Abstract
Persistence is an epigenetic trait that allows a small fraction of bacteria, approximately one in a million, to survive prolonged exposure to antibiotics. In Escherichia coli an increased frequency of persisters, called “high persistence,” is conferred by mutations in the hipA gene, which encodes the toxin entity of the toxin-antitoxin module hipBA. The high-persistence allele hipA7 was originally identified because of its ability to confer high persistence, but little is known about the physiological role of the wild-type hipA gene. We report here that the expression of wild-type hipA in excess of hipB inhibits protein, RNA, and DNA synthesis in vivo. However, unlike the RelE and MazF toxins, HipA had no effect on protein synthesis in an in vitro translation system. Moreover, the expression of wild-type hipA conferred a transient dormant state (persistence) to a sizable fraction of cells, whereas the rest of the cells remained in a prolonged dormant state that, under appropriate conditions, could be fully reversed by expression of the cognate antitoxin gene hipB. In contrast, expression of the mutant hipA7 gene in excess of hipB did not markedly inhibit protein synthesis as did wild-type hipA and yet still conferred persistence to ca. 10% of cells. We propose that wild-type HipA, upon release from HipB, is able to inhibit macromolecular synthesis and induces a bacteriostatic state that can be reversed by expression of the hipB gene. However, the ability of the wild-type hipA gene to generate a high frequency of persisters, equal to that conferred by the hipA7 allele, may be distinct from the ability to block macromolecular synthesis.
Persistence refers to the ability of a small fraction of bacteria within a genetically homogenous population to survive challenge by ostensibly lethal concentrations of antibiotic (5). Persistence develops randomly in a bacterial population and is an epigenetic rather than a genetic trait (3), since the progeny of persistent survivors are as susceptible to subsequent antibiotic exposure as were the parental cells. Recent studies have demonstrated that persistent bacteria survive because they exist in a transient dormant state, which protects persisters from the effects of the antibiotic and allows them to switch back to a growth phase at random intervals (3). The molecular mechanisms necessary to establish the persistent physiological state have been difficult to study because of the low frequency of persisters present in a bacterial population (one in a million in wild-type cells). However, elucidating the mechanism by which persistence is established is of great clinical importance, since persister cells are postulated to contribute to the extraordinary recalcitrance of biofilms to conventional antibiotics (23, 35) and contribute to the increased tolerance of certain bacterial species to antibiotic therapy.
Recent developments in our understanding of toxin-antitoxin (TA) modules have raised the possibility that these genetic elements may be important mediators of persistence. TA modules are two gene operons that encode an unstable antitoxin, which autoregulates expression of the operon, and a stable toxin, which is neutralized by forming a complex with the antitoxin. The Escherichia coli chromosome contains several TA modules, including relBE, mazEF (chpA), chpB, dinJ-yafQ, yefM-yoeB, and hipBA (17). In addition, the chromosomes of a wide range of both gram-positive and gram-negative species contain TA modules, often in multiple copies (28).
In an attempt to explain the presence of cellular toxins and their role in bacterial physiology, two theories have been advanced. First, it has been suggested that TA modules induce programmed cell death (PCD), a genetically programmed “altruistic” suicide process often cued by stress, such as nutrient deprivation or antibiotic treatment. The bulk of evidence for this model comes from studies of mazEF (1, 32), which has been proposed to trigger PCD by exposure to high temperature, DNA damage, and oxidative stress (18). The alternate hypothesis, that TA modules are regulators of macromolecular synthesis under conditions of nutritional stress, arose from studies of RelE and MazF (9, 10, 14, 29). These toxins have been shown to induce a state of bacteriostasis by cleaving mRNA in the ribosomal A site (8, 10, 26, 36), thus inhibiting translation and cell growth. The bacteriostatic state conferred by these toxins can be reversed either by expression of the cognate antitoxin (29) or by overproduction of tmRNA (8, 10). More recently, Keren et al. demonstrated that expression of relE toxin in excess of the antitoxin relB sharply increased the frequency of persistence in E. coli, leading to the proposal that TA modules act as effectors of persistence (21).
Mutations in the toxin gene (hipA) of the TA module hipBA confer the high-persistence phenotype (24). A bacterial population carrying the high-persistence hipA7 allele produces survivors at a frequency of 1% when treated with ampicillin or nalidixic acid compared to the one-in-a-million survival frequency associated with wild-type hipA (20, 22, 24). We previously characterized the hipA7 allele and demonstrated that it contains two point mutations (G22S and D291A), is nontoxic when expressed in the absence of the antitoxin hipB, and requires the alarmone (p)ppGpp to confer high persistence (22). A more recent study of persistence utilizing the hipA7 mutant and a second locus specifying high persistence in E. coli, hipQ, suggested that two different types of persisters are generated, depending on which mutation the strain carries (3). The hipA7 mutants produce type I persisters, which are generated during stationary phase and which remain in a nongrowing phase once the culture is transferred to fresh medium. A second class called type II persisters occur in hipQ mutants (34) and accumulate independently of passage through stationary phase. Interestingly, persisters arising from wild-type cells are continuously generated during exponential growth and increase in frequency as cells pass through the stationary phase, sharing characteristics of both mutants (3).
Although the mutant hipA7 allele was originally identified because it confers persistence, no physiological function has been assigned to the wild-type hipA gene, nor has the wild-type gene been associated with the formation of persisters. We report here that the expression of hipA in excess of hipB inhibits protein, RNA, and DNA synthesis, inducing a dormant state that lasts for more than 3 days in the vast majority of cells (>95%). In a small percentage of cells in which hipA has been expressed, growth is initiated at random intervals, indicating that the dormant state is transient. Consistent with this later proposal, the expression of the cognate antitoxin gene hipB after hipA induction resuscitates the dormant bacteria. Finally, we demonstrate that the hipA7 allele is unable to markedly affect protein synthesis when expressed in excess of hipB and yet confers a high level of persistence regardless of whether it is expressed at high or low levels, suggesting that the ability to confer persistence may be independent of the ability to inhibit macromolecular synthesis.
MATERIALS AND METHODS
Strains and plasmids.
TH1273 is a hipB+ hipA::kan derivative of MG1655 (22). Plasmids pBAD24hipA, pBAD33hipA, pBAD24hipA7, and pBAD33hipA7 have been described previously (22). To generate pBAD24hipB, the hipB gene was PCR amplified from the chromosome of MG1655 by using the primers 5′-TCTCTCTCTCGAATTCACCATGATGAGCTTTCAGAAGATCT ATAGC-3′ and 5′-TCTCTCTCTCCTGCAGTTACCACTCCAGATTTTGCTGTTCTG-3′ and inserted into the EcoRI and PstI sites of pBAD24. The Ptac-lacIq fragment from pLDR21 (American Type Culture Collection [ATCC]) was PCR amplified by using the primers 5′-TTTGACAGCTTATCATCGATAGGATATCC-3′ and 5′-TCTCTCTCTGCTAGCTGTTTCCTGTGTGAAATTGTTATCC-3′ to generate ClaI and NheI sites on either side of the PCR product. The resulting PCR product containing Ptac-lacIq was then inserted into the ClaI and NheI sites of pBAD24 or pBAD24hipB, generating plasmids pTAC24 and pTAC24hipB, respectively. To create a low-copy-number plasmid expressing hipB, the Ptac-lacIq and Ptac-lacIq hipB fragments were excised from pTAC24 and pTAC24hipB, respectively, by using the ClaI and PstI sites and inserted into pWSK29 (33), generating pTAC29 and pTAC29hipB, respectively.
The pET11ftsZ and pET11topA plasmids were constructed by PCR amplification of the ftsZ and topA genes from the chromosome of MG1655 and subsequent insertion into the NdeI and BamHI sites of pET11b (Stratagene). pET11hipA was constructed in a similar manner, except that the hipA gene was PCR amplified from HM21 (24) by using the primers 5′-CTCTCTCTCGAATTCATATGCCTAAACTTGTCACTTGGA-3′ and 5′- TCTCTCTCTGCATGCGGATCCTCACTTACTACCGTATTCTCG-3′ and cloned into pET11b. The pEThipB plasmid was constructed by PCR amplification of the hipB gene from MG1655 by using the primers 5′-TCTCTCTCTCGAATTCGATGATGAGCTTTCAGAAGATC-3′ and 5′-TCTCTCTCTCAAGCTTCCACTCCAGATTTTGCTGTTCTG-3′ into the HindIII and EcoRI sites of pET23b (Novagen). The resulting protein contains an amino-terminal T7 tag and a carboxyl-terminal His6 tag. All plasmids were sequenced to verify that no additional mutations were introduced during amplification.
Media and chemicals.
Bacteria were cultured on LB agar plates, in LB broth, or in morpholinepropanesulfonic acid (MOPS)-minimal medium. The MOPS medium was supplemented with an amino acid mixture containing all amino acids at 40 μg/ml except isoleucine at 120 μg/ml and methionine, which was absent. Appropriate antibiotics and either glucose (0.2%) or arabinose (0.2%) were also added to the MOPS medium. Antibiotics were used at the following concentrations: ampicillin, 100 μg ml−1; chloramphenicol, 50 μg ml−1; and tetracycline, 12.5 μg ml−1. Penicillinase (Sigma) was resuspended at 2,500 U ml−1 in sterile buffer containing 50 mM Tris-Cl (pH 7.5 at 25°C), 50 mM NaCl, 1 mM EDTA, 1 mM dithiothreitol, and 20% glycerol; the mixtures were then filter sterilized, divided into aliquots, and frozen at −80°C. Prior to use, penicillinase was diluted in LB broth to 50 U ml−1.
Persistence assays.
Freshly transformed TH1273 cells carrying appropriate derivatives of the pBAD33 vector (16) were used for all persistence assays. Overnight cultures were diluted to an optical density at 600 nm (OD600) of 0.025 in MOPS-minimal medium supplemented with glucose, amino acids, and chloramphenicol and grown in baffled flasks at 37°C at 200 rpm to an OD600 of ∼0.1. Cultures were split; collected on Millipore HAWP nitrocellulose filters; washed with prewarmed MOPS medium (no carbon source); resuspended in prewarmed MOPS medium containing amino acids, chloramphenicol, and either glucose or arabinose; and returned to 37°C. Aliquots were taken at the indicated times, the OD600 of each sample was measured, samples were diluted and plated on LB or LB-ampicillin, and plates were incubated overnight at 37°C. The following day, the CFU counts were determined from LB plates, and the LB-ampicillin plates were sprayed with a fine mist (∼0.3 ml) of penicillinase (50 U ml−1). The LB-ampicillin plates were returned to 37°C for 24 h (unless stated otherwise) to allow persister cell outgrowth. Each persistence assay was performed at least three times using multiple plates for each time point.
To measure persistence frequencies after treatment with antibiotics that inhibit translation, an overnight culture of MG1655 in LB broth was diluted to an OD600 of 0.01 and grown to an OD600 of 0.1. The culture was then split into three cultures: untreated, chloramphenicol treated (25 μg/ml), or tetracycline treated (12.5 μg/ml). At 0, 30, and 60 min, samples were processed as described above to determine CFU from LB plates or persister CFU from LB-ampicillin plates after treatment with penicillinase.
Microscopy.
For microcolony analysis, slides were prepared by dipping into liquid LB-agar at 55°C and immediately placing the slide on a level surface to allow the agar to solidify. The bottom of the slide was wiped off to remove agar and then cleaned with ethanol. Culture aliquots were diluted, if necessary, to an OD600 of ∼0.15, and 5 μl was spread onto the surface of the prewarmed agar slide by using a pipette tip. A 24-mm-by-40-mm coverslip was placed onto the agar and gently pressed into place. Slides were incubated in a humidity chamber at 37°C and removed at the indicated times for photography.
A LIVE/DEAD BacLight bacterial viability kit (L-7007; Molecular Probes) was used to determine the proportion of cells that retained membrane integrity, and presumably viability, after overexpression of wild-type hipA. TH1273 pBAD33hipA cells were grown as described for persistence assays, except that cells were grown in LB rather than in MOPS-minimal medium. After growth in glucose or arabinose, samples were removed at the indicated intervals, concentrated by spinning for 2 min at 14,000 rpm in an Eppendorf microcentrifuge, and resuspended in 300 μl of LB. Then, 1 μl of SYTO-9-propidium iodide mix in a 1:1 ratio was added to 300 μl of cell suspension and incubated in the dark for 15 min prior to photography. Phase-contrast and fluorescence microscopy were performed with a Nikon Optiphot 2 microscope. Random fields were photographed with a Pixera 600CL CCD digital camera, and cells were counted by using Image-Pro Plus software. The sample size for both microcolony and LIVE/DEAD analysis was greater than 500 cells, and triplicate cultures were analyzed.
Pulse-chase labeling.
Labeling experiments were conducted with fresh transformants of TH1273 containing either pBAD24hipA or pBAD24hipA7. Overnight cultures were diluted to an OD600 of 0.01 in MOPS-minimal medium (with an amino acid mixture minus methionine) supplemented with glucose and ampicillin. Cultures were grown in baffled flasks (200 rpm) at 37°C. At an OD600 of 0.1, cells were collected on Millipore HAWP nitrocellulose filters, washed with MOPS-minimal medium (no carbon source), and resuspended in prewarmed MOPS-minimal medium supplemented with the amino acid mixture minus methionine, ampicillin, and either glucose or arabinose. At the indicated time points, 250-μl samples were removed and labeled with [35S]methionine (2.5 μCi; 200 to 250 mCi/mmol) for 2 min, [3H]thymidine (1 μCi; 49 Ci/mmol) for 1 min, or [3H]uridine (0.1 μCi; 26 Ci/mmol) for 1 min at 37°C. Samples were chased at 37°C for 10 min with 100 μg of cold methionine/ml, 1 mg of cold thymidine/ml, or 1 mg of cold uridine/ml. Aliquots containing 200 μl of labeled cells were added to 1 ml of precipitation solution (90 ml of 0.5 N NaOH plus 10 ml of Carrier mix [5 μg of lysozme/ml, 20 μg of salmon sperm DNA/ml, and 10 μg of bovine serum albumin/ml in 0.5 N NaOH]) and vortexed, and then 1 ml of ice-cold 20% trichloroacetic acid (TCA) was added. Samples were placed on ice for 30 min, and precipitates were collected on Whatman GF/C filters prewet with 10% TCA. Samples were washed twice with 2 ml of ice-cold 10% TCA, once with 3 ml of ice-cold 95% ethanol, dried, and counted in 5 ml of scintillation fluid (Ultima Gold; Packard).
In vitro translation.
The E. coli T7 S30 Extract System for Circular DNA (Promega) was used for in vitro translation assays. Reaction mixtures (25 μl) were prepared according to the manufacturer's instructions using [35S]methionine (>1,000 Ci/mmol; Amersham). For each reaction, a pET vector containing the appropriate gene served as the DNA template and was added in 0.5- to 1.0-μg amounts. The reaction mix plus DNA template was gently vortexed, incubated at 37°C for 2 h, and then transferred to ice for 5 min. Next, 15 μl of the reaction mix was added to 245 μl of 1 M NaOH, followed by incubation at 37°C for 10 min. We then added this to 1.0 ml of ice-cold 25% TCA with 2% Casamino Acids, and the mixture was incubated on ice for 30 min. Precipitates were collected on Whatman GF/A or GF/C filters prewet with 10% TCA. Samples were washed twice with 2 ml of ice-cold 10% TCA and once with 3 to 5 ml of ice-cold 95% ethanol, dried, and then counted in 5 ml of Optima-Gold scintillation cocktail. For sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis, 5 μl of the T7 S30 reaction was acetone precipitated in 20 μl of acetone. Samples were incubated on ice for 15 min, spun at 12,000 × g for 5 min, resuspended in 20 μl of SDS-PAGE sample buffer, and boiled for 5 min. Aliquots were analyzed by SDS-PAGE on a 15% polyacrylamide gel.
HipB resuscitation.
Freshly transformed TH1273 cells carrying pBAD33hipA and either pTAC29 or pTAC29hipB were used. Overnight cultures were diluted to an OD600 of 0.025 in MOPS-minimal medium plus glucose, amino acids, ampicillin, and chloramphenicol and grown in baffled flasks at 37°C to an OD600 of ∼0.1. Cultures were split; cells were washed with prewarmed Minimal-MOPS (no carbon source), resuspended in prewarmed MOPS-minimal medium containing amino acids, ampicillin, chloramphenicol, and either glucose (0.2%) or arabinose (0.4%); and the cultures were returned to 37°C. At the indicated intervals, the OD600 of each sample was measured, and the samples were diluted and plated on LB or LB-IPTG (isopropyl-β-d-thiogalactopyranoside) (1 mM) and incubated overnight at 37°C. The following day, CFU were determined from LB and LB-IPTG plates. Each resuscitation assay was performed a minimum of three times using multiple plates.
RESULTS
Wild-type HipA inhibits cell growth and colony formation.
We reported previously that over expression of hipA in the absence of hipB caused filamentation and cell lysis (22). As noted in that study, there was a great deal of colony-to-colony variation in bacteria that contained a hipA-inducible plasmid in a ΔhipBA background. We suspected that the reproducibility problems were most likely due to variations in the level of wild-type hipA transcription from the pBAD vectors in the absence of the antitoxin gene hipB, leading to chronic exposure of bacteria to the HipA toxin. Consequently, we reexamined the effects of hipA over expression on growth and viability using a strain that contained a chromosomal copy of hipB (TH1273 hipA::Kan). Furthermore, only fresh transformants were tested, to further reduce the possibility of experimental variation. As is shown below, the results reported here suggest that the previous observation regarding hipA-induced filamentation was an artifact.
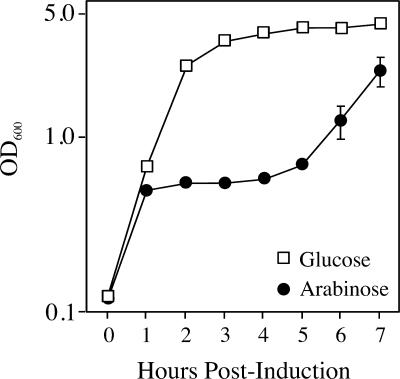
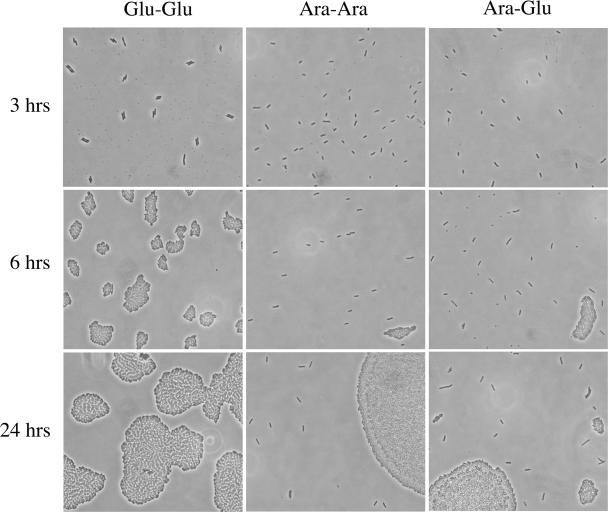
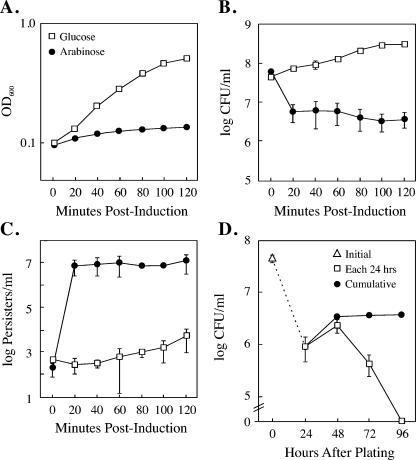
When strain TH1273 carrying pBAD33hipA was cultured in LB containing arabinose, growth continued for one round of division and then leveled off for a period of several hours (Fig. 1). However, the growth inhibition imposed by hipA expression was transient, and culture growth resumed after approximately 5 h, a finding consistent with observations reported previously (22). To determine whether culture outgrowth occurred from a defined subpopulation of cells or if all cells eventually overcome growth inhibition, bacteria were spread on LB agar-coated slides containing arabinose at 3 h postinduction, followed by incubation at 37°C, and the slides were examined for formation of microcolonies. As presented in Fig. 2 (middle panels), a relatively small fraction of cells (0.47% ± 0.15%) can be seen dividing at 6 h postinduction in arabinose. This fraction is only marginally greater at 24 h postinduction (0.79% ± 0.36%). Thus, a small fraction of cells either acquired a mutant plasmid or became either HipA- or arabinose-insensitive, and it was this fraction that was responsible for culture outgrowth in the presence of arabinose.
FIG. 1.
Ectopic expression of hipA causes a transient inhibition of culture growth. TH1273 (hipA::Kan) cells transformed with pBAD33hipA were grown overnight in LB with chloramph-enicol and glucose and subcultured the following morning in the same medium at an OD600 of 0.025. At an OD600 of 0.1, the culture was washed and split into LB-chloramphenicol medium containing glucose or arabinose. The values presented are the average of three independent experiments; error bars represent the standard deviations.
FIG. 2.
A limited number of cells form colonies after expression of hipA. TH1273 (hipA::kan) cells transformed with pBAD33hipA were grown overnight in LB- with chloramph enicol and -glucose and subcultured the following morning in the same medium at an OD600 of 0.025. At an OD600 of 0.1, the culture was washed and split into LB-chloramph enicol medium containing glucose (Glu) or arabinose (Ara). After 180 min of hipA induction, aliquots were transferred to slides coated with LB-arabinose agar (center) or LB-glucose agar (right). The left panels show cells grown only in LB-chloramph enicol-glucose and spread on slides coated with LB-glucose agar.
To determine whether subsequent repression of hipA transcription allowed more cells to recover from arabinose treatment or only the subpopulation that was presumably arabinose insensitive, a similar microcolony analysis was performed by spreading an aliquot from the arabinose-induced culture onto agar-coated slides containing LB-glucose (Fig. 2, right panels). At 6 h postinduction (3 h after plating onto LB-glucose agar), the fraction of cells that had initiated colony formation was only slightly greater, 1.2% ± 0.3%, than what was observed on the LB-arabinose agar. However, by 24 h postinduction, that number had increased to 22.5% ± 1.8%, indicating that once hipA expression from the plasmid had been suppressed and hipB expression from the chromosome had reached a level sufficient to neutralize existing HipA, cell growth resumed. Nonetheless, the majority of the population, almost 80%, failed to resume growth within this time period. It was also noted that microcolonies that formed after arabinose induction and subsequent plating on LB-glucose agar were heterologous in size (Fig. 2, lower right panel), indicating that the initiation of growth following hipA expression was asynchronous.
Wild-type HipA inhibits macromolecular synthesis.
It is well established that toxins from other TA modules, such as relE and mazF, affect macromolecular synthesis (4, 10, 19, 29, 30). To determine whether the cessation of growth that accompanied hipA overexpression was due to the inhibition of macromolecular synthesis, the incorporation of radioactive precursors into DNA, RNA, and protein was examined. For these studies, MOPS minimal medium was substituted for LB, and the duration of incorporation was restricted to 2 h to avoid outgrowth of the arabinose-insensitive subpopulation.
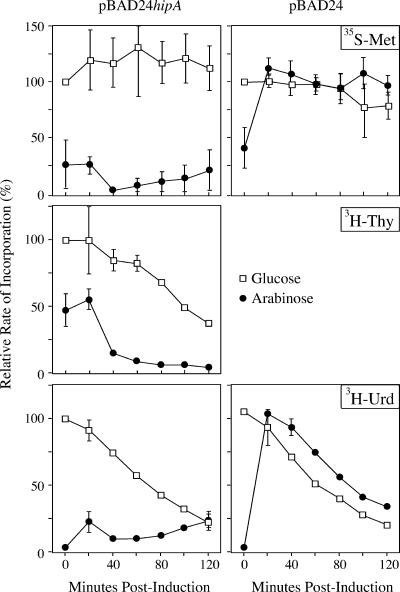
Pulse-chase experiments demonstrated that, after hipA induction, [35S]methionine and [3H]uridine incorporation were sharply reduced at the earliest time point and remained at that level throughout the remainder of the experiment (Fig. 3). In comparison, a control experiment using an empty pBAD24 vector also showed a sharp decrease when cultures were shifted to arabinose, but the cells quickly recovered and incorporation levels at subsequent timepoints matched those seen in the glucose culture. The sharp decrease at the earliest time point is presumably due to the sudden change in the carbon source. Incorporation of [3H]thymidine also decreased after hipA induction, but the decrease was not as great at the initial time points as with protein and RNA synthesis. Continued incorporation of [3H]thymidine at the 20- and 40-min time points most likely reflects the completion of ongoing rounds of DNA replication. Thus, the HipA toxin affects all macromolecular synthesis, with the effects on DNA synthesis probably secondary to the effects on protein and RNA synthesis. The inhibition of both RNA and protein synthesis distinguishes HipA from RelE and MazF, which affects the incorporation of precursors into protein but not RNA (9, 36).
FIG. 3.
Ectopic expression of wild-type hipA inhibits macromolecular synthesis. TH1273 (hipA::kan) was transformed with either pBAD24hipA or pBAD24 without an insert. Overnight cultures grown in MOPS-minimal plus ampicillin and glucose were diluted into the same medium to an OD600 of 0.01. At an OD600 of 0.1, the culture was washed and split into MOPS minimal medium with glucose (squares) or arabinose (circles), and pulse-chase experiments were conducted to monitor the incorporation of radiolabeled precursors into protein (top), DNA (center), or RNA (bottom). The incorporation rates are expressed as the percent relative to the incorporation of precursor into the glucose culture at time zero. The values presented are the averages of three independent experiments; error bars represent the standard deviations.
HipA does not inhibit translation in vitro.
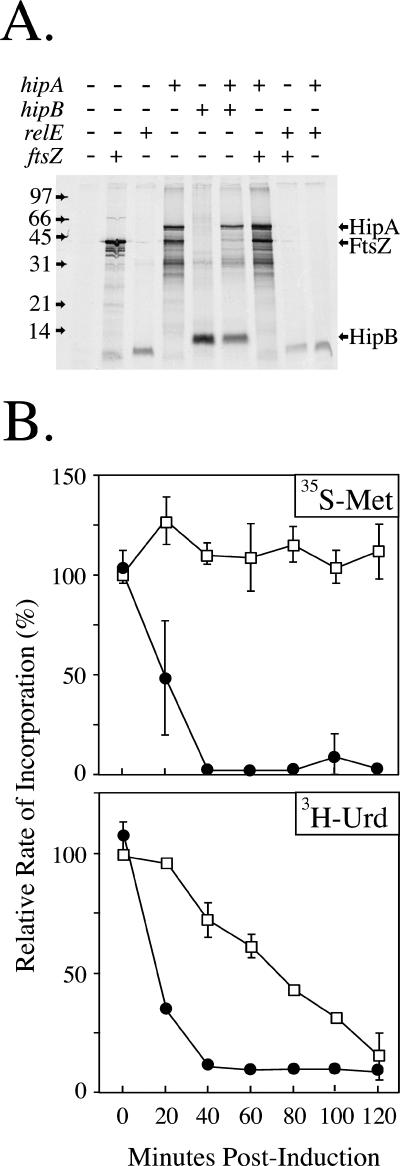
Bacterial toxins, such as RelE and MazF, have been shown to inhibit translation by cleaving mRNA at the ribosomal A-site (8, 10, 26, 36) and inhibit protein synthesis in a cell extract (15, 36). To determine whether wild-type HipA also has a direct effect on translation, the hipA gene was expressed from pEThipA in an in vitro-coupled transcription-translation reaction. As shown in Fig. 4A, HipA was produced in an S30 extract and did not inhibit the production of heterologous proteins (FtsZ and HipB). In contrast, expression of relE inhibited production of both FtsZ and HipA.
FIG. 4.
HipA does not block translation in vitro. (A) T7 S30 in vitro translation system programmed with the DNA templates pET11ftsZ, pET11hipA, pEThipB, or pET11relE. The positions of the individual proteins after SDS-15% PAGE are indicated. (B) BL21(DE3)/pLysS was transformed with pET11hipA, and pulse-chase experiments were conducted in MOPS minimal medium plus glucose as described in Materials and Methods. The incorporation of [35S]methionine and [3H]uridine was measured in the absence (squares) or presence (circles) of 0.05 mM IPTG. The incorporation rate is expressed as the percent relative to the incorporation of precursor into the glucose culture at time zero.
To ensure that the hipA gene in the pET vector was functional, the pEThipA plasmid was introduced into BL21(DE3)/pLysS, and the effects on growth and the incorporation of precursors were examined. Induction of the hipA gene with IPTG inhibited the growth (data not shown) and incorporation of both [35S]methionine and [3H]uridine (Fig. 4B). It was also noted that the initial sharp drop in methionine and uridine incorporation seen in Fig. 3 is absent here, due to the fact that induction was accomplished by adding IPTG rather than switching carbon sources. Thus, the HipA toxin inhibits protein synthesis in vivo but not in vitro.
hipA expressed in excess of hipB generates a high frequency of persisters.
The inhibition of growth after hipA induction (Fig. 1) led us to examine whether the expression of wild-type hipA in excess of hipB conferred a persistence-like state following the shut down of macromolecular synthesis. First, as seen in Fig. 5A and B, the induction of hipA in TH1273 was accompanied by a 90 to 95% decrease in CFU at 20 min, which coincides with the point of growth inhibition. It was also noted that there was no further reduction in CFU, even after 120 min of hipA induction. This is in contrast to the expression of the toxin RelE, which is reported to cause a 106-fold reduction in CFU over a similar time period (29). Second, at 20 min after induction with arabinose, when hipA expression inhibits growth and macromolecular synthesis, the number of persister cells increased by greater than 10,000-fold (Fig. 5C). In contrast, cultures grown in glucose generated persisters at the frequency typically associated with strains carrying a chromosomal copy of the wild-type hipA allele (10−5 to 10−6). Thus, leaky transcription of hipA from the PBAD promoter in glucose is insufficient to confer a high frequency of persistence in the uninduced state. However, if HipA production exceeds production of HipB, the excess HipA protects cells from subsequent ampicillin exposure. This result corroborates previous observations by Falla and Chopra, who showed that low-level expression of wild-type hipA led to a 10- to 100-fold increase in tolerance to fluoroquinones and β-lactamases (13).
FIG. 5.
Expression of wild-type hipA in excess of hipB confers persistence. (A) TH1273 (hipA::kan) cells were transformed with pBAD33hipA. Fresh transformants were grown in MOPS minimal medium containing either glucose (squares) or arabinose (circles). (B and C) Aliquots were diluted and plated on LB to determine the CFU per ml after 24 h (B) or spread on LB-ampicillin plates for 24 h prior to treatment with penicillinase to allow outgrowth of persisters (C). (D) Time course of CFU appearance after a 60-min hipA induction. Symbols: □, number of CFU appearing in each 24-h period; •, total accumulated CFU; ▵, initial CFU present at the time arabinose was added to medium. For all panels, the values presented are the average of three independent experiments; the error bars represent the standard deviations.
The frequency of persisters observed in these experiments (48 h postinduction) was roughly equal to the number of CFU at 24 h after induction of hipA (compare Fig. 5B and C), suggesting that these persisters represented a new wave of colony formers that appeared only after ampicillin was inactivated. To determine how long dormant cells can revive following a short hipA induction, a time course of colony formation was conducted. After 60 min of hipA induction in MOPS-minimal medium, cells were diluted and plated on LB agar plates, and the appearance of colonies was scored every 24 h for 4 days. As shown in Fig. 5D, colonies continued to appear for 3 days after hipA induction, with no new colonies detected on day 4.
Translation inhibition and persister cell development.
If inhibition of translation by HipA or RelE leads to a persistent state, can bacteriostatic antibiotics that inhibit translation also facilitate the development of persister cells? It is conceivable that antibiotic-directed inhibition of translation could cause the cellular concentrations of antitoxins to drop precipitously, thereby promoting release of toxins, which in turn could keep cells in a dormant state following removal of the translation-inhibiting antibiotic. Indeed, it was shown previously that 60 min after challenge with chloramphenicol, the TA module relBE experiences maximum transcription (9). The increase in relBE transcription correlated with degradation of the antitoxin autoregulator RelB and, presumably, with release of the RelB toxin. Also, studies with MazEF showed a similar decrease in MazE antitoxin levels at 60 min after treatment with chloramphenicol (32) and a corresponding drop in CFU, which is indicative of toxin release. If the same happens with HipA and other bacteriostatic toxins, it follows that any generalized inhibitor of translation could potentially lead to cell dormancy.
Chloramphenicol and tetracycline were used to determine whether the frequency of persisters increased after treatment with these translation inhibitors. MG1655 cells were cultured in LB (untreated control) or LB containing either chloramphenicol or tetracycline prior to plating on ampicillin. The addition of either antibiotic halted growth immediately, but after 60 min of exposure the treated cultures did not show a reduction in CFU, thus confirming that the antibiotics were bacteriostatic at the concentrations used (Table 1). Furthermore, bacteria grown in the presence of either chloramphenicol or tetracycline generated the same low frequency of persisters as untreated cells (∼10−6). Thus, in contrast to the translation block imposed by ectopic expression of chromosomal toxins, translation inhibition by antibiotics for a period of time sufficient to cause toxin release is unable to induce persistence or protect cells from the bactericidal effects of ampicillin.
TABLE 1.
Inhibition of protein synthesis does not increase the frequency of persisters
| Antibiotic | CFU60/CFU0 ratio | Frequency of persistersa |
|---|---|---|
| None | 12.1 ± 2.8 | (2.8 ± 1.8) × 10−6 |
| Chloramphenicol | 1.5 ± 0.5 | (5.5 ± 2.0) × 10−6 |
| Tetracycline | 0.9 ± 0.2 | (5.2 ± 1.1) × 10−6 |
That is, the frequency of persisters at 60 min after antibiotic treatment.
The loss of colony-forming ability following hipA induction does not correlate with cell death.
It has been suggested that hipA is a lethal toxin, since cells overexpressing hipA in the absence of hipB exhibited decreased viability (6, 13, 22). However, observations from the persistence assays described above made us reconsider whether hipA was truly bactericidal. Furthermore, Gerdes and coworkers have shown that two other “toxins,” relE and mazF, do not cause cell death but rather induce a bacteriostatic effect (10, 29), although Engleberg-Kulka and coworkers have disputed this claim (2).
Even though only a relatively small fraction of cells expressing hipA in excess of hipB were able to form colonies, it remained a possibility that the remaining 80 to 90% were still viable. To determine the extent to which hipA expression affects cell viability, we used the LIVE/DEAD bacterial viability kit, which measures membrane integrity. Freshly transformed TH1273/pBAD33hipA cells were cultured in LB, and hipA expression was induced by the addition of arabinose. To eliminate the arabinose-insensitive variants that arise spontaneously at later times, ampicillin was added to the culture at 3 h after hipA induction, and incubation was continued in LB in the presence of both arabinose and ampicillin. Any bacteria that begin growing after ampicillin addition should be killed, but dormant cells should survive. The survival of the remaining cells was then determined at intervals using the LIVE/DEAD stain. Although we could detect by using a microscope a small fraction of lysed cells at later times after the addition of ampicillin, we found little evidence for culture growth during the course of the experiment (overall, the culture density increased from 0.54 at the time of ampicillin addition versus 0.73 at 72 h). More importantly, we found that the number of surviving cells remained high after hipA expression (>95%), even after 72 h of continuous induction (data not shown). To determine whether surviving cells were still able to form colonies after 72 h of hipA induction, the remaining cells were washed to remove arabinose, penicillinase was added to eliminate any remaining ampicillin, and the cells were plated on LB-glucose plates. After 24 h of incubation at 37°C, we found that 1.8% of the cells still formed colonies. Thus, we conclude that prolonged exposure of cells to HipA does not kill them but rather causes the vast majority of the cells to become dormant.
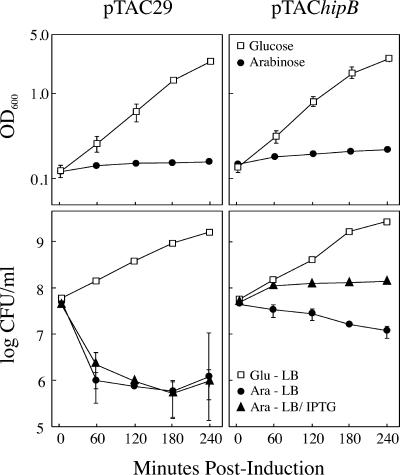
HipA induces bacteriostasis that is reversed by HipB.
It was shown previously for both relBE and mazEF TA pairs that overexpression of either toxin conferred a bacteriostatic effect that was fully reversed by the expression of their cognate antitoxins (10, 29). To determine whether the effects of hipA induction could also be overcome by expression of hipB, TH1273 derivatives carrying pBAD33hipA and either pTAC29 or pTAC29hipB were grown in MOPS-minimal medium supplemented with either glucose or arabinose to repress or induce, respectively, hipA expression. After the expression of hipA, cells were plated on either LB (no hipB induction) or LB-IPTG (hipB induced). Two observations were noteworthy. First, the overexpression of hipA in cells carrying pTAC29hipB resulted in only a 5-fold decrease in CFU at 240 min rather than the 50-fold decrease seen in cells carrying the empty vector, pTAC29 (Fig. 6, bottom panels). We attribute this difference to leaky expression of hipB from the Ptac promoter in pTAC29hipB. Second, inducing hipB expression after expressing hipA for up to 240 min restored growth to cells unable to form colonies on LB plates alone (lower right panel of Fig. 6). Thus, the expression of the antitoxin hipB is sufficient to reverse the bacteriostatic effects of hipA and confirms that the dormant state induced by hipA in all cells is transient.
FIG. 6.
Expression of hipB after hipA overexpression restores cell growth. TH1273 cells carrying pBAD33hipA and either pTAC29 (left panels) or pTAC29hipB (right panels) were grown in MOPS minimal medium in the presence of glucose (□) or arabinose (•) to induce or suppress hipA transcription. At the indicated time points, the cells were removed and plated on LB or LB-IPTG (to induce hipB [▴]), and the numbers of CFU were determined. The values presented are the averages of three independent experiments; error bars represent the standard deviations.
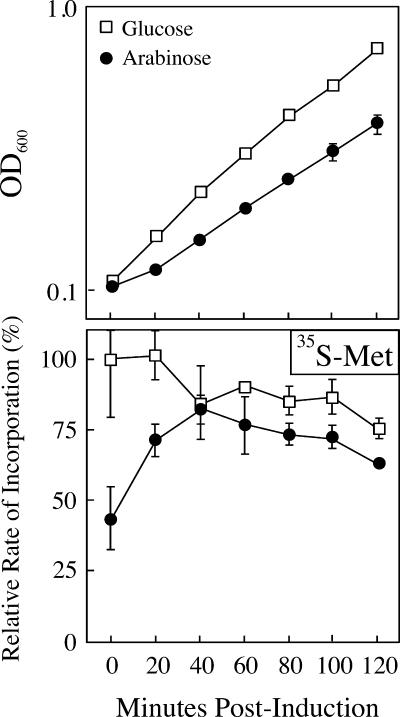
The effect of hipA7 expression on translation is greatly reduced compared to the effect of wild-type hipA.
We have previously shown that the high-persistence mutant hipA7 is nontoxic in the absence of hipB and is recessive to the “toxicity” of wild-type hipA (22). To determine the extent to which the mutant HipA7 inhibits translation, pulse-chase experiments measuring the incorporation of methionine into protein were performed using freshly transformed TH1273 pBAD24hipA7 cells. In agreement with the observation that HipA7 is nontoxic, the expression of the hipA7 allele caused only a minor reduction in growth (generation time of 58.9 min for pBAD24hipA7 versus 47.3 min for the empty vector in arabinose) and a correspondingly small reduction in translation (∼20% from 60 to 120 min) (Fig. 7).
FIG. 7.
Ectopic expression of the hipA7 allele does not markedly inhibit protein synthesis. TH1273 (hipA::Kan) cells were transformed with pBAD24hipA7. Fresh transformants were cultured in MOPS minimal medium in with glucose (□) or arabinose (•) and monitored for growth (upper panel) and [35S]methionine incorporation (lower panel) as described in Materials and Methods. The incorporation rates are expressed as the percent relative to the incorporation of precursor into the glucose culture at time zero. The values presented are the averages of five independent experiments; error bars represent the standard deviations.
The frequency of persistence after hipA7 induction from the pBAD vector was also measured and compared to the generation of persisters following the expression of wild-type hipA. Similar to the results presented in Fig. 5 (which used MOPS-minimal medium), cells carrying a wild-type hipA gene and cultured in LB-glucose showed increased CFU with time (Fig. 8, top panel), but a low frequency of persisters after exposure to ampicillin (frequency of 10−5). Also, as in MOPS-minimal medium, the overexpression of wild-type hipA in LB-arabinose caused a 55-fold decrease in CFU at 30 min postinduction but a 7,500-fold increase in persister cells (Fig. 8, top panel). In contrast, when cultured in glucose, the hipA7 allele conferred protection from ampicillin to a significantly larger fraction of the population (1%) (Fig. 8, bottom panel) even when it was not expressed in excess of hipB. The survival frequencies of 10−2 conferred by the uninduced hipA7 gene are identical to the frequency previously reported for cells carrying a chromosomal copy of the hipA7 allele and an intact hipB gene (20, 22, 24) and may reflect a markedly decreased interaction between the HipA7 and HipB proteins such that the majority of HipA7 is in an unbound state. In the culture supplemented with arabinose, the fraction of persister cells increased from 2% at 0 min to approximately 10% of the total cells at 60 min after hipA7 induction. Thus, hipA7 confers a high level of persistence similar to that conferred by the overexpression of wild-type hipA, despite the fact that the mutant allele has a significantly reduced ability to inhibit macromolecular synthesis.
FIG. 8.
Expression of hipA7 confers persistence regardless of the level of induction. TH1273 cells transformed with either pBAD33hipA or pBAD33hipA7 were grown in LB broth in the presence of glucose (squares) or arabinose (circles). Cells were plated on LB to determine CFU (open symbols) or on LB-ampicillin plates to determine persister CFU (closed symbols). The values presented are the averages of three independent experiments; error bars represent the standard deviations.
DISCUSSION
It is demonstrated here that ectopic expression of the hipA gene in excess of its cognate antitoxin gene, hipB, inhibits macromolecular synthesis and induces a culture-wide state of dormancy. Recovery from the dormant state occurs in a small fraction of cells at random intervals for at least 72 h when hipB is resident on the chromosome or hipA-induced dormancy can be fully reversed by the expression of its cognate antitoxin gene hipB within 4 h of hipA induction, which is similar to what has been shown with the TA modules relBE and mazEF (29). In comparison, ectopic expression of the mutant hipA7 allele has a minor impact on translation inhibition and growth and yet generates a similar number of persister cells as the wild-type hipA gene.
The effects of hipA expression on macromolecular synthesis appear to be distinct from what is observed with other E. coli toxins such as RelE and MazF, which inhibit translation by cleaving mRNA in the ribosomal A site (8-10, 30, 36). Expression of hipA affects incorporation of precursors into both protein and RNA, while similar studies with RelE showed an effect on protein synthesis only (9). Moreover, the inhibition of protein synthesis by RelE is much more rapid than translation inhibition after HipA induction (40 min for HipA [Fig. 4B] versus 5 min for RelE [9, 29]). Also, RelE is able to inhibit protein synthesis in vitro in an S30 extract, either when expressed directly in a coupled transcription-translation system or when purified RelE is added to suppress translation of a heterologous protein (15, 29). In contrast, HipA showed no effect in an S30 extract using either a programmed extract or when purified HipA was added (data not shown). Efforts to identify the target of the HipA toxin are currently under way.
Although HipA-induced persistence is coincident with the inhibition of translation and expression of the translation-inhibiting RelE protein generates persistent bacteria (21), blocking protein synthesis alone is insufficient to develop the persistent state. Treating MG1655 cells with either of two bacteriostatic translation inhibitors, chloramphenicol or tetracycline, did not increase the frequency of persisters above background levels (10−6), even though translation was suppressed for a period of time that correlated with the development of persistence after hipA expression (60 min; Fig. 5C). In some respects, this result is somewhat surprising, because the inhibition of protein synthesis should, in theory, trigger a global release of toxins from TA complexes (21), several of which might be expected to suppress translation even after the antibiotic is removed (e.g., RelE and MazF). Indeed, previous studies have shown that chloramphenicol-induced translation inhibition for 60 min results in the degradation of RelB (9) and MazF (32), which presumably leads to toxin release and subsequent effects on translation. It is possible that another cellular factor, possibly controlled by ppGpp, might be required in addition to translation inhibition to cause cell dormancy. On the other hand, a microarray analysis of E. coli treated for 30 min with four different antibiotic inhibitors of protein synthesis did not show a coordinate increase in the expression of known TA loci, although mazE (chpR) (but not mazF) was increased after treatment with two of the antibiotics (31). These disparate observations emphasize how little we understand of the conditions that might promote toxin release and subsequent establishment of a persistent state.
The observation that a fraction of bacteria exposed to prolonged expression of wild-type hipA can still recover and form colonies after 72 h contrasts with the conclusion of a relatively recent study on mazEF (2), which suggested that a “window of opportunity” for antitoxin-mediated resuscitation of toxin-induced bacteriostasis closes after 6 h in LB medium. Inspection of the data from the previous study reveals that while there is a dramatic decrease in the number of cells that recover after 6 h of mazF expression, a subpopulation of cells (8% in LB and 0.3% in M9 minimal medium) can still be revived by mazE expression up to 24 h, which is not substantially different from the fraction of cells recovered 24 h after hipA induction (2%, Fig. 5D). Thus, regardless of whether the vast majority of cells are killed or merely rendered dormant, a significant percentage of cells are still able to initiate growth and division after extended exposure to chromosomal toxins.
Our results also provide insight as to the fate of the cell population that is unable to form colonies after long-term induction of hipA toxin and may help resolve the controversy that exists as to whether all TA modules function primarily as mediators of programmed cell death (12) or as modulators of macromolecular synthesis during times of nutritional stress (14). We demonstrate that the vast majority of cells unable to form colonies are still viable after 72 h of chronic hipA-induction, as judged by the LIVE/DEAD stain. Thus, over this time interval, the effects of hipA appear to be bacteriostatic rather that bactericidal. Nonetheless, the fact the we were unable to recover CFU from the vast majority of cells suggests that these bacteria remain dormant when plated on fresh medium, similar to cells in a viable but nonculturable (VBNC) state. The VBNC state is considered to be a reversible, genetically programmed state whereby nonsporulating cells protect themselves from adverse environmental conditions (11). The genetic program that underlies the VBNC state has been difficult to elucidate and remains, at best, poorly characterized (27). The discovery here that chronic hipA expression can induce dormancy similar to the VBNC state in a large proportion of cells raises the possibility that the VBNC state, like persistence, might be programmed by TA modules.
The hipA7 mutation has comparatively minor effects on macromolecular synthesis or cell growth when expressed in excess of hipB and yet confers persistence to a similar percentage of cells as when wild-type hipA is expressed. The reduced ability to block the incorporation of precursors into macromolecules is consistent with previous reports that the hipA7 allele is recessive to wild-type hipA (25), suggesting a loss of function by HipA7. However, if hipA7 inhibits protein synthesis or suppresses growth poorly compared to wild-type hipA and the relE toxin, what is the mechanism by which bacteria expressing hipA7 become dormant, and consequently, persisters? We have considered two explanations for this apparent paradox. First, the inhibition of macromolecular synthesis by HipA7 reaches a critical threshold in very few cells, inducing dormancy and leading to persistence in those cells only. The remainder of cells in the culture continue to grow and divide. One could argue that the small differences in the growth and translation rate of cells overexpressing hipA7 (Fig. 7) support this explanation. The alternate possibility is that the high persistence and “toxin” functions of the HipA protein are separate. This explanation would be consistent with the observation that single point mutations, such as D88N (22), S54P, and L327P (T. M. Hill, unpublished results) inactivate the “toxicity” of HipA and yet still confer high persistence. A high-persistence function that is largely independent of the ability to inhibit macromolecular synthesis may explain why Moyed and coworkers, in exhaustive genetic screens, only identified mutations in the hipBA gene pair (7, 24) and not in other TA modules such as relBE and mazEF. Mutational analysis of the HipA protein and the effects of mutations on the high persistence and toxicity functions should help resolve this paradox.
A final point to be made is that passage through the stationary phase does not appear to be a prerequisite for persister formation when either wild-type or mutant hipA is expressed ectopically. Balaban et al., in a study on persistence, concluded that the type I persisters produced by the hipA7 allele originated in stationary phase, since the number of persisters in a culture was proportional to the size of the inoculum from a stationary-phase culture (3). Likewise, Keren et al. demonstrated that a culture of hipA7 cells could be “cured” of persisters if repeatedly subcultured, indicating that persisters are not generated during exponential growth (20). In contrast, we observed either a 10,000-fold or a 5-fold increase in persister cell frequency in cultures growing exponentially after the induction of wild-type hipA or hipA7 expression, respectively. Thus, the overexpression of hipA overcomes whatever factors limit persister formation to stationary phase when the gene is resident on the chromosome.
Acknowledgments
We thank Bridget Johnson for excellent technical assistance. We thank the University of North Dakota Faculty Research Seed Money, the UND School of Medicine and Health Sciences Research Committee, and the Department of Microbiology and Immunology, which have provided full support for this research.
REFERENCES
- 1.Aizenman, E., H. Engelberg-Kulka, and G. Glaser. 1996. An Escherichia coli chromosomal “addiction module” regulated by guanosine-3′5′-bispyrophosphate: a model for programmed cell death. Proc. Natl. Acad. Sci. USA 93:6059-6063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amitai, A., Y. Yassin, and H. Engelberg-Kulka. 2004. MazF-mediated cell death in Escherichia coli: a point of no return. J. Bacteriol. 186:8295-8300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balaban, N. Q., J. Merrin, R. Chait, L. Kowalik, and S. Leibler. 2004. Bacterial persistence as a phenotypic switch. Science 305:1622-1625. [DOI] [PubMed] [Google Scholar]
- 4.Bernard, P., and Couturier. 1992. Cell killing by the F plasmid CcdB protein involves poisioning of DNA-topoisomerase II complexes. J. Mol. Biol. 226:735-745. [DOI] [PubMed] [Google Scholar]
- 5.Bigger, J. W. 1944. Treatment of staphylococcal infections with penicillin by intermittent treatment. Lancet ii:497-500. [Google Scholar]
- 6.Black, D. S., B. Irwin, and H. S. Moyed. 1994. Autoregulation of hip, an operon that affects lethality due to inhibition of peptidoglycan or DNA synthesis. J. Bacteriol. 176:4081-4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Black, D. S., A. J. Kelly, M. J. Mardis, and H. S. Moyed. 1991. Structure and organization of hip, an operon that effects lethality due to inhibition of peptidoglycan or DNA synthesis. J. Bacteriol. 173:5732-5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christensen, S. K., and K. Gerdes. 2003. RelE toxins from Bacteria and Archaea cleave mRNAs on translating ribosomes, which are rescued by tmRNA. Mol. Microbiol. 48:1389-1400. [DOI] [PubMed] [Google Scholar]
- 9.Christensen, S. K., M. Mikkelsen, K. Pedersen, and K. Gerdes. 2001. RelE, a global inhibitor of translation, is activated during nutritional stress. Proc. Natl. Acad. Sci. USA 98:14328-14333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christensen, S. K., K. Pedersen, F. G. Hansen, and K. Gerdes. 2003. Toxin-antitoxin loci as stress-response-elements: ChpAK/MazF and ChpBK cleave translated RNAs and are counteracted by tmRNA. J. Mol. Biol. 332:809-819. [DOI] [PubMed] [Google Scholar]
- 11.Colwell, R. R. 2000. Bacterial death revisited, p. 325-342. In R. Colwell, R. Grimes, and D. Jay (ed.), Nonculturable microorganisms in the environment. ASM Press, Washington, D.C.
- 12.Engelberg-Kulka, H., B. Sat, and R. Hazan. 2001. Bacterial programmed cell death and antibiotics. ASM News 67:617-624. [Google Scholar]
- 13.Falla, T. J., and I. Chopra. 1998. Joint tolerance to β-lactam and fluoroquinilone antibiotics in Escherichia coli results from overexpression of hipA. Antimicrob. Agents Chemother. 42:3282-3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerdes, K. 2000. Toxin-antitoxin modules may regulate synthesis of macromolecules during nutritional stress. J. Bacteriol. 182:561-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gotfredsen, M., and K. Gerdes. 1998. The Escherichia coli relBE genes belong to a new toxin-antitoxin gene family. Mol. Microbiol. 29:1065-1076. [DOI] [PubMed] [Google Scholar]
- 16.Guzman, L.-M., D. Belin, M. J. Carson, and J. Beckwith. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177:4121-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayes, F. 2003. Toxins-antitoxins: plasmid maintenance, programmed cell death, and cell cycle arrest. Science 301:1496-1499. [DOI] [PubMed] [Google Scholar]
- 18.Hazan, R., B. Sat, and H. Engelberg-Kulka. 2004. Escherichia coli mazEF-mediated cell death is triggered by various stressful conditions. J. Bacteriol. 186:3663-3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang, Y., J. Pogliano, D. R. Helinski, and I. Konieczny. 2002. ParE toxin encoded by the broad-host-range plasmid RK2 is an inhibitor of Escherichia coli gyrase. Mol. Microbiol. 44:971-979. [DOI] [PubMed] [Google Scholar]
- 20.Keren, I., N. Kaldalu, A. Spoering, Y. Wang, and K. Lewis. 2004. Persister cells and tolerance to antimicrobials. FEMS Microbiol. Lett. 230:13-18. [DOI] [PubMed] [Google Scholar]
- 21.Keren, I., D. Shah, A. Spoering, Y. Kaldalu, and K. Lewis. 2004. Specialized persister cells and the mechanism of multidrug tolerance in Escherichia coli. J. Bacteriol. 186:8172-8180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Korch, S., T. Henderson, and T. Hill. 2003. Characterization of the hipA7 allele of Escherichia coli and evidence that high persistence is governed by (p)ppGpp synthesis. Mol. Microbiol. 50:1199-1213. [DOI] [PubMed] [Google Scholar]
- 23.Lewis, K. 2001. Riddle of biofilm resistance. Antimicrob. Agents Chemother. 45:999-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moyed, H. S., and K. P. Bertrand. 1983. hipA, a newly recognized gene of Escherichia coli K-12 that affects frequency of persistence after inhibition of murien synthesis. J. Bacteriol. 155:768-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moyed, H. S., and S. H. Broderick. 1986. Molecular cloning and expression of hipA, a gene of Escherichia coli K-12 that affects frequency of persistence after inhibition of murein synthesis. J. Bacteriol. 166:399-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Munoz-Gomez, A. J., S. Santos-Sierra, A. Berzal-Herranz, R. M. Lemonnie, and R. Diaz-Orejas. 2004. Insights into the specificity of RNA cleavage by the Escherichia coli MazF toxin. FEBS Lett. 567:316-320. [DOI] [PubMed] [Google Scholar]
- 27.Nyström, T. 2003. Nonculturable bacteria: programmed survival forms or cells at death's door? Bioessays 25:204-211. [DOI] [PubMed] [Google Scholar]
- 28.Pandey, D. P., and K. Gerdes. 2005. Toxin-antitoxin loci are highly abundant in free-living but lost from host-associated prokaryotes. Nucleic Acids Res. 33:966-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pedersen, K., S. K. Christensen, and K. Gerdes. 2002. Rapid induction and reversal of a bactiostatic condition by controlled expression of toxins and antitoxins. Mol. Microbiol. 45:501-510. [DOI] [PubMed] [Google Scholar]
- 30.Pedersen, K., A. Zavialiv, M. Pavlov, J. Elf, K. Gerdes, and M. Ehrenberg. 2003. The bacterial toxin RelE displays codon-specific cleavage of mRNAs in the ribosomal A site. Cell 112:133-142. [DOI] [PubMed] [Google Scholar]
- 31.Sabina, J., N. Dover, L. J. Templeton, D. R. Smulski, D. Soll, and R. A. LaRossa. 2003. Interfering with different steps of protein synthesis explored by transcriptional profiling of Escherichia coli K-12. J. Bacteriol. 185:6158-6170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sat, B., R. Hazan, T. Fisher, H. Khaner, G. Glaser, and H. Engelberg-Kulka. 2001. Programmed cell death in Escherichia coli: some antibiotics can trigger mazEF lethality. J. Bacteriol. 183:2041-2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang, R. F., and S. R. Kushner. 1991. Construction of versatile low-copy-number vectors for cloning, sequencing, and gene expression in Escherichia coli. Gene 100:195-199. [PubMed] [Google Scholar]
- 34.Wolfson, J. S., D. C. Hooper, G. L. McHugh, M. A. Bozza, and M. N. Swartz. 1990. Mutants of Escherichia coli K-12 exhibiting reduced killing by both quinolone and β-lactam antimictobial agents. Antimicrob. Agents Chemother. 34:1938-1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu, K. D., G. A. McFeters, and P. S. Stewart. 2000. Biofilm resistance to antimicrobial agents. Microbiology 146:547-549. [DOI] [PubMed] [Google Scholar]
- 36.Zhang, Y., J. Zhang, K. Hoeflich, M. Ikura, G. Qing, and M. Inouye. 2003. MazF cleaves cellular mRNAs specifically at ACA to block protein synthesis in Escherichia coli. Mol. Cell 12:913-923. [DOI] [PubMed] [Google Scholar]