Abstract
MukBEF is a bacterial SMC (structural maintenance of chromosome) complex required for chromosome partitioning in Escherichia coli. We report that overproduction of MukBEF results in marked chromosome condensation. This condensation is rapid and precedes the effects of overproduction on macromolecular synthesis. Condensed nucleoids are often mispositioned; however, cell viability is only mildly affected. The overproduction of MukB leads to a similar chromosome condensation, even in the absence of MukE and MukF. Thus, the non-SMC subunits of MukBEF play only an auxiliary role in chromosome condensation. MukBEF, however, was often a better condensin than MukB. Furthermore, the chromosome condensation by MukB did not rescue the temperature sensitivity of MukEF-deficient cells, nor did it suppress the high frequency of anucleate cell formation. We infer that the role of MukBEF in stabilizing chromatin architecture is more versatile than its role in controlling chromosome size. We further propose that MukBEF could be directly involved in chromosome segregation.
SMC (structural maintenance of chromosome) proteins are involved in virtually every genetic event that affects global chromatin structure (7, 20, 35, 50, 52). In solution, SMC proteins form a characteristic V-shaped dimeric molecule with two globular head domains that are connected by two long coiled coils with a hinge domain at the joint (2, 16, 32). The head domains can dimerize by themselves via the shared ATP binding site (17, 22, 29). ATP-modulated conformational changes were proposed to drive the closure of the SMC ring (3, 22, 25) or the oligomerization of SMC proteins into macromolecular assemblies (9, 18).
Eukaryotic SMC proteins comprise the cores of several multisubunit complexes with specialized functions (35, 50, 52). Chromosome condensation prior to cell division is carried out by condensins, which were shown to condense purified plasmid-sized DNA in vitro or entire chromosomes in a cell-free system (19, 24, 49). Another SMC complex, cohesin, is required for proper alignment of the sister chromosomes in dividing cells (28, 33, 36). Among the non-SMC subunits, a conserved family of kleisins has been identified (46). Kleisins bind the head domains of SMC proteins in the vicinity of the ATP binding pocket (17) and apparently mediate the association of the head domains (54, 55). In several cases, a functional interaction between the ATP binding sites of SMC proteins and the non-SMC subunits was detected (18, 21, 61). The role of the remaining non-SMC subunits remains unknown.
In contrast to eukaryotic cells, a single SMC protein supports chromosome segregation in bacteria. In Escherichia coli, the protein is MukB. Two other proteins, MukF and MukE, associate with MukB to form the holoenzyme MukBEF (65, 66). All subunits of MukBEF are encoded within the same operon, smtA-mukF-mukE-mukB, together with a nonessential gene, smtA. smtA encodes S-adenosylmethionine-dependent methyl transferase, which is apparently unrelated to the function of MukBEF (64). Inactivation of any subunit of MukBEF by mutation leads to the same phenotype, and the resulting defects cannot be complemented by the mild overproduction of the other two subunits (65). MukBEF can be purified from crude cell extract using an antibody raised against either subunit of the protein (66). MukF can form a complex with MukB or MukE, whereas MukE associates with MukB only in the presence of MukF (13, 66). Based on structural analysis, MukF was postulated to be a kleisin (13). A recent electron microscopy study found MukEF at the tips of the V-shaped MukB, in association with the globular domains of the protein (31). Furthermore, MukBEF but not MukB was found to form rosette-like clusters, even in the absence of DNA or ATP (31). MukB readily binds DNA in vitro (37, 41); no DNA binding was reported for MukEF (13).
MukBEF plays a central role in organizing nucleoid structure. Mutational inactivation of MukB results in a sharp decline in cell viability above 30°C, chromosome decondensation and cutting, a high frequency of anucleate cell formation (38), an abnormal localization of plasmids and chromosomal origins (40, 59), and the loss of cohesion between sister nucleoids (51). MukB forms clusters at the 1/4 and 3/4 positions within cells; no MukB foci can be found in the absence of MukE or MukF (10, 40). A similar localization pattern has been reported for the Bacillus subtilis SMC protein (26, 58).
The temperature sensitivity of mukB mutants can be suppressed by topoisomerase mutations that increase DNA supercoiling inside cells (45). Since supercoiling makes DNA more compact (20, 44), Sawitzke and Austin argued that the additional compaction by condensins might not be needed for highly supercoiled chromosomes. That, together with the chromosome decondensation phenotype of mukB mutants, led to the suggestion that MukBEF is a condensin (20, 45). Accordingly, purified MukB was found to promote chiral DNA knotting, the signature activity of condensins (41). It is noteworthy that MukB alone was sufficient to support DNA condensation in vitro (41), indicating that the non-SMC subunits of the complex play only an auxiliary role in DNA reconfiguration. We investigated here whether MukBEF can condense chromosomes in vivo. To gain insight into the role of the non-SMC subunits of the complex, we also examined whether chromosome condensation can be induced by MukB alone.
We report that the overproduction of MukBEF or MukB results in a marked chromosome condensation. A similar effect was described earlier for the SMC protein from B. subtilis (58). We found that the physiological consequences of the chromosome condensation varied depending on the growth conditions and the genetic backgrounds of the cells and could sometimes arrest cell growth. However, chromosomes were condensed before we could detect any decline in the rates of macromolecular synthesis. Furthermore, MukB-GFP colocalized with DNA in both overproducing strains. Thus, the overproduced MukBEF and MukB condense chromosomes by directly altering chromatin structure. By quantifying the extent of chromosome condensation, we found that MukBEF is often a better condensin than MukB. However, the overproduced MukB was able to condense chromosomes under all tested conditions, even in the strains that lacked functional MukEF. Remarkably, chromosome condensation induced by MukB overproduction did not restore the viability of ΔmukEF mutants. Furthermore, anucleate cells were readily formed under these conditions. Thus, the role of MukBEF in chromosome segregation goes beyond decreasing chromosome size.
MATERIALS AND METHODS
Plasmids and strains.
Strains GC7528 (38), AZ5381, AZ5450 (65), and OT7 (66) (which lack functional MukB, MukF, and MukE and entire MukBEF, respectively) were a generous gift of Sota Hiraga.
pBB03 encodes the complete smtA-mukF-mukE-mukBHIS10 operon under the control of the arabinose-inducible promoter PBAD. To construct pBB03, the smtA-mukF-mukE fragment was amplified by PCR using genomic DNA of MG1655 as a template. It was then subcloned into the MukB-encoding plasmid pAX814 (kindly provided by Sota Hiraga) to generate the complete smtA-mukF-mukE-mukB operon. The 3′ end of mukB was then modified by PCR to introduce the C-terminal 10-histidine tag to the protein. The entire operon was then inserted between the NcoI and EcoRI sites of pBAD/Myc-HisB (Invitrogen) to yield pBB03. All Muk genes in pBB03 were functionally active, since the plasmid suppressed the temperature sensitivity of GC7528, AZ5381, and AZ5450.
pBB10 contains the mukBHIS10 gene under the control of PBAD. pBB10 was constructed by removing the smtA-mukF-mukE fragment from pBB03 with the help of PCR. pBB08 was constructed similarly and contains the smtA-mukF-mukEHIS9 fragment of the operon under the control of PBAD. The functionality of genes in pBB10 and pBB08 was verified by testing the complementation of the temperature sensitivity of GC7528, AZ5381, or AZ5450 as appropriate. pBB03 and pBB08 were found to be leaky and produced about 20 times the level of endogenous MukBEF in the absence of arabinose and in the presence of repressing glucose (Fig. 1 and data not shown). Furthermore, pBB03 and pBB08 were able to complement the temperature sensitivity of OT7, AZ5381, or AZ5450 cells as appropriate. Perhaps only the first gene in the operon, smtA, is efficiently repressed in these constructs. We found no evidence of leakage from pBB10, which encodes only one gene under the control of PBAD.
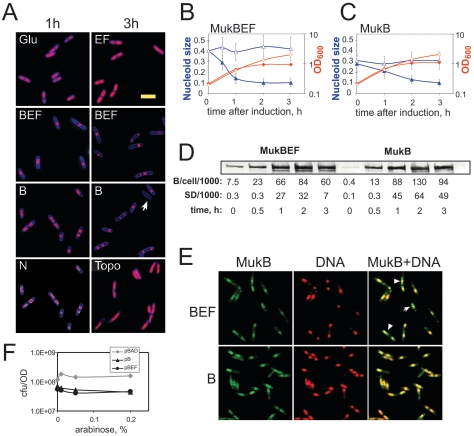
FIG. 1.
Chromosome condensation by overproduced MukBEF and MukB. (A) DH5α cells harboring an appropriate plasmid were grown in LB medium up to an OD600 of 0.2, and the production of MukB (B), MukBEF (BEF), MukEF (EF), or E. coli topoisomerase I (Topo) was induced by the addition of arabinose or repressed by the addition of glucose (Glu). The cells were fixed 1 h or 3 h after induction and analyzed by fluorescence microscopy. The images were quantified using Nucleus. The cell boundaries were determined from SyproOrange staining. The bottom-left panel (N) shows spot recognition by Nucleus for the image obtained for MukB overproducers. The arrow indicates a cell almost completely devoid of DNA. Size bar, 5 μm. (B and C) Growth curves (diamonds) and normalized chromosome sizes (triangles) as functions of time after the MukBEF-overproducing cells (B) or MukB-overproducing cells (C) were supplemented with arabinose (closed symbols) or glucose (open symbols). For any given cell, the sizes of all nucleoids were summed and divided by the area of the cell. The average values for 150 to 200 cells are shown. Error bar, standard deviation. (D) The amount of MukB in MukBEF- and MukB-overproducing strains. OD units (0.001) of cells were mixed with sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel loading buffer, boiled for 5 min, and analyzed by Western blotting using anti-MukB antibody. For the zero time points, we used 0.05 OD units of cells. For each time point, the amount of MukB was quantified as described in Materials and Methods and the data were expressed as the number of MukB monomers per cell. The averages for two independent experiments are shown together with the standard deviations (SDs). (E) Colocalization of the overproduced MukB with DNA. OU103 cells, which carry a MukB-GFP fusion on the chromosome, were transformed with the plasmids that overproduced MukBEF (top row) or MukB (bottom row), grown in LB medium, and induced with arabinose at an OD600 of 0.2. After 3 hours of induction, the cells were stained with Hoechst 33342 and observed by fluorescence microscopy. The left panels (MukB) show GFP fluorescence; the middle panels (DNA) show Hoechst fluorescence; the overlay of these two images is shown on the right panels (MukB+GFP). (F) The overproduction of MukBEF or MukB does not reduce the formation of colonies in LB. DH5α cells harboring pBAD, pBB10, or pBB03 were grown in LB at 37°C up to an OD600 of about 0.2, diluted, and spread on LB plates supplemented with 100 μg/ml ampicillin and the indicated amounts of arabinose, and the plates were further incubated at 37°C overnight.
The ΔmukEF::kan strain OU102 is derived from MG1655 by replacing the smtA-mukF-mukE fragment of the smtA-mukF-mukE-mukB operon with a kanamycin resistance gene. The strain was constructed using the recBC sbcB mutant strain JC7623 (62). The two regions flanking the smtA-mukF-mukE locus of the operon were amplified by PCR using genomic DNA of MG1655 as a template. The kanamycin resistance cassette from pACYC177 (the 1.4-kb AfeI fragment) was inserted between these two PCR products. The resulting construct was transformed into JC7623 at room temperature, and kanamycin-resistant, temperature-sensitive colonies were selected. The mutated muk operon was transduced into MG1655 using the P1vir phage. The resulting OU102 strain could grow at 37°C only if transformed with pBB08. Gene replacement in the muk locus was confirmed by PCR analysis of the chromosomal DNA of OU102.
OU103 was obtained by integrating the MukB-GFP fusion gene under the control of the endogenous PMUK promoter into the chromosomes of DH5α cells by transposition. MukB-GFP fusion was constructed by PCR. In this construct, green fluorescent protein (GFP) is linked to the C terminus of MukB via peptide HHHHHHHHHGG. GFP was amplified by PCR using plasmid pQBIT7-GFP (Quantum) as a template. MukB-GFP was placed under the control of the endogenous PMUK promoter, and a kanamycin-resistance cassette (the 1.4-kb AfeI fragment from pACYC177) was added downstream of the gene. The whole construct was then cloned between transposon Tn5 direct repeats to yield plasmid pBB15KTnPB1. MukB-GFP on this plasmid was functionally active because it complemented the temperature sensitivity of GC7528. The MukB-GFP-kan construct was amplified by PCR, the transposome was assembled using EZ::TN transposase (Epicentre), and the mixture was transformed into DH5α cells by electroporation. Three kanamycin-resistant, GFP-expressing colonies were selected; they were verified to contain no template plasmid and were transformed with pBB10. All three strains gave identical results upon the overexpression of MukB (data not shown). The results for one of the strains, OU103, are shown in Results.
Fluorescence microscopy.
Cells were grown either in LB medium containing 1% NaCl or in M9 medium supplemented with 2 mM MgSO4, 0.1 mM CaCl2, 0.4% Casamino Acids (Difco), 100 μg/ml thiamine, and 0.2% glycerol. Where appropriate, 50 μg/ml ampicillin and 20 μg/ml kanamycin were added. At an optical density at 600 nm (OD600) of 0.2, the protein overproduction was induced by the addition of 0.2% l-arabinose or repressed by the addition of 0.2% d-glucose. To monitor chromosome condensation, we adapted the cell fixation protocol developed by Nordstrom's group (5) because it yields fewer condensed nucleoids and nucleoid fusion events (1, 56). In brief, 400 μl of cells was mixed with 6.4 ml of ice-cold 74% ethanol--phosphate-buffered saline (PBS) mixture (70% final concentration of ethanol) and stored up to 1 week at 4°C. Fixed cells were pelleted, washed with PBS, resuspended in 300 μl of PBS, applied to a poly-l-lysine-coated coverslip for 5 min, rinsed six times in PBS, and gently pressed over a 15-μl drop of SyproOrange (Molecular Probes) and 100 nM DAPI (4′,6′-diamidino-2-phenylindole) in PBS, which was spotted on a clean microscope slide. SyproOrange is a nonspecific protein dye which stains only permealized cells and was used as a control for the even staining of cells. Fluorescence was observed using an Olympus BX-50 microscope equipped with a BX-FLA fluorescent attachment. Photographs were taken using an Insight charge-coupled-device camera (Hitschfel Instruments). Image overlays and color adjustment were done using Adobe Photoshop software. The sizes and subcellular localizations of the nucleoids were quantified using a home-written program, Nucleus.
For comparison, we examined chromosome condensation using two other techniques: (i) staining live cells with Hoechst 33342 and (ii) staining toluene-permealized cells with DAPI (23). All three methods gave essentially the same results for wild-type cells and for cells that overproduced MukBEF or MukB. However, nucleoids of MukEF-overproducing cells appeared condensed when observed on unfixed cells, especially for late time points. We found no evidence that MukEF can bind DNA (data not shown). Furthermore, these nucleoids did not look as regular as they had with MukB or MukBEF. This condensation was apparently caused by crowding effects or another artifact of overproduction. Accordingly, no condensation was found when these cells were fixed with ethanol (Fig. 1A). Thus, the protocol that employs cell fixation gives more-reproducible results. Furthermore, it ensures fairly even staining of all cells. We used this protocol in all chromosome sizing experiments.
Quantification of nucleoid size.
We used Nucleus for high-throughput analysis of the micrographs. The program identifies bright spots in imported image files and performs basic statistical analysis for the found spots. We defined the spot according to the brightness levels of the pixels in the image. All pixels that were at least 60% as bright as the brightest pixel in the spot were counted as part of the spot. Spot recognition errors happen mostly for badly misshapen spots or for closely located cells. Visual review is used to discard such cells from analysis. Once nucleoids and cells are identified, the program calculates their sizes, lengths, and widths and the subcellular localizations of their nucleoids.
Macromolecular synthesis.
The overnight culture grown in LB at 37°C was washed in M9 medium and inoculated into M9 medium that was supplemented with 0.4% Casamino Acids, 2 mM MgSO4, 0.1 mM CaCl2, 100 μg/ml thiamine, 0.2% glycerol, and 50 μg/ml ampicillin. To measure the rate of RNA or DNA synthesis, growth medium was further supplemented with 10 μg/ml uridine or 10 μg/ml thymidine, respectively. The cells were further grown at 37°C and supplemented with 0.2% arabinose at an OD600 of 0.2. At appropriate time points, 1 ml of cell suspension was transferred into a 15-ml Falcon tube and supplemented with either 5μCi of l-[35S]methionine (Amersham, SJ1015), 1 μCi of [5,6-3H]uridine (35 Ci/mmol; MP Biomedicals), or 2 μCi of [methyl-3H]thymidine (48 Ci/mmol; Amersham) mixed with 10 μg/ml of unlabeled thymidine. The incubation continued for 10 min at 37°C; three 300-μl aliquots were mixed with equal volumes of 20% ice-cold trichloroacetic acid and further incubated on ice for at least 30 min. The aliquots were applied to prerinsed glass fiber MultiScreen-FC plates (Millipore), and the filters were washed twice with 250 μl of ice-cold 10% trichloroacetic acid and then twice with ice-cold 70% ethanol. The filters were dried and removed from the filtration plate, and the radioactivity was measured using Beckman Coultier liquid scintillation counter. To measure total radioactivity, 10 μl of cell suspension was removed from each sample and applied to GF/C filters (Whatman); the filters were dried, and the radioactivity was counted as before.
Continuous labeling experiments were done essentially as described above, except that 4 μCi/ml of [35S]methionine, 1 μCi/ml [3H]uridine, or 2 μCi/ml [3H]thymidine was added prior to inoculation.
Western blotting.
Western blotting was done as described previously (4), using rabbit anti-MukBHIS10 antibody (Covance). For quantitative Western blotting, several samples with different concentrations of purified MukB were electrophoresed through the same gel and analyzed in parallel with the unknown samples. Here, purified MukB was supplemented with OT7 cells to match the unknown samples. The intensities of the bands on the blots were quantified using densitometry.
RESULTS
Nucleoid condensation parallels overproduction of MukB and MukBEF.
Overproduction of MukBEF, MukB, or MukEF was performed using plasmids pBB03, pBB10, or pBB08, which encode the complete smtA-mukF-mukE-mukBHIS10 operon, the mukBHIS10 fragment, or the smtA-mukF-mukEHIS9 fragment of the operon, respectively, under the control of the arabinose-inducible promoter PBAD. MukF, MukE, and MukB produced from all these plasmids are functionally active since the plasmids suppressed the temperature sensitivity of the appropriate Muk-deficient cells (see Materials and Methods for details).
DH5α cells harboring plasmid pBB10, pBB03, or pBB08 were grown at 37°C in LB medium up to an OD600 of 0.2, and protein overproduction was induced by the addition of 0.2% arabinose. The aliquots were removed at specific time points, fixed with 70% ethanol, and analyzed by fluorescence microscopy (Fig. 1A). As a control, we used cells that were supplemented with glucose rather than arabinose. Chromosome condensation was evident 1 hour after induction in cells that overproduced either MukB or MukBEF but not in cells that overproduced MukEF (Fig. 1A). Thereafter, chromosomes became progressively more condensed. Many nucleoids were positioned asymmetrically within the cells at later time points (discussed in greater detail below). A decline in growth rate was observed for the overproducing cells (Fig. 1B and C). The overproducing cells, however, remained viable (Fig. 1F).
To quantify the extent of chromosome condensation, we next measured the sizes of DNA spots on fluorescent micrographs as fractions of the sizes of encompassing cells. For cells supplemented with glucose, chromosome size remained constant throughout the experiment. Approximately 30% to 40% of the total cell area was occupied by DNA. Overproduction of MukB resulted in a gradual decrease in nucleoid size. Three hours after induction, DNA occupied 10% of the cell area (Fig. 1C). The overproduction of MukBEF led to a similar decline in chromosome size. In this case, however, chromosome condensation was essentially complete during the first hour and did not progress further after that (Fig. 1B).
Chromosome size in normal, uninduced cells varied somewhat between experiments (Fig. 1B and C). Visual inspection of such images did not reveal any significant differences in cell morphology. This variability in chromosome size arises perhaps due to the inherent lability of the E. coli nucleoid (11, 34, 43). In contrast, the measured sizes of overcondensed chromosomes were highly reproducible. Perhaps the more compact structures of the overcondensed nucleoids made them more resilient to the fixation procedure.
To confirm that nucleoid condensation was not due merely to the protein binding to DNA, we examined cells that overproduce E. coli DNA topoisomerase I using the same pBAD-based expression system. The copy number of overproduced topoisomerase I was greater than 120,000 per cell (Fig. S1A in the supplemental material), which is at least as high as for MukB (Fig. 1D). We found no chromosome condensation in these cells (Fig. 1A). Three hours after induction, DNA occupied 0.4 ± 0.11 of the cell area (averaged for 85 cells), similar to what we observed for uninduced cells (Fig. 1B and C). Plasmid DNA extracted from topoisomerase I-overproducing cells remained highly supercoiled (Fig. S1B in the supplemental material), indicating that the lack of condensation was not due to relaxation activity of topoisomerase I. Thus, the mere binding of a protein to DNA does not suffice to induce chromosome condensation.
As shown in Fig. 1B and 1C, we quantified the extent of chromosome condensation by normalizing DNA spot size to cell size. Since the overproduction of MukBEF could alter the average cell size, this approach could report that the chromosomes are condensed even if the nucleoid sizes remain the same. To validate our approach, we measured the actual sizes of the nucleoids, without any normalization, and then binned the scored nucleoids according to their sizes. The histogram in Fig. 2 shows the distributions of nucleoids according to their sizes found 3 hours after induction of MukBEF or MukB. The two distributions are virtually identical and are very different from those for uninduced cells. The average size of the condensed nucleoids is about one-half that found for short uninduced cells (Fig. 2). The identical distributions found for MukBEF and MukB are in accord with the normalized data presented in Fig. 1. We conclude, therefore, that our initial approach can be used to gauge chromosome condensation.
FIG. 2.
Distribution of the nucleoid sizes following the overproduction of MukBEF and MukB. DH5α cells harboring plasmid pBB10 or pBB03 were supplemented with arabinose (Ara) or glucose (Glu) as appropriate to repress or induce the overproduction of MukB (B) or MukBEF (BEF), further incubated for 3 hours at 37°C in LB, and observed by fluorescence microscopy. Nucleoid size was measured using Nucleus, the nucleoids were binned according to their sizes, and the number of nucleoids in each bin was normalized to the total number of scored nucleoids (between 430 and 700 nucleoids for each data set). The resulting distributions are shown on the plot. The arrow indicates the sizes of single nucleoids found in short, nonoverproducing cells.
The major difference between cells that overproduce MukBEF and MukB can be detected 1 hour after induction, when chromosomes in the MukBEF cells are more condensed than in the MukB cells. The amounts of MukB in the two strains were similar at all time points (Fig. 1D). The dynamics of protein induction roughly paralleled those of chromosome condensation. Three hours after induction, we found (9 ± 4) · 104 and (6 ± 1) · 104 molecules of MukB per cell in cells that overproduced MukB and MukBEF, respectively. This small difference in protein production is expected given the shorter length of the transcript in the MukB strain. Thus, the more efficient chromosome condensation by MukBEF was not due to the greater amount of MukB in this strain but probably reflects the more potent condensation activity of MukBEF.
Overproduced MukB localizes with DNA.
Previous studies established that MukB colocalizes with chromosomes (10, 40). The overproduced MukB, however, could be misfolded and thus could conceivably condense chromosomes via some nonspecific effect, for example, by forming inclusion bodies. To test this possibility, we next examined the distribution of overproduced MukB inside cells. MukB-GFP fusion protein was placed under the control of endogenous PMUK promoter, and the resulting construct was integrated into the chromosome of the DH5α strain via transposition (see Materials and Methods). The resulting OU103 strain was further transformed with plasmid pBB03 or pBB10. MukB-GFP fusion protein was functionally active since it complemented the temperature sensitivity of ΔmukB strain GC7528 when expressed from the plasmid (data not shown).
Upon the addition of arabinose, MukB-GFP formed diffused spots in OU103 strains transformed with either MukB- or MuBEF-overproducing plasmids (Fig. 1E). These spots clearly colocalized with DNA in all examined cells 3 hours after induction (Fig. 1E), indicating that the proteins retained their DNA binding activity. The results were similar for the 1-hour time point, although a significant amount of protein remained unbound to DNA in this case (data not shown). We also detected additional MukB-GFP spots in the MukBEF strain but not in the MukB strain (Fig. 1E, arrows), as well as dispersed fluorescent signals flanking the nucleoids (arrowheads), suggesting that MukBEF might have additional attraction sites within cells. However, the brightest GFP signal was detected at the nucleoids, indicating that MukB-GFP binds primarily to DNA in both MukB and MukBEF cells. Thus, chromosome overcondensation was likely the result of a direct action of MukB on DNA rather than some secondary physiological effect.
Chromosome condensation occurs before effects on macromolecular synthesis.
The size of a bacterial nucleoid is determined by the balance of expansion forces resulting from chromosome attachment to the membrane and condensing forces provided by the architectural proteins (42, 63). If the overproduction of MukBEF triggers a physiological response that tips this balance, a change in nucleoid size is expected, even without direct action by the protein. To address this concern, we next measured the rates of macromolecular synthesis in cells that overproduce MukB and MukBEF.
We measured the rates of protein, RNA, and DNA syntheses by monitoring the incorporation of radioactively labeled [35S]methionine, [3H]uridine, and [3H]thymidine, respectively, into growing DH5α cells that carried pBB03, pBB10, or the unmodified pBAD vector. To facilitate the incorporation, these experiments were done with M9 medium supplemented with Casamino Acids and glycerol. As before, protein production was induced by the addition of arabinose at an OD600 of 0.2.
The effect of protein overproduction was more pronounced in M9 medium than in LB. Both MukBEF and MukB overproducers stopped growing 2.5 h after induction at an OD600 of about 0.5 (Fig. 3). We found, however, no effect of protein overproduction on the rates of transcription and translation during the first hour of induction. The initial declines in the rates of protein and RNA syntheses were observed in all three cultures, including the control. These declines appear perhaps because arabinose, the inducer, is a superior carbon source compared to glycerol, which was used to support cell growth prior to induction. Apparently, the addition of arabinose affected the uptake rates for both overproducing and nonoverproducing strains. In agreement with this view, we found no effect of arabinose on cumulative incorporation of radioactivity at the early time points (Fig. 3, right panels). In contrast, nucleoids were clearly condensed 1 h after induction.
FIG. 3.
Growth curves, extent of chromosome condensation, and rates of macromolecular synthesis in cells that overproduce MukBEF or MukB. DH5α cells harboring pBAD, pBB10 (pB), or pBB03 (pBEF) were induced with arabinose in the synthetic medium, and the rate of (left panels) or cumulative (right panels) incorporation of radioactivity into protein, RNA, or DNA was measured as described in Materials and Methods. Synthesis rates are expressed as the amounts of acid-insoluble radioactivity accumulated after a 10-min pulse, normalized to the total amount of radioactivity in the labeling mixture and further normalized to the OD600 of the cell culture. Cumulative (total) incorporation is measured as the fraction of radioactivity found in acid-insoluble form. The plotted data represent the averages for two independent experiments; error bars show standard deviations. For convenience, the extent of chromosome condensation in M9CA medium is also shown (Fig. 5). The appropriate growth curves are shown in the top panels.
A decline in the rate of protein synthesis could be noted about 2 h after induction. The rate of transcription began to fall off somewhat earlier, about 1.5 h after induction. The first rate to be affected was DNA replication. Compared to those in the control culture, cells that overproduced condensins showed reduced replication rates as early as 1 h after induction (Fig. 3). The pulse-labeling experiments were confirmed for the experiments where radioactive precursors were added to the growth medium prior to protein induction. The amount of acid-insoluble [3H]thymidine began to decrease in the overproducing cells 2 h after induction, before the onset of the growth arrest. For [35S]methionine and [3H]uridine, the incorporation curves paralleled the growth curves (Fig. 3).
These data clearly show that chromosome condensation precedes the effects of the overproduction on transcription and translation. The results for DNA replication are more ambiguous. Judging by continuous-labeling experiments, replication is not affected until after chromosome condensation. The pulse-labeling data indicate that the decline in DNA synthesis might parallel chromosome condensation. Even if the latter is true, it is difficult to rationalize how a termination of DNA replication, which occurs in a handful of places along the chromosome, could produce the dense nucleoids that we see in our micrographs (Fig. 1). It is also unlikely that the overproduction of condensins triggered chromosome condensation by depleting cell resources from the chromosomal genes since we found no changes in cell growth or morphology when we overproduced SmtA under similar conditions (data not shown). We conclude, therefore, that the overproduced MukB and MukBEF condense chromosomes by directly altering chromosome structure. This, in turn, leads to the eventual shutdown of macromolecular synthesis.
Mispositioning of nucleoids follows overcondensation.
The overproduction of MukB and MukBEF results not only in chromosome condensation but also in the mispositioning of nucleoids (Fig. 1A). Even long cells often contained only one nucleoid. Nucleoids were often positioned asymmetrically within a cell. Defects in chromosome segregation became more prominent with time. Three hours after induction, about 3% of the cells contained little if any DNA. The majority of the cells, however, contained one or more well-defined nucleoids (Fig. 1A).
We next quantified the positions of nucleoids at different times after induction for the MukB and MukBEF strains (Fig. 4). The position of every nucleoid, measured as a distance from the middle of the cell, is shown in Fig. 4A as a function of cell length. Short cells are expected to have their single nucleoids located close to the middles of the cells, whereas two-nucleoid cells should have their nucleoids close to the ±1/4 positions. This pattern was indeed observed for cells grown in the presence of glucose (Fig. 4). A small bias in nucleoid position is apparent for the single-nucleoid cells. This bias reflects the convention that we used to assign positive signs to the measured distances rather than actual cell asymmetry.
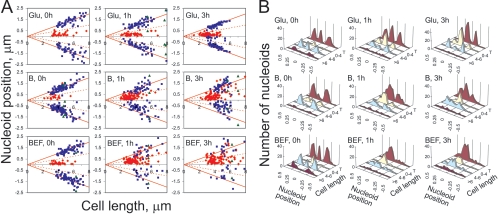
FIG. 4.
Subcellular localization of nucleoids during the overproduction of MukB and MukBEF. The positions of the nucleoids were measured as the distance between the center of the nucleoid and the middle of the cell for DH5α cells that overproduced either MukB (B; middle panels) or MukBEF (BEF; bottom panels) or where the overproduction was repressed by the addition of glucose (Glu; top panels). The cells were collected for microscopy immediately after induction (0 h) or 1 h or 3 h after the addition of a sugar. In panel A, the nucleoid positions are plotted against corresponding cell lengths. The cells with one nucleoid (red diamonds), two nucleoids (blue squares), or three or more nucleoids (green triangles) were scored separately. Solid red lines indicate the +1/4 and −1/4 positions; dashed lines indicate the +1/8 and −1/8 positions of cell length. For panel B, the nucleoid positions were normalized to the cell lengths, and the scored nucleoids were binned according to their normalized positions within the cells. Such histograms were generated separately for cells shorter than 4 μm (0 to 4), with lengths between 4 μm and 6 μm (4-6), or longer than 6 μm (>6) or for all cells regardless of their lengths (T).
The distribution of nucleoids changed dramatically upon the addition of arabinose. For cells with only one nucleoid, the nucleoid could be found anywhere between the middle of the cell and the 1/4 position. Three hours after induction, all cells with one or two nucleoids were affected. For both the MukB and the MukBEF strains, nucleoid distribution appeared random (Fig. 4). Even cells with one nucleoid close to its expected +1/4 position often lacked the sister nucleoid in its rightful −1/4 place (Fig. 1). Thus, excessive chromosome condensation interferes with chromosome segregation.
MukBEF and MukB condense chromosomes under various growth conditions.
Overproduced MukBEF and MukB condensed nucleoids of DH5α cells to the same extent. The only difference between the two proteins was observed 1 hour after induction. At this time point, chromosomes of cells overproducing MukB were somewhat bigger than those for MukBEF (Fig. 1). Twenty-one percent of the cell area was occupied by DNA in the MukB-overproducing strain as opposed to 14% found for MukBEF (Fig. 1BC). Thus, MukBEF might be a better condensin than MukB. To test the generality of this phenomenon, we next explored chromosome overcondensation under various growth conditions. We found that MukBEF and MukB condensed chromosomes under all tested conditions (Fig. 5). The only exception was found for MG1655 cells grown in LB. In this case, almost no chromosome condensation was detected for the MukB strain. MG1655 differs from DH5α in two major respects: (i) MG1655 cells grow faster than DH5α cells (doubling times of 20 min and 30 min, respectively), and therefore, they might differ in overall physiology (6), and (ii) DH5α cells carry several mutations, including recA1, that affect various aspects of DNA metabolism. The different genetic makeup of MG1655 is unlikely the reason for the poor condensation since we found robust condensation by MukB and MukBEF when these cells were grown in M9 medium (Fig. 5). The amounts of MukB and MukBEF were essentially the same under all tested conditions (data not shown). Thus, the efficiency of MukB in chromosome condensation depends on the physiological state of the cell.
FIG. 5.
Chromosome condensation under various growth conditions. Cells harboring the pBB10 (B), pBB03 (BEF), or pBAD vector were grown either at 25°C (labeled 25/37) or at 37°C up to an OD600 of 0.2. The cells were then supplemented with arabinose and further incubated at 37°C for 3 hours. Next, the average cell fraction occupied by DNA was measured using fluorescence microscopy. Between 50 and 100 cells were quantified for each data point. Note that the error bars represent standard deviations rather than standard errors. The error bars describe, therefore, the variation of chromosome sizes in the population rather than the inaccuracy of our technique. wt, wild type.
Importantly, the overproduction of MukBEF resulted in chromosome overcondensation under all tested conditions. With one exception, MG1655 cells grown in LB at 37°C, the same was true for MukB. The extent of chromosome condensation was also similar between MukB and MukBEF 3 hours after induction. After 1 hour of induction, however, MukBEF consistently produced smaller chromosomes than MukB. These results fit into the pattern suggested by our previous experiments (Fig. 1): both MukBEF and MukB can condense chromosomes, yet MukBEF is a better condensin than MukB.
MukB can condense chromosomes in the absence of MukE or MukF.
Our data so far support the view that MukB, at least when overproduced, can perform chromosome condensation almost as effectively as MukBEF. Why then does the inactivation of any of the three subunits of MukBEF result in the same phenotype? Is it because MukB is a weak condensin and thus would not be able to keep chromosomes condensed when produced at its normal level? Or is it possible that MukBEF, besides having a role in chromosome condensation, is involved in other aspects of chromosome segregation? In the former case, chromosome condensation induced by the overproduced MukB should rescue the temperature sensitivity of ΔmukEF mutants, whereas the lack of such complementation would support the latter possibility. We found no such complementation in our next experiment.
Since MukEF-deficient cells do not grow above 30°C (38), we adopted a different protocol to analyze chromosome condensation in a ΔmukEF genetic background. The MukEF-deficient OU102 strain was transformed with either the pBB03 or pBB10 plasmid and grown at room temperature. When the OD600 reached 0.2, cell aliquots were withdrawn and assayed for colony-forming ability in the presence or absence of arabinose (Fig. 6A and B). The remainder of the cell culture was supplemented with arabinose and simultaneously shifted to 37°C to induce chromosome segregation defects. Following 3 hours of incubation, the cells were analyzed using fluorescence microscopy. Using this protocol, we found that the overproduction of MukB and MukBEF in MG1655 cells results in chromosome condensation (Fig. 5).
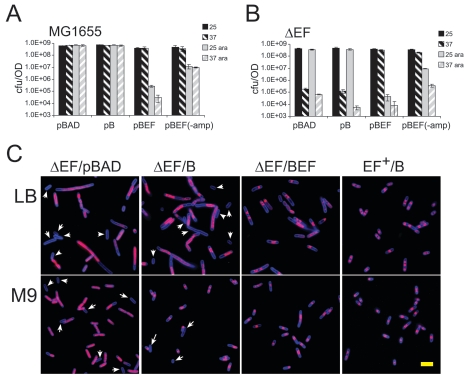
FIG. 6.
Chromosome condensation in the absence of MukEF. (A and B) Colony formation by wild-type and MukEF-deficient cells that overproduce MukB or MukBEF in minimal medium. MG1655 (A) or the ΔEF OU102 cells (B) harboring plasmid pBB10 (pB), pBB03 (pBEF), or pBAD were grown in M9 medium supplemented with 0.2% glycerol, 0.4% Casamino Acids, and 50 μg/ml ampicillin sulfate up to an OD600 of 0.2; diluted as appropriate; and spread onto M9 plates supplemented with 0.4% Casamino Acids, 0.2% glycerol, and 50 μg/ml ampicillin sulfate with (ara) or without 0.2% arabinose. In addition, MukBEF-overproducing cells were also plated without ampicillin (−amp). The plates were incubated at either room temperature (25) or 37°C (37). The averages for at least two separate experiments are shown. Error bars indicate standard deviations. (C) Either OU102 cells harboring plasmid pBAD (ΔEF/pBAD), pBB10 (ΔEF/B), or pBB03 (ΔEF/BEF) or MG1655 cells harboring pBB10 (EF+/B) were grown in M9 medium supplemented with 0.4% Casamino Acids and 0.2% glycerol or in LB at room temperature. At an OD600 of 0.2, the cells were supplemented with 0.2% arabinose, and incubation was continued for 3 hours at 37°C. The cells were then fixed and visualized by fluorescence microscopy. Arrows, anucleate cells; size bar, 5 μm.
The overproduced MukB did condense chromosomes in the absence of MukEF (Fig. 6C). When cultured at the nonpermissive temperature in LB medium, ΔmukEF cells showed a high frequency of anucleate cells (20%) as well as markedly decondensed chromosomes, as was reported previously (38, 45, 60). The overproduction of MukB resulted in partial condensation of the chromosomes; however, the frequency of anucleate cells remained high, about 17% (Fig. 6C). In contrast, we found no anucleate cells upon induction of MukBEF.
The results were essentially the same for M9 medium. In this case, ΔmukEF cells were considerably shorter. Because of this, it was difficult to judge whether the chromosomes of these cells were decondensed; however, anucleate cells were produced with about the same frequency as in LB. Anucleate cell formation was not affected by the induction of MukB, although chromosomes were clearly condensed in this case (Fig. 6C). Thus, chromosome condensation by MukB does not rescue anucleate cell formation.
Similarly, the overproduction of MukB had little effect on the viability of ΔmukEF cells at elevated temperatures (Fig. 6B). To ensure robust chromosome condensation, cells were grown in M9 medium in these experiments. In agreement with previous data (38), the ΔmukEF strain transformed with the vector alone showed a severe decline in CFU at 37°C but not at 25°C. The temperature sensitivity of the ΔmukEF strain was complemented by the production of MukBEF but not MukB protein. We conclude that MukB-induced chromosome condensation does not compensate for the absence of MukEF.
This lack of complementation could alternatively be due to the poisoning effects of overproduced condensins. This did not appear to be true in our case. Indeed, MukB showed no signs of being toxic in the mukE+ mukF+ MG1655 cells (Fig. 6A). We did find that the overproduction of MukBEF in M9 medium reduces colony formation by more than 3 orders of magnitude. However, when we plated MukBEF-overproducing cells in the absence of ampicillin, the selection marker for the plasmid, we observed only a 50-fold decline in CFU (Fig. 5A). For comparison, mutational inactivation of MukBEF results in a decline of more than 4 orders of magnitude in cell viability (38). We found no effect of ampicillin on the colony-forming ability of all other strains (data not shown). Furthermore, the overproduction of MukBEF decreased cell viability to about the same extent at 37°C and 25°C (Fig. 6AB). In contrast, OU102 cells overproducing MukB were deficient in colony formation only at 37°C and not at 25°C.
DISCUSSION
We show here that the overproduction of MukBEF and MukB condenses nucleoids in live E. coli cells. This condensation happens soon after induction of the protein and before we can detect any changes in the rates of transcription or translation. Most of the overproduced MukB colocalizes with the nucleoids (Fig. 1E). MukBEF does not form inclusion bodies since the protein can be found either on the chromosomes or in the cytoplasm after gentle lysis of the cells (data not shown). Finally, the extent of chromosome condensation varied depending on strain or cultivation conditions, although the amounts of the produced protein were virtually the same. No such variation would be expected if chromosome condensation were caused by nonspecific effects. It seems likely, therefore, that the overproduced MukBEF condenses chromosomes by modifying chromatin structure.
The appearance of condensed nucleoids as bright round foci is reminiscent of another example when the overproduction of a DNA binding protein, H-NS, led to chromosome condensation (48). With H-NS, however, all other aspects of protein overproduction were different. The overproduction of H-NS rapidly blocked transcription and translation, eventually killing the cells, whereas the protein itself was found in inclusion bodies (48). Ironically, despite all these differences, MukBEF and H-NS might share the same mechanism of chromosome condensation since H-NS was found to cross-link DNA segments in vitro (8).
One of the goals of this study was to compare the efficiencies of MukBEF and MukB as condensins on their physiological substrate, the chromosome. The central assumption in such comparative analysis is that the amounts of active MukBEF and MukB are the same under appropriate conditions. This assumption held reasonably well in our experiments. Within the accuracy of quantitative Western blotting, the amounts of MukB were up to 50% greater in cells that overproduced MukB rather than MukBEF (Fig. 1D). The greater levels of MukB cannot explain why MukBEF is a better condensin. Furthermore, both MukBEF and MukB appeared to be correctly folded since both proteins colocalized with DNA 3 hours after induction (Fig. 1E). We cannot, however, exclude the possibility that the greater efficiency of MukBEF can be explained in part by its better binding to DNA.
Another challenge is to distinguish between the primary and secondary effects of condensin action. Importantly, we found little difference in how cells reacted to the induction of MukB or MukBEF (Fig. 1 and 3). It seems likely, therefore, that MukB and MukBEF faced essentially the same substrate during the induction and thus that the differences in cell morphology between the two strains do reflect the difference in the activities of the two proteins. Furthermore, the metabolic labeling experiments showed that the rates of macromolecular synthesis are not affected until 1 hour after induction (Fig. 3). Thus, the 1-hour data points most probably reflect direct effects of MukBEF and MukB on chromosome size.
Keeping all these reservations in mind, we still can conclude that MukBEF is a better condensin than MukB. Several lines of evidence support this conclusion. First, chromosome condensation was more pronounced for MukBEF cells at early time points (Fig. 5). Second, MukB induced only modest condensation in MG1655 cells in LB, whereas MukBEF was always effective (Fig. 5). Third, MukBEF was more toxic than MukB under comparable growth conditions (Fig. 6). Fourth, plasmid loss was more pronounced in cells that overproduced MukBEF (Fig. 6A and B). Thus, the role of MukEF might be to stabilize the complex of MukB and DNA.
That does not seem to be a complete answer. We found that chromosome condensation by the overproduced MukB proceeds very differently in the MukEF-deficient strain and in its parental MukEF+ strain, MG1655, which produces the wild-type level of MukEF. Chromosomes of OU102 cells were clearly decondensed when MukB was induced in LB medium, whereas modest condensation was detected for the MG1655 strain under the same conditions (Fig. 6C). Given the disparity in protein levels, the endogenous MukEF is unlikely to directly stimulate the motor properties of the overproduced MukB. A more plausible explanation would be that MukB is presented with different substrates in the two cases; in other words, the structures of the chromosomes are different in these two strains. We propose that MukBEF is the chromatin scaffold protein and that MukEF is essential to maintain correct organization of the scaffold. Perhaps MukEF stimulates oligomerization of MukB. This conclusion agrees well with the findings made by Hiraga's group that MukB forms clusters within cells only in the presence of MukE and MukF (40) and that purified MukBEF but not MukB forms oligomeric, rosette-like structures (31). It is conceivable that such multimolecular MukB-DNA complexes would be more stable and thus would withstand greater forces during chromosome segregation.
Could MukBEF be directly involved in chromosome segregation?
The most provocative of our results is the finding that chromosome condensation by MukB does not suppress the temperature sensitivity or anucleate cell formation of ΔmukEF cells. This result extends the earlier finding of Yamanaka and coworkers that the overproduction of MukB does not suppress the phenotypes of ΔmukE or ΔmukF mutants (65). We show here that MukB does not compensate for the absence of MukEF even when chromosomes are condensed. An intriguing interpretation of this result is that MukBEF might be directly involved in chromosome segregation.
How can we reconcile the role of MukBEF in chromosome segregation with its static place in the chromosome scaffold? A number of actin-like segregation motors that ensure equal partitioning of low-copy-number plasmids or entire chromosomes into daughter cells (reviewed in references 14, 15, 30, and 47) have been recently described. We propose that MukBEF is the link between actin-like segregation motors and chromosomes. In tentative support of this view, we found that the overproduced MukBEF, but not MukB, often forms additional foci that do not strictly colocalize with nucleoids (Fig. 1E). These additional foci might reflect the interaction between MukBEF and the segregation machine. Figure 7 illustrates a possible scenario of how the chromatin scaffold could be involved in chromosome segregation. In this model, the newly replicated stretch of DNA is bound by MukBEF; then, the DNA-bound MukBEF diffuses towards the nearest fragment of the helical actin-like filament. This filament could be made of MreB, SetB (12), or some other as-yet-unidentified protein. MukEF mediates the binding of MukBEF to the motor, either directly or by stabilizing MukB in a conformation that has high affinity for the segregation machine. A possibility for such conformation-dependent interaction has been suggested earlier by the finding that the N-terminal domain of MukB interacts in an ATP-modulated manner with FtsZ (27). The newly bound MukBEF is then translocated towards the cell pole, where it can join the growing scaffold of the daughter chromosome.
FIG. 7.
A possible scheme for MukBEF involvement in chromosome segregation. The primary function of MukBEF is to be the chromatin scaffold. In addition, MukBEF interacts with a putative segregation motor, which is shown as a large helix within the membrane. The motor catalyzes the poleward motion of the DNA-bound MukBEF. The newly synthesized DNA is bound by MukBEF as it emerges from the replisome. This new DNA loop is attached to the motor and transported into the daughter cells to join the growing scaffold.
A satisfying feature of this model is that it naturally explains how chromatin is maintained in order on the global scale. Compelling evidence that such order exists has been reported recently by several groups who found that DNA fragments of several bacteria are localized within cells according to their positions on chromosomes (39, 53, 57). Further studies are needed to test this model.
Supplementary Material
Acknowledgments
We are indebted to Helen Zgurskaya, Hironori Niki, and Sota Hiraga for stimulating discussions and Nicholas R. Cozzarelli for critical reading of the manuscript.
This work was supported by grant GM63786 from NIH and a grant from the American Heart Association.
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Akerlund, T., R. Bernander, and K. Nordstrom. 1992. Cell division in Escherichia coli minB mutants. Mol. Microbiol. 6:2073-2083. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, D. E., A. Losada, H. P. Erickson, and T. Hirano. 2002. Condensin and cohesin display different arm conformations with characteristic hinge angles. J. Cell Biol. 156:419-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arumugam, P., S. Gruber, K. Tanaka, C. H. Haering, K. Mechtler, and K. Nasmyth. 2003. ATP hydrolysis is required for cohesin's association with chromosomes. Curr. Biol. 13:1941-1953. [DOI] [PubMed] [Google Scholar]
- 4.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 1989. Current protocols in molecular biology. Wiley-Interscience, New York, N.Y.
- 5.Botello, E., and K. Nordstrom. 1998. Effects of chromosome underreplication on cell division in Escherichia coli. J. Bacteriol. 180:6364-6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bremer, H., and P. P. Dennis. 1996. Modulation of chemical composition and other parameters of the cell by growth rate, p. 1553-1569. In F. C. Neidhardt (ed.), Escherichia coli and Salmonella: cell and molecular biology, 2nd ed., vol. 2. ASM Press, Washington, D.C. [Google Scholar]
- 7.Cobbe, N., and M. M. Heck. 2004. The evolution of SMC proteins: phylogenetic analysis and structural implications. Mol. Biol. Evol. 21:332-347. [DOI] [PubMed] [Google Scholar]
- 8.Dame, R. T., C. Wyman, and N. Goosen. 2000. H-NS mediated compaction of DNA visualised by atomic force microscopy. Nucleic Acids Res. 28:3504-3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Jager, M., J. van Noort, D. C. van Gent, C. Dekker, R. Kanaar, and C. Wyman. 2001. Human Rad50/Mre11 is a flexible complex that can tether DNA ends. Mol. Cell 8:1129-1135. [DOI] [PubMed] [Google Scholar]
- 10.den Blaauwen, T., A. Lindqvist, J. Lowe, and N. Nanninga. 2001. Distribution of the Escherichia coli structural maintenance of chromosomes (SMC)-like protein MukB in the cell. Mol. Microbiol. 42:1179-1188. [DOI] [PubMed] [Google Scholar]
- 11.Drlica, K., and A. Worcel. 1975. Conformational transitions in the Escherichia coli chromosome: analysis by viscometry and sedimentation. J. Mol. Biol. 98:393-411. [DOI] [PubMed] [Google Scholar]
- 12.Espeli, O., P. Nurse, C. Levine, C. Lee, and K. J. Marians. 2003. SetB: an integral membrane protein that affects chromosome segregation in Escherichia coli. Mol. Microbiol. 50:495-509. [DOI] [PubMed] [Google Scholar]
- 13.Fennell-Fezzie, R., S. D. Gradia, D. Akey, and J. M. Berger. 2005. The MukF subunit of Escherichia coli condensin: architecture and functional relationship to kleisins. EMBO J. 24:1921-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerdes, K., J. Moller-Jensen, G. Ebersbach, T. Kruse, and K. Nordstrom. 2004. Bacterial mitotic machineries. Cell 116:359-366. [DOI] [PubMed] [Google Scholar]
- 15.Graumann, P. L., and H. J. Defeu Soufo. 2004. An intracellular actin motor in bacteria? Bioessays 26:1209-1216. [DOI] [PubMed] [Google Scholar]
- 16.Haering, C. H., J. Lowe, A. Hochwagen, and K. Nasmyth. 2002. Molecular architecture of SMC proteins and the yeast cohesin complex. Mol. Cell 9:773-788. [DOI] [PubMed] [Google Scholar]
- 17.Haering, C. H., D. Schoffnegger, T. Nishino, W. Helmhart, K. Nasmyth, and J. Lowe. 2004. Structure and stability of cohesin's Smc1-kleisin interaction. Mol. Cell 15:951-964. [DOI] [PubMed] [Google Scholar]
- 18.Hirano, M., and T. Hirano. 2004. Positive and negative regulation of SMC-DNA interactions by ATP and accessory proteins. EMBO J. 23:2664-2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirano, T., R. Kobayashi, and M. Hirano. 1997. Condensins, chromosome condensation protein complexes containing XCAP-C, XCAP-E, and a Xenopus homolog of the Drosophila Barren protein. Cell 89:511-521. [DOI] [PubMed] [Google Scholar]
- 20.Holmes, V. F., and N. R. Cozzarelli. 2000. Closing the ring: links between SMC proteins and chromosome partitioning, condensation, and supercoiling. Proc. Natl. Acad. Sci. USA 97:1322-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hopfner, K. P., A. Karcher, D. Shin, C. Fairley, J. A. Tainer, and J. P. Carney. 2000. Mre11 and Rad50 from Pyrococcus furiosus: cloning and biochemical characterization reveal an evolutionarily conserved multiprotein machine. J. Bacteriol. 182:6036-6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hopfner, K. P., A. Karcher, D. S. Shin, L. Craig, L. M. Arthur, J. P. Carney, and J. A. Tainer. 2000. Structural biology of Rad50 ATPase: ATP-driven conformational control in DNA double-strand break repair and the ABC-ATPase superfamily. Cell 101:789-800. [DOI] [PubMed] [Google Scholar]
- 23.Hu, K. H., E. Liu, K. Dean, M. Gingras, W. DeGraff, and N. J. Trun. 1996. Overproduction of three genes leads to camphor resistance and chromosome condensation in Escherichia coli. Genetics 143:1521-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kimura, K., V. Rybenkov, N. Crisona, T. Hirano, and N. Cozzarelli. 1999. 13S condensin actively reconfigures DNA by introducing global positive writhe: implications for chromosome condensation. Cell 98:239-248. [DOI] [PubMed] [Google Scholar]
- 25.Lammens, A., A. Schele, and K. P. Hopfner. 2004. Structural biochemistry of ATP-driven dimerization and DNA-stimulated activation of SMC ATPases. Curr. Biol. 14:1778-1782. [DOI] [PubMed] [Google Scholar]
- 26.Lindow, J. C., M. Kuwano, S. Moriya, and A. D. Grossman. 2002. Subcellular localization of the Bacillus subtilis structural maintenance of chromosomes (SMC) protein. Mol. Microbiol. 46:997-1009. [DOI] [PubMed] [Google Scholar]
- 27.Lockhart, A., and J. Kendrick-Jones. 1998. Interaction of the N-terminal domain of MukB with the bacterial tubulin homologue FtsZ. FEBS Lett. 430:278-282. [DOI] [PubMed] [Google Scholar]
- 28.Losada, A., and T. Hirano. 2001. Intermolecular DNA interactions stimulated by the cohesin complex in vitro: implications for sister chromatid cohesion. Curr. Biol. 11:268-272. [DOI] [PubMed] [Google Scholar]
- 29.Lowe, J., S. C. Cordell, and F. van den Ent. 2001. Crystal structure of the SMC head domain: an ABC ATPase with 900 residues antiparallel coiled-coil inserted. J. Mol. Biol. 306:25-35. [DOI] [PubMed] [Google Scholar]
- 30.Lowe, J., F. van den Ent, and L. A. Amos. 2004. Molecules of the bacterial cytoskeleton. Annu. Rev. Biophys. Biomol. Struct. 33:177-198. [DOI] [PubMed] [Google Scholar]
- 31.Matoba, K., M. Yamazoe, K. Mayanagi, K. Morikawa, and S. Hiraga. 2005. Comparison of MukB homodimer versus MukBEF complex molecular architectures by electron microscopy reveals a higher-order multimerization. Biochem. Biophys. Res. Commun. 333:694-702. [DOI] [PubMed] [Google Scholar]
- 32.Melby, T. E., C. N. Ciampaglio, G. Briscoe, and H. P. Erickson. 1998. The symmetrical structure of structural maintenance of chromosomes (SMC) and MukB proteins: long, antiparallel coiled coils, folded at a flexible hinge. J. Cell Biol. 142:1595-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Michaelis, C., R. Ciosk, and K. Nasmyth. 1997. Cohesins: chromosomal proteins that prevent premature separation of sister chromatids. Cell 91:35-45. [DOI] [PubMed] [Google Scholar]
- 34.Murphy, L. D., and S. B. Zimmerman. 1997. Isolation and characterization of spermidine nucleoids from Escherichia coli. J. Struct. Biol. 119:321-335. [DOI] [PubMed] [Google Scholar]
- 35.Nasmyth, K. 2002. Segregating sister genomes: the molecular biology of chromosome separation. Science 297:559-565. [DOI] [PubMed] [Google Scholar]
- 36.Nicklas, R. B. 1997. How cells get the right chromosomes. Science 275:632-637. [DOI] [PubMed] [Google Scholar]
- 37.Niki, H., R. Imamura, M. Kitaoka, K. Yamanaka, T. Ogura, and S. Hiraga. 1992. E.coli MukB protein involved in chromosome partition forms a homodimer with a rod-and-hinge structure having DNA binding and ATP/GTP binding activities. EMBO J. 11:5101-5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Niki, H., A. Jaffe, R. Imamura, T. Ogura, and S. Hiraga. 1991. The new gene mukB codes for a 177 kd protein with coiled-coil domains involved in chromosome partitioning of E. coli. EMBO J. 10:183-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niki, H., Y. Yamaichi, and S. Hiraga. 2000. Dynamic organization of chromosomal DNA in Escherichia coli. Genes Dev. 14:212-223. [PMC free article] [PubMed] [Google Scholar]
- 40.Ohsumi, K., M. Yamazoe, and S. Hiraga. 2001. Different localization of SeqA-bound nascent DNA clusters and MukF-MukE-MukB complex in Escherichia coli cells. Mol. Microbiol. 40:835-845. [DOI] [PubMed] [Google Scholar]
- 41.Petrushenko, Z. M., C. H. Lai, R. Rai, and V. V. Rybenkov. 2006. DNA reshaping by MukB: right-handed knotting, left-handed supercoiling. J. Biol. Chem. 281:4606-4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pettijohn, D. E. 1996. The nucleoid. In F. C. Neidhardt (ed.), Escherichia coli and Salmonella typhimurium, 2nd ed. ASM Press, Washington, D.C.
- 43.Postow, L., C. D. Hardy, J. Arsuaga, and N. R. Cozzarelli. 2004. Topological domain structure of the Escherichia coli chromosome. Genes Dev. 18:1766-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rybenkov, V. V., C. U. Ullsperger, A. V. Vologodskii, and N. R. Cozzarelli. 1997. Simplification of DNA topology below equilibrium values by type II topoisomerases. Science 277:690-693. [DOI] [PubMed] [Google Scholar]
- 45.Sawitzke, J. A., and S. Austin. 2000. Suppression of chromosome segregation defects of Escherichia coli muk mutants by mutations in topoisomerase I. Proc. Natl. Acad. Sci. USA 97:1671-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schleiffer, A., S. Kaitna, S. Maurer-Stroh, M. Glotzer, K. Nasmyth, and F. Eisenhaber. 2003. Kleisins: a superfamily of bacterial and eukaryotic SMC protein partners. Mol. Cell 11:571-575. [DOI] [PubMed] [Google Scholar]
- 47.Sherratt, D. J. 2003. Bacterial chromosome dynamics. Science 301:780-785. [DOI] [PubMed] [Google Scholar]
- 48.Spurio, R., M. Dürrenberger, M. Falconi, A. La Teana, C. L. Pon, and C. O. Gualerzi. 1992. Lethal overproduction of the Escherichia coli nucleoid protein H-NS: ultramicroscopic and molecular autopsy. Mol. Gen. Genet. 231:201-211. [DOI] [PubMed] [Google Scholar]
- 49.Stray, J. E., and J. E. Lindsley. 2003. Biochemical analysis of the yeast condensin Smc2/4 complex: an ATPase that promotes knotting of circular DNA. J. Biol. Chem. 278:26238-26248. [DOI] [PubMed] [Google Scholar]
- 50.Strunnikov, A. V., and R. Jessberger. 1999. Structural maintenance of chromosomes (SMC) proteins: conserved molecular properties for multiple biological functions. Eur. J. Biochem. 263:6-13. [DOI] [PubMed] [Google Scholar]
- 51.Sunako, Y., T. Onogi, and S. Hiraga. 2001. Sister chromosome cohesion of Escherichia coli. Mol. Microbiol. 42:1233-1241. [DOI] [PubMed] [Google Scholar]
- 52.Swedlow, J. R., and T. Hirano. 2003. The making of the mitotic chromosome: modern insights into classical questions. Mol. Cell 11:557-569. [DOI] [PubMed] [Google Scholar]
- 53.Teleman, A. A., P. L. Graumann, D. C. H. Lin, A. D. Grossman, and R. Losick. 1998. Chromosome arrangement within a bacterium. Curr. Biol. 8:1102-1109. [DOI] [PubMed] [Google Scholar]
- 54.Uhlmann, F., F. Lottspeich, and K. Nasmyth. 1999. Sister-chromatid separation at anaphase onset is promoted by cleavage of the cohesin subunit Scc1. Nature 400:37-42. [DOI] [PubMed] [Google Scholar]
- 55.Uhlmann, F., D. Wernic, M. A. Poupart, E. V. Koonin, and K. Nasmyth. 2000. Cleavage of cohesin by the CD clan protease separin triggers anaphase in yeast. Cell 103:375-386. [DOI] [PubMed] [Google Scholar]
- 56.van Helvoort, J. M., J. Kool, and C. L. Woldringh. 1996. Chloramphenicol causes fusion of separated nucleoids in Escherichia coli K-12 cells and filaments. J. Bacteriol. 178:4289-4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Viollier, P. H., M. Thanbichler, P. T. McGrath, L. West, M. Meewan, H. H. McAdams, and L. Shapiro. 2004. From the cover: rapid and sequential movement of individual chromosomal loci to specific subcellular locations during bacterial DNA replication. Proc. Natl. Acad. Sci. USA 101:9257-9262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Volkov, A., J. Mascarenhas, C. Andrei-Selmer, H. D. Ulrich, and P. L. Graumann. 2003. A prokaryotic condensin/cohesin-like complex can actively compact chromosomes from a single position on the nucleoid and binds to DNA as a ring-like structure. Mol. Cell. Biol. 23:5638-5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weitao, T., S. Dasgupta, and K. Nordstrom. 2000. Role of the mukB gene in chromosome and plasmid partition in Escherichia coli. Mol. Microbiol. 38:392-400. [DOI] [PubMed] [Google Scholar]
- 60.Weitao, T., K. Nordstrom, and S. Dasgupta. 1999. Mutual suppression of mukB and seqA phenotypes might arise from their opposing influences on the Escherichia coli nucleoid structure. Mol. Microbiol. 34:157-168. [DOI] [PubMed] [Google Scholar]
- 61.Weitzer, S., C. Lehane, and F. Uhlmann. 2003. A model for ATP hydrolysis-dependent binding of cohesin to DNA. Curr. Biol. 13:1930-1940. [DOI] [PubMed] [Google Scholar]
- 62.Winans, S. C., S. J. Elledge, J. H. Krueger, and G. C. Walker. 1985. Site-directed insertion and deletion mutagenesis with cloned fragments in Escherichia coli. J. Bacteriol. 161:1219-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Woldringh, C. L., and T. Odijk. 1999. Structure of DNA within the bacterial cell: physics and physiology, p. 171-187. In R. L. Charlebois (ed.), Organization of the prokaryotic genome. ASM Press, Washington, D.C.
- 64.Yamanaka, K., T. Ogura, H. Niki, and S. Hiraga. 1995. Characterization of the smtA gene encoding an S-adenosylmethionine-dependent methyltransferase of Escherichia coli. FEMS Microbiol. Lett. 133:59-63. [DOI] [PubMed] [Google Scholar]
- 65.Yamanaka, K., T. Ogura, H. Niki, and S. Hiraga. 1996. Identification of two new genes, mukE and mukF, involved in chromosome partitioning in Escherichia coli. Mol. Gen. Genet. 250:241-251. [DOI] [PubMed] [Google Scholar]
- 66.Yamazoe, M., T. Onogi, Y. Sunako, H. Niki, K. Yamanaka, T. Ichimura, and S. Hiraga. 1999. Complex formation of MukB, MukE and MukF proteins involved in chromosome partitioning in Escherichia coli. EMBO J. 18:5873-5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.