Abstract
Endoreduplication is an unusual form of cell cycle in which rounds of DNA synthesis repeat in the absence of intervening mitoses. How G1/S cyclin-dependent kinase (Cdk) activity is regulated during the mammalian endocycle is poorly understood. We show here that expression of the G1/S Cdk inhibitor p57Kip2 is induced coincidentally with the transition to the endocycle in trophoblast giant cells. Kip2 mRNA is constitutively expressed during subsequent endocycles, but the protein level fluctuates. In trophoblast giant cells synchronized for the first few endocycles, the p57Kip2 protein accumulates only at the end of S-phase and then rapidly disappears a few hours before the onset of the next S-phase. The protein becomes stabilized by mutation of a C-terminal Cdk phosphorylation site. As a consequence, introduction of this stable form of p57Kip2 into giant cells blocks S-phase entry. These data imply that p57Kip2 is subject to phosphorylation-dependent turnover. Surprisingly, although this occurs in endoreduplicating giant cells, p57Kip2 is stable when ectopically expressed in proliferating trophoblast cells, indicating that these cells lack the mechanism for protein targeting and/or degradation. These data show that the appearance of p57Kip2 punctuates the completion of DNA replication, whereas its turnover is subsequently required to initiate the next round of endoreduplication in trophoblast giant cells. Cyclical expression of a Cdk inhibitor, by terminating G1/S Cdk activity, may help promote the resetting of DNA replication machinery.
INTRODUCTION
The cell cycle is orchestrated by cyclin/cyclin-dependent kinase (Cdk) complexes that function at each phase of the cycle (Sherr, 1993; Hartwell and Kastan, 1994). Different cyclin/Cdk complexes are required at each step: in higher eukaryotes cyclin A/Cdk2 and E/Cdk2 are essential for G1/S transition, whereas cyclin B/Cdc2 is required for mitosis (Sherr, 1993). Progression through a complete cycle depends on stereotypic activation and repression of cyclin/Cdk activities, events that are closely coupled. For example, cyclin E/Cdk2 is essential for activation of cyclin B/Cdc2 later in the cell cycle (Guadagno and Newport, 1996). In turn, G2 activity diminishes expression of G1 cyclins (Amon et al., 1993). A period of low G1/S-phase Cdk activity is required for the correct reestablishment of origins of replication, essential for the maintenance of genome stability (Hartwell and Kastan, 1994). Cdks are modulated by two classes of inhibitors related to the p16INK4 and p21CIP1 proteins (Sherr and Roberts, 1995). The p21 class of Cdk inhibitors includes p21Cip1, p27Kip1, and p57Kip2, which all inhibit cyclin A- and E-associated Cdks and therefore are thought to regulate the G1/S transition and completion of S-phase (Harper et al., 1993; Toyoshima and Hunter, 1994; Lee et al., 1995).
As most cells differentiate during development, they begin to express Cdk inhibitors to arrest cell cycle progression. However, terminal differentiation of some cell types is not associated with cell cycle exit but rather with endoreduplication, a process of repeated rounds of DNA synthesis occurring in the absence of intervening mitoses, resulting in polyploid cells (Edgar and Lehner, 1996; Zybina and Zybina, 1996). Although endoreduplication is unusual mechanistically, in that it bypasses several controls that are fundamental to the regulation of the mitotic cycle, it occurs commonly during the normal development of many cell lineages. In mammals, for example, cardiomyocytes, hepatocytes, megakaryocytes, and trophoblast giant cells all endoreduplicate during terminal cell differentitation (Zybina and Zybina, 1996). It is particularly striking in trophoblast giant cells of the rodent placenta in which the cells can reach ploidies of >1000N, with DNA maintained in a polytene arrangement (Varmuza et al., 1988; Zybina and Zybina, 1996). The transition from the mitotic cycle to the endocycle in trophoblast cells occurs during the G2 phase of a “normal” mitotic cycle. Cyclin B expression is initially induced at the G2 phase (MacAuley et al., 1998; Nakayama et al., 1998), but cyclin B fails to activate Cdk1, and therefore, mitosis is not initiated (MacAuley et al., 1998). Pulses of cyclin A- and E-associated Cdk activities occur before and during each S-phase during the subsequent endocycles (MacAuley et al., 1998). The drop in these activities after each S-phase is presumably required for correct reinitiation of origins of replication, but how these fluctuating levels are achieved during the mammalian endocycle is not understood. We have found previously that the fluctuations in cyclin E levels are less dramatic than the changes in cyclin E-associated kinase activity during the normal endocycle (MacAuley et al., 1998), and that endoreduplication continues even when cyclin E levels are constitutively high (Wang et al., 1999). These data suggest that other factors, besides cyclin abundance, regulate the timing of G1/S Cdk activity during the endocycle.
MATERIALS AND METHODS
Rcho-1 Cell Culture and Transfection
Rcho-1 cells (Faria and Soares, 1991) were cultured as described previously (Cross et al., 1995; MacAuley et al., 1998; Nakayama et al., 1998). Two days after plating trypsin-sensitive “stem” cells at a 1:4 dilution, the cultures reached confluency and were retrypsinized. The cells not removed by trypsin were committed to giant cell fate and are referred to as “day 0” Rcho-1 giant cells (Cross et al., 1995; MacAuley et al., 1998). Giant cells were cultured in supplemented NCTC-135 media (Cross et al., 1995; MacAuley et al., 1998).
Rcho-1 cells were transfected using LipofectAMINE (Life Technologies, Gaithersburg, MD) (Cross et al., 1995). For Kip2 overexpression experiments, cells were transiently cotransfected with a cytomegalovirus promoter expression vector encoding FLAG epitope-tagged wild type (Lee et al., 1995), mutant forms of p57Kip2, or an empty vector (pcDNA-1), plus a β-actin promoter/LacZ vector. DNA synthesis was assessed either by labeling cells for 3 h in media containing 1 μCi/ml [3H]thymidine; subsequently cells were fixed and stained lightly with 5-bromo-4-chloro-3-indolyl β-d-galactopyranoside and then dipped in emulsion and subjected to autoradiography. Alternatively, cells were pulse labeled with bromodeoxyuridine (BrdU) before fixation. BrdU and β-galactosidase (β-Gal) immunoreactivity were observed using indirect immunofluorescence.
mRNA and Protein Analysis
For mRNA blot analysis, 25 μg of total RNA, prepared from tissue or cells, were run on 4-morpholinepropanesulfonic acid/formaldehyde gels, transferred by capillary blotting, UV cross-linked onto nylon membranes (GeneScreen Plus; DuPont New England Nuclear, Boston, MA), and hybridized with 32P-labeled probes. Probes were labeled by random priming of cDNAs for rat glyceraldehyde-3-phosphate dehydrogenase (Fort et al., 1985), murine Cip1 (El-Deiry et al., 1993), Kip2 (Lee et al., 1995), Kip1 (Polyak et al., 1994), and PL-I (Colosi et al., 1987). For immunoblot analysis Rcho-1 stem day 2 and 4 giant cells were washed in PBS and lysed in radioimmunoprecipitation assay buffer (in PBS: 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 1 mM phenylmethylsulfonyl fluoride, 1 mM sodium orthovanadate, and protease inhibitors [1 mg/ml aprotonin, leupeptin, and pepstatin]). Proteins were run on SDS-PAGE gels and transferred to a polyvinylidene difluoride membrane (Schleicher & Schuell, Keene, NH). p57Kip2 was detected using a rabbit polyclonal antibody generated against amino acids 305–324 of mouse p57Kip2 (p57 M-20; Santa Cruz Biotechnology, Santa Cruz, CA) followed by anti-rabbit immunoglobulin G conjugated to horseradish peroxidase (Amersham, Arlington Heights, IL) and an ECL development kit (Amersham) according to the manufacturer's instructions.
For mRNA in situ hybridization experiments, embryonic day 8.5 (E8.5) conceptuses were collected in PBS, fixed in 4% paraformaldehyde, embedded in paraffin, and sectioned. Adjacent sections were hybridized with different 33P-labeled riboprobes (Millen and Hui, 1996). Sections were exposed for 2–6 wk at 4°C in Eastman Kodak (Rochester, NY) NTB-2 emulsion. Immunolocalization of p57Kip2 was performed on cryosections of E8.5 conceptuses fixed in Carnoy's solution. Sections were incubated for 1 h with a 1:100 dilution of an antibody to p57Kip2, followed by 1 h with an FITC-conjugated secondary antibody (Sigma, St. Louis, MO). DNA was stained with bisbenzimide.
BrdU Pulse Labeling
Naturally mated female CD-1 mice were injected through the tail vein at different days postcoitum with BrdU (100 ng/g of body weight) dissolved in sterile saline. Conceptuses were harvested 1 h later, fixed in Carnoy's solution, embedded in OCT compound (Miles, Elkhart, IN), and cryosectioned. Sections were immunostained as above, except a biotintylated anti-BrdU polyclonal antibody (1:000 dilution; Zymed, San Francisco, CA) and an avidin–TRITC-conjugated secondary antibody (Sigma) were included in the incubations. At least 100 giant cells were scored for p57Kip2 and BrdU immunoreactivity in three conceptuses. Cultured Rcho-1 cells were pulse labeled for 1–3 h with BrdU (3 μg/ml), fixed in Carnoy's solution, and immunostained for BrdU and p57Kip2. At least 100 Rcho-1 cell nuclei were scored for each time point.
p57Kip2 Expression Vectors
Oligonucleotide-mediated, site-directed mutagenesis was used to generate point mutations in the Kip2 cDNA using a kit from Promega (Madison, WI). p57Kip2-CC, containing point mutations in the cyclin and Cdk binding sites, was made using the oligonucleotides 5′-CCCGAAGGCGCTAGCGCAGGCGCTGCTACG-3′ and 5′-CACATCCTGCTGGGCGTTGGCGTCCCAG-3′. p57Kip2-T, containing a mutation in the TPRK motif, was made using the oligonucleotide 5′-CAGACGTTTCCGCGGGACCTGCTCCAC-3′. The green fluorescent protein (GFP) fusion proteins were made by subcloning the p57Kip2 cassette into the vector pEGFP-C1 (Clontech, Cambridge, United Kingdom). All constructs were confirmed by DNA sequencing.
RESULTS
Up-Regulation of p57Kip2 Expression during Trophoblast Giant Cell Differentiation
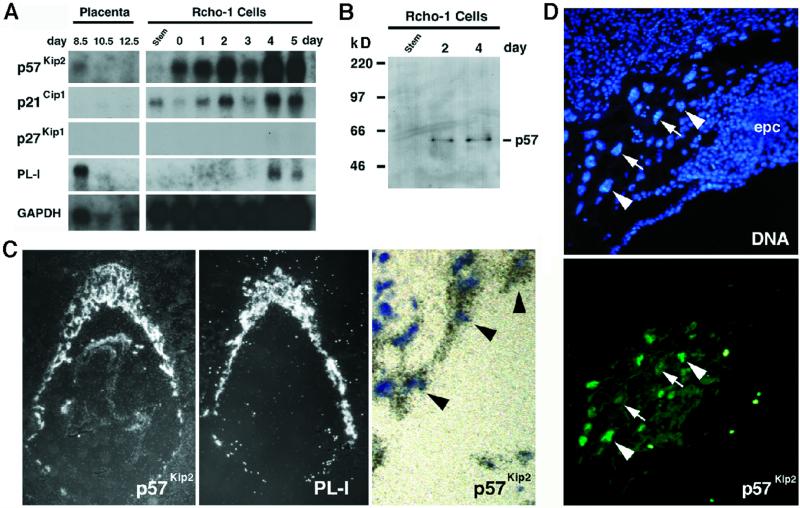
In surveying the expression of cell cycle regulators during the endocycle in trophoblast giant cells, we discovered that transcripts corresponding to Kip2 are strongly expressed in the placenta (Figure 1A). Interestingly, Kip2 mRNA is expressed specifically in trophoblast giant cells but not in their precursors in the ectoplacental cone (Figure 1C). This finding was at first paradoxical, because p57Kip2 is a G1/S Cdk inhibitor, yet the endocycle is, in essence, simply a repetition of G1/S phases. To examine p57Kip2 expression in detail, RNA was harvested from Rcho-1 cells, a cell line that can differentiate into trophoblast giant cells (Faria and Soares, 1991). By differential sensitivity to trypsin, these cells can be segregated into replicating cells and postmitotic cells that endoreduplicate in culture (MacAuley et al., 1998; Nakayama et al., 1998). Kip2 mRNA transcripts were undetectable in proliferating Rcho-1 cells but were abundant in cells that had committed to giant cell differentiation (Figure 1A), consistent with its restricted expression pattern in vivo (Figure 1C). Kip2 mRNA appears to be expressed at similar levels throughout the endocycle. First, the Kip2 mRNA hybridization signal is detectable at similar levels in all giant cells surrounding the conceptus (Figure 1C), even though these cells are at different stages of the endocycle (see Figure 2). Second, the mRNA is present at similar levels in Rcho-1 giant cells over several days of culture (Figure 1A), even though these cultures are synchronized and reflect different stages of the endocycle (MacAuley et al., 1998).
Figure 1.
Expression of the Cdk-inhibitor p57Kip2 in trophoblast giant cells. (A) Northern blot analysis of Cip1, Kip1, and Kip2 expression. RNA from placentas and proliferating stem and differentiating Rcho-1 giant cells was isolated at the indicated times during development, transferred to filters, and hybridized with radiolabeled cDNA probes. Placental lactogen-I (PL-I) is exclusively expressed in trophoblast giant cells. Filters were rehybridized with a glyceraldehyde-3-phosphate dehydrogenase probe to control for RNA loading. (B) Protein immunoblot of p57Kip2. Extracts were prepared from Rcho-1 stem as well as day 2 and 4 giant cells and subjected to immunoblot analysis. A single band migrating at 57 kDa was recognized in trophoblast giant cell extracts. (C) Localization of Kip2 mRNA by in situ hybridization of E8.5 mouse conceptuses. Silver grains, visualized under dark-field microscopy, localized to trophoblast giant cells, as well as specific regions of the embryo. An adjacent section was hybridized with an probe for PL-I, a marker of trophoblast giant cells. Under high-power light microscopy, (far right panel), note a single layer of trophoblast giant cells (black arrowheads), distinguishable by their large nuclei, that all contain silver grains. (D) p57Kip2 protein localization to trophoblast giant cells. Immunolocalization of p57Kip2 protein in trophoblast cells. Unlike the Kip2 mRNA, which was present in all trophoblast giant cells, the protein was detectable in only a subset of giant cell nuclei (arrowheads indicate positive cells, whereas arrows indicate negative cells). In addition, p57Kip2 protein was undetectable in trophoblast cells of the ectoplacental cone (epc).
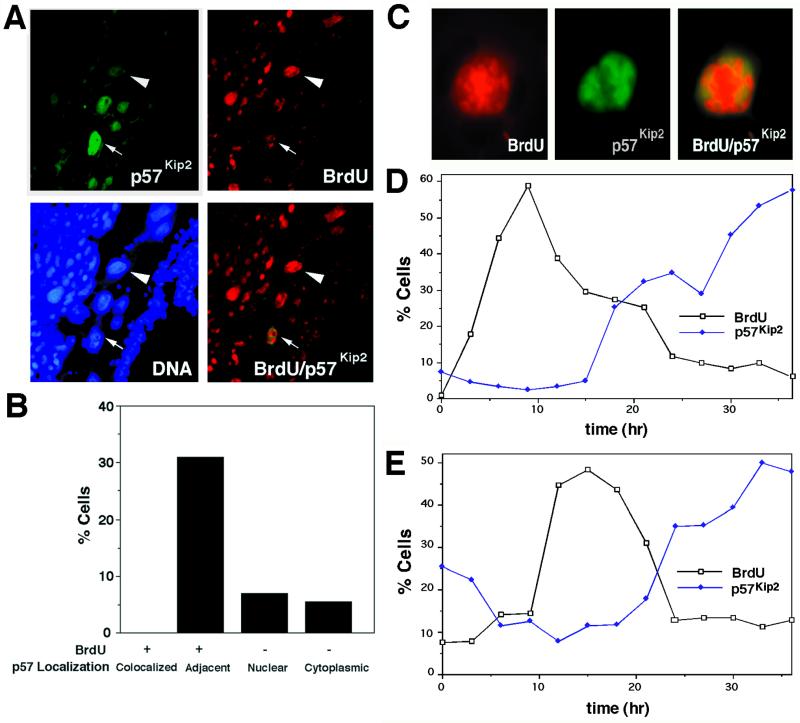
Figure 2.
Endocycle phase-dependent expression of p57Kip2 protein in Rcho-1 giant cells. (A and B) Localization of p57Kip2 and BrdU in trophoblast giant cells visualized by indirect immunofluorescence. Pregnant female mice were injected with BrdU on day 7.5 of gestation, and conceptuses were harvested 1 h later. Trophoblast giant cells with different expression patterns were observed: cells that had incorporated BrdU (red, arrowhead) but were p57Kip2-negative, p57Kip2-positive (green) but BrdU-negative cells, and nuclei in which BrdU- and p57Kip2-positive patches were localized in adjacent regions of the nucleus (red and green, arrow). (C) DNA synthesis and reaccumulation of p57Kip2 occur in exclusive nuclear domains. Rcho-1 giant cells were pulse labeled with BrdU for 2 h before immunostaining. Note that the BrdU-positive region is localized to the central region of the nucleus, whereas p57Kip2 is peripherally localized in the same nucleus. (D and E) Time course of p57Kip2 expression during the endocycle. (D) Rcho-1 stem cells were grown on coverslips for 48 h and then trypsinized, to leave only newly differentiated giant cells, and treated with mimosine (to arrest S-phase) for two 12-h intervals, separated by 12 h, in medium containing 500 μM mimosine. (E) Synchronized giant cells were selected by trypsinization 36 h after seeding of stem cells. Samples were processed every 3 h for 36 h, which first involved pulse labeling with BrdU and then processing for p57Kip2 and BrdU immunostaining.
On immunoblots using a p57Kip2-specific antibody, a single 57-kDa band was detected in extracts from Rcho-1 giant cells but not proliferating cells (Figure 1B). p57Kip2 abundance appeared to vary over the time course of Rcho-1 differentiation (our unpublished results), suggesting that the p57Kip2 protein might vary independently of the mRNA. Using indirect immunofluorescence on histological sections of E8.5 conceptuses, the p57Kip2 protein was readily detectable in giant cells, but, in contrast to Kip2 transcripts, the protein was localized to only a subset of giant cells (Figure 1D). Counting cells from several histological sections revealed that only 39% of giant cells showed the expected nuclear p57Kip2 localization, 6% showed strictly cytoplasmic localization, and protein was undetectable in 55% of giant cells. Thus, the p57Kip2 protein is regulated independently of its transcription in trophoblast giant cells.
The Levels of p57Kip2 Protein Fluctuate during the Endocycle
To investigate the spatial regulation of p57Kip2 during the endocycle, pregnant mice were injected with BrdU to label trophoblast giant cells in S-phase. At E7.5, ∼42% of giant cells were BrdU-positive, but BrdU and p57Kip2 immunoreactivities were never colocalized (Figure 2, A and B). In some cells, only a fraction of the nucleus was labeled with BrdU (Figure 2C), because of the fact that the labeling period was only 1 h, whereas the S-phase of the endocycle lasts ∼10 h (MacAuley et al., 1998). In some of these cells, BrdU and p57Kip2 immunoreactivities were present in the same nucleus, although they appeared in mutually exclusive nuclear domains (Figure 2, A and C). To determine when p57Kip2 disappears from the nucleus relative to the onset of S-phase, Rcho-1 giant cells were synchronized by treatment with the DNA synthesis inhibitor mimosine. After release from the mimosine block, the cells entered an S-phase that peaked at 9 h and was completed by 15 h (Figure 2D). The fraction of cells expressing p57Kip2 protein was low at the early time points but then increased significantly starting in late S-phase and peaking during the following gap phase (Figure 2D). To ensure that the results of the synchronization experiment were not influenced by the use of mimosine, we performed experiments on untreated cells. Resistance to trypsinization was used to select newly committed Rcho-1 giant cells that synchronously enter the first and second endocycles (MacAuley et al., 1998). The results obtained using this protocol also showed that nuclear p57Kip2 protein expression increases in late S-phase (Figure 2E). In addition, because S-phase in this experiment began only 10 h after the start of the time course, we were able to observe the timing of p57Kip2 expression (before S-phase entry. This analysis showed that p57Kip2 protein disappears between 4 and 6 h before the onset of S-phase. We conclude that those giant cells that show immunoreactive BrdU and p57Kip2 in adjacent nuclear domains must be in late S-phase, with the p57Kip2-positive domain likely representing a region in which DNA synthesis was already completed (Figure 2C). We were unable to extend the observations in Rcho-1 cells beyond the first few endocycles, because the cells lose their synchrony (MacAuley et al., 1998). It is likely, however, that the time course of changes in p57Kip2 localization can be extrapolated to subsequent endocycles, because p57Kip2 is not constitutively expressed in giant cells in vivo (Figure 2A), when the cells are known to have higher ploidies (Zybina and Zybina, 1996). In addition, it is clear that these higher ploidy cells in vivo are p57Kip2-negative during S-phase (Figure 2A).
p57Kip2 Turnover is Dependent on Cyclin/Cdk Association
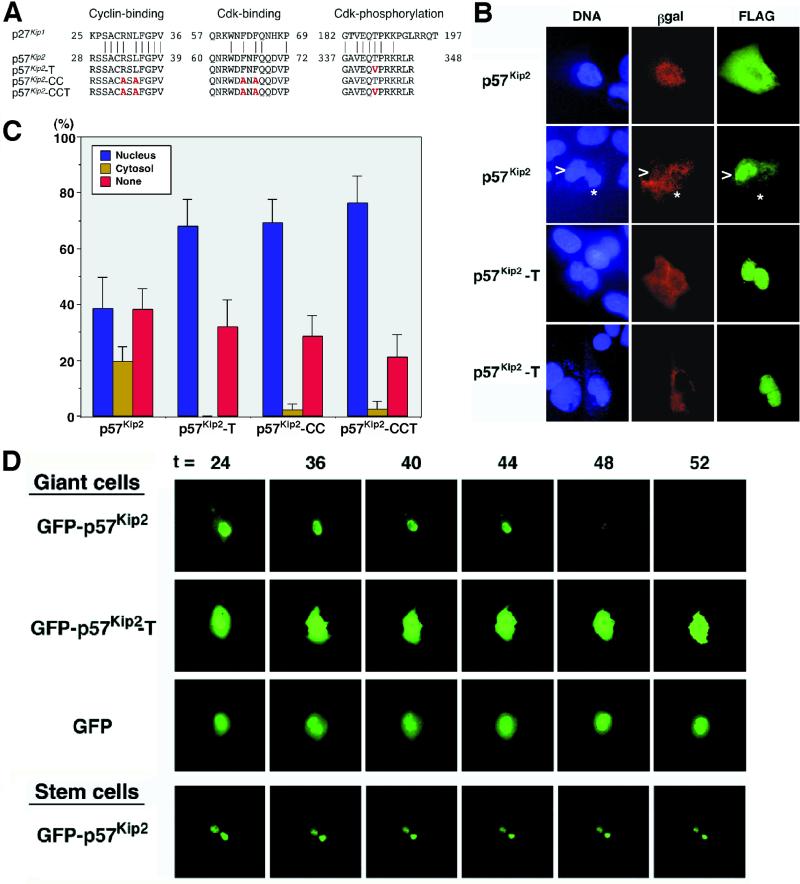
The turnover of p27Kip1 is suppressed by point mutations in the Cdk and cyclin binding domains that inactivate the Cdk inhibitory activity, as well as a C-terminal consensus Cdk phosphorylation site (TPKK amino acid motif) that is linked to targeting the protein for degradation (Vlach et al., 1997). Similar mutations were introduced into p57Kip2 (Figure 3A). To test the effects of the mutations, the mutant proteins were transfected into Rcho-1 giant cells. Transfection with the wild-type p57Kip2 resulted in ∼35–40% of cells showing nuclear localized protein, which was similar to the distribution of the endogenous protein (our unpublished data). Notably, the fraction of cells transfected with wild-type p57Kip2 protein showing cytoplasmic localization was substantially higher than is typically observed for the endogenous protein (20 vs. <5%). This presumably is an artifact of overexpression attributable to transfection. In comparison with the wild-type protein, a mutant protein containing substitutions in the cyclin and Cdk binding domains (p57Kip2-CC) accumulated in the nucleus of transfected cells, implying that cyclin/Cdk binding is essential for loss of p57Kip2 protein from the nucleus (Figure 3C). The increase in nuclear immunoreactivity was balanced by a decrease in the number of immunonegative cells (percent none) and cells showing cytoplasmic localization (percent cytosol) of the FLAG-tagged protein. Mutation of the C-terminal TPRK motif (p57Kip2-T) similarly resulted in an increase in the number of cells showing nuclear localization in transfected giant cells (Figure 3, B and C).
Figure 3.
Stabilized mutants of p57Kip2. (A) Amino acid sequence alignment of p27Kip1, p57Kip2, and mutant proteins. Residues substituted by site-directed mutagenesis in the cyclin binding, Cdk binding, and Cdk phosphorylation domains are indicated by red characters. (B and C) Expression patterns of p57Kip2 and mutant proteins in transfected Rcho-1 stem and giant cells. β-Actin promoter/LacZ vector and FLAG-tagged p57Kip2 expression vector (1:5 ratio) were cotransfected into Rcho-1 cells. After 2 d the expressed proteins were detected by anti-β-Gal and anti-FLAG indirect immunofluorescence. At least 100 β-Gal-positive cells were counted for each transfection experiment, and the data are pooled from at least three different experiments. Representative cells are shown in B. Note that the p57Kip2-T, p57Kip2-CC, and p57Kip2-CCT proteins were stabilized compared with wild-type protein and therefore accumulated in the nucleus. (D) Degradation of GFP-p57Kip2 fusion protein in Rcho-1 giant cells. Trypsin-selected Rcho-1 giant cells (day 0) and stem cells were transfected with the GFP fusion vectors. Groups of cells were photographed from 16 h after trypsin selection, every 4 h, for up to 72 h. Representative cells are shown. In giant cells, the nuclear GFP-p57Kip2 signal rapidly declined between 44 and 48 h, before onset of the next S-phase, whereas GFP and GFP-p57Kip2-T were detected throughout this period.
The most likely explanation for the shift in localization pattern is that the mutant proteins have become stabilized, because the increase in nuclear localization was balanced by a decrease in the both the percent none and the percent cytosol staining patterns. If, by contrast, the mutation affected intracellular (nuclear to cytoplasm) trafficking of p57Kip2, then the percent none should not change. To confirm the interpretation of these experiments, we studied the expression of p57Kip2 within individual cells in real time using a GFP-p57Kip2 fusion protein in Rcho-1 giant cells. Cells were transfected in mid-S-phase and observed every 4 h through the time of the next expected S-phase (∼48 h later). The GFP-p57Kip2 protein was localized to the nucleus of giant cells early in the time course. We subsequently observed a rapid loss of GFP-p57Kip2 throughout the cells between 44 and 48 h, whereas GFP alone was stable through this period (Figure 3D). Expression of the protein reappears 18–24 h later (our unpublished results). The similarity of this time course to that of the endogenous protein (Figure 2E) indicated that the GFP fusion did not affect the regulation of p57Kip2 levels. Together the data indicate that p57Kip2 protein is subject to turnover during the endocycle and is degraded before the onset of S-phase. In contrast to the wild-type protein, GFP-p57Kip2-T was stable throughout the time course (Figure 3D), and levels did not decline even up to 24 h after the decline in the wild-type protein (our unpublished results).
A Stabilized p57Kip2 Mutant Blocks Endocycle Progression in Giant Cells
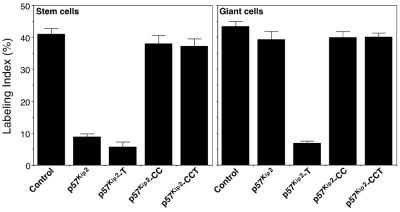
To determine whether degradation of p57Kip2 is essential to proceed through the endocycle, we tested whether expression of the stabilized p57Kip2-T mutant protein affected the progression through the endocycle by pulse labeling cells with BrdU. Whereas overexpression of the wild-type protein had no effect on S-phase progression, the stabilized p57Kip2-T protein functioned as a potent inhibitor to S-phase entry in transfected giant cells (Figure 4). Further mutation of the Cdk and cyclin binding domains (p57Kip2-CCT mutant) abrogated this inhibitory activity (Figure 4). These data show that the turnover of p57Kip2 is essential for progression through the endocycle.
Figure 4.
Cyclic degradation of p57Kip2 is essential for endocycle progression. Cells cotransfected for 48 h with the indicated vectors plus β-actin/LacZ vector were pulse labeled with BrdU for 2 h and then subjected to immunostaining for β-Gal and BrdU. The results indicate the fraction of BrdU-positive cells among transfected β-Gal-positive cells. Note that the stabilized p57Kip2-T mutant, but not wild-type p57Kip2, inhibits DNA synthesis in Rcho-1 giant cells.
Targeted Turnover of p57Kip2 Occurs in Endocycling but Not Proliferating Cells
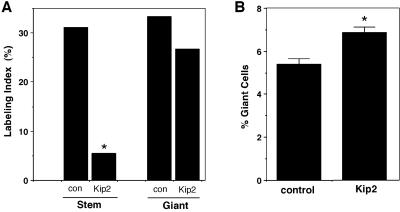
The expression of p57Kip2 has not been previously documented in cycling cells. Likewise, we found that Kip2 transcripts appear in the trophoblast cell lineage only after the cells commit to the endocycle. As a result, it is not clear whether p57Kip2 protein turnover can occur in all cycling cells or rather whether the mechanisms involved are restricted to endocycling cells. To address this, we ectopically expressed Kip2 by transfection of Rcho-1 stem cells. Transfection of giant cells, which already express the endogenous gene, had no effect on the ability of giant cells to enter S-phase (Figure 5A). This result is consistent with the fact that the proportion of transfected cells showing nuclear staining of the epitope-tagged transfected protein was similar to the fraction of cells immunopositive for the endogenous protein (Figure 2), indicating that the capacity for degradation is not exceeded by transfection. In contrast to giant cells, however, ectopic expression of Kip2 in Rcho-1 stem cells caused a significant decrease in the number of cells undergoing DNA synthesis, suggesting that the G1/S transition was effectively blocked in these cells (Figure 5A). This would be expected if p57Kip2 is stable in the cells. To test this directly, Rcho-1 stem cells were transfected with GFP-p57Kip2 and monitorred every 4 h. In contrast to giant cells, the GFP-p57Kip2 signals were persistent for up to 3 d of observation (Figure 3). Therefore, the ability to differentially regulate p57Kip2 protein levels is acquired only at the time of commitment to the giant cell fate. We also noted that the number of giant cells that differentiate after transfection was significantly higher in Kip2-transfected stem cell cultures compared with control transfectants (Figure 5B). In contrast to p57Kip2, transfection of Rcho-1 stem cells with p21Cip1, although having a similar effect to inhibit S-phase progression, did not increase the rate of giant cell differentiation (our unpublished results). Together these data indicate that ectopic expression of Kip2 in proliferating trophoblast cells results in either cell cycle arrest, because of the inability to degrade the protein, or giant cell differentiation.
Figure 5.
Ectopic expression of Kip2 in proliferating Rcho-1 stem cells blocks DNA synthesis and promotes giant cell differentiation. (A) DNA synthesis in Rcho-1 cells transfected with Kip2. Rcho-1 stem cells, which do not normally express Kip2 mRNA, and giant cells were transfected with a Kip2 expression vector. Transfected cells were identified by cotransfection with a β-actin promoter/LacZ vector. After 2 d the cells were labeled with [3H]thymidine for 3 h and then subjected to 5-bromo-4-chloro-3-indolyl β-d-galactopyranoside staining and autoradiography. The labeling index of Kip2-transfected stem cells was significantly reduced (*, p < 0.05). Conversely, overexpression of Kip2 in Rcho-1 giant cells did not significantly reduce the labeling index. (B) Differentiation of Rcho-1 stem cells after Kip2 transfection. Rcho-1 stem cells transfected with a control vector differentiated into giant cells at a frequency of ∼5%, which is similar to nontransfected cells (Cross et al., 1995; MacAuley et al., 1998; Nakayama et al., 1998). Rcho-1 stem cells transfected with Kip2 differentiated into giant cells at a significantly higher rate.
DISCUSSION
Our results show that p57Kip2 expression is regulated during trophoblast cell development both through induction of the Kip2 mRNA upon differentiation and, second, by acquisition of a mechanism in differentiated cells for inducing p57Kip2 protein turnover before the G1/S transition of the endocycle. Compared with other Cdk inhibitors, p57Kip2 has a more restricted pattern of expression and specific functions in development. Kip2 mutant mice show cell differentiation defects in muscle and cartilage, as well as early embryonic mortality (Yan et al., 1997; Zhang et al., 1997). The cause of the embryonic mortality has not been described. However, embryonic mortality is usually due to placental or cardiovascular developmental defects (Cross et al., 1994; Copp, 1995). A role for p57Kip2 in trophoblast cell differentiation was suggested by the fact that p57Kip2 can promote giant cell differentiation when ectopically expressed in proliferating precursor cells (present study). However, trophoblast giant cells are reported to be present in p57Kip2 and p27Kip1/p57Kip2 mutants, indicating that p57Kip2 may not be essential for their formation (Zhang et al., 1998), although their ploidy and cell cycle kinetics have not been assessed.
The immunostaining experiments showed that trophoblast giant cells display one of three patterns of p57Kip2 localization. A large fraction of cells do not express the protein, representing those cells in the gap phase between endocycle S-phases. A small proportion (≤5%) show cytoplasmic staining, whereas most of the p57Kip2-expressing cells show nuclear localization. Mutation of the C-terminal Cdk phosporylation site resulted in a shift in the distribution of p57Kip2 localization in the transfection experiments, with increasing frequency of nuclear and decreasing frequency of immunonegative and cytoplasmic localization. Although it is possible that this mutation alters the nuclear to cytoplasmic (or vice versa) trafficking of the protein, this is not the most likely explanation for the data given the weight of the evidence. First, we observed considerable evidence that the protein disappears from cells rather than simply being transported between compartments. For example, 35–40% of randomly cycling giant cells do not stain in the cytoplasm or nucleus for the endogenous p57Kip2 protein or for the transfected FLAG-p57Kip2 protein, indicating that the protein levels decline. In support of this, we have observed in pulse–chase experiments that the half-life of the protein is shorter during S-phase (<2 h) compared with during the subsequent gap phase when steady-state protein levels are higher (>10 h; our unpublished data). The loss of GFP-p57Kip2 signal in live cells also demonstrates, in real time, the “turnover model.” Second, there is little evidence for nuclear-to-cytoplasmic trafficking that results in export of p57Kip2 from the nucleus before the onset of S-phase. By contrast, in time course experiments, cytoplasmic staining of the endogenous protein tends to appear slightly in advance of the nuclear localization during late S-phase (our unpublished data), suggesting that the cytoplasmic pool may represent the newly synthesized protein not yet localized to the nucleus. However, only ≤5% of giant cells show cytoplasmic p57Kip2 localization of the endogenous protein; therefore, the limited size of this population precludes a precise description of the sequence of events. A distinct “cytoplasmic only” pool was not readily observable in the GFP-p57Kip2 experiments.
Levels of p27Kip1 are regulated by changes in protein turnover and rate of translation during the mitotic cell cycle (Hengst and Reed, 1996). Degradation of p27Kip1 requires the ubiquitin–proteasome pathway and occurs after binding to cyclin E/Cdk2, which phosphorylates a C-terminal TPKK site (Pagano et al., 1995; Sheaff et al., 1997; Vlach et al., 1997; Montagnoli et al., 1999). This phosphorylation site is conserved in p57Kip2, and, therefore, it seems likely that p57Kip2 degradation occurs by a similar general mechanism. Our results showing that mutation of the C-terminal TPRK site results in protein stabilization are consistent with this hypothesis. Furthermore, inhibition of proteasome activity stabilizes the p57Kip2 protein in osteoblast cells (Urano et al., 1999). The Cdk complex that phosphorylates p57Kip2 and targets it for degradation is unknown, although evidence suggests that it is not cyclin E/Cdk, in trophoblast giant cells at least. First, proliferating trophoblast cells lack the ability to target the degradation of p57Kip2, even though cyclin E-associated kinase activity is readily detected in these cells (MacAuley et al., 1998). Second, cyclin E abundance and its associated kinase activity increase only a few hours before the onset of S-phase during the endocycle (MacAuley et al., 1998). Therefore, the demise of the p57Kip2 protein appears to occur before the increase in cyclin E-associated kinase activity rather than the reverse. Finally, p57Kip2 begins to accumulate first in subregions of giant cell nuclei in late S-phase, even though cyclin E expression persists to the end of S-phase (MacAuley et al., 1998).
Progression through both the mitotic cell cycle and the endocycle is dependent on periodic episodes of cyclin E/Cdk activity (Sherr, 1994; Edgar and Lehner, 1996). How this is achieved during endoreduplication is intriguing, because the endocycle lacks mitosis, an event that is central to the resetting of DNA replication machinery during the mitotic cell cycle. Although this issue has been studied in Drosophila, there is clearly a fundamental difference in the regulation of the endocycle between mammals and flies. During the endoreduplication of salivary epithelial cells in Drosophila, cyclin E transcription is confined to short pulses because of a negative feedback loop (Sauer et al., 1995), and progress through the endocycle is blocked if cyclin E is continuously expressed (Follette et al., 1998; Weiss et al., 1998). In trophoblast giant cells, by contrast, cyclin E is present throughout much of the endocycle (MacAuley et al., 1998), indicating that modulation of cyclin E/Cdk activity occurs, beyond simply regulating levels of the constituent proteins. Consistent with such a mechanism, constitutively high cyclin E levels are observed in Cul1 mutant mouse embryos, and yet endoreduplication continues (Wang et al., 1999).
The cyclic expression of p57Kip2 in trophoblast giant cells provides a potential mechanism for modulation of cyclin E/Cdk activity during the endocycle. Its fluctuating levels create two distinct gap phases during the endocycle: a G1-like phase during which p57Kip2 levels drop and stay low for several hours in advance of S-phase, followed by a G2-like phase characterized by accumulation of p57Kip2 upon completion of DNA replication. These fluctuating levels correlate inversely with the levels of cyclin A- and E-associated Cdk activities observed during the trophoblast endocycle (MacAuley et al., 1998). The regulated degradation and reaccumulation of a Cdk inhibitor to modulate increases G1/S Cdk activity, and thus DNA replication, would be a novel mechanism for mammalian cell cycle regulation. Clearly, proof that G1/S Cdk activities are modulated by p57Kip2 levels depends on measuring the association of p57Kip2 with cyclin/Cdk complexes at various stages of the endocycle, as well as examining the effect of p57Kip2 deficiency on cyclin/Cdk activities during the endocycle. If p57Kip2 regulates cyclin E/Cdk during the endocycle, such a mechanism could allow the correct variation in cyclin E/Cdk activity during the endocycle even if cyclin E were constitutively expressed. Cyclin E levels normally fluctuate to some extent during the endocycle (MacAuley et al., 1998), indicating that p57Kip2 (or p57Kip2-like) function could be redundant during a normal endocycle. However, maintaining a balance of cyclin E and p57Kip2 levels is likely critical. The consequences of an increase in cyclin E and/or decrease in G1/S Cdk inhibitor level would be a shortening of the G1 phase and therefore premature resetting of DNA origins of replication. In the case of dividing cells this can result in genome instability (Spruck et al., 1999). In endocycling cells, the result could be a propensity to go through extra rounds of DNA replication, a phenomenon that we have indeed observed in giant cells of Cul1 mutant embryos in which cyclin E levels are significantly elevated (Wang et al., 1999).
ACKNOWLEDGMENTS
We thank J. Massagué and B. Vogelstein for plasmids, I. Scott and H. Nakayama for helpful discussions, and M. Tyers for critical comments on the manuscript. This work was supported by the Medical Research Council of Canada (J.C.C.). J.C.C. is a Medical Research Council Scholar.
REFERENCES
- Amon A, Tyers M, Futcher B, Nasmyth K. Mechanisms that help the yeast cell cycle clock tick: G2 cyclins transcriptionally activate G2 cyclins and repress G1 cyclins. Cell. 1993;74:993–1007. doi: 10.1016/0092-8674(93)90722-3. [DOI] [PubMed] [Google Scholar]
- Colosi P, Talamantes F, Linzer DI. Molecular cloning and expression of mouse placental lactogen I complementary deoxyribonucleic acid. Mol Endocrinol. 1987;1:767–776. doi: 10.1210/mend-1-11-767. [DOI] [PubMed] [Google Scholar]
- Copp AJ. Death before birth: clues from gene knockouts and mutations. Trends Genet. 1995;11:87–93. doi: 10.1016/S0168-9525(00)89008-3. [DOI] [PubMed] [Google Scholar]
- Cross JC, Flannery ML, Blanar MA, Steingrimsson E, Jenkins NA, Copeland NG, Rutter WJ, Werb Z. Hxt encodes a basic helix-loop-helix transcription factor that regulates trophoblast cell development. Development. 1995;121:2513–2523. doi: 10.1242/dev.121.8.2513. [DOI] [PubMed] [Google Scholar]
- Cross JC, Werb Z, Fisher SJ. Implantation and the placenta: key pieces of the development puzzle. Science. 1994;266:1508–1518. doi: 10.1126/science.7985020. [DOI] [PubMed] [Google Scholar]
- Edgar BA, Lehner CF. Developmental control of cell cycle regulators: a fly's perspective. Science. 1996;274:1646–1652. doi: 10.1126/science.274.5293.1646. [DOI] [PubMed] [Google Scholar]
- El-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- Faria TN, Soares MJ. Trophoblast cell differentiation: establishment, characterization, and modulation of a rat trophoblast cell line expression members of the placental prolactin family. Endocrinology. 1991;129:2895–2906. doi: 10.1210/endo-129-6-2895. [DOI] [PubMed] [Google Scholar]
- Follette PJ, Duronio RJ, O'Farrell PH. Fluctuations in cyclin E levels are required for multiple rounds of endocycle S phase in Drosophila. Curr Biol. 1998;8:235–238. doi: 10.1016/s0960-9822(98)70089-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fort P, Marty L, Piechaczyk M, el Sabrouty S, Dani C, Jeanteur P, Blanchard JM. Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate-dehydrogenase multigenic family. Nucleic Acids Res. 1985;13:1431–1442. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guadagno TM, Newport JW. Cdk2 kinase is required for entry into mitosis as a positive regulator of cdc2-cylcin B kinase activity. Cell. 1996;84:73–82. doi: 10.1016/s0092-8674(00)80994-0. [DOI] [PubMed] [Google Scholar]
- Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- Hartwell LH, Kastan MB. Cell cycle control and cancer. Science. 1994;266:1821–1828. doi: 10.1126/science.7997877. [DOI] [PubMed] [Google Scholar]
- Hengst L, Reed SI. Translational control of p27Kip1 accumulation during the cell cycle. Science. 1996;271:1861–1864. doi: 10.1126/science.271.5257.1861. [DOI] [PubMed] [Google Scholar]
- Lee M-H, Reynisdottir I, Massague J. Cloning of p57KIP2, a cyclin-dependent kinase inhibitor with unique domain structure and tissue distribution. Genes Dev. 1995;9:639–649. doi: 10.1101/gad.9.6.639. [DOI] [PubMed] [Google Scholar]
- MacAuley A, Cross JC, Werb Z. Reprogramming the cell cycle for endoreduplication in rodent trophoblast cells. Mol Biol Cell. 1998;9:795–807. doi: 10.1091/mbc.9.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millen K, Hui C. Radioactive hybridization of tissue sections. In: Krieg P, editor. A Laboratory Guide to RNA: Isolation, Analysis and Synthesis. New York: Wiley-Liss; 1996. pp. 339–355. [Google Scholar]
- Montagnoli A, Fiore F, Eytan E, Carrano AC, Draetta GF, Hershko A, Pagano M. Ubiquitination of p27 is regulated by Cdk-dependent phosphorylation and trimeric complex formation. Genes Dev. 1999;13:1181–1189. doi: 10.1101/gad.13.9.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama H, Scott IC, Cross JC. The transition to endoreduplication in trophoblast giant cells is regulated by the mSNA zinc-finger transcription factor. Dev Biol. 1998;199:150–163. doi: 10.1006/dbio.1998.8914. [DOI] [PubMed] [Google Scholar]
- Pagano M, Tam SW, Theodoras AM, Beer-Romero P, Del Sal G, Chau V, Yew PR, Draetta GF, Rolfe M. Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science. 1995;269:682–685. doi: 10.1126/science.7624798. [DOI] [PubMed] [Google Scholar]
- Polyak K, Lee M-H, Erdjument-Bromage H, Koff A, Roberts JM, Tempst P, Massague J. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell. 1994;78:59–66. doi: 10.1016/0092-8674(94)90572-x. [DOI] [PubMed] [Google Scholar]
- Sauer K, Knoblich JA, Richardson H, Lehner CF. Distinct modes of cyclin E/cdc2c kinase regulation and S-phase control in mitotic and endoreduplication cycles of Drosophila embryogenesis. Genes Dev. 1995;9:1327–1339. doi: 10.1101/gad.9.11.1327. [DOI] [PubMed] [Google Scholar]
- Sheaff RJ, Groudine M, Gordon M, Roberts JM, Clurman BE. Cyclin E-CDK2 is a regulator of p27Kip1. Genes Dev. 1997;11:1464–1478. doi: 10.1101/gad.11.11.1464. [DOI] [PubMed] [Google Scholar]
- Sherr CJ. Mammalian G1 cyclins. Cell. 1993;73:1059–1065. doi: 10.1016/0092-8674(93)90636-5. [DOI] [PubMed] [Google Scholar]
- Sherr CJ. G1 phase progression: cycling on cue. Cell. 1994;79:551–555. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- Sherr CJ, Roberts JM. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- Spruck CH, Won K-A, Reed SI. Deregulated cyclin E induces chromosome instability. Nature. 1999;401:297–301. doi: 10.1038/45836. [DOI] [PubMed] [Google Scholar]
- Toyoshima H, Hunter T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell. 1994;78:67–74. doi: 10.1016/0092-8674(94)90573-8. [DOI] [PubMed] [Google Scholar]
- Urano T, Yashiroda H, Muraoka M, Tanaka K, Hosoi T, Inoue S, Ouchi Y, Toyoshima H. p57(Kip2) is degraded through the proteasome in osteoblasts stimulated to proliferation by transforming growth factor beta1. J Biol Chem. 1999;274:12197–12200. doi: 10.1074/jbc.274.18.12197. [DOI] [PubMed] [Google Scholar]
- Varmuza S, Prideaux V, Kothary R, Rossant J. Polytene chromosomes in mouse trophoblast giant cells. Development. 1988;102:127–134. doi: 10.1242/dev.102.1.127. [DOI] [PubMed] [Google Scholar]
- Vlach J, Hennecke S, Amati B. Phosphorylation-dependent degradation of the cyclin-dependent kinase inhibitor p27Kip1. EMBO J. 1997;16:5334–5344. doi: 10.1093/emboj/16.17.5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Penfold S, Tang X, Hattori N, Riley P, Harper JW, Cross JC, Tyers M. Deletion of the Cul1 gene in mice causes arrest in early embryogenesis and accumulation of cyclin E. Curr Biol. 1999;9:1191–1194. doi: 10.1016/S0960-9822(00)80024-X. [DOI] [PubMed] [Google Scholar]
- Weiss A, Herzig A, Jacobs H, Lehner CF. Continuous Cyclin E expression inhibits progression through endoreduplication cycles in Drosophila. Curr Biol. 1998;8:239–242. doi: 10.1016/s0960-9822(98)70090-9. [DOI] [PubMed] [Google Scholar]
- Yan Y, Frisen J, Lee M-H, Massague J, Barbacid M. Ablation of the CDK inhibitor p57Kip2 results in increased apoptosis and delayed differentiation during mouse development. Genes Dev. 1997;11:973–983. doi: 10.1101/gad.11.8.973. [DOI] [PubMed] [Google Scholar]
- Zhang P, Liegeois NJ, Wong C, Finegold M, Hou H, Thompson JC, Silverman A, Harper JW, DePinho RA, Elledge SJ. Altered cell differentiation and proliferation in mice lacking p57KIP2 indicates a role in Beckwith-Wiedemann syndrome. Nature. 1997;387:151–158. doi: 10.1038/387151a0. [DOI] [PubMed] [Google Scholar]
- Zhang P, Wong C, DePinho RA, Harper JW, Elledge SJ. Cooperation between the cdk inhibitors p27(KIP1) and p57(KIP2) in the control of tissue growth and development. Genes Dev. 1998;12:3162–3167. doi: 10.1101/gad.12.20.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zybina EV, Zybina TG. Polytene chromosomes in mammalian cells. Int Rev Cytol. 1996;165:53–119. doi: 10.1016/s0074-7696(08)62220-2. [DOI] [PubMed] [Google Scholar]