Abstract
To examine whether methylation of the GATC sites present in the dnaA promoter region is responsible for the strict temporal coordination of initiation events at oriC as measured by the synchrony of initiation, we introduced point mutations eliminating three (TGW1) and five (TGW2) of the six GATC sites present in the dnaA promoter region. All of the strains containing these mutations, including the one with five GATC sites eliminated, initiated chromosomal replication synchronously.
The initiation of DNA replication at two or more origins is strictly coordinated such that all origins fire simultaneously and do so once per cell cycle (28). Methylation of GATC sites located at oriC and dnaA has been implicated in the strict temporal regulation of initiation timing in Escherichia coli (1). The transfer of a methyl group from S-adenosylmethionine to the adenosine present in 5′-GATC-3′ sites of newly synthesized DNA is catalyzed by deoxyadenosyl methyltransferase or Dam. Most GATC sites become fully methylated 2 to 5 min following the passage of the replication fork. GATC sites present in oriC and the dnaA promoter, however, experience a delay in methylation following initiation at oriC (6). In exponentially growing cells, the genes of the dnaA operon are coordinately expressed from the dnaAp2 promoter (24), and decreased dnaA expression is observed during this period of hemimethylation (31).
This strict temporal coordination of initiation events and expression of dnaA is disrupted in dam mutant strains. Asynchronous initiation timing is observed in dam cells, and initiation events occur randomly throughout the cell cycle (1, 2). Expression from the dnaAp2 promoter in cells grown at 37°C is 2.7- to 4.5-fold lower in dam+ than in dam mutant strains (3). Mutations in seqA greatly reduce the delay in methylation of oriC and the dnaA promoter after initiation (22), and DnaA protein levels are twofold higher in cells carrying a seqA2 mutation (32).
SeqA binds to oriC in vitro (29), to oriR of plasmid P1 (4), to the bacteriophage λ pR promoter (30), and to synthetic oligonucleotides containing multiple GATC sites (4) and may negatively regulate initiation of replication (22). Binding of SeqA to hemimethylated DNA requires the presence of two GATC sites (5) that can be as far apart as 31 bp (18). GATC sites are overrepresented in oriC and the dnaA promoter, and many are separated by less than 31 bp. If GATC sites occurred randomly in the E. coli genome, they would occur, on average, once every 256 bp in the E. coli chromosome, assuming a GC content of 50%. However, the dnaA promoter region contains 6 GATC sites (12) (Fig. 1); 11 are present in the minimal 245-bp oriC gene (20, 33).
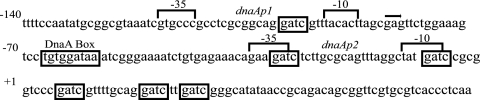
FIG. 1.
The dnaA promoter sequence.
The E. coli K-12 dnaA promoter sequence was compared to those of closely related bacteria (Table 1). A BLAST search was conducted against the microbial database at the National Center for Biotechnology Information with the dnaA sequence from E. coli K-12. Except for two Yersinia pestis strains, all hits for the bacterial strains presented in Table 1 had the expect values of zero. The dnaA promoter sequences that were obtained from hits with low expected values, and therefore a high degree of similarity to the dnaA gene, were uploaded into the Macromolecular Structural Analysis Resource Core, San Diego State University. The GCG (Genetics Computer Group) Wisconsin package of sequence comparison tools was used to perform an alignment of the dnaA sequences. An alignment of the uploaded sequences was performed with the GCG program PILEUP, and the output was then converted with the GCG program PRETTY. Along with the DnaA box (data not shown), the GATC sites located in the −10 and −35 sites of the dnaAp2 promoter are 100% conserved. The most proximal of the three GATC sites located downstream of the dnaAp2 start site is more frequently conserved than the GATC site located in the dnaAp1 promoter.
TABLE 1.
Conserved GATC sites in the dnaA promoter
| Strain | Presence of conserved GATC site at:
|
|||||
|---|---|---|---|---|---|---|
| dnaAp1 | dnaAp2 (−35) | dnaAp2 (−10) | +6 | +19 | +25 | |
| E. coli K-12 dnaA promoter | + | + | + | + | + | + |
| E. coli CFT073 | + | + | + | + | + | + |
| E. coli O157:H7 | + | + | + | + | + | + |
| E. coli O157:H7 EDL933 | + | + | + | + | + | + |
| Shigella flexneri 2a strain 301 | + | + | + | + | + | + |
| Shigella flexneri 2a strain 2457Ta | + | + | + | + | + | + |
| Salmonella enterica serovar Typhimurium | + | + | + | + | + | − |
| Salmonella enterica serovar Typhi TY2 | + | + | + | + | + | − |
| Klebsiella pneumoniae | + | + | + | + | − | − |
| Yersinia pestis CO92 | − | + | + | + | − | − |
| Yersinia pestis KIM | − | + | + | + | − | − |
| Erwinia chrysanthemi 3937 | − | + | + | − | − | − |
| % Conserved | 75 | 100 | 100 | 92 | 66 | 50 |
The superscript T indicates the type strain.
Of the six GATC sites present in the dnaA promoter, those in the −10 and −35 sequences of the dnaAp2 promoter are conserved in all of the gram-negative bacteria examined in Table 1 and are present in a consensus sequence for σ70 transcription factors (14, 15). Strains with the −10 and −35 GATC sites eliminated, alone and in combination, from the dnaAp2 promoter demonstrated synchronous initiation events (12). DnaA protein levels in strains with the −10 and −35 GATC sites eliminated from the dnaAp2 promoter were similar to those in the parental MG1655 strain, in agreement with experiments with a dnaA-chitobiase reporter construct integrated at attB on the E. coli chromosome (19). The amount of dnaA expression is twofold lower in dam+ than in dam mutant strains, which was thought to result from the release from sequestration that occurs through the binding of SeqA to hemimethylated GATC sites (3). However, when DnaA protein levels were compared in dam+ and dam mutant strains possessing a dnaAp2 promoter with the −10 and −35 GATC sites eliminated, the effect was attenuated. The −10 and −35 GATC sites in the dnaAp2 promoter appear not to be necessary for the strict coordination between dnaA expression and initiation timing (7). However, SeqA binding to GATC sites downstream of the pI and paQ promoters in bacteriophage λ has been implicated in transcriptional regulation (30), so we investigated the effect of removing the GATC sites downstream of the dnaAp2 promoter.
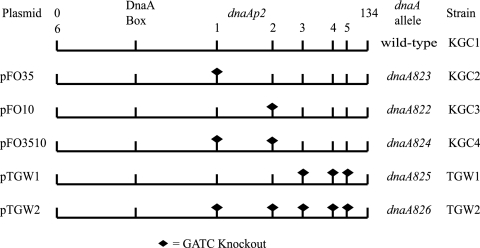
There are three additional GATC sites downstream of the dnaAp2 promoter that are close enough to one another to permit SeqA binding (Fig. 1), and these GATC sites were eliminated in this study by the methods developed by Kedar et al. (19). Two strains, TGW1 and TGW2, were constructed. The TGW1 strain has three GATC-to-GATG mutations introduced downstream of dnaAp2 (Fig. 2) but is otherwise identical to KGC1 (19). The dnaA promoter in the TGW2 strain has five of six GATC sites altered (Fig. 2). The sixth GATC site is located upstream of the dnaAp2 promoter and would not have a neighboring GATC site close enough to permit SeqA binding (5, 18).
FIG. 2.
Strains containing GATC mutations in the dnaA promoter. The first nucleotide of each GATC site and in the DnaA box (represented at the extreme left) are indicated with respect to the relative position to each other and to the 100-bp fragment representing the dnaAp2 promoter (drawn to scale). The GATC sites present in the dnaA promoter sequence are numbered. The dnaAp2 transcriptional start site is present between GATC sites 2 (−10) and 3. The plasmids and the strains ultimately constructed are to the left and extreme right, respectively. Black diamonds indicate the first G in a GATC site that was eliminated by site-specific mutagenesis.
Plasmids and strains were constructed essentially as previously described (19). The pTGW1 and pTGW2 plasmids are derivatives of pKGC3 (8,558 bp) and pKGC4 (8,558 bp), respectively (Table 2). A PCR method of site-specific mutagenesis was used to introduce three C-to-G point mutations and thus eliminate the GATC sites downstream of the dnaAp2 promoter. The mutations were introduced into pKGC3 and pKGC4 by the four-primer method (17, 23). The mutations were present in oligonucleotide primers that were introduced into amplified PCR products, which were ligated into plasmids pKGC3 (7,421 bp) and pKGC4 (7,421 bp). The resulting plasmids, pTGW1 (8,558 bp) and pTGW2 (8,558 bp), respectively, were used to integrate the mutated dnaA promoter into the chromosome of a polA107 (16) mutant in a two-step process. The plasmids contain the dnaA promoter region and part of the dnaA coding region inserted between the Omega cassette (25, 26) and sacB. The Omega cassette provides resistance to streptomycin and spectinomycin. The enzyme levansucrase is encoded by the Bacillus subtilis sacB gene, and its expression in the presence of 5% sucrose results in the accumulation of levans, which is ultimately lethal in E. coli (7, 8, 27). The pTGW1 and pTGW2 plasmids carry a ColE1-derived origin that requires a functional DNA polymerase I for replication and cannot be maintained in a polA mutant (9, 11). Integration of the plasmid, carrying the mutated dnaA promoter, into the chromosome by homologous recombination was selected with spectinomycin. In the second step, excision of the plasmid backbone was selected by addition of 5% sucrose to the culture medium.
TABLE 2.
Strains used in this study
| Strain | Genotype | Source or reference |
|---|---|---|
| DH5α | supE44 lacU169 [φ80 lacZΔM15] hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | Stratagene |
| JZ294 | F−polA1 argH hsdR rpsL thyA36 | 13 |
| JG108 | metE70 thyA deoC2 lacZ lacY14(Am) rhaS rpsL λ− | Cathy Joyce |
| JZ365 | JG108 polA12::Tn10 | Cathy Joyce |
| JZ366 | JG108 polA107::Tn10 | Cathy Joyce |
| JZ1101 | W3110 tnaA262::Tn10 | Charles Yanofsky |
| MG1655 | λ− F− | Mary Berlyn |
| TGW1 | MG1655 with three GATC sites downstream of dnaAp2 eliminated | This study |
| TGW2 | MG1655 with five of six GATC sites in dnaA promoter region eliminated | This study |
The polA107 (16) mutant strain was transformed with either pTGW1 or pTGW2 and incubated at 30°C overnight in 4 ml of SOC buffer containing thymine (25 μg/ml) and spectinomycin (50 μg/ml). The cultures were diluted 1:50 in low-salt LB-5% sucrose-5 mM CaCl2-thymine (25 μg/ml)-spectinomycin (50 μg/ml) and incubated overnight at 30°C. The cultures again were diluted 1:50 in low-salt LB-5% sucrose-5 mM CaCl2-thymine (25 μg/ml)-spectinomycin (50 μg/ml) and incubated overnight at 30°C. The mutated dnaA promoter region was then moved by P1 transduction into MG1655. Transductants were selected on low-salt LB agar plates containing 5% sucrose and spectinomycin (50 μg/ml) and replica plated to LB agar plates containing ampicillin (100 μg/ml) to screen for sensitivity to ampicillin, resulting in strains TGW1 and TGW2 (Table 2).
The resulting Aps Spr strains have three of six (TGW1) or five of six (TGW2) GATC sites eliminated from the dnaA promoter (Fig. 2). PCR of chromosomal DNA, followed by sequencing of the PCR product, verified the absence of intact GATC sites. To demonstrate integration at the wild-type chromosomal location of the Omega cassette (Spr) carrying the mutated dnaA promoter, a tnaA262::Tn10 mutant was transduced with bacteriophage P1 lysates prepared on either TGW1 or TGW2. Transductants were selected on spectinomycin (50 μg/ml)-containing agar plates, replica plated onto agar plates containing both spectinomycin (50 μg/ml) and tetracycline (25 μg/ml), and screened for loss of tetracycline resistance, thereby verifying linkage between the dnaA (Spr) and tnaA::Tn10 (Tcr) loci.
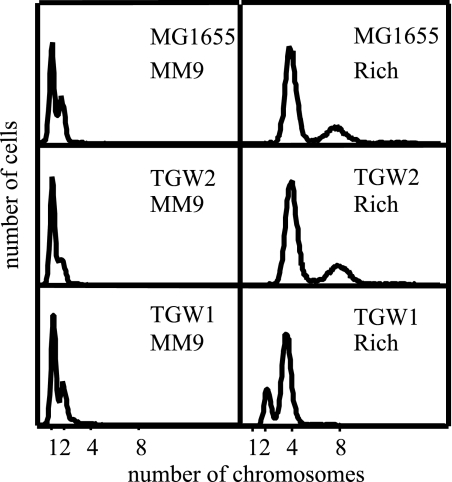
The initiation of DNA replication is precisely coordinated, occurring at a specific time, once per cell cycle, at all origins in the cell simultaneously (28). If initiation is inhibited with rifampin and ongoing rounds of replication are allowed to complete, wild-type cells with coordinated and synchronous initiation of all chromosomes contain 2n (n = 0, 1, 2, 3, 4) chromosomes when examined by flow cytometry (10, 19, 28). Defects in the timing of initiation result in cells that contain “irregular” numbers of completed chromosomes, such as three, five, six, seven, etc. (27). Also, cells that contain chromosomes unable to complete replication because of a DNA lesion or blockage in replication after rifampin treatment show broad peaks of DNA where the amount of DNA per cell does not correspond to the amount of DNA in a single completed chromosome or in multiple numbers of completed chromosomes (10). We examined initiation timing and the completion of chromosomal replication in strains TGW1, TGW2, and MG1655 by flow cytometry (19) in both minimal and enriched media after treatment with rifampin to inhibit initiation and cephalexin to inhibit cell division (Fig. 3). As shown in Fig. 3, the strains with either three (TGW1) or five (TGW2) GATC sites eliminated in the dnaA promoter region contained 2n completed chromosomes, the phenotype of a wild-type E. coli strain such as MG1655. Strain TGW1, with three of the GATC sites eliminated, grew more slowly than either TGW2 or MG1655 (Table 3). Also, strain TGW1 grown in enriched medium had a reduced number of completed chromosomes after rifampin and cephalexin addition. With fewer initiation events occurring at a lower growth rate, the number of completed chromosomes is expected to be lower. The number of chromosomes per cell after runout (4 h of treatment with rifampin and cephalexin) depends on the number of replication cycles per chromosome (n), where n equals C/τ, τ is the generation time in minutes, and C is the C period in minutes; the C period is the duration of chromosomal replication. When cells grow more slowly (τ increases), n decreases, resulting in fewer chromosomes per cell after inhibition of initiation. Although the number of completed chromosomes in TGW1 cells observed by flow cytometry was lower in enriched M9 medium than with MG1655 cells, the number of completed chromosomes in TGW1 was 2n (two and four chromosomes), indicating synchronous initiation at all DNA replication origins in each cell.
FIG. 3.
Strains with three (TGW1) or five (TGW2) GATC sites eliminated from the dnaA promoter initiate DNA replication synchronously. Cells growing exponentially in M9 enriched (rich) or M9 minimal (MM9) medium were treated with rifampin and cephalexin for 4 h. Samples were removed, fixed, stained, and analyzed by flow cytometry as described previously (19).
TABLE 3.
Cell cycle parameters for GATC dnaA mutants
| Growth condition and strain | τa | DNA/cellb | Mass/cellc |
|---|---|---|---|
| M9 minimal medium | |||
| MG1655 | 60 | 1.00 | 1.00 |
| TGW2 | 60 | 1.00 | 1.00 |
| TGW1 | 75 | 0.94 | 0.93 |
| M9 enriched medium | |||
| MG1655 | 32 | 1.00 | 1.00 |
| TGW2 | 34 | 1.00 | 0.98 |
| TGW1 | 52 | 0.80 | 0.88 |
τ, generation time (min).
DNA/cell, mean value of fluorescence in exponentially growing cells compared to MG1655.
Mass/cell, mean value of light scattering in exponentially growing cells compared to MG1655.
The methylation state of the GATC sites in the dnaA promoter appears to be of little consequence to initiation timing, because elimination of the two GATC sites in the dnaAp2 promoter (19), the three GATC sites downstream of the dnaAp2 promoter (TGW1, this study), or all five GATC sites (TGW2, this study) did not result in initiation timing defects detectable by flow cytometry (Fig. 3). Either proper initiation timing does not require cell cycle-dependent dnaA expression, or cell cycle control of dnaA expression is maintained by a mechanism other than sequestration. The concentration of the active ATP-bound form of DnaA protein may be the critical event regulating initiation events, and this concentration may be regulated in a cell cycle-dependent manner that is independent of the sequestration of the dnaA promoter (21).
Acknowledgments
We thank our colleagues Moselio Schaechter, Joe Mahaffy, and Anca Segall for valuable input during discussions concerning the manuscript. We thank Mary Berlyn, Cathy Joyce, Walter Messer, Andrew Wright, Charles Yanofsky, and C. Peter Wolk for strains and plasmids.
These studies were supported by National Science Foundation grant MCB-9507209 to J.W.Z. and by Ministerio de Ciencia y Tecnología grant BMC2002-00830 and Junta de Extremadura grant 2PR04A036 to E.C.G.
REFERENCES
- 1.Bakker, A., and D. W. Smith. 1989. Methylation of GATC sites is required for precise timing between rounds of DNA replication in Escherichia coli. J. Bacteriol. 171:5738-5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boye, E., and A. Lobner-Olesen. 1990. The role of Dam methyltransferase in the control of DNA replication in E. coli. Cell 62:981-989. [DOI] [PubMed] [Google Scholar]
- 3.Braun, R. E., and A. Wright. 1986. DNA methylation differentially enhances the expression of one of the two E. coli dnaA promoters in vivo and in vitro. Mol. Gen. Genet. 202:246-250. [DOI] [PubMed] [Google Scholar]
- 4.Brendler, T., A. Abeles, and S. Austin. 1995. A protein that binds to the P1 origin core and the oriC 13mer region in a methylation-specific fashion is the product of the host seqA gene. EMBO J. 14:4083-4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brendler, T., and S. Austin. 1999. Binding of SeqA protein to DNA requires interaction between two or more complexes bound to separate hemimethylated GATC sequences. EMBO J. 18:2304-2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell, J. L., and N. Kleckner. 1990. E. coli oriC and the dnaA gene promoter are sequestered from dam methyltransferase following the passage of the chromosomal replication fork. Cell 62:967-979. [DOI] [PubMed] [Google Scholar]
- 7.Gay, P., D. Le Coq, M. Steimetz, T. Berkelman, and C. I. Kado. 1985. Positive selection procedure for entrapment of insertion sequence elements in gram-negative bacteria. J. Bacteriol. 164:918-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gay, P., D. Le Coq, M. Steinmetz, E. Ferrari, and J. A. Hoch. 1983. Cloning structural gene sacB, which codes for exoenzyme levansucrase of Bacillus subtilis: expression of the gene in Escherichia coli. J. Bacteriol. 153:1424-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glickman, B. W., C. A. van Sluis, H. L. Heijneker, and A. Rorsch. 1973. A mutant of Escherichia coli K12 deficient in the 5′-3′ exonucleolytic activity of DNA polymerase I. I. General characterization. Mol. Gen. Genet. 124:69-82. [DOI] [PubMed] [Google Scholar]
- 10.Grigorian, A. V., R. B. Lustig, E. C. Guzman, J. M. Mahaffy, and J. W. Zyskind. 2003. Escherichia coli cells with increased levels of DnaA and deficient in recombinational repair have decreased viability. J. Bacteriol. 185:630-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grindley, N. D., and W. S. Kelley. 1976. Effects of different alleles of the E. coli K12 polA gene on the replication of non-transferring plasmids. Mol. Gen. Genet. 143:311-318. [DOI] [PubMed] [Google Scholar]
- 12.Hansen, F. G., E. B. Hansen, and T. Atlung. 1982. The nucleotide sequence of the dnaA gene promoter and of the adjacent rpmH gene, coding for the ribosomal protein L34, of Escherichia coli. EMBO J. 1:1043-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harding, N. E., J. M. Cleary, D. W. Smith, J. J. Michon, W. S. Brusilow, and J. W. Zyskind. 1982. Chromosomal replication origins (oriC) of Enterobacter aerogenes and Klebsiella pneumoniae are functional in Escherichia coli. J. Bacteriol. 152:983-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harley, C. B., and R. P. Reynolds. 1987. Analysis of E. coli promoter sequences. Nucleic Acids Res. 15:2343-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hawley, D. K., and W. R. McClure. 1983. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 11:2237-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heijneker, H. L., D. J. Ellens, R. H. Tjeerde, B. W. Glickman, B. van Dorp, and P. H. Pouwels. 1973. A mutant of Escherichia coli K12 deficient in the 5′-3′ exonucleolytic activity of DNA polymerase I. II. Purification and properties of the mutant enzyme. Mol. Gen. Genet. 124:83-96. [DOI] [PubMed] [Google Scholar]
- 17.Higuchi, R., B. Krummel, and R. K. Saiki. 1988. A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res. 16:7351-7367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang, S., J. S. Han, K. P. Kim, H. Y. Yang, K. Y. Lee, C. B. Hong, and D. S. Hwang. 2005. Dimeric configuration of SeqA protein bound to a pair of hemi-methylated GATC sequences. Nucleic Acids Res. 33:1524-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kedar, G. C., F. Ozcan, E. C. Guzman, D. W. Smith, V. G. Newman, and J. W. Zyskind. 2000. Role of DNA methylation at GATC sites in the dnaA promoter, dnaAp2. J. Mol. Microbiol. Biotechnol. 2:301-310. [PubMed] [Google Scholar]
- 20.Kornberg, A., and T. A. Baker. 1992. DNA replication, 2nd ed. W. H. Freeman & Co., New York, N.Y.
- 21.Kurokawa, K., S. Nishida, A. Emoto, K. Sekimizu, and T. Katayama. 1999. Replication cycle-coordinated change of the adenine nucleotide-bound forms of DnaA protein in Escherichia coli. EMBO J. 18:6642-6652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu, M., J. L. Campbell, E. Boye, and N. Kleckner. 1994. SeqA: a negative modulator of replication initiation in E. coli. Cell 77:413-426. [DOI] [PubMed] [Google Scholar]
- 23.Old, R. W., and S. B. Primrose. 1994. Principles of gene manipulation: an introduction to genetic engineering, 5th ed. Blackwell Scientific Publications, Oxford, England.
- 24.Perez-Roger, I., M. Garcia-Sogo, J. P. Navarro-Avino, C. Lopez-Acedo, F. Macian, and M. E. Armengod. 1991. Positive and negative regulatory elements in the dnaA-dnaN-recF operon of Escherichia coli. Biochimie 73:329-334. [DOI] [PubMed] [Google Scholar]
- 25.Prentki, P., A. Binda, and A. Epstein. 1991. Plasmid vectors for selecting IS1-promoted deletions in cloned DNA: sequence analysis of the omega interposon. Gene 103:17-23. [DOI] [PubMed] [Google Scholar]
- 26.Prentki, P., and H. M. Krisch. 1984. In vitro insertional mutagenesis with a selectable DNA fragment. Gene 29:303-313. [DOI] [PubMed] [Google Scholar]
- 27.Reyrat, J., V. Pelicic, B. Gicquel, and R. Rappuoli. 1998. Counterselectable markers: untapped tools for bacterial genetics and pathogenesis. Infect. Immun. 66:4011-4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skarstad, K., E. Boye, and H. B. Steen. 1986. Timing of initiation of chromosome replication in individual Escherichia coli cells. EMBO J. 5:1711-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slater, S., S. Wold, M. Lu, E. Boye, K. Skarstad, and N. Kleckner. 1995. E. coli SeqA protein binds oriC in two different methyl-modulated reactions appropriate to its roles in DNA replication initiation and origin sequestration. Cell 82:927-936. [DOI] [PubMed] [Google Scholar]
- 30.Slominska, M., G. Konopa, S. Baranska, G. Wegrzyn, and A. Wegrzyn. 2003. Interplay between DnaA and SeqA proteins during regulation of bacteriophage lambda pR promoter activity. J. Mol. Biol. 329:59-68. [DOI] [PubMed] [Google Scholar]
- 31.Theisen, P. W., J. E. Grimwade, A. C. Leonard, J. A. Bogan, and C. E. Helmstetter. 1993. Correlation of gene transcription with the time of initiation of chromosome replication in Escherichia coli. Mol. Microbiol. 10:575-584. [DOI] [PubMed] [Google Scholar]
- 32.von Freiesleben, U., K. V. Rasmussen, and M. Schaechter. 1994. SeqA limits DnaA activity in replication from oriC in Escherichia coli. Mol. Microbiol. 14:763-772. [DOI] [PubMed] [Google Scholar]
- 33.Zyskind, J. W., and D. W. Smith. 1986. The bacterial origin of replication, oriC. Cell 46:489-490. [DOI] [PubMed] [Google Scholar]