formation of the terminator helix and transcription termination. SAM binding also promotes a tertiary interaction (dashed line) between residues (green) in the terminal loop of helix 2 and the unpaired region between helices 3 and 4. SAM (*) is generated from methionine and ATP by MetK (SAM synthetase).
Recent studies revealed that addition of SAM to an in vitro transcription termination system is sufficient to promote termination of transcription at S-box leader region terminators in the absence of cellular factors other than RNA polymerase (RNAP) (4, 20, 39). The S-box RNAs were shown to interact directly and specifically with SAM, and binding of SAM results in a structural rearrangement that includes stabilization of helix 1. Mutations that result in loss of repression during growth in the presence of methionine also result in loss of SAM-directed transcription termination and SAM binding in vitro (20, 21). Overexpression of SAM synthetase in vivo results in increased repression of S-box gene expression, supporting the model that SAM is the effector in vivo (3, 20). These results suggested that S-box RNAs directly sense SAM and are therefore members of the family of RNA elements termed “riboswitches,” which monitor regulatory signals without a requirement for accessory factors such as RNA-binding proteins or ribosomes (12, 23, 33).
While SAM is sufficient for RNA binding and transcription termination in vitro, it was not clear whether other factors might be involved in S-box gene regulation in vivo. We therefore attempted to identify trans-acting mutations that would lead to loss of repression of S-box gene expression during growth in the presence of methionine. We predicted that we could isolate mutations in genes that affect the molecular effector (SAM) or in genes involved in the folding of the leader RNA, the SAM/leader RNA interaction, or transcription termination in response to SAM. We identified a single trans-acting mutation that specifically affected S-box gene expression, and the mutation was identified as a nucleotide substitution in the coding region of metK, the gene encoding SAM synthetase. This mutation was shown to result in decreased SAM synthetase activity and reduced SAM pools during growth in the presence of methionine. These results are therefore consistent with the model that SAM is the key effector both in vivo and in vitro.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The Bacillus subtilis strains used in this study were 168 (trpC2); BR151 (lys-3 metB10 trpC2); BR151MA (lys-3 trpC2); ZB307Spc (SPβc2del2::Tn917erm/spc::pSK10Δ6), a derivative of strain ZB307A (SPβc2del2::Tn917::pSK10Δ6) (41) in which the Tn917 erm gene is replaced by a spectinomycin resistance cassette (31); and IS56B (lys-3 metB relA1 sra-3 strA), a derivative of strain IS56 (lys-3 relA1 trpC2) (30) containing a mutation (sra-3) that suppresses the partial methionine auxotrophy conferred by the relA1 allele (unpublished results). Strains containing lacZ fusions are designated with the original strain name followed by the description of the fusion (e.g., IS56B::yitJ-lacZ). B. subtilis mapping strains 1A627 through 1A645 (34) were obtained from the Bacillus Genetic Stock Center (Ohio State University), and generalized transducing phage PBS-1 was obtained from P. Setlow (University of Connecticut Health Center, Farmington). B. subtilis strains were grown on tryptose blood agar base medium (TBAB; Difco), Spizizen minimal medium (2), 2XYT broth (22), or A3 medium (antibiotic medium 3; Difco). Antibiotics were added as indicated at the following concentrations: chloramphenicol, 5 μg/ml; erythromycin, 1 μg/ml; lincomycin, 25 μg/ml; neomycin, 5 μg/ml; spectinomycin, 25 μg/ml. X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside; Gold Biotechnologies) was used at 40 μg/ml as an indicator of β-galactosidase activity. All growth was at 37°C.
Genetic techniques.
Transformation of B. subtilis was carried out as described previously (14). Chromosomal DNA was prepared using the DNeasy tissue kit (QIAGEN). Wizard columns (Promega) were used for plasmid preparations. Oligonucleotide primers were purchased from Integrated DNA Technologies (Coralville, IA). Restriction endonucleases and DNA-modifying enzymes were purchased from New England Biolabs and used as described by the manufacturer. Plasmid pGEM7Zf(+) (Promega) was used for cloning of metK gene fragments. Mutations were identified by DNA sequencing (Genewiz Inc., North Brunswick, NJ).
Transcriptional fusions were generated in plasmid pFG328 (13), which contains a cat gene conferring resistance to chloramphenicol, and integrated in single copy into the B. subtilis chromosome by transformation of strain ZB307Spc, which contains a variant of specialized transducing phage SPβ that carries a spectinomycin resistance cassette. Strains containing lacZ fusions were grown in the presence of chloramphenicol or spectinomycin. Transformation of strains containing lacZ fusion constructs with plasmid pFGneo, a derivative of plasmid pFG328 that contains a neomycin resistance gene cassette in place of the cat gene, was used to replace both the cat gene and the adjacent promoter/leader region directing lacZ expression with a neomycin resistance cassette, by using flanking plasmid sequences to direct homologous recombination.
Methanesulfonic acid ethyl ester (EMS; Sigma) was used for generalized mutagenesis. Cells were grown in 2XYT medium to early exponential growth phase. EMS (1% final concentration) was added, and cells were incubated with shaking for an additional 100 min at 37°C, pelleted by centrifugation, and washed twice with Davis salts [0.7% K2HPO4, 0.3% KH2PO4, 0.1% (NH4)2SO4, and 0.005% MgSO4 · 7H2O]. The cells were then resuspended in 2XYT medium and incubated with shaking for 3 h at 37°C before addition of 10% glycerol and storage at −70°C. EMS-mutagenized cells were diluted in 2XYT medium, grown for 30 min, and plated on TBAB containing X-Gal as an indicator of lacZ expression.
Preparation of PBS-1 transducing lysates and transductions were carried out as described previously (14). PBS-1 transducing lysates were generated from 19 B. subtilis mapping strains containing chromosomal insertions of the transposon Tn917 (34). The PBS-1 phage lysates were passed through the donor strains at least three times before transduction of the recipient strain. Erythromycin and lincomycin were used to select for resistance to macrolide, lincomycin, and streptogramin B antibiotics (MLSr) encoded by the transposon.
MetK sequence comparisons.
The tblastn program of the BLAST suite of sequence analysis programs (1) was used to identify MetK homologs in unannotated sequence data. Signature amino acid patterns for SAM synthetases were retrieved from the PROSITE database of protein families and domains (6).
β-Galactosidase measurements.
Strains containing lacZ fusions were grown in Spizizen minimal medium containing all required amino acids at 50 μg/ml until early exponential growth. The cells were harvested by centrifugation and then resuspended in fresh Spizizen minimal medium in the presence or absence of methionine. Samples were collected at 1-h intervals and assayed for β-galactosidase activity using toluene permeabilization (22). All starvation experiments and assays were carried out at least twice, and variation was <10%.
SAM synthetase assay.
Cells (400 ml) were grown in Spizizen minimal medium containing the required amino acids until the A600 reached 0.8. Cell extracts were prepared by passage through a French pressure cell (8,000 lb/in2) followed by removal of cell debris by centrifugation (22,000 × g for 20 min at 4°C). Protein concentration of cell extracts was measured by the Bradford protein assay (Bio-Rad) using a bovine serum albumin standard. Cell extracts were assayed for SAM synthetase activity by measuring incorporation of [35S]methionine (1,175 Ci/mmol; Perkin-Elmer) into [35S]SAM using a protocol adapted from the work of Ochi and Freese (24). Samples were filtered through P81 phosphocellulose paper (Upstate Biotechnology) and washed with distilled water. Bound [35S]SAM was quantitated in Packard Bioscience Ultima Gold scintillation fluid using a Packard Tri-Carb 2100TR liquid scintillation counter. The recovery of SAM after the wash with distilled water was 67% as determined with a [methyl-14C]SAM (ICN) standard; the measured amounts of [35S]SAM were therefore multiplied by 1.5. The amount of [35S]SAM generated in the reaction mixture was measured at 10-min intervals and used to determine the specific activity of SAM synthetase in the crude cell extract by calculating the amount of [35S]SAM (in pmol) generated per mg of protein in the cell extract per minute.
SAM-dependent transcription termination in vitro.
DNA templates containing the B. subtilis yitJ leader region fused to the glyQS promoter were generated by PCR, and single-round transcription reactions were carried out using His-tagged B. subtilis RNAP as previously described (20, 21). Transcription reactions were terminated by extraction with phenol, and products were resolved by denaturing polyacrylamide gel electrophoresis and visualized by PhosphorImager (Molecular Dynamics) analysis. Percent termination was calculated from the percentage of product in the terminated band relative to the total of the terminated and readthrough products. Reproducibility was ±5%. Extracts were prepared from cells grown to late logarithmic growth phase in minimal medium in the presence of methionine. Cells were collected by filtration and extracted with 1.5 ml of 0.5 M formic acid, and the formic acid was removed by lyophilization as described by Ochi et al. (25). Extracts were neutralized with KOH prior to addition to the in vitro transcription termination assay. A standard curve for SAM-dependent transcription termination was generated using diluted SAM stocks neutralized with KOH.
RESULTS
Isolation of mutants exhibiting increased expression of S-box genes during growth in the presence of methionine.
The observation that B. subtilis relA mutants exhibit partial methionine auxotrophy (37) and reduced S-box gene expression (unpublished results) suggested that the stringent response might influence S-box gene regulation. Since a possible requirement for the stringent response could prevent S-box gene expression in the absence of amino acid starvation, we identified a suppressor mutant (sra-3, suppressor of RelA) in which the methionine auxotrophy of a relA1 mutation was relieved but S-box gene regulation was maintained. Four independent pools of B. subtilis strain IS56B::yitJ-lacZ (lys-3 metB relA1 sra-3 strA SPβ::yitJ-lacZ) were randomly mutagenized with EMS. The mutagenized cells were screened for isolates that formed blue colonies on TBAB (a rich medium that contains methionine) containing X-Gal, indicating loss of repression of the yitJ-lacZ fusion during growth in the presence of methionine. Rich medium was chosen to allow growth of mutants that might potentially affect multiple riboswitch systems and therefore might affect growth in minimal medium. At least one mutant exhibiting high β-galactosidase activity was obtained from each pool, with a total of 10 isolates from approximately 7,000 colonies.
Previous studies demonstrated that mutations in the helix 1 to 4 region or the terminator of the yitJ-lacZ fusion result in loss of repression during growth in the presence of methionine (9, 21, 38; unpublished results). To screen for isolates containing cis-acting mutations, chromosomal DNA was prepared for each IS56B variant and introduced into a clean genetic background (B. subtilis strain ZB307Spc) by transformation, selecting for recombination of the SPβ sequences flanking the yitJ-lacZ fusion in the chromosome of the mutant strains with the resident SPβ prophage in strain ZB307Spc. Eight of the 10 isolates were eliminated at this stage since introduction of the yitJ-lacZ fusion from these strains into ZB307Spc produced variants that formed blue colonies on TBAB containing X-Gal, indicating that the mutation leading to expression during growth in the presence of methionine was in the yitJ-lacZ fusion.
The two remaining isolates were tested to determine whether the β-galactosidase activity was derived from the yitJ-lacZ fusion rather than from expression of lacA, a cryptic β-galactosidase gene carried on the B. subtilis chromosome (26). The yitJ-lacZ fusion in these isolates was replaced with a neomycin resistance gene cassette by homologous recombination, and the resulting colonies were screened for lacZ expression. One isolate formed blue colonies on TBAB containing X-Gal after removal of the yitJ-lacZ fusion, indicating that lacZ expression in this variant was independent of the fusion and probably resulted from expression of lacA. The mutation in the remaining isolate was designated “S-box-derepressed mutation 1” (SBD1), as it resulted in derepression of S-box gene expression during growth in the presence of methionine.
Specificity of the SBD1 allele.
The specificity of the effect of the SBD1 allele on S-box gene expression was tested by introducing a series of transcriptional fusions into the chromosome of IS56B-SBD1 from which the yitJ-lacZ fusion had been removed. Reintroduction of an unmutagenized yitJ-lacZ fusion into the mutant strain background resulted in high expression during growth in rich medium (Table 1), confirming that the SBD1 mutation acts in trans to confer increased expression of a wild-type fusion. Similarly, a second S-box gene fusion (ykrT-lacZ) (9) exhibited a 100-fold increase in expression relative to the wild-type parent, which indicates that the SBD1 mutation is not specific for yitJ expression. In contrast, the SBD1 allele had no effect on expression of a PtyrS-TrpsD-lacZ fusion containing a heterologous terminator (from the B. subtilis rpsD gene, which encodes ribosomal protein S4) or a tyrS-lacZ fusion, which is regulated by the T-box transcription termination control mechanism and responds to tRNATyr (8), demonstrating that the SBD1 allele does not generally increase readthrough of leader region terminators.
TABLE 1.
Specificity of the SBD1 allele
| lacZ fusion construct | β-Galactosidase activity for straina:
|
|
|---|---|---|
| IS56B | IS56B-SBD1 | |
| yitJ-lacZ | 0.10 | 60 |
| ykrT-lacZ | 0.14 | 14 |
| PtyrS-TrpsD-lacZ | 5.3 | 7.4 |
| tyrS-lacZ | 32 | 19 |
| yitJ-Pst3-lacZ | 0.11 | 0.13 |
Fusions were integrated in single copy in strains IS56B and IS56B-SBD1 (metK10). Cells were grown in 2XYT medium until late logarithmic growth and assayed for β-galactosidase activity (Miller units) (22).
The effect of the SBD1 allele on expression of a yitJ-lacZ fusion containing a mutation in the antiterminator element was tested to see whether the increased expression is dependent on the antitermination control system. The yitJ-Pst3 mutation (which alters residues in the 3′ side of helix 1, preventing formation of the antiterminator and leaving the terminator intact) results in loss of yitJ-lacZ expression under any growth condition (9). The SBD1 allele did not increase expression of a yitJ-lacZ fusion containing the yitJ-Pst3 allele, demonstrating that the effect of the SBD1 allele is dependent on the normal S-box termination control mechanism.
Effect of the SBD1 allele on growth and yitJ-lacZ expression.
The SBD1 allele was introduced into strains BR151 (metB10) and BR151MA (Met+) to test the effect of the mutation on growth and yitJ-lacZ expression levels in an unmutagenized background and in the absence of the relA1 and sra-3 alleles. In the Met− strain BR151, expression of the yitJ-lacZ fusion was high in cells starved for methionine for both the wild type and the SBD1 mutant; the major difference between the strains was that repression during growth in the presence of methionine was reduced from >300-fold for the wild-type strain to 3.5-fold for the SBD1 mutant (Table 2). In the Met+ strain BR151MA, removal of methionine from the growth medium resulted in a transient induction of yitJ-lacZ expression (64 Miller units), followed by adjustment to a steady-state level of expression of approximately 15 to 20 Miller units, as previously reported (9, 20), while continuous growth in the presence of methionine resulted in very low expression (<0.2 Miller units). In contrast, introduction of the SBD1 allele resulted in constitutive high expression (Table 2). These results indicate that the SBD1 allele is sufficient to confer loss of repression of S-box gene expression during growth in the presence of methionine. The SBD1 allele also resulted in a modest reduction in growth rate in the BR151MA strain background, and this effect was not suppressed by addition of methionine. Growth was not impaired in the BR151 background, which suggests that the metB10 allele, which causes a defect in homoserine O-acetyltransferase and blocks the methionine biosynthesis pathway (10), suppresses the growth defect caused by the SBD1 mutation.
TABLE 2.
Effect of the SBD1 allele on expression of a yitJ-lacZ transcriptional fusion and growth
| Strain | metB | Methionine addition | yitJ-lacZ expressiona | Doubling time (min) |
|---|---|---|---|---|
| BR151 | − | − | 120 | NDb |
| − | + | 0.38 | 58 | |
| BR151-SBD1 | − | − | 180 | ND |
| − | + | 50 | 60 | |
| BR151MA | + | − | 64 | 84 |
| + | + | 0.14 | 76 | |
| BR151MA-SBD1 | + | − | 44 | 130 |
| + | + | 40 | 134 |
Cells of strains BR151 and BR151-SBD1 (metB10) or BR151MA and BR151MA-SBD1 (Met+) were grown in minimal medium containing methionine, harvested by centrifugation, and resuspended in minimal medium in the presence (+) or absence (−) of methionine. yitJ-lacZ fusion activity is in Miller units (22) and is reported for the time points at which maximal expression was observed (4 h after the cultures were split for BR151 and BR151-SBD1 and 1 h after the cultures were split for BR151MA and BR151MA-SBD1).
ND, not determined.
Identification of the SBD1 allele.
The SBD1 allele was genetically mapped using a set of B. subtilis mapping strains, each of which has an insertion of Tn917 (conferring MLSr) at a unique location in the chromosome (34). Generalized transduction using phage PBS-1 was employed to determine the linkage between the SBD1 allele and the MLSr marker from each mapping strain. PBS-1 transducing lysates were generated in each of the mapping strains and used to transduce BR151-SBD1::yitJ-lacZ. MLSr transductants were screened for formation of white colonies on TBAB containing X-Gal, indicating replacement of the SBD1 allele in the recipient strain with the wild-type allele by cotransduction with the MLSr marker from the donor strain. All of the MLSr transductants obtained formed blue colonies with the exception of transductants obtained with donor strain 1A642, in which the Tn917 insertion is 85% linked to ald (34), located at 3,277.3 kb on the B. subtilis chromosome (16). Approximately 60% of the MLSr transductants obtained using lysates from strain 1A642 formed white colonies on TBAB containing X-Gal, indicating that the mutation responsible for loss of repression during growth in rich medium is located in the region of the chromosome near the Tn917 insertion site of strain 1A642. Examination of the B. subtilis genome sequence for candidate regulatory genes in this region revealed that the metK gene, encoding SAM synthetase, is located at 3,128.1 kb (16). Since mutations in metK (which is itself an S-box gene) might be predicted to affect S-box gene expression by affecting SAM pools, we tested whether the SBD1 allele represents an alteration in the metK gene.
PCR products covering the entire metK gene, including its promoter and leader region, were generated from chromosomal DNA from strains BR151 and BR151-SBD1, and the DNA sequence was determined. The only change observed was a single nucleotide substitution in the metK coding region that corresponds to replacement of alanine with threonine at amino acid position 83 (A83T). A 350-bp fragment of the metK coding sequence containing the A83T substitution was isolated from strain BR151-SBD1 by PCR and cloned into plasmid pGEM7Zf(+), and the resulting construct was introduced into BR151::yitJ-lacZ by transformation. Transformants were screened for formation of blue colonies on TBAB containing X-Gal, indicating introduction of the SBD1 allele by homologous recombination. Chromosomal DNA was isolated from transformants that exhibited either high or low yitJ-lacZ expression, and the metK locus was isolated by PCR and screened for the presence of the A83T substitution by DNA sequencing. All transformants that formed white colonies on TBAB containing X-Gal were wild type for metK, while all transformants that formed blue colonies (indicating loss of repression during growth in the presence of methionine) contained the A83T substitution. These results demonstrated that the A83T substitution in metK is sufficient to confer the phenotype observed for the SBD1 allele. We therefore designate this mutation metK10.
MetK homologs were identified by tblastn searches (1) in 69 additional bacterial species representing 46 different genera. The sequences present in the region surrounding A83 from B. subtilis MetK and the MetKs from other representative species are shown in Fig. 2. The identity of the amino acid analogous to A83 in B. subtilis MetK was alanine in 28 species representing 16 genera, serine in 32 species representing 25 genera, threonine in 8 species representing 4 genera, and glycine in one organism. Of the 28 species containing alanine at this position in MetK, 20 are gram positive, including six additional Bacillus species. All MetK variants containing serine at this position are from gram-negative organisms. Of the eight species containing threonine at this position, six species are gram negative; the two gram-positive species are Streptococcus mutans and Streptococcus pneumoniae, both of which use a SAM-binding riboswitch (the SMK box) distinct from the S box to regulate metK expression in response to SAM (5). The A83T mutation in the metK10 allele appears to be a conservative change, as threonine is found at the corresponding position in other metK genes.
FIG. 2.
Sequence comparison of bacterial MetK homologs. The region of B. subtilis MetK surrounding residue A83 is aligned with the analogous MetK regions from other bacterial species. Sequences were obtained by searching the NCBI database with signature sequences for SAM synthetases (6) by using the tblastn program (1). A83 in B. subtilis MetK and the analogous residues from the other bacterial MetKs are boxed. Residues that differ from the B. subtilis sequence are shown in boldface. Bsu, B. subtilis; Ban, Bacillus anthracis; Sau, Staphylococcus aureus; Lpl, Lactobacillus plantarum; Spn, Streptococcus pneumoniae; Sty, Salmonella enterica serovar Typhimurium; Bma, Burkholderia mallei; Pae, Pseudomonas aeruginosa; Bpe, Bordetella pertussis; Eco, E. coli. (+), gram-positive species; (−), gram-negative species. Amino acid identities to B. subtilis MetK were as follows: 86%, B. anthracis; 77%, S. aureus; 68%, L. plantarum; 68%, S. pneumoniae; 63%, S. enterica serovar Typhimurium; 59%, B. mallei; 59%, P. aeruginosa; 58%, B. pertussis; 57%, E. coli.
Measurement of SAM synthetase activity in BR151-SBD1.
The identification of the SBD1 mutation as a single amino acid substitution in metK suggested that the reduced repression of S-box gene expression could be due to a reduction in SAM synthetase activity. The level of SAM synthetase activity in strains BR151 and BR151-SBD1 grown in minimal medium containing methionine was therefore measured. SAM synthetase activity in strain BR151 was 150 ± 7 pmol min−1 mg of protein−1, consistent with the range of activity (128 to 200 pmol min−1 mg of protein−1) previously reported for metK+ strains of B. subtilis (24, 35, 40). Activity in strain BR151-SBD1 was reduced 15-fold to 10 ± 2 pmol min−1 mg of protein−1. As noted above, the SBD1 allele had no effect on growth rate in the BR151 strain background (Table 2). Other metK mutant strains with a similar reduction of SAM synthetase activity also exhibited normal growth in medium containing methionine (35), suggesting that the amount of SAM produced under these conditions is sufficient for growth but not for repression of S-box gene expression.
Effect of the metK10 allele on SAM pools in vivo.
Transcription termination at the B. subtilis yitJ leader region terminator occurs in response to the addition of SAM to an in vitro transcription assay (20, 21). We used this assay to monitor the SAM concentration in cell extracts of wild-type and metK10 mutant cells grown in the presence of methionine by comparing the termination efficiency promoted by addition of these cell extracts to the transcription termination assay to the termination efficiency promoted by known concentrations of SAM. As reported previously (21), addition of 0.15 μM SAM resulted in 50% termination (data not shown). The wild-type cell extract was fourfold more efficient in promotion of transcription termination than was the mutant cell extract (Fig. 3), corresponding to SAM pools of 250 μM for the wild-type strain versus 59 μM for the metK10 mutant. The SAM concentration (measured by high-pressure liquid chromatography) for metK+ strains grown in the presence of methionine was previously reported at 400 μM (35), a value similar to that obtained using the in vitro transcription termination assay. This assay therefore provides a convenient method for monitoring SAM concentrations in crude cell extracts.
FIG. 3.
Measurement of SAM pools in cell extracts of metK+ and metK10 strains by SAM-dependent transcription termination. Cell extracts were generated from strains BR151MA and BR151MA-SBD1 by formic acid extraction of cells grown in minimal medium in the presence of methionine. Extracts were neutralized by the addition of 1 N KOH, and samples (standardized by cell numbers used in preparation of the extracts) were added to a B. subtilis RNAP in vitro transcription reaction mixture containing a Pgly-yitJ DNA template. The percent termination was calculated as the fraction of the terminated RNA product relative to the total of the terminated and readthrough products. Reproducibility was ±5%. Measurement of termination efficiency in response to addition of various amounts of SAM resulted in generation of a standard curve in which 50% termination was observed at 0.15 μM SAM. The amount of cell extract required to confer 50% termination was compared to the SAM standard curve. An average internal cell volume of 0.535 ± 0.13 μl A600−1 (35) was used to calculate the intracellular SAM concentrations (250 μM for BR151MA and 59 μM for BR151MA-SBD1) from the cell equivalents and A600 of cell cultures used in preparation of the extracts.
DISCUSSION
SAM has been shown to cause transcription termination of S-box genes in vitro, and overexpression of SAM synthetase results in decreased S-box gene expression in vivo (20). In this study we attempted to isolate trans-acting mutations that lead to loss of repression of S-box gene expression during growth in the presence of methionine and predicted that these mutations could occur in genes that affect SAM pools or in genes encoding other unknown factors that are involved in S-box gene regulation in vivo. A single trans-acting mutation (SBD1 or metK10) was isolated that led to derepression of S-box gene expression, and this mutation was identified as a single nucleotide substitution in the metK gene, which encodes SAM synthetase. The observation that this mutation results in reduced SAM synthetase activity and SAM pools during growth in the presence of methionine further supports the model that the S-box RNAs directly monitor SAM in vivo. No evidence was obtained for participation of additional cellular factors in the S-box mechanism, but it is possible that mutations of other types were missed.
The metK gene product converts methionine and ATP into SAM, which is required for methyltransferase reactions in the cell as well as for polyamine biosynthesis (19). The availability of methionine in the cell can therefore be measured indirectly by sensing SAM levels, as methionine must be present for SAM to be synthesized. SAM is the key effector controlling methionine biosynthesis gene expression in Escherichia coli at the level of transcription initiation (7), indicating that SAM is the effector of choice in a variety of biological systems despite variability in the regulatory mechanism. The metK10 allele results in a 15-fold reduction in SAM synthetase activity and a 4-fold reduction in SAM pools. Mutations with reduced SAM synthetase activity were previously shown to result in an increase in intracellular methionine, while overexpression of SAM synthetase results in partial methionine auxotrophy (40). These results are consistent with the prediction that SAM is the molecular effector for S-box gene expression both in vivo and in vitro (3, 20).
Inactivation of the metK gene is a lethal event in B. subtilis, as some level of SAM synthetase activity is essential for viability (40). It was therefore possible only to isolate mutations that decrease SAM synthetase activity, not abolish it. Wabiko et al. (35) identified metK mutant strains of B. subtilis that resulted in a 25- to 200-fold decrease in SAM synthetase activity compared to the parent strain, yet these strains exhibited only a three- to fourfold decrease in SAM pools during growth in the presence of methionine, consistent with the results obtained for the metK10 allele. It therefore appears that a significant reduction in SAM synthetase activity can be tolerated, as only the most severe mutations result in a growth defect. A major decrease in SAM pools has a variety of effects in E. coli, including poor growth and increased mutation rate, due at least in part to defects in DNA methylation (27, 36). The metK10 mutation resulted in a reduction in SAM pools and growth rate that was not suppressed by supplementation of the growth medium with extra methionine. The observation that the growth defect occurred only in a metB+ strain and not in a metB10 mutant suggests that this effect is not due to limitation for SAM but instead results from a metabolic imbalance that occurs as a consequence of deregulation of the methionine biosynthesis pathway by disruption of SAM-dependent repression of multiple S-box genes; this imbalance, which could result in both accumulation of toxic intermediates and draining of key precursors, is absent in a metB mutant that lacks homoserine O-acetyltransferase and is therefore blocked at the first step of the pathway (10). The B. subtilis metK gene is also predicted to be a member of the S-box regulon (9), suggesting that metK expression is likely to increase when SAM pools are low. B. subtilis metK expression increases during methionine limitation (3, 40), and metK genes from members of the Lactobacillales are regulated by SAM via the SMK box, a SAM-responsive riboswitch distinct from the S box (5). Increased expression of metK in metK10 strains may partially compensate for the reduction in SAM synthetase activity caused by the mutation.
The amino acid sequence of SAM synthetase enzymes is highly conserved, and B. subtilis and E. coli SAM synthetases exhibit 57% amino acid identity. E. coli SAM synthetase has been characterized in detail and consists of four identical subunits (17). Two subunits form a tight dimer with two active sites located between them, and two dimers interact in an asymmetrical arrangement to form a peanut-shaped tetrameric enzyme (32). The interactions between the dimers appear to be less extensive than the subunit-subunit interactions within each dimer (15, 32). Residue S80 in E. coli MetK, which corresponds to the position of the A83T substitution of the B. subtilis metK10 allele, is located at the tetramer interface and is the only residue that participates in hydrogen bond formation between the dimers (32) (Fig. 4). Substitution of the nonpolar alanine residue with a polar threonine residue at position 83 in B. subtilis MetK could affect activity by affecting tetramerization, which is required for full activity (18). The presence of threonine at this position in MetK proteins from certain species suggests that sequence context may influence its role. Modification of both C90, a highly conserved residue located at the tetramer interface, and C240 by N-ethylmaleimide results in dissociation of E. coli SAM synthetase active tetramers into inactive dimers. Site-directed mutagenesis of C90 to alanine or serine results in a mixture of dimers and tetramers and a reduction in SAM synthetase activity; the purified dimers exhibit approximately 20-fold-lower activity than the purified tetramers (28), a reduction in activity similar to that observed for the metK10 allele. Further characterization will be necessary to determine if the reduction in SAM synthetase activity conferred by the metK10 allele is due to an effect on oligomerization, on some other functional property of the enzyme, or on protein stability.
FIG. 4.
Location of S80 in the crystal structure of the E. coli SAM synthetase ternary complex. The crystal structure of E. coli SAM synthetase (15) with the ATP analog AMPPNP and methionine in each active site is shown. The image was created using the Cn3D v4.1 three-dimensional structure viewer (NCBI), using coordinates from the work of Komoto et al. (15). The tetrameric enzyme is a dimer of dimers, with two active sites located between the two subunits in each dimer. The location of S80 (in tan) in each subunit is indicated by red arrows. S80 is the only residue that participates in hydrogen bond formation between the dimers of E. coli SAM synthetase.
The metK10 mutation was the only trans-acting mutation identified in this study. While it is possible that SAM, which is sufficient to cause transcription termination in vitro, may be the only factor involved in S-box gene regulation in vivo, it remains possible that other cellular components are involved. We predicted that mutations resulting in a specific defect in S-box gene expression would not be lethal, as the resulting overexpression of genes involved in methionine biosynthesis should be tolerated, especially in a metB10 genetic background in which the methionine biosynthesis pathway is blocked; this prediction is supported by the modest growth defect caused by the metK10 allele even in a metB+ background, despite significant derepression of S-box gene expression. However, the possibility remains that additional required gene products could not be identified because they play essential roles in other cellular processes.
Acknowledgments
This work was supported by National Institutes of Health grant GM63615 and by National Institutes of Health predoctoral fellowship F32 GM20923 (to B.A.M.).
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Anagnostopoulos, C., and J. Spizizen. 1961. Requirements for transformation in Bacillus subtilis. J. Bacteriol. 81:741-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Auger, S., A. Danchin, and I. Martin-Verstraete. 2002. Global expression profile of Bacillus subtilis grown in the presence of sulfate or methionine. J. Bacteriol. 184:5179-5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Epshtein, V., A. S. Mironov, and E. Nudler. 2003. The riboswitch-mediated control of sulfur metabolism in bacteria. Proc. Natl. Acad. Sci. USA 100:5052-5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuchs, R. T., F. J. Grundy, and T. M. Henkin. 2006. The SMK box is a new SAM-binding RNA for translational regulation of SAM synthetase. Nat. Struct. Mol. Biol. 13:226-233. [DOI] [PubMed]
- 6.Gasteiger, E., A. Gattiker, C. Hoogland, I. Ivanyi, R. D. Appel, and A. Bairoch. 2003. ExPasy: the proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 31:3784-3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greene, R. C. 1996. Biosynthesis of methionine, p. 542-560. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaecter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. American Society for Microbiology, Washington, D.C.
- 8.Grundy, F. J., and T. M. Henkin. 1993. tRNA as a positive regulator of transcription antitermination in B. subtilis. Cell 74:475-482. [DOI] [PubMed] [Google Scholar]
- 9.Grundy, F. J., and T. M. Henkin. 1998. The S box regulon: a new global transcription termination control system for methionine and cysteine biosynthesis genes in Gram-positive bacteria. Mol. Microbiol. 30:737-749. [DOI] [PubMed] [Google Scholar]
- 10.Grundy, F. J., and T. M. Henkin. 2002. Synthesis of serine, glycine, cysteine, and methionine, p. 245-254. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives: from genes to cells. American Society for Microbiology, Washington, D.C.
- 11.Grundy, F. J., and T. M. Henkin. 2003. The T box and S box transcription termination control systems. Front. Biosci. 8:d20-d31. [DOI] [PubMed] [Google Scholar]
- 12.Grundy, F. J., and T. M. Henkin. 2004. Regulation of gene expression by effectors that bind to RNA. Curr. Opin. Microbiol. 7:126-131. [DOI] [PubMed] [Google Scholar]
- 13.Grundy, F. J., D. A. Waters, S. H. Allen, and T. M. Henkin. 1993. Regulation of the Bacillus subtilis acetate kinase gene by CcpA. J. Bacteriol. 175:348-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henkin, T. M., and G. H. Chambliss. 1984. Genetic mapping of a mutation causing an alteration in Bacillus subtilis ribosomal protein S4. Mol. Gen. Genet. 193:364-369. [DOI] [PubMed] [Google Scholar]
- 15.Komoto, J., T. Yamada, Y. Takata, G. D. Markham, and F. Takusagawa. 2004. Crystal structure of the S-adenosylmethionine synthase ternary complex: a novel catalytic mechanism of S-adenosylmethionine synthesis from ATP and Met. Biochemistry 43:1821-1831. [DOI] [PubMed] [Google Scholar]
- 16.Kunst, F., N. Ogasawara, I. Moszer, A. M. Albertini, G. Alloni, et al. 1997. The complete genome sequence of the Gram-positive bacterium Bacillus subtilis. Nature 390:249-256. [DOI] [PubMed] [Google Scholar]
- 17.Markham, G. D., E. W. Hafner, C. W. Tabor, and H. Tabor. 1980. S-Adenosylmethionine synthetase from Escherichia coli. J. Biol. Chem. 255:9082-9092. [PubMed] [Google Scholar]
- 18.Markham, G. D., and C. Satishchandran. 1988. Identification of the reactive sulfhydryl groups of S-adenosylmethionine synthetase. J. Biol. Chem. 263:8666-8670. [PubMed] [Google Scholar]
- 19.Matthews, R. G. 1996. One-carbon metabolism, p. 600-611. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaecter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. American Society for Microbiology, Washington, D.C.
- 20.McDaniel, B. A. M., F. J. Grundy, I. Artsimovitch, and T. M. Henkin. 2003. Transcription termination control of the S box system: direct measurement of S-adenosylmethionine by the leader RNA. Proc. Natl. Acad. Sci. USA 100:3083-3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDaniel, B. A., F. J. Grundy, and T. M. Henkin. 2005. A tertiary structural element in S box leader RNAs is required for S-adenosylmethionine-directed transcription termination. Mol. Microbiol. 57:1008-1021. [DOI] [PubMed] [Google Scholar]
- 22.Miller, J. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 23.Nudler, E., and A. S. Mironov. 2004. The riboswitch control of bacterial metabolism. Trends Biochem. Sci. 29:11-17. [DOI] [PubMed] [Google Scholar]
- 24.Ochi, K., and E. Freese. 1982. A decrease in S-adenosylmethionine synthetase activity increases the probability of spontaneous sporulation. J. Bacteriol. 152:400-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ochi, K., J. C. Kandala, and E. Freese. 1981. Initiation of Bacillus subtilis sporulation by the stringent response to partial amino acid deprivation. J. Biol. Chem. 256:6866-6875. [PubMed] [Google Scholar]
- 26.Ogura, M., S. Hirao, Y. Ohshiro, and T. Tanaka. 1999. Positive regulation of Bacillus subtilis sigD by C-terminal truncated LacR at translational level. FEBS Lett. 457:112-116. [DOI] [PubMed] [Google Scholar]
- 27.Posnick, L. M., and L. D. Samson. 1999. Influence of S-adenosylmethionine pool size on spontaneous mutation, Dam methylation, and cell growth of Escherichia coli. J. Bacteriol. 181:6756-6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reczkowski, R. S., and G. D. Markham. 1995. Structural and functional roles of cysteine 90 and cysteine 240 in S-adenosylmethionine synthetase. J. Biol. Chem. 270:18484-18490. [DOI] [PubMed] [Google Scholar]
- 29.Rodionov, D. A., A. G. Vitreschak, A. A. Mironov, and M. S. Gelfand. 2004. Comparative genomics of the methionine metabolism in Gram-positive bacteria: a variety of regulatory systems. Nucleic Acids Res. 32:3340-3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith, I., P. Paress, K. Cabane, and E. Dubnau. 1980. Genetics and physiology of the rel system of Bacillus subtilis. Mol. Gen. Genet. 178:272-279. [DOI] [PubMed] [Google Scholar]
- 31.Steinmetz, M., and R. Richter. 1994. Plasmids designed to alter the antibiotic resistance expressed by insertion mutations in Bacillus subtilis. Gene 142:79-83. [DOI] [PubMed] [Google Scholar]
- 32.Takusagawa, F., S. Kamitori, S. Misaki, and G. D. Markham. 1996. Crystal structure of S-adenosylmethionine synthetase. J. Biol. Chem. 271:136-147. [PubMed] [Google Scholar]
- 33.Tucker, B. J., and R. R. Breaker. 2005. Riboswitches as versatile control elements. Curr. Opin. Struct. Biol. 15:342-348. [DOI] [PubMed] [Google Scholar]
- 34.Vandeyar, M. A., and S. A. Zahler. 1986. Chromosomal insertions of Tn917 in Bacillus subtilis. J. Bacteriol. 167:530-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wabiko, H., K. Ochi, D. M. Nguyen, E. R. Allen, and E. Freese. 1988. Genetic mapping and physiological consequences of metE mutations of Bacillus subtilis. J. Bacteriol. 170:2705-2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wei, Y., and E. B. Newman. 2002. Studies on the role of the metK gene product of Escherichia coli K-12. Mol. Microbiol. 43:1651-1656. [DOI] [PubMed] [Google Scholar]
- 37.Wendrich, T. M., and M. A. Marahiel. 1997. Cloning and characterization of a relA/spoT homologue from Bacillus subtilis. Mol. Microbiol. 26:65-79. [DOI] [PubMed] [Google Scholar]
- 38.Winkler, W. C., F. J. Grundy, B. A. Murphy, and T. M. Henkin. 2001. The GA motif: an RNA element common to bacterial antitermination systems, rRNA, and eukaryotic RNAs. RNA 7:1165-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Winkler, W. C., A. Nahvi, N. Sudarsen, J. E. Barrick, and R. R. Breaker. 2003. An mRNA structure that controls gene expression by binding S-adenosylmethionine. Nat. Struct. Biol. 10:701-707. [DOI] [PubMed] [Google Scholar]
- 40.Yocum, R. R., J. B. Perkins, C. L. Howitt, and J. Pero. 1996. Cloning and characterization of the metE gene encoding S-adenosylmethionine synthetase from Bacillus subtilis. J. Bacteriol. 178:4604-4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zuber, P., and R. Losick. 1987. Role of AbrB in Spo0A- and Spo0B-dependent utilization of a sporulation promoter in Bacillus subtilis. J. Bacteriol. 169:2223-2230. [DOI] [PMC free article] [PubMed] [Google Scholar]