Abstract
Butanediol fermentation in two Serratia species is shown to be affected by N-acyl-l-homoserine lactone-dependent quorum sensing. Knockout of quorum-sensing signal production caused a shift towards enhanced acid production, resulting in early growth arrest, which was reversible by the addition of synthetic signal molecules.
The Enterobacteriaceae are commonly divided into two groups with different fermentation pathways (4). Members of genera such as Escherichia, Salmonella, and Shigella use the mixed-acid pathway, causing strong acidification of their environment due to the production of large amounts of acids, including acetate, lactate, succinate, and formate. In contrast, members of Klebsiella, Enterobacter, Serratia, and a number of other genera ferment glucose predominantly to 2,3-butanediol (18). In the latter case, the production of acidic products is limited because a significant amount of pyruvate from glycolysis is channeled into the butanediol pathway (6, 9). The production of neutral compounds is ecologically relevant since it allows butanediol fermenters to prevent lethal acidification as cells approach stationary phase. In Klebsiella terrigena, the LysR-type transcriptional activator BudR appears to regulate at least part of the 2,3-butanediol pathway (10). Recently, microarray analysis showed that the production of 2,3-butanediol in Vibrio cholerae is also regulated by the transcriptional activator AphA, in addition to the LysR-type transcriptional activator AlsR (8). The AphA activator is, in turn, regulated by multiple quorum-sensing systems that act in parallel. System 1 uses the CAI-1 autoinducer of unknown structure, while system 2 uses AI-2, a furanosyl borate diester, to trigger a phosphorelay circuit (1, 2, 7, 11, 19). These systems are homologues of the Vibrio harveyi systems (11) but differ from the LuxIR paradigm in Vibrio fischeri, in which LuxI synthesizes the N-acyl-l-homoserine lactone (AHL) signaling molecules and the AHL-activated LuxR protein functions as a transcriptional activator triggering a response (5).
We recently initiated a study of biofilm-forming bacteria from a food-processing environment (16). One isolate, designated RVH1, was identified as Serratia plymuthica (17), and we recently characterized its LuxIR-homologous quorum-sensing system, SplIR. The SplIR quorum-sensing system regulates the production of an extracellular chitinase, protease, nuclease, and antibacterial compound (R. Van Houdt, P. Moons, A. Aertsen, A. Jansen, K. Vanoirbeek, M. Daykin, P. Williams, and C. W. Michiels, submitted for publication). In the current study, we report that 2,3-butanediol production in this strain is quorum sensing regulated via the same AHL-dependent system. Furthermore, we confirmed our observations in Serratia marcescens MG1 (previously Serratia liquefaciens MG1), the type species of the Serratia genus. The demand and manufacture of 2,3-butanediol are still increasing worldwide (annual rate of 4 to 7%) due to a variety of applications, such as its use as a liquid fuel additive, and to the increased demand for polybutylene terephthalate resin, gamma-butyrolactone, spandex, and its precursors (14). Furthermore, rising petroleum prices revived significant interest in producing feedstock chemicals, including 2,3-butanediol, from biomass. These results therefore may also be useful for improving the yield of 2,3-butanediol fermentation via metabolic engineering.
Effect of carbohydrates on growth and acid production of S. plymuthica RHV1 and its isogenic quorum-sensing mutants.
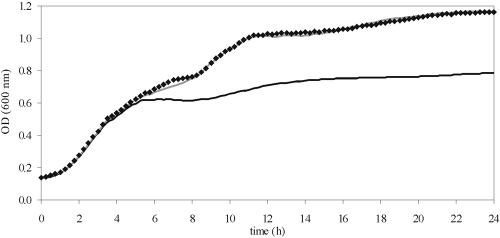
We observed incidentally that the splI mutant of S. plymuthica RVH1, which is deficient in the production of N-acyl-l-homoserine lactone quorum-sensing signals, grew less well than its parent strain in Luria-Bertani (LB) broth supplemented with glucose. To investigate this effect more systematically, we recorded growth curves for both strains in LB broth at 30°C, with or without the addition of several sugars, using a Bioscreen C growth analyzer (Thermo Life Sciences, Brussels, Belgium). This equipment incubates up to 200 cultures in a microplate format and automatically measures their optical densities by using a wide-band filter (405 to 600 nm). Measurements were taken every 15 min after shaking of cultures for 60 s. No growth differences between the strains were observed in unamended LB broth (data not shown). However, in the presence of 0.5% of glucose, galactose, arabinose, fructose, or mannose, the splI mutant entered more rapidly into stationary phase than the parent strain. In LB broth supplemented with 0.5% galactose (Fig. 1), the onset of stationary phase occurred at an optical density of about 1.0 for the parent strain, compared to 0.6 for the splI mutant, and the corresponding cell densities at the end of the experiment (24 h) were 9.5 and 8.7 log CFU/ml, respectively. Furthermore, there was an important difference in the pHs of the spent culture media after 24 h, with values of 7.48 and 4.87, respectively, for the parent and the mutant. Interestingly, the apparent growth defect of the splI mutant could be nullified by the addition of 10 μM synthetic N-(3-oxo-hexanoyl)-l-homoserine lactone (3-oxo-C6-HSL) (Sigma, Bornem, Belgium), the major AHL produced by S. plymuthica RVH1 (Van Houdt et al., submitted for publication). Growth curves for the mutant in LB broth with galactose and 3-oxo-C6-HSL were indistinguishable from those of the parent strain, and the cell density (9.5 log CFU/ml) and medium pH (7.37) after 24 h of growth were also restored to those of the parent strain. Growth of the splR and splI splR mutants in LB broth, with and without added sugar, was indistinguishable from that of the parent strain (data not shown). The same experiment was repeated with S. marcescens MG1, in which AHL-dependent quorum sensing has been studied in much detail. In this strain, quorum sensing affects phenotypes such as swarming, biofilm formation, and production of exoenzymes (3, 12, 13). Like the case in S. plymuthica, we observed an early onset of stationary phase accompanied by acidification in LB broth supplemented with sugars for the S. marcescens swrI mutant, which is deficient in the production of the signals N-butanoyl-l-homoserine lactone (C4-HSL) and N-hexanoyl-l-homoserine lactone (C6-HSL).
FIG. 1.
Growth of S. plymuthica RVH1 (gray line) and its isogenic splI mutant with (diamonds) or without (black line) the addition of 10 μM 3-oxo-C6-HSL in LB medium supplemented with 0.5% galactose.
We next investigated whether the observed acidification and early growth arrest in S. plymuthica would also take place in methyl red-Voges-Proskauer (MR-VP) medium, which is a glucose-containing broth used in the classical test to distinguish between the mixed-acid and butanediol fermentation types in the Enterobacteriaceae family. The same effect was indeed noticeable after 24 h of growth in glass tubes (4 ml) but was somewhat less pronounced than that in LB-based media, probably because MR-VP medium is buffered. The effect was also reversible by the addition of 10 μM synthetic 3-oxo-C6-HSL (Table 1). Similar results were obtained with the methyl red test for S. marcescens MG1 and its swrI mutant, either complemented or not with C4-HSL (data not shown). Together, these results suggested that activation of the butanediol pathway at the end of the exponential growth phase is quorum sensing regulated, a hypothesis that was further investigated.
TABLE 1.
Plate count determinations and pH measurements for cultures grown for 24 h
| Strain and supplement | Data for cultures grown in indicated mediuma
|
|||
|---|---|---|---|---|
| LB + 0.5% galactose
|
MR-VP broth
|
|||
| Log CFU/ml | pH | Log CFU/ml | pH | |
| RVH1 | 8.89 ± 0.05b | 6.03 ± 0.05b | 8.95 ± 0.04b | 6.50 ± 0.01b |
| RVH1 splI | 8.51 ± 0.07c | 4.94 ± 0.01c | 8.58 ± 0.04c | 5.04 ± 0.05c |
| RVH1 splI + 3-oxo-C6-HSL | 9.02 ± 0.02b | 5.96 ± 0.02b | 9.01 ± 0.31b | 6.45 ± 0.05b |
Data are means ± standard deviations. Different superscripts indicate significant differences (P < 0.05) between values in the same column.
Acetoin production by S. plymuthica RVH1 and its isogenic quorum-sensing mutants.
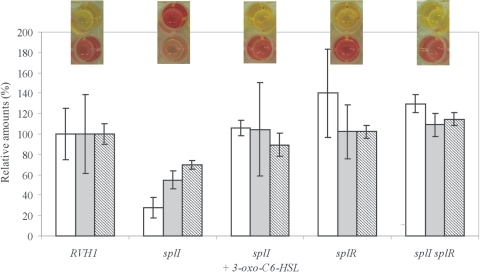
The Voges-Proskauer test was used as a qualitative method to demonstrate 2,3-butanediol fermentation by the detection of acetoin, a precursor of 2,3-butanediol. To 100 μl of bacterial culture grown for 24 h in MR-VP medium, 30 μl freshly prepared 5% α-naphthol in absolute ethanol and 10 μl of 40% KOH were added, and the mixture was stirred vigorously. The formation of a red color is indicative of the presence of acetoin. As shown in Fig. 2, an intensive red color was obtained for S. plymuthica RVH1 and the splI mutant grown in the presence of 10 μM 3-oxo-C6-HSL, while only a pale pink color developed for the splI mutant in the absence of the quorum signal. Furthermore, an intensive red color was also observed for the splR and splI splR mutants. Similarly, the test indicated acetoin production by S. marcescens MG1 and also by its swrI mutant in the presence of 10 μM C4-HSL, but not in its absence (data not shown). A more specific analysis of 2,3-butanediol and its precursors diacetyl and acetoin by gas chromatography coupled to mass spectrometry was performed as previously described (15). Briefly, gas chromatography was performed by using a Fisons GC 8000 gas chromatograph (Fisons, Mainz, Germany) equipped with a Chrompack CP-WAX-52-CB column (Varian, Palo Alto, CA), and total ion mass chromatograms were obtained with a Fisons MD 800 quadrupole mass spectrometer (Fisons, Mainz, Germany) and analyzed using the Masslab software program (ThermoQuest, Manchester, United Kingdom) for identification and quantification of volatiles. This analysis showed decreased levels of all three compounds in the splI mutant, which were restored to parental levels in the presence of 10 μM 3-oxo-C6-HSL, and comparable or slightly increased levels for the splR and splI splR mutants. The results for the splR and splI splR mutants are in accordance with our previous observation that SplR acts as a repressor, since the knockout of splR results in comparable levels of butanediol fermentation products to those in the parent strain and since the reduced amounts of these products in the absence of 3-oxo-C6-HSL (splI mutant) could be fully restored by inactivation of SplR (splI splR mutant).
FIG. 2.
Voges-Proskauer (top images, bottom row) and methyl red (top images, top row) assays and semiquantitative determination of amounts of acetoin (white bars), 2,3-butanediol (gray bars), and diacetyl (diagonally striped bars) for the S. plymuthica RVH1 wild type (left) and its isogenic splI mutant, isogenic splI mutant complemented with 10 μM 3-oxo-C6-HSL, isogenic splR mutant, and isogenic splI splR double mutant. Amounts are expressed relative to the levels in RVH1 (100%). The experiment was performed in duplicate.
In conclusion, butanediol fermentation is dependent on AHL-dependent quorum sensing in S. plymuthica RVH1 and S. marcescens MG1, and although butanediol fermentation has been well studied, it was not known to be regulated by AHL-dependent quorum sensing in any bacterium until now. Inactivation of the quorum-sensing system leads to continued production of acidic end products at the end of the exponential phase and throughout the stationary growth phase, which in turn leads to early growth arrest in the presence of fermentable sugars. Although the precise mechanism of regulation remains to be investigated, this finding may have important implications for the ecology of these bacteria. For example, Rice et al. (12) observed that in minimal medium supplemented with glucose and Casamino Acids, an swrI mutant formed thin, undifferentiated biofilms, in contrast to the well-developed biofilms formed by its parent strain, MG1, whereas in 0.1× LB medium, both strains formed biofilms that resembled the wild-type architecture. Their proposed model for quorum-sensing-controlled biofilm development should take into account these new findings, which demonstrate additional metabolic regulation that could lead to differential acidification of the environment in the presence of fermentable sugars.
Acknowledgments
We thank Michael Givskov (Technical University of Denmark) for providing S. marcescens strains MG1 and MG44 (swrI mutant) and Luk Daenen and Freddy Delvaux for the gas chromatography-mass spectrometry analysis.
REFERENCES
- 1.Bassler, B. L., M. Wright, and M. R. Silverman. 1994. Multiple signalling systems controlling expression of luminescence in Vibrio harveyi: sequence and function of genes encoding a second sensory pathway. Mol. Microbiol. 13:273-286. [DOI] [PubMed] [Google Scholar]
- 2.Chen, X., S. Schauder, N. Potier, A. Van Dorsselaer, I. Pelczer, B. L. Bassler, and F. M. Hughson. 2002. Structural identification of a bacterial quorum-sensing signal containing boron. Nature 405:545-549. [DOI] [PubMed] [Google Scholar]
- 3.Eberl, L., S. Molin, and M. Givskov. 1999. Surface motility of Serratia liquefaciens MG1. J. Bacteriol. 181:1703-1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eisenstein, B. I. 1990. Enterobacteriaceae, p. 1658-1673. In G. L. Mandell, R. G. Douglas, and J. E. Bennett (ed.), Principles and practice of infectious disease, 3rd ed. Churchill Livingstone, New York, N.Y.
- 5.Fuqua, C., S. C. Winans, and E. P. Greenberg. 1996. Census and consensus in bacterial ecosystems: the LuxR-LuxI family of quorum-sensing transcriptional regulators. Annu. Rev. Microbiol. 50:727-751. [DOI] [PubMed] [Google Scholar]
- 6.Johansen, L., K. Bryn, and F. C. Stormer. 1975. Physiological and biochemical role of the butanediol pathway in Aerobacter (Enterobacter) aerogenes. J. Bacteriol. 123:1124-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kovacikova, G., and K. Skorupski. 2002. Regulation of virulence gene expression in Vibrio cholerae by quorum sensing: HapR functions at the aphA promoter. Mol. Microbiol. 46:1135-1147. [DOI] [PubMed] [Google Scholar]
- 8.Kovacikova, G., W. Lin, and K. Skorupski. 2005. Dual regulation of genes involved in acetoin biosynthesis and motility/biofilm formation by the virulence activator AphA and the acetate-responsive LysR-type regulator AlsR in Vibrio cholerae. Mol. Microbiol. 57:420-433. [DOI] [PubMed] [Google Scholar]
- 9.Magee, R. J., and N. Kosaric. 1987. The microbial production of 2,3-butanediol. Adv. Appl. Microbiol. 32:89-161. [Google Scholar]
- 10.Mayer, D., V. Schlensog, and A. Bock. 1995. Identification of the transcriptional activator controlling the butanediol fermentation pathway in Klebsiella terrigena. J. Bacteriol. 177:5261-5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller, M. B., K. Skorupski, D. H. Lenz, R. K. Taylor, and B. L. Bassler. 2002. Parallel quorum sensing systems converge to regulate virulence in Vibrio cholerae. Cell 110:303-314. [DOI] [PubMed] [Google Scholar]
- 12.Rice, S. A., K. S. Koh, S. Y. Queck, M. Labbate, K. W. Lam, and S. Kjelleberg. 2005. Biofilm formation and sloughing in Serratia marcescens are controlled by quorum sensing and nutrient cues. J. Bacteriol. 187:3477-3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riedel, K., T. Ohnesorg, K. A. Krogfelt, T. S. Hansen, K. Omori, M. Givskov, and L. Eberl. 2001. N-Acyl-l-homoserine lactone-mediated regulation of the lip secretion system in Serratia liquefaciens MG1. J. Bacteriol. 183:1805-1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Syu, M.-J. 2001. Biological production of 2,3-butanediol. Appl. Microbiol. Biotechnol. 55:10-18. [DOI] [PubMed] [Google Scholar]
- 15.Vanderhaegen, B., H. Neven, L. Daenen, K. J. Verstrepen, H. Verachtert, and G. Derdelinckx. 2004. Furfuryl ethyl ether: important aging flavor and a new marker for the storage conditions of beer. J. Agric. Food Chem. 52:1661-1668. [DOI] [PubMed] [Google Scholar]
- 16.Van Houdt, R., A. Aertsen, A. Jansen, A. L. Quintana, and C. W. Michiels. 2003. Biofilm formation and cell-to-cell signalling in gram-negative bacteria isolated from a food processing environment. J. Appl. Microbiol. 96:177-184. [DOI] [PubMed] [Google Scholar]
- 17.Van Houdt, R., P. Moons, A. Jansen, K. Vanoirbeek, and C. W. Michiels. 2005. Genotypic and phenotypic characterization of a biofilm-forming Serratia plymuthica isolate from a raw vegetable processing line. FEMS Microbiol. Lett. 15:265-272. [DOI] [PubMed] [Google Scholar]
- 18.White, D. 2000. Fermentations, p. 363-383. In D. White (ed.), The physiology and biochemistry of prokaryotes. Oxford University Press, Inc., New York, N.Y.
- 19.Zhu, J., M. B. Miller, R. E. Vance, M. Dziejman, B. L. Bassler, and J. J. Mekalanos. 2002. Quorum-sensing regulators control virulence gene expression in Vibrio cholerae. Proc. Natl. Acad. Sci. USA 99:3129-3134. [DOI] [PMC free article] [PubMed] [Google Scholar]