Abstract
We determined that LVS and Schu S4 strains of the human pathogen Francisella tularensis express a siderophore when grown under iron-limiting conditions. We purified this siderophore by conventional column chromatography and high-pressure liquid chromatography and used mass spectrometric analysis to demonstrate that it is structurally similar to the polycarboxylate siderophore rhizoferrin. The siderophore promoted the growth of LVS and Schu S4 strains in iron-limiting media. We identified a potential siderophore biosynthetic gene cluster encoded by fslABCD in the F. tularensis genome. The first gene in the cluster, fslA, encodes a member of the superfamily of nonribosomal peptide synthetase-independent siderophore synthetases (NIS synthetases) characterized by the aerobactin synthetases IucA and IucC. We determined that fslA is transcribed as part of an operon with downstream gene fslB and that the expression of the locus is induced by iron starvation. A targeted in-frame nonpolar deletion of fslA in LVS resulted in the loss of siderophore expression and in a reduced ability of F. tularensis to grow under conditions of iron limitation. Siderophore activity and the ability to grow under iron limitation could be regained by introducing the fslA+ gene on a complementing plasmid. Our results suggest that the fslA-dependent siderophore is important for survival of F. tularensis in an iron-deficient environment.
Francisella tularensis, a deeply divergent gram-negative γ-proteobacterium, is the causative agent of tularemia, a zoonosis transmissible from infected arthropods, rodents, and lagomorphs to humans (reviewed recently in references 36 and 41). F. tularensis strains are classified into four main subspecies of varying virulence; the most virulent subspecies, F. tularensis subsp. tularensis, and the less virulent subspecies F. tularensis subsp. holarctica are predominantly the human pathogens. Infection may be acquired by aerosol with an extremely low dose (<10 CFU) of bacteria, and F. tularensis subsp. tularensis is considered to be a significant threat as a bioweapon (9). The live vaccine strain (LVS), a nonvirulent F. tularensis subsp. holarctica derivative, is nevertheless virulent for mice and has been extensively used to examine immune responses to the pathogen.
F. tularensis is an intracellular pathogen infecting multiple cell types, including macrophages and hepatocytes. Genetic studies on the relatively nonpathogenic subspecies F. tularensis subsp. novicida have been instrumental in the identification of potential pathogenicity factors such as those encoded on a pathogenicity island (35), but the biology of the pathogen remains poorly understood. Techniques for the manipulation of the LVS and the strain Schu S4 (F. tularensis subsp. tularensis) genomes have more recently been established (20, 26, 42). With the genome sequencing of these strains complete (30; http://bbrp.llnl.gov/bbrp/bin/f.tularensis_blast), it is now possible to take a bioinformatics-based approach toward understanding the basic biology of the organism.
The acquisition of essential nutrients within the host is a key requirement for the success of a pathogen. Iron is one of the limiting nutrients in the mammalian host, being largely sequestered within enzymes and cofactors or in ferritin storage complexes. Pathogenic bacteria acquire ferric iron from host sources by various mechanisms expressed in response to iron limitation (reviewed in reference 37). One such strategy is the use of siderophores. These small molecules, secreted by many bacteria and fungi, are able to chelate iron from inorganic and host sources (43). The iron-siderophore complexes are bound by specific receptors on the bacterial surface and subsequently taken up by the microorganism.
Several lines of evidence indicate the importance of iron in the virulence of F. tularensis. Intracellular replication of F. tularensis was shown to be iron dependent, with deferroxamine having inhibitory effects in a tissue culture model of infection (17). A derivative of LVS attenuated for virulence in mice was shown to gain increased virulence after growth in iron-limited media (2). Host iron metabolism also may regulate susceptibility to F. tularensis. The product of the nramp1 gene is thought to mediate endosomal pH and iron availability within the phagosome. The mouse bcg(s) mutation, which is linked to nramp1, confers susceptibility to a variety of intracellular pathogens, including Salmonella and Mycobacterium spp. (reviewed in reference 16). Paradoxically, the bcg(s) mutation renders murine cells resistant to infection with the LVS strain of F. tularensis (28).
To our knowledge, there have been no reports on mechanisms for acquisition of iron in F. tularensis. A growth-initiating substance (GIS) that promoted the growth of dilute cultures of F. tularensis in specific media was reported in the 1960s; this secreted factor showed some characteristics of a siderophore (21, 22). We report here that F. tularensis strains do indeed express a siderophore under conditions of iron limitation. We have purified the siderophore and found it to be structurally similar to the polycarboxylate siderophore rhizoferrin (12). We show that the siderophore promotes the growth of both LVS and Schu S4 under conditions of iron limitation. We have identified the fslA locus as necessary for the production of siderophore and have determined its expression to be iron regulated. Our results demonstrate that loss of siderophore attenuates the ability of the organism to grow under iron limitation.
MATERIALS AND METHODS
Bacterial strains and culture.
F. tularensis strains Schu S4 (obtained from the Centers for Disease Control and Prevention [Colorado]) and LVS (live vacccine strain; from K. Elkins, CBER) were grown at 37°C on cystine heart agar (Difco) plates with 5% defibrinated horse blood (CHAB) or on modified Mueller-Hinton agar (Difco) supplemented with ferric pyrophosphate, horse serum, and Isovitalex (BBL). Isolates were passaged up to five times on CHAB before restreaking from stock cultures maintained at −80°C. Chamberlain's defined media containing 2 μg of FeSO4/ml (CDM [6]) or tryptic soy broth supplemented with 0.1% cysteine (TSB/C) were used for routine liquid culture. CDM prepared without the addition of FeSO4 (CDM-Fe) was used as an iron-limiting medium in some experiments. For CDM with defined levels of iron, the medium was stirred at 4°C overnight with 1% (wt/vol) Chelex-100 resin (Bio-Rad). After Chelex treatment, the medium was supplemented with essential divalent cations (MgSO4 [0.55 mM], ZnCl2 [1.5 μM], CuSO4 [0.2 μM], MnCl2 [1 μM], and CaCl2 [5 μM]) and is referred to as che-CDM. Defined levels of iron as FeSO4 or FeCl3 were added to the che-CDM; iron-limited che-CDM contained 0.1 μg of FeCl3/ml (0.37 μM) or FeSO4/ml (0.36 μM), whereas iron-replete medium contained 2 μg of FeCl3/ml (7.4 μM) or FeSO4/ml (7.19 μM). Kanamycin, when used, was present at 15 μg/ml. All solutions and media were prepared in Milli-Q (Millipore) water.
Escherichia coli strain MC1061.1 [Δ(ara-leu)7696 araD139 Δ(lac)X74 galK16 galE15 mcrA mcrB1 rpsL(Strr) hsdR2 λ− F− recA], obtained from Chang Hahn (University of Virginia), was used for routine cloning and was grown in Luria broth (LB). Ampicillin was added to liquid cultures, when needed, at 50 μg/ml and to agar plates at 100 μg/ml.
Growth in iron-limiting liquid culture.
F. tularensis cells grown to logarithmic phase in CDM were pelleted, washed three times in che-CDM with no added iron, and then diluted into che-CDM containing defined levels of iron to an optical density at 595 nm (OD595) of 0.01 (∼3 × 107 CFU/ml). Growth was monitored as the change in OD595 of the liquid cultures over a period of 50 to 60 h.
Liquid culture bioassay for siderophore.
Bacterial cultures were inoculated from CHAB plate culture and grown overnight in iron-rich CDM. The bacteria from the log-phase cultures were pelleted, washed three times in che-CDM, and then inoculated in 2.5 ml of iron-limiting che-CDM to an OD595 of 0.01. Then, 50 μl of partially purified LVS siderophore (corresponding to siderophore from 1 ml of culture) or a mock preparation from siderophore-deficient mutant GR7 was added to cultures. The OD595 was measured every 6 h using a 96-well microtiter plate on a Bio-Rad microplate reader 680.
Growth on iron-limiting agar.
Iron-limiting CDM agar plates were prepared by leaving out iron salts (CDM-Fe agar). An overnight culture of the indicator strain GR7 in CDM was washed, and ∼3 × 105 CFU were spread on the CDM-Fe plates. LVS and other test strains were grown overnight in CDM-Fe to logarithmic phase, and the cells were pelleted and washed once in che-CDM and resuspended to an OD595 of 1.0 (∼3 × 109 CFU/ml). Next, 2.5 μl of these test strains was spotted onto the GR7-seeded plates, and the plates were incubated at 37°C for 3 days. A growth halo of indicator colonies around a test strain spot demonstrated the ability of the indicator strain to grow on the iron-limiting plate utilizing the siderophore secreted by the test strain (cross-feeding).
Siderophore detection.
Cultures were grown in iron-limiting CDM (either CDM-Fe or che-CDM with defined levels of iron). The production of siderophore in supernatants of LVS and Schu S4 cultures was detected by using a chromazurol-S (CAS) assay (38). The assay was adapted to a microtiter plate format. A total of 100 μl of culture supernatants was mixed with 100 μl of the CAS reagent and 2 μl of the shuttle solution. The absorbance at 630 nm was read after 30 min. The CAS activity was normalized to the cell density (OD595) to obtain a specific CAS activity. The supernatants were diluted as necessary with Milli-Q water or with phosphate-buffered saline to maintain the reaction in the linear range.
Siderophore purification.
Bacterial cultures were grown for 48 h in iron-limiting CDM. Cultures were centrifuged to pellet the cells, and the supernatants were filter sterilized. The supernatant was shaken for 15 min with 1/10 volume XAD-2 (Amberlite) beads that had been prewet in methanol and washed with Milli-Q water. The beads were filtered out, and the flowthrough was then applied to a 1/20 volume Dowex 50W-X8 column packed in water and washed with 3 volumes of water before use. The flowthrough of this column was brought to neutral pH with NaOH. This flowthrough was then applied to a 1/20 volume AG1-X8 (formate form) column (Bio-Rad). The column was washed with 5 volumes of water and eluted with 3 volumes of 1.5 M ammonium formate or 40% formic acid. The eluate was lyophilized and resuspended in water at 1/20 the original volume. The formic acid eluate was further treated with NaOH to neutralize the pH. Eluate was also Chelex treated to remove any excess iron. Purification of the siderophore was followed at every step with the liquid CAS assay.
HPLC purification.
Ion-exchange high-pressure liquid chromatography (HPLC) was carried out by using a Luna amino column (Phenomenex, 250 by 4 mm, 5-μm particles) in the Biomolecular Research Facility at the University of Virginia. The buffer used was 20 mM phosphate (pH 7.0) and a sodium chloride gradient of 0 to 1 M applied from 6 to 21 min at a flow rate of 1 ml/min at room temperature. An absorbance at 210 nm was used for detection. To desalt the eluted siderophore, the CAS-active fraction was diluted 10-fold in water and bound to AG1-X8 resin. The siderophore was eluted with ammonium formate, lyophilized, and resuspended in water.
Mass spectrometry.
Positive-ion spectra were acquired on a ThermoFinnigan (San Jose, CA) LCQ classic ion trap mass spectrometer equipped with electrospray ionization (ESI). A 2-μl sample was loaded under pressure (500 lb/in2 helium) onto an in-house-prepared microcapillary resolving column (6 cm of 5-μm C18 reversed-phase packing material; YMC; Waters Corp., Milford, MA). After loading, the microcapillary column was interfaced to an HP1100 HPLC apparatus (Agilent Technologies, Palo Alto, CA) with a flow-splitter directing a flow of 100 nl/min through the column. A gradient of 0 to 100% solvent B (70% acetonitrile in 0.1 M acetic acid) was applied over 17 min, ending with 10 min of reequilibration to solvent A (0.1 M acetic acid).
Spectra were acquired in a data-dependent manner, using the Xcalibur data system with which the instrument is equipped. The top five most abundant ions in each full MS spectra were isolated for fragmentation, and tandem mass spectrometry (MS/MS) data were acquired. This process was repeated before each of the top five ions was placed on an exclusion list for 2 min. The subsequent five most abundant ions were then isolated for fragmentation, and MS/MS spectra were acquired. This process continued throughout the 40-min analysis.
Construction of ΔfslA mutant and complementing plasmid.
A suicide vector, pGIR457, based on the pUC plasmid pSL1180 (4) was used to generate the chromosomal fslA deletion in LVS. A 2.6-kb stretch of DNA upstream of and including 55 bp at the 5′ coding region of the fslA gene was amplified from Schu S4 chromosomal DNA by using Turbo Pfu DNA polymerase (Stratagene) and the primers 5′-ctactggctagcGTCCATATAACCAATACTTTGG-3′ and 5′-ctactgggtacCAAGAGTCTCTATATTAAGTGG-3′ (uppercase letters indicate sequences encoded in the F. tularensis genome). This 5′-flanking region was cloned by using NheI and KpnI sites on the plasmid. The 3′-flanking sequence including that encoding the terminal 12 residues and stop codon of fslA was obtained by amplification from LVS DNA using the primers 5′-ctactgggtaccGGAGATATCTATATCAACCTC-3′ and 5′-ctactggagctcACCTAAGTCTATCCATTCTAG-3′ and introduced as a 2-kb KpnI-SacI fragment. The suicide plasmid also carried between XhoI and PmlI sites the Bacillus subtilis sacB gene (19) obtained by PCR amplification from the plasmid pKO3 (31; provided by George Church, Harvard Medical School) using the primers 5′-ctactggcggccgcctcgagACCCATCACATATACCTG-3′ and 5′-ctactggagctccacgtgTCGGCATTTTCTTTTGCG-3′. The RK2 mob sequence carried on plasmid pRK290 (10; provided by Noriko Ohta, Princeton University) was amplified using the primers 5′-ctactggaattcCTTTTTGTCCGGTGTTGGGTTG-3′ and 5′-ctactgggtaccggatccGCGCTCGGTCTTGCCTTGC-3′ and was cloned between the EcoRI and BamHI sites. A fragment containing the Tn5 kanamycin resistance marker was obtained from plasmid pCK155 (13; provided by Kevin Young, University of North Dakota) by digestion with NdeI, filling in the ends with Klenow, and this was followed by digestion with BamHI. This fragment was cloned across EcoRV and BamHI sites so that transcription from the nuo promoter encoded in the 5′-flanking region of fslA would read through into the kanamycin resistance gene. This final plasmid construct, pGIR457, was introduced into LVS by electroporation.
LVS was grown as a lawn on CHAB for 18 h. The cells were collected and washed in 0.5 M sucrose, and 2 μg of plasmid pGIR457 was electroporated into the cells in a Bio-Rad micropulser set at 2.5 kV, 600-Ω resistance, and 10-μF conductance. Kanamycin-resistant colonies were selected for on CHAB containing 15 μg of kanamycin/ml. The presence of plasmid integrated in the genome was verified by PCR with genomic DNA by using two sets of primers where one primer lay within the integrative plasmid and the other was outside of the sequences cloned in the plasmid (see Fig. 5B). Primers a (5′-GCCATCACATAAGCATGCTC-3′) and b (5′-ctactggagctcCTGCTATGATTATAAGCTGAC-3′) were used to analyze recombination in the 5′ flanking region of fslA, whereas primers c (5′-ctactggcggccgcTGTTAAATGCAAATCCTGTCG-3′) and d (5′-TCGACCAAACTACGTCCTAG-3′) were used to analyze the 3′-flanking region for recombination events. Strain GR6, with plasmid integrated in the 3′-flanking region, was streaked out onto CHAB containing sucrose. Colonies growing on sucrose were screened for loss of kanamycin resistance, and isolates with the fslA deletion were identified by PCR of genomic DNA using the primer sets a-b and c-d. The PCR product obtained from one deletion isolate, GR7, was sequenced to confirm that the deletion was correct. The complete sequence of Pfu DNA polymerase-generated PCR products from LVS and Schu S4 genomic DNA using primers c and b was obtained using a set of internal primers in order to confirm the fslA sequences of the two strains.
FIG. 5.
fslABCD locus, ΔfslA mutant, and transcription. (A) Organization of the fslABCD region of the chromosome. The ORFs in the fslABCD locus are identified by GenBank accession numbers, and the closest homologs in the GenBank database are indicated. Also shown is the fur box sequence upstream of fslABCD. (B) PCR analysis of ΔfslA mutant. fslA and flanking sequences are depicted, along with locations of primers used for diagnostic PCR analysis of recombinants. Shown beneath is the extent of sequences carried on the deletion plasmid construct pGIR457. Primers a and b were used to generate PCR products from genomic DNAs of isolates in lanes 1, 2, and 5 to 7, while primers c and d were used for lanes 3, 4, and 8 to 10. The isolates used in reactions were as follows: GR5 (fsl+ control), lanes 1 and 3; GR6 (plasmid integrant), lanes 2, 4, 7, and 10; GR8 (fsl+ derivative of GR6), lanes 5 and 8; and GR7 (ΔfslA derivative of GR6), lanes 6 and 9. M indicates lanes with the 1-kb DNA ladder (New England Biolabs). (C) RT-PCR analysis of fslA and fslB expression. The structure of the fslAB region is shown along with the primers used in PCR. The hatched region in fslA represents the sequences deleted in strain GR7. cDNAs from LVS and GR7 were analyzed for expression of fslA using primers 1 and 2, fslB using primers 3 and 4, and fslAB operonic product using primers 5 and 4. The −RT reactions represent negative controls where no reverse transcriptase was present in the cDNA synthesis reactions. (D) qPCR of cDNA prepared from LVS after growth in iron-replete and iron-limiting media. The fslA levels were normalized to two different internal standards, polA and trpB. The change in relative fslA transcription was analyzed after 6 h of iron limitation. Reactions were run in triplicate. The results of two independent experiments were averaged and are plotted with the standard deviations.
For complementation of the mutant strain GR7, the fslA gene, along with the promoter region, was amplified from Schu S4 using primers 5′-ctactggcggccgcTGTTAAATGCAAATCCTGTCG-3′ and 5′-ctactggagctcCTGCTATGATTATAAGCTGAC-3′. This 2.275-kb fragment was cloned as a NotI-SacI fragment in an intermediate pSL1180 vector, from which it was excised as a NotI-XhoI fragment and cloned into the shuttle plasmid pFNLTP6 (32; provided by Tom Zahrt, Medical College of Wisconsin). This fslA+ clone pGIR458 and parent vector pFNLTP6 were each introduced into LVS and into GR7 by electroporation, and transformants were selected on CHAB containing15 μg of kanamycin/ml.
RNA isolation and reverse transcription (RT).
F. tularensis cultures were grown overnight in CDM, washed three times in che-CDM, and inoculated into iron-limited or iron-replete che-CDM to an OD595 of 0.05 or 0.01 for 6 h of growth and 24 h of growth, respectively. RNA was isolated from cells by using TRIzol (Invitrogen) and cleaned up on an RNeasy column (QIAGEN) according to the manufacturer's protocols. A total of 1 to 3 μg of RNA was reverse transcribed at 42°C for 50 min by using Superscript II (Invitrogen). Random hexamers were used as primers for the reverse transcriptase reaction. Negative control reactions lacked only reverse transcriptase. Resultant cDNAs were used in standard PCRs and quantitative PCR (qPCR) experiments.
Standard PCR of cDNAs.
cDNA samples were used as a template for PCRs with HotStar Taq polymerase (QIAGEN) and primers located within the fsl locus (see Fig. 5C). Detection of fslA transcripts was done with primers 1 (5′-ctactgggatccATGCATATTGAACAACTCCAAAAAAAC-3′) and 2 (5′-ctactggaattcAAGCTAATGGATTTTTGAGGTTGATATAG-3′). Primers 3 (5′-ctactgATGAATAATCATATAAAAGCACAAATACTG-3′) and 4 (5′-TAGATGATTTAAGGTCAAATAGATAAAGTAG-3′) were used to detect fslB transcripts. Primer 4 (see above) was used in conjunction with primer 5 (5′-TTACCAAGCTTTGAAGCAAGCG-3′) to detect an fslAB operonic product.
qPCR.
cDNA samples were used as a template for qPCR of fslA. polA and trpB were used as internal standards for standardizing transcript levels between different RNA preparations. For each gene, a standard curve was generated with serial dilutions of PCR products from a genomic DNA template. The primers used for the real-time reaction were 5′-ATCTGAAATGCAAGGCTGCT-3′ and 5′-ATCCGTTTCCGTGTCAAAAG-3′ for polA. The primers for trpB were 5′-TCAGCTGGTCTGGATTTTCC-3′ and 5′-GCTAGGGCGTGAGATGATTC-3′. The fslA-specific primers were 5′-ATCACGATGATTGGCAACAA-3′ and 5′-AACTGCTCCCCATTGCTCTA-3′. HotStar Taq polymerase (QIAGEN) was used for the amplification reactions, with activation at 94°C for 15 min. The reactions were carried out in a DNA Engine Opticon 2 real-time thermocycler from MJ Research. The program used was as follows: denaturation at 94°C for 20 s, annealing at 58°C for 30 s, and extension at 72°C for 20 s, followed by a plate read, a pulse at 76°C for 10 s, and a plate read for 40 cycles. Reactions included 0.15% Triton X-100, and SYBR Green dye (Bio-Rad) was used for the detection of PCR products.
Nucleotide sequence accession numbers.
The DNA sequences for fslA from Schu S4 and from LVS have been deposited in GenBank under accession numbers DQ447633 and DQ447634, respectively.
RESULTS
F. tularensis strains produce a siderophore under conditions of iron limitation.
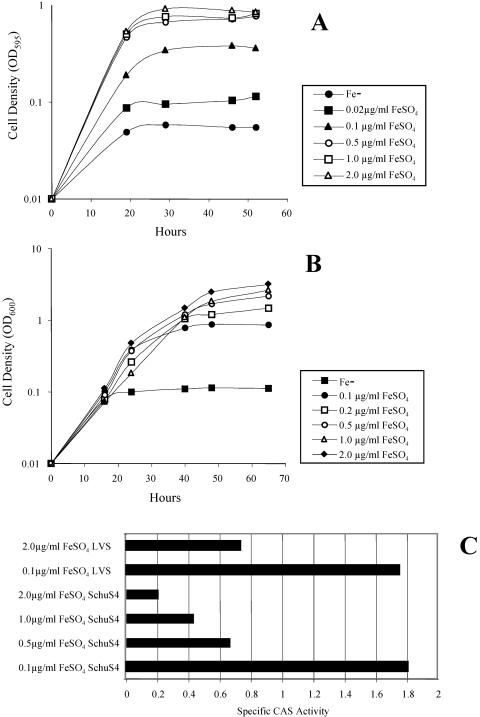
To detect siderophore expression by F. tularensis, we first defined the conditions for iron-limited growth in CDM. We monitored the growth of Schu S4 and LVS after supplementation of che-CDM with various levels of FeSO4. As shown in Fig. 1A and B, both strains showed a dependence on added iron for growth. At 0.1 μg of FeSO4/ml, the strains grew to about a third of the density obtained with 2 μg of FeSO4/ml; these levels were therefore chosen to represent iron-limiting and iron-replete conditions, respectively. We also observed a difference in the growth kinetics of the two strains. Schu S4 cultures showed the effect of iron starvation from the very start of the growth experiment, but LVS cultures started to show iron dependence only at about 24 h into the experiment. This was reproducibly observed and may reflect larger internal iron stores in LVS. Higher levels of bacterioferritin have been reported in LVS and other F. tularensis subsp. holarctica strains relative to F. tularensis subsp. tularensis (24, 25).
FIG. 1.
Iron-dependent growth and expression of siderophore in F. tularensis. Growth of Schu S4 (A) and LVS (B) in che-CDM with various levels of FeSO4, measured as the change in the OD. (C) Production of siderophore, expressed as specific CAS activity, in supernatants after 55 h of growth in che-CDM with various levels of FeSO4.
We assessed the culture supernatants for CAS activity indicative of siderophore after 55 h of growth. As shown in Fig. 1C, we detected CAS-active material in the supernatants of both strains. The specific CAS activity was dependent on the extent of iron starvation, with maximum siderophore expression at the lowest iron levels.
The siderophore of F. tularensis is similar to rhizoferrin.
We used diagnostic assays for the detection of catechol or hydroxamate liganding groups commonly found in siderophores (1, 7), but our tests of the CAS-reactive supernatants were negative. We were able to partially purify the siderophore using a series of chromatographic steps similar to those used for purification of the polycarboxylate siderophore rhizoferrin from Rhizopus spp. and other fungal species (12, 40). The siderophore was retained on an anion-exchange column AG1-X8 (formate form) and could be eluted using formic acid or ammonium formate (Fig. 2A). Extracts from both Schu S4 and LVS behaved similarly on the columns.
FIG. 2.
Siderophore purification. (A) Partial purification of siderophore. CAS-active supernatant from an LVS culture grown for 48 h in CDM-Fe was passed through a series of columns, and CAS activity was used to determine whether the siderophore was retained on the matrix. The eluate from the AG1-X8 column was evaporated to dryness and resuspended in water. (B) HPLC purification of the partially pure siderophore from LVS. The peak with an asterisk at 18.4 min on the chromatogram corresponds to the CAS-active siderophore. (C) HPLC of extracts from the ΔfslA strain GR7 processed as described for panel A. The chromatogram lacked the 18.4-min CAS-active peak.
Since our siderophore activity proved to be acid-labile, we developed an ion-exchange protocol for HPLC purification of the siderophore in place of reversed-phase HPLC previously described for rhizoferrin (12). A single CAS-reactive peak corresponding to the LVS siderophore was eluted at 18.4 min corresponding to ∼0.8 M NaCl (Fig. 2B); this peak was absent in similarly processed extracts from siderophore-deficient LVS mutant GR7 (see below; Fig. 2C).
The HPLC-pure siderophore was subjected to ESI-MS analysis. As shown in Fig. 3A, the major peaks corresponded to masses of 459 and 437 Da, a finding consistent with the sodiated and protonated forms of rhizoferrin (436 Da), respectively (12). The product ion spectra of each of these peaks (MS/MS) were consistent with the structure of rhizoferrin, which comprises two citric acid moieties linked by a putrescine residue (Fig. 3). The major product ions of the m/z 437 peak (Fig. 3B) included several species showing loss of an H2O moiety; m/z values of 419 and 401 corresponding to the -H2O and -2H2O forms of rhizoferrin, respectively, peaks of m/z 391 and 373 corresponding to (rhizoferrin-H2O-CO) and (rhizoferrin-2H2O-CO), and an m/z 355 peak corresponding to (rhizoferrin-3H2O-CO). We additionally observed a (rhizoferrin-citryl) ion of mass 263 Da and a (rhizoferrin-citryl-H2O) ion of 245 Da. The MS/MS spectra of the sodiated rhizoferrin ion of 459 Da are shown in Fig. 3C. The (rhizoferrin-citryl) species was a major peak at m/z 285, as reported previously (12). In addition, peaks corresponding to -H2O (441 Da), -2H2O (423 Da), and -citryl-H2O (267 Da) were also evident.
FIG. 3.
MS of HPLC-purified LVS siderophore. (A) Full mass spectrum, showing major peaks at m/z 459 and m/z 437, a finding consistent with sodiated and protonated forms of rhizoferrin, respectively. (B) MS/MS of the m/z 437 peak showing several dehydrated and decarboxylated species and a peak at m/z 263 consistent with protonated (rhizoferrin-citryl) species. (C) MS/MS of the m/z 459 peak showing several dehydrated and decarboxylated species and a peak at m/z 285 consistent with sodiated (rhizoferrin-citryl) ion. The structure of rhizoferrin (12) is shown as a reference.
Schu S4 siderophore extracts had peaks identical to the protonated and sodiated rhizoferrin ions from LVS (data not shown), indicating that the two strains expressed the same siderophore. We also determined, by using MS, that the acid lability of the siderophore resulted from its conversion to dehydro and didehydro forms after exposure to acid (data not shown). Our analysis of the F. tularensis siderophore showed it to be structurally similar to, if not identical to, rhizoferrin.
Siderophore enhances the growth of F. tularensis strains under iron limitation.
Having demonstrated siderophore production by the Schu S4 and LVS strains, we next determined whether the strains could utilize the siderophore. Specifically, we tested the ability of the siderophore to promote growth of the strains in iron-limited liquid culture. We examined growth of Schu S4 and of LVS in iron-limiting CDM after supplementation with either partially purified siderophore or a parallel extract from a siderophore-deficient mutant, GR7 (see below). As shown in Fig. 4, the addition of LVS siderophore enhanced the growth of both strains, resulting in a culture density closer to that seen in iron-replete media. As expected, the control extract from the siderophore-deficient mutant did not promote growth of the cells. These results suggested that the siderophore is important for growth of cells under iron limitation.
FIG. 4.
Growth promotion of Schu S4 and LVS in iron-limiting media by exogenous siderophore. Washed cells from 24-h cultures in iron-replete CDM were inoculated into iron-limiting che-CDM or into iron-limiting che-CDM supplemented with LVS siderophore extract or with a mock preparation from siderophore-deficient mutant GR7. Parallel cultures were grown in iron-replete che-CDM. Cultures were grown in triplicate, and the means and standard deviations are indicated in the growth plot.
Generation of ΔfslA mutant.
We examined the genomes of Schu S4 and LVS for coding sequences with similarity to other known bacterial siderophore biosynthetic genes and identified a gene encoding an open reading frame (ORF; annotated as a hypothetical protein for Schu S4, GenBank accession no. YP169105.1; FTT0029, www.biocyc.org/FRANT/) with homology to the superfamily of NRPS (nonribosomal peptide synthetase)-independent siderophore synthetases (NIS synthetases [5]). On the basis of our analysis of this locus, described below, we designated this gene fslA (for Francisella siderophore locus A). The closest homolog to FslA in GenBank is the product of frgA from Coxiella burnetii (GenBank accession no. NP_819459; 37% identity, 49% similarity); other related ORFs include the product of Legionella pneumophila frgA (23) and the aerobactin biosynthetic proteins IucA and IucC encoded on the enterobacterial plasmid pColV-K30 (33).
A candidate Shine-Dalgarno sequence, AGGGA, is located 8 bp upstream of the ATG start codon. The fslA gene is followed by the fslB locus, with only 6 bp separating the stop codon from the presumptive start codon of fslB. The fslC and fslD ORFs are also closely clustered. The structure of the presumptive operon fslABCD is diagrammed in Fig. 5A, and the closest homologs of the ORFs on the basis of BLAST searches are indicated. A fur homolog is located 312 bp upstream of the fslA gene, and a canonical fur box sequence lies 22 bp upstream of the fslA start codon, suggesting that fslA is subject to Fur-mediated regulation in response to iron (14).
The fslA locus is highly conserved between the Schu S4 and LVS genomes, with only four nucleotide differences resulting in a single amino acid difference across the 1,917-nucleotide coding region of fslA. The 32% G+C content of the coding region is similar to that of the whole genome (30).
We generated a mutant of LVS carrying an unmarked deletion of fslA in the chromosome by using a two-step method similar to that used previously (20). We used PCR of the genomic DNA with primer sets a-b and c-d to characterize recombinant isolates at each step of the mutagenesis procedure, where primers a and d lie outside of the region contained in the deletion plasmid construct (Fig. 5B). DNA from one of the kanamycin-resistant isolates obtained at the first step, GR6, generated an ∼4.6-kb band in PCR with primer set a-b (Fig. 5B, lanes 2 and 7), similar to a control fslA+ LVS derivative, GR5, that we used as a standard (Fig. 5B, lane 1). However, with primer set c-d, GR6 DNA yielded an ∼2.5-kb band (Fig. 5B, lanes 4 and 10) compared to the ∼4.3 kb with the control DNA (Fig. 5B, lane 3). This indicated that in GR6 the plasmid had integrated by recombination at the 3′-flanking sequences. We isolated sucrose-resistant, kanamycin-sensitive derivatives of GR6 and characterized them by using the same sets of primers. Shown in Fig. 5B are representatives of a deletion mutant, GR7, and a wild-type revertant, GR8, obtained in this screen: the deletion mutant yielded an ∼2.8-kb PCR product with primer set a-b and an ∼2.5-kp product with primer set c-d (Fig. 5B, lanes 6 and 9, respectively), while the wild-type revertant generated ∼4.6- and ∼4.3-kb bands, respectively (lanes 5 and 8).
We designed the ΔfslA mutation to be an in-frame deletion, encoding 18 residues at the amino terminus and 12 amino acids at the carboxy terminus of the FslA ORF. Our goal was to minimize the probability of polar effects by the presence of the mutation on downstream genes of the putative operon. We determined the sequence of the a-b PCR product of GR7 across the deletion site and found that the deletion was correct and in frame.
The fslA gene is necessary for the production of siderophore.
We compared siderophore expression in the parent LVS strain and in the ΔfslA derivative GR7 after growth in CDM-Fe. As shown in Fig. 6A, the LVS supernatants had CAS activity, but in the GR7 supernatants the activity was close to background. MS analysis of culture supernatants from GR7 failed to detect any LVS siderophore-associated peaks (data not shown). These results demonstrated that fslA was required for siderophore expression.
FIG. 6.
Complementation of ΔfslA mutant. (A) Siderophore expression. GR7 and transformants with vector alone (pFNLTP6) or with the fslA+ plasmid pGIR458 were grown overnight in CDM-Fe, and the specific CAS activity of culture supernatants was determined. LVS transformants were tested as a control. (B and C) Promotion of growth on iron-limiting agar by cross-feeding of siderophore. GR7 was seeded as an indicator strain on iron-limiting CDM agar, and cultures of test strains were spotted on the plate. Growth halo formation around the test strains was recorded after 3 days at 37°C. The test strains used were LVS and GR7 (B) and GR7 carrying vector plasmid pFNLTP6 or fslA+ complementing plasmid pGIR458, and LVS carrying pFNLTP6 (C).
fslA is part of an operon that is transcribed under conditions of iron limitation.
In order to determine whether fslA was transcribed as part of an operon, we used RT-PCR with primers specific to gene segments fslA (primers 1 and 2) and fslB (primers 3 and 4), as well as primers spanning part of fslA in addition to fslB (primers 5 and 4) (Fig. 5C). We extracted RNA from LVS and GR7 cultures after iron starvation and analyzed the cDNA. To rule out genomic DNA contamination, we confirmed that no PCR products were obtained in the absence of RT. As shown in Fig. 5C, cDNA from LVS yielded a 1.917-kp product corresponding to fslA and a 1.219-kb fslB product. In addition, the cDNA from LVS showed the presence of a 1.614-kb fslAB product, indicating that fslA and fslB are part of the same transcription unit. As expected, GR7 cDNA lacked the full-length fslA product and instead had a small ∼0.1-kp product consistent with the internal deletion. Expression of fslB could be detected in GR7, but a product corresponding to fslAB was not observed. This was expected, since the sequences corresponding to primer 5 were deleted in the strain. Our results demonstrated that fslA and the adjacent fslB are part of an operonic transcript and also confirmed that the fslA deletion in strain GR7 did not have a polar effect on expression of downstream fslB.
The presence of a fur box sequence upstream of fslA suggested that the expression of the locus is iron regulated. We used qRT-PCR to analyze expression of fslA after exposure to iron-replete and iron-limiting conditions. We normalized fslA expression in each case to two different internal standards that should be unaffected by iron levels: polA (encoding DNA polymerase I) and trpB (encoding tryptophan synthase beta subunit). As shown in Fig. 5D, iron limitation resulted in induction of fslA expression. With the expression in iron-replete media set at 100%, we observed a 2.25-fold increase in fslA transcription with polA as the internal standard and a 2.75-fold increase relative to trpB as the internal standard after 6 h in iron-limiting media. These results demonstrated that fslA expression is induced in response to iron limitation.
fslA-dependent siderophore is necessary for growth under iron limitation.
In order to test the importance of siderophore for survival of cells under iron limitation, we compared the growth of iron-starved LVS and that of the ΔfslA GR7 mutant in liquid culture. Cultures grown in iron-limiting che-CDM to deplete internal iron stores were then used as an inoculum for comparison of the growth in iron-limiting or iron-replete che-CDM. As shown in Fig. 7, there was not much difference between the growth of LVS and GR7 in iron-replete media. However, a distinct difference was seen in iron-limiting media, with GR7 only growing to about one-sixth the density of LVS.
FIG. 7.
Growth deficiency in liquid culture of iron-starved ΔfslA mutant. Washed cells of LVS and GR7 from a 24-h culture in CDM were grown for 24 h in iron-limiting che-CDM to deplete internal iron stores. These cultures were then inoculated into fresh iron-replete or iron-limiting che-CDM, and the growth was plotted. Cultures were grown in triplicate, and the means and standard deviations are depicted.
We also examined the importance of siderophore for promoting colony formation on iron-limiting CDM agar. GR7 cells were spread on this iron-limiting plate as an indicator strain, and LVS or GR7 cells were spotted onto the plate. A halo of GR7 colonies grew around the LVS spot, demonstrating that GR7 cells can utilize exogenous siderophore and form colonies on the agar (Fig. 6B). Only insignificant growth was observed around the GR7 spot, reflecting the inability of GR7 to provide the siderophore required for the cross-feeding.
Complementation of ΔfslA mutant by plasmid-borne fslA.
In order to correlate the siderophore expression and growth defects of GR7 specifically with the ΔfslA mutation, we introduced plasmid pGIR458 carrying the fslA+ gene along with its promoter into GR7. Although transformants of GR7 harboring the vector pFNLTP6 had only background levels of CAS activity, significant siderophore activity was recovered in the supernatants of the pGIR458 transformants (Fig. 6A). The high level of expression (four times that of LVS) was seen also in LVS transformed with pGIR458 and was likely due to the increased copy number of the plasmid-borne fslA gene.
We then tested the ability of the complemented strain to promote growth of GR7 on iron-limiting media. Plasmid vector pFNLTP6-transformed GR7 was unable to support growth halo formation in the plate bioassay, but the complementing plasmid pGIR458 conferred the ability to promote growth halos, similar to pFNLTP6-transformed LVS (Fig. 6C).
These results indicated that fslA was specifically required for siderophore production and that this siderophore was important for the growth of cells under iron-limiting conditions.
DISCUSSION
We have characterized the siderophore expressed by F. tularensis and shown it to be structurally similar to rhizoferrin, a polycarboxylate siderophore made by Rhizopus spp. and other fungal species (12, 40). We believe that our siderophore is identical to the “GIS” characterized by Halmann and coworkers, who also noted that a Rhizopus contaminant produced GIS (21, 22). We determined that the same siderophore was produced both by Schu S4 and by LVS, and it is likely that this siderophore is expressed by other F. tularensis subsp. tularensis and F. tularensis subsp. holarctica strains as well.
The structure of rhizoferrin is relatively simple, consisting of two citric acid moieties linked by amide bonds to a putrescine backbone (12). A bacterium, Ralstonia pickettii, has been previously shown to produce an S,S enantiomeric form of rhizoferrin, whereas the fungal siderophore adopts an R,R configuration of the citryl residues (34). A structurally related siderophore, staphyloferrin A from Staphylococcus aureus, has ornithine linking the two citric acid moieties in place of putrescine, and the citryl chiral centers in this molecule are also in the S,S configuration (11, 27). This enantiomeric configuration was proposed to be a characteristic of the bacterially encoded biosynthetic pathways, and we speculate that the F. tularensis siderophore also may possess an S,S configuration.
We found Francisella siderophore production to be dependent on fslA, the first gene in a locus, fslABCD, that is highly conserved between Schu S4 and LVS. FslA is similar to the NIS synthetases, which are enzymes mediating the assembly of nonpeptide siderophores involving dicarboxylic acids and diamines or amino-alcohols (5). In the case of the F. tularensis siderophore, FslA likely mediates amide bond formation between citric acid and putrescine. The NIS synthetases have been further classified as type A, B, or C enzymes based on sequence relationships (5). FslA appears to be a type B enzyme, like its closest homolog, the C. burnetii FrgA sequence, and ORFs from other siderophore biosynthetic loci, PvsB (Vibrio parahemolyticus vibrioferrin), SbnC (S. aureus staphylobactin), and AcsA (Erwinia chrysanthemi achromobactin) (5).
Siderophore biosynthesis is induced under iron limitation. We demonstrated that fslA transcription is subject to iron regulation. The presence of the fur box sequence in the vicinity of the promoter makes it highly likely that the Fur repressor is responsible for regulation of fslA transcription, as occurs commonly with siderophore biosynthesis in other bacteria. Siderophore biosynthetic genes in bacteria are generally organized in clusters as operons. We demonstrated here that fslA is cotranscribed with the downstream gene fslB. There is an 8-bp overlap between the end of fslB and the fslC ORFs. A total of 15 bp separate the fslC and fslD ORFs, and there is no identifiable Shine-Dalgarno sequence preceding fslD. We believe that the four genes are transcribed as part of an operon and, consistent with this idea, have determined that fslC is cotranscribed with fslB as a single transcript and that fslD is cotranscribed with fslC (data not shown). The FslB and FslD ORFs are most similar to efflux transporters, while the most closely related sequence to the FslC ORF is diaminopimelate decarboxylase. Sequences similar to these ORFs are present in biosynthetic loci for other siderophores such as achromobactin (18), vibrioferrin (39), and staphylobactin (8), suggesting that the fslABCD genes are all involved in F. tularensis siderophore biosynthesis and/or export. Pathways for synthesis of the bacterial siderophores are frequently conserved, as with staphyloferrin B biosynthetic genes in S. aureus and Ralstonia solanacaerum (3), and it is possible that pathways for synthesis of bacterial rhizoferrin are similarly conserved.
Siderophore-iron complexes are bound and taken up by specific receptor proteins in the outer membrane of gram-negative bacteria. The only genes known to be involved in the uptake of rhizoferrin are the rumA and rumB genes of Morganella morganii (29). We do not find homologs of these genes in the F. tularensis genome. Uptake of the siderophore-iron complexes is an energy-dependent process, facilitated by transient interaction of the receptor with the inner membrane-associated TonB-ExbB-ExbD complex (reviewed in reference 15). Surprisingly, although F. tularensis can synthesize and utilize siderophore, we have been unable to find sequences in the genome encoding the ubiquitous TonB, ExbB, and ExbD proteins. The mechanism of siderophore-mediated iron uptake in this organism appears to be unusual, perhaps involving novel proteins.
Iron uptake from the environment is a vital function for most organisms, and bacteria commonly use several redundant pathways to safeguard this ability. The F. tularensis genome is relatively small and considered degenerate with regard to many metabolic pathways (30). Bioinformatics-based analysis of the genome identified only one other iron uptake pathway (ferrous iron transporter, feoB) in addition to the fslABCD locus, suggesting that a limited number of iron uptake mechanisms are operational in this organism. The F. tularensis siderophore may be an important factor for the survival and growth of the pathogen in an iron-limiting host environment.
. . . . .
Acknowledgments
We thank Siv Andersson at Uppsala University for providing access to the Schu S4 genome sequence prior to publication. We are grateful to Carol A. Gilchrist for help with qPCR. We thank Kevin Young, George Church, Noriko Ohta, and Tom Zahrt for providing plasmids. We thank members of the B. J. Mann and W. A. Petri labs for discussions and suggestions.
This study was supported by R21 grant AI056227 from the National Institute of Allergy and Infectious Diseases to G.R.
Footnotes
This paper is dedicated to the memory of the late Robert J. Kadner, who was a source of advice and encouragement through the progress of this investigation.
REFERENCES
- 1.Arnow, L. E. 1937. Colorimetric determination of the components of 3,4 hydroxyphenylalanine-tyrosine mixtures. Annu. Rev. Biochem. 118:531-537. [Google Scholar]
- 2.Bhatnagar, N. B., K. L. Elkins, and A. H. Fortier. 1995. Heat stress alters the virulence of a rifampin-resistant mutant of Francisella tularensis. Infect. Immun. 63:154-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhatt, G., and T. P. Denny. 2004. Ralstonia solanacearum iron scavenging by the siderophore staphyloferrin B is controlled by PhcA, the global virulence regulator. J. Bacteriol. 186:7896-7904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brosius, J. 1989. Superpolylinkers in cloning and expression vectors. DNA 8:759-777. [DOI] [PubMed] [Google Scholar]
- 5.Challis, G. L. 2005. A widely distributed bacterial pathway for siderophore biosynthesis independent of nonribosomal peptide synthetases. ChemBioChem 6:601-611. [DOI] [PubMed] [Google Scholar]
- 6.Chamberlain, R. E. 1965. Evaluation of live tularemia vaccine prepared in a chemically defined medium. Appl. Microbiol. 13:232-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Csaky, T. 1948. On the estimation of bound hydroxylamines in biological materials. Acta Chem. Scand. 2:450-454. [Google Scholar]
- 8.Dale, S. E., A. Doherty-Kirby, G. Lajoie, and D. E. Heinrichs. 2004. Role of siderophore biosynthesis in virulence of Staphylococcus aureus: identification and characterization of genes involved in production of a siderophore. Infect. Immun. 72:29-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dennis, D. T., T. V. Inglesby, D. A. Henderson, J. G. Bartlett, M. S. Ascher, E. Eitzen, A. D. Fine, A. M. Friedlander, J. Hauer, M. Layton, S. R. Lillibridge, J. E. McDade, M. T. Osterholm, T. O'Toole, G. Parker, T. M. Perl, P. K. Russell, et al. 2001. Tularemia as a biological weapon: medical and public health management. JAMA 285:2763-2773. [DOI] [PubMed] [Google Scholar]
- 10.Ditta, G., S. Stanfield, D. Corbin, and D. R. Helinski. 1980. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc. Natl. Acad. Sci. USA 77:7347-7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drechsel, H., and G. Winkelmann. 2005. The configuration of the chiral carbon atoms in staphyloferrin A and analysis of the transport properties in Staphylococcus aureus. Biometals 18:75-81. [DOI] [PubMed] [Google Scholar]
- 12.Drechsel, H., J. Metzger, S. Freund, G. Jung, J. R. Boelaert, and G. Winkelmann. 1991. Rhizoferrin: a novel siderophore from the fungus Rhizopus microsporus var. rhizopodiformis. Biol. Metals 4:238-243. [Google Scholar]
- 13.Eberl, L., C. S. Kristensen, M. Givskov, E. Grohmann, M. Gerlitz, and H. Schwab. 1994. Analysis of the multimer resolution system encoded by the parCBA operon of broad-host-range plasmid RP4. Mol. Microbiol. 12:131-141. [DOI] [PubMed] [Google Scholar]
- 14.Escolar, L., J. Perez-Martin, and V. de Lorenzo. 1999. Opening the iron box: transcriptional metalloregulation by the Fur protein. J. Bacteriol. 181:6223-6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faraldo-Gomez, J. D., and M. S. Sansom. 2003. Acquisition of siderophores in gram-negative bacteria. Nat. Rev. Mol. Cell Biol. 4:105-116. [DOI] [PubMed] [Google Scholar]
- 16.Forbes, J. R., and P. Gros. 2001. Divalent-metal transport by NRAMP proteins at the interface of host-pathogen interactions. Trends Microbiol. 9:397-403. [DOI] [PubMed] [Google Scholar]
- 17.Fortier, A. H., D. A. Leiby, R. B. Narayanan, E. Asafoadjei, R. M. Crawford, C. A. Nacy, and M. S. Meltzer. 1995. Growth of Francisella tularensis LVS in macrophages: the acidic intracellular compartment provides essential iron required for growth. Infect. Immun. 63:1478-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franza, T., B. Mahe, and D. Expert. 2005. Erwinia chrysanthemi requires a second iron transport route dependent of the siderophore achromobactin for extracellular growth and plant infection. Mol. Microbiol. 55:261-275. [DOI] [PubMed] [Google Scholar]
- 19.Gay, P., D. Le Coq, M. Steinmetz, T. Berkelman, and C. I. Kado. 1985. Positive selection procedure for entrapment of insertion sequence elements in gram-negative bacteria. J. Bacteriol. 164:918-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golovliov, I., A. Sjostedt, A. Mokrievich, and V. Pavlov. 2003. A method for allelic replacement in Francisella tularensis. FEMS Microbiol. Lett. 222:273-280. [DOI] [PubMed] [Google Scholar]
- 21.Halmann, M., M. Benedict, and J. Mager. 1967. Nutritional requirements of Pasteurella tularensis for growth from small inocula. J. Gen. Microbiol. 49:451-460. [Google Scholar]
- 22.Halmann, M., and J. Mager. 1967. An endogenously produced substance essential for growth initiation of Pasteurella tularensis. J. Gen. Microbiol. 49:461-468. [Google Scholar]
- 23.Hickey, E. K., and N. P. Cianciotto. 1997. An iron- and Fur-repressed Legionella pneumophila gene that promotes intracellular infection and encodes a protein with similarity to the Escherichia coli aerobactin synthetases. Infect. Immun. 65:133-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hubalek, M., L. Hernychova, J. Havlasova, I. Kasalova, V. Neubauerova, J. Stulik, A. Macela, M. Lundqvist, and P. Larsson. 2003. Towards proteome database of Francisella tularensis. J. Chromatogr. B 787:149-177. [DOI] [PubMed] [Google Scholar]
- 25.Hubalek, M., L. Hernychova, M. Brychta, J. Lenco, J. Zechovska, and J. Stulik. 2004. Comparative proteome analysis of cellular proteins extracted from highly virulent Francisella tularensis ssp. tularensis and less virulent F. tularensis ssp. holarctica and F. tularensis ssp. mediaasiatica. Proteomics 4:3048-3060. [DOI] [PubMed] [Google Scholar]
- 26.Kawula, T. H., J. D. Hall, J. R. Fuller, and R. R. Craven. 2004. Use of transposon-transposase complexes to create stable insertion mutant strains of Francisella tularensis LVS. Appl. Environ. Microbiol. 70:6901-6904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Konetschny-Rapp, S., G. Jung, J. Meiwes, and H. Zahner. 1990. Staphyloferrin A: a structurally new siderophore from staphylococci. Eur. J. Biochem. 191:65-74. [DOI] [PubMed] [Google Scholar]
- 28.Kovarova, H., L. Hernychova, M. Hajduch, M. Sirova, and A. Macela. 2000. Influence of the bcg locus on natural resistance to primary infection with the facultative intracellular bacterium Francisella tularensis in mice. Infect. Immun. 68:1480-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuhn, S., V. Braun, and W. Koster. 1996. Ferric rhizoferrin uptake into Morganella morganii: characterization of genes involved in the uptake of a polyhydroxycarboxylate siderophore. J. Bacteriol. 178:496-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larsson, P., P. C. Oyston, P. Chain, M. C. Chu, M. Duffield, H. H. Fuxelius, E. Garcia, G. Halltorp, D. Johansson, K. E. Isherwood, P. D. Karp, E. Larsson, Y. Liu, S. Michell, J. Prior, R. Prior, S. Malfatti, A. Sjostedt, K. Svensson, N. Thompson, L. Vergez, J. K. Wagg, B. W. Wren, L. E. Lindler, S. G. Andersson, M. Forsman, and R. W. Titball. 2005. The complete genome sequence of Francisella tularensis, the causative agent of tularemia. Nat. Genet. 37:153-159. [DOI] [PubMed] [Google Scholar]
- 31.Link, A. J., D. Phillips, and G. M. Church. 1997. Methods for generating precise deletions and insertions in the genome of wild-type Escherichia coli: application to open reading frame characterization. J. Bacteriol. 179:6228-6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maier, T. M., A. Havig, M. Casey, F. E. Nano, D. W. Frank, and T. C. Zahrt. 2004. Construction and characterization of a highly efficient Francisella shuttle plasmid. Appl. Environ. Microbiol. 70:7511-7519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martinez, J. L., M. Herrero, and V. de Lorenzo. 1994. The organization of intercistronic regions of the aerobactin operon of pColV-K30 may account for the differential expression of the iucABCD iutA genes. J. Mol. Biol. 238:288-293. [DOI] [PubMed] [Google Scholar]
- 34.Munzinger, M., K. Taraz, H. Budzikiewicz, H. Drechsel, P. Heymann, G. Winkelmann, and J. M. Meyer. 1999. S,S-rhizoferrin (enatio-rhizoferrin)—a siderophore of Ralstonia (Pseudomonas) pickettii DSM6297—the optical antipode of R-rhizoferrin isolated from fungi. BioMetals 12:189-193. [Google Scholar]
- 35.Nano, F. E., N. Zhang, S. C. Cowley, K. E. Klose, K. K. Cheung, M. J. Roberts, J. S. Ludu, G. W. Letendre, A. I. Meierovics, G. Stephens, and K. L. Elkins. 2004. A Francisella tularensis pathogenicity island required for intramacrophage growth. J. Bacteriol. 186:6430-6436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oyston, P. C., A. Sjostedt, and R. W. Titball. 2004. Tularaemia: bioterrorism defense renews interest in Francisella tularensis. Nat. Rev. Microbiol. 2:967-978. [DOI] [PubMed] [Google Scholar]
- 37.Ratledge, C., and L. G. Dover. 2000. Iron metabolism in pathogenic bacteria. Annu. Rev. Microbiol. 54:881-941. [DOI] [PubMed] [Google Scholar]
- 38.Schwyn, B., and J. B. Neilands. 1987. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 160:47-56. [DOI] [PubMed] [Google Scholar]
- 39.Tanabe, T., T. Funahashi, H. Nakao, S. Miyoshi, S. Shinoda, and S. Yamamoto. 2003. Identification and characterization of genes required for biosynthesis and transport of the siderophore vibrioferrin in Vibrio parahaemolyticus. J. Bacteriol. 185:6938-6949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thieken, A., and G. Winkelmann. 1992. Rhizoferrin: a complexone type siderophore of the Mucorales and Entomophthorales (Zygomycetes). FEMS Microbiol. Lett. 73:37-41. [DOI] [PubMed] [Google Scholar]
- 41.Titball, R. W., A. Johansson, and M. Forsman. 2003. Will the enigma of Francisella tularensis virulence soon be solved? Trends Microbiol. 11:118-123. [DOI] [PubMed] [Google Scholar]
- 42.Twine, S., M. Bystrom, W. Chen, M. Forsman, I. Golovliov, A. Johansson, J. Kelly, H. Lindgren, K. Svensson, C. Zingmark, W. Conlan, and A. Sjostedt. 2005. A mutant of Francisella tularensis strain SCHU S4 lacking the ability to express a 58-kilodalton protein is attenuated for virulence and is an effective live vaccine. Infect. Immun. 73:8345-8352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Winkelmann, G. 2002. Microbial siderophore-mediated transport. Biochem. Soc. Trans. 30:691-696. [DOI] [PubMed] [Google Scholar]