Abstract
Dipicolinic acid (DPA) comprises ∼10% of the dry weight of spores of Bacillus species. Although DPA has long been implicated in spore resistance to wet heat and spore stability, definitive evidence on the role of this abundant molecule in spore properties has generally been lacking. Bacillus subtilis strain FB122 (sleB spoVF) produced very stable spores that lacked DPA, and sporulation of this strain with DPA yielded spores with nearly normal DPA levels. DPA-replete and DPA-less FB122 spores had similar levels of the DNA protective α/β-type small acid-soluble spore proteins (SASP), but the DPA-less spores lacked SASP-γ. The DPA-less FB122 spores exhibited similar UV resistance to the DPA-replete spores but had lower resistance to wet heat, dry heat, hydrogen peroxide, and desiccation. Neither wet heat nor hydrogen peroxide killed the DPA-less spores by DNA damage, but desiccation did. The inability to synthesize both DPA and most α/β-type SASP in strain PS3664 (sspA sspB sleB spoVF) resulted in spores that lost viability during sporulation, at least in part due to DNA damage. DPA-less PS3664 spores were more sensitive to wet heat than either DPA-less FB122 spores or DPA-replete PS3664 spores, and the latter also retained viability during sporulation. These and previous results indicate that, in addition to α/β-type SASP, DPA also is extremely important in spore resistance and stability and, further, that DPA has some specific role(s) in protecting spore DNA from damage. Specific roles for DPA in protecting spore DNA against damage may well have been a major driving force for the spore's accumulation of the high levels of this small molecule.
Spores of Bacillus species are dormant and extremely resistant to many environmental stresses including, heat, desiccation, radiation, and a variety of toxic chemicals (9, 17, 33). As a consequence, spores can survive for extremely long periods, certainly hundreds of years and perhaps much longer (5, 15, 37). Spore resistance is due to a variety of factors, including the outer spore coats and the relative impermeability of the spore's inner membrane (6, 9, 17). There are also novel features of the spore's central region or core, the site of spore DNA, which play major roles in spore resistance. These include the relatively low content of core water and the saturation of spore DNA with a group of small acid-soluble proteins (SASP) of the α/β type (9, 11, 23, 31). The low core water protects spores against wet heat, while the α/β-type SASP protect DNA against damage due to wet and dry heat, desiccation, and genotoxic chemicals (6, 17, 31, 33). The binding of the α/β-type SASP also drastically alters the DNA's UV photochemistry, and this change is a major factor in the elevated resistance of dormant spores to UV light (17, 18).
Another novel feature of the spore core is the presence of high levels (∼20% of core dry weight) of pyridine-2,6-dicarboxylic acid (dipicolinic acid [DPA]), that likely exists in the core as a 1:1 chelate with divalent cations, predominantly Ca2+ (11). One role of DPA is to lower the core water content, since DPA loss early in germination is paralleled by its replacement with water, and genetically DPA-less dormant spores have more core water than wild-type dormant spores (10, 21, 32). The role of DPA in spore properties has long been unclear. There are data indicating that increasing spore DPA levels are associated with increased spore wet heat resistance (11, 21). However, mutants have been isolated that produce heat-resistant but DPA-less spores (4, 12). Unfortunately, there has never been a thorough genetic analysis of these strains, and they may well have had multiple mutations.
DPA-less spores of B. subtilis can also be generated by mutation of the spoVF operon that encodes DPA synthetase (7, 11, 21). However, the DPA-less spoVF spores are unstable and lyse during sporulation (21, 36). In contrast, when sporulated with exogenous DPA, spoVF spores accumulate near wild-type DPA levels and are stable (21, 36). DPA-less spoVF spores can also be stabilized somewhat, albeit not completely, by deleting the genes encoding the three receptors that trigger spore germination in response to nutrient germinants (termed the ger3 mutations) (21). DPA-less and DPA-replete ger3 spoVF spores have relatively similar resistance to UV radiation, but the DPA-less spores are more sensitive to wet heat and hydrogen peroxide (21).
Although the latter differences in resistance between spores with or without DPA are striking, it is not clear whether these differences are due only to the higher core water content of the DPA-less spores or to specific effects of DPA on spore resistance. Indeed, DPA can have striking effects on spore properties, in particular spore DNA properties (8, 26). In particular, UV irradiation of spores lacking DPA is much less efficient in generating the thyminyl-thymine adduct termed the spore photoproduct in DNA than is UV irradiation of spores containing normal DPA levels (8). This difference is even larger in spores with or without DPA that also lack α/β-type SASP (8). These latter studies were carried out with spores of a strain lacking both spoVF and sleB. The latter gene encodes one of the two redundant enzymes responsible for the hydrolysis of the spore's peptidoglycan cortex in the first min of germination (32). The second cortex-lytic enzyme, CwlJ, is present in sleB spoVF spores. However, CwlJ requires DPA for its activation (20). Consequently, DPA-less sleB spoVF spores do not germinate either spontaneously or with nutrients and are much more stable than ger3 spoVF spores (20, 21). However, DPA-less sleB spoVF spores can be germinated either by exogenous Ca2+-DPA or by lysozyme treatment of decoated spores in a hypertonic medium (20, 21).
Given the extreme stability of the sleB spoVF spores that lack DPA, we have reexamined the role of DPA in the resistance of these spores. We have also examined the survival and resistance of spores that lack both DPA and α/β-type SASP. We report here the results of these studies.
MATERIALS AND METHODS
Strains used.
All strains used are isogenic with PS832, a prototrophic laboratory strain of B. subtilis 168 and are listed in Table 1. Strain PS3664 (8) lacks the sspA and sspB genes which encode SASP-α and -β that together constitute ∼80% of the spore's pool of α/β-type SASP; consequently, PS3664 spores are termed α−β−. Strains PS3664 and FB122 also lack the spoVF operon that encodes DPA synthetase, and the DPA-less spores produced by these strains are stabilized by the loss of the sleB gene that encodes one of the spore's cortex lytic enzymes (8, 20). Strains made in current study were constructed by transformation to appropriate drug resistance with chromosomal DNA (1). Transformants were screened to ensure that they retained all antibiotic markers and, in the case of strains PS3747 and PS3755, that the spores of these strains lacked SASP-α and -β.
TABLE 1.
B. subtilis strains used
| Strain | Genotype | Phenotypea | Constructionb and/or source or reference |
|---|---|---|---|
| AD28 | ΔcotE::cam | Cot− | A. Driks (41) |
| FB122 | ΔsleB::spc ΔspoVF::tet | DPA− Spr Tcr | 20 |
| PS683 | ΔsigG | Asporogenous | 35 |
| PS832 | Wild type | Laboratory stock | |
| PS2318 | ΔrecA::cam | Rep− Cmr | 30 |
| PS3664 | ΔsleB::spc ΔspoVF::tet ΔsspA ΔsspB | α−β− DPA− Spr Tcr | 8 |
| PS3747 | ΔcotE::cam ΔsleB::spc ΔspoVF::tet ΔsspA ΔsspB | α−β− Cot− DPA− Cmr Spr Tcr | AD28→PS3664 |
| PS3754 | ΔrecA::cam ΔsleB::spc ΔspoVF::tet | DPA− Rep− Cmr Spr Tcr | PS2318→FB122 |
| PS3755 | ΔrecA::cam ΔsleB::spc ΔspoVF::tet ΔsspA ΔsspB | α−β− DPA− Rep− Cmr Spr Tcr | PS2318→PS3664 |
α−β−, spores lack SASP-α and -β and thus ∼80% of the spore's pool of α/β-type SASP; Cmr, resistance to chloramphenicol (3 mg/ml); Cot−, spores have a defective coat; DPA−, spores lack DPA; Rep−, lacks the capacity for much DNA repair; Spr, resistance to spectinomycin (100 μg/ml); Tcr, resistance to tetracycline (10 μg/ml).
Strains to the right of the arrow were transformed to appropriate drug resistance with chromosomal DNA from the strain to the left of the arrow.
Spore formation.
Spores of FB122 were routinely prepared by growth at 37°C on 2xSG medium agar plates (19) without antibiotics for 72 h. The plates were then incubated at 23°C for 3 to 4 days, and spores were harvested, cleaned over 10 to 14 days at 4°C, and stored as described previously (19). In some experiments strain FB122 was sporulated on 2xSG medium plates containing 200 μg of DPA/ml. FB122 spores made without added DPA had <2% of the DPA of wild-type spore DPA levels, whereas spores made with DPA had ∼85% of wild-type spore DNA levels, as found previously (20, 21). Spores of strain FB112 used in this study were free (>98%) of growing or sporulating cells, germinated spores, or cell debris as observed in a phase-contrast microscope. In one experiment FB122 spores were prepared at 37°C in liquid 2xSG medium (19) without DPA.
Spores of strain PS3664 were initially prepared on plates with or without DPA as described above. However, while spores of this strain made with DPA were fully viable after cleaning, the DPA-less spores were not (see Results). Consequently, in further work this strain was grown at 37°C in liquid 2xSG medium (19) with or without DPA. At various times in sporulation 1-ml aliquots were harvested by centrifugation in a microcentrifuge and treated with decoating solution at an optical density at 600 nm (OD600) of ∼5 (see below). The decoated preparation was washed, suspended in hypertonic medium at an OD600 of 1, and germinated with lysozyme, and viable counts were determined as described below. In other experiments, washed decoated preparations of sporulating cells of various strains were subjected to wet heat treatment at an OD600 of 2, and viable counts were determined as described below.
Measurement of spore resistance.
Spores of strain FB122 or PS3664 with or without DPA were decoated and washed (3) prior to measurement of resistance to heat and oxidizing agents to allow spore germination by lysozyme treatment in a hypertonic medium. This method was chosen for spore germination rather than germination with Ca2+-DPA, since CwlJ, the enzyme activated by Ca2+-DPA, is relatively sensitive to treatments such as wet heat and oxidizing agents, more sensitive than is spore viability (2, 39, 40). The decoating procedure was as described previously (3) but at 45°C for 60 min. The spores at an OD600 of 1 to 20 in hypertonic medium were germinated with lysozyme (22), diluted serially in hypertonic medium, and aliquots were spotted onto LB medium plates (21) containing an appropriate antibiotic, generally spectinomycin at 100 μg/ml. The plates were incubated for ∼36 h at 37°C, and colonies were counted, although generally >90% of colonies appeared in 24 h. Decoating has no significant effect on B. subtilis spore resistance to wet or dry heat or UV radiation but does decrease spore resistance to hydrogen peroxide slightly (17, 18, 24).
For wet heat resistance, decoated spores at an OD600 of 2 were incubated in water at various temperatures. At various times samples (0.5 ml) were diluted with 0.5 ml of cold water, centrifuged, and suspended in 1 ml of hypertonic medium. For UV resistance, decoated spores at an OD600 of 1 in water were irradiated in small petri dishes rotating beneath a UVS-11 lamp (UVP, Inc., San Gabriel, CA) with a maximum output at 254 nm. At various times aliquots (1 ml) were centrifuged and suspended in 1 ml of hypertonic medium. For hydrogen peroxide resistance, spores at an OD600 of 1 were incubated at 23°C in 25 mM KPO4 buffer (pH 7.4) with 1.5% H2O2. At various times samples (1 ml) were centrifuged (1 min) and rapidly suspended in 1 ml of hypertonic medium containing catalase (40 μg/ml) as described previously (21, 27). For dry heat resistance, decoated spores (200 μl of a suspension with an OD600 of 10) were lyophilized in glass tubes and exposed to dry heat in an oil bath. After heating, the tubes were cooled on ice, 1 ml of hypertonic medium added, and the suspension sonicated in a bath sonicator to disperse the spores, followed by lysozyme treatment.
For desiccation resistance, 2 ml of intact spores at an OD600 of 10 were pelleted in a 2-ml plastic centrifuge tube and freeze-dried overnight. The dried spores were hydrated in 2 ml of water in a cold room at 4°C overnight, and the OD600 of the suspension measured to assess spore recovery. An aliquot of the spores was diluted 1/10 in 50 mM Ca2+-DPA at pH 8.3 and incubated for 2 h at 23°C (20), and viability was assessed by plating as described above. The centrifugation, freeze-drying, and hydration steps were then repeated.
Analytical procedures.
Extraction of SASP from spores and their analysis by polyacrylamide gel electrophoresis at low pH was as described previously (21). Spore core water content was determined on decoated spores by isopycnic density gradient centrifugation as described previously (11). The level of mutations in spores of different strains with or without various treatments was determined by transferring colonies of either untreated or treated spore preparations obtained from LB medium plates to either 2xSG medium plates or Spizizen minimal medium plates without Casamino Acids as described previously (10, 34). A lack of growth on the minimal medium plates indicates an auxotrophic mutation, while the colonies on 2xSG medium plates were examined for sporulation.
RESULTS
Characterization of DPA-less sleB spoVF spores.
Previous work on DPA-less spores (i.e., spoVF spores) of Bacillus species has shown that such spores lyse readily and are thus extremely difficult if not impossible to work with (21, 36). Deletion of the genes encoding the three functional nutrient germinant receptors (termed the ger3 mutations) stabilizes spoVF spores to some degree, such that they can be isolated and characterized (21). However, even these ger3 spoVF spores germinate spontaneously much more readily than wild-type spores (21). In contrast to the ger3 mutations, a sleB mutation stabilizes spoVF spores completely, since sleB spoVF spores exhibit no spontaneous germination (20; data not shown). Indeed, these spores even germinate extremely poorly with nutrients, because the remaining spore cortex-lytic enzyme, CwlJ, requires DPA for its activation (20). However, DPA-less sleB spoVF spores do germinate with exogenous Ca2+-DPA, and decoated sleB spoVF spores can also be germinated by lysozyme treatment in a hypertonic medium (20).
Previous work found that DPA-less ger3 spoVF spores had very low levels of SASP-γ compared to SASP-γ levels in wild-type or DPA-replete ger3 spoVF spores (21). SASP-γ was also absent from DPA-less sleB spoVF spores (Fig. 1). In contrast, the levels of the DNA binding SASP-α and -β were similar in DPA-less and DPA-replete sleB spoVF spores (Fig. 1), as found previously with ger3 spoVF spores (21). The DPA-less sleB spoVF spores also had ∼20% higher levels of core water than did DPA-replete spores (data not shown), similar to results with ger3 spoVF spores (21).
FIG. 1.
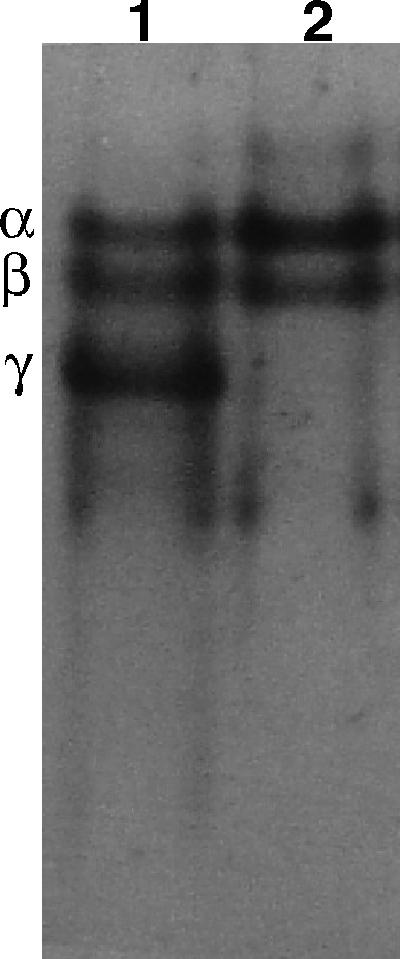
Levels of SASP in sleB spoVF spores prepared with or without DPA. SASP were extracted from spores of strain FB122 (sleB spoVF) prepared at 37°C on plates with or without DPA. Extracts were prepared and subjected to polyacrylamide gel electrophoresis at low pH, and the gel was stained with Coomassie blue as described in Materials and Methods. The samples run in the lanes are from 0.5 mg of dry spores and are presented as spores with DPA (lane 1) and spores without DPA (lane 2). The symbols α, β, and γ denote the migration positions of SASP-α, -β, and -γ, respectively.
Resistance of sleB spoVF spores.
The higher level of core water in DPA-less sleB spoVF spores made it likely that these spores would be more sensitive to wet heat than their DPA-replete counterparts (11, 16), and this was the case (Fig. 2A). The DPA-less spores were also more sensitive to hydrogen peroxide and dry heat than were the DPA-replete spores (Fig. 2C and D). Since the levels of SASP-α and -β were similar in spores with or without DPA, it seemed likely that these spores would have similar UV resistance (17, 18, 31), and this was again the case (Fig. 2B). While DPA-less sleB spoVF spores were more sensitive to wet heat and hydrogen peroxide that their DPA-replete brethren, the DPA-less spores were not killed by DNA damage. Analysis of auxotrophic and asporogenous mutations in survivors of DPA-less sleB spoVF spores killed ∼95% by wet heat or hydrogen peroxide revealed no significant increase in mutation frequency (Table 2). In contrast, 15 to 20% of the survivors of α−β− spores treated with hydrogen peroxide or wet heat, treatments that kill these spores via DNA damage, have auxotrophic or asporogenous mutations (10, 27). Dry heat is also known to kill even wild-type spores by DNA damage (28).
FIG. 2.
Resistance of sleB spoVF spores prepared with or without DPA. Spores of strain FB122 (sleB spoVF) prepared on plates at 37°C with or without DPA were decoated (A, C, and D) or not (B) and treated with wet heat (70°C) at an OD600 of 2 (A), UV radiation at an OD600 of 1 (B), hydrogen peroxide at an OD600 of 1 (C), or dry heat (200 μl at an OD600 of 10) at 105°C (D). Spores were germinated with lysozyme in hypertonic medium (A, C, and D) or by Ca2+-DPA treatment (B), and viability was determined as described in Materials and Methods. Symbols: ○, spores with DPA; •, spores without DPA.
TABLE 2.
Level of mutations in DPA-less sleB spoVF spores exposed to various treatments
| Treatment | Level of killing (%) | No. of colonies tested | No. of mutationsa
|
%b | ||
|---|---|---|---|---|---|---|
| aux | spo | aux and spo | ||||
| 70°C, 30 min | 85 | 416 | 0 | 1 | 0 | 0.2 |
| H2O2, 20 min | 94 | 416 | 1 | 0 | 0 | 0.2 |
| Four freeze-dry cycles | 95 | 432 | 8 | 20 | 1 | 6.7 (<0.2) |
| None | 0 | 355 | 0 | 0 | 0 | 0 |
Spores of strain FB122 prepared on plates without DPA were treated in various ways, and survivors were tested for mutations to auxotrophy (aux) or asporogeny (spo) as described in Materials and Methods. One colony surviving freeze-drying was both auxotrophic and asporogenous (aux and spo). Spores treated with wet heat at an OD600 of 2 and with hydrogen peroxide at an OD600 of 1 were decoated prior to treatment and recovered by lysozyme germination in hypertonic medium. Samples subjected to no treatment or to freeze-drying were not decoated and were recovered by Ca2+-DPA treatment.
This value is the percentage of mutants in the survivors and was calculated as: 100 × (the number of colonies with mutations/the total number of colonies tested). The value in parentheses is the percentage of mutations in DPA-replete FB122 spores given four cycles of freeze-drying.
The greater sensitivity of the DPA-less sleB spoVF spores to dry heat suggested that these spores might also be sensitive to desiccation alone. Indeed, while DPA-replete sleB spoVF spores were resistant to at least 10 cycles of freezing, desiccation, and rehydration, the DPA-less spores lost ∼50% of their viability in each cycle (Fig. 3). However, these DPA-less spores were not sensitive to freezing and thawing (data not shown). Examination of the survivors of four freeze-dry cycles showed that the DPA-replete spores had acquired no mutations, whereas the survivors of the DPA-less spores had (Table 2 and data not shown). These results suggested that desiccation had killed DPA-less sleB spoVF spores by DNA damage. This was suggested further by the greater sensitivity of DPA-less recA sleB spoVF spores to desiccation, while DPA-replete recA sleB spoVF spores remained resistant (Fig. 3). Previous work found that a recA mutation alone does not sensitize B. subtilis spores to desiccation (30).
FIG. 3.
Effect of freezing, desiccation, and rehydration on spores prepared with or without DPA. Spores of strains FB122 (sleB spoVF) (circles) or PS3754 (squares) (recA sleB spoVF) (2 ml at an OD600 of 10) prepared at 37°C with (open symbols) or without (solid symbols) DPA were subjected to freeze drying cycles as described in Materials and Methods. Spores were germinated by Ca2+-DPA treatment, and viability was determined as described in Materials and Methods. This procedure gave 2 × 107 to 8 × 107 CFU of spores/ml at an OD600 of 1 at time zero for the four types of spores.
Loss of viability of DPA-less sspA sspB sleB spoVF spores.
Another factor that is important in spore resistance and long-term survival is the α/β-type SASP (9, 17, 18, 31, 33). Consequently, it was of interest to analyze spores that lacked both DPA and α/β-type SASP. A strain, PS3664, that produces such spores has been generated and has been used to show that both DPA and α/β-type SASP play major roles in determining the UV photochemistry of spore DNA (8). However, initial attempts to measure the resistance properties of cleaned DPA-less PS3664 spores made on plates failed because these cleaned spores at an OD600 of 1 routinely gave ≤0.001% of the CFU/ml given by the DPA-replete spores at the same concentration (Table 3). The low viability of the DPA-less PS3664 spores when the spores were plated directly on nutrient plates was not surprising, since these spores are expected to germinate very poorly with nutrients, as noted above (20). However, these DPA-less spores were also not viable when either germinated with Ca2+-DPA or first decoated and then germinated with lysozyme in a hypertonic medium (Table 3). In contrast, DPA-replete PS3664 spores at an OD600 of 1 gave ∼107 CFU/ml when decoated and germinated with lysozyme (Table 3). This latter value is similar to that obtained in the present study and previously with spores of several B. subtilis strains germinated by this procedure (Table 3) (22, 25). Purified spores of strain PS3747 (cotE sspA sspB sleB spoVF) prepared without DPA were also not viable when germinated with lysozyme in a hypertonic medium (Table 3). Spores of cotE strains are germinated by lysozyme without prior decoating since, due to the lack of the morphogenic CotE protein, their defective coat is permeable to lysozyme (9). Thus, the lack of recovery of viable spores of DPA-less PS3664 spores was not due to their destruction by the decoating regimen used. Despite the extremely low viability of the DPA-less PS3664 spores, the spores were bright and looked normal in the phase-contrast microscope (data not shown).
TABLE 3.
Viability of spores of various strains prepared with or without DPAa
| Strain (genotype) | Sporulated with DPA | CFU/ml of spores at an OD600 of 1 as determined by various methods for the assessment of spore viabilityb
|
||
|---|---|---|---|---|
| Nutrients | CaDPA | Decoat/lysozyme | ||
| FB122 (sleB spoVF) | Yes | 108 | 108 | 107 |
| FB122 (sleB spoVF) | No | 104 | 107 | 9 × 106 |
| PS3664 (sspA sspB sleB spoVF) | Yes | 9 × 107 | 108 | 8 × 106 |
| PS3664 (sspA sspB sleB spoVF) | No | <103 | <10−3 (<10−3) | <10−3 (<10−3) |
| PS3747 (cotE sspA sspB sleB spoVF) | No | NT | <10−3 | <10−3 |
Spores of strains FB122 and PS3664 were prepared on plates at 37oC with or without DPA and were cleaned as described in Materials and Methods. The viability of the cleaned spores was measured by plating on LB plates alone (Nutrients), on LB plates after pretreatment with Ca2+-DPA (CaDPA), or on LB plates after decoating and germinating with lysozyme in hypertonic medium (Decoat/lysozyme).
NT, not tested. Values in parentheses are for spores prepared in liquid medium at 37°C and then cleaned as described in Materials and Methods.
Although the cleaned DPA-less PS3664 spores were not viable, it was possible that these spores were simply extremely labile and died during the ∼72 h of sporulation on plates at 37°C, the several days of incubation of the plates at 23°C, and the ∼10 days of incubation at 4°C after the spores were harvested and were being cleaned. Spores of strain PS3664 harvested after 36 h of sporulation at 37°C in liquid medium without DPA and then cleaned over a period of ∼10 days were also not viable (Table 3 [values in parentheses]). However, we did recover viable spores early in the sporulation of such cultures by decoating, followed by lysozyme treatment in hypertonic medium (Fig. 4). In contrast, no viable forms (≤103 CFU/ml at an OD600 of 1; data not shown) were recovered in this manner from cultures of strain PS683, an asporogenous B. subtilis strain blocked late in sporulation (data not shown). The maximum viable count recovered from a culture of strain PS3664 sporulated without DPA (1.4 × 106 CFU/ml at an OD600 of 1; Fig. 4) was only ∼4-fold lower than for this strain sporulated with DPA recovered similarly (5 × 106 CFU/ml at an OD600 of 1; data not shown). However, the DPA-replete PS3664 spores maintained their viability throughout sporulation, harvesting, and cleaning (Table 3), whereas the DPA-less spores lost viability continually as sporulation proceeded (Fig. 4). In contrast to strain PS3664 sporulated without DPA, strain FB122 sporulated without DPA did give fully viable spores after harvesting and cleaning (Table 3).
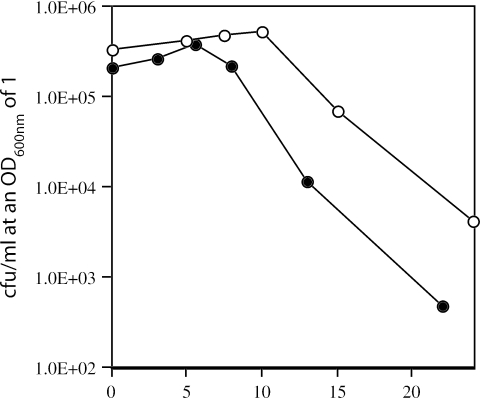
FIG. 4.
Viability of DPA-less PS3664 spores during sporulation. Strain PS3664 (sspA sspB sleB spoVF)) was sporulated at 37°C in liquid without DPA, at various times samples (2 ml) were harvested, decoated, and germinated with lysozyme at an OD600 of 1 in hypertonic medium, a process that took ≤3 h, and viability was determined as described in Materials and Methods. Cultures were also examined in the phase-contrast microscope. The culture was inoculated ∼10 h prior to time zero, and arrows a and b denote the times at which surviving spores were tested for mutations. The number of phase bright spores in the culture did not decrease (≤20%) after 75 h as determined by phase-contrast microscopy.
Analysis of mutations in the PS3664 spores prepared in liquid medium with or without DPA showed that <1% of the DPA-replete spores had auxotrophic or asporogenous mutations (Table 4). However, of the colonies from DPA-less PS3664 spores harvested at several times after the point of maximum spore viability, 12 to 34% had obvious mutations (Table 4). This finding suggested that DPA-less PS3664 spores were dying because of DNA damage. Consistent with this suggestion, when sporulated without DPA, the recA derivative of strain PS3664 (strain PS3755) lost viability more rapidly than did strain PS3664 (Fig. 5).
TABLE 4.
Levels of mutations in spores of strain PS3664 prepared with or without DPAa
| Time (h) in sporulation (+/−DPA)b | No. of colonies tested | Mutation(s)
|
%c | ||
|---|---|---|---|---|---|
| aux | spo | aux and spo | |||
| 35 (+DPA) | 416 | 1 | 0 | 0 | <0.2 |
| 8 (−DPA) | 290 | 10 | 19 | 8 | 13 |
| 25 (−DPA) | 237 | 18 | 49 | 11 | 33 |
Samples (2 ml) were harvested from cultures of strain PS3664 (sspA sspB sleB spoVF) sporulating at 37°C in liquid with or without DPA. The spores were decoated, germinated with lysozyme at an OD600 of 1, and plated on LB medium plates as described in Materials and Methods. Colonies on LB medium plates were picked onto appropriate plates for identification of auxotrophic (aux) or asporogenous (spo) mutants, including colonies that had acquired both auxotrophic and asporogenous mutations (aux and spo).
For the culture sporulated without DPA, the sporulation times are as denoted by the arrows in Fig. 4. For the culture sporulated with DPA, the time is equivalent to hour 35 in Fig. 4.
That is, the percentage of mutants in survivors, was calculated as 100 × (the number of colonies with mutations/the total number of colonies tested).
FIG. 5.
Effect of a recA mutation on the viability of DPA-less α−β− spores during sporulation. Strains PS3664 (sspA sspB sleB spoVF) and PS3755 (recA sspA sspB sleB spoVF) were sporulated at 37°C in liquid 2xSG medium without DPA. At various times samples were harvested and decoated, spores were germinated at an OD600 of 1 with lysozyme in hypertonic medium, and the viability was determined as described in Materials and Methods. Cultures were inoculated ∼10 h prior to time zero, and the times of development in the two cultures were aligned by examination with a phase-contrast microscope. Symbols: ○, strain PS3664; •, strain PS3755.
Wet heat resistance of DPA-less PS3664 spores.
Given the relatively rapid loss in viability of PS3664 spores during sporulation without DPA, it was reasonable to assume that these spores would have much lower resistance than DPA-replete PS3664 spores or DPA-less spores that contained α/β-type SASP. Indeed, when sporulating cultures were harvested shortly after the achievement of maximum spore viability, DPA-less PS3664 spores died rapidly upon incubation at 65°C, a temperature to which the DPA-replete PS3664 spores were relatively resistant (Fig. 6). DPA-less FB122 spores were also much more resistant to this temperature than were the DPA-less PS3664 spores (Fig. 6; see also Fig. 2). Given the significant DNA damage in DPA-less PS3664 spores even just after their release from the sporangium, we carried out no further studies of the resistance of these DPA-less spores.
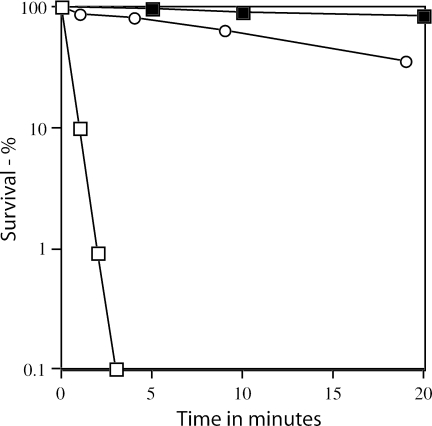
FIG. 6.
Resistance of spores of various strains to wet heat. Strains FB122 (sleB spoVF) and PS3664 (sspA sspB sleB spoVF) were sporulated with or without DPA, and samples were harvested after ∼80% of spores had been released from the sporangium, treated with decoating solution, washed, treated with wet heat at 65°C, and germinated with lysozyme in hypertonic medium. The spore viability was determined as described in Materials and Methods. Symbols: ▪, PS3664 sporulated with DPA; □, PS3664 sporulated without DPA; ○, FB122 sporulated without DPA.
DISCUSSION
Some of the results presented here with DPA-less spores of strain FB122 (sleB spoVF) confirm results obtained with DPA-less spores of a ger3 spoVF strain (21). These confirmatory results with DPA-less FB122 spores include (i) the absence of SASP-γ, (ii) the presence of normal levels of SASP-α and -β, and (iii) their increased sensitivity to wet heat and hydrogen peroxide compared to that of their DPA-replete brethren. It was suggested previously (21) that the increased sensitivity to wet heat and hydrogen peroxide of DPA-less ger3 spoVF spores is due to the increased water content of these DPA-less spores, since increased core water content increases spore sensitivity to both wet heat and hydrogen peroxide (11, 23). The increased core water content of DPA-less FB122 spores is certainly consistent with this suggestion. The increased core water content is also the likely reason for the absence of SASP-γ from DPA-less FB122 spores. Presumably, the SASP-specific germination protease (GPR) that becomes activated late in forespore development is able to digest SASP-γ in the developing DPA-less forespore due to these forespore's higher levels of hydration (14, 21, 28). However, the binding of SASP-α and -β to DNA protects these proteins against GPR digestion (29, 31). In contrast, SASP-γ does not bind to spore DNA and is thus not well protected against GPR digestion (31).
Previous work showed that DPA-less ger3 spoVF spores were slightly more UV resistant than were DPA-replete spores, but DPA-less sleB spoVF spores exhibited similar UV resistance to the comparable DPA-replete spores. The retention of spore UV resistance in the DPA-less spores is not surprising, since these spores retain their α/β-type SASP, the major factor causing spore UV resistance (17, 18, 31, 33). Previous work (21) also found very little difference in the dry heat resistance of DPA-less and DPA-replete ger3 spoVF spores, while we now observed significantly lower dry heat resistance of DPA-less sleB spoVF spores. We do not know the reason for the differences between previous and current findings. Possible contributing factors may be that (i) our current measurements were more extensive than those made previously and (ii) the DPA-less sleB spoVF spores are much more stable than the DPA-less ger3 spoVF spores.
Another new finding was that DPA-less sleB spoVF spores were sensitive to repeated desiccation, a treatment that was not investigated thoroughly with DPA-less ger3 spoVF spores (21). The high level of mutations in survivors of DPA-less sleB spoVF spores subjected to multiple freeze-drying cycles suggests that desiccation is killing these spores by DNA damage. This is consistent with the sensitization of these DPA-less spores to desiccation by a recA mutation that eliminates much DNA repair in B. subtilis (30, 38).
Certainly the most striking finding in the present study was that spores that lacked both DPA and α/β-type SASP rapidly lost viability during sporulation and storage. In contrast, spores that lacked only DPA or only α/β-type SASP are stable for weeks to years during sporulation and subsequent storage, as found previously and in current study (10, 20, 21; data not shown). α−β− spores stored in water at 12°C do lose viability on extended storage, since 90% viability is lost in ∼2 years (10). However, DPA-less α−β− spores lost ∼90% viability in 10 to 15 h at 37°C. The high level of mutations in DPA-less α−β− spores recovered early in sporulation suggests that these spores were dying largely because of DNA damage, and again this was consistent with the more rapid decline in viability of DPA-less recA α−β− spores. α−β− spores do accumulate DNA damage during sporulation, harvesting, and cleaning (10, 39, 40). However, the levels of obvious mutations in α−β− spores just after the spores are cleaned, a process that can take up to 2 weeks, are only ∼1% of the population (39, 40), a value more than 10-fold lower than for DPA-less α−β− spores immediately after their release from the sporangium. The heightened sensitivity of DPA-less α−β− spores to wet heat is a further indication of the potential lability of DNA in DPA-less spores, since wet heat treatment kills α−β− spores by DNA damage (10, 28, 30).
Although DPA was identified as a major component of spores of Bacillus species over 50 years ago, the role of this compound in spores has been difficult to clarify. This has been in large part because of the instability of spores from strains whose only lesion is that they cannot make DPA. The results in this communication as well as previous work indicate that stable DPA-less spores can be prepared from B. subtilis strains that have defined mutations in genes of known function (20, 21). In current and previous work the analysis of these spores has shown that DPA plays a number of roles in spores including the following. (i) DPA in the spore core displaces water such that DPA-replete spores have less core water than isogenic DPA-less spores (21). This is a major reason for the role of DPA in spore wet heat resistance, since there is an inverse correlation between core water content and spore wet heat resistance (11). (ii) DPA plays a major role in the UV photochemistry of spore DNA, affecting both the photoreactivity of spore DNA and the nature of the photoproducts produced (8, 26). Since at least some of the effects of DPA on spore DNA are most likely via energy transfer from an excited state of DPA to DNA (8), DPA must be in close proximity to spore DNA. (iii) The likely close proximity of DPA and DNA noted above is consistent with spore protection against dry heat damage by DPA, since dry heat kills spores by DNA damage (28). (iv) The loss in viability of DPA-less spores upon desiccation, with killing of these DPA-less spores due in large part to DNA damage, is further evidence that DPA plays a major role in stabilizing spore DNA. (v) Finally, the extreme lability of DPA-less α−β− spores indicates again that DPA is an important factor in protecting spore DNA from damage, since in α−β− spores the strong protective effect of α/β-type SASP on DNA is lost.
DPA in spores thus has two main general functions. One is to lower the content of core water by replacing some core water with DPA. This elevates spore wet heat resistance by protecting core proteins from inactivation or denaturation by wet heat. The second function of DPA in spores is to contribute to the protection of spore DNA against many types of damage. In wild-type spores, the protection of DNA against wet heat by DPA may not be especially important, since this is provided by α/β-type SASP (10). However, when α/β-type SASP are absent, DNA protection by DPA becomes important even in hydrated spores. In dry spores, DNA is not completely protected against heat damage by the α/β-type SASP (28). Consequently, DPA protects the DNA in dry spores from heat damage and also against desiccation. The mechanism whereby DPA protects DNA against dry heat and desiccation is not known. However, the DNA damage caused by dry heat and desiccation treatment of spores is different than that caused by wet heat or spontaneous mutations (13).
Since core water content is significantly higher in DPA-less spores, this raises the possibility of there being a low level of aerobic metabolic activity in such spores, and aerobic metabolism can generate compounds such as superoxide and hydrogen peroxide that directly or indirectly can cause DNA damage. In otherwise wild-type spores, the α/β-type SASP would protect DNA against such oxidative damage (27), but in the absence of these protective proteins such DNA damage could lead to spore death. Currently available evidence suggests that metabolism in dormant spores, even DPA-less spores, is minimal, if it takes place at all (25). However, a very low level of metabolism in DPA-less spores would be extremely difficult to detect.
Spores of most Bacillus species are soil organisms and thus are likely to spend a large amount of time in the dry state. Consequently, the protection of spore DNA against damage due to dry heat and desiccation may be extremely important in spore survival in the environment. The ability of DPA to contribute significantly to spore DNA protection may thus have been a major driving force in the evolution of the capacity for DPA synthesis during sporulation and the accumulation of high DPA levels in spores.
Acknowledgments
This study was supported by a grant from the Army Research Office.
REFERENCES
- 1.Anagnostopoulos, C., and J. Spizizen. 1961. Requirements for transformation in Bacillus subtilis. J. Bacteriol. 81:74-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atrih, A., and S. J. Foster. 2001. In vivo roles of the germination-specific lytic enzymes of Bacillus subtilis. Microbiology 147:2925-2932. [DOI] [PubMed] [Google Scholar]
- 3.Bagyan, I., M. Noback, S. Bron, M. Paidhungat, and P. Setlow. 1998. Characterization of yhcN, a new forespore-specific gene of Bacillus subtilis. Gene 212:179-188. [DOI] [PubMed] [Google Scholar]
- 4.Balassa, G., P. Milhand, E. Raulet, M. T. Silva, and J. C. F. Sousa. 1979. A Bacillus subtilis mutant requiring dipicolinic acid for the development of heat-resistant spores. J. Gen. Microbiol. 110:365-379. [DOI] [PubMed] [Google Scholar]
- 5.Cano, R. J., and M. K. Borucki. 1995. Revival and identification of bacterial spores in 25-40-million-year-old Dominican amber. Science 268:1060-1061. [DOI] [PubMed] [Google Scholar]
- 6.Cortezzo, D. E., and P. Setlow. 2005. Analysis of factors influencing the sensitivity of spores of Bacillus subtilis to DNA damaging chemicals. J. Appl. Microbiol. 98:606-617. [DOI] [PubMed] [Google Scholar]
- 7.Daniel, R. A., and J. Errington. 1993. Cloning, DNA sequence, functional analysis and transcriptional regulation of the genes encoding dipicolinic acid synthetase required for sporulation in Bacillus subtilis. J. Mol. Biol. 232:468-483. [DOI] [PubMed] [Google Scholar]
- 8.Douki, T., B. Setlow, and P. Setlow. 2005. Photosensitization of DNA by dipicolinic acid, a major component of spores of Bacillus species. Photochem. Photobiol. Sci. 4:893-896. [DOI] [PubMed] [Google Scholar]
- 9.Driks, A. 2002. Proteins of the spore core and coat, p. 527-535. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives: from genes to cells. ASM Press, Washington, D.C.
- 10.Fairhead, H., B. Setlow, and P. Setlow. 1993. Prevention of DNA damage in spores and in vitro by small, acid-soluble proteins from Bacillus species. J. Bacteriol. 175:1367-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerhardt, P., and R. E. Marquis. 1989. Spore thermoresistance mechanisms, p. 43-63. In I. Smith, R. A. Slepecky, and P. Setlow (ed.), Regulation of prokaryotic development. American Society for Microbiology, Washington, D.C.
- 12.Hanson, R. S., M. V. Curry, J. V. Garner, and H. O. Halvorson. 1972. Mutants of Bacillus cereus strain T that produce thermoresistant spores lacking dipicolinic acid have low levels of calcium. Can. J. Microbiol. 18:1139-1143. [DOI] [PubMed] [Google Scholar]
- 13.Huesca-Espitia, L. del, C., C. Caley, I. Bagyan, and P. Setlow. 2002. Base-change mutations induced by various treatments of Bacillus subtilis spores with or without DNA protective small, acid-soluble spore proteins. Mutation Res. 503:77-84. [DOI] [PubMed] [Google Scholar]
- 14.Illades-Aguiar, B., and P. Setlow. 1994. Autoprocessing of the protease that degrades small, acid-soluble proteins of spores of Bacillus species is triggered by low pH, dehydration, and dipicolinic acid. J. Bacteriol. 176:4721-4729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kennedy, M. J., S. L. Reader, and L. M. Swierczynski. 1994. Preservation records of microorganisms: evidence of the tenacity of life. Microbiology 140:2513-2529. [DOI] [PubMed] [Google Scholar]
- 16.Melly, E., P. C. Genest, M. E. Gilmore, S. Little, D. L. Popham, A. Driks, and P. Setlow. 2002. Analysis of the properties of spores of Bacillus subtilis prepared at different temperatures. J. Appl. Microbiol. 92:1105-1115. [DOI] [PubMed] [Google Scholar]
- 17.Nicholson, W. L., N. Munakata, G. Horneck, H. J. Melosh, and P. Setlow. 2000. Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol. Mol. Biol. Rev. 64:548-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nicholson, W. L., A. C. Schuerger, and P. Setlow. 2005. The solar UV environment and bacterial spore UV resistance: considerations for panspermia and planetary protection. Mutation Res. 571:249-264. [DOI] [PubMed] [Google Scholar]
- 19.Nicholson, W. L., and P. Setlow. 1990. Sporulation, germination, and outgrowth, p. 391-450. In C. R. Harwood and S. M. Cutting (ed.), Molecular biological methods for Bacillus. John Wiley and Sons, Sussex, England.
- 20.Paidhungat, M., K. Ragkousi, and P. Setlow. 2001. Genetic requirements for induction of germination of spores of Bacillus subtilis by Ca2+-dipicolinate. J. Bacteriol. 183:4886-4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paidhungat, M., B. Setlow, A. Driks, and P. Setlow. 2000. Characterization of spores of Bacillus subtilis which lack dipicolinic acid. J. Bacteriol. 182:5505-5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Popham, D. L., J. Helin, C. E. Costello, and P. Setlow. 1996. Analysis of the peptidoglycan structure of Bacillus subtilis endospores. J. Bacteriol. 178:6451-6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Popham, D. L., S. Sengupta, and P. Setlow. 1995. Heat, hydrogen peroxide, and UV resistance of Bacillus subtilis spores with increased core water content and with or without major DNA-binding proteins. Appl. Environ. Microbiol. 61:3633-3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riesenman, P. J., and P. Setlow. 2000. Role of the spore coat layer in Bacillus subtilis spore resistance to hydrogen peroxide, artificial UV-C, UV-B, and solar UV radiation. Appl. Environ. Microbiol. 66:620-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Setlow, B., E. Melly, and P. Setlow. 2001. Properties of spores of Bacillus subtilis blocked at an intermediate stage of spore germination. J. Bacteriol. 183:4894-4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Setlow, B., and P. Setlow. 1993. Dipicolinic acid greatly enhances the production of spore photoproduct in bacterial spores upon ultraviolet irradiation. Appl. Environ. Microbiol. 59:640-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Setlow, B., and P. Setlow. 1993. Binding of small, acid-soluble spore proteins to DNA plays a significant role in the resistance of Bacillus subtilis spores to hydrogen peroxide. Appl. Environ. Microbiol. 59:3418-3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Setlow, B., and P. Setlow. 1995. Small acid-soluble proteins bound to DNA protect Bacillus subtilis spores from killing by dry heat. Appl. Environ. Microbiol. 61:2787-2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Setlow, B., and P. Setlow. 1995. Binding to DNA protects a/b-type small, acid-soluble spore proteins of Bacillus subtilis against digestion by their specific protease as well as by other proteases. J. Bacteriol. 177:4149-4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Setlow, B., and P. Setlow. 1996. Role of DNA repair in Bacillus subtilis spore resistance. J. Bacteriol. 178:3486-3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Setlow, P. 1995. Mechanisms for the prevention of DNA damage to the DNA in spores of Bacillus species. Annu. Rev. Microbiol. 49:29-54. [DOI] [PubMed] [Google Scholar]
- 32.Setlow, P. 2003. Spore germination. Curr. Opin. Microbiol. 6:550-556. [DOI] [PubMed] [Google Scholar]
- 33.Setlow, P. 2006. Spores of Bacillus subtilis: their resistance to radiation, heat and chemicals. J. Appl. Microbiol. In press. [DOI] [PubMed]
- 34.Spizizen, J. 1958. Transformation of biochemically deficient strains of Bacillus subtilis by deoxyribonucleate. Proc. Natl. Acad. Sci. USA 44:1072-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun, D., R.-M. Cabrera-Martinez, and P. Setlow. 1991. Control of transcription of the Bacillus subtilis spoIIIG gene, which codes for the forespore-specific transcription factor σG. J. Bacteriol. 173:2977-2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tovar-Rojo, F., M. Chander, B. Setlow, and P. Setlow. 2002. The products of the spoVA operon are involved in dipicolinic acid uptake into developing spores of Bacillus subtilis. J. Bacteriol. 184:584-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vreeland, R. H., W. D. Rosenweig, and D. W. Powers. 2000. Isolation of a 250 million-year-old halotolerant bacterium from a primary salt crystal. Nature 407:897-900. [DOI] [PubMed] [Google Scholar]
- 38.Yasbin, R., D. Cheo, and D. Bol. 1993. DNA repair systems, p. 529-538. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and molecular genetics. American Society for Microbiology, Washington, D.C.
- 39.Young, S. B., and P. Setlow. 2003. Mechanisms of killing of Bacillus subtilis spores by hypochlorite and chlorine dioxide. J. Appl. Microbiol. 95:54-67. [DOI] [PubMed] [Google Scholar]
- 40.Young, S. B., and P. Setlow. 2004. Mechanisms of Bacillus subtilis spore resistance to and killing by aqueous ozone. J. Appl. Microbiol. 96:1133-1142. [DOI] [PubMed] [Google Scholar]
- 41.Zheng, L., W. P. Donovan, P. C. Fitz-James, and R. Losick. 1988. Gene encoding a morphogenic protein required in the assembly of the outer coat of the Bacillus subtilis endospore. Genes Dev. 2:1047-1054. [DOI] [PubMed] [Google Scholar]