Abstract
Aer, a low-abundance signal transducer in Escherichia coli, mediates robust aerotactic behavior, possibly through interactions with methyl-accepting chemotaxis proteins (MCP). We obtained evidence for interactions between Aer and the high-abundance aspartate (Tar) and serine (Tsr) receptors. Aer molecules bearing a cysteine reporter diagnostic for trimer-of-dimer formation yielded cross-linking products upon treatment with a trifunctional maleimide reagent. Aer also formed mixed cross-linking products with a similarly marked Tar reporter. An Aer trimer contact mutation known to abolish trimer formation by MCPs eliminated Aer trimer and mixed trimer formation. Trimer contact alterations known to cause epistatic behavior in MCPs also produced epistatic properties in Aer. Amino acid replacements in the Tar trimer contact region suppressed an epistatic Aer signaling defect, consistent with compensatory conformational changes between directly interacting proteins. In cells lacking MCPs, Aer function required high-level expression, comparable to the aggregate number of receptors in a wild-type cell. Aer proteins with clockwise (CW)-biased signal output cannot function under these conditions but do so in the presence of MCPs, presumably through formation of mixed signaling teams. The Tar signaling domain was sufficient for functional rescue. Moreover, CW-biased lesions did not impair aerotactic signaling in a hybrid Aer-Tar transducer capable of adjusting its steady-state signal output via methylation-dependent sensory adaptation. Thus, MCPs most likely assist mutant Aer proteins to signal productively by forming collaborative signaling teams. Aer evidently evolved to operate collaboratively with high-abundance receptors but can also function without MCP assistance, provided that it can establish a suitable prestimulus swimming pattern.
Aer, an unorthodox member of the methyl-accepting chemotaxis protein (MCP) family of chemoreceptors, is the major oxygen-sensing transducer in Escherichia coli (5, 19). Aer and the conventional MCPs share a highly conserved C-terminal signaling domain that forms a ternary complex with two cytoplasmic proteins, CheA, a histidine kinase, and CheW, which couples CheA activity to receptor control. CheA activity in turn modulates the phosphorylation state of the response regulator CheY, which controls the direction of rotation of the cell's flagellar motors. Orthodox MCPs have an N-terminal periplasmic ligand-binding domain that monitors chemoeffector levels and triggers changes in output activity of the signaling domain (10). Unlike MCPs, Aer has a cytoplasmic N-terminal sensing domain (PAS) that is thought to monitor environmental oxygen status through associated redox changes in a flavin adenine dinucleotide prosthetic group (25, 26). Moreover, MCPs have two membrane-spanning segments that flank their sensing domain, whereas Aer is anchored to the inner side of the cytoplasmic membrane by a central hydrophobic hairpin (3). However, the most significant difference between Aer and the MCPs is their method of sensory adaptation. MCPs undergo reversible methylation at specific signaling domain sites to tune signal output to ambient chemoeffector levels. Aer, in contrast, is not methylated and does not require the MCP-specific methyltransferase (CheR) or methylesterase (CheB) to mediate aerotactic behavior (6).
E. coli has four MCPs: Tar (aspartate and maltose sensor) and Tsr (serine) comprise about 90% of the cell's receptor molecules, and Tap (dipeptides) and Trg (ribose and galactose), along with Aer, comprise the remainder (13). Receptor signaling complexes cluster at the cell pole(s) (15, 22) and appear to form an interconnected array with cooperative, high-gain signaling properties (17, 21). Trimers of receptor homodimers, first observed in the crystal structure of the Tsr signaling domain (11), may be an important structure/function component of these signaling arrays (2, 17, 23). The four MCPs and Aer have identical trimer contact residues in their signaling domains, and Tar, Tsr, and Trg have been shown to form mixed trimers of dimers in vivo that are believed to comprise discrete signaling teams within the receptor array (23). The highly conserved nature of its trimer contact sites predicts that Aer molecules might form mixed trimers with conventional MCPs, which could promote functional interactions between the two transducer types.
In a companion study, we described mutations in the PAS, HAMP, and HAMP-proximal signaling domains of Aer (see Fig. 2) that produced a strong clockwise (CW) flagellar rotational bias, presumably reflecting constitutively high CheA activity (8). Although nonfunctional as the cell's sole transducer, these CW-biased mutant Aer proteins regained the ability to mediate aerotactic responses in the presence of MCPs, an effect we term functional rescue. We postulate that MCP-rescuable Aer defects (designated Aer-CW) do not abrogate signal transmission through the Aer molecule but rather distort Aer's prestimulus signal output, producing an inappropriate (CW-biased) swimming pattern. According to this model, functional rescue could occur in either of two ways: (i) the presence of other transducers could simply return the cell's overall rotational bias to the normal range, allowing the mutant Aer molecules to operate more effectively, or (ii) MCPs might alter Aer signaling properties through formation of mixed, trimer-based signaling teams.
FIG. 2.
Transducer constructs and mutations used in this study. The different functional domains shown in the figure are not drawn to scale. The large shaded circle enclosed by the PAS domain represents the flavin adenine dinucleotide (FAD) cofactor. Mutation symbols: black diamonds, CW biased; black squares, epistatic; gray squares, epistasis suppressors. Other symbols: white circle, TMEA cross-linking site; gray and black circles, methylation sites. SD, signaling domain; F1, segment of unknown function (8); HAMP, segment for PAS input (8, 14, 27); M, membrane segment (3).
In this study we explore the mechanism of Aer-CW functional rescue by MCPs, in particular the possibility that these transducers can form mixed signaling teams. To establish the minimum MCP requirements for rescue of CW-biased Aer defects, we examined the ability of various mutant MCP molecules to rescue Aer-CW function. To investigate mixed trimer formation, we used a trifunctional cross-linking reagent [Tris-(2-maleimidoethyl-amide) (TMEA)] to detect physical interactions between Aer and MCP molecules in vivo. We also tested mutations at Aer trimer contact sites for epistatic effects on MCP signaling and to determine whether those Aer lesions were suppressible by mutations in the MCP trimer contact region. Our results indicate that Aer is able to function in the presence of high-abundance, methylation-dependent chemoreceptors by forming mixed signaling teams that may serve to amplify Aer-initiated sensory signals.
MATERIALS AND METHODS
Bacterial strains.
The following strains used are derivatives of E. coli K-12 strain RP437 (18), and their markers relevant to this study are given in brackets or parentheses: RP3098 [Δ(flhD-flhB)4] (20), UU1250 [Δaer-1 Δtsr-7028 Δ(tar-tap)5201 Δtrg-100] (2), UU1535 [Δaer-1 Δ(tar-cheB)2234 Δtsr-7028 Δtrg-1005] (6), UU1604 [tar(S364C) Δtsr-7028 Δtrg-100 Δ(tap-cheB)2241] (24), UU1613 [tar(S364C) Δtsr-7028 Δtrg-100 Δ(tap-cheB)2241 Δ(cheA-cheW)2167] (24), UU1615 [Δaer-1 Δ(tar-tap)5201 Δtrg-100] (P. Ames and J. S. Parkinson, unpublished data), and UU1624 (Δaer-1 Δtsr-7028 Δtap-3654 Δtrg-100) (Ames and Parkinson, unpublished).
Plasmids.
Aer expression plasmids used in this work were pBR322 derivatives (7) inducible by isopropyl-β-d-thiogalactopyranoside (IPTG) and conferring ampicillin resistance: pMB1 Aer-M112V, pDM17 Aer-K221E, pDM17 Aer-M252T, pDM17 Aer-Q263L (8), pDM17 Aer-Q263am (M. Burón-Barral and J. S. Parkinson, unpublished data), pSB90 (Aer-C193A/C203A/C253A), and pKG122 (Aear-269) (6). pKG106 (wild-type Aer) carries the PCR-amplified aer coding region from pSB20 (5), flanked by introduced NdeI and BamHI sites, inserted between the NdeI and BamHI sites of the pNP1 cloning vector (6).
Tar expression plasmids were pACYC184 (9) derivatives inducible by sodium salicylate and conferring chloramphenicol resistance: pLC113 (wild-type Tar) (2), pLC113 Tar-L376F, pLC113 Tar-A380V, pLC113 Tar-G393V (Ames and Parkinson, unpublished), pPA790 [TarΔ(44-183)] (Tar with residues 44 to 183 deleted), and pPA791 [TarΔ(44-183)/T303I] (Ames and Parkinson, unpublished).
Chemotaxis assays.
Aer and Tar plasmids were assessed for function on tryptone soft agar plates (16) or minimal succinate soft agar plates (4, 5) containing appropriate antibiotics (ampicillin [50 μg/ml] and/or chloramphenicol [12.5 μg/ml]) and inducers (IPTG and/or salicylate). Plates were incubated for 17 to 20 h at 30°C or 32°C.
Immunoblotting Aer.
Polyclonal rabbit antisera by Covance Technologies (Denver, PA) were raised against Aer residues 1 to 262 (Aer[1-262]). To prepare Aer[1-262] fragments, RP3098 cells carrying plasmid pDM17 Aer-Q263am were grown in H1 Casamino Acids at 30°C to mid-log phase and induced with 1 mM IPTG, as described previously (1). After three additional hours of growth, the cells were harvested by centrifugation and lysed by two passes through a French press at 10,000 lb/in2. Membranes containing Aer[1-262] were pelleted by centrifugation at 100,000 × g for 1 h, and then the pellet was homogenized and solubilized in 8 M urea, 0.1 M NaH2PO4, 0.01 M Tris-HCl (pH 8) by being rocked at room temperature for 2 h. The sample was partially clarified by centrifugation at 7,800 × g for 20 min and fractionated on preparative 15% acrylamide-sodium dodecyl sulfate (SDS) gels. Gel slices containing the Aer[1-262] band were used for antiserum production.
Cell extracts containing Aer proteins were prepared and analyzed as described previously (8). Aer[1-262] antiserum was treated with an acetone powder of strain UU1250 to reduce nonspecific cross-reactivity. Western blot analyses were performed with a 1/600 dilution of the primary antibody in a 5% milk solution. Aer bands were visualized with a goat anti-rabbit secondary antibody conjugated to a Cy5 fluorescent reporter (GE/Amersham Biosciences, Piscataway, NJ).
TMEA cross-linking.
An Aer derivative with alanine replacements at each of the three native cysteines (C193A/C203A/C253A), which had previously been shown to support aerotaxis in the presence of MCPs (strain UU1117 carrying pSB90 [pSB90/UU1117] [6]), failed to do so in the absence of MCPs (pSB90/UU1250 [data not shown]). We found that the C253A replacement was responsible for this defect. To construct a fully functional Aer derivative lacking all three native cysteine residues, we randomized codon 253 in pSB90 by QuikChange mutagenesis (2) and selected on tryptone soft agar for aerotactic transformants of host UU1250. A C253L derivative with full Aer function (pKG151 [Aer-C193A/C203A/C253L]) was chosen as the parental plasmid for in vivo cross-linking studies. A reporter site (S356C) and subsequent trimer contact lesion (I367P) were created in pKG151 by site-directed mutagenesis, yielding pKG158 (pKG151-S356C) and pKG193 (pKG158-I367P).
Strains carrying pKG158 or pKG193 were grown in tryptone broth plus 50 μg/ml ampicillin at 30°C to early log phase (optical density at 600 nm of ∼0.2), and Aer synthesis was induced with 15 or 60 μM IPTG, respectively. After an additional 4 h of incubation at 30°C, the cells were harvested by centrifugation and resuspended in KEP buffer (2) at an optical density at 600 nm of 2. Cell samples (0.5 ml) were treated with 100 μM TMEA for 5 min at 30°C, and reactions were quenched with 10 mM N-ethylmaleimide. The treated cells were pelleted, resuspended in 50 μl of SDS-polyacrylamide gel electrophoresis sample buffer (12) containing 10 mM N-ethylmaleimide, and analyzed by SDS-polyacrylamide gel electrophoresis as previously described (23). Aer was visualized by immunoblotting with a 1:600 dilution of anti-Aer[1-262]. Aer and Tar were visualized on the same blot with a 1:1,000 dilution of anti-Tsr[290-470] (1).
RESULTS
Optimal Aer stoichiometry in the presence and absence of MCPs.
Aer comprises only a few percent of the receptor population in wild-type cells (13), where the Tar and Tsr receptors predominate, and probably has little direct control over steady-state CheA activity and the cell's unstimulated swimming pattern. Yet, Aer mediates robust aerotactic responses in wild-type cells, presumably with assistance from the high-abundance receptors. With no collaborating receptors, Aer itself would need to establish an appropriate swimming pattern in order to promote aerotactic behavior.
To determine the optimal stoichiometry for Aer function in the absence of other receptors, we examined the aerotactic behavior and Aer expression level of transducerless strain UU1250 carrying pKG106, an IPTG-inducible Aer expression plasmid (Fig. 1). With no induction, pKG106 expressed Aer at roughly sixfold the chromosomal level but produced little increase in colony size on tryptone soft agar over the vector control plasmid (pNP1). Maximal aerotactic expansion occurred with 50 μM IPTG, which leads to a level of Aer expression about 350-fold higher than that supported by the chromosomal gene. Aer expression at 900-fold above the chromosomal level (500 μM IPTG) caused a modest decline in colony size (Fig. 1). These findings indicate that as the cell's sole transducer, Aer functions optimally at an expression level that is roughly comparable to the aggregate number of transducer molecules in a wild-type cell.
FIG. 1.
Relationship of Aer function to Aer expression level. Measurements were made with the transducerless strain UU1250 carrying pKG106. Aerotactic ability was assessed on tryptone soft agar containing 50 μg/ml ampicillin and various IPTG concentrations. Fresh transformants were toothpicked to soft agar, and colony diameters (filled circles) were measured after incubation at 30°C for 17 h. Data points represent the mean values for four colonies; standard errors (not shown) were smaller than the size of the data symbol. The dashed line indicates the size of UU1250 colonies carrying a control vector (pNP1). Aer expression levels were determined in tryptone cell cultures grown to mid-log phase at 30°C as described in Materials and Methods. Aer expression levels in pKG106/UU1250 were normalized to that for RP437 (wild type) measured in parallel.
Functional rescue of CW-biased Aer defects.
Mutant Aer proteins with CW-biased (CheA-activating) output signals cannot mediate aerotactic responses as the cell's sole transducer, regardless of their expression level (8). However, even at roughly chromosomal expression levels, CW-biased Aer proteins function normally in cells containing a full complement of MCP receptors (8). To explore the mechanistic basis of this effect, we examined variants of the high-abundance aspartate receptor Tar for their ability to rescue the function of Aer-CW proteins. We chose Tar as the rescuing receptor because the other high-abundance receptor, Tsr (serine), is known to mediate an aerotactic response that could confound assessment of Aer function (19). The various receptor constructs and mutations used in these studies are listed in Fig. 2.
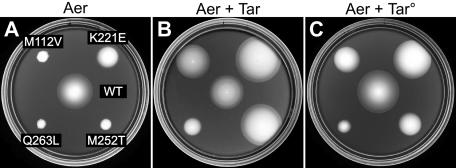
We first tested whether wild-type Tar alone was capable of rescuing Aer-CW mutations (M112V, K221E, M252T, and Q263L) previously shown to regain function in a strain containing all four MCPs (8). Mutant Aer plasmids were tested in strain UU1624, which expresses wild-type Tar from the chromosomal tar gene and lacks the genes for other MCPs and Aer. Because Tar mediates aspartate taxis on tryptone media, we evaluated aerotaxis on minimal succinate soft agar plates (4, 5) (Fig. 3B). At uninduced Aer expression levels (roughly sixfold over chromosomal Aer levels), Tar alone rescued all but the Aer-Q263L mutation (Fig. 3). Several of the Tar-rescued mutants, particularly K221E and M252T, formed colonies that were consistently larger than those of cells expressing wild-type Aer (Fig. 3B). These behaviors appear to be examples of a “super-swarmer” phenomenon first noted by Ma et al. (14). In the Discussion, we offer an explanation for this interesting effect.
FIG. 3.
Functional rescue of CW-biased Aer mutants by Tar and Tar° transducers. (A) Transducerless strain UU1250 carrying both pPA791 (Tar°-T303I) and CW-biased derivatives of pKG106 (Aer) on tryptone soft agar containing 50 μM IPTG. WT, wild type. (C) Same strains as for panel A, except induced with both 50 μM IPTG and 1 μM salicylate. The plates in panels A and C contained 50 μg/ml ampicillin and 12.5 μg/ml chloramphenicol and were incubated at 30°C for 17 h. (B) Strain UU1624 (wild-type Tar) carrying CW-biased derivatives of pKG106 on minimal succinate soft agar containing no IPTG. The plate in panel B contained 50 μg/ml ampicillin and was incubated at 30°C for 20 h.
Functional rescue by the Tar signaling domain.
We asked whether the Tar signaling domain was sufficient for rescue by testing a Tar derivative lacking the periplasmic aspartate-binding domain (Tar° [Fig. 2]). These rescue experiments were performed in a receptorless host strain (UU1250) carrying two compatible plasmids, an IPTG-inducible Aer plasmid and a salicylate-inducible Tar° plasmid. Because Tar° cannot mediate an aspartate response, we assessed aerotactic behavior on tryptone soft agar plates. The initial Tar° construct could not induce tumbling episodes in UU1250 and failed to rescue any Aer mutants (data not shown). However, a single amino acid replacement (T303I) that restores CW-signaling ability to Tar° (Ames and Parkinson, unpublished) enabled it to rescue all but the Aer-Q263L defect (Fig. 3C). The extent of rescue was more variable and less complete with Tar°-T303I than with wild-type Tar. Aer-K221E was fully rescued by both, whereas Aer-M112V and Aer-M252T were only partly rescued by Tar°-T303I: colonies increased in size but lacked edges with the sharp ring of aerotactic cells characteristic of wild-type Aer colonies (Fig. 3C). The differential rescuability of CW-biased Aer defects may reflect their degrees of CW bias and how effectively the rescuing receptor can impose a normal swimming pattern on the cell. Wild-type Tar is capable of adjusting its signal output via methylation changes, whereas Tar° constructs could be less efficient in this regard. In any event, it is clear that a nonfunctional receptor (Tar°) with a membrane-associated signaling domain can rescue Aer-CW defects. A fully soluble Tar signaling domain might also rescue Aer-CW mutants, but we did not test this prediction in the current study.
Aer functional rescue and mixed trimer formation.
The Aer signaling domain is quite similar to that of MCPs, with fully conserved trimer contact residues. Functional rescue of CW-biased Aer proteins could conceivably occur through formation of mixed trimers of dimers with the rescuing receptors. To assess Aer trimer-of-dimer formation in vivo, we used a trifunctional thiol-reactive cross-linker (TMEA) and Aer molecules with a cysteine residue near the predicted trimer interface (23). In Tar, Tsr, and Trg, a single cysteine reporter at the position corresponding to Aer-S356 allowed efficient capture of the three axial subunits in trimers of dimers upon TMEA treatment of intact cells (23). The Aer reporter protein (Aer [C193A/C203A/C253L/S356C]; hereafter, Aer-S356C) (see Materials and Methods) mediated aerotactic responses on tryptone soft agar but with some degradation in the sharpness of the aerotactic ring at the colony edge caused by the S356C alteration (data not shown).
Cells expressing Aer-S356C readily yielded two- and three-subunit cross-linking products upon TMEA treatment, consistent with trimer-of-dimer formation (Fig. 4). Moreover, a null mutation at one of the putative contact residues (I367P) greatly reduced the yield of three-subunit products, confirming their trimer-dependent origin (Fig. 4). Note that Aer-S356C/I367P still formed some two-subunit cross-linking products (Fig. 4, lane 4). In other receptors, the equivalent trimer contact lesion prevents trimer formation but also seems to enhance collisional cross-linking between subunits within a dimer, probably by destabilizing the local structure of the signaling domain (23). In summary, it appears that Aer can form trimers of dimers.
FIG. 4.
TMEA cross-linking of Aer and Tar. Cells expressing various combinations of Tar and Aer cross-linking reporters were grown in tryptone broth at 30°C, treated with TMEA, and analyzed as described in Materials and Methods. About 50% of Aer and Tar subunits become cross-linked under these conditions (not shown; see reference 23). The gel regions containing two-subunit (e.g., Tar∼Tar) and three-subunit (e.g., Tar∼Tar∼Tar) cross-linking products are shown; uncrosslinked Aer and Tar molecules are not. The gel on the left (first three lanes) was blotted with a combination of anti-Aer and anti-Tsr; the gel on the right was blotted with anti-Aer. Lane 1, pNP1 (Aer vector control)/UU1604 (Tar-S364C); lane 2, pKG158 (Aer-S356C)/UU1604; lane 3, pKG193 (Aer-S356C/I367P)/UU1604; lane 4, pKG193 (Aer-S356C/I367P)/UU1250 (no transducers); lane 5, pKG158 (Aer-S356C)/UU1250. Strains containing pKG158 were induced at 15 μM IPTG; strains containing pKG193 were induced with 60 μM IPTG to compensate for lower expression of Aer proteins with the I367P lesion. The faint bands at the Tar∼Tar position, most apparent in lanes 4 and 5, are unidentified cellular proteins that cross-react with the anti-Aer serum.
To look for evidence of mixed trimer formation, Aer-S356C was expressed in strain UU1604, which encodes Tar-S364C from the chromosome (17). Upon TMEA treatment of the cells, we observed both pure and mixed two- and three-subunit cross-linking products (Fig. 4, lanes 1 to 3). The I367P trimer contact lesion eliminated the mixed products, demonstrating that their formation was trimer dependent (Fig. 4). Trimer formation by Tar and other MCPs occurs efficiently in strains lacking the CheA or CheW components of receptor signaling complexes (17, 23). We found that the cross-linking patterns of Aer-S356C were unchanged in strain UU1613, which carries a cheA-cheW deletion, demonstrating that Aer trimer formation is also independent of the CheA and CheW proteins (data not shown).
Aer interference with Tar and Tsr signaling.
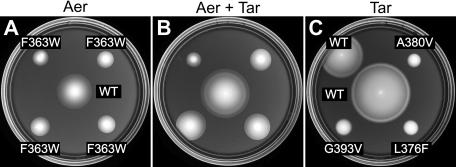
Our cross-linking results demonstrate that Aer forms trimers and mixed trimers in vivo. Accordingly, Aer lesions that allow formation of mixed, but defective, trimers might block signaling by heterologous receptors. A variety of trimer contact mutations in Tsr are known to exert epistatic effects on Tar function (2). We chose two such lesions, Tsr-F373W and Tsr-N376W, and created the comparable mutations in Aer (Aer-F363W and Aer-N366W). As expected, both alterations severely impaired Aer function, although Aer-F363W evinced some residual activity in soft agar assays (see Fig. 6A). When expressed in Tar-only (UU1624) and Tsr-only (UU1615) host strains, the mutant Aer plasmids blocked aspartate and serine chemotaxis (Fig. 5). Epistasis over Tsr, the most abundant MCP species in E. coli, was evident only upon Aer induction (Fig. 5A and B), whereas the Aer mutants impaired Tar function without explicit induction (Fig. 5C and D). We also noted that coexpression of wild-type Aer with Tar augmented the rate of colony expansion, possibly due to synergy between the aspartate and aerotactic responses (Fig. 5C). Upon induction with 50 μM IPTG, corresponding to a modest stoichiometric excess, wild-type Aer caused slight reductions in Tar and Tsr function that were much less dramatic than the epistatic Aer defects. We conclude that alterations at the Aer trimer contact sites can block signaling by heterologous receptors and propose that these epistatic effects occur in mixed trimers of receptor dimers.
FIG. 6.
Suppression of epistatic Aer defects by mutant Tar receptors. Transducerless strain UU1250 was transformed with compatible plasmids (pKG106 derivative plus pLC113 derivative) and tested for Aer and Tar function on tryptone soft agar. The genotypes of the Aer plasmids are listed in panel A; those of the Tar plasmids are listed in panel C. Plates contained various combinations of inducers to express Aer alone (A) (50 μM IPTG, 0 μM salicylate), Aer plus Tar (B) (50 μM IPTG, 0.7 μM salicylate), or Tar alone (C) (0 μM IPTG, 0.7 μM salicylate). Incubation was at 30°C for 12 h. WT, wild type.
FIG. 5.
Epistatic effects of mutant Aer proteins on Tar and Tsr function. Plasmids (pNP1 [Aer vector control], pKG106 [wild-type Aer], pKG106-F363W, and pKG106-N366W) were introduced into strains expressing only Tsr (UU1615) or Tar (UU1624) from the chromosome. Transformant cells were tested for serine chemotaxis (32°C) or aspartate chemotaxis (30°C) on tryptone soft agar after incubation for the following times: (A) 7 h, (B) 12 h, (C) 16 h, and (D) 16 h. WT, wild type.
Suppression of epistatic Aer defects by mutant Tar receptors.
If Aer epistasis occurs in mixed trimers, it might be possible to suppress Aer signaling defects, analogous to the functional rescue phenomenon, through mutations in other receptors that create compensatory alterations in the conformation and signaling properties of the mixed trimers. For example, mutations in the trimer contact region of Tar can act as allele-specific suppressors of the epistatic Tsr-F373W defect (17). We tested several of those Tar mutations (Tar-A380V, Tar-L376W, and Tar-G393V) for ability to suppress the aerotaxis defect of Aer-F363W (Fig. 6). All three Tar mutations enhanced colony expansion and restored ring formation to the Aer mutant, in contrast to wild-type Tar, which had no effect on Aer function (Fig. 6B). Note that the mutant Tar receptors were not able to mediate aspartate taxis, either alone (Fig. 6C) or in combination with Aer-F363W (Fig. 6B), consistent with a compensatory conformational change in a Tar/Aer structural complex.
Functional rescue of CW-biased defects in an Aer/Tar chimera.
Functional rescue of Aer-CW defects by Tar° molecules that lack a sensing domain implies that the mutant Aer proteins retain aerosensing and output control capability. Thus, the role of the rescuing receptor might be simply to adjust the prestimulus output activity of the mutant Aer signaling domains through mixed trimer formation. This rescue mechanism predicts that the mutant Aer molecules alone might mediate aerotactic responses if their CW-biased prestimulus output could somehow be attenuated. To test this idea, we transferred CW-biased Aer lesions into an Aer/Tar chimera (Aear-269) that has the input-output module of Aer joined to the signaling domain of Tar (6). Like Tar, Aear transducers are able to modulate their signal output through feedback control of their methylation state (6). In Aear, the effects of the CW-biased Aer input-output module should be offset by the sensory adaptation ability of the Tar signaling domain.
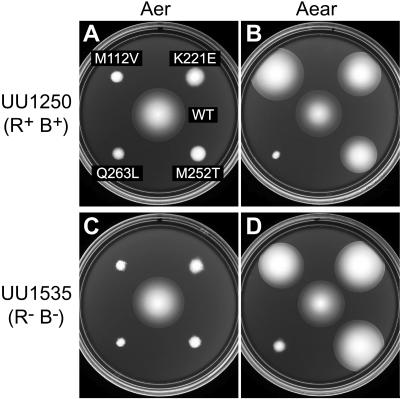
CW-biased Aer mutations were introduced into Aear-269, and the mutant chimeras were tested for ability to promote aerotaxis in receptorless host strains (Fig. 7). Three CW lesions (M112V, K221E, and M252T) exhibited functional rescue in Aear; the Q263L defect did not (Fig. 7). The rescue phenotypes were similar in strains with (UU1250 [Fig. 7A and B]) and without (UU1535 [Fig. 7C and D]) the CheR/CheB functions, suggesting that the mutant chimeras functioned efficiently with two unmethylated sites and two undeamidated sites, which minic methylated sites.
FIG. 7.
Phenotypic suppression of CW-biased signaling defects in the Aear-269 chimeric transducer. Strains containing plasmids with CW-biased mutations in Aer (pKG106 derivatives [A and C]) or Aear-269 (pKG122 derivatives [B and D]) were tested for aerotactic ability on tryptone soft agar (50 μM IPTG) at 30°C. Incubation times were 17 h (A, C, and D) and 12 h (B). WT, wild type; R+ B+, strain with CheR/CheB functions; R− B−, strain without CheR/CheB functions.
DISCUSSION
Aer normally operates as a low-abundance transducer.
In wild-type E. coli, Aer molecules comprise at most a few percent of the chemoreceptor population yet produce robust motility responses to aerotactic stimuli. Because Aer communicates with the flagellar motors by the same phosphorelay pathway as conventional MCP receptors, the high-abundance transducers presumably command the majority of CheA signaling molecules and must somehow augment Aer signal output. One possible mechanism for collaborative signaling between different MCP-type receptors is formation of mixed trimer-of-dimer signaling teams (2, 23). In this study we obtained several lines of evidence that Aer forms mixed signaling teams with the aspartate receptor Tar and the serine receptor Tsr.
Evidence for mixed Aer signaling teams.
Aer molecules bearing a cysteine reporter diagnostic for the trimer-of-dimers geometry (23) yielded two- and three-subunit cross-linking products upon treatment with the trifunctional reagent TMEA. In the presence of corresponding Tar reporter molecules, Aer also formed mixed cross-linking products. A trimer contact site lesion (I367P) known to abolish trimer formation by MCPs (23) eliminated both Aer signaling and trimer/mixed trimer formation. Although we did not explicitly investigate cross-linking interactions between Aer and Tsr in this report, we assume that Tar is not unique in this regard. Moreover, Aer exhibited no evident preference for self-association in these cross-linking assays, which implies that at its normal relative expression level, Aer dimers seldom interact as part of the same trimer team.
Trimer contact alterations known to cause epistatic behavior in other receptors (F363W and N366W) also produced epistatic properties in Aer. These Aer lesions prevented not only aerotactic signaling but also chemotactic signaling by wild-type Tar and Tsr molecules. Amino acid replacements in the Tar trimer contact region (L376F, A380V, and G393V) suppressed the Aer-F363W signaling defect, consistent with compensatory conformational changes between directly interacting proteins.
Aer also functions as a solitary transducer.
Aer can mediate aerotactic responses in cells that lack MCP receptors but must be expressed at high levels to do so. We found that the optimal Aer expression level for function in the absence of MCPs approximated the aggregate number of Aer and chemoreceptor molecules in a wild-type cell. We assume that this level of Aer expression yields satisfactory stoichiometries with the CheA/CheW components of ternary Aer signaling complexes and in turn imparts a steady-state run/tumble swimming pattern suitable for aerotactic responses. However, episodes of CW flagellar rotation are less frequent in Aer-only cells than in cells containing other transducers (8), which may reflect the lack of a methylation-dependent adaptation system in Aer or an intrinsically different efficiency of CheA activation in Aer signaling complexes.
The behavior and underlying signaling mechanisms of Aer-only cells could be distinctly different from those of wild-type cells. In the absence of other transducers, Aer molecules presumably form homogeneous trimer-of-dimer signaling teams. Such Aer complexes most likely do not exist in wild-type cells, owing to mixed trimer formation with the vast excess of MCP molecules. The MCP-dependent functional rescue of mutant Aer proteins with CW-biased signal output provides a striking example of these differences. In the absence of MCPs, CW-biased Aer proteins cannot mediate aerotactic responses, regardless of expression level (8). However, in the presence of MCPs, even very low levels of CW-biased Aer molecules produce robust aerotactic behavior (8).
We found that a Tar molecule lacking the periplasmic aspartate-binding domain (Tar°) also restored function to CW-biased Aer proteins, supporting the idea that rescue occurs in mixed teams based on trimer-of-dimer interactions between receptor signaling domains. We suggest that CW-biased Aer molecules spend more time in the counterclockwise conformation when interacting with MCPs in trimers of dimers. This shift in counterclockwise-CW equilibrium in turn enables Aer to modulate team signal output in response to aerotactic stimuli, thereby restoring aerotactic behavior. Consistent with this team-based model of functional rescue, we also found that CW-biased Aer lesions did not impair aerotactic signaling in a hybrid Aer-Tar transducer capable of adjusting its steady-state signal output via methylation-dependent sensory adaptation. The extent of rescue seemed to be correlated with the severity of the CW-biased defects, which might reflect residual stimulus sensitivity and output signaling efficiency. CW-biased lesions in PAS (M112V) and HAMP (M252T), which may interact to elicit CW rotation (8, 27), were rescued less effectively than a lesion in the HAMP connector (K221E), which has been proposed to play a less direct role in the production of CW output signals in Aer (8). Similarly, a CW-biased lesion in the proximal signaling domain (Q263L), which is downstream of the proposed sensory input control point, was not effectively rescued in any test.
Functional rescue of CW-biased Aer defects and the “super-swarmer” phenomenon.
Ma et al. have described mutations in the HAMP domain of Aer that cause rapidly spreading colonies (“super swarms”) on minimal succinate soft agar (14). Those plasmid-borne Aer lesions were characterized in a host that contained Tar and the low-abundance Tap and Trg transducers. More recently, several of those mutations (K221E, A223V, and V264A) were shown to be MCP-rescuable, CW-biased Aer lesions (8). We suggest that the super-swarming behavior of these mutants depends on bias adjustment by MCPs and reflects altered sensitivity to oxygen gradients in the succinate plates. This explanation predicts that some of the other super-swarmer mutations described by Ma et al. may also exhibit CW-biased signaling defects in a strain lacking MCPs.
The super-swarming effect implies that band formation at the perimeter of aerotactic colonies may involve a different behavioral strategy than that for other chemoattractants. Cells travel toward conventional attractants by suppressing CW rotation whenever they head up-gradient, whereas down-gradient movements have no effect on their run/tumble bias. In contrast, aerotactic migrations in spatial oxygen gradients might involve bidirectional movement control: suppression of CW bias when cells head up-gradient and enhancement of CW bias when cells head down-gradient. Cells, such as the super-swarmers, with inherently CW-biased Aer proteins should be more sensitive than wild-type cells to down-gradient movements, which might readily elicit tumbling responses, thereby reducing down-gradient travel time and speeding colony expansion.
Acknowledgments
This work was supported by research grant GM62940 from the National Institute of General Medical Sciences. The Protein-DNA Core Facility at the University of Utah receives support from National Cancer Institute grant CA42014 to the Huntsman Cancer Institute.
REFERENCES
- 1.Ames, P., and J. S. Parkinson. 1994. Constitutively signaling fragments of Tsr, the Escherichia coli serine chemoreceptor. J. Bacteriol. 176:6340-6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ames, P., C. A. Studdert, R. H. Reiser, and J. S. Parkinson. 2002. Collaborative signaling by mixed chemoreceptor teams in Escherichia coli. Proc. Natl. Acad. Sci. USA 99:7060-7065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amin, D. N., B. L. Taylor, and M. S. Johnson. 2006. Topology and boundaries of the aerotaxis receptor Aer in the membrane of Escherichia coli. J. Bacteriol. 188:894-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bibikov, S. I., L. A. Barnes, Y. Gitin, and J. S. Parkinson. 2000. Domain organization and flavin adenine dinucleotide-binding determinants in the aerotaxis signal transducer Aer of Escherichia coli. Proc. Natl. Acad. Sci. USA 97:5830-5835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bibikov, S. I., R. Biran, K. E. Rudd, and J. S. Parkinson. 1997. A signal transducer for aerotaxis in Escherichia coli. J. Bacteriol. 179:4075-4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bibikov, S. I., A. C. Miller, K. K. Gosink, and J. S. Parkinson. 2004. Methylation-independent aerotaxis mediated by the Escherichia coli Aer protein. J. Bacteriol. 186:3730-3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bolivar, F., R. Rodriguez, P. J. Greene, M. C. Betlach, H. L. Heyneker, and H. W. Boyer. 1977. Construction and characterization of new cloning vehicles. Gene 2:95-113. [PubMed] [Google Scholar]
- 8.Burón-Barral, M. D. C., K. K. Gosink, and J. S. Parkinson. 2006. Loss- and gain-of-function mutations in the F1-HAMP region of the Escherichia coli aerotaxis transducer Aer. J. Bacteriol. 188:3477-3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang, A. C. Y., and S. N. Cohen. 1978. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the p15A cryptic miniplasmid. J. Bacteriol. 134:1141-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Falke, J. J., and G. L. Hazelbauer. 2001. Transmembrane signaling in bacterial chemoreceptors. Trends Biochem. Sci. 26:257-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim, K. K., H. Yokota, and S. H. Kim. 1999. Four-helical-bundle structure of the cytoplasmic domain of a serine chemotaxis receptor. Nature 400:787-792. [DOI] [PubMed] [Google Scholar]
- 12.Laemmli, U. K. 1970. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 13.Li, M., and G. L. Hazelbauer. 2004. Cellular stoichiometry of the components of the chemotaxis signaling complex. J. Bacteriol. 186:3687-3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma, Q., M. S. Johnson, and B. L. Taylor. 2005. Genetic analysis of the HAMP domain of the Aer aerotaxis sensor localizes flavin adenine dinucleotide-binding determinants to the AS-2 helix. J. Bacteriol. 187:193-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maddock, J. R., and L. Shapiro. 1993. Polar location of the chemoreceptor complex in the Escherichia coli cell. Science 259:1717-1723. [DOI] [PubMed] [Google Scholar]
- 16.Parkinson, J. S. 1976. cheA, cheB, and cheC genes of Escherichia coli and their role in chemotaxis. J. Bacteriol. 126:758-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parkinson, J. S., P. Ames, and C. A. Studdert. 2005. Collaborative signaling by bacterial chemoreceptors. Curr. Opin. Microbiol. 8:116-121. [DOI] [PubMed] [Google Scholar]
- 18.Parkinson, J. S., and S. E. Houts. 1982. Isolation and behavior of Escherichia coli deletion mutants lacking chemotaxis functions. J. Bacteriol. 151:106-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rebbapragada, A., M. S. Johnson, G. P. Harding, A. J. Zuccarelli, H. M. Fletcher, I. B. Zhulin, and B. L. Taylor. 1997. The Aer protein and the serine chemoreceptor Tsr independently sense intracellular energy levels and transduce oxygen, redox, and energy signals for Escherichia coli behavior. Proc. Natl. Acad. Sci. USA 94:10541-10546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith, R. A., and J. S. Parkinson. 1980. Overlapping genes at the cheA locus of E. coli. Proc. Natl. Acad. Sci. USA 77:5370-5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sourjik, V. 2004. Receptor clustering and signal processing in E. coli chemotaxis. Trends Microbiol. 12:569-576. [DOI] [PubMed] [Google Scholar]
- 22.Sourjik, V., and H. C. Berg. 2000. Localization of components of the chemotaxis machinery of Escherichia coli using fluorescent protein fusions. Mol. Microbiol. 37:740-751. [DOI] [PubMed] [Google Scholar]
- 23.Studdert, C. A., and J. S. Parkinson. 2004. Crosslinking snapshots of bacterial chemoreceptor squads. Proc. Natl. Acad. Sci. USA 101:2117-2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Studdert, C. A., and J. S. Parkinson. 2005. Insights into the organization and dynamics of bacterial chemoreceptor clusters through in vivo crosslinking studies. Proc. Natl. Acad. Sci. USA 102:15623-15628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor, B. L., and I. B. Zhulin. 1999. PAS domains: internal sensors of oxygen, redox potential, and light. Microbiol. Mol. Biol. Rev. 63:479-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor, B. L., I. B. Zhulin, and M. S. Johnson. 1999. Aerotaxis and other energy-sensing behavior in bacteria. Annu. Rev. Microbiol. 53:103-128. [DOI] [PubMed] [Google Scholar]
- 27.Watts, K. J., Q. Ma, M. S. Johnson, and B. L. Taylor. 2004. Interactions between the PAS and HAMP domains of the Escherichia coli aerotaxis receptor Aer. J. Bacteriol. 186:7440-7449. [DOI] [PMC free article] [PubMed] [Google Scholar]