Abstract
The hyperthermophilic archaeon Pyrobaculum islandicum uses the citric acid cycle in the oxidative and reductive directions for heterotrophic and autotrophic growth, respectively, but the control of carbon flow is poorly understood. P. islandicum was grown at 95°C autotrophically, heterotrophically, and mixotrophically with acetate, H2, and small amounts of yeast extract and with thiosulfate as the terminal electron acceptor. The autotrophic growth rates and maximum concentrations of cells were significantly lower than those in other media. The growth rates on H2 and 0.001% yeast extract with and without 0.05% acetate were the same, but the maximum concentration of cells was fourfold higher with acetate. There was no growth with acetate if 0.001% yeast extract was not present, and addition of H2 to acetate-containing medium greatly increased the growth rates and maximum concentrations of cells. P. islandicum cultures assimilated 14C-labeled acetate in the presence of H2 and yeast extract with an efficiency of 55%. The activities of 11 of 19 enzymes involved in the central metabolism of P. islandicum were regulated under the three different growth conditions. Pyruvate synthase and acetate:coenzyme A (CoA) ligase (ADP-forming) activities were detected only in heterotrophically grown cultures. Citrate synthase activity decreased in autotrophic and acetate-containing cultures compared to the activity in heterotrophic cultures. Acetylated citrate lyase, acetate:CoA ligase (AMP forming), and phosphoenolpyruvate carboxylase activities increased in autotrophic and acetate-containing cultures. Citrate lyase activity was higher than ATP citrate synthase activity in autotrophic cultures. These data suggest that citrate lyase and AMP-forming acetate:CoA ligase, but not ATP citrate synthase, work opposite citrate synthase to control the direction of carbon flow in the citric acid cycle.
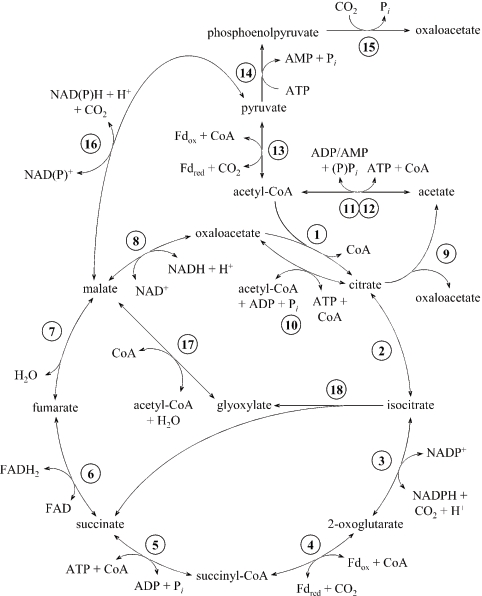
The citric acid cycle consists of eight enzymatic steps that oxidize acetyl coenzyme A (acetyl-CoA) into two molecules of CO2 per turn of the cycle (Fig. 1). The reductive citric acid cycle reverses this process and is an alternative to the Calvin cycle for CO2 fixation in many anaerobic autotrophic prokaryotes. One of the key steps in the reductive cycle is the conversion of citrate into oxaloacetate and acetyl-CoA. This step complements the irreversible citrate synthase step of the oxidative cycle, which controls the direction of carbon flow (Fig. 1). In Chlorobium limicola and Desulfobacter hydrogenophilus, the step is catalyzed by ATP citrate synthase (Fig. 1) (2, 24, 34). Alternatively, it has been proposed that this conversion is also catalyzed by some photosynthetic bacteria in two enzyme steps (Fig. 1), using citrate lyase and AMP-forming acetate:CoA ligase (http://www.genome.jp/kegg/pathway/map/map00720.html). AMP-forming acetate:CoA ligase was characterized from Rhodospirillum rubrum (13), which uses the reductive citric acid cycle for CO2 fixation (10), and the genome sequence of this organism contains genes encoding two homologs of the catalytic β subunit of citrate lyase (RruA0217 and RruA1200; accession number NC007643). In Hydrogenobacter thermophilus, the conversion is catalyzed in two other steps by citryl-CoA synthase (citrate + ATP + CoASH → citryl-CoA + ADP + Pi) and citryl-CoA lyase (citryl-CoA → oxaloacetate + acetyl-CoA) (3, 4).
FIG. 1.
Proposed citric acid cycle and related enzyme reactions in P. islandicum. The enzymes are as follows: 1, citrate synthase; 2, aconitate hydratase; 3, isocitrate dehydrogenase; 4, 2-oxoglutarate synthase; 5, succinate:CoA ligase (ADP forming); 6, succinate dehydrogenase; 7, fumarate hydratase; 8, malate dehydrogenase; 9, citrate lyase; 10, ATP citrate synthase; 11, acetate:CoA ligase (ADP forming); 12, acetate:CoA ligase (AMP forming); 13, pyruvate synthase; 14, pyruvate-water dikinase; 15, phosphoenolpyruvate carboxylase; 16, malate dehydrogenase (decarboxylating); 17, malate synthase; and 18, isocitrate lyase. Fd, electron carrier ferredoxin; FAD, flavin adenine dinucleotide; FADH2, reduced flavin adenine dinucleotide.
Pyrobaculum islandicum is an archaeon that grows optimally at 100°C and is a facultative autotroph (20). All eight citric acid cycle enzyme activities and pyruvate synthase activity were measured previously in P. islandicum cultures grown on peptides (36). The presence of ATP citrate synthase, pyruvate synthase, and 2-oxoglutarate synthase activities and the absence of other CO2 fixation enzyme activities in autotrophically grown P. islandicum cultures led to the suggestion that this organism fixes CO2 via the reductive citric acid cycle (21). This was consistent with the operation of this pathway in Thermoproteus neutrophilus, a member of the same family, as determined by 13C nuclear magnetic resonance and enzyme activity studies (6, 32, 33, 39). However, for both P. islandicum and T. neutrophilus, the activities of ATP citrate synthase were estimated to be too low to account for the activity needed to maintain CO2 fixation (6, 21). It was also reported previously that some Pyrobaculum species (e.g., Pyrobaculum aerophilum) can oxidize acetate for growth (42), while other species (e.g., P. islandicum) cannot (20). Other workers have since reported that they were unable to grow either P. aerophilum or P. islandicum via acetate oxidation (1, 40).
In this study, we defined conditions for the growth of P. islandicum on H2 and CO2 and on H2, acetate, and low concentrations of organic compounds. The activities of the eight citric acid cycle enzymes and 11 other central metabolic enzymes (Fig. 1) in P. islandicum cultures grown under autotrophic, mixotrophic, and heterotrophic conditions were measured. The data suggest that citrate is converted into oxaloacetate and acetyl-CoA primarily in two steps, using acetylated citrate lyase and an AMP-forming acetate:CoA ligase. Pyruvate synthase activity was found only in heterotrophically grown cultures, suggesting that there was an alternative but unknown mechanism for pyruvate synthesis in acetate-grown and autotrophically grown cultures.
MATERIALS AND METHODS
Organisms used.
P. islandicum DSM 4184 was used for this study and was a gift from Kazem Kashefi and Derek Lovley (Department of Microbiology, University of Massachusetts, Amherst). Sulfolobus acidocaldarius DSM 639 was grown and used as a positive control for measurement of isocitrate lyase activity.
Growth conditions.
P. islandicum was grown in a 20-liter fermentor in medium described previously unless indicated otherwise (36). With all media, cultures were grown anaerobically using 0.2% (wt/vol) Na2S2O3 as the terminal electron acceptor (36). For heterotrophic growth, 0.05% (wt/vol) casein hydrolysate (enzymatic; Difco) and 0.02% (wt/vol) yeast extract (Difco) were added, and the medium was sparged throughout growth with Ar at a rate of 30 ml min−1. For growth with acetate, 0.05% (wt/vol) sodium acetate plus 0.001% yeast extract were added, and the medium was sparged with H2 at a rate of 40 ml min−1. For autotrophic growth, no organic compounds were added, and the medium was sparged with an H2-CO2 mixture (80:20) at a rate of 30 ml min−1. Cysteine-HCl (0.5 mM) was added as the reducing agent to remove residual O2. There was no cell growth in autotrophic medium lacking CO2 and containing cysteine as the reducing agent (i.e., there was no growth on cysteine). The pH of the medium was adjusted to 6.00 ± 0.05, and the medium was heated to 95°C and stirred at 120 to 150 rpm throughout growth. At 95°C, the pH values of the heterotrophic and autotrophic media were maintained at 5.7 ± 0.1 by automatic addition of 1 N H2SO4 (which inhibited growth with acetate).
The fermentor was inoculated with a logarithmic-phase culture that had been grown and transferred at least three times in bottles containing the medium used. At various times during growth, a sample was removed to determine the concentration of cells using a Petroff-Hausser counting chamber and phase-contrast light microscopy. The specific growth rate (k) of the culture was determined by using a best-fit curve for the logarithmic portion of the growth data. Cells were harvested from the fermentor when they reached the late logarithmic growth phase, as described previously (16). Cultures were grown four times in the autotrophic medium, three times with acetate, and four times in the heterotrophic medium.
Serum bottle experiments.
The headspace of cultures grown heterotrophically in sealed serum bottles was checked for H2 following growth using a gas chromatograph (GC-8A; Shimadzu) fitted with a 5A 80/100 molecular sieve column (Alltech) and Ar as the carrier and reference gas. Cultures were grown on 0.001% yeast extract with and without 0.05% acetate and with either H2 or Ar in the headspace to determine the effects of these compounds on growth. The proportion of acetate that was either oxidized to CO2 or assimilated into biomass was determined by adding 1 μCi (1.2 μM) of 14CH3COONa (Sigma Chemical Co.) to 20 ml of acetate medium in a 60-ml serum bottle. After the cultures were incubated at 95°C until they reached the late logarithmic growth phase, 0.1 ml of 5 N NaOH (enough to bring the pH of the samples to 10) was added to a pair of bottles. To measure the amount of 14CO2 produced, a plastic center well (Kontes Glass Co.) containing a folded strip of Whatman no. 1 chromatography paper soaked with 0.2 ml of β-phenethylamine was suspended from a stopper above each sample, and 0.2 ml of 4 N H2SO4 (enough to bring the pH of the sample to 1) was added to the sample (18). After the samples were gently shaken in the dark for 90 min, the chromatography paper was removed, and the amount of radioactive carbon was determined using a scintillation counter. To determine the amount of radiolabeled carbon assimilated into cell biomass, the liquid from a separate pair of bottles incubated without acid or base added (the acid caused cell lysis) was filtered through a 0.45-μm-pore-size membrane filter and rinsed with 2 volumes of sterile medium salts solution, and the amount of radioactivity was determined as described above.
Enzyme assays.
All sample transfers and manipulations were performed in an anoxic chamber, and all sample buffers were degassed and flushed with Ar and contained 2 mM sodium dithionite and 2 mM dithiothreitol. Each cell suspension was thawed, and DNase I was added to a final concentration of 0.0002% (wt/vol). The cells were then disrupted on ice by sonication in an anoxic chamber. Cell lysis was verified using phase-contrast light microscopy, and an aliquot of the solution was used as the whole-cell extract. The remainder was spun at 100,000 × g for 45 min in a centrifuge, and the supernatant was used for the assays unless indicated otherwise. Protein fractions that were not used immediately for enzyme assays were frozen in liquid N2 and stored at −80°C. The protein concentrations in the whole-cell extract and spun fractions were determined spectrophotometrically using a protein determination kit (Bio-Rad) based on the Bradford assay (7). Bovine serum albumin was used as a protein standard.
Twenty-two different enzyme activities were assayed for each of the 11 fermentor runs. All enzyme activities were measured at 80°C unless indicated otherwise. The activities of the following enzymes were measured under anaerobic conditions in rubber stopper-sealed glass and quartz cuvettes that were degassed and flushed with Ar: citrate lyase (30, 31), ATP citrate synthase (21), citryl-CoA synthetase (3), aconitate hydratase and isocitrate lyase (41), 2-oxoglutarate synthase and pyruvate synthase (35), succinate dehydrogenase (6), fumarate hydratase (27), malate synthase (37), hydrogenase with H2 in the headspace (25), and formate dehydrogenase (26). The activities of the following enzymes were measured under aerobic conditions: citrate synthase at 55°C (12), isocitrate dehydrogenase and malate dehydrogenase (38), succinate:CoA ligase (ADP forming) and pyruvate-water dikinase (19, 22), decarboxylating malate dehydrogenase (5), phosphoenolpyruvate carboxylase (14), pyruvate carboxykinase (29), and ADP- and AMP-forming acetate:CoA ligases (8, 9).
Statistical analyses.
The culture growth rate data and each enzyme activity measurement were subjected to statistical analyses as described previously (43). The growth rates were compared using a linear regression analysis, analysis of covariance, and a Tukey test (α = 0.05). The enzyme activities were compared by using analysis of variance and a Tukey test (α = 0.05). Individual enzyme activities for each condition were expressed as the mean ± standard deviation. The results of the enzyme activity Tukey test are shown in Table 1.
TABLE 1.
Specific activities of citric acid cycle enzymes and other enzymes in cells grown on various media
| Enzyme | EC no. | Sp act (nmol min−1 mg−1) on the following growth mediaa:
|
||
|---|---|---|---|---|
| H2 + CO2 | H2 + acetate + yeast extract | Tryptone + yeast extract | ||
| Citric acid cycle enzymes | ||||
| Citrate synthase | 2.3.3.1 | 101 ± 21 | 137 ± 31 | 217 ± 60c |
| Aconitate hydratase | 4.2.1.3 | 342 ± 72 | 221 ± 28 | 525 ± 140 |
| Isocitrate dehydrogenase | 1.1.1.42 | 430 ± 34 | 626 ± 87 | 711 ± 224 |
| 2-Oxoglutarate synthase | 1.2.7.3 | 112 ± 23 | 148 ± 12 | 130 ± 17 |
| Succinate:CoA ligase (ADP forming) | 6.2.1.5 | 99 ± 16 | 90 ± 1 | 90 ± 11 |
| Succinate dehydrogenase | 1.3.99.1 | 192 ± 41 | 115 ± 8 | 161 ± 88 |
| Succinate dehydrogenaseb | 1.3.99.1 | 195 ± 77 | 148 ± 109 | 201 ± 76 |
| Fumarate hydratase | 4.2.1.2 | 316 ± 10 | 339 ± 96 | 428 ± 52 |
| Malate dehydrogenase | 1.1.1.37 | 1,176 ± 193 | 1,747 ± 646 | 2,221 ± 118 |
| Other enzymes | ||||
| Citrate lyase | 4.1.3.6 | 25 ± 6 | 9 ± 3 | 3 ± 0 |
| ATP citrate synthase | 2.3.3.8 | 12 ± 16 | 51 ± 8 | 25 ± 3 |
| Acetate:CoA ligase (ADP forming) | 6.2.1.13 | 0 | 0 | 93 ± 6 |
| Acetate:CoA ligase (AMP forming) | 6.2.1.1 | 95 ± 8 | 32 ± 6 | 0 |
| Pyruvate synthase | 1.2.7.1 | 0 | 0 | 59 ± 19 |
| Pyruvate-water dikinase | 2.7.9.2 | 32 ± 5 | 19 ± 4 | 22 ± 5 |
| Phosphoenolpyruvate carboxylase | 4.1.1.31 | 123 ± 20 | 126 ± 2 | 0 |
| Malate dehydrogenase (decarboxylating) | 1.1.1.39 | 319 ± 49 | 293 ± 33 | 558 ± 76 |
| Hydrogenase | 1.12.7.2 | 165 ± 20 | 130 ± 11 | 210 ± 34 |
| Hydrogenaseb | 1.12.7.2 | 474 ± 110 | 1,005 ± 80 | 520 ± 65 |
| Formate dehydrogenase | 1.2.2.- | 299 ± 199 | 245 ± 160 | 524 ± 200 |
| Formate dehydrogenaseb | 1.2.2.- | 2,818 ± 1,674 | 1,842 ± 1,253 | 5,743 ± 152 |
| Malate synthase | 2.3.3.9 | 19 ± 12 | 30 ± 7 | 21 ± 9 |
Most enzyme activities were measured using only the cytoplasmic protein fraction. The exceptions were the succinate dehydrogenase, hydrogenase, and formate dehydrogenase activities, which were also measured using whole-cell extracts. The values are means ± standard deviations.
Enzyme activity measured using the whole-cell extract.
Boldface type indicates values that are significantly different (P < 0.05) from one or both values for the same enzyme that are not in boldface type. The statistical trends are as follows: for citrate synthase, peptides > H2-CO2; for aconitate hydratase, peptides > acetate; for malate dehydrogenase, peptides > H2-CO2; for citrate lyase, H2-CO2 > acetate and peptides; for ATP citrate synthase, acetate > peptides and H2-CO2; for acetate:CoA ligase (ADP forming), peptides > acetate and H2-CO2; for acetate:CoA ligase (AMP forming), H2-CO2 > acetate > peptides; for pyruvate synthase, peptides > acetate and H2-CO2; for phosphoenolpyruvate carboxylase, acetate and H2-CO2 > peptides; for malate dehydrogenase (decarboxylating), peptides > acetate and H2-CO2; and for hydrogenase in whole-cell extracts, acetate > peptides and H2-CO2.
RESULTS
P. islandicum growth versus carbon source.
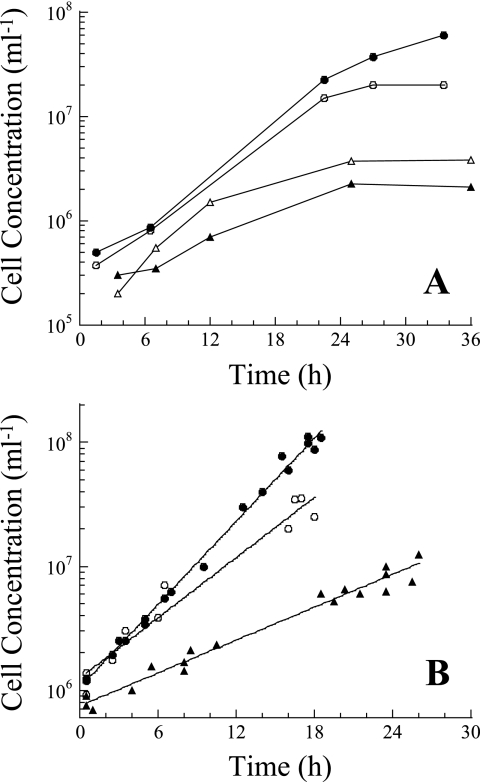
P. islandicum used both H2 and CO2 for autotrophic growth. Cultures did not grow on acetate alone. Hydrogen significantly enhanced growth, with and without acetate, when the yeast extract concentration was 0.001% (Fig. 2A). The growth rates of cultures grown on H2 and 0.001% yeast extract with and without 0.05% acetate were not significantly different; however, the maximum concentration of cells was fourfold higher for cultures grown with acetate (Fig. 2A). Acetate uptake experiments using 14C-labeled acetate showed that acetate was used by the cultures and that the percentages of acetate assimilated into biomass and respired as CO2 were 55% and 45%, respectively. No H2 was detected in the headspace of heterotrophically grown cultures.
FIG. 2.
(A) P. islandicum growth in 60-ml serum bottles on H2, 0.05% acetate, and 0.001% yeast extract (•), on H2 and 0.001% yeast extract (○), on Ar, 0.05% acetate, and 0.001% yeast extract (▴), and on Ar and 0.001% yeast extract (▵). (B) Growth in the 20-liter fermentor on H2 and CO2 (▴), on H2, 0.05% acetate, and 0.001% yeast extract (○), and on 0.05% casein hydrolysate and 0.02% yeast extract (•).
For cultures grown in the 20-liter fermentor, all growth curves demonstrated that the cultures were in the logarithmic growth phase throughout the experiment (Fig. 2B). The doubling times of P. islandicum grown autotrophically, with acetate, and heterotrophically were 6.8 h (k = 0.102 ± 0.009 h−1 [95% confidence interval]), 3.7 h (k = 0.187 ± 0.032 h−1), and 2.7 h (k = 0.258 ± 0.009 h−1), respectively. These values were all significantly different from each other (P < 0.05). The maximum concentrations of cells obtained were highest and lowest for cultures grown heterotrophically and autotrophically, respectively, and there was a 10-fold difference between these concentrations (Fig. 2B).
Enzyme activities.
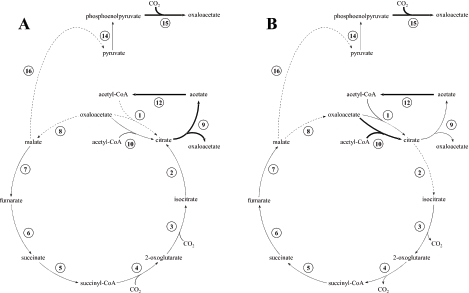
The activities of all eight enzymes of the citric acid cycle plus 11 other central metabolic enzymes in cells grown under the three growth conditions were measured (Table 1). No activities were detected for pyruvate carboxykinase, citryl-CoA synthetase, and isocitrate lyase. Notably, for 11 of the 19 active enzymes there were significant differences (P < 0.05) in activity when there were differences in the growth conditions. The specific activities of citrate synthase, aconitate hydratase, malate dehydrogenase, ADP-forming acetate:CoA ligase, pyruvate synthase, and decarboxylating malate dehydrogenase were significantly higher in cells grown heterotrophically than in cells grown in one or both of the other media (Fig. 3). The activities of hydrogenase in the whole-cell extract and ATP citrate synthase were higher in cells grown with acetate (Fig. 3B). The activities of citrate lyase, AMP-forming acetate:CoA ligase, and phosphoenolpyruvate carboxylase were higher in cells grown with acetate and on H2 and CO2 than in cells grown on peptides (Fig. 3), and the AMP-forming acetate:CoA ligase activity was higher in cells grown on H2 and CO2 than in cells grown with acetate. There was no citrate lyase activity if 0.03% (vol/vol) acetic anhydride in dimethyl sulfoxide was not added to activate the enzyme by acetylation (31). The activities that were essentially the same under the three growth conditions were the isocitrate dehydrogenase, 2-oxoglutarate synthase, succinate:CoA ligase, succinate dehydrogenase, fumarate hydratase, pyruvate-water dikinase, formate dehydrogenase, and malate synthase activities (Fig. 3).
FIG. 3.
Models for carbon flow during autotrophic growth (A) and growth with acetate (B). The boldface arrows and dashed arrows indicate enzyme activities that were significantly higher and lower, respectively, than the activities in heterotrophically grown cells. Pyruvate synthase and acetate:CoA ligase (ADP-forming) activities (reactions 11 and 13) are not present in either model. The numbers indicate enzymes as described in the legend to Fig. 1.
DISCUSSION
It was proposed previously that P. islandicum grows autotrophically using the reductive citric acid cycle based on measurement of the ATP citrate synthase, pyruvate synthase, and 2-oxoglutarate synthase activities in cells reportedly grown autotrophically (21). This was consistent with the operation of this pathway in T. neutrophilus, a member of the same family (6, 32, 33, 39). Our results support this idea with the caveat that the carbon flow appears to be different than the carbon flow at key regulatory steps suggested previously.
The growth rates and maximum concentrations of cells increased with increasing amounts of organic compounds present. P. islandicum grew heterotrophically on remarkably low concentrations of organic compounds (0.001% yeast extract), albeit to low concentrations of cells. Cultures did not grow on acetate alone, but the maximum concentrations of cells increased up to fourfold in medium containing 0.001% yeast extract and H2 when acetate was added. Growth on acetate without H2 was poor. 14C-labeled acetate uptake studies showed that acetate was used by the cells, but apparently cells were unable to meet all of their biosynthetic needs without another source of organic compounds, and H2 appeared to ameliorate the need to use acetate as the sole source of electrons. Similarly, acetate assimilation in R. rubrum was enhanced by addition of pyruvate and was further enhanced by addition of H2 (23).
The activity of citrate lyase was more than eight times higher in autotrophically grown cultures than in heterotrophically grown cultures, suggesting that this activity was regulated. ATP citrate synthase activities were not significantly different in autotrophic and heterotrophic samples and were apparently not regulated by the change in conditions. Citrate lyase activity was higher than ATP citrate synthase activity in autotrophic samples. Citrate lyase in mesophilic bacteria requires covalent modification via acetylation for activation (11, 17, 30). In P. islandicum, citrate lyase activity was observed only when an acetylating compound was present in the assay mixture, suggesting that acetylation may further regulate the activity of this enzyme.
ADP-forming acetate:CoA ligase activity was observed in heterotrophically grown cultures, but AMP-forming acetate:CoA ligase activity was not detected. Conversely, AMP-forming acetate:CoA ligase activity was observed in acetate-grown and autotrophically grown cultures but not in heterotrophically grown cultures. This suggests that ADP-forming acetate:CoA ligase could be used in reverse to make ATP and acetate during heterotrophic growth, but energy in the form of ATP was required to form acetyl-CoA from acetate during growth with acetate and autotrophic growth. During autotrophic growth, the acetate for the reaction came from citrate lyase, and the AMP-forming acetate:CoA ligase reaction completed the conversion of citrate into oxaloacetate and acetyl-CoA (Fig. 3A). The ATP citrate synthase activity was highest in acetate-grown cells. Therefore, during growth with acetate, the acetyl-CoA formed by AMP-forming acetate:CoA ligase appeared to enter the citric acid cycle for further oxidation in part by ATP citrate synthase, perhaps to generate ATP from ADP (Fig. 3B).
The activities of malate dehydrogenase, decarboxylating malate dehydrogenase, pyruvate synthase, citrate synthase, and ADP-forming acetate:CoA ligase, which catalyze adjacent metabolic steps, were all significantly lower in autotrophically grown cultures than in cultures grown heterotrophically (Fig. 3A). The activities of citrate lyase, AMP-forming acetate:CoA ligase, and phosphoenolpyruvate carboxylase were all significantly higher in autotrophically grown cultures (Fig. 3A). These nine enzymes share many of the same reactants and products. Therefore, their activities appear to be generally coordinated to control carbon flow in the citric acid cycle. In particular, it appeared that acetylated citrate lyase and AMP-forming acetate:CoA ligase, but not ATP citrate synthase, function in a coordinated manner with citrate synthase to regulate the direction of carbon flow. The activities of the other five enzymes of the citric acid cycle, including two enzymes that use CO2, were not affected by a change in the carbon source. The hydrogenase and formate dehydrogenase activities were high in all whole-cell extracts, and phosphoenolpyruvate carboxylase activity was observed only in acetate-grown and autotrophically grown cultures. These results suggest that there may be CO2 uptake at these steps, as well as CO2 reduction to formate via the putatively membrane-bound hydrogenase and formate dehydrogenase. No H2 was detected in the headspace of heterotrophically grown cultures grown in sealed serum bottles, suggesting that P. islandicum did not use pyruvate-formate lyase.
The lack of pyruvate synthase activity when growth was not heterotrophic is interesting since this enzyme is a key enzyme for biosynthesis in the reductive citric acid cycle (10, 15). It is not known how pyruvate was formed and how biosynthesis from acetyl-CoA occurred in P. islandicum during autotrophic growth or during growth with acetate without pyruvate synthase. However, one possibility is that the citramalate cycle was used; in this cycle acetyl-CoA is converted into glyoxylate, which enters the citric acid cycle via malate synthase and can be used to make various biosynthetic precursors. It was proposed previously that this pathway is used for biosynthesis in R. rubrum, which, like P. islandicum, lacks pyruvate synthase and isocitrate lyase activities when cultures are grown with acetate (23).
In conclusion, P. islandicum CO2 fixation via the reductive citric acid cycle may be more like the pathway proposed for some purple photosynthetic bacteria than like the pathways found in green photosynthetic bacteria and Hydrogenobacter species. The primary enzymes for the formation of oxaloacetate and acetyl-CoA in P. islandicum appear to be citrate lyase and AMP-forming acetate:CoA ligase and not ATP citrate synthase. It was suggested previously that the reductive citric acid cycle was the origin of intermediary metabolism based on the chemistry of the intermediates (28). Therefore, further study of this pathway in hyperthermophilic archaea, which themselves may have many ancient traits, may provide insight into the natural history of this pathway.
Acknowledgments
We thank K. Kashefi, B. Blunt, and D. R. Lovley for kindly providing the P. islandicum strain used in this study and for their assistance with the isotope analyses.
This research was funded by grant MAS00897 from the U.S. Department of Agriculture.
REFERENCES
- 1.Afshar, S., C. Kim, H. G. Monbouquette, and I. Schröder. 1998. Effect of tungstate on nitrate reduction by the hyperthermophilic archaeon Pyrobaculum aerophilum. Appl. Environ. Microbiol. 64:3004-3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antranikian, G., C. Herzberg, and G. Gottschalk. 1982. Characterization of ATP citrate lyase from Chlorobium limicola. J. Bacteriol. 152:1284-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aoshima, M., M. Ishii, and Y. Igarashi. 2004. A novel enzyme, citryl-CoA synthetase, catalyzing the first step of the citrate cleavage reaction in Hydrogenobacter thermophilus TK-6. Mol. Microbiol. 52:751-761. [DOI] [PubMed] [Google Scholar]
- 4.Aoshima, M., M. Ishii, and Y. Igarashi. 2004. A novel enzyme, citryl-CoA lyase, catalyzing the second step of the citrate cleavage reaction in Hydrogenobacter thermophilus TK-6. Mol. Microbiol. 52:763-770. [DOI] [PubMed] [Google Scholar]
- 5.Bartolucci, S., R. Rella, A. Guagliardi, C. A. Raia, A. Gambacorta, M. De Rosa, and M. Rossi. 1987. Malic enzyme from archaebacterium Sulfolobus solfataricus: purification, structure and kinetic properties. J. Biol. Chem. 262:7725-7731. [PubMed] [Google Scholar]
- 6.Beh, M., G. Strauss, R. Huber, K. O. Stetter, and G. Fuchs. 1993. Enzymes of the reductive citric acid cycle in the autotrophic eubacterium Aquifex pyrophilus and in the archaebacterium Thermoproteus neutrophilus. Arch. Microbiol. 160:306-311. [Google Scholar]
- 7.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 8.Bräsen, C., and P. Schönheit. 2004. Unusual ADP-forming acetyl-coenzyme A synthetases from the mesophilic halophilic euryarchaeon Haloarcula marismortui and from the hyperthermophilic crenarchaeon Pyrobaculum aerophilum. Arch. Microbiol. 182:277-287. [DOI] [PubMed] [Google Scholar]
- 9.Bräsen, C., C. Urbanke, and P. Schönheit. 2005. A novel octameric AMP-forming acetyl-CoA synthetase from the hyperthermophilic crenarchaeon Pyrobaculum aerophilum. FEBS Lett. 579:477-482. [DOI] [PubMed] [Google Scholar]
- 10.Buchanan, B. B., M. C. W. Evans, and D. I. Arnon. 1967. Ferredoxin-dependent carbon assimilation in Rhodospirillum rubrum. Arch. Microbiol. 59:32-40. [DOI] [PubMed] [Google Scholar]
- 11.Buckel, W., V. Buschmeier, and H. Eggerer. 1971. Der Wirkungsmechanismus der Citrate-Lyase aus Klebsiella aerogenes. Hoppe-Seyler's Z. Physiol. Chem. 352:1195-1205. [PubMed] [Google Scholar]
- 12.Danson, M. J., and D. W. Hough. 2001. Citrate synthase from hyperthermophilic Archaea. Methods Enzymol. 331:3-12. [DOI] [PubMed] [Google Scholar]
- 13.Eisenberg, M. A. 1955. The acetate-activating enzyme of Rhodospirillum rubrum. Biochim. Biophys. Acta 16:58-65. [DOI] [PubMed] [Google Scholar]
- 14.Ettema, T. J. G., K. S. Makarova, G. L. Jellema, H. J. Gierman, E. V. Koonin, M. A. Huynen, W. M. de Vos, and J. van der Oost. 2004. Identification and functional verification of archaeal-type phosphoenolpyruvate carboxylase, a missing link in archaeal central carbohydrate metabolism. J. Bacteriol. 186:7754-7762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans, M. C. W., B. B. Buchanan, and D. I. Arnon. 1966. A new ferredoxin-dependent carbon reduction cycle in a photosynthetic bacterium. Proc. Natl. Acad. Sci. USA 55:928-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feinberg, L. F., and J. F. Holden. 2006. Characterization of dissimilatory Fe(III) versus NO3− reduction in the hyperthermophilic archaeon Pyrobaculum aerophilum. J. Bacteriol. 188:525-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gifforn, F., N. Beuscher, and G. Gottschalk. 1972. Regulation of citrate lyase activity in Rhodopseudomonas gelatinosa. Biochem. Biophys. Res. Commun. 49:467-472. [DOI] [PubMed] [Google Scholar]
- 18.Griffiths, R. P., S. S. Hayasaka, T. M. McNamara, and R. Y. Morita. 1977. Comparison between two methods of assaying relative activity in marine environments. Appl. Environ. Microbiol. 34:801-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hasegawa, H., M. Parniak, and S. Kaufman. 1982. Determination of the phosphate content of purified proteins. Anal. Biochem. 120:360-364. [DOI] [PubMed] [Google Scholar]
- 20.Huber, R., J. K. Kristjansson, and K. O. Stetter. 1987. Pyrobaculum gen. nov., a new genus of neutrophilic, rod-shaped archaebacteria from continental solfataras growing optimally at 100°C. Arch. Microbiol. 149:95-101. [Google Scholar]
- 21.Hügler, M., H. Huber, K. O. Stetter, and G. Fuchs. 2003. Autotrophic CO2 fixation pathways in archaea (Crenarchaeota). Arch. Microbiol. 179:160-173. [DOI] [PubMed] [Google Scholar]
- 22.Hutchins, A. M., J. F. Holden, and M. W. W. Adams. 2001. Phosphoenolpyruvate synthetase from the hyperthermophilic archaeon Pyrococcus furiosus. J. Bacteriol. 183:709-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ivanovsky, R. N., E. N. Krasilnikova, and I. A. Berg. 1997. A proposed citramalate cycle for acetate assimilation in the purple non-sulfur bacterium Rhodospirillum rubrum. FEMS Microbiol. Lett. 153:399-404. [Google Scholar]
- 24.Ivanovsky, R. N., N. V. Sintsov, and E. N. Kondratieva. 1980. ATP-linked citrate lyase activity in the green sulfur bacterium Chlorobium limicola forma thiosulfatophilum. Arch. Microbiol. 128:239-241. [Google Scholar]
- 25.Ma, K., and M. W. W. Adams. 2001. Hydrogenases I and II from Pyrococcus furiosus. Methods Enzymol. 331:208-216. [DOI] [PubMed] [Google Scholar]
- 26.Ma, K., H. Loessner, J. Heider, M. K. Johnson, and M. W. W. Adams. 1995. Effects of elemental sulfur on the metabolism of the deep-sea hyperthermophilic archaeon Thermococcus strain ES-1: characterization of a sulfur-regulated, nonheme iron alcohol dehydrogenase. J. Bacteriol. 177:4748-4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mizobata, T., T. Fujioka, F. Yamasaki, M. Hidaka, J. Nagai, and Y. Kawata. 1998. Purification and characterization of a thermostable class II fumarase from Thermus thermophilus. Arch. Biochem. Biophys. 355:49-55. [DOI] [PubMed] [Google Scholar]
- 28.Morowitz, H. J., J. D. Kostelnik, J. Yang, and G. D. Cody. 2000. The origin of intermediary metabolism. Proc. Natl. Acad. Sci. USA 97:7704-7708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mukhopadhyay, B., V. J. Patel, and R. S. Wolfe. 2000. A stable archaeal pyruvate carboxylase from the hyperthermophile Methanococcus jannaschii. Arch. Microbiol. 174:406-414. [DOI] [PubMed] [Google Scholar]
- 30.Quentmeier, A., and G. Antranikian. 1985. Characterization of citrate lyase from Clostridium sporosphaeroides. Arch. Microbiol. 141:85-90. [DOI] [PubMed] [Google Scholar]
- 31.Quentmeier, A., A. Holzenburg, F. Mayer, and G. Antranikian. 1987. Reevaluation of citrate lyase from Escherichia coli. Biochim. Biophys. Acta 913:60-65. [DOI] [PubMed] [Google Scholar]
- 32.Schäfer, S., C. Barkowski, and G. Fuchs. 1986. Carbon assimilation by the autotrophic thermophilic archaebacterium Thermoproteus neutrophilus. Arch. Microbiol. 146:301-308. [Google Scholar]
- 33.Schäfer, S., M. Götz, W. Eisenreich, A. Bacher, and G. Fuchs. 1989. 13C-NMR study of autotrophic CO2 fixation in Thermoproteus neutrophilus. Eur. J. Biochem. 184:151-156. [DOI] [PubMed] [Google Scholar]
- 34.Schauder, R., F. Widdel, and G. Fuchs. 1987. Carbon assimilation pathways in sulfate-reducing bacteria. II. Enzymes of a reductive citric acid cycle in the autotrophic Desulfobacter hydrogenophilus. Arch. Microbiol. 148:218-225. [Google Scholar]
- 35.Schut, G. J., A. L. Menon, and M. W. W. Adams. 2001. 2-Keto acid oxidoreductases from Pyrococcus furiosus and Thermococcus litoralis. Methods Enzymol. 331:144-158. [DOI] [PubMed] [Google Scholar]
- 36.Selig, M., and P. Schönheit. 1994. Oxidation of organic compounds to CO2 with sulfur or thiosulfate as electron acceptor in the anaerobic hyperthermophilic archaea Thermoproteus tenax and Pyrobaculum islandicum proceeds via the citric acid cycle. Arch. Microbiol. 162:286-294. [Google Scholar]
- 37.Serrano, J. A., M. Camacho, and M. J. Bonete. 1998. Operation of glyoxylate cycle in halophilic archaea: presence of malate synthase and isocitrate lyase in Haloferax volcanii. FEBS Lett. 434:13-16. [DOI] [PubMed] [Google Scholar]
- 38.Steen, I. H., H. Hvoslef, T. Lien, and N.-K. Birkeland. 2001. Isocitrate dehydrogenase, malate dehydrogenase, and glutamate dehydrogenase from Archaeoglobus fulgidus. Methods Enzymol. 331:13-26. [DOI] [PubMed] [Google Scholar]
- 39.Strauss, G., W. Eisenreich, A. Bacher, and G. Fuchs. 1992. 13C-NMR study of autotrophic CO2 fixation pathways in the sulfur-reducing archaebacterium Thermoproteus neutrophilus and in the phototrophic eubacterium Chloroflexus aurantiacus. Eur. J. Biochem. 205:853-866. [DOI] [PubMed] [Google Scholar]
- 40.Tor, J. M., K. Kashefi, and D. R. Lovley. 2001. Acetate oxidation coupled to Fe(III) reduction in hyperthermophilic microorganisms. Appl. Environ. Microbiol. 67:1363-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uhrigshardt, H., M. Walden, H. John, and S. Anemüller. 2001. Purification and characterization of the first archaeal aconitase from the thermoacidophilic Sulfolobus acidocaldarius. Eur. J. Biochem. 268:1760-1771. [PubMed] [Google Scholar]
- 42.Völkl, P., R. Huber, E. Drobner, R. Rachel, S. Burggraf, A. Trincone, and K. O. Stetter. 1993. Pyrobaculum aerophilum sp. nov., a novel nitrate-reducing hyperthermophilic archaeum. Appl. Environ. Microbiol. 59:2918-2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zar, J. H. 1996. Biostatistical analysis, 3rd ed. Prentice Hall, Upper Saddle River, N.J.