Abstract
Microcolony formation is one of the initial steps in biofilm development, and in enteropathogenic Escherichia coli (EPEC) it is mediated by several adhesins, including the bundle-forming pilus (BFP) and the EspA filament. Here we report that EPEC forms biofilms on plastic under static conditions and a flowthrough continuous culture system. The abilities of several EPEC isogenic mutants to form biofilms were assessed. Adhesins such as BFP and EspA, important in microcolony formation on epithelial cells, are also involved in bacterial aggregation during biofilm formation on abiotic surfaces. Mutants that do not express BFP or EspA form more-diffuse biofilms than does the wild type. We also determined, using gfp transcriptional fusions, that, consistent with the role of these adhesins in biofilms, the genes encoding BFP and EspA are expressed during biofilm formation. Finally, expression of espA is controlled by a quorum-sensing (QS) regulatory mechanism, and the EPEC qseA QS mutant also forms altered biofilms, suggesting that this signaling mechanism plays an important role in EPEC biofilm development. Taken together, these studies allowed us to propose a model of EPEC biofilm formation.
Biofilms have been associated with chronic infections in microorganisms such as Pseudomonas aeruginosa (51), Staphylococcus aureus (12), and Burkholderia cepacia (73), among others. Biofilms have also been implicated as an important step in the life cycle and transmission of Vibrio cholerae in regions where it is endemic (88). Enteropathogenic Escherichia coli (EPEC) is a major cause of infant diarrhea in children in developing countries (53). EPEC causes protracted and chronic diarrhea, and the severity of this disease may require extensive hospitalization (34, 47, 60). EPEC colonizes the proximal small intestine, where it adheres to epithelial cells forming microcolonies. This adhesion pattern has been referred to as localized adherence (LA) (67). EPEC forms microcolonies on cultured epithelial cells, cultures of pediatric small intestinal tissue, and biopsy samples from EPEC patients (3, 33). This adhesion pattern is multifactorial, involving the products of genes encoded within the locus of enterocyte effacement (LEE) pathogenicity island (33), the bundle-forming pili (BFP; type IV pili) (24), and flagella (25). LA is essential for EPEC virulence, given that mutants hindered for microcolony formation have been shown to be highly attenuated for virulence in human volunteers (3).
EPEC also forms attaching and effacing (AE) lesions on the intestinal epithelial cells. These lesions are characterized by the destruction of the microvilli and the rearrangement of the cytoskeleton, culminating in a pedestallike structure which cups each bacterium (42, 49, 82). All the genes involved in the formation of the AE lesion are encoded on the LEE pathogenicity island (36). This region contains the following: (i) genes encoding a type III secretion system (TTSS) (36); (ii) the eae gene encoding the adhesin intimin, responsible for the intimate attachment of the bacteria to the epithelial cell (37); (iii) the espA, espB, and espD genes that encode proteins that are secreted by the TTSS (20, 40, 44), where EspA form a filamentous structure involved in protein translocation and adhesion (43); and (iv) tir, which encodes the translocated intimin receptor (39). The LEE region contains 41 genes, the majority of them organized in five major operons: LEE1, LEE2, LEE3, LEE5, and LEE4. The LEE genes are directly activated by the LEE-encoded regulator (Ler), which is encoded by the first gene in the LEE1 operon (5, 21, 28, 48, 65, 78).
EPEC also contains a large plasmid, referred to as the EPEC adherence factor (EAF) plasmid (52). The EAF plasmid encodes a regulator of virulence genes called Per (plasmid-encoded regulator) consisting of three open reading frames: perA, perB, and perC (26). PerA is an AraC homologue (26) and activates the expression of the bfp operon (81). The per loci also activate the expression of ler, which then activates expression of the other LEE genes in a regulatory cascade (28, 48, 78). The LEE genes are also activated through quorum sensing (QS) (75), which is a mechanism of cell-to-cell signaling via the production of chemical compounds known as autoinducers. QS allows bacteria to “sense” their own population as well as the population of other bacteria in a given environment. This intercellular bacterial communication results in a coordinated population behavior, resulting in a group response to specific environmental cues.
QS was first described in the regulation of bioluminescence in Vibrio fischeri (54) and since then has been shown to be a widespread gene regulation mechanism. One of the most widely distributed QS systems is the luxS system, first described in Vibrio harveyi (79). The presence of luxS is necessary for the synthesis of two autoinducers: AI-2 (79) and AI-3 (76). The AI-3 signal is the actual autoinducer involved in the QS regulation of the LEE and flagellar genes in enterohemorrhagic E. coli and EPEC (76). EPEC virulence genes are repressed by GadX through acid pH (passage into the stomach) and activated in alkaline pH (small intestine environment). GadX, through Per, activates transcription of the bfp operon and the LEE genes (71). Upon activation of the LEE genes, there is formation of AE lesions and later, through BFP and flagella, formation of microcolonies (71). QS regulation is involved in AE lesion formation and microcolony development (72). QS activates transcription of QS E. coli regulator A (QseA) (72, 74), which activates transcription of ler. Furthermore, QS activates expression of the flagellar regulon through the QseBC two-component system (77).
Here we report that EPEC is capable of forming robust biofilms under flowthrough-continuous conditions. Structural appendages, such as BFP and EspA, which are important for microcolony formation, are also involved in bacterial aggregation during biofilm development. Even though EPEC causes persistent infections, nothing is known about EPEC's ability to form a biofilm. Because biofilms are resistant to antimicrobials, a better understanding of EPEC biofilm formation may shed light in its potential role in disease and transmission.
MATERIALS AND METHODS
Strains and plasmids.
All strains and plasmids used in this study are described in Table 1. All mutants are isogenic to wild-type strain E2348/69; mutants CVD206 (eae) (19), UMD872 (espA) (41), 31-6-1 (bfpA) (18), AGT01 (fliC) (25), VS102 (luxS) (72), and VS193 (qseA) (72) have been previously published (16, 17, 24, 61). The bacterial strains were grown overnight in Luria-Bertani (LB) broth or LB agar (LBA) at 37°C. Antibiotics were added in the following concentrations: ampicillin, 100 μg/ml; kanamycin, 50 μg/ml; chloramphenicol, 30 μg/ml; and streptomycin, 100 μg/ml.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant description or genotype | Reference or source |
|---|---|---|
| E2348/69 | EPEC wild type (O127:H6) | 46 |
| CVD206 | EPEC eaeA mutant | 19 |
| UMD872 | EPEC espA mutant | 41 |
| 31-6-1 | EPEC bfpA mutant | 18 |
| AGT01 | EPEC fliC mutant | 25 |
| VS102 | EPEC luxS mutant | 72 |
| VS104 | EPEC luxS complement | 72 |
| VS193 | EPEC qseA mutant | 74 |
| MPS150 | EPEC qseA complement | 72 |
| DH5α | supE44 ΔlacU169 (φ80 lacZΔM15) hsdR17 | Stratagene |
| CGM201 | EPEC fimA mutant | This study |
| CGM211 | EPEC flu mutant | This study |
| pFPV25 | GFP reporter fusion vector | 83 |
| pBlueScript KSII | Cloning vector | Stratagene |
| TOPO PCR blunt | PCR blunt cloning vector with topoisomerase | Invitrogen |
| pd2EGFP | Commercial GFP vector | Clontech |
| pKD3 | Red recombinase expression plasmid for λ Red mutagenesis | 15 |
| pTP223 | λ Red-Gam-producing plasmid for λ Red mutagenesis | 57 |
| pCGM11 | LEE1::gfp in pFPV25 | This study |
| pCGM21 | LEE2::gfp in pFPV25 | This study |
| pCGM31 | LEE3::gfp in pFPV25 | This study |
| pCGM41 | LEE4::gfp in pFPV25 | This study |
| pCGM51 | LEE5::gfp in pFPV25 | This study |
| pCGM61 | perA::gfp in pFPV25 | This study |
| pCGM71 | bfpA::gfp in pFPV25 | This study |
| pCGM81 | fliA::gfp in pFPV25 | This study |
| pCGM91 | fliC::gfp in pFPV25 | This study |
| pCGM101 | flhDC::gfp in pFPV25 | This study |
| pCGM111 | bla::gfp in pFPV25 | This study |
Recombinant DNA techniques.
All molecular biology techniques, such as plasmid purification, PCR, digestion, ligation, transformation, and DNA gel electrophoresis, were performed using standard methods (64). DNA purification and extraction were performed with QIAGEN kits, according to the manufacturer's instructions. Sequences of all oligonucleotide primers used in this study can be found in Table 2 at http://www3.utsouthwestern.edu/microbiology/pages/faculty/sperandio.html.
Western blotting.
The Western blotting analyses of this study were performed using whole-cell lysates from planktonic cultures. Briefly, 3 ml of culture was pelleted (13,000 rpm for 5 min at 4°C) and resuspended in 300 μl lysis buffer (50 mM Tris-HCl [pH 7.5], 50 mM NaCl, 5% glycerol, 1 mM dithiothreitol, and 30 mM phenylmethylsulfonyl fluoride); lysozyme was added to a final concentration of 300 μg/ml; the mixture was incubated at 4°C for 4 h and DNase I treated for 45 min at 4°C; and cell debris was pelleted (13,000 rpm for 10 min at 4°C), and supernatant containing whole-cell protein removed. Protein concentration was measured using the Lowry assay (64). Equal amounts of total proteins were subjected to 12% or 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and blotted to polyvinylidene difluoride membranes. The membranes were later stained with amido black to ensure equal loading of proteins. Western blotting procedures were performed as previously described (64). The membranes were probed with polyclonal antisera directed against antigen 43 (Ag43), kindly provided by Alfredo Torres (University of Texas Medical Branch).
Biofilm formation under static conditions.
The ability of EPEC and the isogenic mutants to form biofilms was assessed in experiments performed in triplicate using Dulbecco's modified Eagle medium (DMEM; Invitrogen). Overnight bacterial cultures grown under static conditions were inoculated into fresh medium in a 1:100 dilution in 24-well cell culture plates (Falcon). These plates were incubated at 37°C in a CO2 atmosphere, using five different time points: 3, 6, 9, 12, and 24 h. The medium in plates was changed every 3 h up to 12 h and then extended further for 12 h to complete the 24-h time point. Biofilm tests were performed on different surfaces: polystyrene and glass.
Quantification of biofilm formation.
Biofilm formation was quantified by calculating CFU of bacteria (9, 61-63). After the test time periods, the biofilms were rinsed three times with phosphate-buffered saline and disrupted with a solution of Triton X-100 (1%) in phosphate-buffered saline for 20 min. Serial dilutions were performed and plated on LBA for CFU counts.
Expression of EPEC virulence genes during biofilm formation using GFP fusions.
We constructed transcriptional fusions of the constitutive bla (β-lactamase) promoter, LEE promoters (LEE1, -2, -3, -4, and -5 or tir), bfpA, perA, fliA, flhDC, and fliC with the promoterless gene encoding the green fluorescent protein (gfp) using vector pFPV25 (83). Briefly, these regulatory regions were amplified using Pfx polymerase (Invitrogen) from EPEC wild-type strain E2348/69 and primers (listed in Table 2 at http://www3.utsouthwestern.edu/microbiology/pages/faculty/sperandio.html) containing EcoRI and BamHI restriction sites. These amplified fragments were cloned into pFPV25 digested with EcoRI and BamHI and transformed into wild-type (WT) EPEC strain E2348/69. Green fluorescent protein (GFP) expression was monitored at the same time points as biofilm quantification. Biofilm assays from E2348/69 harboring the different GFP transcriptional fusions were performed on plastic surfaces in 24-well plates. The biofilms were then disrupted with a Triton X-100 solution, and GFP expression was measured in triplicate with a λ485 excitation and λ520 emission in black 96-well plates with 5% gain in a fluorimeter (Fluostar OPTIMA; BMG Labtechnologies). These readings were blanked against strain CGM01 (E2348/69 containing the promoterless pFVF25 vector). The bacterial cells were then plated on LBA and CFU determined. Relative fluorescence units are expressed as fluorescence divided by CFU.
Light microscopy.
The biofilm formation assays also were performed in triplicate in glass coverslips in 24-well plates using DMEM (Invitrogen). Overnight bacterial cultures grown in static conditions were inoculated into fresh medium in a 1:100 dilution in 24-well cell culture plates (Falcon). These plates were incubated at 37°C in a CO2 atmosphere, using five different time points: 3, 6, 9, 12, and 24 h. The medium in plates was changed every 3 h up to 12 h and then extended for 12 h more to complete the 24-h time point. The procedure was identical to the quantification assay described above. At the different incubation time points, the biofilms were fixed with methanol, stained with Giemsa stain (Sigma), and visualized and photographed in an inverted microscope (Axiovert 200; Carl Zeiss, Inc.).
Lambda Red mutagenesis.
The Lambda Red technology was used in this study for easy PCR-mediated generation of deletion mutants (15, 50, 57) of type 1 fimbriae (fimA gene) and antigen 43 (flu gene) of EPEC prototype E2348/69 based on sequences from the Sanger Institute Database (http://www.sanger.ac.uk/Projects/Escherichia_Shigella/). Briefly, wild-type strain E2348/69 was transformed with plasmid pTP223 (50), expressing the γ, β, and exo Lambda Red genes. Expression of these genes was induced with IPTG (isopropyl-β-d-thiogalactopyranoside). The cat gene from plasmid pKD3 (15) was amplified with primers fimAI and fimAII for fimA deletion and with fluI and fluII for flu deletion (Table 2 at http://www3.utsouthwestern.edu/microbiology/pages/faculty/sperandio.html). These PCR products were then electroporated into E2348/69 (pTP223). Recombinant colonies were plated on LBA with chloramphenicol.
Flow cell chamber biofilm assays.
Biofilms were grown using a once-flowthrough-continuous culture biofilm system (32). The EPEC prototype and the isogenic mutants expressing GFP from the plasmid pD2EGFP (Clontech) were inoculated ∼1 cm upstream of a three-chamber flow cell system (7) by injection of 400 μl of an overnight culture directly into the silicon tubing (internal diameter, 3 mm; Fisher) attached to the flow cell. The bacteria were injected using a 1-ml polypropylene syringe with a 25-gauge needle. The injection area was immediately sealed with silicone sealant, and the system was closed upstream with tubing clamps. The flow cells were incubated inverted for 1 h at 25°C to allow for initial attachment. DMEM (Invitrogen) was then flowed through the flow cell at 60 μl min−1 using a Watson Marlowe 205S peristaltic pump. Biofilms were imaged every 24 h afterwards using an epifluorescence or confocal laser scanning microscope as described above, with a 40× or 60× oil immersion lens. Scanning images were taken with a step size of 1.0 μm in the z axis, and image analysis was performed using the Leica Confocal Software Lite package (Leica Microsystems) (59). Biofilm tests for each strain were performed in triplicate, and six image stacks were acquired randomly at each time point per chamber. Hence, for each time point and each condition, 18 random images were analyzed. Simulated three-dimensional images were generated using the Leica Confocal Software Lite package (Leica Microsystems) (59).
RESULTS AND DISCUSSION
EPEC biofilm formation under static conditions.
Biofilms have been extensively studied in E. coli K-12 strains (13, 58, 68, 69); however, there are no reports on the ability of EPEC to form biofilms. The E. coli genome differs ∼25% among different E. coli serotypes, as revealed by the published genomes of E. coli K-12 (4), 2 enterohemorrhagic E. coli strains (31, 56), 1 uropathogenic E. coli strain (87), and the as yet nonannotated EPEC genome (http://www.sanger.ac.uk/Projects/Escherichia_Shigella/), together with data being generated by three consortia in the United States, Europe, and Japan to sequence ∼20 different E. coli isolates. Furthermore, studies on biofilm formation by different serotypes of V. cholerae have established that these strains use different appendages and strategies to form biofilms (84-86). Considering that EPEC has about an extra megabase of DNA in its genome compared to K-12 (http://www.sanger.ac.uk/Projects/Escherichia_Shigella/), the mechanisms by which EPEC and K-12 form biofilms will probably be quite disparate.
The ability of the WT EPEC to form biofilms was initially addressed under static conditions by assessing the biomass of the biofilm. This can be achieved either by performing CFU counts (9) or by using a crystal violet colorimetric assay (85), which indirectly assesses bacterial cell numbers based on staining of peptidoglycan. The crystal violet colorimetric assay is less labor intensive but is prone to variation due to dehydration of the samples (9). Indeed, we observed that the crystal violet assessment was quite variable; we therefore decided to assess bacterial cell numbers by directly counting the CFU within these biofilms. Using this methodology, we obtained highly reproducible results to assess the ability of EPEC to form biofilms.
The majority of the biofilm studies in E. coli K-12 to date were performed using abiotic surfaces such as plastic (14, 22). Therefore, we initially addressed EPEC biofilm formation on abiotic surfaces under static conditions, and we observed identical biofilms by EPEC on both plastic (polystyrene) and glass (data not shown) surfaces, suggesting that biofilm formation by EPEC was not substratum specific, for at least these two surfaces tested. These assays were then performed on polystyrene in DMEM at 37°C in 5% CO2 (Fig. 1). This medium was chosen because expression of the EPEC virulence genes has been extensively reported to be induced during growth in DMEM (5, 38, 65, 75). On polystyrene, EPEC formed a biofilm with a biomass of 107 CFU/cm2 by 3 h. The biomass of this biofilm diminished 2 orders of magnitude (105 CFU/cm2) by 6 h. This biofilm then started to increase its biomass again to 106 CFU/cm2 at 12 h and a biomass of 108 CFU/cm2 by 24 h (Fig. 1).
FIG. 1.
Measurement of biofilm biomass during different incubation time points (3, 6, 9, 12, and 24 h) by WT EPEC (filled diamonds) and isogenic mutants in espA (filled squares), bfp (open triangles), qseA (×'s), fimA (filled circles), and flu (open circles) in DMEM on an abiotic surface under static conditions. Biomass is expressed as CFU/cm2. Error bars indicate standard errors of the means.
Expression of EPEC virulence genes during biofilm formation.
It has been well documented that E. coli K-12 expresses type 1 fimbriae and antigen 43 (13, 58, 68, 69) during biofilm formation. Given that EPEC produces additional adhesins such as the BFP (which is a type IV fimbria involved in microcolony formation), the EspA filament (involved in adhesion and protein translocation through the TTSS), and intimin (which is the adhesin responsible for the intimate attachment of EPEC to the epithelial cells), we decided to address gene expression patterns of these and other EPEC virulence factors during biofilm formation on abiotic surfaces in DMEM under static conditions (Fig. 2). For this purpose, we constructed transcription fusions with a reporter gfp gene. Among the promoter regions tested were LEE1, LEE2, LEE3, LEE4, LEE5, per, bfp, fliA, fliC, and flhDC. LEE1 encodes Ler (the transcriptional regulator of all LEE genes) (48), a chaperone for EspA (11), and inner membrane components of the TTSS (23). LEE2 encodes the outer membrane components of the TTSS, and LEE3 encodes inner membrane TTSS proteins and the EscN ATPase (23). LEE5 encodes Tir (the translocated intimin receptor) and intimin (37, 39). LEE4 encodes the TTSS needle (EscF) (70) and the EspA filament (43). Both bfp and per are carried within the EAF plasmid. The Per proteins activate transcription of the bfp operon and ler (26, 48, 81). The genes from the flagellar regulon tested were flhDC (master flagellar regulator); fliA, which is a class 2 gene encoding the alternative sigma factor σ28; and fliC, which encodes flagellin (class 3 genes) (6). We used as a negative control the bla::gfp (β-lactamase promoter) fusion, which is constitutive and, as expected, did not have its transcription changed in any of the conditions tested (Fig. 2). Transcription of all promoters was normalized by the number of bacterial cells and subtracted from basal gfp expression levels expressed by the promoterless gfp vector (pFPV25).
FIG. 2.
Transcription expression of EPEC virulence genes (LEE1, LEE2, LEE3, LEE4, LEE5, per, bfp, fliA, fliC, and flhDC) during biofilm formation on plastic in DMEM using transcription fusions with a reporter gfp gene (plasmid pFPV25). We used as a negative control the bla::gfp (β-lactamase promoter) fusion, which is constitutive. Transcription of all promoters was normalized by the number of bacterial cells and subtracted from basal gfp expression levels expressed by the promoterless gfp vector (pFPV25).
During biofilm formation on a plastic surface, the LEE2 operon showed the highest expression, peaking at 9 h and diminishing by 24 h (Fig. 2). Transcription of LEE2 is activated by Ler (encoded within LEE1) (48, 78) and GrlRA (17; R. Russell and V. Sperandio, unpublished results). The grlRA genes comprise a small operon within the LEE region. It has been proposed that GrlA activates transcription of the LEE genes through ler, while GrlR represses it (17). Finally, there is another level of feedback regulation, in which transcription of the grlRA operon is activated by Ler (2, 21). GrlA has been reported to interact with itself in yeast two-hybrid experiments, suggesting that this transcription regulator may function at least as a dimer. Furthermore, GrlRA have also been reported to interact with each other, adding yet another level of complexity to this regulatory network (10). GrlRA activate the transcription of LEE2 in a Ler-independent manner (R. Russell and V. Sperandio, unpublished results), suggesting that there are two levels of induction of LEE2 expression, one through Ler and another through GrlRA.
We could not detect expression of LEE1 (Fig. 2); however, it has been recently demonstrated that transcription of LEE1 occurs as early as 10 min (45), and it was almost undetectable by real-time reverse transcription-PCR (a more sensitive technique than gfp fusions) by 5 h. Thus, the observation that we could not detect LEE1 transcription using gfp fusions after 3 h is consistent with the results reported by Leverton and Kaper (45). The high levels of LEE2 transcription compared to the other LEE genes could be a consequence of the additional regulation through GrlRA. Transcription of LEE3, LEE4, and LEE5 could be detected only after 9 h (Fig. 2). The differential kinetics of LEE gene transcription during biofilm formation is reflective of the complex regulation of the LEE, with many transcription factors affecting the final outcome.
Expression of both per and bfp operons was elevated, reaching maximal expression at 9 h and then diminishing by 24 h (Fig. 2). The product of the perA gene from the per operon directly activates transcription of the bfp genes (35). Therefore, the correlation of expression of per and bfp was predicted. Transcription of fliA was observed only at 24 h. The fliA gene encodes the flagellar alternative sigma factor σ28, which is essential for the expression of flagellin and motility genes (6). Transcription of all other flagellar genes tested was too low to be detected (Fig. 2). These data suggest that key EPEC virulence genes, such as the LEE and bfp genes, are expressed during biofilm formation and may also play a role in biofilm development.
Biofilm formation by EPEC isogenic mutants.
To test the above hypothesis further, we next assessed the ability of several EPEC mutants to form biofilms under static conditions. We tested mutants in fimA (encodes type 1 fimbria subunit, which is the E. coli adhesin involved in early biofilm attachment), flu (encodes antigen 43, involved in cell-to-cell aggregation during biofilm formation), and fliC (encodes flagellin), which have been shown to be extensively involved in biofilm formation in K-12 (13, 58, 68, 69). We also tested several mutants in EPEC-specific adhesins, such as those with mutations of bfp, espA (carried within LEE4), and eae (carried within LEE5), shown to be expressed during biofilm formation (Fig. 2). Finally, given that quorum-sensing gene regulation is required for biofilm maturation and virulence in other pathogens (16, 27, 29, 88, 89) and, in EPEC QS, is also involved in regulation of the LEE genes (72), we tested whether QS mutants were also altered in biofilm formation. We tested two QS mutants, one with a mutation in luxS (enzyme necessary for the synthesis of the QS signaling molecules AI-2 and AI-3) and one in qseA (transcription regulator of the QS cascade that activates transcription of the LEE genes) (72, 74).
Biofilms in DMEM on plastic showed bacterial cell numbers between 1 × 105 and 1 × 108 CFU/cm2 (Fig. 1). The biofilms formed by eae, fliC, and luxS mutants were similar to the WT (data not shown). However, biofilm formation was higher in the espA, bfp, qseA, fimA, and flu mutants than in the WT at earlier time points (Fig. 1). The bfp and espA mutants formed the highest-density biofilms between 6- and 12-h time points. The espA mutant biofilm had 3 orders of magnitude more bacterial cells than the WT at 6 h, and 2 orders of magnitude more cells between 9 and 12 h. This biofilm started at a 108-CFU/cm2 bacterial cell density and kept it steady throughout the test. The bfp mutant biofilm had 2 orders of magnitude more cells than the WT between 6 and 12 h. Both espA and bfp mutant biofilms still had almost 1 order of magnitude more bacterial cells than WT biofilms by 24 h. Biofilms by fimA and qseA mutants both had 1 order of magnitude more bacterial cells than the WT between 6 to 12 h. The flu mutant biofilm began to have 1 order of magnitude more bacterial cells at 6 h and then its results became similar to that for the WT. By 24 h, WT biofilms had the same density as the biofilms formed by these three latter mutants (Fig. 1). Biofilm formation by the WT and bfp, espA, and qseA mutants in this condition is illustrated in Fig. 3, showing biofilm formation at 6, 12, and 24 h, respectively. The WT forms a very weak biofilm at 6 h and starts organizing these biofilms into microcolonies at 12 h, having more defined microcolonies by 24 h. The qseA mutant forms a very compacted biofilm comprised of large microcolonies by 6 and 12 h, showing dispersal of these microcolonies by 24 h. The espA mutant already showed a very dense biofilm by 6 h and kept it steady until 24 h, in agreement with the CFU density measurements depicted in Fig. 1. However, this biofilm was more diffuse and did not organize into microcolonies. The bfp mutant showed a progressive increase in biofilm formation with a very dense and diffuse biofilm already at 6 h and becoming denser by 24 h (Fig. 1). The growth rate of all mutants is identical to the WT (data not shown); hence, the differences observed in the biofilm biomass are not due to differential growth among these strains.
FIG. 3.
Light microscopy of biofilm formation by WT EPEC and isogenic mutants (of espA, bfpA, and qseA) during different incubation time points (6, 12, and 24 h) in DMEM on an abiotic surface under static conditions. Magnification, ×100.
Our results thus far suggest that WT EPEC does not form a very dense biofilm on abiotic surfaces (under static conditions) (Fig. 1 and 3) and that EPEC is mostly in the microcolony state of biofilm development by 24 h (Fig. 3). Within biofilms, EPEC expresses several virulence genes, namely LEE and bfp (Fig. 2). BFP is expressed as early as 3 h and is expressed until 24 h. EspA (LEE4) expression is first detected at 9 h and increases until 24 h (Fig. 2). Both BFP and EspA play a role in microcolony formation in EPEC adhesion to epithelial cells (8). The observation that both the bfp and espA mutants form disperse biofilms, lacking bacterial clustering (Fig. 3), suggests that the role of these two adhesins in biofilm development is in bacterial aggregation. The clustered nature of the qseA mutant biofilm suggested that QseA may have a repressive role on the expression of bacterial autoaggregation appendages. We had previously reported that QseA did not affect expression of BFP and activated expression of the LEE genes and, consequently, espA (72). Hence, the autoaggregation appendages down-regulated by QseA were likely not BFP and EspA. Antigen 43 is a self-recognizing surface protein that confers autoaggregation of bacteria (30), whose expression is important during biofilm development in E. coli. We initially observed that the qseA mutant autoaggregates during planktonic growth in LB (Fig. 4A) and DMEM (data not shown), and this phenotype could be complemented with qseA in trans. In agreement with this phenotype, we observed that the qseA mutant overexpressed antigen 43 (Fig. 4B), while both WT and complemented strains did not. Overexpression of antigen 43 by the qseA mutant could be responsible for the extensive aggregative phenotype in qseA biofilms (Fig. 3).
FIG. 4.
A) Autoaggregation of bacterial cells during planktonic growth in LB. Autoaggregation was measured by absorbance at an optical density at 600 nm (OD600) of supernatants from WT (open diamonds), qseA mutant (filled squares) and qseA mutant complemented (filled triangles) EPEC strains of static cultures during a period of 360 min. B) Western blot of whole-cell lysates of WT (left lane), qseA mutant (middle lane), and qseA mutant complemented (right lane) EPEC strains grown to an OD600 of 1.0 in LB; a cross-reactive band was used as a loading control.
EPEC biofilm formation on abiotic surfaces in a flowthrough-continuous culture system.
The study of static biofilms allows the observation of the initial stages in biofilm development (up to 24 h) but does not allow the study of long-term biofilms. The use of flow chambers allows for longer incubation times and for a less static environmental condition due to the continuous reposition of nutrients by the continuous flow of fresh medium. In this system, a glass coverslip is fixed to the top of the flow cell chamber. This glass coverslip is the surface on which the biofilm development is monitored using confocal laser scanning microscopy (CLSM) of GFP-expressing bacteria. CLSM images were taken within 2 h of inoculation and at 24-h intervals up to 72 h (55). EPEC had already formed a mature 40-μm-thick biofilm by 24 h (Fig. 5), and this biofilm was maintained until 72 h (data not shown). This was in contrast to the biofilms formed in static conditions, which were still in the microcolony stage by 24 h (Fig. 3).
FIG. 5.
CLSM of flow cell assays on plastic in DMEM. Shown are two-dimensional transparent projections of series images in the z axis in green channels in the z, x, and y axes, their respective projection transparent stereo images at a 180o angle, and the three-dimensional view projection of image topography in the z, x, and y axes. Bars, 40 μm. A) WT EPEC 24 h; B) EPEC fimA mutant 48 h; C) EPEC flu mutant 48 h; D) EPEC bfp mutant 24 h.
Using the flow chamber system, the fimA (type 1 fimbriae) mutant was delayed for biofilm formation due to impaired initial attachment, forming a 42-μm biofilm comparable to that of the WT only after 48 h (Fig. 5B). The flu (Ag43) mutant was developmentally impaired for biofilm formation and, after 48 h, only formed a flat 20-μm biofilm (Fig. 5C), never forming an organized three-dimensional structure. In the flow chamber system the bfp mutant also formed denser biofilms than the WT, having a 52-μm thick biofilm at 24 h (Fig. 5D), compared to the 40-μm thick WT biofilm (Fig. 5A). In this system, the bfp biofilm is also more diffuse than that of the WT (Fig. 5A and D), suggesting again that BFP is playing a role in interbacterial aggregation during biofilm development.
The flowthrough system is a more dynamic and nutrient-rich environment than static conditions for biofilm formation. EPEC seems to form very dense and developed biofilms in this system by 24 h, reaching 40 μm and having a defined three-dimensional structure (Fig. 5A). This is in contrast with the weak biofilm formed in static conditions, which reached only 15 μm (data not shown) and was arrested at the microcolony developmental state (Fig. 1 and 3). In both static and flowthrough conditions (Fig. 3 and 5), BFP was involved in microcolony formation during biofilm development. BFP has previously been shown to be involved in epithelial-cell adhesion during microcolony formation by EPEC (24), but no role has ever been established for this pilus during biofilm formation on abiotic surfaces. Our data suggest that during biofilm formation by EPEC, the major role of BFP is in bacterial autoaggregation.
Concluding remarks.
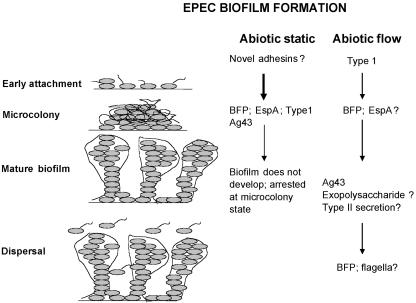
The data accumulated thus far allow us to propose a model for EPEC biofilm formation on abiotic surfaces under static and flowthrough conditions (Fig. 6). On abiotic surfaces under static conditions, EPEC probably uses novel (as yet undescribed) adhesins for initial attachment. This biofilm then develops into a microcolony using structures such as BFP, EspA, type 1 fimbriae, and antigen 43. Under static conditions, this biofilm is arrested at the microcolony state and does not develop into a mature three-dimensional structure. On abiotic surfaces under flowthrough conditions, EPEC uses type 1 fimbriae for the initial adhesion. BFP is needed for microcolony development, and EspA might be also; development of a mature biofilm requires antigen 43 and maybe exopolysaccharide and type II secretion. In the EPEC genome (http://www.sanger.ac.uk/Projects/Escherichia_Shigella/), we found homologues of the enterotoxigenic E. coli gsp genes. These genes encode a type II secretory pathway involved in secretion of the heat-labile enterotoxin (LT) (80). This secretory system is also a homologue of the V. cholerae eps system involved in secretion of the cholera toxin (66). The V. cholerae eps system is also involved in the secretion of the exopolysaccharide (1), necessary for biofilm formation (29, 89). This biofilm is then later dispersed, probably using flagella, which are expressed only at 24 h (Fig. 2).
FIG. 6.
Proposed model of biofilm formation by EPEC.
Whether biofilms are involved in the virulence or the epidemiological spread of EPEC in regions where it is endemic remains to be established. This study reports for the first time the ability of EPEC to form dense biofilms, especially under flowthrough conditions. We also report that virulence traits of EPEC such as BFP and EspA are involved in biofilm development.
Acknowledgments
We thank Alfredo G. Torres for providing the anti-antigen 43 serum.
This work has been supported by NIH grants AI054468 and AI053067 to V.S. C.G.M. was supported by fellowships from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
REFERENCES
- 1.Ali, A., J. A. Johnson, A. A. Franco, D. J. Metzger, T. D. Connell, J. G. Morris, Jr., and S. Sozhamannan. 2000. Mutations in the extracellular protein secretion pathway genes (eps) interfere with rugose polysaccharide production in and motility of Vibrio cholerae. Infect. Immun. 68:1967-1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barba, J., V. H. Bustamante, M. A. Flores-Valdez, W. Deng, B. B. Finlay, and J. L. Puente. 2005. A positive regulatory loop controls expression of the locus of enterocyte effacement-encoded regulators Ler and GrlA. J. Bacteriol. 187:7918-7930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bieber, D., S. W. Ramer, C. Y. Wu, W. J. Murray, T. Tobe, R. Fernandez, and G. K. Schoolnik. 1998. Type IV pili, transient bacterial aggregates, and virulence of enteropathogenic Escherichia coli. Science 280:2114-2118. [DOI] [PubMed] [Google Scholar]
- 4.Blattner, F. R., G. Plunkett III, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1474. [DOI] [PubMed] [Google Scholar]
- 5.Bustamante, V. H., F. J. Santana, E. Calva, and J. L. Puente. 2001. Transcriptional regulation of type III secretion genes in enteropathogenic Escherichia coli: Ler antagonizes H-NS-dependent repression. Mol. Microbiol. 39:664-678. [DOI] [PubMed] [Google Scholar]
- 6.Chilcott, G. S., and K. T. Hughes. 2000. Coupling of flagellar gene expression to flagellar assembly in Salmonella enterica serovar Typhimurium and Escherichia coli. Microbiol. Mol. Biol. Rev. 64:694-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christensen, B. B., C. Sternberg, J. B. Andersen, R. J. Palmer, Jr., A. T. Nielsen, M. Givskov, and S. Molin. 1999. Molecular tools for study of biofilm physiology. Methods Enzymol. 310:20-42. [DOI] [PubMed] [Google Scholar]
- 8.Cleary, J., L. C. Lai, R. K. Shaw, A. Straatman-Iwanowska, M. S. Donnenberg, G. Frankel, and S. Knutton. 2004. Enteropathogenic Escherichia coli (EPEC) adhesion to intestinal epithelial cells: role of bundle-forming pili (BFP), EspA filaments and intimin. Microbiology 150:527-538. [DOI] [PubMed] [Google Scholar]
- 9.Costerton, J. W. 2004. A short history of the development of the biofilm concept, p. 4-19. In M. Ghannoum and G. A. O'Toole (ed.), Microbial biofilms, 1st ed. ASM Press, Washington, D.C.
- 10.Creasey, E. A., R. M. Delahay, S. J. Daniell, and G. Frankel. 2003. Yeast two-hybrid system survey of interactions between LEE-encoded proteins of enteropathogenic Escherichia coli. Microbiology 149:2093-2106. [DOI] [PubMed] [Google Scholar]
- 11.Creasey, E. A., D. Friedberg, R. K. Shaw, T. Umanski, S. Knutton, I. Rosenshine, and G. Frankel. 2003. CesAB is an enteropathogenic Escherichia coli chaperone for the type-III translocator proteins EspA and EspB. Microbiology 149:3639-3647. [DOI] [PubMed] [Google Scholar]
- 12.Cucarella, C., C. Solano, J. Valle, B. Amorena, I. Lasa, and J. R. Penades. 2001. Bap, a Staphylococcus aureus surface protein involved in biofilm formation. J. Bacteriol. 183:2888-2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Danese, P. N., L. A. Pratt, S. L. Dove, and R. Kolter. 2000. The outer membrane protein, antigen 43, mediates cell-to-cell interactions within Escherichia coli biofilms. Mol. Microbiol. 37:424-432. [DOI] [PubMed] [Google Scholar]
- 14.Danese, P. N., L. A. Pratt, and R. Kolter. 2000. Exopolysaccharide production is required for development of Escherichia coli K-12 biofilm architecture. J. Bacteriol. 182:3593-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davies, D. G., M. R. Parsek, J. P. Pearson, B. H. Iglewski, J. W. Costerton, and E. P. Greenberg. 1998. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 280:295-298. [DOI] [PubMed] [Google Scholar]
- 17.Deng, W., J. L. Puente, S. Gruenheid, Y. Li, B. A. Vallance, A. Vazquez, J. Barba, J. A. Ibarra, P. O'Donnell, P. Metalnikov, K. Ashman, S. Lee, D. Goode, T. Pawson, and B. B. Finlay. 2004. Dissecting virulence: systematic and functional analyses of a pathogenicity island. Proc. Natl. Acad. Sci. USA 101:3597-3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donnenberg, M. S., J. A. Giron, J. P. Nataro, and J. B. Kaper. 1992. A plasmid-encoded type IV fimbrial gene of enteropathogenic Escherichia coli associated with localized adherence. Mol. Microbiol. 6:3427-3437. [DOI] [PubMed] [Google Scholar]
- 19.Donnenberg, M. S., and J. B. Kaper. 1991. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect. Immun. 59:4310-4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Donnenberg, M. S., J. Yu, and J. B. Kaper. 1993. A second chromosomal gene necessary for intimate attachment of enteropathogenic Escherichia coli to epithelial cells. J. Bacteriol. 175:4670-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elliott, S. J., V. Sperandio, J. A. Giron, S. Shin, J. L. Mellies, L. Wainwright, S. W. Hutcheson, T. K. McDaniel, and J. B. Kaper. 2000. The locus of enterocyte effacement (LEE)-encoded regulator controls expression of both LEE- and non-LEE-encoded virulence factors in enteropathogenic and enterohemorrhagic Escherichia coli. Infect. Immun. 68:6115-6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrieres, L., and D. J. Clarke. 2003. The RcsC sensor kinase is required for normal biofilm formation in Escherichia coli K-12 and controls the expression of a regulon in response to growth on a solid surface. Mol. Microbiol. 50:1665-1682. [DOI] [PubMed] [Google Scholar]
- 23.Garmendia, J., G. Frankel, and V. F. Crepin. 2005. Enteropathogenic and enterohemorrhagic Escherichia coli infections: translocation, translocation, translocation. Infect. Immun. 73:2573-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giron, J. A., A. S. Ho, and G. K. Schoolnik. 1991. An inducible bundle-forming pilus of enteropathogenic Escherichia coli. Science 254:710-713. [DOI] [PubMed] [Google Scholar]
- 25.Giron, J. A., A. G. Torres, E. Freer, and J. B. Kaper. 2002. The flagella of enteropathogenic Escherichia coli mediate adherence to epithelial cells. Mol. Microbiol. 44:361-379. [DOI] [PubMed] [Google Scholar]
- 26.Gomez-Duarte, O. G., and J. B. Kaper. 1995. A plasmid-encoded regulatory region activates chromosomal eaeA expression in enteropathogenic Escherichia coli. Infect. Immun. 63:1767-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gonzalez Barrios, A. F., R. Zuo, Y. Hashimoto, L. Yang, W. E. Bentley, and T. K. Wood. 2006. Autoinducer 2 controls biofilm formation in Escherichia coli through a novel motility quorum-sensing regulator (MqsR, B3022). J. Bacteriol. 188:305-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haack, K. R., C. L. Robinson, K. J. Miller, J. W. Fowlkes, and J. L. Mellies. 2003. Interaction of Ler at the LEE5 (tir) operon of enteropathogenic Escherichia coli. Infect. Immun. 71:384-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hammer, B. K., and B. L. Bassler. 2003. Quorum sensing controls biofilm formation in Vibrio cholerae. Mol. Microbiol. 50:101-104. [DOI] [PubMed] [Google Scholar]
- 30.Hasman, H., T. Chakraborty, and P. Klemm. 1999. Antigen-43-mediated autoaggregation of Escherichia coli is blocked by fimbriation. J. Bacteriol. 181:4834-4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hayashi, T., K. Makino, M. Ohnishi, K. Kurokawa, K. Ishii, K. Yokoyama, C. G. Han, E. Ohtsubo, K. Nakayama, T. Murata, M. Tanaka, T. Tobe, T. Iida, H. Takami, T. Honda, C. Sasakawa, N. Ogasawara, T. Yasunaga, S. Kuhara, T. Shiba, M. Hattori, and H. Shinagawa. 2001. Complete genome sequence of enterohemorrhagic Escherichia coli O157:H7 and genomic comparison with a laboratory strain K-12. DNA Res. 8:11-22. [DOI] [PubMed] [Google Scholar]
- 32.Heydorn, A., A. T. Nielsen, M. Hentzer, C. Sternberg, M. Givskov, B. K. Ersboll, and S. Molin. 2000. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology 146:2395-2407. [DOI] [PubMed] [Google Scholar]
- 33.Hicks, S., G. Frankel, J. B. Kaper, G. Dougan, and A. D. Phillips. 1998. Role of intimin and bundle-forming pili in enteropathogenic Escherichia coli adhesion to pediatric intestinal tissue in vitro. Infect. Immun. 66:1570-1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hill, S. M., A. D. Phillips, and J. A. Walker-Smith. 1991. Enteropathogenic Escherichia coli and life threatening chronic diarrhoea. Gut 32:154-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ibarra, J. A., M. I. Villalba, and J. L. Puente. 2003. Identification of the DNA binding sites of PerA, the transcriptional activator of the bfp and per operons in enteropathogenic Escherichia coli. J. Bacteriol. 185:2835-2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jarvis, K. G., J. A. Giron, A. E. Jerse, T. K. McDaniel, M. S. Donnenberg, and J. B. Kaper. 1995. Enteropathogenic Escherichia coli contains a putative type III secretion system necessary for the export of proteins involved in attaching and effacing lesion formation. Proc. Natl. Acad. Sci. USA 92:7996-8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jerse, A. E., J. Yu, B. D. Tall, and J. B. Kaper. 1990. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc. Natl. Acad. Sci. USA 87:7839-7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kenny, B., A. Abe, M. Stein, and B. B. Finlay. 1997. Enteropathogenic Escherichia coli protein secretion is induced in response to conditions similar to those in the gastrointestinal tract. Infect. Immun. 65:2606-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kenny, B., R. DeVinney, M. Stein, D. J. Reinscheid, E. A. Frey, and B. B. Finlay. 1997. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell 91:511-520. [DOI] [PubMed] [Google Scholar]
- 40.Kenny, B., and B. B. Finlay. 1995. Protein secretion by enteropathogenic Escherichia coli is essential for transducing signals to epithelial cells. Proc. Natl. Acad. Sci. USA 92:7991-7995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kenny, B., L. C. Lai, B. B. Finlay, and M. S. Donnenberg. 1996. EspA, a protein secreted by enteropathogenic Escherichia coli, is required to induce signals in epithelial cells. Mol. Microbiol. 20:313-323. [DOI] [PubMed] [Google Scholar]
- 42.Knutton, S., M. M. Baldini, J. B. Kaper, and A. S. McNeish. 1987. Role of plasmid-encoded adherence factors in adhesion of enteropathogenic Escherichia coli to HEp-2 cells. Infect. Immun. 55:78-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knutton, S., I. Rosenshine, M. J. Pallen, I. Nisan, B. C. Neves, C. Bain, C. Wolff, G. Dougan, and G. Frankel. 1998. A novel EspA-associated surface organelle of enteropathogenic Escherichia coli involved in protein translocation into epithelial cells. EMBO J. 17:2166-2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lai, L. C., L. A. Wainwright, K. D. Stone, and M. S. Donnenberg. 1997. A third secreted protein that is encoded by the enteropathogenic Escherichia coli pathogenicity island is required for transduction of signals and for attaching and effacing activities in host cells. Infect. Immun. 65:2211-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leverton, L. Q., and J. B. Kaper. 2005. Temporal expression of enteropathogenic Escherichia coli virulence genes in an in vitro model of infection. Infect. Immun. 73:1034-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levine, M. M., E. J. Bergquist, D. R. Nalin, D. H. Waterman, R. B. Hornick, C. R. Young, and S. Sotman. 1978. Escherichia coli strains that cause diarrhoea but do not produce heat-labile or heat-stable enterotoxins and are non-invasive. Lancet i:1119-1122. [DOI] [PubMed] [Google Scholar]
- 47.Levine, M. M., and R. Edelman. 1984. Enteropathogenic Escherichia coli of classic serotypes associated with infant diarrhea: epidemiology and pathogenesis. Epidemiol. Rev. 6:31-51. [DOI] [PubMed] [Google Scholar]
- 48.Mellies, J. L., S. J. Elliott, V. Sperandio, M. S. Donnenberg, and J. B. Kaper. 1999. The Per regulon of enteropathogenic Escherichia coli: identification of a regulatory cascade and a novel transcriptional activator, the locus of enterocyte effacement (LEE)-encoded regulator (Ler). Mol. Microbiol. 33:296-306. [DOI] [PubMed] [Google Scholar]
- 49.Moon, H. W., S. C. Whipp, R. A. Argenzio, M. M. Levine, and R. A. Giannella. 1983. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect. Immun. 41:1340-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Murphy, K. C., and K. G. Campellone. 2003. Lambda Red-mediated recombinogenic engineering of enterohemorrhagic and enteropathogenic E. coli. BMC Mol. Biol. 4:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nagata, T., H. Mukae, J. Kadota, T. Hayashi, T. Fujii, M. Kuroki, R. Shirai, K. Yanagihara, K. Tomono, T. Koji, and S. Kohno. 2004. Effect of erythromycin on chronic respiratory infection caused by Pseudomonas aeruginosa with biofilm formation in an experimental murine model. Antimicrob. Agents Chemother. 48:2251-2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nataro, J. P., M. M. Baldini, J. B. Kaper, R. E. Black, N. Bravo, and M. M. Levine. 1985. Detection of an adherence factor of enteropathogenic Escherichia coli with a DNA probe. J. Infect. Dis. 152:560-565. [DOI] [PubMed] [Google Scholar]
- 53.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nealson, K. H., T. Platt, and J. W. Hastings. 1970. Cellular control of the synthesis and activity of the bacterial luminescent system. J. Bacteriol. 104:313-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parsek, M. R., and E. P. Greenberg. 1999. Quorum sensing signals in development of Pseudomonas aeruginosa biofilms. Methods Enzymol. 310:43-55. [DOI] [PubMed] [Google Scholar]
- 56.Perna, N. T., G. Plunkett III, V. Burland, B. Mau, J. D. Glasner, D. J. Rose, G. F. Mayhew, P. S. Evans, J. Gregor, H. A. Kirkpatrick, G. Posfai, J. Hackett, S. Klink, A. Boutin, Y. Shao, L. Miller, E. J. Grotbeck, N. W. Davis, A. Lim, E. T. Dimalanta, K. D. Potamousis, J. Apodaca, T. S. Anantharaman, J. Lin, G. Yen, D. C. Schwartz, R. A. Welch, and F. R. Blattner. 2001. Genome sequence of enterohaemorrhagic Escherichia coli O157:H7. Nature 409:529-533. [DOI] [PubMed] [Google Scholar]
- 57.Poteete, A. R., and A. C. Fenton. 1984. Lambda red-dependent growth and recombination of phage P22. Virology 134:161-167. [DOI] [PubMed] [Google Scholar]
- 58.Pratt, L. A., and R. Kolter. 1998. Genetic analysis of Escherichia coli biofilm formation: roles of flagella, motility, chemotaxis and type I pili. Mol. Microbiol. 30:285-293. [DOI] [PubMed] [Google Scholar]
- 59.Ramsey, M. M., and M. Whiteley. 2004. Pseudomonas aeruginosa attachment and biofilm development in dynamic environments. Mol. Microbiol. 53:1075-1087. [DOI] [PubMed] [Google Scholar]
- 60.Rothbaum, R., A. J. McAdams, R. Giannella, and J. C. Partin. 1982. A clinicopathologic study of enterocyte-adherent Escherichia coli: a cause of protracted diarrhea in infants. Gastroenterology 83:441-454. [PubMed] [Google Scholar]
- 61.Ryu, J.-H., and L. R. Beuchat. 2005. Biofilm formation by Escherichia coli O157:H7 on stainless steel: effect of exopolysaccharide and curli production on its resistance to chlorine. Appl. Environ. Microbiol. 71:247-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ryu, J.-H., and L. R. Beuchat. 2003. Development of method to quantify extracellular carbohydrate complexes produced by Escherichia coli O157:H7. J. Appl. Microbiol. 95:1304-1314. [DOI] [PubMed] [Google Scholar]
- 63.Ryu, J.-H., and L. R. Beuchat. 2004. Factors affecting production of extracellular carbohydrate complexes by Escherichia coli O157:H7. Int. J. Food Microbiol. 95:189-204. [DOI] [PubMed] [Google Scholar]
- 64.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 65.Sánchez-SanMartín, C., V. H. Bustamante, E. Calva, and J. L. Puente. 2001. Transcriptional regulation of the orf19 gene and the tir-cesT-eae operon of enteropathogenic Escherichia coli. J. Bacteriol. 183:2823-2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sandkvist, M., L. O. Michel, L. P. Hough, V. M. Morales, M. Bagdasarian, M. Koomey, and V. J. DiRita. 1997. General secretion pathway (eps) genes required for toxin secretion and outer membrane biogenesis in Vibrio cholerae. J. Bacteriol. 179:6994-7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Scaletsky, I. C., M. L. Silva, and L. R. Trabulsi. 1984. Distinctive patterns of adherence of enteropathogenic Escherichia coli to HeLa cells. Infect. Immun. 45:534-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schembri, M. A., L. Hjerrild, M. Gjermansen, and P. Klemm. 2003. Differential expression of the Escherichia coli autoaggregation factor antigen 43. J. Bacteriol. 185:2236-2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schembri, M. A., and P. Klemm. 2001. Biofilm formation in a hydrodynamic environment by novel fimH variants and ramifications for virulence. Infect. Immun. 69:1322-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sekiya, K., M. Ohishi, T. Ogino, K. Tamano, C. Sasakawa, and A. Abe. 2001. Supermolecular structure of the enteropathogenic Escherichia coli type III secretion system and its direct interaction with the EspA-sheath-like structure. Proc. Natl. Acad. Sci. USA 98:11638-11643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shin, S., M. P. Castanie-Cornet, J. W. Foster, J. A. Crawford, C. Brinkley, and J. B. Kaper. 2001. An activator of glutamate decarboxylase genes regulates the expression of enteropathogenic Escherichia coli virulence genes through control of the plasmid-encoded regulator, Per. Mol. Microbiol. 41:1133-1150. [DOI] [PubMed] [Google Scholar]
- 72.Sircili, M. P., M. Walters, L. R. Trabulsi, and V. Sperandio. 2004. Modulation of enteropathogenic Escherichia coli virulence by quorum sensing. Infect. Immun. 72:2329-2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Speert, D. P. 2002. Advances in Burkholderia cepacia complex. Paediatr. Respir. Rev. 3:230-235. [DOI] [PubMed] [Google Scholar]
- 74.Sperandio, V., C. C. Li, and J. B. Kaper. 2002. Quorum-sensing Escherichia coli regulator A: a regulator of the LysR family involved in the regulation of the locus of enterocyte effacement pathogenicity island in enterohemorrhagic E. coli. Infect. Immun. 70:3085-3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sperandio, V., J. L. Mellies, W. Nguyen, S. Shin, and J. B. Kaper. 1999. Quorum sensing controls expression of the type III secretion gene transcription and protein secretion in enterohemorrhagic and enteropathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA 96:15196-15201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sperandio, V., A. G. Torres, B. Jarvis, J. P. Nataro, and J. B. Kaper. 2003. Bacteria-host communication: the language of hormones. Proc. Natl. Acad. Sci. USA 100:8951-8956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sperandio, V., A. G. Torres, and J. B. Kaper. 2002. Quorum sensing Escherichia coli regulators B and C (QseBC): a novel two-component regulatory system involved in the regulation of flagella and motility by quorum sensing in E. coli. Mol. Microbiol. 43:809-821. [DOI] [PubMed] [Google Scholar]
- 78.Sperandio, V. V. 2000. How the bacterial flora and the epithelial cell get along. Trends Microbiol. 8:544. [DOI] [PubMed] [Google Scholar]
- 79.Surette, M. G., M. B. Miller, and B. L. Bassler. 1999. Quorum sensing in Escherichia coli, Salmonella typhimurium, and Vibrio harveyi: a new family of genes responsible for autoinducer production. Proc. Natl. Acad. Sci. USA 96:1639-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tauschek, M., R. J. Gorrell, R. A. Strugnell, and R. M. Robins-Browne. 2002. Identification of a protein secretory pathway for the secretion of heat-labile enterotoxin by an enterotoxigenic strain of Escherichia coli. Proc. Natl. Acad. Sci. USA 99:7066-7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tobe, T., G. K. Schoolnik, I. Sohel, V. H. Bustamante, and J. L. Puente. 1996. Cloning and characterization of bfpTVW, genes required for the transcriptional activation of bfpA in enteropathogenic Escherichia coli. Mol. Microbiol. 21:963-975. [DOI] [PubMed] [Google Scholar]
- 82.Tzipori, S., I. K. Wachsmuth, C. Chapman, R. Birden, J. Brittingham, C. Jackson, and J. Hogg. 1986. The pathogenesis of hemorrhagic colitis caused by Escherichia coli O157:H7 in gnotobiotic piglets. J. Infect. Dis. 154:712-716. [DOI] [PubMed] [Google Scholar]
- 83.Valdivia, R. H., and S. Falkow. 1996. Bacterial genetics by flow cytometry: rapid isolation of Salmonella typhimurium acid-inducible promoters by differential fluorescence induction. Mol. Microbiol. 22:367-378. [DOI] [PubMed] [Google Scholar]
- 84.Watnick, P. I., K. J. Fullner, and R. Kolter. 1999. A role for the mannose-sensitive hemagglutinin in biofilm formation by Vibrio cholerae El Tor. J. Bacteriol. 181:3606-3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Watnick, P. I., and R. Kolter. 1999. Steps in the development of a Vibrio cholerae El Tor biofilm. Mol. Microbiol. 34:586-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Watnick, P. I., C. M. Lauriano, K. E. Klose, L. Croal, and R. Kolter. 2001. The absence of a flagellum leads to altered colony morphology, biofilm development and virulence in Vibrio cholerae O139. Mol. Microbiol. 39:223-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Welch, R. A., V. Burland, G. Plunkett III, P. Redford, P. Roesch, D. Rasko, E. L. Buckles, S. R. Liou, A. Boutin, J. Hackett, D. Stroud, G. F. Mayhew, D. J. Rose, S. Zhou, D. C. Schwartz, N. T. Perna, H. L. Mobley, M. S. Donnenberg, and F. R. Blattner. 2002. Extensive mosaic structure revealed by the complete genome sequence of uropathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA 99:17020-17024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhu, J., and J. J. Mekalanos. 2003. Quorum sensing-dependent biofilms enhance colonization in Vibrio cholerae. Dev. Cell 5:647-656. [DOI] [PubMed] [Google Scholar]
- 89.Zhu, J., M. B. Miller, R. E. Vance, M. Dziejman, B. L. Bassler, and J. J. Mekalanos. 2002. Quorum-sensing regulators control virulence gene expression in Vibrio cholerae. Proc. Natl. Acad. Sci. USA 99:3129-3134. [DOI] [PMC free article] [PubMed] [Google Scholar]