Abstract
Enterococcus faecium 664.1H1 is multiply antibiotic resistant and mercury resistant. In this study, the genetic support for the tetracycline resistance of E. faecium 664.1H1 was characterized. The tet(S) gene is responsible for tetracycline resistance, and this gene is located on the chromosome of E. faecium 664.1H1, on a novel conjugative transposon. The element is transferable to Enterococcus faecalis, where it integrates into a specific site. The element was designated EfcTn1. The integrase of EfcTn1 is related to the integrase proteins found on staphylococcal pathogenicity islands. We show that the transposon is flanked by an 18-bp direct repeat, a copy of which is also present at the target site and at the joint of a circular form, and we propose a mechanism of insertion and excision.
Antibiotic-resistant enterococcal species have become an increasingly common nosocomial threat in recent years. Among the most common enterococcal infections are those of the urinary tract, wounds, bloodstream, and endocardium (23). Enterococcus faecalis accounts for more than 70% of infections, and Enterococcus faecium is responsible for the majority of the remainder (13). However, infections due to E. faecium are on the increase. The emergence of E. faecalis and E. faecium as major nosocomial pathogens mimics, but is distinct from, the increased reports of antibiotic resistance in these species (16).
Tetracycline resistance in enterococcal strains has been identified in organisms originating from humans (1, 6), animals (1, 2), and foods (9). Most tetracycline resistance is mediated by tet(M), which is usually found on conjugative transposons of the Tn916 family (20). In E. faecalis, the tet(S) gene has also been shown to be responsible for tetracycline resistance (4). This gene was originally discovered in Listeria monocytogenes strain BM4210 (3) on the self-transferable plasmid pIP811. It has also been found on plasmid pK214 in Lactococcus lactis (19). The E. faecalis tet(S) resistance determinant has been shown to transfer from chromosome to chromosome (4). In L. lactis and Listeria monocytogenes, tet(S) is closely linked to homologues of orf6, orf9, orf10, and orf7 from the conjugative transposon Tn916 (8, 19). Additionally, a transferable Tn916-like conjugative transposon containing tet(S) in place of tet(M) has recently been found (15).
This work shows that tet(S) is responsible for the transferable tetracycline resistance in E. faecium 664.1H1 and that it is contained within a novel conjugative transposon. Furthermore, a mechanism for insertion and excision of this element is proposed.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
All strains used during this study are shown in Table 1. E. faecalis and E. faecium were grown in or on brain heart infusion medium at 37°C. Escherichia coli strains were grown at 37°C on or in LB medium. Antibiotics were used at concentrations of 12.5 μg ml−1 (chloramphenicol), 10 μg ml−1 (tetracycline), 25 μg ml−1 (rifampin), and 50 μg ml−1 (ampicillin). Filter-mating experiments were carried out as previously described (21). Transconjugant E. faecalis cells were selected on brain heart infusion agar containing tetracycline and rifampin. Partial sequencing of the 16S rRNA gene was carried out with putative transconjugants to confirm their identity and to rule out contamination or spontaneous mutation of the donor.
TABLE 1.
Bacterial strains used in this study
| Strain | Propertiesa | Reference or source |
|---|---|---|
| E. faecium 664.1H1 | Merr Smr Tcr; isolated from primate study | 24; gift from A. O. Summers |
| E. faecalis JH2-2 | Rifr; recipient for filter-mating studies | 14 |
| E. coli DH5α MCR | Used for general cloning | Gibco-BRL |
| E. coli TransforMax EPI300 | Used for BAC cloning | Epicentre |
| E. coli H12 | TransforMax EPI300 containing 50-kb BAC clone H12 [tet(S) positive] | This work |
| Filter-mating studies | ||
| E. faecalis T1A | E. faecium 664.1H1 × E. faecalis JH2-2 Tcr Rifr | This work |
| E. faecalis T2A | E. faecium 664.1H1 × E. faecalis JH2-2 Tcr Rifr | This work |
| E. faecalis T3A | E. faecium 664.1H1 × E. faecalis JH2-2 Tcr Rifr | This work |
| E. faecalis T4A | E. faecium 664.1H1 × E. faecalis JH2-2 Tcr Rifr | This work |
| E. faecalis Ttet38 | E. faecium 664.1H1 × E. faecalis JH2-2 Tcr Rifr | This work |
Merr, mercury resistant; Smr, streptomycin resistant; Tcr, tetracycline resistant; Rifr, rifampin resistant.
Molecular cloning techniques.
Genomic DNA preparations were carried out with the Yeast and Gram Positive Bacteria genomic DNA isolation kit (Gentra, Minneapolis, Minn., supplied through Flowgen) according to the manufacturer's instructions. E. coli plasmid DNA was isolated with the QIAGEN Miniprep kit (QIAGEN, Basingstoke, United Kingdom) according to the manufacturer's instructions. Plasmid isolation from Enterococcus spp. was carried out with the QIAprep Midiprep kit (QIAGEN) according to the manufacturer's instructions, with the following changes. A 10-ml starter culture was inoculated and incubated at 37°C for 8 h with shaking at 200 rpm; 300 μl of this starter culture was used to inoculate 150 ml of prewarmed broth and was incubated for 16 h under the above conditions. The cells were centrifuged at 1,500 × g at 4°C for 10 min and resuspended in 12 ml of P1 buffer (supplied with the QIAGEN kit). Then, 600 μl of mutanolysin (10,000 U ml−1) (Sigma, Poole, United Kingdom) and 600 μl of lysozyme (10 mg ml−1) (Sigma) were added and incubated for 1 h at 37°C. The manufacturer's instructions were followed from this point forward.
Southern blotting and colony hybridization were carried out with ECL kits (Amersham, Little Chalfont, United Kingdom). All restriction enzymes and other molecular biology enzymes were obtained from Promega (Southampton, United Kingdom).
PCR assays for tetracycline resistance genes were carried out as described previously (17). All primers were purchased from Sigma-Genosys (Heverhill, United Kingdom) and are shown in Table 2.
TABLE 2.
Primers used in this study
| Primer | Sequence 5′→3′ | Amplicona |
|---|---|---|
| GWEF1 | AACTTAAACTGATACCTGTGTGGTCAG | sspPCR of BAC H12; excisionase- and integrase-containing fragment |
| GWEF2 | GATACCTTGTTTCATAAGGGATTAGCC | sspPCR of BAC H12 |
| GWEF3 | TATGGAAGGAGGAAAAACGA | sspPCR of BAC H12 |
| GWEF4 | CCATTCCGTATTGTCAGAAAC | sspPCR of BAC H12 |
| GWEF5 | GCATATGACTGTTCATGG | sspPCR of BAC H12; right genome-Tn junction, CI joint |
| GWEF8 | CCCATTCTACACTGGTCGTC | Right genome-Tn junction; target site-, excisionase-, and integrase-containing fragment |
| GWEF7 | GTTCGATTAAAGACTACCAG | Left genome-Tn junction; CI joint |
| GWEF9 | ATACGGTATACGGACTAGCTATAC | Left genome-Tn junction; target site |
CI, circular intermediate; Tn, transposon.
PCR products were cleaned with the QIAGEN PCR clean-up kit and cloned into the pGEM-T Easy PCR cloning vector (Promega). The recombinant plasmid was used to transform competent E. coli cells as described previously (5). Transformants were selected on ampicillin-containing LB agar plates. DNA sequencing was carried out with the Big Dye Terminator Ready Reaction Mix, version 3.1 (Applied Biosystems, Foster City, Calif.), and analyzed on an ABI 310 Genetic Analyzer (Applied Biosystems) or was carried out by Lark Technologies Inc. (Takeley, United Kingdom).
Construction of the BAC library.
A bacterial artificial chromosome (BAC) library was prepared by ligating E. faecuim DNA, partially digested with HindIII, into the predigested vector pCC1BAC (Epicentre, Madison, Wis.). The ligation was used in an electroporation with E. coli TransforMax EPI300 cells according to the manufacturer's instructions. Colony hybridizations were carried out with the ECL hybridization system according to the manufacturer's instructions.
sspPCR.
Single-specific-primer PCR (sspPCR) was carried out in order to facilitate genome walking along the length of the element in the donor and to compare donor, recipient, and transconjugants in order to delineate the ends of the element. Genomic DNA and pUC18 were digested with a restriction endonuclease (either BamHI, HindIII, HincII, AatI, or EcoRI). The digested pUC18 was dephosphorylated with calf intestine alkaline phosphatase. Both the pUC18 and genomic restriction digests were cleaned by being passed through a QIAGEN Miniprep column. Ligations were carried out overnight with T4 DNA ligase, and 5 μl of the ligation mixture was used as a template in PCR with a specific primer (designed from the donor DNA sequence) and either M13 forward or M13 reverse primer (situated in pUC18). Products were either gel or PCR purified with the relevant QIAGEN kit and sequenced as described above.
Bioinformatics.
In silico analyses of the sequence data were carried out with Chromas, version 1.45 (http://www.technelysium.com.au/chromas.html), DNAMAN, version 5.2.2 (Lynnon Biosoft, Quebec, Canada), and various sequence manipulation tools accessed through Bioinformatics.Net (http://bioinformatics.vg/). Sequence database searches were carried out with NCBI tools (http://www.ncbi.nlm.nih.gov/) and the E. faecium genome project website (http://www.hgsc.bcm.tmc.edu/projects/microbial/Efaecium/). Multiple alignments were carried out with the ClustalW service of the European Bioinformatics Institute (http://www.ebi.ac.uk/clustalw).
Nucleotide sequence accession numbers.
The following sequences have been deposited in the GenBank database: tet(S), DQ295784; EfcTn1 left end, DQ370177; EfcTn1 excisionase, integrase, and right end; DQ370176.
RESULTS AND DISCUSSION
The tetracycline resistance gene tet(S) is present on the chromosome of E. faecium 664.1H1.
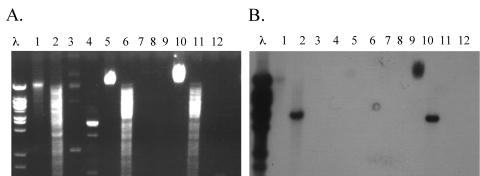
PCR specific for tet(K), tet(L), tet(M), tet(O), and tet(S) was carried out on the genomic DNA of E. faecium 664.1H1 with primers described previously (17). Only the PCR for tet(S) produced a product of the expected size; this product was sequenced and shown to be identical to the corresponding portion of the tet(S) gene from L. monocytogenes (accession number L09756) (3). PCR primers were designed to amplify the entire gene. The sequence of this PCR product (accession number DQ295784) revealed a tet(S) gene identical to the tet(S) gene from L. monocytogenes, 99% similar to tet(S) from L. lactis plasmid pK214 (accession number X92946) (19), and 98% similar to tet(S) from the recently discovered Tn916S element (accession number AY534326) (15). The PCR product of the internal fragment of the tet(S) gene from E. faecium 664.1H1 was used as a probe in Southern hybridization of E. faecium 664.1H1 genomic and plasmid DNA restricted with HindIII. Hybridization to the chromosomal DNA was observed. No hybridization to the plasmid DNA in the donor was observed (Fig. 1), inferring a chromosomal location for the tet(S) gene in this strain.
FIG. 1.
Southern hybridization of donor, recipient, and transconjugant DNA to a probe derived from the tet(S) gene present in the donor. Panel A shows the agarose gel of the DNA, and panel B shows the blotting performed on this gel. λ, lambda HindIII ladder; lanes 1 to 4, E. faecium 664.1H1; lanes 5 to 8, E. faecalis JH2-2; lanes 9 to 12, E. faecalis T38. Lanes 1, 5, and 9, undigested genomic DNA; lanes 2, 6, and 10, HindIII-digested genomic DNA; lanes 3, 7, and 11, undigested plasmid DNA; lanes 4, 8, and 12, HindIII-digested plasmid DNA. Plasmid DNA could be isolated only from the original donor strain, and no hybridization to this DNA was detected.
The tetracycline resistance of E. faecium 664.1H1 is transferable.
Transfer of the tet(S) determinant from E. faecium 664.1H1 to E. faecalis JH2-2 was detected at 1 × 10−9 transconjugants per recipient. No spontaneous mutations of the donor to rifampin resistance were detected on the control plates. PCR of the tet(S) gene confirmed that it had transferred (data not shown). A Southern blot of genomic DNA restricted with HindIII inferred that the element is located on the chromosome of the E. faecalis transconjugant (Fig. 1). It is present on a fragment of a different size than in the E. faecium donor, and plasmid DNA could not be isolated from any of the transconjugants. This element was designated EfcTn1 (for “Enterococcus faecium conjugative transposon 1”). A total of five independently derived transconjugants (from separate filters) were chosen for further study (Table 1).
Characterization of the ends of the element.
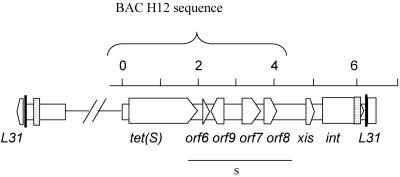
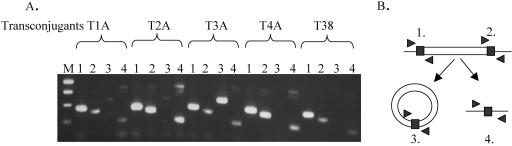
A BAC library was constructed from E. faecium 664.1H1 DNA. The library was screened with the tet(S) probe. A clone that contained an approximately 50-kb insert was isolated. The insert from this clone was partially sequenced on a single strand with primers reading out from the tet(S) gene. Additionally, end sequencing of the clone was carried out. This single-strand sequencing data showed that the ends of the transposon were not likely to be contained within this clone; however, the data did show that downstream of tet(S) were homologues of orf6, orf9, orf10, orf7, and orf8 in the same arrangement as in Tn916. The BAC insert terminated following the orf8 homologue (Fig. 2). Therefore, sspPCR was carried out with primers (Table 2) designed from the right end of the sequence of the BAC clone (H12) (Table 1) on donor and transconjugant genomic DNA. Sequential sspPCRs of the genomic DNA of the original donor and the transconjugants allowed us to extend the single-strand sequence data obtained from the BAC H12 clone into and beyond an excisionase and an integrase gene in both the 664.1H1 donor and one of the transconjugants (T1A). The sequence data suggested that EfcTn1 had inserted into the ribosomal gene L31. Additionally and fortuitously, one of the sspPCR products resulting from the original donor contained sequence that was different from the L31 gene. Due to similarity with the right-end sequence and the presence of a 57-bp repeat region, this amplicon was thought to contain the joint of a circular form of EfcTn1. This allowed us to determine the right end of the element and the right half of the target sequence (in the ribosomal gene L31) in the transconjugant. Subsequent sspPCRs carried out on the recipient DNA with a primer designed from the region downstream of the integrase and reading back across the target site allowed the target site sequence in the E. faecalis recipient to be determined. Further sspPCR of transconjugant DNA with a primer designed from the flanking region of the left side of the target site and reading into the left side of the element allowed the sequence of the left-hand transposon-genome junction to be determined in this transconjugant. The sequence of the left end was the same as the divergent sequence in the previously mentioned sspPCR product from the donor, showing that we had amplified the joint of a circular form in this strain. PCR primers were subsequently designed to amplify the joint of the circular form of EfcTn1 (Fig. 3). PCRs to amplify both the left and right transposon-genome junctions, the empty target site, and the joint of the circular form of the element were carried out on DNA from all five transconjugants. Products of the same size were obtained in each transconjugant (Fig. 3). Additionally, only the empty target site could be amplified in E. faecalis JH2-2, the recipient (data not shown), indicating that the element inserts into the same target site in each transconjugant.
FIG. 2.
Schematic overview of the ends of EfcTn1. The area underlined by the line labeled “s” is single-stranded sequence from the BAC H12 clone. The region cloned within BAC H12 is indicated above the schematic. All other sequence has been determined on both strands. The thick vertical lines represent the 18-bp direct repeat region, and the striped boxes represent the position of the 57-bp direct repeat. The unfilled arrows represent the predicted orfs; they point in the probable direction of transcription. The scale above is in kilobases.
FIG. 3.
Site specificity of insertion in E. faecalis JH2-2. (A) Lanes: M, 100-bp benchtop ladder; 1, PCR of the left junction; 2, PCR of the right junction; 3, PCR of the circular-form junction; 4, PCR of the empty target site. (B) Schematic showing the positions of the primers for each PCR. The unfilled box represents the transposon. The filled box represents the 18-bp repeat sequence.
The putative excisionase and integrase genes are situated at the right end of EfcTn1 (GenBank accession number DQ370176). The integrase gene ends 51 bp before the end of the terminal 18-bp repeat sequence (Fig. 2). The amino acid sequence of the integrase shows homology to a group of tyrosine integrases that are found in a variety of mobile elements (pathogenicity islands, phage, transposons, and plasmids) isolated from many different bacteria. It is most closely related to those found in a group of related Staphylococcus aureus pathogenicity islands, namely, Int (42% identical and 62% similar) from SaPIbov (accession number AAG29618) and Sip (41% identical and 63% similar) from SaPIbov2 (accession numbers AAP51267 and AAP55251) (7, 25). The protein sequence contains the conserved catalytic pentad R-K-H-R-H (highlighted in Fig. 4) and the conserved tyrosine residue that is essential for function of this type of integrase (11, 18). It is predicted that Tyr-357 within Sip from SaPIbov2 is the catalytic residue involved in the nucleophilic attack of the phosphate group on each DNA target site (25); this corresponds to Tyr-360 in Int from EfcTn1. This protein is also extended at the C-terminal domain by 29 amino acids compared to the other integrases in this group (Fig. 4). The amino acid sequence of the putative excisionase shows similarity to a Fis-like Xis protein (27% identical and 50% similar) involved in DNA binding and unwinding in phage genomes. Additionally, it is related to Orf18 (20% identical and 50% similar) from the staphylococcal pathogenicity island SaPIbov. However, it is present in the opposite orientation: it appears to be transcribed in the same direction as the EfcTn1 integrase in EfcTn1, but orf18 is transcribed in the opposite orientation to its cognate integrase in SaPIbov (7). The S. aureus pathogenicity islands are flanked by direct repeats of various sizes, 74 bp for SaPIbov (7) and 149 bp for SaPIbov2 (25). EfcTn1 is flanked by the directly repeated 18-bp sequence discussed below (Fig. 5). There is also another imperfect (53 of 57 bp identical) direct repeat of 57 bp located at each end of the transposon. This direct repeat is situated 5 bp from the terminus of the right end of EfcTn1 (accession number DQ370176) and 174 bp from the terminus of the left end of EfcTn1 (accession number DQ370177).
FIG. 4.
Alignment of EfcTn1 with the integrase from the S. aureus pathogenicity islands SaPIbov and SaPIbov2. The labels of the amino acid sequence include the respective pathogenicity islands and strains of S. aureus. The conserved catalytic pentad R-K-H-R-H and the conserved tyrosine residue are shaded in gray (11, 18). *, identical amino acids; “:”, conserved amino acid substitutions; ., semiconserved amino acid substitutions.
FIG. 5.
Sequences of the ends of EfTn1 and the target site. The ends of EfcTn1 are shown in bold and are underlined, the 18-bp repeat is shown in bold, and the chromosomal sequence is in plain text.
Proposed mechanism of transfer.
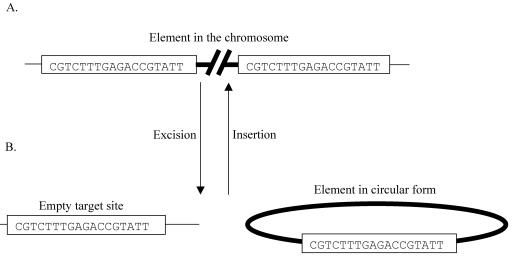
The sequence data show that the element is delineated by an 18-bp direct repeat, one copy each of which is also situated at the joint of the circular form and at the genomic target site (Fig. 5). The target site in both E. faecium 664.1H1 and E. faecalis JH2-2 is located within the gene encoding the ribosomal protein L31 (Cog EF1171; GenBank accession number AE016950). Interestingly, the target site for the staphylococcal SaPIbov2 is also an 18-bp sequence that is directly repeated on either side of the integrated element and is present at the joint of a circular form of a mini-SaPIbov2 element (25). Based on the sequence data for the ends of the element (Fig. 5) and on comparison to the staphylococcal integrases, the following model for insertion and excision of EfcTn1 is proposed (Fig. 6). EfcTn1 Int mediates the excision of EfcTn1 by introducing staggered cuts, presumably within the 18-bp direct repeats flanking the element. Previous biochemical evidence for a variety of tyrosine integrase proteins strongly suggests that the staggered cleavage of the DNA will occur on either side of a 6- to 8-bp spacer region (10). However, we have no biochemical evidence to support this at this time. By comparison to other tyrosine integrases, such as IntTn from Tn916, it is likely that this cleavage will result in 5′ hydroxyl protruding ends (22). Strand exchange and recombination occurs, resulting in excision of the transposon as a circular molecule and regeneration of the original target site, with one copy of the 18-bp repeat present at each site (the joint of the circular form and the chromosomal target site). The circular form then transfers to a new host cell, probably as a single strand. The single-stranded circular molecules are then repaired by the host's DNA replication machinery, where they can transpose into the genome, providing that the 18-bp target site is present. Integration occurs when Int recognizes the target site (18-bp sequence within L31) and promotes site-specific recombination with the joint of the circular form. Strand exchange and ligation result in the insertion of EfcTn1, flanked by directly repeated 18-bp sequences (Fig. 6). Additionally, by comparison with other Xis proteins, such as the one from lambda, it is predicted that Xis will be required for the excisive site-specific recombination event (26) and may, as is the case with the Tn916 XisTn, lead to some directional control of the excision insertion reaction (12). The mechanism of insertion and excision described here is essentially identical to that previously described for other members of the tyrosine integrase family (11).
FIG. 6.
Model of insertion and excision of EfcTn1 from the chromosome. The chromosome of the host is represented as a thin line, and EfcTn1 is represented as a thick line. (A) The element in the chromosome with one copy of the 18-bp direct repeat at each end of the element. (B) After excision, the joint of the circular form of the element and the target site in the chromosome each contain a single copy of the 18-bp sequence. Insertion is thought to be the reverse of this process.
In conclusion, we have shown tet(S) is responsible for tetracycline resistance in E. faecium 664.1H1 and that this gene is located on a novel mobile element integrated into the chromosome within the ribosomal L31 gene. Additionally, it can transfer to E. faecalis JH2-2, where it also integrates into the ribosomal L31 gene. The element has some homology with Tn916; however, the excisionase and integrase are more closely related to those found on the S. aureus pathogenicity islands SaPIbov and SaPIbov2.
Acknowledgments
This work was supported by a grant from the BBSRC (D15925) and a Proctor and Gamble Oral & Dental Research Trust Award (A.P.R.). L.S. is funded by the European Union as part of the ARTRADI project (QLK2-CT2002-00843).
Thanks go to A. O. Summers (Athens, Ga.) for the gift of E. faecium 664.1H1 and to two anonymous reviewers for insightful suggestions.
REFERENCES
- 1.Aarestrup, F. M., Y. Agerso, P. Gerner-Smidt, M. Madsen, and L. B. Jensen. 2000. Comparison of antimicrobial resistance phenotypes and resistance genes in Enterococcus faecalis and Enterococcus faecium from humans in the community, broilers, and pigs in Denmark. Diagn. Microbiol. Infect. Dis. 37:127-137. [DOI] [PubMed] [Google Scholar]
- 2.Butaye, P., L. A. Devriese, and F. Haesebrouck. 2001. Differences in antibiotic resistance patterns of Enterococcus faecalis and Enterococcus faecium strains isolated from farm and pet animals. Antimicrob. Agents Chemother. 45:1374-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Charpentier, E., G. Gerbaud, and P. Courvalin. 1993. Characterization of a new class of tetracycline-resistance gene tet(S) in Listeria monocytogenes BM4210. Gene 131:27-34. [DOI] [PubMed] [Google Scholar]
- 4.Charpentier, E., G. Gerbaud, and P. Courvalin. 1994. Presence of the Listeria tetracycline resistance gene tet(S) in Enterococcus faecalis. Antimicrob. Agents Chemother. 38:2330-2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chung, C. T., S. L. Niemela, and R. H. Miller. 1989. One-step preparation of competent Escherichia coli: transformation and storage of bacterial cells in the same solution. Proc. Natl. Acad. Sci. USA 86:2172-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dahlen, G., W. Samuelsson, A. Molander, and C. Reit. 2000. Identification and antimicrobial susceptibility of enterococci isolated from the root canal. Oral Microbiol. Immunol. 15:309-312. [DOI] [PubMed] [Google Scholar]
- 7.Fitzgerald, J. R., S. R. Monday, T. J. Foster, G. A. Bohach, P. J. Hartigan, W. J. Meaney, and C. J. Smyth. 2001. Characterization of a putative pathogenicity island from bovine Staphylococcus aureus encoding multiple superantigens. J. Bacteriol. 183:63-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flannagan, S. E., L. A. Zitzow, Y. A. Su, and D. B. Clewell. 1994. Nucleotide sequence of the 18-kb conjugative transposon Tn916 from Enterococcus faecalis. Plasmid 32:350-354. [DOI] [PubMed] [Google Scholar]
- 9.Giraffa, G. 2002. Enterococci from foods. FEMS Microbiol. Rev. 26:163-171. [DOI] [PubMed] [Google Scholar]
- 10.Gopaul, D. N., and G. D. van Duyne. 1999. Structure and mechanism in site-specific recombination. Curr. Opin. Struct. Biol. 9:14-20. [DOI] [PubMed] [Google Scholar]
- 11.Guo, F., D. N. Gopaul, and G. D. van Duyne. 1997. Structure of Cre recombinase complexed with DNA in a site-specific recombination synapse. Nature 389:40-46. [DOI] [PubMed] [Google Scholar]
- 12.Hinerfeld, D., and G. Churchward. 2001. Xis protein of the conjugative transposon Tn916 plays dual opposing roles in transposon excision. Mol. Microbiol. 41:1459-1467. [DOI] [PubMed] [Google Scholar]
- 13.Huycke, M. M., D. F. Sahm, and M. S. Gilmore. 1998. Multiple-drug resistant enterococci: the nature of the problem and an agenda for the future. Emerg. Infect. Dis. 4:239-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacob, A. E., and S. J. Hobbs. 1974. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J. Bacteriol. 117:360-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lancaster, H., A. P. Roberts, R. Bedi, M. Wilson, and P. Mullany. 2004. Characterization of Tn916S, a Tn916-like element containing the tetracycline resistance determinant tet(S). J. Bacteriol. 186:4395-4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mundy, L. M., D. F. Sahm, and M. Gilmore. 2000. Relationships between enterococcal virulence and antimicrobial resistance. Clin. Microbiol. Rev. 13:513-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ng, L. K., I. Martin, M. Alfa, and M. Mulvey. 2001. Multiplex PCR for the detection of tetracycline resistant genes. Mol. Cell. Probes 15:209-215. [DOI] [PubMed] [Google Scholar]
- 18.Nunes-Düby, S. E., H. J. Kwon, R. S. Tirumalai, T. Ellenberger, and A. Landy. 1998. Similarities and differences among 105 members of the Int family of site-specific recombinases. Nucleic Acids Res. 26:391-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perreten, V., F. Schwarz, L. Cresta, M. Boeglin, G. Dasen, and M. Teuber. 1997. Antibiotic resistance spread in food. Nature 389:801-802. [DOI] [PubMed] [Google Scholar]
- 20.Rice, L. B. 1998. Tn916 family conjugative transposons and dissemination of antimicrobial resistance determinants. Antimicrob. Agents Chemother. 42:1871-1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roberts, A. P., P. Mullany, and M. Wilson. 2001. Gene transfer in bacterial biofilms. Methods Enzymol. 336:60-65. [DOI] [PubMed] [Google Scholar]
- 22.Rudy, C., K. L. Taylor, D. Hinerfeld, J. R. Scott, and G. Churchward. 1997. Excision of a conjugative transposon in vitro by the Int and Xis proteins of Tn916. Nucleic Acids Res. 25:4061-4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shepard, B. D., and M. S. Gilmore. 2002. Antibiotic-resistant enterococci: the mechanisms and dynamics of drug introduction and resistance. Microbes Infect. 4:215-224. [DOI] [PubMed] [Google Scholar]
- 24.Summers, A. O., J. Wireman, M. J. Vimy, F. L. Lorscheider, B. Marshall, S. B. Levy, S. Bennett, and L. Billard. 1993. Mercury released from dental “silver” fillings provokes an increase in mercury- and antibiotic-resistant bacteria in oral and intestinal floras of primates. Antimicrob. Agents Chemother. 37:825-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ubeda, C., M. A. Tormo, C. Cucarella, P. Trotonda, T. J. Foster, I. Lasa, and J. R. Penades. 2003. Sip, an integrase protein with excision, circularization and integration activities, defines a new family of mobile Staphylococcus aureus pathogenicity islands. Mol. Microbiol. 49:193-210. [DOI] [PubMed] [Google Scholar]
- 26.Wu, Z., R. I. Gumport, and J. F. Gardner. 1998. Defining the structural and functional roles of the carboxyl region of the bacteriophage lambda excisionase (Xis) protein. J. Mol. Biol. 281:651-661. [DOI] [PubMed] [Google Scholar]