Abstract
The insertional inactivation of the dlt operon from Lactobacillus plantarum NCIMB8826 had a strong impact on lipoteichoic acid (LTA) composition, resulting in a major reduction in d-alanyl ester content. Unexpectedly, mutant LTA showed high levels of glucosylation and were threefold longer than wild-type LTA. The dlt mutation resulted in a reduced growth rate and increased cell lysis during the exponential and stationary growth phases. Microscopy analysis revealed increased cell length, damaged dividing cells, and perforations of the envelope in the septal region. The observed defects in the separation process, cell envelope perforation, and autolysis of the dlt mutant could be partially attributed to the L. plantarum Acm2 peptidoglycan hydrolase.
Teichoic acids (TAs) are essential polymers found in gram-positive bacteria and represent up to 50% of the cell wall dry weight (17). Lactobacillus plantarum contains two types of TAs: lipoteichoic acids (LTA) and wall teichoic acids (WTA). The L. plantarum LTA are polyglycerophosphate polymers anchored in the membrane through a glycolipid. They are highly substituted with d-alanyl esters (d-Ala:phosphate [P] ratio of 0.89) and to a minor extent with glucose (Glc:P ratio of 0.11) (2). L. plantarum WTA are polyribitolphosphates covalently bound to the peptidoglycan via a linkage unit (22). They also carry d-Ala and Glc residues but in strain-dependent variable ratios (14).
Despite their essentiality, our understanding of the physiological role(s) of TAs is still incomplete (17). d-Alanyl substitutions strongly contribute to the function of TAs, and the study of mutants deficient in d-alanylation is of interest to expand our insight into the physiological role of these substitutents. The positively charged amino groups of d-alanyl esters partially counteract the negative charges of the backbone phosphate groups. Consequently, d-alanyl esters can modulate cell envelope properties and the function of several extracellular proteins (for a review, see reference 24). The biosynthesis of d-alanyl-LTA was extensively studied with Lactobacillus. rhamnosus (formerly casei) (10, 18, 19, 24, 25) and with Bacillus subtilis (27). d-Alanylation requires four proteins (DltA/Dcl, DltB, DltC/Dcp, and DltD) encoded in the dlt operon. In B. subtilis, which contains both LTA and WTA, inactivation of dlt genes prevents d-alanylation of both types of TAs (27). The dlt mutants exhibit an extended variety of phenotypes (for a review, see reference 24). For L. plantarum, it was recently demonstrated that the dlt mutant analyzed in this work displayed an enhanced anti-inflammatory capacity in a murine model of colitis (16). A large number of phenotypes can be associated with the higher-density of negative charges in the cell wall resulting from the lack of d-alanyl esters (for a review, see reference 24). As an example, TAs are thought to be involved in the control of autolysins through electrostatic interactions (12, 29). Autolysins play an important role in autolysis, cell separation, and peptidoglycan turnover, and a stimulatory effect of d-alanyl ester deprivation on autolysis has been observed in B. subtilis, Staphylococcus aureus, and Lactococcus lactis (23, 32, 33). In L. lactis, the increased autolysis of a dltD mutant was clearly shown to be mediated by AcmA, which is the major lactococcal autolysin involved in cell separation and autolysis in stationary phase (32).
We have recently reported the construction and the phenotypic analysis of an alanine racemase (alr) mutant of L. plantarum, which is auxotrophic for d-alanine (20, 26). Blocking d-alanine production has a pleiotropic effect, since this residue is involved in the biosynthesis of both the peptidoglycan and TAs. The phenotype of the alr mutant strongly suggests an activation or increased binding of autolysins. Under d-alanine starvation, the alr mutation resulted in loss of cell envelope integrity associated with perforations of the cell wall in the septal region, ultimately leading to cell lysis (26).
Here, we report the characterization of the L. plantarum dlt mutant and the implication of d-alanylation in LTA structure, cell morphology, growth, and autolysis. Since the dlt mutant displays similarities in terms of lysis with the previously characterized alr mutant, the hypothesis that the lack of d-alanylation results in the activation or increased binding of autolysins was further verified by the analysis of a double mutant deficient in both d-alanyl esters and the autolysin Acm2.
Genetic analysis of the dlt locus of L. plantarum in the wild-type and dlt mutant strains.
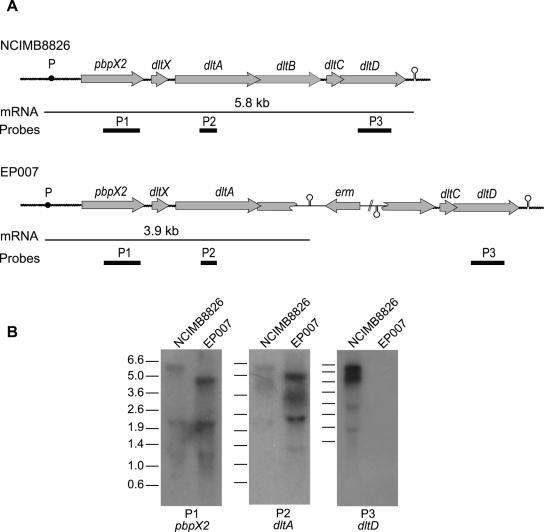
Sequence analysis of the dlt locus of L. plantarum WCFS1 (NCIMB8826 [21]) revealed the presence of two additional genes (pbpX2 [lp_2021] and dltX [lp_2020]) upstream of the four dlt genes (dltABCD) previously found in other gram-positive bacteria (Fig. 1A) (1, 3, 9, 18, 25, 27, 28, 30). The presence of short intergenic sequences and the absence of putative terminators among the six genes strongly suggest that they belong to the same polycistronic unit. pbpX2 encodes a protein showing homology to various low-molecular-weight penicillin binding proteins (PBPs) that have a d,d-carboxypeptidase activity. dltX encodes a putative peptide of 49 amino acids. A dltX homologue was systematically found upstream of dltA in all dlt clusters available from public databases. To validate cotranscription of the six genes (pbpX2, dltXABCD), total RNAs extracted from cells harvested in the exponential phase of growth were independently hybridized with three 32P-labeled probes corresponding to internal fragments of pbpX2, dltA, and dltD (P1, P2, and P3; Fig. 1A and B). The same transcript of the predicted size (ca. 5.8 kb) was detected with the three different probes, confirming that the six genes belong to the dlt operon. The additional bands below the 5.8-kb mRNA band most probably represent degradation products or nonspecific hybridization with rRNAs. A similar Northern blot analysis was performed on RNA extracted from the L. plantarum dlt mutant strain (EP007; dltB::pGIE007, erythromycin-resistant (16) (Fig. 1B). The genetic organization of the dlt operon in this mutant strain is depicted in Fig. 1A. A 3.9-kb transcript was detected with the probes hybridizing with pbpX2 (P1) and dltA (P2), while no transcript could be detected using the dltD probe (P3). The 1.9-kb mRNA truncation is in agreement with the insertion of the plasmid-borne transcriptional terminator (phage λ oop RNA terminator) from pJDC9 (8) located downstream of the dltB internal fragment (Fig. 1A). Both the decreased mRNA size and the lack of dltD transcript detection confirmed the mutant genotype. In addition, these results indicated that strain EP007 also lacks dltC and dltD transcription but retains pbpX2 and dltX transcription. Complementation of the dlt mutant was performed using a plasmid bearing the complete copy of the dlt operon (dltXABCD) of L. rhamnosus under control of its own promoter (pNZ123/dlt) (11). The growth defect observed in the dlt mutant (see below) was suppressed in the complemented strain (data not shown).
FIG. 1.
Characterization and transcriptional analysis of the dlt operon of L. plantarum. (A) Genetic organization of the dlt operon in NCIMB8826 (wild type) and EP007 (dlt mutant). The probes P1, P2, and P3, used for the Northern blot experiments (see panel B), are indicated as heavy black lines. The transcripts are depicted by thin horizontal lines, and their expected sizes are indicated. The black dot represents the putative promoter (P), and the stem-loop structure represents the transcription terminator. (B) Transcriptional analysis of the dlt operon of the NCIMB8826 and EP007 strains by Northern blotting (13). α-32P-radiolabeled PCR fragments were used as specific probes for pbpX (P1, primers NCIPBPX1 [5′-GGACGTCAGACAACACTTCG-3′] and NCIPBPXR2 [5′-ATAGTAATTACTCAACAGTACG-3′)], dltA (P2, primers LPDLT1 [5′-TCGGGATCCTCATGATAACTTGGTCAGTTACG-3′] and INVDLT3 [5′CGTATAGTGTCCCACCTAACGC-3′]), and dltD (P3, primers NCIDLTD1 [5′-CAAGGAACGCGGAAAGATGC-3′] and NCIDLTDR2 [5′-ACCCTTGACTCTTCAACTGG-3′]) (see panel A). DNA fragments corresponding to the reference ladder are given in kb.
Characterization of the d-alanylation defect of LTAs.
A preliminary characterization of LTA from the wild-type strain (NCIMB8826) and the dlt mutant (EP007) was previously reported (16). It was shown that LTA from NCIMB8826 is constituted of polyglycerophosphates (Gro-P) with d-alanyl esters as unique detectable substituents (41.7% d-Ala:Gro-P). A strong reduction in d-alanyl esters of LTA from EP007 was observed (16). During this work, a more extensive proton nuclear magnetic resonance analysis of the LTA structure from cells harvested in exponential growth phase was achieved. LTA from NCIMB8826 contain chains of 21 to 22 glycerophosphate (Gro-P) residues. Concerning the EP007 mutant, two LTA fractions were eluted from the chromatography column. The major fraction (85% of total LTA) contains threefold-longer LTA (62 to 63 Gro-P, 1.1% d-Ala:Gro-P) compared to the minor fraction (15% of total LTA, 22 to 23 Gro-P, 5.0% d-Ala:Gro-P) and to wild-type LTA. The percentage of d-alanyl esters in the EP007 mutant was strongly reduced in both LTA fractions (8- to 40-fold) compared to the wild type. Notably, the LTA from an L. lactis dltD mutant characterized by nuclear magnetic resonance analysis showed only a fivefold reduction in d-alanylation (5.8% d-Ala:Gro-P) compared to the wild type (28.5% d-Ala:Gro-P) (32). Interestingly, in L. plantarum, the longest LTA found in the major fraction is also the least d-alanylated. These results support a role of d-alanylation in polymer chain length control in LTA biosynthesis in L. plantarum. Strikingly, 24.2% of Gro-P residues in both fractions from the mutant are substituted with glucose, while glucose substituents were undetectable in wild-type LTA.
Growth characteristics, lysis, and cell morphology of the dlt mutant.
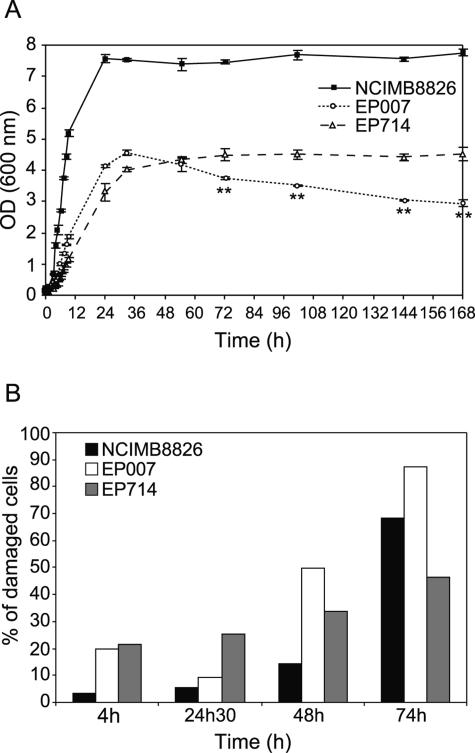
The dlt mutant displayed different growth characteristics in comparison to the wild-type strain (as shown in Fig. 2A). The dlt mutant showed a lower growth rate during exponential phase, a nearly twofold-lower optical density at 600 nm (OD600) at the entry of stationary phase, and a decreasing OD600 during stationary phase, suggesting a lytic process. During stationary phase (between 24 and 72 h), the number of CFU/ml for the dlt mutant decreased dramatically (60%), while it remained almost unchanged for the parent strain (data not shown). In order to confirm this lytic process, the integrity of the membrane in the cell population was investigated by previously described fluorescence labeling with a mixture of SYTO-9 (5 μM) and propidium iodide (30 μM), which differentially labels all bacteria and injured bacteria with damaged membranes, respectively (6) (Fig. 2B and 3A and B). The percentage of injured cells (labeled in red in Fig. 3) in the dlt mutant was higher than in the wild type at every growth stage (Fig. 2B). The twofold decrease in injured cells observed for the dlt mutant at the entry of stationary phase (24 h 30 min) is probably due to a higher number of disrupted cells that are too severely damaged to be labeled at all.
FIG. 2.
Effect of dlt and dlt Acm2 mutations on growth (A) and cell integrity (B). (A) Growth was measured by monitoring the OD600 of the NCIMB8826 strain (wild type), the EP007 mutant (dlt), and the EP714 mutant (dlt acm2) grown in MRS broth (Difco 0881, Detroit, MI) at 30°C without shaking and in the absence of antibiotics. The presence or absence of antibiotics (Em or Cm), used to maintain the integrated plasmid(s) in the chromosome, had no impact on the growth curve of the mutant strains (data not shown). Error bars represent standard deviations from the means (n = 3). Statistical analysis of OD600 values between EP007 and EP714 was performed with the Student t test. **, highly significant difference (P < 0.01). (B) Percentage of damaged cells was measured at different growth stages for NCIMB8826, EP007, and EP714 by epifluorescence microscopy using propidium iodide (red; labels damaged cells) and SYTO-9 (green; labels all cells). Cell samples were washed once with sterile distilled water, resuspended to about 1010 cells/ml, and mounted with Mowiol 4-88 medium prepared according to the manufacturer's instructions (Calbiochem-Novabiochem Corp., San Diego, Calif.). Epifluorescence microscopy was performed with a Reichert-Jung Polyvar microscope using filters B2 (fluorescein isothiocyanate, 901124) and G2 (901220). The percentage of damaged cells labeled with propidium iodide was calculated with respect to the total number of cells labeled with SYTO-9. Enumeration was done for a minimum of 300 cells for each strain. Measurements were performed in the exponential growth phase (4h), at the entry of stationary phase (24h30), and in stationary phase (48h and 74h).
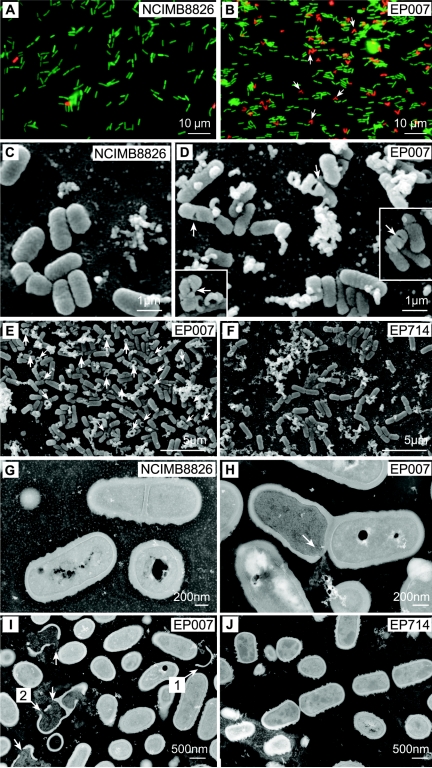
FIG. 3.
Epifluorescence, scanning electron, and transmission electron micrographs of cells from NCIMB8826 (wild type; A, C, and G), EP007 (dlt; B, D, F, H, and I), or EP714 (dlt acm2; F and J). (A and B) Cells for epifluorescence microscopy were collected in exponential growth phase. Growth conditions and cell preparation were as described in the Fig. 2 legend. The arrows indicate particular V-shaped dividing cells (B). (C to F) Scanning electron microscopy was performed with cells collected in exponential growth (C and D) and in late stationary growth phase (E and F). The arrows show perforations of EP007 cells (D) that are absent in wild-type cells (C). (G to J) Transmission electron microscopy was performed with cells collected in stationary growth phase. The arrow in panel H shows a perforation located at the septum of an EP007 cell. In panel I, arrow no. 1 shows a fragment of an empty envelope and arrow no. 2 indicates a perforation at the putative next division site. The perforations visible in EP007 cells (H, I) disappear in EP714 (J).
Epifluorescence and electron microscopy revealed that the dlt disruption had an impact on cell morphology. During the exponential growth phase, EP007 cells displayed an increased length in comparison to the wild type (data not shown). A similar observation has previously been reported for the dltD mutant of L. rhamnosus (11). Epifluorescence microscopy observations revealed that a majority of damaged cells of the mutant (labeled in red) showed a V shape during exponential phase (Fig. 3B), suggesting that a lethal event occurs during the division process. Scanning and transmission electron microscopy (SEM and TEM), performed as previously reported (26), clearly showed perforations of cell envelopes (Fig. 3D, H, and I), the release of cytoplasmic material from perforated cells, empty cells, and cell envelope fragments (Fig. 3E and I). The cell perforations became more numerous in stationary phase (data not shown), corroborating the above observations on cell lysis. When the cell damage is limited to a hole (50 cells examined), the perforation is localized in the septal region (see Fig. 3H for an example); also, in some cells, additional perforations were observed at the position of the next division site (see Fig. 3I for an example).
Inactivation of the gene encoding the autolysin Acm2.
It was recently shown that lysis of a dltD mutant of L. lactis clearly involved the major autolysin AcmA, which plays a role in cell separation and autolysis in stationary phase (32). Acm2 from L. plantarum WCFS1 (21) displayed the highest level of identity (34%) with the lactococcal AcmA protein. In addition, Acm2 contains a C-terminal domain with five repeats and an N-terminal region rich in Ala, Ser, and Thr. These two regions might be involved in cell wall binding, as previously reported for other peptidoglycan hydrolases from L. lactis (4, 31).
To confirm functional orthology between L. plantarum Acm2 and L. lactis AcmA, the acm2 gene was expressed in the acmA mutant of L. lactis (5). Heterologous expression of acm2 in this background resulted in complete suppression of the cell separation defect in the L. lactis mutant (data not shown), confirming the role of Acm2 as a peptidoglycan hydrolase involved in cell separation.
In order to investigate the functional role of Acm2 as a peptidoglycan hydrolase, the acm2 gene was disrupted by a single crossover in the wild-type strain (20). A 622-bp fragment of acm2 was PCR amplified (primers NCNAM1F1 [5′-GATCTGCAGTGAGCTGCGACTAAGGGAAACAG-3′] [PstI] and NCNAM1R2 [5′-TGCGGTACCTGACTAGTCATTGCCCGCG-3′] [KpnI]) and cloned between PstI and KpnI of the suicide plasmid pGIM008 (pACYC184 derivative containing the pC194 chloramphenicol resistance gene; M. Deghorain, unpublished), yielding pGIE014. Chromosomal integration of pGIE014 results in a 3′-end-deleted acm2 copy encoding the putative N-terminal cell wall binding domain and a second 5′-truncated acm2 copy, which is preceded by stop codons in the three frames to avoid any translation. Correct integration of pGIE014 at the acm2 locus was confirmed by PCR, and the resulting mutant was designated EP114 (acm2::pGIE014, Cmr).
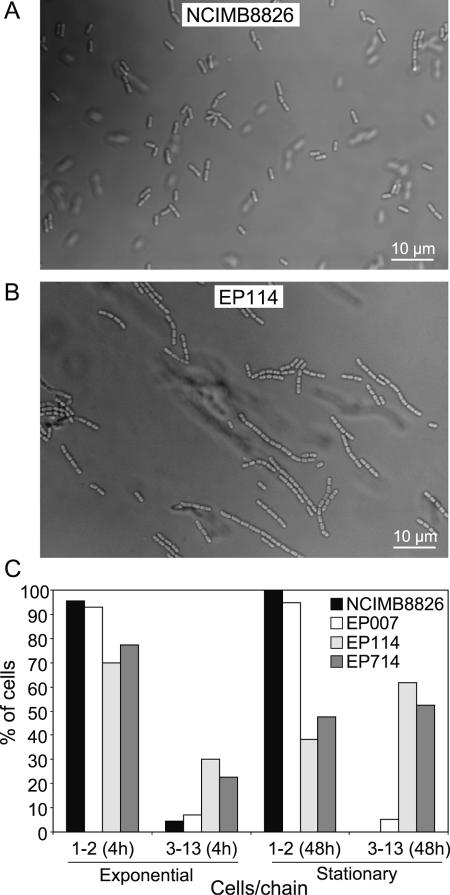
The major characteristic of the acm2 mutant was the appearance of chains of incompletely separated cells (Fig. 4). During the exponential phase (4 h) and the stationary phase (48 h), 30% and 60% of EP114 cells are associated in chains (3 to 13 cells), respectively (Fig. 4C), while more than 95% of the wild-type cells at any growth phase are isolated or associated in pairs (Fig. 4C). Analysis of EP114 cells by SEM and TEM showed a cell length similar to that of the wild type and a capacity to form a complete septum (data not shown).
FIG. 4.
Effect of dlt, acm2, and dlt acm2 mutations on cell chain formation. Contrast phase micrographs of cells from NCIMB8826 (wild type; A) and EP114 (acm2; B) collected in stationary growth phase (growth conditions as described in Fig. 2 legend). (C) The number of cells per chain was measured for NCIMB8826, EP007, EP114, and EP714 in exponential (4 h) and stationary (48 h) growth phases. Percentage of cells corresponds to the number of individual cells over total cells that are present either isolated or in pairs (category 1-2) or included in chains of 3 to 13 cells (category 3-13). Measurements were performed on micrographs for a minimum of 300 cells per strain.
Contribution of Acm2 to dlt mutant phenotype.
In order to investigate the contribution of Acm2 to the lytic phenotype of the dlt mutant, a dlt acm2 double mutant was constructed by the integration of pGIE007 (16) in the acm2 mutant strain EP114 and was designated EP714.
Inactivation of Acm2 had a slight negative effect on growth during exponential phase, since the generation time of EP714 (150 min) is higher than that of EP007 (137 min) (Fig. 2A). Although the maximal OD600 at the entry of the stationary phase is similarly low in both mutant strains compared to that of the wild type, the decrease in OD600 observed during the stationary phase of the EP007 strain was suppressed by the inactivation of Acm2 in EP714 (Fig. 2A). In exponential phase (4 h), the same numbers of damaged cells were observed in the two strains (Fig. 2B), while during stationary phase (48 and 74 h), the number of EP714 injured cells was clearly reduced relative to that for EP007 (Fig. 2B). These data revealed that Acm2 is involved in the autolytic process that takes place during the stationary phase of growth of the dlt mutant.
Microscopy analysis of the double mutant revealed similar cell chains in both exponential and stationary phases as was observed for the single acm2 mutant (Fig. 4C), indicating that Acm2 also performed the final separation of EP007 cells. Notably, no cell wall perforations were observed at any stage of growth in EP714 cells examined by SEM (Fig. 3F) or TEM (Fig. 3J), while dead V-shaped cells observed by epifluorescence microscopy were nearly absent in the EP714 cell population (data not shown). These observations demonstrate that Acm2 plays a key role in the formation of damage to the envelope of EP007 cells.
Concluding remarks.
In most gram-positive species, four genes (dltABCD) are responsible for the incorporation of d-Ala into TAs. In L. plantarum, the dlt cluster is cotranscribed with the functionally distinct pbpX2 gene, which is predicted to encode a d,d-carboxypeptidase involved in peptidoglycan biosynthesis. An in silico investigation of dlt loci from gram-positive bacterial genomes showed that the presence of pbpX2 upstream of dltA is unique to L. plantarum. However, a pbpX homologue was found downstream of the dlt genes in Lactibacillus acidophilus, Lactobacillus gasseri, and Lactobacillus johnsonii, but experimental data with regard to cotranscription of these genes is unavailable to date. Interestingly, transcription of pbpX and that of of the dlt operon were recently shown to be under the control of the σX factor in B. subtilis (7). Besides the mutual function in cell wall biosynthesis of pbpX2 and dlt, the functional importance of their genetic linkage and/or cotranscription in L plantarum and other species remains to be established (7).
The inactivation of dlt in L. plantarum NCIMB8826 resulted in a deficiency of d-alanylation of LTA. Unexpectedly, this lack of d-Ala on LTA was partially replaced by glucose substitution, which was undetectable in wild-type LTA. The only similar observation reported in the literature was a doubling of the level of N-acetylglucosamine TA substituents in dlt mutants of B. subtilis (27). Moreover, the majority of LTA extracted from the dlt mutant of L. plantarum were threefold longer than LTA of the wild-type strain. Finally, the longest LTA were the least d-alanylated, suggesting a link between the level of d-alanylation and the elongation process, which might be correlated with the perturbation of the ionic environment of the LTA biosynthesis machinery. To the best of our knowledge, a similar alteration of the LTA length in other dlt mutants has never been reported before.
The major physiological changes displayed by the dlt mutant of L plantarum were a slower exponential growth rate accompanied by cell elongation and lysis during the exponential and stationary phases. These results show that the d-alanylation defect in L plantarum results in lysis in stationary phase and confirm previous observations with some other gram-positive bacteria, like B. subtilis (33) and L. lactis (32). However, in L. plantarum, in contrast to these bacteria, the lytic process had already started during exponential growth. Perforations of the cell envelope in the dlt mutant appeared in 20% of dividing cells (red V-shaped cells) in exponential phase and became gradually more numerous at the end of the exponential growth phase and during the stationary phase. These “holes” in the envelope are localized in the septal region, which suggests that the defect is linked to the cell separation process and the formation of the septum. By comparison, cell perforations were not observed during the exponential phase of the dlt mutant of L. lactis (data not shown). In addition, we showed that the predicted autolysin Acm2, necessary for the final separation of wild-type cells, was responsible for these cell perforations observed by electron microscopy in the dlt mutant of L. plantarum. Moreover, Acm2 involvement in lysis of the dlt mutant during the stationary phase could be demonstrated. Nevertheless, inactivation of Acm2 suppressed neither the slower growth, nor the presence of 20% of dead cells (propidium iodide labeled) during exponential phase, nor the detection of low numbers of dead cells in the stationary phase. In contrast, the growth defect and enhanced autolysis in stationary growth phase were completely suppressed by AcmA inactivation in the L. lactis dltD mutant (32). Although many proteins involved in the cell division and separation processes could be affected by the absence of d-alanylation, other autolysins predicted to be encoded in the L. plantarum genome (lp_2162, lp_3093, and lp_3421) (21) may be involved in this phenotype.
The characterization of the L. plantarum dlt mutant allowed us to evaluate the specific contribution of d-alanyl esters of TAs compared to the complete absence of d-Ala in both peptidoglycan and TAs, as has previously been described for the L. plantarum alr mutant (26). In this mutant, similar perforated cells were observed under d-alanine starvation, suggesting that the origin of these perforations was the lack of d-alanyl esters on TAs and depended on Acm2 activity. In contrast to the alr mutant, the dlt mutant does not show a thinning of the cell envelope, suggesting that this alteration is due to the absence of d-Ala in the peptidoglycan precursors, leading to a defect in peptidoglycan assembly. The recent construction of an L. plantarum cell wall mutant strictly deficient in the incorporation of d-Ala in peptidoglycan precursors confirms these observations. This mutant, unable to synthesize the d-Ala-d-Lac depsipeptide under d-lactate deprivation (15), shows a thinning of the cell envelope but does not display cell wall perforations (P. S. Cocconcelli, P. Goffin, and P. Hols, unpublished).
Future work will aim at investigating the functional role of the two new genes discovered in the dlt operon, as well as the topological distribution of Acm2 and TA subtituents into the cell envelope of L. plantarum.
Acknowledgments
This research was carried out with financial support from the Commission of the European Communities, specific RTD project DEPROHEALTH (QLK1-2000-00146). It does not necessarily reflect its views and in no way anticipates the commission's future policy in this area.
We thank R. Leer for providing PCR products of the dlt operon of L. plantarum at the start of this work. We thank R. R. Giorno for her help in the initial characterization of the dlt mutant. We warmly thank J. Delcour, P. Rouxhet, and F. C. Neuhaus for fruitful discussions and scientific advice. We thank D. Prozzi for critically reading the manuscript. E.P. holds a doctoral fellowship from FRIA. P.H. is Research Associate at FNRS.
REFERENCES
- 1.Abachin, E., C. Poyart, E. Pellegrini, E. Milohanic, F. Fiedler, P. Berche, and P. Trieu-Cuot. 2002. Formation of D-alanyl-lipoteichoic acid is required for adhesion and virulence of Listeria monocytogenes. Mol. Microbiol. 43:1-14. [DOI] [PubMed] [Google Scholar]
- 2.Archibald, A. R., and J. Baddiley. 1966. The teichoic acids. Adv. Carbohydr. Chem. Biochem. 21:323-375. [DOI] [PubMed] [Google Scholar]
- 3.Boyd, D. A., D. G. Cvitkovitch, A. S. Bleiweis, M. Y. Kiriukhin, D. V. Debabov, F. C. Neuhaus, and I. R. Hamilton. 2000. Defects in d-alanyl-lipoteichoic acid synthesis in Streptococcus mutans results in acid sensitivity. J. Bacteriol. 182:6055-6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buist, G., H. Karsens, A. Nauta, D. van Sinderen, G. Venema, and J. Kok. 1997. Autolysis of Lactococcus lactis caused by induced overproduction of its major autolysin, AcmA. Appl. Environ. Microbiol. 63:2722-2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buist, G., J. Kok, K. J. Leenhouts, M. Dabrowska, G. Venema, and A. J. Haandrikman. 1995. Molecular cloning and nucleotide sequence of the gene encoding the major peptidoglycan hydrolase of Lactococcus lactis, a muramidase needed for cell separation. J. Bacteriol. 177:1554-1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bunthof, C. J., S. van Schalkwijk, W. Meijer, T. Abee, and J. Hugenholtz. 2001. Fluorescent method for monitoring cheese starter permeabilization and lysis. Appl. Environ. Microbiol. 67:4264-4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao, M., and J. D. Helmann. 2004. The Bacillus subtilis extracytoplasmic-function σX factor regulates modification of the cell envelope and resistance to cationic antimicrobial peptides. J. Bacteriol. 186:1136-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, J. D., and D. A. Morrison. 1988. Construction and properties of a new insertion vector, pJDC9, that is protected by transcriptional terminators and useful for cloning of DNA from Streptococcus pneumoniae. Gene 64:155-164. [DOI] [PubMed] [Google Scholar]
- 9.Clemans, D. L., P. E. Kolenbrander, D. V. Debabov, Q. Zhang, R. D. Lunsford, H. Sakone, C. J. Whittaker, M. P. Heaton, and F. C. Neuhaus. 1999. Insertional inactivation of genes responsible for the d-alanylation of lipoteichoic acid in Streptococcus gordonii DL1 (Challis) affects intrageneric coaggregations. Infect. Immun. 67:2464-2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Debabov, D. V., M. P. Heaton, Q. Zhang, K. D. Stewart, R. H. Lambalot, and F. C. Neuhaus. 1996. The d-alanyl carrier protein in Lactobacillus casei: cloning, sequencing, and expression of dltC. J. Bacteriol. 178:3869-3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Debabov, D. V., M. Y. Kiriukhin, and F. C. Neuhaus. 2000. Biosynthesis of lipoteichoic acid in Lactobacillus rhamnosus: role of DltD in d-alanylation. J. Bacteriol. 182:2855-2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delcour, J., T. Ferain, M. Deghorain, E. Palumbo, and P. Hols. 1999. The biosynthesis and functionality of the cell-wall of lactic acid bacteria. Antonie Leeuwenhoek 76:159-184. [PubMed] [Google Scholar]
- 13.Derzelle, S., B. Hallet, K. P. Francis, T. Ferain, J. Delcour, and P. Hols. 2000. Changes in cspL, cspP, and cspC mRNA abundance as a function of cold shock and growth phase in Lactobacillus plantarum. J. Bacteriol. 182:5105-5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Douglas, L. J., and M. J. Wolin. 1971. Cell wall polymers and phage lysis of Lactobacillus plantarum. Biochemistry 10:1551-1555. [DOI] [PubMed] [Google Scholar]
- 15.Goffin, P., M. Deghorain, J. L. Mainardi, I. Tytgat, M. C. Champomier-Verges, M. Kleerebezem, and P. Hols. 2005. Lactate racemization as a rescue pathway for supplying d-lactate to the cell wall biosynthesis machinery in Lactobacillus plantarum. J. Bacteriol. 187:6750-6761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grangette, C., S. Nutten, E. Palumbo, S. Morath, C. Hermann, J. Dewulf, B. Pot, T. Hartung, P. Hols, and A. Mercenier. 2005. Enhanced antiinflammatory capacity of a Lactobacillus plantarum mutant synthesizing modified teichoic acids. Proc. Natl. Acad. Sci. USA 102:10321-10326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hancock, I. C. 2001. Teichoic acids of Gram-positive bacteria, p. 79-92. In M. Sussman (ed.), Molecular medical microbiology. Academic Press, San Diego, Calif.
- 18.Heaton, M. P., and F. C. Neuhaus. 1992. Biosynthesis of d-alanyl-lipoteichoic acid: cloning, nucleotide sequence, and expression of the Lactobacillus casei gene for the d-alanine-activating enzyme. J. Bacteriol. 174:4707-4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heaton, M. P., and F. C. Neuhaus. 1994. Role of the d-alanyl carrier protein in the biosynthesis of d-alanyl-lipoteichoic acid. J. Bacteriol. 176:681-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hols, P., C. Defrenne, T. Ferain, S. Derzelle, B. Delplace, and J. Delcour. 1997. The alanine racemase gene is essential for growth of Lactobacillus plantarum. J. Bacteriol. 179:3804-3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kleerebezem, M., J. Boekhorst, R. van Kranenburg, D. Molenaar, O. P. Kuipers, R. Leer, R. Tarchini, S. A. Peters, H. M. Sandbrink, M. W. Fiers, W. Stiekema, R. M. Lankhorst, P. A. Bron, S. M. Hoffer, M. N. Groot, R. Kerkhoven, M. de Vries, B. Ursing, W. M. de Vos, and R. J. Siezen. 2003. Complete genome sequence of Lactobacillus plantarum WCFS1. Proc. Natl. Acad. Sci. USA 100:1990-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kojima, N., Y. Araki, and E. Ito. 1985. Structural studies on the linkage unit of ribitol teichoic acid of Lactobacillus plantarum. Eur. J. Biochem. 148:29-34. [DOI] [PubMed] [Google Scholar]
- 23.Nakao, A., S. Imai, and T. Takano. 2000. Transposon-mediated insertional mutagenesis of the d-alanyl-lipoteichoic acid (dlt) operon raises methicillin resistance in Staphylococcus aureus. Res. Microbiol. 151:823-829. [DOI] [PubMed] [Google Scholar]
- 24.Neuhaus, F. C., and J. Baddiley. 2003. A continuum of anionic charge: structures and functions of d-alanyl-teichoic acids in gram-positive bacteria. Microbiol. Mol. Biol. Rev. 67:686-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neuhaus, F. C., M. P. Heaton, D. V. Debabov, and Q. Zhang. 1996. The dlt operon in the biosynthesis of D-alanyl-lipoteichoic acid in Lactobacillus casei. Microb. Drug Resist. 2:77-84. [DOI] [PubMed] [Google Scholar]
- 26.Palumbo, E., C. F. Favier, M. Deghorain, P. S. Cocconcelli, C. Grangette, A. Mercenier, E. E. Vaughan, and P. Hols. 2004. Knockout of the alanine racemase gene in Lactobacillus plantarum results in septation defects and cell wall perforation. FEMS Microbiol. Lett. 233:131-138. [DOI] [PubMed] [Google Scholar]
- 27.Perego, M., P. Glaser, A. Minutello, M. A. Strauch, K. Leopold, and W. Fischer. 1995. Incorporation of D-alanine into lipoteichoic acid and wall teichoic acid in Bacillus subtilis. Identification of genes and regulation. J. Biol. Chem. 270:15598-15606. [DOI] [PubMed] [Google Scholar]
- 28.Peschel, A., M. Otto, R. W. Jack, H. Kalbacher, G. Jung, and F. Götz. 1999. Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J. Biol. Chem. 274:8405-8410. [DOI] [PubMed] [Google Scholar]
- 29.Peschel, A., C. Vuong, M. Otto, and F. Götz. 2000. The d-alanine residues of Staphylococcus aureus teichoic acids alter the susceptibility to vancomycin and the activity of autolytic enzymes. Antimicrob. Agents Chemother. 44:2845-2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poyart, C., M. C. Lamy, C. Boumaila, F. Fiedler, and P. Trieu-Cuot. 2001. Regulation of d-alanyl-lipoteichoic acid biosynthesis in Streptococcus agalactiae involves a novel two-component regulatory system. J. Bacteriol. 183:6324-6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steen, A., G. Buist, K. J. Leenhouts, M. El Khattabi, F. Grijpstra, A. L. Zomer, G. Venema, O. P. Kuipers, and J. Kok. 2003. Cell wall attachment of a widely distributed peptidoglycan binding domain is hindered by cell wall constituents. J. Biol. Chem. 278:23874-23881. [DOI] [PubMed] [Google Scholar]
- 32.Steen, A., E. Palumbo, M. Deghorain, P. S. Cocconcelli, J. Delcour, O. P. Kuipers, J. Kok, G. Buist, and P. Hols. 2005. Autolysis of Lactococcus lactis is increased upon d-alanine depletion of peptidoglycan and lipoteichoic acids. J. Bacteriol. 187:114-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wecke, J., K. Madela, and W. Fischer. 1997. The absence of D-alanine from lipoteichoic acid and wall teichoic acid alters surface charge, enhances autolysis and increases susceptibility to methicillin in Bacillus subtilis. Microbiology 143:2953-2960. [DOI] [PubMed] [Google Scholar]