Abstract
During bacteriophage T7 infection, the Escherichia coli RNA polymerase β′ subunit is phosphorylated by the phage-encoded kinase Gp0.7. Here, we used proteolytic degradation and mutational analysis to localize the phosphorylation site to a single amino acid, Thr1068, in the evolutionarily hypervariable segment of β′. Using a phosphomimetic substitution of Thr1068, we show that phosphorylation of β′ leads to increased ρ-dependent transcription termination, which may help to switch from host to viral RNA polymerase transcription during phage development.
Bacteriophage T7 is a prototypic member of a large family of phages that use host RNA polymerase (RNAP) for expression of their early genes but rely on their own, single-subunit RNAP for middle and late viral gene expression. The early T7 genes include, in addition to gene 1 coding for T7 RNAP, several genes whose products participate in host transcription shutoff. The shutoff occurs concurrently with the beginning of transcription of viral genes by T7 RNAP.
The products of two viral genes, Gp0.7 and Gp2, are implicated in host transcription shutoff (33-35, 39). Gp0.7 is a serine/threonine protein kinase (31, 36). Among the identified targets are components of translation (IF1, IF2, IF3, elongation factor G, and ribosomal proteins S1 and S6 [37, 38]) and transcription (RNAP β′ subunit [47]) machineries; RNase III (24), the enzyme responsible for mRNA processing; and RNase E (23), which controls mRNA decay. Several lines of evidence suggest that Gp0.7 plays an important role in bacteriophage development. First, even though the 0.7 gene is dispensable for T7 growth under standard laboratory conditions, it is essential at elevated temperatures or in nutrient-poor media (19), as well as in the presence of certain extrachromosomal genetic elements (16). Second, the protein kinase activity appears to be conserved: it is present in T7-like coliphages (25, 41), as well as in phages that infect Yersinia enterocolitica (32), Kluyvera cryocrescens (15), Klebsiella pneumoniae (D. Savalia, I. J. Molineux, and K. Severinov, unpublished observation), and Caulobacter crescentus (20).
Gp2 is a 64-amino-acid-long protein that binds to and abolishes promoter recognition by host RNAP (28). Gene 0.7 becomes essential when Gp2 function is attenuated by a mutation in its binding site in a functionally dispensable domain of RNAP β′ (18), supporting the idea that phosphorylation of β′ by Gp0.7 is functionally relevant and suggesting that Gp2 and Gp0.7 may cooperate with each other during bacteriophage development.
Phosphorylation is a common covalent modification of proteins. In eukaryotes, core components of all three nuclear RNAPs are phosphorylated (4, 7). Phosphorylation of the largest subunit, A190, which is homologous to β′, may regulate the function of yeast (Saccharomyces cerevisiae) RNAP I (14). However, with the exception of the largest RNAP II subunit C-terminal domain phosphorylation, RNAP phosphorylation sites have not been mapped and functional consequences of RNAP phosphorylation have not been established. Here, we used protein chemistry and mutational analysis to localize the Escherichia coli RNAP β′ phosphorylation site by T7 Gp0.7 and to study the functional consequences of phosphorylation using a phosphomimetic substitution in β′.
MATERIALS AND METHODS
Bacterial strains, plasmids, T7 bacteriophage, and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1.
TABLE 1.
Strains and plasmids used in this work
| Strain/plasmid | Description | Reference or source |
|---|---|---|
| E. coli strains | ||
| MG1655 | Wild-type cells | 12 |
| JE1134 | MG1655 rpoC (β′ Δ1149-1190) | 12 |
| 397c | Temperature sensitive, carries rpoC397 deletion | 10 |
| RL211 | Increased termination at trp attenuator | 21 |
| Plasmids | ||
| pRL663 | rpoC+ expression vector, Ampr | 30 |
| pRL663/T1068E | Same as pRL663 but with rpoC(T1068E) | This work |
| pRL663/T1054E | Same as pRL663 but with rpoC(T1054E) | This work |
| pRL663T1054E/ T1068E | Same as pRL663 but with rpoC(T1054E T1068E) | This work |
| pRL6633504 | Same as pRL663 but with rpoC(T1238I) | 43 |
Plasmids expressing mutant β′ were constructed by site-specific mutagenesis. DNA fragments with desirable mutations were obtained by PCR and exchanged with the corresponding rpoC fragment of the pRL663 plasmid. Mutations were confirmed by DNA sequencing performed at the Rockefeller University DNA Sequencing Resource Center.
T7+ bacteriophage was grown and purified as described by Studier (40). Phage stocks (∼1012 PFU/ml) were stored at 4°C.
To monitor T7-induced phosphorylation of bacterial proteins in vivo, the following medium (P medium) was used: 120 mM Tris-HCl, pH 7.4, 80 mM NaCl, 20 mM KCl, 20 mM NH4Cl, 3 mM Na2SO4, 1 mM MgCl2, 0.2 mM CaCl2, 2 mM FeCl3, 0.2% glucose, supplemented with 100 mM Na3PO4 and 1 mM of each of the 20 natural l-amino acids. A screen for increased-termination phenotypes was performed in Vogel-Bonner minimal medium as described previously (43). For protein purification, E. coli was grown in a standard Luria-Bertani medium, supplemented, when necessary, with 100 μg/ml ampicillin to maintain plasmids.
Phosphorylation of proteins in vivo upon T7 infection.
Cells were grown at 30°C in P medium until the optical density at 550 nm reached a value of 0.25 to 0.4. Cells were collected by centrifugation at room temperature at 4,000 rpm for 5 min. Cell pellets were resuspended in P medium without Na3PO4 to obtain the initial optical density. Cells were incubated at 30°C for 30 min and infected with T7 at a multiplicity of infection of 5 to 10 PFU. Surviving colony counts were determined 3 minutes after infection and were always below 1%. Two minutes after the infection, 20 μCi of [32P]orthophosphoric acid (8,500 Ci/mmol) was added to 100 μl of cell suspension and incubation was continued for an additional 15 min at 30°C. Reactions were terminated by transferring the cells on ice and adding an equal volume of ice-cold P medium. Cells were collected by centrifugation at 2°C. To reveal phosphorylated proteins, cell pellets were resuspended in 20 μl of Laemmli sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer (125 mM Tris-HCl, pH 6.8, 1% SDS, 10% glycerol, 350 mM β-mercaptoethanol, and 0.025% bromophenol blue), heated at 100°C for 3 min, and then cooled on ice. RNase A (5 mg) was added to the samples, followed by a 1-hour incubation at 30°C. The samples were next heated at 100°C for 1 min and analyzed by SDS-PAGE in 4.5% gels.
Proteins.
Wild-type and β′T1068E (β′ harboring the T1068E substitution) RNAPs were purified by the method of Hager et al. (17), with minor modifications. Radioactively labeled RNAP was purified by a combination of the initial steps of the procedure described in reference 17 with the immunoaffinity chromatography step using a monoclonal anti-β′ antibody attached to agarose beads (42). Purified RNAP was at least 95% pure judging by silver staining of overloaded SDS gels. Protein concentrations were determined by the Bradford assay (6a), with lysozyme as a standard.
DNA.
DNA fragments containing promoters and intrinsic or ρ-dependent terminators were obtained by PCR amplification of corresponding templates (sequences of the primers are available upon request) followed by purification from agarose gels using a QIAEX gel extraction kit (QIAGEN). DNA concentrations were estimated by running purified DNA fragments against similar-sized fragments with known concentration on agarose gels.
Protein chemistry.
Partial or complete degradation of the RNAP β′ subunit by CNBr and 2-nitro-5-thiocyanobenzoic acid (NTCBA) was performed as described in reference 27 and references therein. Phosphoamino acid analysis was performed as described previously (6). Prior to analysis, 32P-phosphorylated β′ was separated from other proteins by SDS-PAGE in 4.5% gels, the corresponding band was identified by autoradiography and excised from the gel, and the β′ polypeptide was eluted and concentrated by acetone precipitation.
In vitro transcription reactions.
The reaction mixtures contained, in 10 μl, 1.25 pmo of RNAP holoenzyme, 0.625 pmo of DNA template (λ PR promoter followed by the cro gene followed by the tR1 terminator), 75 μM of CpA primer, 5 μM of unlabeled nucleoside triphosphates (NTPs) and an additional 250 μM of ATP (ρ factor requires ATP for its activity), and 5 μCi of [α-32P]UTP in transcription buffer (20 mM HEPES, pH 7.8, 50 mM NaCl, 10 mM MgCl2, 20 mM ZnCl2, and 5 mM β-mercaptoethanol). When required, 20 pmo of ρ (a generous gift of Smita Patel) was added to transcription reaction mixtures prior to the addition of NTPs. Reactions proceeded at 30°C and were either terminated by the addition of ice-cold stop solution (20 mM Tris-HCl, pH 8, 2 mM EDTA, 1 mg/ml heparin, 95% formamide, and 0.025% bromophenol blue) or chased by the addition of 1 mM of all four unlabeled NTPs, incubated for 1 min at 30°C, and then terminated as described above. Transcription reaction products were resolved by 10% 7 M urea-Tris-borate-EDTA PAGE and revealed with a phosphorimager.
RESULTS
Mapping of the β′ phosphorylation site.
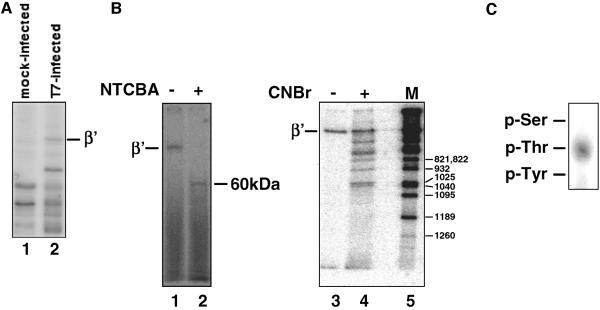
E. coli MG1655 cells were grown in minimal low-phosphate medium at 30°C and infected with wild-type T7. 32P-labeled phosphoric acid was added 2 min postinfection, and cells were harvested 15 min later. Mock-infected 32P-labeled cells were prepared as a control. Analysis of whole-cell lysates by SDS-PAGE and autoradiography revealed a radioactive band that comigrated with the β′ subunit of E. coli RNAP in the lysates of infected cells but was absent from the mock-infected cell lysates, as expected (Fig. 1A). To localize the β′ phosphorylation site, RNAP from infected cell lysates was purified to homogeneity and subjected to degradation with either of two chemical proteases, cysteine-specific NTCBA or methionine-specific CNBr. Exhaustive NTCBA treatment of RNAP from T7-infected cells resulted in the disappearance of the radioactive full-sized β′ band and the appearance of an ∼60-kDa radioactive band (Fig. 1B, left panel). Longer incubations with the cleaving agent did not result in further shortening of the 60-kDa fragment, suggesting that it is a terminal product of degradation. Inspection of the β′ sequence reveals that the 60-kDa radioactive fragment must correspond to the β′ segment C terminal of Cys888 (calculated molecular weight of 55,723), since there are no cysteine residues between Cys888 and the β′ C terminus (5). Therefore, the site of β′ phosphorylation by Gp0.7 must reside within β′ amino acids 889 to 1407.
FIG. 1.
Localization of the β′ phosphorylation site by use of chemical proteases. (A) E. coli MG1655 cells were infected with bacteriophage T7 in the presence of radioactive orthophosphate as described in Materials and Methods. Cells were collected and lysed, proteins were resolved by SDS-PAGE, and phosphoproteins were revealed by autoradiography. Lane 1 shows phosphoproteins in control, mock-infected cells. The position of the E. coli RNAP β′ subunit is indicated. (B) 32P-labeled RNAP was purified from T7-infected cells prepared as described for panel A and subjected to complete proteolysis with Cys-specific NTCBA (left panel) or limited proteolysis with Met-specific CNBr (right panel). Met residues are labeled at right (right panel). The products were resolved by SDS-PAGE and revealed by autoradiography. (C) 32P-labeled RNAP β′ from T7-infected cells was subjected to complete acid hydrolysis, and phosphoamino acids were revealed, after thin-layer chromatography, by autoradiography. Positions of phosphoamino acid markers are indicated.
Limited proteolysis of 32P-labeled β′ with CNBr revealed a more complex pattern (Fig. 1B, right panel). To identify CNBr cleavage fragments, internal markers were prepared by limited CNBr digestion of β′ 32P labeled at the C-terminal heart muscle kinase (HMK) phosphorylation tag. Preliminary experiments demonstrated that (i) there were no natural HMK recognition sequences in E. coli β′ and (ii) HMK-tagged β′ was phosphorylated by HMK with high efficiency. Partial chemical degradation of terminally labeled β′ generated a set of nested radioactive fragments that were easily identified after SDS-PAGE separation based on their electrophoretic mobility and the known locations of Met residues within the β′ sequence. The smallest radioactive band observed in the CNBr-treated RNAP sample from T7-infected cells corresponded to the β′ fragment from Met1040 to the C terminus. The absence of radioactive fragment corresponding to cleavage at the next methionine residue, Met1095, indicates that the site of β′ phosphorylation by T7 Gp0.7 is localized between β′ Met1040 and Met1095.
Previously, it was reported that Gp0.7, a serine/threonine kinase, phosphorylates only β′ threonines (47). The results of our phosphoamino acid analysis confirm this (Fig. 1C). Inspection of the E. coli β′ sequence between Met1040 and Met1095 revealed six threonine residues that could be targets of Gp0.7 phosphorylation (Fig. 2). The absence of convenient proteolytic sites between Met1040 and Met1095 prevented us from a more precise localization of the β′ phosphorylation site by biochemical means.
FIG. 2.
Genetic context of the Gp0.7 phosphorylation site in the E. coli RNAP β′ subunit. The thick bar at the top represents the 1,407-amino-acid-long β′ subunit of E. coli RNAP. Hatched boxes labeled A to H represent segments highly conserved in evolution (1). The E. coli hypervariable region that contains the Gp0.7 phosphorylation site and that is absent from β′ homologues from gram-positive bacteria (46) is shown by an open box, and its sequence is expanded underneath. The E. coli sequence (E. c.) is aligned with homologous sequences from Yersinia pestis (Y. p.), Kluyvera cryocrescens (K. c.), Caulobacter crescentus (C. c.), and Bacillus subtilis (B. s.). Dots indicate identity to the E. coli sequence, and dashes indicate gaps. Methionine residues that flank the CNBr fragment that contains the phosphorylation site are indicated above the E. coli sequence, threonine residues that are contained within this CNBr fragment are italicized, and two phosphomimetic substitutions used in this work are indicated. The bar above the E. coli sequence shows the deletion of the β′ downstream jaw in the E. coli JE1144 cells.
To further narrow down the phosphorylation site, we took advantage of the fact that several T-odd phages infecting bacteria other than E. coli phosphorylate host RNAP β′ or encode Gp0.7 homologues. Gp0.7 homologues have been found in T7-like phages infecting Yersinia pestis (Savalia, Molineux, and Severinov, unpublished), Yersinia enterocolitica (32), Caulobacter crescentus (44), and Kluyvera cryocrescens (15). In the case of Caulobacter crescentus, a threonine residue(s) in the RNAP β′ subunit is phosphorylated during phage φCd1 infection (20). Since φCd1 appears to be highly similar to T7 (2), it is highly likely that (i) during φCd1 infection, phosphorylation of the C. crescentus β′ residue(s) is performed by a Gp0.7-like protein and (ii) the phosphorylated β′ residue is identical to the E. coli β′ residue(s) phosphorylated by T7 Gp0.7. In Fig. 2, a segment of the E. coli β′ sequence containing the T7 Gp0.7 phosphorylation site is aligned with the corresponding segments of publicly available deduced β′ sequences from Y. pestis and C. crescentus, as well as the K. cryocrescens sequence determined by us. The corresponding sequence from gram-positive Bacillus subtilis is also included in the alignment. As can be seen, the β′ segment between Met1040 and Met1095 is part of a long hypervariable segment of β′ that is missing in homologues from gram-positive bacteria (46). In Y. pestis and K. cryocrescens sequences, all six threonines that may be targeted by Gp0.7 are present, and thus no new information about the phosphorylation site(s) can be derived. In contrast, in Caulobacter β′, only two threonines, corresponding to E. coli Thr1054 and Thr1068, are present (Fig. 2), suggesting that one (or both) of these residues may be phosphorylated by Gp0.7.
To locate the Gp0.7 target, we created derivatives of the rpoC expression plasmid pRL663 that overproduced β′ harboring substitutions T1054E and/or T1068E. The mutant genes were functional, as judged by their ability to complement E. coli 397c rpoC(Ts) cells (30) at a restrictive temperature of 42°C (data not shown). E. coli JE1134 cells harboring a chromosomal deletion removing rpoC codons 1149 to 1190 (12) were transformed with pRL663 or its derivatives and infected with T7. JE1134 is poorly infected by wild-type T7 since deletion of β′ residues 1149 to 1190 destroys the Gp2-binding site (28). However, infections of JE1134 harboring either the wild-type or mutant rpoC expression plasmids proceeded normally (data not shown), indicating that plasmid-encoded β′ subunits efficiently competed with chromosomally encoded β′ for entry in the RNAP enzyme and interacted with Gp2. Due to the 42-amino-acid deletion, the chromosomal copy of JE1134 β′ can be distinguished from full-sized, plasmid-borne β′ on 4.5% SDS gels (Fig. 3, compare, for example, lane 1 with lane 6). The truncated chromosomal copy of β′ was efficiently phosphorylated during T7 infection (Fig. 3, lane 1′). During infection of JE1134 cells carrying plasmids expressing the wild-type or rpoC(T1054E) alleles, a radioactive band migrating slightly slower than the JE1134 β′ band and comigrating with full-sized β′ was also present (Fig. 3, lanes 5′ and 4′, respectively). In contrast, no such band was present during infection of JE1134 cells harboring plasmids expressing the rpoC(T1068E) or rpoC(T1054E T1068E) alleles (Fig. 3, lanes 2′ and 3′, respectively). This experiment proves that Thr1068 is phosphorylated by Gp0.7; moreover, Thr1068 is the only β′ residue phosphorylated by Gp0.7.
FIG. 3.
β′ Thr1068 is the target of Gp0.7 phosphorylation. JE1134 cells harboring a chromosomal deletion of rpoC codons 1149 to 1190 were transformed with plasmids expressing the indicated alleles of rpoC. Cells were grown and infected with T7 phage as described in the legend for Fig. 1A. Proteins were resolved by SDS-PAGE, the gel was silver stained (left panel), and phosphoproteins were revealed by autoradiography (autorad) (right panel). Plasmid-free JE1134 and wild-type (wt) MG1655 cells were used as controls (lanes 1 and 1′ and 6 and 6′). Lane 7 is a marker lane (RNAP σ70 holoenzyme).
Functional consequences of β′ phosphorylation.
Since the degree of RNAP phosphorylation during T7 infection is difficult to control, we used the β′ substitution T1068E to understand the properties of RNAP phosphorylated by Gp0.7. While the locations of the charged and methyl groups in phosphorylated threonine and in glutamate do to not match and the numbers of charges are not identical, the substitution is considered “phosphomimetic” and such substitutions were previously used to understand the effects of phosphorylation in proteins (see, for example, reference 13 and references cited therein).
Transcription termination properties of the mutant rpoC gene were tested in vivo using the RL211 E. coli tester strain (21). Strain RL211 becomes resistant to 5-methylanthranilic acid (5-MAA) when transcription termination in the trp operon leader region increases, since the synthesis of toxic 5-methyltryptophan is decreased. The results of plating on 5-MAA are presented in Fig. 4. As a control, we used pRL663 plasmid expressing the allele of rpoC3504 (substituting β′ Thr1328 for Ile), which was previously shown to increase transcription termination in vivo (43). As can be seen, plasmids expressing rpoC(T1328I), rpoC(T1068E), and rpoC(T1054E T1068E) alleles allowed RL211 growth in the presence of 5-MAA. In contrast, cells harboring the wild-type pRL663 or pRL663/T1054E did not grow on the selective medium. Therefore, we conclude that β′ substitution T1068E and, by extension, phosphorylation of β′ Thr1068 by Gp0.7 lead to increased transcription termination in vivo.
FIG. 4.
β′ substitution T1068E leads to increased transcription termination in vivo. E. coli RL211 cells were transformed with pRL663 or its indicated derivatives and plated as shown schematically in the center of the figure. The plate on the left contained rich unselective medium, and the plate on the right contained minimal medium with 5-MAA. The results of overnight growth at 30°C are presented. T1328I is a point substitution in β′ that was previously shown to increase RL211 survival in the presence of 5-MAA due to increased transcription termination (43). IPTG, isopropyl-β-d-thiogalactopyranoside; VB, Vogel-Bonner; Amp, ampicillin; wt, wild type.
Previous work on termination-altering RNAP mutants had shown that some mutations cause complex, apparently nonspecific termination phenotypes, both increasing and decreasing transcription termination, depending on the context (43). Strain RL211 contains a chromosomal cat gene located downstream of an intrinsic terminator. RL211 becomes resistant to chloramphenicol when transcription termination at this terminator decreases, allowing sufficient amounts of cat mRNA to be synthesized. None of the rpoC expression plasmids used in this work allowed RL211 growth in the presence of chloramphenicol (data not shown), while a control plasmid overproducing antiterminating protein CspE resulted in chloramphenicol resistance, as expected (3). Therefore, phosphomimetic β′ substitution T1068E and, by extension, phosphorylation of β′ Thr1068 by Gp0.7 appear to specifically increase transcription termination in vivo.
To determine whether increased cell survival in the presence of 5-MAA was due to a change in intrinsic termination properties of β′T1068E RNAP or was mediated by a transcription factor(s), we purified wild-type and β′T1068E RNAPs from 397c cells transformed with corresponding rpoC expression plasmids. The 397c rpoC mutation removes 29 C-terminal residues of β′ (10). The deletion destabilizes RNAP, and as a consequence, no RNAP harboring chromosomally encoded β′ can be purified (our unpublished observations). Therefore, RNAP purified from 397c cells carrying plasmid-borne rpoC contains only plasmid-encoded β′.
No obvious differences between the mutant and the wild-type RNAP were detected in several promoter-dependent transcription initiation assays, though the mutant enzyme had a consistently lower specific activity in these assays (a three- to fivefold difference compared to that of the identically purified wild-type enzyme; data not shown). No change in termination efficiency for several ρ-independent transcription terminators was observed (data not shown). Therefore, the effect of the β′T1068E substitution on ρ-dependent transcription termination was determined using a ρ-dependent λ tR1 terminator, which contains three termination sites, tr1a, tr1b, and tr1c (22). In the absence of ρ, ∼60% of wild-type RNAP transcribed to the end of the template and only a fraction of RNAP molecules stalled at tr1a (15%), tr1b (10%), and tr1c (10%) (Fig. 5, lane 1). In contrast, under identical conditions, 65% of β′T1068E RNAP was stalled at the tr1a site (Fig. 5, lane 3). The shorter transcripts corresponded to transcriptional pauses, since the addition of NTPs at high concentration chased, almost quantitatively, the wild-type RNAP transcripts to the runoff transcript (Fig. 5, lane 2); in contrast, ∼15% of β′T1068E RNAP remained at tr1a even after the chase (Fig. 5, lane 4) and must therefore have resulted from transcription termination or arrest. The addition of ρ resulted in increased production of the tr1a, tr1b, and tr1c transcripts by the wild-type RNAP (Fig. 5, compare lanes 1 and 5) (5% runoff; 55, 30, and 10% of elongation complexes stalled at tr1a, tr1b, and tr1c, respectively) but had little effect on the β′T1068E RNAP, which efficiently stalled at tr1a even in the absence of ρ (Fig. 5, compare lanes 3 and 7). Only a fraction (20% for wild-type RNAP, 10% for β′T1068E RNAP) of transcripts produced in the presence of ρ was chased in the presence of high concentrations of NTPs, indicating that ρ-dependent transcription termination had occurred, as expected. The amount of transcripts terminated at tr1a was significantly higher for β′T1068E RNAP than for wild-type RNAP (70% versus 35%, respectively) (Fig. 5, compare lanes 8 and 6). We conclude that the T1068E substitution in β′ leads to increased pausing at ρ-dependent terminators, which in turn leads to increased ρ-dependent termination. We note that this effect is independent of transcription elongation rate, which was the same, at least at undersaturating concentrations of NTPs, for both the mutant and the wild-type enzyme (data not shown).
FIG.5.
β′ substitution T1068E leads to increased ρ-dependent termination in vitro. Transcription reactions were performed on a template containing the λ tR1 ρ-dependent terminator, as described in Materials and Methods, in the presence (+) or in the absence (−) of the ρ factor. Where indicated, transcription reactions were chased (+) with 1 mM NTPs. The numbers at the right indicate expected sizes of the terminated and runoff transcription products. The gel shown is representative of three independent experiments. wt, wild type.
DISCUSSION
In this work, we mapped the site of phosphorylation of the E. coli RNAP β′ subunit by bacteriophage T7-encoded kinase Gp0.7 to Thr1068 in the evolutionarily variable region of β′. The structure of this region, the GNCD (for region G nonconserved domain) was very recently solved (9). The GNCD structure consists of a tandem of SBHM (sandwich-barrel hybrid motif) folds, SBMHa and SBMHb. Thr1068 is located in loop b1 of the SMBHb domain. A charge-altering posttranslational modification of Thr1068 is expected to either change the SMBHb domain structure and/or alter its ability to interact with transcription factors, other RNAP domains, or nucleic acids. At present, the precise positioning of GNCD in the model of the Thermus aquaticus. RNAP transcription complex is not possible. However, fitting the GNCD structure into the low-resolution E. coli RNAP structure obtained by means of electron crystallography, followed by superposition of the resulting structure with the model of the Thermus aquaticus. RNAP transcription complex allowed Chlenov and coworkers to make a plausible structural model of a transcription complex that includes GNCD (9). In this model, SMBHa forms an extension of the secondary channel and may interact with the 3′ end of the nascent RNA in backtracked elongation complexes. SMBHb likely contacts downstream DNA and forms, together with the downstream jaw domain of β′, the downstream DNA-binding trough. The downstream jaw is the binding site of T7 Gp2, a protein whose biologically significant function is to lower the stability of host RNAP complexes on viral DNA (8, 11, 26, 29, 45; Savalia, Molineux, and Severinov, unpublished). Though we did not test this expressly, it is likely that a negative charge introduced by phosphorylation of Thr1068 weakens SMBHb interaction with downstream DNA and also destabilizes transcription complexes directly or indirectly (this would explain the lower specific activity of β′T1068E RNAP). Bacteriophage T7 harboring deletions in gene 0.7 is viable on wild-type laboratory strains but cannot plate on E. coli BR3 (18). RNAP from BR3 cells harbors a substitution of β′ Glu1158 in the downstream jaw that prevents RNAP interaction with Gp2 (28). It is tempting to speculate that Gp0.7 phosphorylation of GCND and Gp2 binding to the downstream jaw cooperate in destabilization of host RNAP transcription complexes.
Due to difficulties in obtaining RNAP quantitatively phosphorylated by Gp0.7, here we studied RNAP harboring a phosphomimetic substitution at the site of Gp0.7 phosphorylation. The successful use of phosphomimetic substitutions to study effects of phosphorylation has been reported before (see, for example, reference 13). Our results with T1068E RNAP suggest that phosphorylation of Thr1068 could lead to increased termination in vivo. The result is fully consistent with earlier work that suggested that RNAP phosphorylation by Gp0.7 affects viral gene expression by promoting efficient transcription termination at sites located between the T7 class I genes (transcribed by E. coli RNAP) and class II genes (transcribed by viral RNAP) (33). Decreasing host RNAP readthrough into class II genes may be beneficial for the phage since it helps to avoid interference from slow host RNAP with fast viral RNAP.
The results of in vitro analysis suggest that the termination defect of β′T1068E RNAP is limited to ρ-dependent transcription terminators. It appears that the T1068E substitution causes increased pausing at ρ-dependent terminators, which allows the ρ factor to catch up with the mutant RNAP more efficiently. The mechanism of this effect remains to be investigated and may be complex. For example, increased pausing at the terminator site can be caused by decreased interaction of phosphorylated GCND with downstream DNA (which should promote backtracking) or can result from alterations in the position of SMBHa that may make it more prone to interact with the 3′ end of the nascent RNA and cause backtracking. Future structure-based analysis of RNAP mutants with alterations in GCND shall clarify these questions.
Acknowledgments
This work was supported by the Burroughs Wellcome Career Award and NIH R01 grant GM59295 to K.S.
We thank Alexei Ryazanov for help with phosphoamino acid analysis, Charles Yanofsky for the gift of 5-MAA, Juana Maria Navarro-Llorens for the gift of K. cryocrescens DNA, and Smita Patel for the gift of the ρ factor.
REFERENCES
- 1.Allison, L. A., M. Moyle, M. Shales, and C. J. Ingles. 1985. Extensive homology among the largest subunits of eukaryotic and prokaryotic RNA polymerases. Cell 42:599-610. [DOI] [PubMed] [Google Scholar]
- 2.Amemiya, K., and L. Shapiro. 1982. In vitro transcription of the early region of Caulobacter phage phi Cd1 deoxyribonucleic acid by host RNA polymerase. Biochemistry 21:4707-4713. [DOI] [PubMed] [Google Scholar]
- 3.Bae, W., B. Xia, M. Inouye, and K. Severinov. 2000. Escherichia coli CspA-family RNA chaperones are transcription antiterminators. Proc. Natl. Acad. Sci. USA 97:7784-7789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bell, G. I., P. Valenzuela, and W. J. Rutter. 1977. Phosphorylation of yeast DNA-dependent RNA polymerases in vivo and in vitro. Isolation of enzymes and identification of phosphorylated subunits. J. Biol. Chem. 252:3082-3091. [PubMed] [Google Scholar]
- 5.Borukhov, S., J. Lee, and A. Goldfarb. 1991. Mapping of a contact for the RNA 3′ terminus in the largest subunit of RNA polymerase. J. Biol. Chem. 266:23932-23935. [PubMed] [Google Scholar]
- 6.Boyle, W. J., P. van der Geer, and T. Hunter. 1991. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 201:110-149. [DOI] [PubMed] [Google Scholar]
- 6a.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 7.Breant, B., J. M. Buhler, A. Sentenac, and P. Fromageot. 1983. On the phosphorylation of yeast RNA polymerases A and B. Eur. J. Biochem. 130:247-251. [DOI] [PubMed] [Google Scholar]
- 8.Buchstein, S., and D. C. Hinkle. 1982. Genetic analysis of two bacterial RNA polymerase mutants that inhibit the growth of bacteriophage T7. Mol. Gen. Genet. 188:211-218. [DOI] [PubMed] [Google Scholar]
- 9.Chlenov, M., S. Masuda, K. S. Murakami, V. Nikiforov, S. A. Darst, and A. Mustaev. 2005. Structure and function of lineage-specific sequence insertions in the bacterial RNA polymerase beta′ subunit. J. Mol. Biol. 353:138-154. [DOI] [PubMed] [Google Scholar]
- 10.Christie, G. E., S. B. Cale, L. A. Isaksson, D. J. Jin, M. Xu, B. Sauer, and R. Calendar. 1996. Escherichia coli rpoC397 encodes a temperature-sensitive C-terminal frameshift in the β′ subunit of RNA polymerase that blocks growth of bacteriophage P2. J. Bacteriol. 178:6991-6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeWyngaert, M. A., and D. C. Hinkle. 1979. Bacterial mutants affecting phage T7 DNA replication produce RNA polymerase resistant to inhibition by the T7 gene 2 protein. J. Biol. Chem. 254:11247-11253. [PubMed] [Google Scholar]
- 12.Ederth, J., L. A. Isaksson, and F. Abdulkarim. 2002. Origin-specific reduction of ColE1 plasmid copy number due to mutations in a distinct region of the Escherichia coli RNA polymerase. Mol. Genet. Genomics 267:587-592. [DOI] [PubMed] [Google Scholar]
- 13.Ellerbroek, S. M., K. Wennerberg, and K. Burridge. 2003. Serine phosphorylation negatively regulates RhoA in vivo. J. Biol. Chem. 278:19023-19031. [DOI] [PubMed] [Google Scholar]
- 14.Fath, S., P. Milkereit, G. Peyroche, M. Riva, C. Carles, and H. Tschochner. 2001. Differential roles of phosphorylation in the formation of transcriptional active RNA polymerase I. Proc. Natl. Acad. Sci. USA 98:14334-14339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gadaleta, P., and J. Zorzopulos. 1997. Kluyvera bacteriophage Kvp1: a new member of the Podoviridae family phylogenetically related to the coliphage T7. Virus Res. 51:43-52. [DOI] [PubMed] [Google Scholar]
- 16.Gomez, B., and L. Nualart. 1977. Requirement of the bacteriophage T7 0.7 gene for phage growth in the presence of the Col 1b factor. J. Gen. Virol. 35:99-106. [DOI] [PubMed] [Google Scholar]
- 17.Hager, D. A., D. J. Jin, and R. R. Burgess. 1990. Use of Mono Q high-resolution ion-exchange chromatography to obtain highly pure and active Escherichia coli RNA polymerase. Biochemistry 29:7890-7894. [DOI] [PubMed] [Google Scholar]
- 18.Hesselbach, B. A., and D. Nakada. 1977. “Host shutoff” function of bacteriophage T7: involvement of T7 gene 2 and gene 0.7 in the inactivation of Escherichia coli RNA polymerase. J. Virol. 24:736-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirsch-Kauffmann, M., P. Herrlich, H. Ponta, and M. Schweiger. 1975. Helper function of T7 protein kinase in virus propagation. Nature 255:508-510. [DOI] [PubMed] [Google Scholar]
- 20.Hodgson, D., L. Shapiro, and K. Amemiya. 1985. Phosphorylation of the β′ subunit of RNA polymerase and other host proteins upon φCD1 infection of Caulobacter crescentus. J. Virol. 55:238-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Landick, R., J. Stewart, and D. N. Lee. 1990. Amino acid changes in conserved regions of the beta-subunit of Escherichia coli RNA polymerase alter transcription pausing and termination. Genes Dev. 4:1623-1636. [DOI] [PubMed] [Google Scholar]
- 22.Lau, L. F., J. W. Roberts, and R. Wu. 1983. RNA polymerase pausing and transcript release at the lambda tR1 terminator in vitro. J. Biol. Chem. 258:9391-9397. [PubMed] [Google Scholar]
- 23.Marchand, I., A. W. Nicholson, and M. Dreyfus. 2001. Bacteriophage T7 protein kinase phosphorylates RNase E and stabilizes mRNAs synthesized by T7 RNA polymerase. Mol. Microbiol. 42:767-776. [DOI] [PubMed] [Google Scholar]
- 24.Mayer, J. E., and M. Schweiger. 1983. RNase III is positively regulated by T7 protein kinase. J. Biol. Chem. 258:5340-5343. [PubMed] [Google Scholar]
- 25.Mertens, H., and R. Hausmann. 1982. Coliphage BA14: a new relative of phage T7. J. Gen. Virol. 62:331-341. [DOI] [PubMed] [Google Scholar]
- 26.Mooney, P. Q., R. North, and I. J. Molineux. 1980. The role of bacteriophage T7 gene 2 protein in DNA replication. Nucleic Acids Res. 8:3043-3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mustaev, A., E. Zaychikov, M. Grachev, M. Kozlov, K. Severinov, V. Epshtein, N. Korzheva, O. Bereshchenko, V. Markovtsov, V. Lukhtanov, I. Tsarev, T. Maximova, M. Kashlev, I. Bass, V. Nikiforov, and A. Goldfarb. 2003. Studies of methods of cross-linking of RNA polymerase active center. Methods Enzymol. 371:191-206. [DOI] [PubMed] [Google Scholar]
- 28.Nechaev, S., and K. Severinov. 1999. Inhibition of E. coli RNA polymerase by bacteriophage T7 gene 2 protein. J. Mol. Biol. 289:815-826. [DOI] [PubMed] [Google Scholar]
- 29.Nechaev, S., and K. Severinov. 2003. Bacteriophage-induced modifications of host RNA polymerase. Annu. Rev. Microbiol. 57:301-322. [DOI] [PubMed] [Google Scholar]
- 30.Nedea, E. C., D. Markov, T. Naryshkina, and K. Severinov. 1999. Localization of Escherichia coli rpoC mutations that affect RNA polymerase assembly and activity at high temperature. J. Bacteriol. 181:2663-2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pai, S. H., H. J. Rahmsdorf, H. Ponta, M. Hirsch-Kauffmann, P. Herrlich, and M. Schweiger. 1975. Protein kinase of bacteriophage T7. 2. Properties, enzyme synthesis in vitro and regulation of enzyme synthesis and activity in vivo. Eur. J. Biochem. 55:305-314. [DOI] [PubMed] [Google Scholar]
- 32.Pajunen, M. I., S. J. Kiljunen, M. E.-L. Söderholm, and M. Skurnik. 2001. Complete genomic sequence of the lytic bacteriophage φYeO3-12 of Yersinia enterocolitica serotype O:3. J. Bacteriol. 183:1928-1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pfennig-Yeh, M. L., H. Ponta, M. Hirsch-Kauffmann, H. J. Rahmsdorf, P. Herrlich, and M. Schweiger. 1978. Early T7 gene expression: rates of RNA synthesis and degradation, protein kinase dependent termination of transcription, and efficiency of translation. Mol. Gen. Genet. 166:127-140. [DOI] [PubMed] [Google Scholar]
- 34.Ponta, H., H. J. Rahmsdorf, S. H. Pai, M. Hirsch-Kauffmann, P. Herrlich, and M. Schweiger. 1974. Control of gene expression in bacteriophage T7: transcriptional controls. Mol. Gen. Genet. 134:281-297. [DOI] [PubMed] [Google Scholar]
- 35.Ponta, H., H. J. Rahmsdorf, S. H. Pai, P. Herrlich, and M. Schweiger. 1974. Control of gene expression in bacteriophage T7. Isolation of a new control protein and mechanism of action. Mol. Gen. Genet. 134:29-38. [DOI] [PubMed] [Google Scholar]
- 36.Rahmsdorf, H. J., S. H. Pai, H. Ponta, P. Herrlich, R. Roskoski, Jr., M. Schweiger, and F. W. Studier. 1974. Protein kinase induction in Escherichia coli by bacteriophage T7. Proc. Natl. Acad. Sci. USA 71:586-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robertson, E. S., and A. W. Nicholson. 1990. Protein kinase of bacteriophage T7 induces the phosphorylation of only a small number of proteins in the infected cell. Virology 175:525-534. [DOI] [PubMed] [Google Scholar]
- 38.Robertson, E. S., L. A. Aggison, and A. W. Nicholson. 1994. Phosphorylation of elongation factor G and ribosomal protein S6 in bacteriophage T7-infected Escherichia coli. Mol. Microbiol. 11:1045-1057. [DOI] [PubMed] [Google Scholar]
- 39.Rothman-Denes, L. B., S. Muthukrishan, R. Haselkorn, and F. W. Studier. 1973. A T7 gene function required for shutoff of host and early T7 transcription. p. 227-239. In C. F. Fox and W. S. Robinson (ed.), Virus research. Academic Press, New York, N.Y.
- 40.Studier, F. W. 1973. Genetic analysis of non-essential bacteriophage T7 genes. J. Mol. Biol. 79:227-236. [DOI] [PubMed] [Google Scholar]
- 41.Studier, F. W., and N. R. Movva. 1976. SAMase gene of bacteriophage T3 is responsible for overcoming host restriction. J. Virol. 19:136-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thompson, N. E., D. A. Hager, and R. R. Burgess. 1992. Isolation and characterization of a polyol-responsive monoclonal antibody useful for gentle purification of Escherichia coli RNA polymerase. Biochemistry 31:7003-7008. [DOI] [PubMed] [Google Scholar]
- 43.Weilbaecher, R., C. Hebron, G. Feng, and R. Landick. 1994. Termination-altering amino acid substitutions in the beta′ subunit of Escherichia coli RNA polymerase identify regions involved in RNA chain elongation. Genes Dev. 8:2913-2927. [DOI] [PubMed] [Google Scholar]
- 44.West, D., C. Lagenaur, and N. Agabian. 1976. Isolation and characterization of Caulobacter crescentus bacteriophage φCd1. J. Virol. 17:568-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamada, Y., J. Silnutzer, and D. Nakada. 1978. Mutant of Escherichia coli which blocks T7 bacteriophage assembly: accumulation of short T7 DNA. J. Mol. Biol. 121:95-111. [DOI] [PubMed] [Google Scholar]
- 46.Zakharova, N., I. A. Bass, E. Arsenieva, V. Nikiforov, and K. Severinov. 1998. Mutations in and monoclonal antibody binding to evolutionary hypervariable region of E. coli RNA polymerase b′ subunit inhibit transcript cleavage and transcript elongation. J. Biol. Chem. 273:19371-19374. [DOI] [PubMed] [Google Scholar]
- 47.Zillig, W., H. Fujiki, W. Blum, D. Janekovic, M. Schweiger, H. J. Rahmsdorf, H. Ponta, and M. Hirsch-Kauffmann. 1975. In vivo and in vitro phosphorylation of DNA-dependent RNA polymerase of Escherichia coli by bacteriophage-T7-induced protein kinase. Proc. Natl. Acad. Sci. USA 7:2506-2510. [DOI] [PMC free article] [PubMed] [Google Scholar]