Abstract
The naphthalene-catabolic (nah) genes on the incompatibility group P-9 (IncP-9) self-transmissible plasmid NAH7 from Pseudomonas putida G7 are some of the most extensively characterized genetic determinants for bacterial aerobic catabolism of aromatic hydrocarbons. In contrast to the detailed studies of its catabolic cascade and enzymatic functions, the biological characteristics of plasmid NAH7 have remained unclear. Our sequence determination in this study together with the previously deposited sequences revealed the entire structure of NAH7 (82,232 bp). Comparison of NAH7 with two other completely sequenced IncP-9 catabolic plasmids, pDTG1 and pWW0, revealed that the three plasmids share very high nucleotide similarities in a 39-kb region encoding the basic plasmid functions (the IncP-9 backbone). The backbone of NAH7 is phylogenetically more related to that of pDTG1 than that of pWW0. These three plasmids carry their catabolic gene clusters at different positions on the IncP-9 backbone. All of the NAH7-specified nah genes are located on a class II transposon, Tn4655. Our analysis of the Tn4655-encoded site-specific recombination system revealed that (i) a novel tyrosine recombinase, TnpI, catalyzed both the intra- and intermolecular recombination between two copies of the attI site, (ii) the functional attI site was located within a 119-bp segment, and (iii) the site-specific strand exchange occurred within a 30-bp segment in the 41-bp CORE site. Our results and the sequence data of other naphthalene-catabolic plasmids, pDTG1 and pND6-1, suggest a potential role of the TnpI-attI recombination system in the establishment of these catabolic plasmids.
Bacterial catabolic plasmids carry various genes that enable their host cells to utilize a number of natural and xenobiotic compounds as sole sources of carbon, nitrogen, and energy (9, 35, 60). Most such plasmids are large (>50 kb) and carry genes for their conjugal transfer to other bacterial strains (9, 35, 60). Such transfer events across taxa, and subsequent mutations and rearrangements of the catabolic genes, have contributed to the rapid adaptation of bacteria to novel chemical compounds (46, 52). Recent studies have shown that genes for the degradation of xenobiotic compounds, such as atrazine (31), haloacetate (44), and 2,4-dichlorophenoxyacetate (58), are predominantly carried on the incompatibility group P-1 (IncP-1) plasmids, whereas genes that encode the degradation of natural aromatic hydrocarbons, such as phenol (41), naphthalene (10, 28), and toluene/xylenes (18), are usually found on IncP-2, IncP-7, and IncP-9 plasmids (9, 51). Large catabolic gene clusters are also found on mobile elements integrated into bacterial chromosomes as genomic islands or conjugative transposons (8, 14, 53, 56).
The IncP-9 plasmid NAH7 from Pseudomonas putida G7 (11) (Fig. 1A) has been extensively characterized with respect to its naphthalene degradation (nah) genes. The gene products encoded by the upper nah operon (nahAa to nahD) convert naphthalene to salicylate, and those encoded by the lower nah operon (nahG to nahY) convert salicylate to pyruvate and acetaldehyde via the meta-cleavage pathway (Fig. 1A) (67). The overall structures of these two nah operons are very similar to those that encode the aerobic degradation of several aromatic compounds via the meta-cleavage pathway in various strains (Fig. 2), indicating that these operons must have been distributed by horizontal transfer (61). Both the upper and lower nah operons on NAH7 have been shown to be situated within a class II (Tn3-like) transposon designated Tn4655 (54) (Fig. 1). Class II transposons usually transpose by a two-step mechanism (40). In the first step, a tnpA product (transposase) acts at the terminal inverted repeats (IRs) to generate a cointegrate of the donor and target molecules connected by two directly repeated copies of the transposon, one at each junction. In the second step, the cointegrate resolves at the resolution (res) sites using a site-specific recombination system encoded by the transposon. Tn4655 lacked its tnpA gene but was able to form a cointegrate when the tnpA gene from Tn4653, the larger mobile unit of two toluene-catabolic transposons from pWW0 (see Fig. 3B), was supplied in trans (54). In contrast to its defective cointegration function, Tn4655 encodes a functional site-specific recombination system for the resolution of its cointegrate within a 2.5-kb region (see Fig. 1A and 4A) (54). Interestingly, this recombination system was shown to be different from those encoded by other typical class II transposons (54).
FIG. 1.
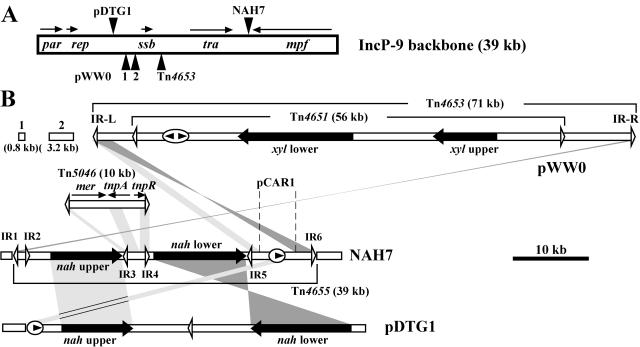
(A) Overall linear map of NAH7. The bar above the map represents the nucleotide position of NAH7, and the sections in the bar show regions of NAH7 that share high nucleotide sequence identity with other genomic sequences. The pentagons indicate the sizes and transcriptional orientations of the genes: yellow, genes for naphthalene degradation; green, genes for replication and maintenance of plasmid; blue, genes for conjugative transfer of plasmid; purple, genes for resistance to UV light as well as DNA polymerase V; red, genes for site-specific recombination; and white, genes for other functions. The circles represent the DNA sequences required in cis: green, origin of vegetative replication of plasmid (oriV); blue, origin of conjugative transfer of plasmid (oriT); and red, the TnpI-mediated site-specific resolution/integration site (attI). Abbreviations 1 to 37 indicate the orf1 to orf37 genes (see the table in the supplemental material); see references 10 and 18 and the table in the supplemental material for the details of the predicted functions of these products. The white arrowheads represent the 38-bp IRs highly homologous to those of the class II transposons. The DNA region flanked by IR1 and IR6 has been defined as Tn4655 (54). Below the functional map are shown the Tn4655 and IncP-9 backbone regions. (B) IR sequences located on Tn4655. The dots indicate the nucleotides identical to those of IR1, and the hyphens represent absent ones. The IR sequences of Tn4653, which belongs to the same subgroup as Tn4655 (54), are also shown, and the left (IR-L) and right (IR-R) ends of Tn4653 are defined as those located proximal and distal, respectively, to the lower xyl operon (Fig. 3B).
FIG. 2.
Comparison of catabolic genes in the meta-cleavage operons. The percent amino acid identity of NAH7-specified gene products to the corresponding gene products from the other operons is shown. nah and xyl are genes for degradation of naphthalene and toluene/xylenes, respectively. The tnpA and tnpA2 genes in pDTG1 and AN10, respectively, are ISPre1 (29) derivatives, and tnp in S-47 is a putative transposase gene. Tn5501 is a cryptic class II transposon identified in P. putida H (26). Accession numbers of the sequence data are as follows: P. putida NCIB9816-4(pDTG1), AF491307; Pseudomonas sp. ND6(pND6-1), AY208917; P. stutzeri AN10, AF039534; P. putida mt-2(pWW0), AJ344068; and Pseudomonas sp. S-47, AF320981. The genes in P. stutzeri AN10 and Pseudomonas sp. S-47 are located on their chromosomes.
FIG. 3.
Comparison of three IncP-9 catabolic plasmids. The comparison was performed with Artemis Comparison Tool software (36), and the figure represents the results schematically. The nucleotide A of the start codon for the parA gene in each plasmid is defined as position 1. (A) Shown is the 39-kb IncP-9 backbone with insertion of various DNA fragments. Horizontal arrows above the bar indicate the transcriptional direction of the indicated genes or gene clusters. The vertical black arrowheads represent the insertion sites of additional genes (see panel B). (B) Additional DNA fragments in the three catabolic plasmids. The white arrowheads indicate the IR and IR-like sequences of the class II transposons. The ovals indicate the region encoding the site-specific recombination system, and the black arrowhead in the oval represents the recombinase gene and its transcriptional direction. mer represents genes for resistance to mercury. Homologous regions are shown by light (same strand) and dark (different strand) shadings. pWW0 has three insertions; the insertions 1 and 2 correspond to the regions containing ORF7 and ORF20 to ORF24 of the plasmid, respectively (18). The pDTG1 fragment (47 kb) contains orf77 to int genes of the plasmid (10). pDTG1 has a Tn501-like IR between the nah operons but not any other Tn501-like sequences. See the text for details of the NAH7 fragments.
FIG. 4.
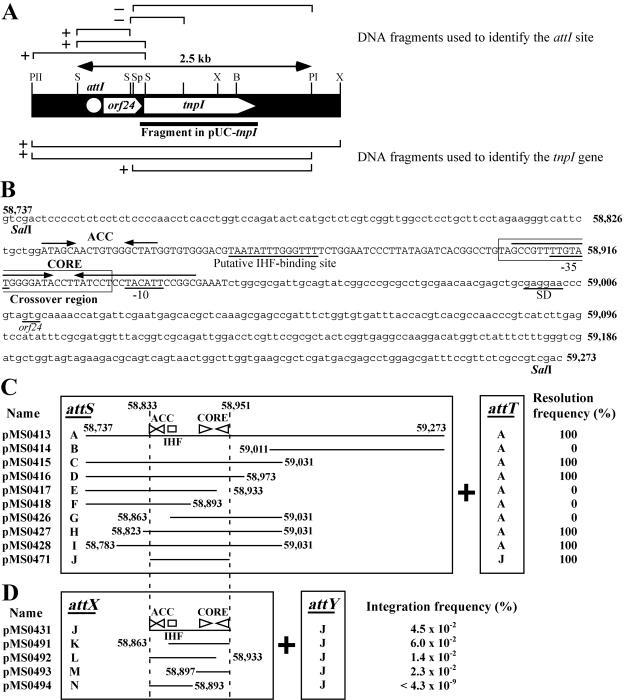
Structure of the NAH7-derived region containing the tnpI gene and structures of the attI derivatives used for the resolution and integration experiments. (A) Organization of the attI-orf24-tnpI region of NAH7. Abbreviations for restriction enzymes: B, BglII; S, SalI; Sp, SphI; PI, PvuI; PII, PvuII; and X, XhoI. The DNA fragments used to identify the attI site and tnpI gene in our previous study (54) are shown above and below the map, respectively. The DNA fragments having the predicted functions are marked by “+” and those not having such functions by “−”. The DNA region cloned in pUC-tnpI (Table 1) is also depicted. (B) Nucleotide sequence of the 537-bp SalI fragment carrying the attI site. The numbers are positions in the NAH7 map in Fig. 1A. The capital letters indicate the 119-bp sequence defined as the attI site. The ACC and CORE sites important for the TnpI-mediated resolution/integration reactions are presented. The putative IHF-binding sequence as well as the putative GTG start codon, promoter (−35 and −10), and Shine-Dalgarno (SD) sequence of orf24 are underlined. The boxed sequence represents the 30-bp crossover region. (C) DNA fragments used for the resolution experiments. Fragment A represents the 537-bp sequence shown in panel B. Each plasmid had two attI-derived fragments, one as attS and another as attT, and a Kmr gene to form the structure depicted in Fig. 5. The ACC and CORE sites are shown by a set of facing triangles, and the putative IHF-binding site is depicted by a rectangle. The resolution frequency is expressed as the number of Kms colonies per all colonies examined. (D) DNA fragments used for the integration experiments. Each plasmid had an attI-derived fragment as attX (Fig. 5). The 119-bp attI fragment (panel B) is cloned in R388attKm as attY (Fig. 5). The integration frequency is expressed as the number of Cmr Smr transconjugants per number of Kmr Smr transconjugants (see Materials and Methods). The values are averages from three independent experiments.
Prokaryotic site-specific recombination systems are ubiquitous and play important roles in the movement of bacteriophage genomes, plasmids, transposons, and integrons (8, 22, 33). The site-specific recombinases involved in these movements are categorized into two major groups, serine recombinases and tyrosine recombinases (17, 33, 43). The majority of serine recombinases, e.g., resolvases and invertases, contain a conserved serine residue as the catalytic center at their N-terminal regions and catalyze only the intramolecular recombination between two copies of the specific recombination sites on the same replicon (43). The tyrosine recombinases are structurally diverse but share a conserved R-H-R-Y motif at their C-terminal regions, the tyrosine residue of which works as the catalytic center (17). In contrast to serine recombinases, tyrosine recombinases catalyze both the intra- and intermolecular recombination between two recombination sites (attachment [att] sites) (see Fig. 5) (33). Several class II transposons carry tyrosine recombinases to resolve their cointegrates (3, 30), and we have also identified and characterized a tyrosine recombinase, TnpS, in the smaller toluene-catabolic transposon Tn4651 from pWW0 (Fig. 3B) (15).
FIG. 5.
Detection of TnpI-mediated site-specific recombination. In the resolution experiment, a pSTV29-based plasmid carrying a Kmr determinant between two attI sequences (A: the pMS0413 derivatives depicted in Fig. 4C) was used as a substrate for TnpI. When resolution occurs, plasmid A resolves to a plasmid that lacks the Kmr gene but has a hybrid att site (attX) (B) and a circular DNA with the Kmr gene and another hybrid att site (attY) (C). As a result, the host cells lose their Kmr phenotype so that the resolution can be detected by the loss of the phenotype. In the integration experiment, a pSTV29-based plasmid carrying an attI derivative (attX) (B: pMS0431 derivatives shown in Fig. 4D) and a self-transmissible R388 derivative carrying a Kmr gene and the 119-bp attI site (attY) (C: R388attKm) were used as substrates for TnpI in the donor cells. When integration occurs, the two plasmids generate a cointegrate (A), and the Cmr phenotype encoded by a pMS0431 derivative can be transferred to the recipient cells by conjugation. Thus, integration can be detected by the presence of the Cmr transconjugants.
Although most information on naphthalene catabolism has been based on the analysis of NAH7 (12, 16, 37, 66, 68, 69), until now the complete nucleotide sequence of this IncP-9 plasmid has not been reported. In addition, further detailed molecular features have remained unclear with respect to the unique site-specific resolution system of Tn4655. This paper reports the complete genome sequence of NAH7 and its structural comparison with two other completely sequenced IncP-9 plasmids, pWW0 (18) and naphthalene-catabolic plasmid pDTG1 (10). We also show for the first time that the site-specific resolution of the Tn4655-mediated cointegrate is carried out at the attI site, formerly designated the res site (54), by a tyrosine recombinase, TnpI (formerly designated TnpR), which is also able to catalyze the intermolecular site-specific integration reaction.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
Escherichia coli strains used in this study were DH5α [F− φ80dlacZΔM15 Δ(lacZYA-argF)U169 deoR recA1 endA1 hsdR17 phoA supE44 thi-1 gyrA96 relA1] and HB101 (hsdS20 recA13 ara-14 proA2 lacY1 galK2 rpsL20 xyl-5 mtl-1 supE44) (1). Strains were cultivated at 37°C. Plasmids used are listed in Table 1. Luria broth (LB) and LB agar (1) were used throughout this study. The antibiotics added to the media were as follows: ampicillin (Ap), 100 μg/ml; chloramphenicol (Cm), 50 μg/ml; kanamycin (Km), 50 μg/ml; and streptomycin (Sm), 100 μg/ml.
TABLE 1.
Plasmids used in this study
| Plasmid name | Relevant characteristics | Reference(s) or source |
|---|---|---|
| NAH7 | IncP-9, naphthalene degradation, Tn4655 | 54, 65 |
| pDTG1 | IncP-9, naphthalene degradation, intA gene is nearly identical with tnpI of NAH7 | 10 |
| pSTV29 | Cmr, cloning vector, p15Aori | TAKARA BIO |
| pUC18 | Apr, cloning vector, pMB1ori | 64 |
| pUC19 | Apr, cloning vector, pMB1ori | 64 |
| pUC4K | Apr Kmr, pMB1ori, the Kmr cassette is flanked by EcoRI, BamHI, SalI, and PstI sites | 50 |
| pUC-tnpI | Apr, pUC18 derivative carrying the tnpI gene of Tn4655 | This study |
| pMS0411 | Cmr, pSTV29 derivative carrying the 537-bp fragment located upstream of the tnpI gene (see Fig. 4A and B) at the SalI site | This study |
| pMS0412 | Cmr, pMS0411 derivative carrying another copy of the 537-bp fragment at the KpnI site | This study |
| pMS0413a | Cmr Kmr, pMS0412 derivative in which the Kmr determinant at the BamHI site is flanked by two copies of the 537-bp fragment (see Fig. 4C and 5) | This study |
| pMS0431b | Cmr, pSTV29 derivative carrying the 119-bp attI site between the SacI and KpnI sites (see Fig. 4D) | This study |
| pMS0435 | Cmr, pMS0431 derivative carrying another copy of the 119-bp attI site between the SalI and SphI sites | This study |
| pMS0471 | Cmr Kmr, pMS0435 derivative in which the Kmr determinant at the BamHI site is flanked by two copies of the 119-bp attI site (see Fig. 4C and 5) | This study |
| pMS0477 | Cmr Kmr, pMS0471 derivative in which the 119-bp attI site between the SalI and SphI sites was replaced with a similar fragment from pDTG1 | This study |
| R388c | Tpr Sur, IncW, tra+ | 59 |
| R388attKm | Tpr Kmr, tra+, R388 derivative carrying the 119-bp attI site and a Kmr determinant between the EcoRI and SacI sites | This study |
Various deletion derivatives were constructed with PCR to investigate the resolution function of the tnpI gene product and attI site. The names and schematic structures of these derivatives are shown in Fig. 4C.
Various deletion derivatives were constructed with PCR to investigate the integration function of the tnpI gene product and attI site. The names and schematic structures of these derivatives are shown in Fig. 4D.
Tpr, trimethoprim resistance; Sur, sulfonamide resistance.
DNA methodology.
Standard methods were used for extraction of the plasmid DNA, DNA digestion with restriction endonucleases, ligation, gel electrophoresis, and transformation of E. coli cells (1). The PCR was carried out with ExTaq DNA polymerase (TAKARA BIO). Purification of the PCR-amplified DNA fragments was done with GFX PCR DNA and Gel Band purification kit (Amersham Biosciences) according to the manufacturer's protocol. Nucleotide sequencing was performed with an ABI PRISM model 310 sequencer (Applied Biosystems).
PCR amplification and construction of plasmids.
Various NAH7 fragments obtained by digestion with appropriate restriction endonucleases were cloned in pUC18 or pUC19 (64), and the resulting plasmids were used as templates for DNA sequencing. A 1.3-kb fragment containing the tnpI gene of Tn4655 (see Fig. 4A) was amplified by PCR and cloned between the BamHI and PstI sites of pUC18 so that the tnpI gene was transcribed from the lac promoter on the vector sequence. The resulting plasmid was designated pUC-tnpI. The 537-bp SalI fragment located upstream of the tnpI gene (base positions [BP] 58737 to 59273 in NAH7) (see Fig. 4A and B) was cloned at the SalI site in pSTV29 to construct pMS0411. The same 537-bp fragment but flanked by KpnI sites was amplified by PCR and cloned to the KpnI site in pMS0411 to generate pMS0412. A BamHI-flanked Km resistance (Kmr) gene from pUC4K (50) was subsequently inserted into the BamHI site in pMS0412 to obtain pMS0413, in which the Kmr gene was flanked by directly repeated copies of the 537-bp fragment (Fig. 5). Various segments located within this fragment were amplified by PCR so as to be bracketed by KpnI and SacI sites, and the KpnI-flanked 537-bp fragment on pMS0413 was replaced with either one of these amplified segments. Such replacement gave rise to a series of pMS plasmids, pMS0414 to pMS0428, depicted in Fig. 4C. A 119-bp segment (BP 58833 to 58951) of the 537-bp fragment (see Fig. 4B and C) and its four deletion derivatives were amplified by PCR and cloned between the SacI and KpnI sites in pSTV29 to obtain pMS0431 and four other pMS plasmids (see Fig. 4D). Insertion of another copy of the 119-bp fragment between the SalI and SphI sites of pMS0431 generated pMS0435, and subsequent insertion of the pUC4K-derived Kmr gene into the BamHI site of pMS0435 gave rise to pMS0471 (see Fig. 4C). The NAH7-derived 119-bp segment located between the SalI and SphI sites of pMS0471 was replaced with a pDTG1-derived 119-bp fragment whose sequence differed from that of NAH7 at only nine positions (10) (see Fig. 6B). The resulting plasmid was designated pMS0477. The 119-bp fragment and the pUC4K-derived Kmr gene were cloned together between the EcoRI and SacI sites in the self-transmissible IncW plasmid R388 (59) to construct R388attKm.
FIG. 6.
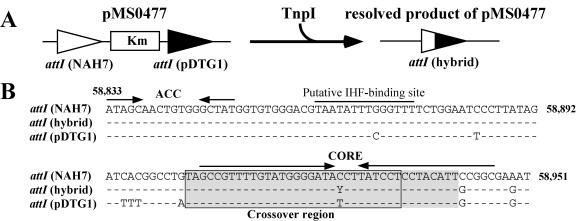
Determination of the crossover region. (A) Strategy for determination of the crossover region. Plasmid pMS0477 had the 119-bp attI site from Tn4655 and its nearly identical 119-bp sequence from pDTG1 (9-bp mismatch). Resolution of this plasmid resulted in generation of a hybrid attI site, and then its sequence was determined. (B) Comparison of three attI sequences. Hyphens represent the nucleotides identical to those of the attI site in Tn4655, and the nucleotide Y indicates the nucleotide C or T. The ACC, CORE, and putative IHF-binding sites are shown. The crossover region identified using the above strategy is shaded (38 bp), and that identified from this 38 bp and the result of integration experiments is boxed (30 bp).
Site-specific recombination assays.
The analysis of the site-specific recombination system of the Tn4655-specified tyrosine recombinase TnpI was performed as follows. To investigate the resolution function, a strain DH5α derivative harboring one of the pMS plasmids listed in Fig. 4C was transformed with pUC-tnpI. The resulting transformants were cultivated in LB containing Ap and Cm for 1 h and then plated on an LB agar plate containing Ap and Cm. The colonies thus obtained were examined for resistance to Km and for the profiles of the residing plasmids (see Fig. 5). The resolution frequency was expressed as the number of Km-sensitive (Kms) colonies per number of examined colonies. To investigate the integration function, the strain DH5α derivative carrying R388attKm, pUC-tnpI, and one of the pMS plasmids depicted in Fig. 4D was mated with strain HB101, and Cmr Smr transconjugants were selected to detect the mobilization of the pMS plasmid (see Fig. 5). The integration frequency was expressed as the number of Cmr Smr transconjugants per number of Kmr Smr transconjugants.
Sequencing analysis.
The computer analysis of the NAH7 sequence was performed with the software programs GENETYX 13 (SDC Inc.) and BLAST (National Institute of Genetics, Mishima, Japan). Whole-plasmid DNA comparison was performed with sequence comparison software Artemis Comparison Tool (www.sanger.ac.uk/Software/ACT) (36).
Nucleotide sequence accession number.
The complete nucleotide sequence of NAH7 (82,232 bp) has been deposited in the DDBJ/EMBL/GenBank databases under the accession number AB237655.
RESULTS
Overview of NAH7 structure.
We completed the determination of the entire NAH7 sequence, portions of which (ca. 13.5 kb total within the two nah operons) had been previously sequenced (12, 16, 23-25, 38, 39, 68). Plasmid NAH7 was found to be 82,232 bp in size, and the nucleotide A of the start codon for the parA gene of NAH7 was defined as nucleotide position 1. The average G+C content of the whole NAH7 sequence was 55.8%. This value is lower than those for the chromosomes of Pseudomonas strains such as P. aeruginosa PAO1 (67.1%) (47), P. putida KT2440 (62.3%) (34), and P. syringae DC3000 (59.2%) (7), although IncP-9 plasmids have been identified only from Pseudomonas strains. A total of 84 open reading frames (ORFs) were postulated to be located on NAH7 (Fig. 1A and the table in the supplemental material). BLAST searches in public databases revealed 59 ORFs with known or predicted functions and the remaining 25 ORFs with hypothetical functions. Among the former 59 ORFs, 23 ORFs were predicted to be involved in naphthalene degradation (nah), 6 in plasmid replication and maintenance (ssb, rep, and par), 4 in plasmid transfer (tra), 10 in mating pair formation (mpf), 2 in resistance to UV light as well as the DNA polymerase Pol V function (rul) (49), 2 in site-specific recombination systems (tnpI and rsv), and 12 in other various functions (orf12, orf14, orf18, orf19, orf20, orf22, orf23, orf25, orf26, orf29, orf31, and orf33) (see the table in the supplemental material). Seventy-eight of the 84 ORF products (exceptions were products from orf18, orf19, orf20, orf28, orf29, and orf30) showed very high similarities to products encoded by two other IncP-9 catabolic plasmids, pDTG1 and pWW0, and the IncP-7 carbazole-degrading plasmid pCAR1 (29). The two nah operons on NAH7 showed very high similarity (>85% nucleotide sequence identity) to those of pDTG1 and the IncP-7 naphthalene-catabolic plasmid pND6-1 (28) (see below for details).
IncP-9 backbone of NAH7.
The three backbone segments of NAH7 (BP 1 to 10875, 13434 to 25678, and 68860 to 82232 in Fig. 1A), with a total size of 36.4 kb, were conserved in pDTG1 (10) and pWW0 (18). The 2.6-kb portion carrying orf7 and orf8 (BP 10876 to 13433) did not show high similarity to any nucleotide sequences in the databases, but the products had 54% and 40% amino acid identities, respectively, to two putative products encoded by pWW0. Therefore, we defined the contiguous 39.0-kb sequence (BP 68860 to 82232 and 1 to 25678) of NAH7 as the IncP-9 backbone that contained the genes and sites for replication, transfer, and partitioning of this group of plasmids. The backbone of NAH7 showed 94% and 70% identities with those of pDTG1 and pWW0, respectively. This closer phylogenetic relationship of NAH7 to pDTG1 than to pWW0 was also clear from the comparison of the Rep proteins of the three plasmids: 96% and 78% amino acid identities between NAH7 and pDTG1 and between NAH7 and pWW0, respectively. As is the case in other IncP-9 plasmids, such as pDTG1, pWW0, and pM3 (10, 18, 19), the putative origins of plasmid transfer (oriT) and replication (oriV) were situated between traA and traD and between rep and rsv, respectively (Fig. 1A and the table in the supplemental material).
Definition of the IncP-9 backbone allowed us to locate the various insertions at the different positions in the backbones of NAH7, pDTG1, and pWW0 (Fig. 3). Two regions unique to NAH7 were located just upstream and downstream of Tn4655: the 0.6-kb sequence containing orf18 and the 3.3-kb sequence containing three ORFs, orf28 to orf30, respectively. This continuous 43.2-kb segment containing Tn4655 was located between orf17 and orf31, whereas the 47-kb fragment containing all of the nah genes on pDTG1 was inserted into the rulB gene (on the NAH7 map) (Fig. 1A and 3). In contrast, pWW0 contained the following three inserts: a 0.8-kb insert at orf3, a 3.2-kb insert between orf6 and orf7, and a 71-kb insert of transposon Tn4653 between orf12 and orf14 (on the NAH7 map) (Fig. 1A and 3). None of these inserts on pWW0, except Tn4653, contained structural characteristics at their extreme ends that supported the direct involvement of mobile genetic elements in the insertion events. The backbone of NAH7 was compared with that of the IncP-7 plasmid pND6-1 in detail, because both plasmids have very similar nah genes (see below). The orf28 product of NAH7 showed 62.3% amino acid identity to the hypothetical protein (ND073) of pND6-1, but no significant similarity was found in any other regions. These results indicate that (i) NAH7 is an IncP-9 plasmid that is phylogenetically closer to pDTG1 than to pWW0 and (ii) the catabolic genes in the three plasmids have most likely been acquired independently, resulting in their present plasmid structures.
Naphthalene-catabolic genes.
The structure and DNA sequence of the upper nah operon on NAH7 were compared with five other complete upper-pathway operons involved in the degradation of naphthalene and/or phenanthrene to salicylate that have so far been deposited in the public databases: three upper nah operons on pDTG1, pND6-1, and the P. stutzeri AN10 chromosome and two phenanthrene-degrading (pah) upper operons on the chromosomes of P. putida OUS82 and P. aeruginosa PaK1 (5, 10, 28, 48). The order of the 10 genes in the upper nah operon of NAH7 was conserved in the five other upper nah/pah operons, except that the AN10 operon lacked the nahQ gene, a gene with unknown function. All of the gene products encoded by the NAH7 upper nah operon shared 86 to 99% amino acid identities with the corresponding gene products encoded by the five operons (data not shown).
The lower nah operon of NAH7 consisted of 12 genes (Fig. 2). The first nahG gene is involved in the conversion of salicylate to catechol, and the following nahTHINLOMKJ cluster is involved in the conversion of catechol to pyruvate and acetyl-coenzyme A via the meta-cleavage pathway (61). This cluster was also highly similar to the corresponding gene clusters of three other naphthalene degraders and to the xylTEGFJQKIH clusters in the toluene/xylene degraders (Fig. 2), although some gene products in the xyl gene clusters (e.g., xylT, xylQ, and xylK on pWW0) shared rather low (<60%) amino acid identities. The lower nah operon on NAH7 also carried the nahX and nahY genes just downstream of the conserved cluster. The nahY gene involved in chemotaxis to naphthalene (20) was also found in pDTG1 and pND6-1, but the nahX gene with unknown function was so far unique for NAH7. The DNA regions upstream of nahG in the four naphthalene degraders in Fig. 2 carried a nahR gene, which encodes a LysR-type transcriptional activator for the expression of both the upper and lower nah operons. Although the nahR gene product of NAH7 showed high amino acid identity to those of pDTG1, pND6-1, and AN10 (>81% in their N-terminal 284 residues), its size (374 residues) was much larger than the three other NahR proteins (300 residues). The regions upstream of nahTHINLOMKJ showed structural diversity due to the presence of transposable elements: ISPre1 derivatives on pDTG1 and AN10 (tnpA and tnpA2 in Fig. 2, respectively) and a cryptic class II transposon, Tn5501, on pND6-1 (4, 10, 26, 28). P. stutzeri AN10 has an additional gene, nahW, which is a functional homologue of nahG (6).
Almost all of the nah gene products on NAH7 showed high amino acid identities (>86%) with those of pDTG1 and pND6-1 (Fig. 2 and the table in the supplemental material). The latter two plasmids carry the two nah operons and their intervening sequence in 39-kb fragments that are almost identical to each other (28). Interestingly, the relative orientation of the upper nah operon to the nahR-lower nah operon cluster on NAH7 was opposite to that on pDTG1 and pND6-1 (Fig. 3B). Furthermore, the intervening region between the upper and lower nah operons of NAH7 did not show any homology with the corresponding regions of pDTG1 and pND6-1. These results suggest that the nah operons on NAH7 have been acquired differently from those of pDTG1 and pND6-1.
Transposon Tn4655.
Tn4655, with a size of 39,116 bp, carried 33 ORFs, 23 of which were involved in naphthalene degradation. We have previously demonstrated that Tn4655 has terminal IRs (IR1 and IR6) showing high similarity to those of Tn4653 on pWW0 (Fig. 1B) but lacks the DNA region that hybridizes with the tnpA and tnpR genes of Tn4653 (54). The complete absence of these two genes was confirmed by the sequence determination in this study. Based on our previous genetic analysis of the Tn4655-encoded site-specific resolution system (54), its resolution site, attI, was found to be located within the 537-bp SalI fragment (BP 58737 to 59273), and the recombinase gene, tnpI, was within the 1,886-bp SphI-PvuI fragment (BP 59316 to 61201) (Fig. 4). We have now shown that the tnpI gene spans from BP 59414 to 60661. A 405-bp ORF (orf24) was located between attI and tnpI and overlapped with tnpI by four bases. A putative promoter sequence within attI might contribute to transcription of both orf24 and tnpI (Fig. 4B). The TnpI protein (415 amino acid residues) had an R-H-R-Y motif at its C-terminal region that is commonly conserved in tyrosine recombinases (17). Database searches revealed that genes very similar to the NAH7 tnpI gene are found on pDTG1 (intA, 94% identity in amino acid sequence, AF491307), pND6-1 (intA, 94%, AY208917), and pCAR1 (orf72, 94%, AB088420). It is noteworthy that (i) the latter three tnpI homologues are also preceded by sequences that are highly similar to attI-orf24 of Tn4655 and (ii) these attI-orf24-tnpI homologues are located in close proximity to the corresponding catabolic genes of these plasmids. This suggests possible involvement of the attI-orf24-tnpI homologues in the dissemination of catabolic genes.
Tn4655 was shown to have four additional copies of IR-like sequences (IR2 to IR5) that showed high similarity with the IRs of class II transposons (Fig. 1B). IR5 and IR6 were similar to one IR (IR-L) of Tn4653 on pWW0 (Fig. 1B and 3B). At both ends of the 9,575-bp region flanked by IR5 and IR6, two nearly identical but inversely oriented fragments, which shared more than 90% nucleotide identity to the orf41-, orf43-, and orf44-containing region (2.6 kb) of Tn4653, were found (Fig. 3B and the table in the supplemental material). The remaining internal fragment (BP 57363 to 62999) within the 9,575-bp region carried the attI-orf24-tnpI cluster and was more than 85% identical to the corresponding sequence on pCAR1. In contrast to the IR6 end of Tn4655, the homology of its IR1 end to the IR-R end of Tn4653 was limited to their IR sequences (Fig. 1B and 3B). The region bracketed by IR3 and IR4 resembled a mercury resistance class II transposon, Tn5046 (32). However, its tnpA and tnpR genes were truncated, and no mercury resistance genes were found between IR3 and IR4 (Fig. 3B and the table in the supplemental material).
It is known that class II transposons generate a 5-bp duplication of their target sequences upon transposition (40). However, such a 5-bp duplication was found only just outside of IR1 and IR6, suggesting that transposition of the entire Tn4655 transposon or its ancestor into an IncP-9 plasmid resulted in the generation of NAH7.
Site-specific recombination activity of TnpI.
As described above, the Tn4655-mediated cointegrate resolves by TnpI within the 537-bp SalI-flanked sequence (attI site) that is located just upstream of orf24 (Fig. 4A) (54). To analyze this resolution system in detail under an E. coli recA background, the pSTV29-based plasmids depicted in Fig. 4C, in which a Kmr gene was bracketed by two copies of various fragments derived from the 537-bp sequence, were constructed. An E. coli DH5α derivative harboring one of these pSTV29-based plasmids was transformed with pUC-tnpI, a pUC18 derivative carrying the Tn4655 tnpI gene. The resulting transformants were cultured in LB containing Ap and Cm for 1 h and subsequently plated on LB agar plates containing Ap and Cm. At least 150 colonies from each DH5α derivative were examined for resistance to Km. pMS0413 and pMS0415, but not pMS0414, contained two copies of the tnpI-distal 295-bp portion (BP 58737 to 59031) of the 537-bp sequence. While use of pMS0413 and pMS0415 gave rise to Kms colonies at a frequency of 100%, use of pMS0414 generated no Kms colonies (Fig. 4C). Based on these results, we further constructed six pSTV29-based plasmids (pMS0416 to pMS0428 in Fig. 4C), in each of which the Kmr gene was bracketed by a deletion fragment of the 295-bp sequence (designated attS in this study) and the intact 537-bp sequence (designated attT) (Fig. 4C and 5). One hundred percent resolution was observed when the substrate plasmid was pMS0416, pMS0427, or pMS0428 (Fig. 4C). However, use of pMS0417, pMS0418, or pMS0426 resulted in the complete loss of the resolution reaction (Fig. 4C). These results indicate that the functional attI site is located within a 151-bp region (BP 58823 to 58973) of the 537-bp sequence and that the two segments BP 58823 to 58862 and 58934 to 58973 are crucial for the resolution reaction (Fig. 4C).
Studies on various site-specific recombination systems have clarified that each of their efficient recombination reactions requires one or two characteristic structures within the DNA fragment that covers the recombination crossover site. One common structure is the recombinase-binding site (core site), which is composed of two approximately 10-bp sequences arranged as inverted repeats (2, 33, 57). Such a core site is often but not always associated with a neighboring accessory sequence(s) for the binding of (i) host factors, such as integration host factor (IHF) and Fis (factor for inversion stimulation), (ii) auxiliary proteins like Xis, and/or (iii) the arm-type domain of integrases (2, 33, 57). Searching for such sites within the 151-bp region of Tn4655 revealed two inverted repeat sequences (the 18-bp sequence [BP 58833 to 58850] having 5-bp inverted repeats with an 8-bp spacer [designated the ACC site] and the 41-bp sequence [BP 58906 to 58946] having 19-bp inverted repeats with a 3-bp spacer [designated the CORE site]) and a putative IHF-binding site (BP 58862 to 58875) (Fig. 4B). Plasmid pMS0471 carried two copies of the 119-bp sequence (BP 58833 to 58951) containing these three sites, and the resolution experiment with this plasmid gave rise to the Kms colonies at a 100% frequency (Fig. 4C). Therefore, we conclude that the 119-bp sequence includes the functional attI site for TnpI.
Since TnpI was structurally categorized as a member of the tyrosine recombinases, we also investigated its site-specific integration activity (Fig. 5). For this purpose, we constructed strain DH5α derivatives harboring the following three plasmids: (i) pUC-tnpI (supplier of TnpI), (ii) R388attKm, a self-transmissible IncW plasmid R388 (59) derivative carrying a Kmr gene together with the 119-bp attI sequence (designated attY) (Fig. 5), and (iii) a pSTV29 derivative carrying the 119-bp attI site or its deletion derivative (designated attX) (Fig. 4D and 5). These strains were mated with strain HB101, and Cmr Smr transconjugants were selected. In contrast to the results of the resolution experiments, use of pMS0491, pMS0492, or pMS0493, each of which carried a deleted mutation in the attI site, still allowed us to detect Cmr Smr transconjugants at a frequency similar to that of pMS0431 carrying intact attI (Fig. 4D). No Cmr Smr transconjugants were obtained when the CORE site was absent in attX (pMS0494). Control experiments using pUC18 instead of pUC-tnpI did not produce any Cmr Smr transconjugants (<10−8). These results indicate that (i) TnpI is able to mediate the site-specific integration reaction and (ii) at least one of the inverted repeat sequences in the CORE site is required for site-specific integration.
Determination of the crossover region.
It is known that tyrosine recombinases generally make a cleavage at the 5′ end of the crossover region (a generally 6- to 8-bp asymmetry sequence) on both top and bottom strands to perform the strand exchange (branch migration) reaction (2, 33, 57). Two heteroduplex strands of the crossover regions are formed between two copies of a core site after the reaction. To identify the crossover region in the attI site, we constructed pMS0477 that had a Kmr gene flanked by the 119-bp attI sequence of Tn4655 and the 119-bp attI-like sequence of pDTG1 that differs from the Tn4655 attI sequence at nine positions (Fig. 6) and performed the resolution experiment described above. The strain DH5α(pMS0477)(pUC-tnpI) gave rise to Kms colonies at a 100% frequency. Sequence determination of 11 resolved products revealed that the hybrid attI formation occurred within the 38-bp sequence (BP 58904 to 58941) around the CORE site and that two kinds of the hybrid attI site were generated: one had the nucleotide C derived from NAH7 (three samples), and the other had the nucleotide T from pDTG1 (eight samples) at the position corresponding to 58925 of NAH7 (Fig. 6B). The sequence data strongly suggested that the crossover region in attI of NAH7 contains the 58,925th nucleotide. These results and the integrative activity of the 101-bp attI derivative (BP 58833 to 58933) on pMS0492 (Fig. 4D) indicate that (i) the TnpI-mediated crossover region is located within the 30-bp sequence (BP 58904 to 58933) in the CORE site (Fig. 4B and 6B) and (ii) one of the strand cleavage/exchange sites is present between BP 58926 and 58933.
DISCUSSION
The complete sequence determination of plasmid NAH7 and its comparison with two other completely sequenced catabolic IncP-9 plasmids helped to elucidate the structure and composition of the IncP-9 backbone. The NAH7 backbone was found to be phylogenetically more closely related to that of pDTG1 than that of pWW0, and this result agrees with the phylogenetic analysis of IncP-9 plasmids (Y. R. Sevastsyanovich and C. M. Thomas, personal communication). The comparison also revealed that the major DNA fragments that carry the catabolic gene clusters of these plasmids are inserted at various positions within the backbone (Fig. 3), suggesting that this backbone carries various DNA fragments that are not really essential to the basic plasmid functions, such as replication, maintenance, and conjugation. This contrasts with the situation in IncP-1 plasmids, in which foreign DNA fragments are inserted mostly at two specific sites in the backbone without interrupting the basic functions of the plasmids (9, 44). Sequence determination of other IncP-9 plasmids will reveal the more definitive IncP-9 backbone.
Tn4655 exhibits a patchwork structure containing portions of pCAR1, Tn4653, and Tn5046, all of which are bracketed by the IRs highly similar to those of Tn4653 (Fig. 1 and 3B). Tn4655 must have been derived from the same ancestor as Tn4653, since the tnpA product of the latter transposon is able to mediate the cointegration reaction of Tn4655 (54). Tn4655 completely lacks the structural homologues of the tnpA and tnpR genes of Tn4653 but instead encodes a site-specific recombination system whose evolutionary origin is most probably the same as that of the system encoded in pCAR1. These findings suggest that different site-specific recombination systems might have been incorporated into an ancestral class II transposon that lacked such a recombination system, leading to the structural diversification of class II transposons. This is compatible with the proposals that the present members of class II transposons might have evolved by the insertion of various site-specific resolution systems into an ancestral element carrying only a tnpA gene and IRs (21, 45, 63).
Notable features of the CORE site are its longer (19-bp) inverted repeats and shorter (3-bp) spacer sequence than those of the so far known core sequences (8- to 13-bp inverted repeats and 6- to 8-bp crossover sequences) (27, 55). Whether the whole 19-bp sequence is required for TnpI binding and whether the crossover region is actually the 3-bp sequence CCT remain to be investigated. However, it is most likely that TnpI mediates its recombination reaction by a mechanism similar to those of known tyrosine recombinases and that the strand exchange reaction occurs within the 30-bp crossover sequence. More detailed biochemical analysis will clarify this plausibility. The accessory sequence of attI, the ACC site, is also a noteworthy feature of the TnpI-mediated recombination system in that it is necessary only for the resolution reaction (Fig. 4). The most extensively studied examples of the accessory sequences are the P and P′ arms of the attP site in the bacteriophage λ DNA, which have five binding sites for the small N-terminal domain of λ integrase (2). Comparison of the secondary structure of TnpI with those of several tyrosine recombinases using a Web-based software, jpred (http://www.compbio.dundee.ac.uk/∼www-jpred/submit.html), revealed that TnpI is similar to arm-type integrases such as those from λ and a conjugative transposon, Tn916 (13), in that each of them has a three-stranded β-sheet as a DNA-binding domain in their N-terminal regions (data not shown) (62). These observations could explain our results, in that (i) the ACC site possibly includes a binding site of the TnpI arm domain and (ii) deletion of this site (pMS0426 in Fig. 4C) might result in the inefficient binding of TnpI to attI, leading to the inability to form a correct synapse for the resolution reaction. Both λ and Tn916 code for a small protein, Xis, which is located next to the integrase and is essential for the excision of both elements (2). Interestingly, a small protein (orf24 product of NAH7 or its homologues) is encoded just upstream of the tnpI gene or its homologues in NAH7, pDTG1 (10), pND6-1 (28), and pCAR1 (29), but we demonstrated that the orf24 product of NAH7 was not necessary for either resolution or integration. Moreover, these orf24-like gene products did not show any significant similarity to the Xis proteins or any proteins in databases (data not shown).
Database searches revealed that the attI-orf24-tnpI sequence is found only on large catabolic plasmids NAH7, pDTG1, pND6-1, and pCAR1 in close proximity to their catabolic gene clusters. The two naphthalene-catabolic plasmids pDTG1 (IncP-9) (10) and pND6-1 (IncP-7) (28, 42) have, in spite of their different plasmid backbones, almost identical 39-kb sequences containing the attI-orf24-tnpI homologues as well as all of the nah operons. The nucleotide sequences outside the attI-like sequences on these two plasmids did not share any homology with each other (data not shown). Furthermore, no clear footprints are observed around the 39-kb sequences, which indicates that they were located on some kind of transposons (data not shown). Based on these findings, we postulate the following hypotheses: (i) that the large nah-containing segments on pDTG1 and pND6-1 might have been incorporated into the present positions by the integration function of the attI-TnpI system and (ii) that such an incorporation event of catabolic genes together with the genes for site-specific recombination systems into a class II transposon might have resulted in the establishment of large catabolic transposons. These ideas are consistent with the observations that tyrosine recombinases are able to translocate large (>20-kb) DNA fragments (e.g., phage genomes and conjugative transposons) from one replicon to the other (2, 13, 33).
Supplementary Material
Acknowledgments
We are grateful to E. L. Madsen (Cornell University) for his kind gift of pDTG1. We also appreciate C. M. Thomas and Y. R. Sevastsyanovich (University of Birmingham) for providing their unpublished information on the IncP-9 plasmids. We also thank M. Nagai (Institute for Environmental Sciences) for his experimental assistance.
This work was carried out under contract with the Ministry of Education, Culture, Sports, Science and Technology, Japan. This work was also supported by a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Agriculture, Forestry, and Fisheries (HC-06-2323), Japan, and by NIH grant number P20 RR 16448 from the Center of Biomedical Research Excellence (COBRE).
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 1988. Current protocols in molecular biology. Wiley, New York, N.Y.
- 2.Azaro, M. A., and A. Landy. 2002. λ integrase and the λ Int family, p. 118-148. In N. L. Craig, R. Craigie, M. Gellert, and A. M. Lambowitz (ed.), Mobile DNA II. ASM Press, Washington, D.C.
- 3.Baum, J. A. 1994. Tn5401, a new class II transposable element from Bacillus thuringiensis. J. Bacteriol. 176:2835-2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bosch, R., E. Garcia-Valdes, and E. R. Moore. 2000. Complete nucleotide sequence and evolutionary significance of a chromosomally encoded naphthalene-degradation lower pathway from Pseudomonas stutzeri AN10. Gene 245:65-74. [DOI] [PubMed] [Google Scholar]
- 5.Bosch, R., E. Garcia-Valdes, and E. R. Moore. 1999. Genetic characterization and evolutionary implications of a chromosomally encoded naphthalene-degradation upper pathway from Pseudomonas stutzeri AN10. Gene 236:149-157. [DOI] [PubMed] [Google Scholar]
- 6.Bosch, R., E. R. Moore, E. Garcia-Valdes, and D. H. Pieper. 1999. NahW, a novel, inducible salicylate hydroxylase involved in mineralization of naphthalene by Pseudomonas stutzeri AN10. J. Bacteriol. 181:2315-2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buell, C. R., V. Joardar, M. Lindeberg, J. Selengut, I. T. Paulsen, M. L. Gwinn, R. J. Dodson, R. T. Deboy, A. S. Durkin, J. F. Kolonay, R. Madupu, S. Daugherty, L. Brinkac, M. J. Beanan, D. H. Haft, W. C. Nelson, T. Davidsen, N. Zafar, L. Zhou, J. Liu, Q. Yuan, H. Khouri, N. Fedorova, B. Tran, D. Russell, K. Berry, T. Utterback, S. E. Van Aken, T. V. Feldblyum, M. D'Ascenzo, W. L. Deng, A. R. Ramos, J. R. Alfano, S. Cartinhour, A. K. Chatterjee, T. P. Delaney, S. G. Lazarowitz, G. B. Martin, D. J. Schneider, X. Tang, C. L. Bender, O. White, C. M. Fraser, and A. Collmer. 2003. The complete genome sequence of the Arabidopsis and tomato pathogen Pseudomonas syringae pv. tomato DC3000. Proc. Natl. Acad. Sci. USA 100:10181-10186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burrus, V., G. Pavlovic, B. Decaris, and G. Guedon. 2002. Conjugative transposons: the tip of the iceberg. Mol. Microbiol. 46:601-610. [DOI] [PubMed] [Google Scholar]
- 9.Dennis, J. J. 2005. The evolution of IncP catabolic plasmids. Curr. Opin. Biotechnol. 16:291-298. [DOI] [PubMed] [Google Scholar]
- 10.Dennis, J. J., and G. J. Zylstra. 2004. Complete sequence and genetic organization of pDTG1, the 83 kilobase naphthalene degradation plasmid from Pseudomonas putida strain NCIB 9816-4. J. Mol. Biol. 341:753-768. [DOI] [PubMed] [Google Scholar]
- 11.Dunn, N. W., and I. C. Gunsalus. 1973. Transmissible plasmid coding early enzymes of naphthalene oxidation in Pseudomonas putida. J. Bacteriol. 114:974-979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eaton, R. W. 1994. Organization and evolution of naphthalene catabolic pathways: sequence of the DNA encoding 2-hydroxychromene-2-carboxylate isomerase and trans-o-hydroxybenzylidenepyruvate hydratase-aldolase from the NAH7 plasmid. J. Bacteriol. 176:7757-7762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franke, A. E., and D. B. Clewell. 1981. Evidence for a chromosome-borne resistance transposon (Tn916) in Streptococcus faecalis that is capable of “conjugal” transfer in the absence of a conjugative plasmid. J. Bacteriol. 145:494-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaillard, M., T. Vallaeys, F. J. Vorhölter, M. Minoia, C. Werlen, V. Sentchilo, A. Pühler, and J. R. van der Meer. 2006. The clc element of Pseudomonas sp. strain B13, a genomic island with various catabolic properties. J. Bacteriol. 188:1999-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Genka, H., Y. Nagata, and M. Tsuda. 2002. Site-specific recombination system encoded by toluene catabolic transposon Tn4651. J. Bacteriol. 184:4757-4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghosal, D., I. S. You, and I. C. Gunsalus. 1987. Nucleotide sequence and expression of gene nahH of plasmid NAH7 and homology with gene xylE of TOL pWWO. Gene 55:19-28. [DOI] [PubMed] [Google Scholar]
- 17.Grainge, I., and M. Jayaram. 1999. The integrase family of recombinase: organization and function of the active site. Mol. Microbiol. 33:449-456. [DOI] [PubMed] [Google Scholar]
- 18.Greated, A., L. Lambertsen, P. A. Williams, and C. M. Thomas. 2002. Complete sequence of the IncP-9 TOL plasmid pWW0 from Pseudomonas putida. Environ. Microbiol. 4:856-871. [DOI] [PubMed] [Google Scholar]
- 19.Greated, A., M. Titok, R. Krasowiak, R. J. Fairclough, and C. M. Thomas. 2000. The replication and stable-inheritance functions of IncP-9 plasmid pM3. Microbiology 146:2249-2258. [DOI] [PubMed] [Google Scholar]
- 20.Grimm, A. C., and C. S. Harwood. 1999. NahY, a catabolic plasmid-encoded receptor required for chemotaxis of Pseudomonas putida to the aromatic hydrocarbon naphthalene. J. Bacteriol. 181:3310-3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grindley, N. D. F. 2002. The movement of Tn3-like elements: transposition and cointegrate resolution, p. 272-302. In N. L. Craig, R. Craigie, M. Gellert, and A. M. Lambowitz (ed.), Mobile DNA II. ASM Press, Washington, D.C.
- 22.Hall, R. M., C. M. Collis, M. J. Kim, S. R. Partridge, G. D. Recchia, and H. W. Stokes. 1999. Mobile gene cassettes and integrons in evolution. Ann. N. Y. Acad. Sci. 870:68-80. [DOI] [PubMed] [Google Scholar]
- 23.Harayama, S., A. Polissi, and M. Rekik. 1991. Divergent evolution of chloroplast-type ferredoxins. FEBS Lett. 285:85-88. [DOI] [PubMed] [Google Scholar]
- 24.Harayama, S., and M. Rekik. 1989. Bacterial aromatic ring-cleavage enzymes are classified into two different gene families. J. Biol. Chem. 264:15328-15333. [PubMed] [Google Scholar]
- 25.Harayama, S., M. Rekik, A. Wasserfallen, and A. Bairoch. 1987. Evolutionary relationships between catabolic pathways for aromatics: conservation of gene order and nucleotide sequences of catechol oxidation genes of pWW0 and NAH7 plasmids. Mol. Gen. Genet. 210:241-247. [DOI] [PubMed] [Google Scholar]
- 26.Lauf, U., C. Müller, and H. Herrmann. 1998. The transposable elements resident on the plasmids of Pseudomonas putida strain H, Tn5501 and Tn5502, are cryptic transposons of the Tn3 family. Mol. Gen. Genet. 259:674-678. [DOI] [PubMed] [Google Scholar]
- 27.Lavigne, J. P., A. C. Vergunst, G. Bourg, and D. O'Callaghan. 2005. The IncP island in the genome of Brucella suis 1330 was acquired by site-specific integration. Infect. Immun. 73:7779-7783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li, W., J. Shi, X. Wang, Y. Han, W. Tong, L. Ma, B. Liu, and B. Cai. 2004. Complete nucleotide sequence and organization of the naphthalene catabolic plasmid pND6-1 from Pseudomonas sp. strain ND6. Gene 336:231-240. [DOI] [PubMed] [Google Scholar]
- 29.Maeda, K., H. Nojiri, M. Shintani, T. Yoshida, H. Habe, and T. Omori. 2003. Complete nucleotide sequence of carbazole/dioxin-degrading plasmid pCAR1 in Pseudomonas resinovorans strain CA10 indicates its mosaicity and the presence of large catabolic transposon Tn4676. J. Mol. Biol. 326:21-33. [DOI] [PubMed] [Google Scholar]
- 30.Mahillon, J., and D. Lereclus. 1988. Structural and functional analysis of Tn4430: identification of an integrase-like protein involved in the co-integrate-resolution process. EMBO J. 7:1515-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinez, B., J. Tomkins, L. P. Wackett, R. Wing, and M. J. Sadowsky. 2001. Complete nucleotide sequence and organization of the atrazine catabolic plasmid pADP-1 from Pseudomonas sp. strain ADP. J. Bacteriol. 183:5684-5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mindlin, S., G. Kholodii, Z. Gorlenko, S. Minakhina, L. Minakhin, E. Kalyaeva, A. Kopteva, M. Petrova, O. Yurieva, and V. Nikiforov. 2001. Mercury resistance transposons of gram-negative environmental bacteria and their classification. Res. Microbiol. 152:811-822. [DOI] [PubMed] [Google Scholar]
- 33.Nash, H. A. 1996. Site-specific recombination: integration, excision, resolution, and inversion of defined DNA segments, p. 2363-2376. In F. C. Neidhardt (ed.), Escherichia coli and Salmonella, 2nd ed., vol. 2. ASM Press, Washington, D.C. [Google Scholar]
- 34.Nelson, K. E., C. Weinel, I. T. Paulsen, R. J. Dodson, H. Hilbert, V. A. Martins dos Santos, D. E. Fouts, S. R. Gill, M. Pop, M. Holmes, L. Brinkac, M. Beanan, R. T. DeBoy, S. Daugherty, J. Kolonay, R. Madupu, W. Nelson, O. White, J. Peterson, H. Khouri, I. Hance, P. Chris Lee, E. Holtzapple, D. Scanlan, K. Tran, A. Moazzez, T. Utterback, M. Rizzo, K. Lee, D. Kosack, D. Moestl, H. Wedler, J. Lauber, D. Stjepandic, J. Hoheisel, M. Straetz, S. Heim, C. Kiewitz, J. A. Eisen, K. N. Timmis, A. Düsterhöft, B. Tümmler, and C. M. Fraser. 2002. Complete genome sequence and comparative analysis of the metabolically versatile Pseudomonas putida KT2440. Environ. Microbiol. 4:799-808. [DOI] [PubMed] [Google Scholar]
- 35.Ogawa, N., A. M. Chakrabarty, and O. Zaborina. 2004. Degradative plasmids, p. 341-376. In B. E. Funnell and G. J. Phillips (ed.), Plasmid biology. ASM Press, Washington, D.C.
- 36.Rutherford, K., J. Parkhill, J. Crook, T. Horsnell, P. Rice, M. A. Rajandream, and B. Barrell. 2000. Artemis: sequence visualization and annotation. Bioinformatics 16:944-945. [DOI] [PubMed] [Google Scholar]
- 37.Schell, M. A. 1983. Cloning and expression in Escherichia coli of the naphthalene degradation genes from plasmid NAH7. J. Bacteriol. 153:822-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schell, M. A. 1986. Homology between nucleotide sequences of promoter regions of nah and sal operons of NAH7 plasmid of Pseudomonas putida. Proc. Natl. Acad. Sci. USA 83:369-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schell, M. A., and P. E. Wender. 1986. Identification of the nahR gene product and nucleotide sequences required for its activation of the sal operon. J. Bacteriol. 166:9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sherratt, D. 1989. Tn3 and related transposable elements: site-specific recombination and transposition, p. 163-184. In D. E. Berg and M. M. Howe (ed.), Mobile DNA. American Society for Microbiology, Washington, D.C.
- 41.Shingler, V., F. C. Franklin, M. Tsuda, D. Holroyd, and M. Bagdasarian. 1989. Molecular analysis of a plasmid-encoded phenol hydroxylase from Pseudomonas CF600. J. Gen. Microbiol. 135:1083-1092. [DOI] [PubMed] [Google Scholar]
- 42.Shintani, M., H. Yano, H. Habe, T. Omori, H. Yamane, M. Tsuda, and H. Nojiri. 2006. Characterization of the replication, maintenance, and transfer features of the IncP-7 plasmid pCAR1, which carries genes involved in carbazole and dioxin degradation. Appl. Environ. Microbiol., in press. [DOI] [PMC free article] [PubMed]
- 43.Smith, M. C., and H. M. Thorpe. 2002. Diversity in the serine recombinases. Mol. Microbiol. 44:299-307. [DOI] [PubMed] [Google Scholar]
- 44.Sota, M., H. Kawasaki, and M. Tsuda. 2003. Structure of haloacetate-catabolic IncP-1β plasmid pUO1 and genetic mobility of its residing haloacetate-catabolic transposon. J. Bacteriol. 185:6741-6745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sota, M., H. Yano, Y. Nagata, Y. Ohtsubo, H. Genka, H. Anbutsu, H. Kawasaki, and M. Tsuda. 2006. Functional analysis of unique class II insertion sequence IS1071. Appl. Environ. Microbiol. 72:291-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Springael, D., and E. M. Top. 2004. Horizontal gene transfer and microbial adaptation to xenobiotics: new types of mobile genetic elements and lessons from ecological studies. Trends Microbiol. 12:53-58. [DOI] [PubMed] [Google Scholar]
- 47.Stover, C. K., X. Q. Pham, A. L. Erwin, S. D. Mizoguchi, P. Warrener, M. J. Hickey, F. S. Brinkman, W. O. Hufnagle, D. J. Kowalik, M. Lagrou, R. L. Garber, L. Goltry, E. Tolentino, S. Westbrock-Wadman, Y. Yuan, L. L. Brody, S. N. Coulter, K. R. Folger, A. Kas, K. Larbig, R. Lim, K. Smith, D. Spencer, G. K. Wong, Z. Wu, I. T. Paulsen, J. Reizer, M. H. Saier, R. E. Hancock, S. Lory, and M. V. Olson. 2000. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406:959-964. [DOI] [PubMed] [Google Scholar]
- 48.Takizawa, N., N. Kaida, S. Torigoe, T. Moritani, T. Sawada, S. Satoh, and H. Kiyohara. 1994. Identification and characterization of genes encoding polycyclic aromatic hydrocarbon dioxygenase and polycyclic aromatic hydrocarbon dihydrodiol dehydrogenase in Pseudomonas putida OUS82. J. Bacteriol. 176:2444-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tark, M., A. Tover, K. Tarassova, R. Tegova, G. Kivi, R. Horak, and M. Kivisaar. 2005. A DNA polymerase V homologue encoded by TOL plasmid pWW0 confers evolutionary fitness on Pseudomonas putida under conditions of environmental stress. J. Bacteriol. 187:5203-5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taylor, L. A., and R. E. Rose. 1988. A correction in the nucleotide sequence of the Tn903 kanamycin resistance determinant in pUC4K. Nucleic Acids Res. 16:358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Top, E. M., Y. Moenne-Loccoz, T. Pembroke, and C. M. Thomas. 2000. Phenotypic traits conferred by plasmids, p. 249-286. In C. M. Thomas (ed.), The horizontal gene pool. Harwood Academic Publishers, Amsterdam, The Netherlands.
- 52.Top, E. M., and D. Springael. 2003. The role of mobile genetic elements in bacterial adaptation to xenobiotic organic compounds. Curr. Opin. Biotechnol. 14:262-269. [DOI] [PubMed] [Google Scholar]
- 53.Toussaint, A., C. Merlin, S. Monchy, M. A. Benotmane, R. Leplae, M. Mergeay, and D. Springael. 2003. The biphenyl- and 4-chlorobiphenyl-catabolic transposon Tn4371, a member of a new family of genomic islands related to IncP and Ti plasmids. Appl. Environ. Microbiol. 69:4837-4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsuda, M., and T. Iino. 1990. Naphthalene degrading genes on plasmid NAH7 are on a defective transposon. Mol. Gen. Genet. 223:33-39. [DOI] [PubMed] [Google Scholar]
- 55.van der Meer, J. R., W. M. de Vos, S. Harayama, and A. J. Zehnder. 1992. Molecular mechanisms of genetic adaptation to xenobiotic compounds. Microbiol. Rev. 56:677-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van der Meer, J. R., and V. Sentchilo. 2003. Genomic islands and the evolution of catabolic pathways in bacteria. Curr. Opin. Biotechnol. 14:248-254. [DOI] [PubMed] [Google Scholar]
- 57.Van Duyne, G. D. 2002. A structural view of tyrosine recombinase site-specific recombination, p. 93-117. In N. L. Craig, R. Craigie, M. Gellert, and A. M. Lambowitz (ed.), Mobile DNA II. ASM Press, Washington, D.C.
- 58.Vedler, E., M. Vahter, and A. Heinaru. 2004. The completely sequenced plasmid pEST4011 contains a novel IncP1 backbone and a catabolic transposon harboring tfd genes for 2,4-dichlorophenoxyacetic acid degradation. J. Bacteriol. 186:7161-7174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ward, J. M., and J. Grinsted. 1982. Physical and genetic analysis of the Inc-W group plasmids R388, Sa, and R7K. Plasmid 7:239-250. [DOI] [PubMed] [Google Scholar]
- 60.Williams, P. A., R. M. Jones, and G. Zylstra. 2004. Genomics of catabolic plasmids, p. 165-195. In J. L. Ramos (ed.), Pseudomonas, vol. 1. Plenum Publishing Corporation, New York, N.Y. [Google Scholar]
- 61.Williams, P. A., and J. R. Sayers. 1994. The evolution of pathways for aromatic hydrocarbon oxidation in Pseudomonas. Biodegradation 5:195-217. [DOI] [PubMed] [Google Scholar]
- 62.Wojciak, J. M., D. Sarkar, A. Landy, and R. T. Clubb. 2002. Arm-site binding by λ-integrase: solution structure and functional characterization of its amino-terminal domain. Proc. Natl. Acad. Sci. USA 99:3434-3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wyndham, R. C., A. E. Cashore, C. H. Nakatsu, and M. C. Peel. 1994. Catabolic transposons. Biodegradation 5:323-342. [DOI] [PubMed] [Google Scholar]
- 64.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]
- 65.Yen, K. M., and I. C. Gunsalus. 1982. Plasmid gene organization: naphthalene/salicylate oxidation. Proc. Natl. Acad. Sci. USA 79:874-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yen, K. M., and I. C. Gunsalus. 1985. Regulation of naphthalene catabolic genes of plasmid NAH7. J. Bacteriol. 162:1008-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yen, K. M., and C. M. Serdar. 1988. Genetics of naphthalene catabolism in pseudomonads. Crit. Rev. Microbiol. 15:247-268. [DOI] [PubMed] [Google Scholar]
- 68.You, I. S., D. Ghosal, and I. C. Gunsalus. 1988. Nucleotide sequence of plasmid NAH7 gene nahR and DNA binding of the nahR product. J. Bacteriol. 170:5409-5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.You, I. S., and I. C. Gunsalus. 1986. Regulation of the nah and sal operons of plasmid NAH7: evidence for a new function in nahR. Biochem. Biophys. Res. Commun. 141:986-992. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.