Abstract
The Borrelia burgdorferi genome exhibits redundancy, with many plasmid-carried genes belonging to paralogous gene families. It has been suggested that certain paralogs may be necessary in various environments and that they are differentially expressed in response to different conditions. The chromosomally located p13 gene which codes for a channel-forming protein belongs to paralog family 48, which consists of eight additional genes. Of the paralogous genes from family 48, the BBA01 gene has the highest homology to p13. Herein, we have inactivated the BBA01 gene in B. burgdorferi strain B31-A. This mutant shows no apparent phenotypic difference compared to the wild type. However, analysis of BBA01 in a C-terminal protease A (CtpA)-deficient background revealed that like P13, BBA01 is posttranslationally processed at its C terminus. Elevated BBA01 expression was obtained in strains with the BBA01 gene introduced on the shuttle vector compared to the wild-type strain. We could further demonstrate that BBA01 is a channel-forming protein with properties surprisingly similar to those of P13. The single-channel conductance, of about 3.5 nS, formed by BBA01 is comparable to that of P13, which together with the high degree of sequence similarity suggests that the two proteins may have similar and interchangeable functions. This is further strengthened by the up-regulation of the BBA01 protein and its possible localization in the outer membrane in a p13 knockout strain, thus suggesting that P13 can be replaced by BBA01.
Borrelia burgdorferi is the etiologic agent of Lyme borreliosis, an infection transmitted by Ixodes ticks (18, 60). Borrelia spirochetes are host-propagated bacteria that cycle between vertebrate and arthropod hosts and have therefore developed advanced strategies to sense and survive in these environments (57, 63). The unique genome organization of B. burgdorferi consists of a linear chromosome of ∼910 kb and 21 plasmids (12 linear and 9 circular) comprising ∼611 kb (21, 26). Many genes responsible for adaptation of Borrelia to different environments (63) as well as for infectivity are located on plasmids (29, 47, 61). It is also well documented that in vitro passage is associated with loss of various plasmids (7, 31). The B. burgdorferi genome exhibits extensive genetic redundancy, with 70% of plasmid-encoded genes belonging to paralogous gene families (21, 26). The biological advantage of maintaining so many paralogs, an energetically expensive process, is unclear. Several studies have implied that specific paralogs may be necessary in different environments and that they are differentially expressed in response to environmental signals (43, 48, 63). Conditions such as temperature shift, serum deprivation, tick feeding, and the mammalian environment influence the regulation of several paralogous gene family members, including mlp, vlsE, bdr, and family 54 genes (16, 20, 49, 63, 65, 66).
Regulation of porin expression in response to different environmental signals is well understood in Escherichia coli, particularly for OmpF and OmpC (33, 37, 39). In B. burgdorferi, previous studies (16, 63) have suggested that some mammalian factors present in the incoming blood during tick feeding may drive the repression of the Oms28 porin (58). Without these factors, temperature alone seems to increase its transcription (16, 43, 48). It has been suggested that Oms28 porin is preferentially expressed in unfed ticks and may play a role in the acquisition of small molecules while Borrelia is in the nutrient-poor midgut environment (63).
Outer membrane proteins fulfill a number of tasks that are crucial for bacterial cells, such as solute and protein transport, signal transduction, and interaction with other cells (1). Substances can be taken into bacterial cells by diffusion through the outer membrane via porins (9) or by receptor-mediated uptake (38). Porin proteins often have a β-barrel structure and are located in the outer membrane, where they, depending on their physicochemical properties, form trimers and allow the diffusion of molecules across the lipid bilayer membranes (9, 30). Variation in their structures confers specificity, with some porins functioning as general diffusion pores while others act as substrate-specific channels (1, 9).
Porin function and regulation are poorly understood in Borrelia spirochetes. Only three porins have been characterized so far (45, 58, 59), and the implication exists that many more uncharacterized channel-forming activities are present in B. burgdorferi (45). One well-studied porin gene, p13 (40, 41, 45, 46), is a chromosomally carried member of gene family 48, which consists of eight other plasmid-encoded paralogs. BBA01, the closest paralog, shares 40.9% identity and 54.1% similarity to P13 on the amino acid level. The corresponding proteins, P13 and BBA01, are also similar in apparent molecular mass (13.9 and 13.5 kDa, respectively) and also in predicted trans-membrane-spanning domain pattern (41), suggesting they are structurally alike. Recently, we demonstrated that BBA01 is overexpressed in a p13 knockout mutant (46), which led us to hypothesize that these paralogs have compensatory function.
To test this possibility, we analyzed the channel-forming capability of BBA01 as well as its cellular expression and localization in relation to P13. In this article, we show that BBA01 is remarkably similar to its closest paralog. Like P13, BBA01 is a channel-forming protein which is posttranslationally cleaved by C-terminal processing protease A (CtpA). Further, both proteins form channels exhibiting similar conductance, indicating that they potentially allow passage of the same solutes. To investigate the relationship between the BBA01 and p13 genes, we created mutants in various B. burgdorferi strain backgrounds for analysis of protein expression and localization. Interestingly, BBA01 seems to be present but not preferentially found in the outer membrane protein preparation, except when P13 is absent. These data together with the previously published finding that BBA01 is up-regulated in a p13 knockout mutant (46) support the idea that BBA01 may act as a substitute for P13 in the latter's absence.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The B. burgdorferi strains examined in this study are described in Table 1. The strains were grown at 35°C in Barbour-Stoenner-Kelly (BSK) H medium (Sigma, St. Louis, Mo.), with 6% rabbit serum. Single colonies were isolated from solid medium (P-BSK) as described elsewhere (51).
TABLE 1.
Description of Borrelia strains used in this study
| Strain | Description | Antibiotic resistance | Reference |
|---|---|---|---|
| B31-A | Noninfectious, high-passage clone of B31 | None | 15 |
| B313-D | Transformable clone of B313, a mutant lacking major Ospsa | None | 53 and present study |
| B313-C | Nontransformable clone of B313, a mutant lacking major Ospsa | None | 53 and present study |
| Δp13 | P13-18, a p13 knockout, PflaB-kan insertion in p13 | Kanr | 45 |
| ΔctpA | C-terminal protease A (ctpA) knockout, PflaB-kan insertion in ctpA | Kanr | 44 |
| Δbba01 | BBA01 knockout in B31-A, PflaB-kan insertion in BBA01 gene | Kanr | Present study |
| Δbba01c | Complementation of Δbba01 with pBSV2G+bba01 | Kanr Gmr | Present study |
| B313-D+bba01 | Introduction of pBSV2G+bba01 in B313-D | Gmr | Present study |
| Δp13+bba01 | Introduction of pBSV2G+bba01 in Δp13 | Kanr Gmr | Present study |
Outer surface proteins, OspA, OspB, OspC, OspD, and decorin-binding proteins, DbpA, DbpB.
Competent E. coli Top10 (Carlsbad, Calif.) and Rosetta (DE3) pLysS (Novagen, Madison, Wis.) cells were used for cloning and expression. E. coli cells were grown in Luria-Bertani (LB) broth or on agar plates with 50 mg/ml carbenicillin, 50 μg/ml kanamycin, or 34 μg/ml chloramphenicol (Sigma) when required.
Construction of plasmid for inactivation of the BBA01 gene.
To inactivate the B. burgdorferi BBA01 gene, a recombinant plasmid was constructed (Fig. 1A). The primer pair a01-1_FOR and a01-2_REV (Table 2) was used to amplify a 1.89-kb fragment of lp54 from strain B31. The PCR conditions were 35 cycles at 94°C for 30 s, 55°C for 1 min, and 72°C for 1 min. The fragment was cloned into pGEM-T Easy (Promega, Madison, Wis.) to obtain pGEM-T Easy/bba01. Primers a01-3_FOR and a01-4_REV containing KpnI restriction sites (Table 2) were used to amplify the region of the pGEM-T Easy/bba01 plasmid (Fig. 1A). The PCR conditions were 35 cycles at 94°C for 30 s, 60°C for 1 min, and 72°C for 4 min. The PCR fragment was cleaved with KpnI and subsequently ligated to itself, resulting in a plasmid with a partially deleted BBA01 gene. A 1.2-kb PflaB-kan resistance cassette described elsewhere (15) was previously cloned in pGEM-T Easy (45), resulting in plasmid pKanR, and was excised using the restriction enzyme KpnI. The PflaB-kan fragment was inserted in the KpnI-cleaved plasmid with the partially deleted BBA01 gene. To prevent introduction of ampicillin resistance (Ampr) into B. burgdorferi, the Ampr gene was removed by digestion with BspHI, and the resulting plasmid was denoted pEN-Δa01 (Fig. 1A).
FIG. 1.
Inactivation of the BBA01 gene. (A) Construction of plasmid used for BBA01 gene inactivation. (B) Overview of inactivated BBA01 gene with PflaB insertion. Oligonucleotides used for analysis of the BBA01 mutant are indicated by arrows. The figure is not drawn to scale. (C) PCR analysis of the B31-A and B31 strains and the kanamycin-resistant Δbba01 mutant with the KNaA and KNaC primer pairs (Table 2). (D) PCR analysis of the B31-A and Δbba01 strains with KNaB and a01-1rev primer pairs (Table 2). Plasmid pEN-Δa01, the construct used for inactivation of the BBA01 gene, was used as a positive control. Sterile water was used as negative control. DNA molecular size standards in bp are indicated on the left.
TABLE 2.
Oligonucleotides used in this study
| Oligonucleotide | Sequence (5′ to 3′)a | Application |
|---|---|---|
| a01-1_FOR | AATACAAAAATAAAAATTACTACATTATCAAAGAAC | Gene inactivation |
| a01-2_REV | ATAAATTTCAGACTTGGATTTGCTATATAGTGTC | Gene inactivation |
| a01-3_FOR | CAGCGGTACCCTTGCAGGCTTTGAGCCCAA | Gene inactivation |
| a01-4_REV | TCTCGGTACCTAAGAATAATTCCAGTTCCC | Gene inactivation |
| KnaA | GTTATTCATTCGTGATTGCGCC | Mutant analysis |
| KnaC | CCGCGATTAAATTCCAACATGG | Mutant analysis |
| A01-1 rev | TTGGTGCGAATACTTTCTCTGTTGCCAAATTG | Mutant analysis |
| A01-M6F | TTATGGTACCCAAAGAATTTCGAATTAATTATA | Complementation |
| A01-M6R | ATTTTCTGCAGATTTGATAAGCCAACAGA | Complementation |
| A01-M1 | ACGAGCTCAATCCAAACTTTATTTGCTTGC | Overexpression |
| A01-M1Rev | TTGGTGCGAATACTTTCTCTGTTGCCAAATTG | Overexpression |
| A01-M5F | TAAAATCATGAAAAAAATTTTCACATTAATATTAAT | Overexpression |
| A01-M5R | TTATGGTACCATTAACTTTTTTTAAACGATAAT | Overexpression |
| P13-M1F | CTTTGCCATGGCTAATGATTCTAAAAATGG | Overexpression |
| P13-M1R | TCAAGGTACCCTTAAGCTACATTAAGGCTA | Overexpression |
Restriction sites are indicated in boldface.
Construction of a plasmid used for genetic complementation.
The shuttle vector pBSV2G (24), which contains a gentamicin resistance (Gmr) cassette, was used to construct plasmid pBSV2G+bba01 for genetic complementation. The region covering the BBA01 gene and 260-bp upstream and 130-bp downstream regions was amplified with primers A01-M6F and A01-M6R (Table 2), which contain KpnI and PstI restriction sites, respectively. The PCR conditions were as follows: 5 cycles at 94°C for 30 s, 45°C for 1 min, and 72°C for 1 min followed by 35 cycles at 94°C for 30 s, 55°C for 1 min, and 72°C for 1 min. The resulting 860-bp product was cleaved with the corresponding restriction enzymes, ligated into PstI- and KpnI-digested pBSV2G, and used to transform E. coli TOP10. Gmr clones were screened, and the clone containing the correct construct was further subjected to large-scale plasmid preparation using QIAfilter Plasmid Midi kit (QIAGEN).
Electroporation of B. burgdorferi and screening for transformants.
Preparation of competent B. burgdorferi cells, electroporation, and plating of transformants were done as described previously (54, 55, 62). Single colonies were obtained as described elsewhere (51). For each transformation, 30 to 40 μg of plasmid DNA was used. Clones were screened by PCR to detect allelic exchange of the BBA01 locus or the presence of the shuttle vector. Primers A01-M2 and A01-M2rev (46) were used to detect BBA01, primers KNaA and KNaC (Table 2), as well as KNaB (45) were used to detect the kanamycin resistance (Kanr) cassette, and primers A01-M2 and 4-pCR2-3fgen-MluI (24) were used to detect the presence of the BBA01 gene along with the aacC1 gene which confers Gmr. The PCR conditions were as follows: 5 cycles at 94°C for 30 s, 45°C for 1 min, and 72°C for 2 min and 15 s followed by 35 cycles at 94°C for 30 s, 55°C for 1 min, and 72°C for 2 min and 15 s. The resulting BBA01 knockout mutant was designated the Δbba01 strain; in this mutant, nucleotides 269 to 467 of the BBA01 gene were deleted and replaced by the PflaB-kan resistance cassette.
Determination of plasmid profile and recovery of shuttle vectors.
The Wizard genomic DNA purification kit (Promega) was used to prepare total DNA from B. burgdorferi strains B31-A (WT BBA01 gene) and B313-D (no BBA01 gene), as well as the B313-D+bba01, Δbba01, Δbba01c, Δp13, and Δp13+bba01 strains (Table 1). Plasmid content was then determined as previously described (25). All PCR products were analyzed by 1% Tris-borate-EDTA (TBE)-agarose gel stained with ethidium bromide (5 μg/ml). Total DNA was used to transform competent E. coli TOP10 in order to detect the presence of the vector. Plasmids were prepared from E. coli transformants using a mini plasmid purification kit (QIAGEN) and were analyzed by 1% agarose gel.
Protein preparation, electrophoresis, immunoblotting, and antibodies.
For gel electrophoresis, protein samples were boiled for 5 min in Novex 4× NuPage sample buffer (Invitrogen, Carlsbad, Calif.) in the presence of 2.5% β-mercapthoethanol and separated through Bis-Tris 4 to 12% polyacrylamide gradient NuPage gels using the Novex XCell Sure Lock electrophoresis cell (Invitrogen). B. burgdorferi total proteins were prepared as described previously (8, 46). Outer membrane proteins (B-fractions) of B. burgdorferi B313 were prepared by octyl-β-d-glucopyranoside (OGP) fractionation as described elsewhere (34). For immunoblotting, proteins were transferred to a polyvinylidene difluoride (PVDF) membrane (PALL Corporation, Ann Arbor, Mich.) and probed with antibodies (Abs). Bound antibodies were detected using peroxidase-conjugated antirabbit or antimouse Abs (DAKOI A/S, Glostrup, Denmark) and enhanced chemiluminescence (ECL) reagents according to the manufacturer's instructions (Amersham Pharmacia Biotech, Buckinghamshire, England).
The polyclonal Abs against BBA01 are described elsewhere (46). Monoclonal antibodies (MAbs) H9724, H914, and 15G6, recognizing flagellin B, P66, and P13, respectively, were described previously (2, 52, 53).
Protease treatment of Borrelia cells.
Cell surface proteolysis of intact Borrelia was performed by proteinase K digestion as described previously (8) with some minor changes. Briefly, freshly harvested spirochetes were washed once with PBS-5 mM MgCl2 (PBS-Mg) and, after centrifugation at 2,000 × g for 10 min at 4°C, resuspended in PBS-Mg to a final concentration of 2 × 109 cells/ml. To 0.5 ml of cell suspension was added 25 ml proteinase K (Boehringer, Mannheim, Germany) in sterile water to a final concentration of 12.5 to 100 μg/ml. As a negative control, sterile water was added to the cell suspension. After incubation for 1 h at 20°C, the reactions were stopped by addition of 10 μl of the peptidase inhibitor phenylmethylsulfonyl fluoride (Sigma) (50 mg/ml in isopropanol). The suspensions were then centrifuged at 2,500 × g for 10 min and washed twice with PBS-Mg.
Expression of rP13 and rBBA01 in E. coli.
To obtain recombinant P13 and BBA01 proteins, we cloned the BBA01 open reading frame (ORF) into expression vector pETM-11 (EMBL, Germany, Heidelberg). DNA corresponding to mature P13 protein without the N and C termini (45) was cloned in the vector pETM-20 (EMBL). All primer sequences are listed in Table 2; the restriction sites included were Acc65I for P13-M1R and A01-M5R, BspHI for primer A01-M5, and NcoI for primer P13-M1F. Primer pair P13-M1F and P13-M1R was used for amplifying p13 and the PCR product from the BBA01 gene was obtained in a two-step PCR. First, primers A01-1 M with A01-1MRev were used to amplify the BBA01 gene plus upstream and downstream sequences. For the second step, the PCR product from the first step served as template and internal primers A01-M5F with A01-M5R were used to obtain the full-length BBA01 gene. The Expand High Fidelity PCR System (Roche) was used, and the PCR conditions were 5 cycles at 94°C for 30 s, 45°C for 1 min, and 72°C for 1 min and 35 cycles at 94°C for 30 s, 55°C for 1 min, and 72°C for 1 min. PCR products were digested with Acc65I and NcoI or Acc65I and BspHI (New England Biolabs, Beverly, Mass.) for p13 or the BBA01 gene, respectively. Afterwards the products were ligated to Acc65I- and BspHI-digested vectors pETM-20 (for p13) and pETM-11 (for the BBA01 gene). The plasmids were used to transform E. coli Top10 and purified using the Qiaprep plasmid miniprep kit (QIAGEN). After confirming by restriction enzyme digestion that the plasmids had obtained the inserts, the plasmids were used to transform competent E. coli Rosetta (DE3)pLysS. Cultures were grown to an optical density at 600 nm (OD600) of ∼0.7, and then protein expression was induced by adding isopropyl-β-d-thiogalactopyranoside (IPTG) to a final concentration of 1 mM. After 3 h, the cultures were harvested and protein concentration was estimated by Bio-Rad Protein Assay kit (Bio-Rad, Hercules, Calif.).
Purification of recombinant P13 and BBA01 proteins.
His-tag fusion of rP13 and rBBA01 were purified under native conditions with Ni-nitrilotriacetic acid (NTA) agarose (QIAGEN) according to the manufacturer's instructions in the QIAexpressionist manual (QIAGEN). Protein purity in eluted fractions was monitored by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting. When impurities were present, samples were subjected to an additional round of Ni-NTA agarose batch purification. Before applying the samples to the second Ni-NTA round, buffer exchange using Microcon-10 Microspin columns (Millipore, Billerica, Mass.) was performed. For rP13, the His tag and thioredoxin reductase (TrxA) fusion were eliminated by cleavage with rTEV protease (kindly provided by Stefan Bäckström, Umeå University, Sweden). rBBA01 was used in all assays with an N-terminal His tag.
Planar lipid bilayer assay.
The method for the black lipid bilayer assay has been described previously (11). The instrumentation consists of a Teflon chamber with two compartments separated by a thin wall and connected by a small circular hole with an area of about 0.2 mm2. The membranes were formed from a 1% (wt/vol) solution of diphytanoyl phosphatidylcholine (PC) (Avanti Polar Lipids, Alabaster, AL) in n-decane. The outer membrane fraction of the Δp13+bba01 strain was solubilized in 1% Genapol (Fluka, Buchs, Switzerland) to a concentration of ∼0.05 μg/ml, and purified rP13 (50 ng) and rBBA01 (30 ng) were diluted 1:1 in 1% Genapol and added to the aqueous phase either immediately before membrane formation or after the membrane had turned black. All salt solutions were used unbuffered at a pH of ∼6. The membrane current was measured with a pair of Ag/AgCl electrodes with salt bridges switched in series with a voltage source and a highly sensitive current amplifier (Keithley 427). The applied voltage was 20 mV, and temperature was kept at 20°C.
For SDS-sensitivity testing, SDS (Sigma) was added to purified rP13 and rBBA01 to a final concentration of 1%. After incubation at room temperature for 10 min, channel-forming activity was tested in the lipid bilayer assay.
RESULTS
Inactivation of the BBA01 gene in B. burgdorferi.
In previous studies, we investigated the function of P13 (41, 45). In the present study, we wanted to analyze the role of its paralog, BBA01. We inactivated the BBA01 gene by allelic exchange (Fig. 1) in high-passage B. burgdorferi strain B31-A. Integration of a Kanr cassette within the BBA01 gene was confirmed by PCR (Fig. 1B to D). Using the KNaA and KNaC primers which hybridize within the Kanr gene (Fig. 1B), the expected 448-bp PCR product was amplified from the mutant and the control plasmid but not from wild-type (WT) B31-A, or low-passage B31 (Fig. 1C). To confirm that allelic exchange occurred only at the BBA01 locus, the primer pair KNaB and a01-1rev was used (Fig. 1B). A 540-bp PCR product confirming allelic exchange was obtained from the mutant but not from B31-A, as expected (Fig. 1D). The PCR results also showed that the Kanr gene was inserted in the same direction as the inactivated gene (Fig. 1B and D).
No phenotypic differences between WT B31-A and the Δbba01 mutant were observed when the morphology of the spirochetes was compared by microscopy, and no differences in growth rate were observed (data not shown). This lack of discernible phenotype may be explained by the presence of P13, which is highly expressed in B. burgdorferi strains, including B31-A. Due to the high degree of sequence similarity, it would not be surprising if P13 and BBA01 shared a common function. We were unable to assess the effect of BBA01 gene deletion in a strain lacking p13 because multiple attempts to make such a mutant were unsuccessful. No detectable change in expression of two other paralogs (P13, BBH41) was observed in the Δbba01 strain (data not shown).
Complementation of the Δbba01 strain and introduction of the BBA01 gene into the B313-D and Δp13 strains.
The BBA01 gene was cloned into the shuttle vector pBSV2G and used to transform different B. burgdorferi strains for complementation and protein overexpression. In strain B313-D, which lacks lp54 and therefore the BBA01 gene, pBSV2G+bba01 was introduced in order to assess the effect of BBA01 gene overexpression in the absence of the major Osps (OspA to -D and DbpA and -B) (53). Previously, it has been shown that Osps mask the surface exposure of integral membrane proteins P13 and P66 (17, 41); it was therefore of interest to us to understand the influence of Osps on BBA01. The Δp13 strain was transformed with pBSV2G+bba01 in order to analyze the effect of BBA01 gene overexpression in the absence of p13 and to create a strain for functional studies in which BBA01 is not obscured by P13, a protein of very similar properties (46).
Gmr clones appeared after 8 to 10 days and were analyzed by PCR for the presence of the BBA01 gene and the shuttle vector. Oligonucleotides A01-M2 and A01-M2 rev (Table 2), which amplify a 254-bp region within the BBA01 gene, were used to detect introduced BBA01 gene in the Δbba01 and B313-D strains. Oligonucleotides A01-M2 (46) and 4-pCR2-3fgen-MluI (24) were used to confirm that the BBA01 gene was successfully introduced into the Δp13 strain. The 1,698-bp-long PCR product, consisting of part of the BBA01 gene together with part of the pBSV2G vector, was amplified. Thus, these PCR analyses confirmed the presence of pBSV2G+bba01 in transformed strains and its absence in the parental strains (data not shown). The transformants were examined for plasmid content, since loss of plasmids during in vitro cultivation is a well-known feature of Borrelia (6, 19, 28, 31, 36, 42). To analyze plasmid profiles before and after the genetic manipulations, the B31-A, B313-D, Δp13, Δbba01, Δbba01c, Δp13+bba01, and B313-D+Δbba01 strains were analyzed by PCR as previously described (25). The B31-A, Δbba01, and Δp13 strains had identical plasmid profiles, whereas the additional plating step used to obtain the genetically complemented Δbba01c strain resulted in loss of lp56 (Table 3). The same result was obtained using the Δp13+bba01 strain (Table 3).
TABLE 3.
Determination of the plasmid contents
| Plasmida | Result for B. burgdorferi strain:
|
||||||
|---|---|---|---|---|---|---|---|
| B31-A | Δbba01 | Δbba01c | B313-D | B313-D+ bba01 | Δp13 | Δp13+ bba01 | |
| Lp54 | + | + | + | − | − | + | + |
| cp26 | + | + | + | + | + | + | + |
| Lp17 | + | + | + | + | + | + | + |
| lp28-2 | + | + | + | + | + | + | + |
| lp28-3 | + | + | + | − | − | + | + |
| lp38 | + | + | + | − | − | + | + |
| cp32-8 | + | + | + | + | + | + | + |
| cp32-9 | + | + | + | − | − | + | + |
| cp32-7 | + | + | + | + | + | + | + |
| cp32-1 | + | + | + | + | + | + | + |
| lp56 | + | + | − | − | − | + | − |
| cp32-4 | + | + | + | + | + | + | + |
| cp32-3 | + | + | + | + | + | + | + |
Plasmids cp9, lp25, lp28-1, lp28-4, lp36, cp32-6, lp5, and lp21 were absent in all strains investigated.
To investigate whether the pBSV2G+bba01 plasmids were maintained as stable replicons rather than becoming integrated into the chromosome of B. burgdorferi, the plasmids were successfully rescued by transformation of genomic DNA from the Δbba01c, B313-D+bba01, and Δp13+bba01 strains into E. coli (data not shown).
Analysis of BBA01 protein expression in various B. burgdorferi strains.
Production of BBA01 in cultures of B. burgdorferi B31-A, Δbba01, Δbba01c, B313-D, B313-D+bba01, Δp13, Δp13+bba01, and ΔctpA cells was examined by immunoblotting using polyclonal rabbit antiserum against BBA01 (Fig. 2). As expected, BBA01 was detected in WT B31-A, but not in the Δbba01 (Fig. 2A) strain nor in B313-D (lacking the BBA01 gene) (Fig. 2B). As we have shown previously, a higher expression level of BBA01 compared to WT B31-A was detected in the Δp13 strain (46). Herein we could show by Western blot analysis that BBA01 production is much higher in strains where the BBA01 gene is expressed from pBSV2G+bba01 (Fig. 2B). It should be noted that BBA01 in WT B31-A is almost undetectable by immunoblotting. Despite applying equal amounts of total protein (10 μg) to the gels, it was necessary to expose the membranes in Fig. 2A and Fig. 3 to X-ray film 10 times longer than the membrane in Fig. 2B, indicating that the amount of BBA01 produced from lp54 is significantly lower than that produced from pBSV2G+bba01.
FIG. 2.
Immunoblot analysis of BBA01 protein expression. Ten micrograms of total protein from different B. burgdorferi strains used in this study was separated by SDS-PAGE (Bis-Tris 4 to 12% NuPage gel; Novex), blotted to PVDF membrane, and probed with rabbit immune serum against BBA01. (A) Analysis of inactivation of the BBA01 gene. (B) Analysis of complementation of the BBA01 mutant. In panel A, the membrane was exposed 10 times longer than in panel B. The position of the molecular mass standard (in kDa) is indicated to the left.
FIG. 3.
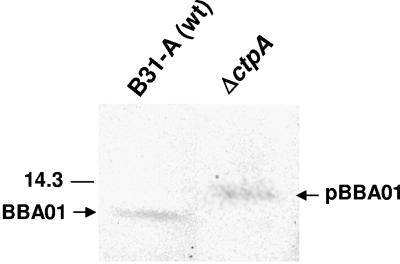
Analysis of C-terminal cleavage of BBA01. Ten milligrams of total protein was separated by SDS-PAGE (Bis-Tris 4 to 12% NuPage gel; Novex), blotted to PVDF membrane, and probed with rabbit immune serum against BBA01. The mature form of BBA01 (BBA01) and the precursor form of BBA01 (pBBA01) are indicated by arrows. The position of the molecular mass standard (in kDa) is indicated to the left.
C-terminal processing of BBA01.
Intriguingly, in the carboxyl-terminal processing enzyme A (CtpA) knockout (ΔctpA) strain, the size of BBA01 was larger and the precursor form of BBA01 was visible (Fig. 3), suggesting that the protein is processed by this enzyme in a similar manner to what has been shown for P13 (44). Thus, we demonstrate herein that BBA01 is a substrate for CtpA. The CtpA cleavage site remains to be elucidated, but considering the similarity between the two paralogs, it is possible that cleavage occurs following an alanine residue, as previously shown for P13 (44). In P13, 28 amino acids are removed from the C terminus by CtpA (44). By analysis of the amino acid sequence of BBA01 (41) and the size of the protein by SDS-PAGE (Fig. 3), it is likely that cleavage occurs after alanine at amino acid position 137, resulting in the removal of 24 amino acids.
Analysis of the cellular localization of BBA01.
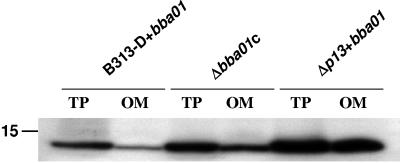
To analyze expression and cellular localization of BBA01 in different strain backgrounds, we compared only strains with shuttle vector containing the BBA01 gene due to the extreme difficulty in detecting BBA01 otherwise. Cellular localization was analyzed by comparing the amounts of BBA01 present in total protein preparations and outer membrane protein fractions (B-fractions) by SDS-PAGE and Western blotting (Fig. 4). We consider B-fractions (34) to contain the outer membrane proteins based on our observation that this fraction has no detectable periplasmic FlaB protein but is enriched for several experimentally verified outer membrane proteins (such as OspA to -D, P66, and Oms28) (data not shown). We have seen previously that P13 is enriched in outer membrane fractions (B-fractions) (41) and therefore expected that the same phenomenon would be observed for BBA01. In contrast, we found that BBA01 is present, but not enriched in B-fractions. The differences in the amount of BBA01 in B-fractions purified from the Δp13+bba01, B313-D+bba01, and Δbba01c strains is noticeable; the majority of BBA01 is found in the B-fraction from the Δp13+bba01 strain, but the protein is present only at a low level in the B-fraction from strain B313-D+bba01 (Fig. 4).
FIG. 4.
Localization of the BBA01 protein. Comparison of the amounts of BBA01 in total protein (TP) and outer-membrane (OM) fractions (B-fractions). Ten micrograms of total protein or outer-membrane fraction from B. burgdorferi strains with introduced pBSV2G+bba01 was separated by SDS-PAGE (Bis-Tris 4 to 12% NuPage gel; Novex), blotted to PVDF membrane, and probed with rabbit immune serum against BBA01. The position of the molecular mass standard (in kDa) is indicated to the left.
Further analysis of BBA01 surface exposure involved proteinase K treatment. Protease-treated Borrelia cells (Δp13+bba01 and B313-D+bba01 strains) were analyzed by SDS-PAGE and immunoblotting. To investigate whether high concentrations of protease would cleave proteins not exposed on the surface, different concentrations of protease were used. Incomplete cleavage of BBA01 occurs in the Δp13+bba01 strain, indicating that only a proportion (∼65%) of the total cellular BBA01 protein is surface exposed (Fig. 5A). However, the possibility that BBA01 is protease resistant due to complexing with itself or other outer membrane proteins cannot be ruled out. In strain B313-D+bba01, where P13 is present, no detectable cleavage of BBA01 was observed, indicating that the protein is most likely not located in the outer membrane of this strain (Fig. 5B). The relative amount of BBA01 cleaved was independent of proteinase K concentration, which differs from what has been observed previously for P13 (41). P66, a known surface-exposed protein (17), was used as a positive control and it showed the typical cleavage pattern of a surface-exposed protein (Fig. 5). Periplasmic flagellum used as a negative control was slightly susceptible to cleavage in some samples, which we attribute to experimental variability since it was not concentration dependent (Fig. 5). Furthermore, data representative of reproducible experiments show no correlation between flagellum cleavage and increased proteolysis of either BBA01 or P66 (Fig. 5).
FIG. 5.
Immunoblot analysis of BBA01 localization in cells. Whole intact spirochetes were incubated with different concentrations of proteinase K, separated by SDS-PAGE (Bis-Tris 4 to 12% NuPage gel; Novex), transferred to a PVDF membrane, and probed with polyclonal rabbit antiserum against BBA01 (α-A01 RS) or monoclonal antibodies against flagellin (α-Fla MAb) and P66 (α-P66 MAb). (A) Proteinase K-treated B. burgdorferi Δp13+bba01 proteins. (B) Proteinase K-treated B. burgdorferi B313-D+bba01 proteins. The positions of molecular mass standards (in kDa) are indicated to the left. The amounts of proteins applied on the gels were equal and were verified by SDS-PAGE and Coomassie staining (data not shown). For quantification of the proportion of total cellular BBA01 being surface exposed in the Δp13+bba01 strain, the membrane was scanned in a Fluor-S MultiImager (Bio-Rad) and the image processing was performed using “Quantity One” software in concert with “Quantitation tool” (Bio-Rad).
Channel-forming activity of BBA01 in the outer membrane fraction.
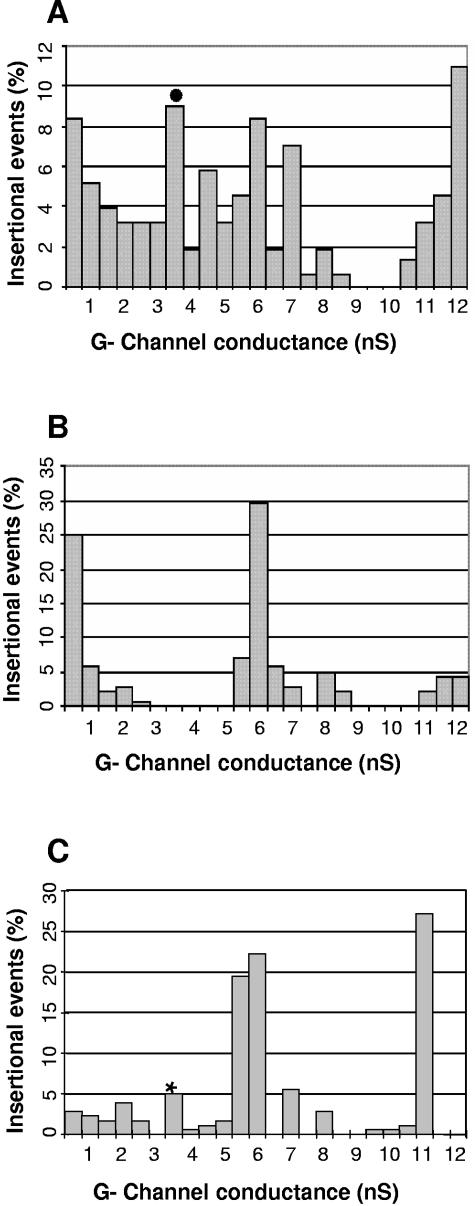
In a recent study, we showed that P13 has channel-forming activity when purified in native form from the outer membrane fraction (B-fraction) of B. burgdorferi (45). Therefore, we wanted to investigate whether native BBA01 would exhibit similar channel-forming activity. The channel-forming activities of the B-fractions from the B31-A (WT), Δp13 (45), and Δp13+bba01 strains were compared in planar lipid bilayer assays (Fig. 6). Comparisons in a p13 knockout background were done because the presence of the highly expressed P13 protein obscures the activity of other channels in the same conductance range. Elimination of 3.5 nS of channel-forming activity due to the absence of P13 (45) was restored in the Δp13+bba01 strain (Fig. 6), indicating that the BBA01 gene can compensate for the p13 deletion. The frequency of channel reconstitution in lipid bilayer membranes is influenced by the relative amounts of certain proteins in the outer membrane protein fraction as well as by the possible interplay between different porins. Thus, differences in the number of insertional events for a certain porin might be due to the absence of another porin (for example, P13). The frequency in lipid bilayer measurements is also dependent on the efficiency with which a certain porin is reconstituted in the lipid bilayer, i.e., the channel-forming activity (45). However, the numbers of insertional events at 3.5 nS due to P13 and BBA01 are somewhat similar: 9% and 5%, respectively (Fig. 6). Together, these data provide evidence that BBA01 is a channel-forming protein which can potentially allow the passage of substrates of similar sizes as the channel formed by P13.
FIG. 6.
Porin activities of outer membrane proteins. (A) Outer membrane proteins (B-fraction) from the B31-A (45), (B) Δp13 (panels A and B are reproduced with permission from reference 45), and (C) Δp13+bba01 strains in PC-n-decane membranes were analyzed in black lipid bilayer assay. •, single-channel conductance due to P13; *, single channel conductance due to BBA01. The total numbers of insertional events were 143 for B31-A, 139 for the Δp13 strain, and 180 for the Δp13+bba01 strain. The aqueous phase contained 1 M KCl. The temperature was 20°C, and the applied voltage was 20 mV.
Channel-forming activities of rP13 and rBBA01 proteins.
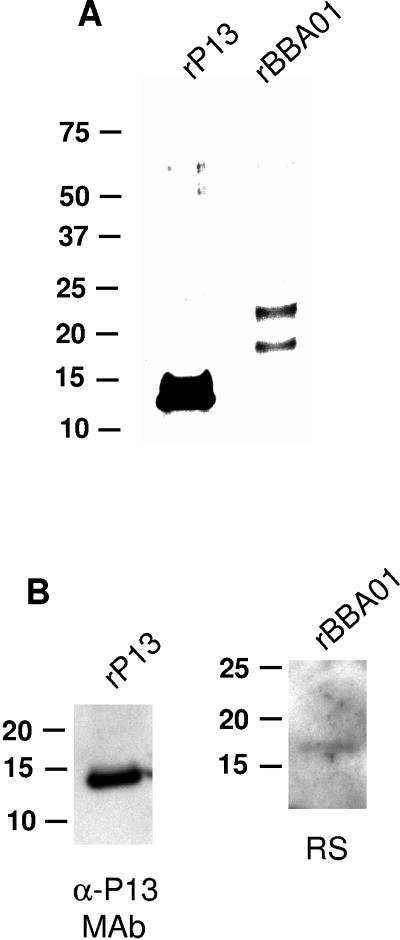
To analyze the channel-forming abilities of pure P13 and BBA01 proteins, we expressed and isolated the mature form of P13 and full-length BBA01 as recombinant proteins in E. coli (Fig. 7). Purified rP13 and rBBA01 were tested for contamination with E. coli proteins by SDS-PAGE, silver staining (Fig. 7A), and immunoblotting (Fig. 7B). Aside from one copurifying protein band present in the rBBA01 sample (Fig. 7A), our recombinant proteins were highly pure. The lipid bilayer assay showed that both proteins exhibit channel-forming activity, with membrane conductance increasing in a step-like fashion (Fig. 8). Both proteins showed an average single-channel conductance of 4 nS. Some conductance steps of 1 and 2 nS were observed; however, these could be explained by minor contamination with E. coli porins. Both rP13 and rBBA01 displayed high specific activity, since a 100,000-fold dilution yielded 98 and 96 individual stepwise insertion events over approximately 30 min for rP13 and 50 min for rBBA01, respectively (Fig. 8A and B).
FIG. 7.
Analysis of purified rP13 and rBBA01 proteins. (A) Silver staining of the Ni-NTA agarose-purified rP13 and rBBA01 proteins (3 μg) separated by SDS-PAGE (Bis-Tris 4 to 12% NuPage gel; Novex). (B) Immunoblot analysis of the purified rP13 (200 ng) and rBBA01 (50 ng) probed either with MAb 15G6 or polyclonal rabbit serum (RS) against BBA01. α-P13, anti-P13 antibody. Molecular mass standards (in kDa) are indicated to the left.
FIG. 8.
Channel-forming activity associated with recombinant P13 and BBA01 proteins. (A) Single-channel conductance observed for rP13 in lipid bilayer membranes. Purified rP13 was added to PC-n-decane membranes bathed in 1 M KCl. The histogram illustrates the individual single-channel conductance events observed for rP13. The average single-channel conductance for a total of 98 insertional events was 4 nS. (B) Single-channel conductance observed for rBBA01 in lipid bilayer membranes. Purified rBBA01 was added to PC-n-decane membranes bathed in 1 M KCl. The histogram illustrates the individual single-channel conductance events observed for rBBA01. The average single-channel conductance for a total of 96 events was 4 nS.
In our previous study, we showed that P13 channel-forming activity is disrupted by SDS (45), and therefore we expected BBA01 to also be SDS sensitive. In E. coli strains derived from BL21 (Novagen), an SDS-resistant OmpF-like porin having an ∼4-nS channel-forming activity has been observed (R. Benz, unpublished results). Since the Rosetta (DE3) pLysS strain used for expression of rP13 and rBBA01 is a derivative of BL21, we wanted to confirm that the channel-forming activity observed for rP13 and rBBA01 was not due to contamination with this E. coli porin. Because of the expected discrepancy in SDS sensitivity of P13, BBA01, and OmpF-like porin, we tested the SDS sensitivity of rP13 and rBBA01. After adding SDS-treated rP13 and rBBA01 to the lipid bilayer, the channel-forming activity was completely disrupted in comparison with that of untreated samples (data not shown). Moreover, to exclude any possibility that the 4-nS channel-forming activity was due to contamination by OmpF-like porin, we performed SDS-PAGE with purified rP13 and rBBA01, sliced the gel into thin sections, and eluted the proteins. No channel-forming activities were found in the fractions eluted from these gel slices (data not shown), demonstrating that neither OmpF-like porin nor any other SDS-resistant porin was a contaminant. It is possible that an SDS-sensitive porin could have been present; however, we consider this unlikely, since no other porins in E. coli are known to have channel-forming activities similar to those of P13 and BBA01 (3-5, 10, 12-14, 22, 32, 35, 56, 64). Together, these data confirm that the channel-forming activities observed for rP13 and rBBA01 were due to their channel-forming properties and not to contamination with E. coli OmpF-like porin.
DISCUSSION
The phenomenon of Borrelia spirochetes harboring so many paralogous genes in their genomes is a paradigm which is still unsolved. Paralogous genes may serve to increase genetic diversity by providing a pool of sequences similar enough to undergo recombination. The encoded proteins may exhibit sufficient functional overlap such that they can act as backups in the event of variation and loss of the primary gene. Alternatively, some paralogs may vary in functional specificity to suit the demands of changing environments. Our previous studies revealing that the BBA01 gene, the closest paralog to p13 in gene family 48, is up-regulated in a p13 knockout, suggested a possible redundancy in function (46). Therefore, we wanted to elucidate the potential significance of paralogous genes through analyzing the relationship between the p13 and BBA01 genes. In this study, we functionally characterized BBA01 and examined its expression and localization pattern in relation to P13. We present data on genetic manipulations of the BBA01 gene by allelic-exchange mutagenesis and gene complementation, as well as the posttranslational processing, membrane localization, and channel-forming capability of native and recombinant BBA01.
Interestingly, in the absence of P13, some proportion of the total cellular BBA01 protein is transported to the outer membrane, where it is surface exposed, while the remainder localizes elsewhere. In contrast, when P13 is present, BBA01 remains subsurface (Fig. 5B). Noteworthy, outer membrane proteins do not hinder accessibility to proteinase K since BBA01 is fully protease resistant in strain B313-D+bba01, which is devoid of the major Osps. Cellular localization experiments were done using strains in which the BBA01 gene is overexpressed from a plasmid to circumvent difficulties in detecting WT-level BBA01. Therefore, the possibility that overexpression could lead to unnatural partitioning of BBA01 exists; nevertheless, our aim was to investigate differential behavior of BBA01 in the absence of P13, which was not feasible in the WT setting. Differential cellular localization of proteins from the same paralog family have been previously implicated for OspE/F/Elp and the Bdr paralogs (29, 50). The spatial regulation of OspE/F/Elp lipoproteins throughout the enzootic cycle leads to dual (outer surface and periplasm) localization of these paralogs in B. burgdorferi, and host-specific signals can alter the expression patterns and final cellular location of these lipoproteins (29). If a similar mechanism occurs in gene family 48, circumstances may exist in which P13 is down-regulated and replaced by BBA01.
To assess whether P13 and BBA01 could potentially have compensatory functions, we determined the functional similarity between the two paralogs. P13 is a known channel-forming protein (45); thus, we examined the capability of BBA01 to create channel-forming pores by analyzing both bacterial membrane preparations and purified recombinant proteins in lipid bilayer assays (Fig. 6 and 8). By comparing the Δp13 strain, in which the 3.5-nS channel-forming activity due to P13 is abolished (45), with the Δp13+bba0 strain, we found that a 3.5-nS channel-forming activity was restored (Fig. 6). Essentially, the Δp13+bba01 strain exhibited the same pore-forming activities as strain B31-A, although the relative numbers of insertional events were slightly variable (Fig. 6A and C). Purified recombinant P13 and BBA01 displayed similar channel-forming activities of 4 nS (Fig. 8), compatible with the 3.5-nS conductance steps observed for native, purified P13 (45) and BBA01 in membrane protein preparations (Fig. 6). Because we were unable to purify rBBA01 to homogeneity (Fig. 7A), we went to great lengths to ensure that the channel formation activities measured were not due to contaminants, particularly E. coli OmpF-like porin, which has a similar conductance, or any other SDS-sensitive E. coli porins. Since both porins form channels of similar size, BBA01 and P13 may have overlapping function, although additional experiments are necessary to determine whether they share substrate specificity.
The high level of sequence homology and the same conductance steps for both P13 and BBA01 indicates that both proteins have very similar structures. Conventional E. coli porins, such as OmpF and LamB, contain 16 to 18 β-sheets that form β-barrel structures spanning the outer membrane (23, 27). Our previous studies (41, 44-46) revealed that P13 has an unusual structure, different from other known porins because of its low molecular mass, C-terminal cleavage, and lack of sufficient β-sheets to form channels by conventional pore-forming mechanisms (9, 30). Instead, three hydrophobic α-helices are predicted to span the membrane (41, 46). The same prediction is valid for the BBA01 protein (41), and therefore it is evident that this paralog also belongs to an unusual porin group.
The results presented here support the idea that one purpose of B. burgdorferi's many paralogous gene families is to be able to replace or regulate certain protein functions, a capacity perhaps necessitated in the event of genetic alterations or as the spirochete enters different environments. We present data demonstrating that an interplay exists between paralog proteins P13 and BBA01, whereby expression level and alternative localization of one, BBA01, are dependent upon the presence of the other, P13. Moreover, because these two paralogs are remarkably similar channel-forming proteins, functional interchangeability may be possible, an idea consistent with the lack of phenotype of a Δbba01 knockout. As Borrelia organisms encounter different environments during their life cycle, there are circumstances in which the function of one protein may need to be substituted for or be more favorably performed by another (paralog) protein (49, 65). We therefore propose that BBA01 plays a minor role in B. burgdorferi physiology under conditions in which P13 dominates, but acts as a reserve under conditions in which p13 is repressed or nonfunctional. Additional studies are needed to investigate the expression of the paralogs in different environments: for example, in ticks, in order to provide a better understanding of the biology of Borrelia spirochetes and Lyme disease pathogenesis.
Acknowledgments
This study was supported by Swedish Research Council grant 07922; the Swedish Council for Environment, Agricultural Sciences and Spatial Planning grant 23.0161; Swedish Foundation for Strategic Research, Infection and Vaccinology; MicMan; The Swedish Foundation for International Cooperation in Research and Higher Education (STINT); and the J. C. Kempe foundation. K.D. and R.B. were supported by the Deutsche Forschungsgemeinschaft and the Fonds der Chemischen Industrie.
We thank Betty Guo for carefully reading the manuscript, A. G. Barbour for providing MAb 15G6, Yngve Östberg for helpful discussions, Elke Maier and Bettina Schiffler for technical assistance in the lipid bilayer measurements, and Stefan Bäckström for providing the rTEV protease.
REFERENCES
- 1.Achouak, W., T. Heulin, and J. M. Pages. 2001. Multiple facets of bacterial porins. FEMS Microbiol. Lett. 199:1-7. [DOI] [PubMed] [Google Scholar]
- 2.Akins, D. R., K. W. Bourell, M. J. Caimano, M. V. Norgard, and J. D. Radolf. 1998. A new animal model for studying Lyme disease spirochetes in a mammalian host-adapted state. J. Clin. Investig. 101:2240-2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersen, C., D. Krones, C. Ulmke, K. Schmid, and R. Benz. 1998. The porin RafY encoded by the raffinose plasmid pRSD2 of Escherichia coli forms a general diffusion pore and not a carbohydrate-specific porin. Eur. J. Biochem. 254:679-684. [DOI] [PubMed] [Google Scholar]
- 4.Andersen, C., B. Rak, and R. Benz. 1999. The gene bglH present in the bgl operon of Escherichia coli, responsible for uptake and fermentation of beta-glucosides encodes for a carbohydrate-specific outer membrane porin. Mol. Microbiol. 31:499-510. [DOI] [PubMed] [Google Scholar]
- 5.Arora, A., D. Rinehart, G. Szabo, and L. K. Tamm. 2000. Refolded outer membrane protein A of Escherichia coli forms ion channels with two conductance states in planar lipid bilayers. J. Biol. Chem. 275:1594-1600. [DOI] [PubMed] [Google Scholar]
- 6.Barbour, A. G. 1989. The molecular biology of Borrelia. Rev. Infect. Dis. 11(Suppl. 6):S1470-S1474. [DOI] [PubMed] [Google Scholar]
- 7.Barbour, A. G. 1988. Plasmid analysis of Borrelia burgdorferi, the Lyme disease agent. J. Clin. Microbiol. 26:475-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barbour, A. G., S. L. Tessier, and S. F. Hayes. 1984. Variation in a major surface protein of Lyme disease spirochetes. Infect. Immun. 45:94-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benz, R. 2001. Porins—structure and function, p. 227-246. In G. Winkelmann (ed.), Microbial transport systems. Wiley-VCH Verlag GmbH, Weinheim, Germany.
- 10.Benz, R., R. P. Darveau, and R. E. Hancock. 1984. Outer-membrane protein PhoE from Escherichia coli forms anion-selective pores in lipid-bilayer membranes. Eur. J. Biochem. 140:319-324. [DOI] [PubMed] [Google Scholar]
- 11.Benz, R., K. Janko, W. Boos, and P. Lauger. 1978. Formation of large, ion-permeable membrane channels by the matrix protein (porin) of Escherichia coli. Biochim. Biophys. Acta 511:305-319. [DOI] [PubMed] [Google Scholar]
- 12.Benz, R., E. Maier, and I. Gentschev. 1993. TolC of Escherichia coli functions as an outer membrane channel. Zentbl. Bakteriol. 278:187-196. [DOI] [PubMed] [Google Scholar]
- 13.Benz, R., A. Schmid, T. Nakae, and G. H. Vos-Scheperkeuter. 1986. Pore formation by LamB of Escherichia coli in lipid bilayer membranes. J. Bacteriol. 165:978-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benz, R., H. Tokunaga, and T. Nakae. 1984. Properties of chemically modified porin from Escherichia coli in lipid bilayer membranes. Biochim. Biophys. Acta 769:348-356. [DOI] [PubMed] [Google Scholar]
- 15.Bono, J. L., A. F. Elias, J. J. Kupko III, B. Stevenson, K. Tilly, and P. Rosa. 2000. Efficient targeted mutagenesis in Borrelia burgdorferi. J. Bacteriol. 182:2445-2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brooks, C. S., P. S. Hefty, S. E. Jolliff, and D. R. Akins. 2003. Global analysis of Borrelia burgdorferi genes regulated by mammalian host-specific signals. Infect. Immun. 71:3371-3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bunikis, J., and A. G. Barbour. 1999. Access of antibody or trypsin to an integral outer membrane protein (P66) of Borrelia burgdorferi is hindered by Osp lipoproteins. Infect. Immun. 67:2874-2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burgdorfer, W., A. G. Barbour, S. F. Hayes, J. L. Benach, E. Grunwaldt, and J. P. Davis. 1982. Lyme disease—a tick-borne spirochetosis? Science 216:1317-1319. [DOI] [PubMed] [Google Scholar]
- 19.Busch, U., G. Will, C. Hizo-Teufel, B. Wilske, and V. Preac-Mursic. 1997. Long-term in vitro cultivation of Borrelia burgdorferi sensu lato strains: influence on plasmid patterns, genome stability and expression of proteins. Res. Microbiol. 148:109-118. [DOI] [PubMed] [Google Scholar]
- 20.Carroll, J. A., R. M. Cordova, and C. F. Garon. 2000. Identification of 11 pH-regulated genes in Borrelia burgdorferi localizing to linear plasmids. Infect. Immun. 68:6677-6684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Casjens, S., N. Palmer, R. van Vugt, W. M. Huang, B. Stevenson, P. Rosa, R. Lathigra, G. Sutton, J. Peterson, R. J. Dodson, D. Haft, E. Hickey, M. Gwinn, O. White, and C. M. Fraser. 2000. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol. Microbiol. 35:490-516. [DOI] [PubMed] [Google Scholar]
- 22.Conlan, S., Y. Zhang, S. Cheley, and H. Bayley. 2000. Biochemical and biophysical characterization of OmpG: a monomeric porin. Biochemistry 39:11845-11854. [DOI] [PubMed] [Google Scholar]
- 23.Dutzler, R., Y. F. Wang, P. Rizkallah, J. P. Rosenbusch, and T. Schirmer. 1996. Crystal structures of various maltooligosaccharides bound to maltoporin reveal a specific sugar translocation pathway. Structure 4:127-134. [DOI] [PubMed] [Google Scholar]
- 24.Elias, A. F., J. L. Bono, J. J. Kupko III, P. E. Stewart, J. G. Krum, and P. A. Rosa. 2003. New antibiotic resistance cassettes suitable for genetic studies in Borrelia burgdorferi. J. Mol. Microbiol. Biotechnol. 6:29-40. [DOI] [PubMed] [Google Scholar]
- 25.Elias, A. F., P. E. Stewart, D. Grimm, M. J. Caimano, C. H. Eggers, K. Tilly, J. L. Bono, D. R. Akins, J. D. Radolf, T. G. Schwan, and P. Rosa. 2002. Clonal polymorphism of Borrelia burgdorferi strain B31 MI: implications for mutagenesis in an infectious strain background. Infect. Immun. 70:2139-2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fraser, C. M., S. Casjens, W. M. Huang, G. G. Sutton, R. Clayton, R. Lathigra, O. White, K. A. Ketchum, R. Dodson, E. K. Hickey, M. Gwinn, B. Dougherty, J. F. Tomb, R. D. Fleischmann, D. Richardson, J. Peterson, A. R. Kerlavage, J. Quackenbush, S. Salzberg, M. Hanson, R. van Vugt, N. Palmer, M. D. Adams, J. Gocayne, J. C. Venter, et al. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390:580-586. [DOI] [PubMed] [Google Scholar]
- 27.Garavito, R. M., and J. P. Rosenbusch. 1980. Three-dimensional crystals of an integral membrane protein: an initial x-ray analysis. J. Cell Biol. 86:327-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grimm, D., A. F. Elias, K. Tilly, and P. A. Rosa. 2003. Plasmid stability during in vitro propagation of Borrelia burgdorferi assessed at a clonal level. Infect. Immun. 71:3138-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hefty, P. S., S. E. Jolliff, M. J. Caimano, S. K. Wikel, and D. R. Akins. 2002. Changes in temporal and spatial patterns of outer surface lipoprotein expression generate population heterogeneity and antigenic diversity in the Lyme disease spirochete, Borrelia burgdorferi. Infect. Immun. 70:3468-3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koebnik, R., K. P. Locher, and P. Van Gelder. 2000. Structure and function of bacterial outer membrane proteins: barrels in a nutshell. Mol. Microbiol. 37:239-253. [DOI] [PubMed] [Google Scholar]
- 31.Labandeira-Rey, M., and J. T. Skare. 2001. Decreased infectivity in Borrelia burgdorferi strain B31 is associated with loss of linear plasmid 25 or 28-1. Infect. Immun. 69:446-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lakey, J. H., J. P. Watts, and E. J. Lea. 1985. Characterisation of channels induced in planar bilayer membranes by detergent solubilised Escherichia coli porins. Biochim. Biophys. Acta 817:208-216. [DOI] [PubMed] [Google Scholar]
- 33.Liu, X., and T. Ferenci. 1998. Regulation of porin-mediated outer membrane permeability by nutrient limitation in Escherichia coli. J. Bacteriol. 180:3917-3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Magnarelli, L. A., J. F. Anderson, and A. G. Barbour. 1989. Enzyme-linked immunosorbent assays for Lyme disease: reactivity of subunits of Borrelia burgdorferi. J. Infect. Dis. 159:43-49. [DOI] [PubMed] [Google Scholar]
- 35.Maier, C., E. Bremer, A. Schmid, and R. Benz. 1988. Pore-forming activity of the Tsx protein from the outer membrane of Escherichia coli. Demonstration of a nucleoside-specific binding site. J. Biol. Chem. 263:2493-2499. [PubMed] [Google Scholar]
- 36.McDowell, J. V., S. Y. Sung, M. Labandeira-Rey, J. T. Skare, and R. T. Marconi. 2001. Analysis of mechanisms associated with loss of infectivity of clonal populations of Borrelia burgdorferi B31MI. Infect. Immun. 69:3670-3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mizuno, T., A. Shinkai, K. Matsui, and S. Mizushima. 1990. Osmoregulatory expression of porin genes in Escherichia coli: a comparative study on strains B and K-12. FEMS Microbiol. Lett. 56:289-293. [DOI] [PubMed] [Google Scholar]
- 38.Nikaido, H. 2003. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 67:593-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nikaido, H., and T. Nakae. 1979. The outer membrane of Gram-negative bacteria. Adv. Microb. Physiol. 20:163-250. [DOI] [PubMed] [Google Scholar]
- 40.Nilsson, C. L., H. J. Cooper, K. Håkansson, A. G. Marshall, Y. Östberg, M. Lavrinovicha, and S. Bergström. 2002. Characterization of the P13 membrane protein of Borrelia burgdorferi by mass spectrometry. J. Am. Soc. Mass Spectrom. 13:295-299. [DOI] [PubMed] [Google Scholar]
- 41.Noppa, L., Y. Östberg, M. Lavrinovicha, and S. Bergström. 2001. P13, an integral membrane protein of Borrelia burgdorferi, is C-terminally processed and contains surface-exposed domains. Infect. Immun. 69:3323-3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Norris, S. J., J. K. Howell, S. A. Garza, M. S. Ferdows, and A. G. Barbour. 1995. High- and low-infectivity phenotypes of clonal populations of in vitro-cultured Borrelia burgdorferi. Infect. Immun. 63:2206-2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ojaimi, C., C. Brooks, S. Casjens, P. Rosa, A. Elias, A. Barbour, A. Jasinskas, J. Benach, L. Katona, J. Radolf, M. Caimano, J. Skare, K. Swingle, D. Akins, and I. Schwartz. 2003. Profiling of temperature-induced changes in Borrelia burgdorferi gene expression by using whole genome arrays. Infect. Immun. 71:1689-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Östberg, Y., J. A. Carroll, M. Pinne, J. G. Krum, P. Rosa, and S. Bergström. 2004. Pleiotropic effects of inactivating a carboxyl-terminal protease, CtpA, in Borrelia burgdorferi. J. Bacteriol. 186:2074-2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Östberg, Y., M. Pinne, R. Benz, P. Rosa, and S. Bergström. 2002. Elimination of channel-forming activity by insertional inactivation of the p13 gene in Borrelia burgdorferi. J. Bacteriol. 184:6811-6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pinne, M., Y. Östberg, P. Comstedt, and S. Bergström. 2004. Molecular analysis of the channel-forming protein P13 and its paralogue family 48 from different Lyme disease Borrelia species. Microbiology 150:549-559. [DOI] [PubMed] [Google Scholar]
- 47.Purser, J. E., and S. J. Norris. 2000. Correlation between plasmid content and infectivity in Borrelia burgdorferi. Proc. Natl. Acad. Sci. USA 97:13865-13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Revel, A. T., A. M. Talaat, and M. V. Norgard. 2002. DNA microarray analysis of differential gene expression in Borrelia burgdorferi, the Lyme disease spirochete. Proc. Natl. Acad. Sci. USA 99:1562-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roberts, D. M., M. Caimano, J. McDowell, M. Theisen, A. Holm, E. Orff, D. Nelson, S. Wikel, J. Radolf, and R. T. Marconi. 2002. Environmental regulation and differential production of members of the Bdr protein family of Borrelia burgdorferi. Infect. Immun. 70:7033-7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roberts, D. M., M. Theisen, and R. T. Marconi. 2000. Analysis of the cellular localization of Bdr paralogs in Borrelia burgdorferi, a causative agent of Lyme disease: evidence for functional diversity. J. Bacteriol. 182:4222-4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosa, P., D. S. Samuels, D. Hogan, B. Stevenson, S. Casjens, and K. Tilly. 1996. Directed insertion of a selectable marker into a circular plasmid of Borrelia burgdorferi. J. Bacteriol. 178:5946-5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sadziene, A., P. A. Rosa, P. A. Thompson, D. M. Hogan, and A. G. Barbour. 1992. Antibody-resistant mutants of Borrelia burgdorferi: in vitro selection and characterization. J. Exp. Med. 176:799-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sadziene, A., D. D. Thomas, and A. G. Barbour. 1995. Borrelia burgdorferi mutant lacking Osp: biological and immunological characterization. Infect. Immun. 63:1573-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Samuels, D. S., K. E. Mach, and C. F. Garon. 1994. Genetic transformation of the Lyme disease agent Borrelia burgdorferi with coumarin-resistant gyrB. J. Bacteriol. 176:6045-6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Samuels, D. S., R. T. Marconi, W. M. Huang, and C. F. Garon. 1994. gyrB mutations in coumermycin A1-resistant Borrelia burgdorferi. J. Bacteriol. 176:3072-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schulein, K., C. Andersen, and R. Benz. 1995. The deletion of 70 amino acids near the N-terminal end of the sucrose-specific porin ScrY causes its functional similarity to LamB in vivo and in vitro. Mol. Microbiol. 17:757-767. [DOI] [PubMed] [Google Scholar]
- 57.Schwan, T. G., and J. Piesman. 2000. Temporal changes in outer surface proteins A and C of the Lyme disease-associated spirochete, Borrelia burgdorferi, during the chain of infection in ticks and mice. J. Clin. Microbiol. 38:382-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Skare, J. T., C. I. Champion, T. A. Mirzabekov, E. S. Shang, D. R. Blanco, H. Erdjument-Bromage, P. Tempst, B. L. Kagan, J. N. Miller, and M. A. Lovett. 1996. Porin activity of the native and recombinant outer membrane protein Oms28 of Borrelia burgdorferi. J. Bacteriol. 178:4909-4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Skare, J. T., T. A. Mirzabekov, E. S. Shang, D. R. Blanco, H. Erdjument-Bromage, J. Bunikis, S. Bergström, P. Tempst, B. L. Kagan, J. N. Miller, and M. A. Lovett. 1997. The Oms66 (p66) protein is a Borrelia burgdorferi porin. Infect. Immun. 65:3654-3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Steere, A. C., R. L. Grodzicki, A. N. Kornblatt, J. E. Craft, A. G. Barbour, W. Burgdorfer, G. P. Schmid, E. Johnson, and S. E. Malawista. 1983. The spirochetal etiology of Lyme disease. N. Engl. J. Med. 308:733-740. [DOI] [PubMed] [Google Scholar]
- 61.Thomas, V., J. Anguita, S. Samanta, P. A. Rosa, P. Stewart, S. W. Barthold, and E. Fikrig. 2001. Dissociation of infectivity and pathogenicity in Borrelia burgdorferi. Infect. Immun. 69:3507-3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tilly, K., A. F. Elias, J. L. Bono, P. Stewart, and P. Rosa. 2000. DNA exchange and insertional inactivation in spirochetes. J. Mol. Microbiol. Biotechnol. 2:433-442. [PubMed] [Google Scholar]
- 63.Tokarz, R., J. M. Anderton, L. I. Katona, and J. L. Benach. 2004. Combined effects of blood and temperature shift on Borrelia burgdorferi gene expression as determined by whole genome DNA array. Infect. Immun. 72:5419-5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Whitfield, C., R. E. W. Hancock, and J. W. Costerton. 1983. Outer membrane protein K of Escherichia coli: purification and pore-forming properties in lipid bilayer membranes. J. Bacteriol. 156:873-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang, X., A. Hübner, T. G. Popova, K. E. Hagman, and M. V. Norgard. 2003. Regulation of expression of the paralogous Mlp family in Borrelia burgdorferi. Infect. Immun. 71:5012-5020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang, J.-R., and S. J. Norris. 1998. Kinetics and in vivo induction of genetic variation of vlsE in Borrelia burgdorferi. Infect. Immun. 66:3689-3697. [DOI] [PMC free article] [PubMed] [Google Scholar]