Abstract
Clostridium difficile produces two toxins, A and B, which act together to cause pseudomembraneous colitis. The genes encoding these toxins, tcdA and tcdB, are part of the pathogenicity locus, which also includes tcdC, a putative negative regulator of the toxin genes. In this study, we demonstrate that TcdC is a membrane-associated protein in C. difficile.
Clostridium difficile is the etiologic agent of C. difficile-associated disease (CDAD). This disease is unique in that it is often initiated by antibiotic therapy (1). In susceptible individuals, disruption of the normal colonic microflora by antibiotic treatment leads to colonization by C. difficile. Pathogenic C. difficile produces two toxins, toxin A and toxin B, which modify the actin cytoskeleton of intestinal epithelial cells, compromising the barrier function of the epithelium and leading to fluid loss and diarrhea (2, 19).
The pathogenicity locus of C. difficile contains tcdA, tcdB, tcdR, tcdE, and tcdC (3, 7). Genes tcdA and tcdB code for toxins A and B, respectively. The gene tcdR (previously txeR) has been shown to encode an alternate sigma factor that positively regulates tcdA and tcdB as well as itself (13, 14). Negative regulators of tcdR and the toxin genes are unknown, although it has been postulated that TcdC might be involved in the negative regulation of toxin gene expression (9). The gene for TcdC is divergently transcribed from the toxin genes and codes for a 231-amino-acid protein. To further characterize TcdC, we raised polyclonal anti-TcdC antibodies in a rabbit and used them to determine that TcdC is localized in the C. difficile cytoplasmic membrane and is specifically expressed during the logarithmic phase of growth.
Production of anti-TcdC polyclonal antibodies.
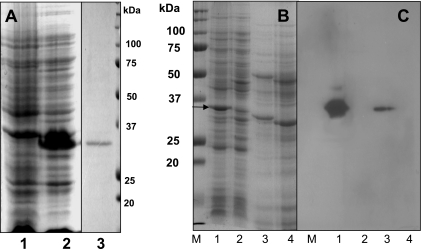
The TcdC gene was cloned from C. diffcile strain VPI 10463 into the NdeI and BamHI sites of pET22b (Novagen), using PCR and the oligonucleotide primers TcdC (forward), 5′-GGTCGTCATATGTTTTCTAAAAAAAATGAGGG-3′, and TcdC (reverse), 5′-GGCCCGGGGATCCTTAATTTTCTCTA-3′. The cloned tcdC gene was expressed from the vector-derived T7 promoter and ribosomal binding site in the Escherichia coli strain BL21 (DE3). The expressed protein carried a six-carboxy-terminal histidine (six-His) tag. TcdC contains 231 amino acid residues with a calculated molecular mass of 25.7-kDa. However, the expressed TcdC has an apparent molecular mass of 34 kDa, as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Fig. 1A). The overexpressed soluble protein was purified, using a Ni2+ affinity column (13). The affinity-purified protein (Fig. 1A, lane 3) was then digested in gel with trypsin (Promega) and analyzed by liquid chromatography-mass spectrometry (data not shown). Two peptides (VIQVIEDGDEVQIR and VLEDDYITIR) were detected, which confirmed that the protein was TcdC. The purified TcdC was used to raise anti-TcdC antibodies in a rabbit. Two hundred fifty micrograms of TcdC was mixed with the adjuvant Titermax (Sigma) and injected subcutaneously into rabbits. After two booster injections, anti-TcdC antibodies were collected and preadsorbed against crude cell extracts of E. coli strain BL21(DE3) harboring pET22b (without tcdC).
FIG. 1.
(A) Expression and purification of TcdC. Analysis by SDS-PAGE of protein extracts from E. coli BL21(DE3) carrying either the pET22b vector or pET22b expressing TcdC. Lanes: 1, crude cell extract from E. coli carrying the vector pET22b; 2, crude cell extract from E. coli carrying the vector expressing TcdC; 3, His6-purified TcdC (5 μg). Proteins were stained by Coomassie brilliant blue. (B) Analysis by SDS-PAGE of protein extracts from E. coli and C. difficile strains (from 4-h-old exponentially growing cultures). Lanes: 1, crude cell extract from E. coli carrying the pET22b vector expressing TcdC; 2, crude cell extract from E. coli carrying the vector only; 3, crude cell extract from C. difficile strain VPI 10463; 4, crude cell extract from tcdC-negative nonpathogenic C. difficile strain VPI 11186. Proteins were stained with Coomassie brilliant blue. The arrow indicates the over-expressed TcdC. (C) Specificity of anti-TcdC antibody. Immunodetection of TcdC was carried out by using anti-TcdC antibody (dilution, 1:500). Antigen-antibody complexes were detected using anti-rabbit horseradish peroxidase-conjugated antibody (dilution, 1:10,000) and ECL Western blotting detection reagents (Amersham Biosciences). The protein samples in each lane correspond to those in the same-numbered lane in panel B. The optical densities of both E. coli and C. difficile strains were adjusted to 0.1 at 550 nm, and the cells were then sonicated and boiled with SDS-PAGE sample buffer before being loaded onto the gels.
Specificity of TcdC antibody.
The anti-TcdC antibodies were used to develop Western blots of proteins from cell extracts of C. difficile strains VPI 10463 and VPI 11186 and E. coli strains with or without tcdC (Fig. 1C). The anti-TcdC antibody reacted with a single protein band of similar molecular weight in the extracts of E. coli and C. difficile cells expressing TcdC but not with proteins from E. coli or C. difficile cells which do not express TcdC.
Subcellular localization of TcdC in C. difficile cells.
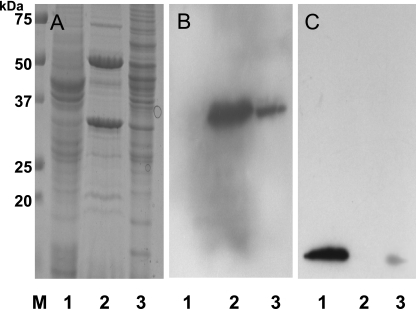
To determine the subcellular location of TcdC, we separated the cell proteins from C. difficile strain VPI 10463 into cytosolic and membrane fractions and probed these fractions for TcdC by Western blotting. Briefly, the cells were harvested by centrifugation, resuspended in Tris buffer (0.05 M Tris-HCl, pH 7.5) containing a protease inhibitor cocktail (Sigma), and disrupted by passage through a French pressure cell (no. 43398; Aminco) at 1,000 Kg/cm2. After being incubated with a mixture of DNase and RNase (50 μg each) (100 μg/ml) for 30 min, the cell lysates were centrifuged at low speed (4,000 × g) for 15 min at 4°C to remove unbroken cells. The supernatant was centrifuged at 200,000 × g for 60 min at 4°C to separate the cytosolic proteins (supernatant) from the membrane and peptidoglycan-associated proteins (pellet). The pellet was processed by two different methods. For the first, we separated the cytoplasmic membrane proteins from those associated with the peptidoglycan according to the method of Candela and Fouet (4). This process involved resuspending the pellet in Tris-HCl (pH 7.4)-5 mM EDTA with 2% Triton X-100 for 30 min at room temperature to solubilize the membrane proteins, followed by centrifugation (20, 000 × g for 1 h at 4°C) to pellet the peptidoglycan and its associated proteins. In the second method, the pellet was resuspended in Tris-HCl (pH 7.4) with 10% sucrose, loaded onto a step gradient consisting of 2 ml of 15, 30, 40, 50, and 60% sucrose in the same buffer, and centrifuged at 200,000 × g overnight before 0.5-ml fractions were collected for further analysis (8). Equal amounts of Triton X-100-soluble and -insoluble membranes and cytosolic proteins (30 μg) from isolated fractions were separated on an SDS-PAGE gel and analyzed by probing Western blots with anti-TcdC and rabbit antibodies raised against the streptococcal ribosomal proteins L7 and L12 (10). It has previously been shown that anti-L7/L12 antibodies cross-react with ribosomal proteins from a variety of bacteria (10). The anti-TcdC antibody reacted against a protein band in both the Triton X-100-soluble and -insoluble membrane fractions but not against proteins in the cytosolic fraction (Fig. 2B). As expected, the anti-L7/L12 antibodies detected a protein band in the cytoplasmic fraction. However, a weak reaction of anti-L7/L12 against the Triton X-100-insoluble membrane fraction (Fig. 2C) was detected. This may be due to a small contamination of the membrane fraction with polysomes resulting from the centrifugation steps. While anti-TcdC serum detected a protein fraction in both the soluble and insoluble membrane factions, the majority of TcdC was found in the Triton X-100-soluble fraction, suggesting that TcdC is associated with the cytoplasmic membrane.
FIG. 2.
Subcellular localization of TcdC in C. difficile cells. (A) Analysis by SDS-PAGE of membrane and cytosolic proteins harvested from C. difficile strain VPI 10463 grown in tryptone-yeast extract-glucose medium for 4 h. Lanes: 1, cytosolic proteins; 2, Triton X-100-soluble membrane proteins; 3, Triton X-100-insoluble proteins. The proteins were stained with Coomassie brilliant blue. (B) Immunodetection of TcdC by using anti-TcdC antibody. (C) Immunodetection of L7/L12 ribosomal protein using monoclonal anti-L7/L12 streptococcal ribosomal proteins (dilution, 1:1,000). The protein samples in panels B and C correspond to those in the same-numbered lanes in panel A.
Fractions collected after sucrose gradient centrifugation were separated by SDS-PAGE, transferred onto polyvinylidene difluoride membranes, and probed with two different antibodies: anti-TcdC and the anti-ATPase E. coli β subunit (6). The anti-ATPase antibodies reacted with a C. difficile protein in fractions 16 to 19, and the anti-TcdC antibodies reacted with a protein in fractions 11 to 20. Hence, both ATPase (a cytoplasmic membrane protein) and TcdC in higher abundance were found in the same sucrose fractions (Fig. 3, lanes 16 to 19), indicating that TcdC is associated with the cytoplasmic membrane. Note that bioinformatic software TMPRED (http://www.ch.embnet.org/software/TMPRED_form.html) has predicted a putative transmembrane domain in the N-terminal region of TcdC (amino acids 31 to 51).
FIG. 3.
Localization of TcdC and ATPase in membrane fractions collected after sucrose density gradient centrifugation. Lanes are marked with the collected-fraction number. (A) Proteins were stained with Coomassie brilliant blue. (B) TcdC was revealed by anti-TcdC antibody. (C) Immunoblot using anti-ATP synthetase β-subunit antibody (dilution, 1:50,000).
Expression analysis of TcdC in C. difficile cells.
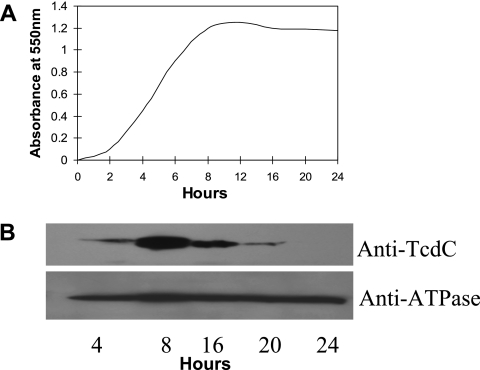
Cells from a growing culture of VPI 10463 were harvested at different time points, and the relative amount of TcdC per cell (i.e., optical density at 550 nm [OD550]) at each time point was determined by Western blotting. Maximum levels of TcdC were found during the exponential phase of growth, and levels decreased as the culture approached stationary phase (Fig. 4). That equal numbers of cells were employed in this analysis is shown by the equal levels of ATPase detected in the samples. Hundsberger et al. (9) have demonstrated a similar pattern for the transcription of tcdC, which is at maximum levels during exponential growth and decreases as the cells go into stationary phase. Hence, the amounts of TcdC per cell during the exponential and stationary phases of growth mimic the transcriptional activity of the TcdC gene.
FIG. 4.
(A) Growth curve of C. difficile strain VPI 10463 grown anaerobically in tryptone-yeast extract-glucose medium. Aliquots were removed at different time points for OD550 readings. (B) Expression analysis of TcdC in C. difficile cells. Lanes are marked with the times at which the cells were collected. The absorbance of the harvested cells was adjusted to 0.1 at 550 nm, and the cells were sonicated and boiled with SDS-PAGE sample buffer before being loaded onto the gels. ATPase and TcdC were detected, using specific antibodies.
In summary, we have cloned and expressed TcdC in E. coli cells and raised polyclonal antibodies against this protein. These antibodies were used to demonstrate that TcdC is membrane associated and to characterize its expression during growth in culture. Recently, there have been several reports concerning increases in the rate and severity of CDAD that may be associated with the emergence of a highly toxic, antibiotic-resistant strain of C. difficile (12, 15, 20). The predominant strain in these outbreaks has become resistant to fluoroquinolones, carries the binary toxin genes, and has a partial (18-bp) deletion in tcdC. Although the importance of the binary toxin and the 18-bp deletion to the virulence of C. difficle is unknown, the finding that the prevalence of these factors is much higher in isolates from outbreaks associated with increased morbidity suggests that they may affect the severity of CDAD.
Three different mutant alleles of tcdC have been identified thus far, and it has been suggested that these tcdC variants may have influenced the increased elaboration of toxins A and B in these strains (16, 20). For example, C. difficile strain 8864 carries a mutation resulting in a truncated TcdC protein (22 amino acids), which has been postulated to contribute to the extreme cytotoxicity of this strain (17). In a different study involving comparative analysis of pathogenicity loci in clinical isolates, two variant tcdC genes were identified: one variant codes for a truncated TcdC protein of only 61 amino acids, and the other carries an 18-bp deletion (18). Both variants produce increased amounts of toxins A and B. Finally, the 18-bp deletion in tcdC that has been found in C. difficile strains isolated from recent outbreaks in the United States and Canada (toxin type III) produced 16-fold more toxin A and 23-fold more toxin B than the control strains isolated from the same geographical regions at the same time (toxin type 0) (20). Although it is not known whether these mutant alleles render their tcdC products nonfunctional, the increased toxin production associated with tcdC deletions has led to the speculation that TcdC acts as a negative regulatory protein with respect to toxin expression. Localization of transcription factors to membranes would appear to be incompatible with the control of gene expression. However, similar membrane-associated transcriptional regulators have been reported for other bacteria. Some examples are ToxR of Vibrio cholerae (11) and the RpoE sigma factor of E. coli (5). Only with further genetic and physiological studies of TcdC will we begin to understand its role in the regulation of the expression of the toxin genes of this very important pathogen. Our finding that TcdC resides in the C. difficile membrane may have important implications for such studies.
Acknowledgments
We thank G. Deckers-Hebestreit and K. Altendorf for kindly providing the anti-ATPase β subunit and J. Kolberg for anti-L7/L12 antibodies. We also thank A. Sonenshein for his very helpful suggestions.
This research was supported in part by NIH grant R03DK054816-01.
REFERENCES
- 1.Bartlett, J. G. 1985. Treatment of Clostridium difficile colitis. Gastroenterology 89:1192-1195. [DOI] [PubMed] [Google Scholar]
- 2.Bongaerts, G. P., and D. M. Lyerly. 1994. Role of toxins A and B in the pathogenesis of Clostridium difficile disease. Microb. Pathog. 17:1-12. [DOI] [PubMed] [Google Scholar]
- 3.Braun, V., T. Hundsberger, P. Leukel, M. Sauerborn, and C. von Eichel-Streiber. 1996. Definition of the single integration site of the pathogenicity locus in Clostridium difficile. Gene 181:29-38. [DOI] [PubMed] [Google Scholar]
- 4.Candela, T., and A. Fouet. 2005. Bacillus anthracis CapD, belonging to the gamma-glutamyltranspeptidase family, is required for the covalent anchoring of capsule to peptidoglycan. Mol. Microbiol. 57:717-726. [DOI] [PubMed] [Google Scholar]
- 5.Dartigalongue, C., D. Missiakas, and S. Raina. 2001. Characterization of the Escherichia coli sigma E regulon. J. Biol. Chem. 276:20866-20875. [DOI] [PubMed] [Google Scholar]
- 6.Deckers-Hebestreit, G., and K. Altendorf. 1986. Accessibility of F0 subunits from Escherichia coli ATP synthase. A study with subunit specific antisera. Eur. J. Biochem. 161:225-231. [DOI] [PubMed] [Google Scholar]
- 7.Hammond, G. A., and J. L. Johnson. 1995. The toxigenic element of Clostridium difficile strain VPI 10463. Microb. Pathog. 19:203-213. [DOI] [PubMed] [Google Scholar]
- 8.Hofmeister, A. 1998. Activation of the proprotein transcription factor pro-σE is associated with its progression through three patterns of subcellular localization during sporulation in Bacillus subtilis. J. Bacteriol. 180:2426-2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hundsberger, T., V. Braun, M. Weidmann, P. Leukel, M. Sauerborn, and C. von Eichel-Streiber. 1997. Transcription analysis of the genes tcdA-E of the pathogenicity locus of Clostridium difficile. Eur. J. Biochem. 244:735-742. [DOI] [PubMed] [Google Scholar]
- 10.Kolberg, J., E. A. Hoiby, R. Lopez, and K. Sletten. 1997. Monoclonal antibodies against Streptococcus pneumoniae detect epitopes on eubacterial ribosomal proteins L7/L12 and on streptococcal elongation factor Ts. Microbiology 143:55-61. [DOI] [PubMed] [Google Scholar]
- 11.Krukonis, E. S., R. R. Yu, and V. J. Dirita. 2000. The Vibrio cholerae ToxR/TcpP/ToxT virulence cascade: distinct roles for two membrane-localized transcriptional activators on a single promoter. Mol. Microbiol. 38:67-84. [DOI] [PubMed] [Google Scholar]
- 12.Loo, V. G., L. Poirier, M. A. Miller, M. Oughton, M. D. Libman, S. Michaud, A. M. Bourgault, T. Nguyen, C. Frenette, M. Kelly, A. Vibien, P. Brassard, S. Fenn, K. Dewar, T. J. Hudson, R. Horn, P. Rene, Y. Monczak, and A. Dascal. 2005. A predominantly clonal multiinstitutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N. Engl. J. Med. 353:2442-2449. [DOI] [PubMed] [Google Scholar]
- 13.Mani, N., and B. Dupuy. 2001. Regulation of toxin synthesis in Clostridium difficile by an alternative RNA polymerase sigma factor. Proc. Natl. Acad. Sci. USA 98:5844-5849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mani, N., D. Lyras, L. Barroso, P. Howarth, T. Wilkins, J. I. Rood, A. L. Sonenshein, and B. Dupuy. 2002. Environmental response and autoregulation of Clostridium difficile TxeR, a sigma factor for toxin gene expression. J. Bacteriol. 184:5971-5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDonald, L. C., G. E. Killgore, A. Thompson, R. C. Owens, Jr., S. V. Kazakova, S. P. Sambol, S. Johnson, and D. N. Gerding. 2005. An epidemic, toxin gene-variant strain of Clostridium difficile. N. Engl. J. Med. 353:2433-2441. [DOI] [PubMed] [Google Scholar]
- 16.Moncrief, J. S., L. Zheng, L. M. Neville, and D. M. Lyerly. 2000. Genetic characterization of toxin A-negative, toxin B-positive Clostridium difficile isolates by PCR. J. Clin. Microbiol. 38:3072-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soehn, F., A. Wagenknecht-Wiesner, P. Leukel, M. Kohl, M. Weidmann, C. von Eichel-Streiber, and V. Braun. 1998. Genetic rearrangements in the pathogenicity locus of Clostridium difficile strain 8864—implications for transcription, expression and enzymatic activity of toxins A and B. Mol. Gen. Genet. 258:222-232. [DOI] [PubMed] [Google Scholar]
- 18.Spigaglia, P., and P. Mastrantonio. 2002. Molecular analysis of the pathogenicity locus and polymorphism in the putative negative regulator of toxin production (TcdC) among Clostridium difficile clinical isolates. J. Clin. Microbiol. 40:3470-3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Voth, D. E., and J. D. Ballard. 2005. Clostridium difficile toxins: mechanism of action and role in disease. Clin. Microbiol. Rev. 18:247-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Warny, M., J. Pepin, A. Fang, G. Killgore, A. Thompson, J. Brazier, E. Frost, and L. C. McDonald. 2005. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet 366:1079-1084. [DOI] [PubMed] [Google Scholar]