Abstract
The bactericidal activity of Streptococcus pneumoniae toward Staphylococcus aureus is mediated by hydrogen peroxide. Catalase eliminated this activity. Pneumococci grown anaerobically or genetically lacking pyruvate oxidase (SpxB) were not bactericidal, nor were nonpneumococcal streptococci. These results provide a possible mechanistic explanation for the interspecies interference observed in epidemiologic studies.
Nasal Staphylococcus aureus and nasopharyngeal Streptococcus pneumoniae colonization serve as a source for infection of other sites and for transmission between humans (12, 24). Epidemiological studies have suggested that nasopharyngeal carriage of S. pneumoniae may inhibit nasal carriage of S. aureus (4, 17). Hydrogen peroxide produced by S. pneumoniae has been reported to be toxic in vitro to other bacteria (11, 15) and to mammalian cells (2, 5, 9). H2O2 is produced by pyruvate oxidase (SpxB) as a by-product of aerobic metabolism (19). We sought to test the hypothesis that S. pneumoniae directly inhibits S. aureus in vitro by an H2O2-dependent mechanism.
(The results of this study were partially presented at the 45th Interscience Conference of Antimicrobial Agents and Chemotherapy, Washington, D.C., October 2005.)
Bacterial strains used in this study are described in detail in Table 1. S. pneumoniae strains used were TIGR4 (21), Rx1 (16), and Pn-20. In addition, SpxB-negative variants of TIGR4 and Pn-20 and LytA-negative variants of Rx1 and Pn-20 were created using the Janus cassette (20). Strains of three other Streptococcus spp.—S. gordonii, group A streptococcus, and group C streptococcus—were also tested. Staphylococcal species used in the study were the following: S. aureus strains Newman (NCTC 8178) and ALR and coagulase-negative staphylococci (CNS) (Staphylococcus epidermidis, Staphylococcus xylosus, and Staphylococcus sciuri). Bovine liver catalase (1,000 U/ml; MP Biomedicals, Inc., Solon, OH) was added to growth medium when specified. Bacterial H2O2 production was measured colorimetrically in culture supernatants (8).
TABLE 1.
Bacterial strains and primers used in this study
| Strain or primer | Description | Source or reference |
|---|---|---|
| S. pneumoniae | ||
| TIGR4 | 21 | |
| TIGR4S | TIGR4 but streptomycin resistant by selection of mutants | 22 |
| CP1296 | R6 derivative, cbp3::kan-rpsL+ | 20 |
| TIGR4ΔspxB | TIGR4S but spxB::kan-rpsL+ by transformation with ligation product of PCR fragments amplified with primer pairs TTM211-TTM212 in TIGR4, DAM406-DAM351 in CP1296, and TTM213-TTM214 in TIGR4, and prior to ligation digested with BamHI, BamHI and ApaI, and ApaI, respectively | This study |
| Pn-20 | Serotype 35B, nasopharyngeal human isolate | This study |
| Pn-20ΔspxB | Pn-20s but spxB::kan-rpsL+ by transformation with TTM211-TTM214 PCR product of TIGR4ΔspxB | This study |
| Pn-20ΔlytA | Pn-20s but lytA::kan-rpsL+ by transformation with LAD4-LAD1 PCR product of Rx1ΔlytA | This study |
| Rx1 | 18 | |
| R6 | 10 | |
| Rx1S | Rx1 but streptomycin resistant by selection of mutants | |
| Rx1ΔlytA | Rx1S but lytA::kan-rpsL+ by transformation with ligation product of PCR fragments amplified with primer pairs LAD4-LAD3 in R6, DAM406-DAM351 in CP1296, and LAD2-LAD1 in R6 and prior to ligation digested with BamHI, BamHI and ApaI, and ApaI, respectively | This study |
| S. gordonii strain GP251 | 13 | |
| Group A streptococcus strain 771 | Kindly supplied by Michael Wessels | |
| Group C streptococcus | This study | |
| S. aureus | ||
| Strain Newman | NCTC 8178 | |
| Strain ALR | Nasal isolate from a Wistar rat | Kindly supplied by Jean Lee |
| S. sciuri | Nasal isolate from an ICR mouse | Kindly supplied by Jean Lee |
| S. epidermidis strain RP62a | ATCC 35984 | |
| S. xylosus | Nasal isolate from a C57BL/6 mouse | This study |
| Primers | ||
| DAM406 | TCTATGCCTATTCCAGAGGAAATGGAT | 22 |
| DAM351 | CTAGGGCCCCTTTCCTTATGCTTTTGGAC | 20 |
| TTM211 | CGTTAAGCGAGCGAGTGA corresponds to positions 1002-985 upstream of spxB in TIGR4 | This study |
| TTM212 | TTTGGATCCGATGCAGTAATTTTCCCTTGAGTC positions 10-34 correspond to positions 26-3 within spxB in TIGR4 | This study |
| TTM213 | AAAGGGCCCTCTCGCCGAAAATCAAATA positions 8-28 correspond to positions 3-23 downstream of spxB in TIGR4 | This study |
| TTM214 | CGCCACGTCCAGAACATCC corresponds to positions 1199-1181 downstream of spxB in TIGR4 | This study |
| LAD1 | CAAGGTATCCATCATTCC corresponds to 1011-993 downstream of lytA in R6 | This study |
| LAD2 | CGCGGATCCACAGTAGAGCCAGATGGC positions 9-27 correspond to positions 921-939 within lytA in R6 | This study |
| LAD3 | TTGGGCCCGTTGCACGCCGACTTGAGG positions 10-27 correspond to positions 37-54 within lytA in R6 | This study |
| LAD4 | CTTTGCTTCTCAGAATCTAGG corresponds to 850-830 upstream of lytA in R6 | This study |
To quantify bactericidal activity, we cocultured bacterial strains in brain heart infusion (BHI) and evaluated their survival on selective media. Serial twofold dilutions of the staphylococcal strain were mixed with twofold serial dilutions of the streptococcal strain in a final volume of 100 μl (see legend to Fig. 1 for more details). Cultures with and without catalase were incubated at 37°C in 5% CO2. At time zero and 6 h, approximately 2 μl of each culture was plated on selective medium using a replica plater (Sigma, Aldrich).
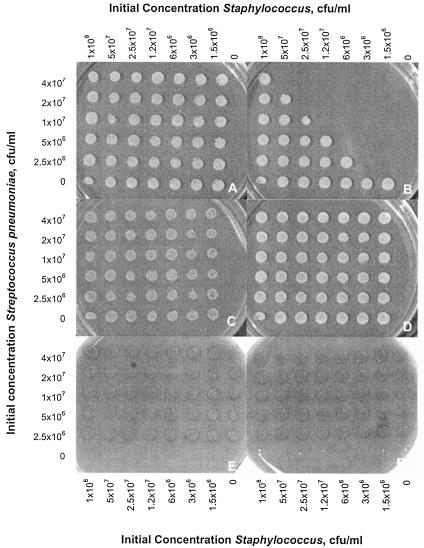
FIG. 1.
Growth of S. aureus and S. pneumoniae on selective media after replica plating from cocultures. Columns of the plate contain twofold dilutions of the staphylococcal strain beginning with 108 CFU/ml in the left column. Rows contain twofold dilutions of the streptococcal strain beginning with 4 × 107 CFU/ml. The right column contains the staphylococcal strain only, and the bottom row contains the streptococcal strain only. The bottom right corner well contains uninoculated BHI. A and B—S. aureus (Newman) after 0 and 6 h, respectively, grown on selective medium (tryptic soy agar supplemented with 5 μg/ml optochin); C and D—S. aureus (Newman) after 0 and 6 h in coculture supplemented with catalase (anaerobic conditions yielded identical results to those shown in panels C to D); E and F—S. pneumoniae (Pn-20) after 0 and 6 h, respectively, grown on selective medium (tryptic soy agar supplemented with 5% sheep blood and 8 μg/ml gentamicin).
For a given S. aureus inoculum, a higher S. pneumoniae inoculum led to greater killing (Fig. 1A and B). The diagonal pattern of killing (Fig. 1B) suggests that the inoculum of S. aureus that could be killed completely was approximately linearly proportional to the pneumococcal inoculum. Killing was eliminated by the addition of catalase (Fig. 1C to D). No inhibition of S. pneumoniae by S. aureus was observed (Fig. 1E to F).
To quantify bactericidal efficiency, we report the maximum staphylococcal inoculum “killed” (reduced below the detection limit of 50 CFU/well) by 106 CFU of streptococci at 6 h (IK6). Tables 2 and 3 compare the killing efficiencies of different streptococci and the susceptibilities of different staphylococci. SpxB-negative strains did not produce detectable amounts of H2O2 and were minimally bactericidal to S. aureus. The maximum inoculum of S. aureus killed by S. pneumoniae Pn-20ΔspxB was 1.2 log10 CFU (IK6 = 1.2), 5 orders of magnitude lower than that of the parental strains (Table 2); catalase abolished the residual killing activity. Other streptococcal species that did not produce detectable levels of H2O2 demonstrated little or no bactericidal effect (Table 2).
TABLE 2.
Various bactericidal efficiencies of different streptococcus strains against S. aureus strain Newmana
| Streptococcus strain | Bactericidal efficiency (IK6) (log10 CFU ± SD) | Concn of H2O2 (mM) in supernatant of 7.5 log10 CFU of S. pneumoniae culture |
|---|---|---|
| S. pneumoniae (Pn-20) | 6.4 ± 0.3 | 1.60 ± 0.58 |
| S. pneumoniae (Pn-20ΔspxB) | 1.2 ± 0.8 | <0.05 |
| S. pneumoniae (Pn-20ΔlytA) | 6.2 ± 0.1 | 1.60 ± 0.87 |
| S. pneumoniae (TIGR4) | 6.3 ± 0.3 | 0.12 ± 0.07 |
| S. pneumoniae (TIGR4ΔspxB) | 1.4 ± 0.2 | <0.05 |
| S. pneumoniae (Rx1) | 5.8 ± 0.5 | 0.20 ± 0.17 |
| S. pneumoniae (Rx1ΔlytA) | 5.8 ± 0.6 | 0.43 ± 0.05 |
| S. gordonii | 3.8 ± 0.6 | <0.05† |
| Group A streptococcus | 0* | <0.05† |
| Group C streptococcus | 0* | <0.05† |
IK6, maximal S. aureus Newman inoculum killed by 106 CFU of streptococci at 6 h. IK values are averages from at least three independent experiments±SD. *, detection level = 50 CFU/well; †, detection level = 0.05 mM.
TABLE 3.
Susceptibilities of various staphylococci to S. pneumoniae strain Pn-20 bactericidal effect and to external H2O2 and their catalase activities
| Staphylococcus strain | Susceptibility to S. pneumoniae killing IK6 (log10 CFU ± SD)a | H2O2 susceptibility (Δlog10 CFU)b | Catalase activity (mU per 108 CFU) |
|---|---|---|---|
| S. aureus (Newman) | 6.4 ± 0.3 | −2.5 | 161 |
| S. aureus (Newman) in medium supplemented with catalase | 0* | +1.5 | ND |
| S. aureus (Newman) under anaerobic conditions | 0* | NDc | ND |
| S. aureus (ALR) | 5.35 ± 0.93 | −1.5 | ND |
| S. epidermidis | <2 | +0.3 | 524 |
| S. xylosus | <2 | +0.2 | 1,050 |
| S. sciurii | <2 | +0.4 | 7,334 |
IK6 = maximal staphylococcal inoculum killed by 106 CFU of S. pneumoniae (Pn-20) at 6 h. *, detection level = 50 CFU/well. IK values are averages from at least three independent experiments ± SD.
Net reduction in log10 CFU at 6 h compared to the initial 7.7 log10 CFU of S. aureus in medium supplemented with H2O2 periodically.
ND, not done.
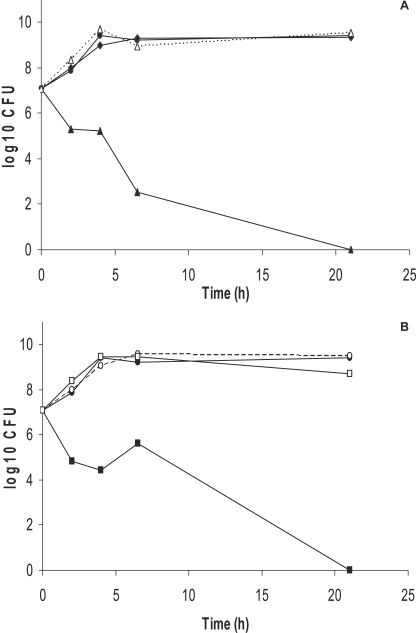
To verify that S. aureus is inhibited by a soluble factor produced by S. pneumoniae and that this factor is H2O2, we evaluated the growth of S. aureus (Newman) in filtered pneumococcal supernatant and compared it to growth in fresh BHI and in coculture with S. pneumoniae (Pn-20). Figure 2 demonstrates a bactericidal effect of sterile supernatant from aerobic cultures of S. pneumoniae to S. aureus within 6 h (Fig. 2A). This effect was mitigated either when catalase was added to the medium or with supernatant from anaerobically grown S. pneumoniae, which does not produce H2O2 (14) (Fig. 2B).
FIG. 2.
S. aureus growth (A) in coculture with S. pneumoniae under aerobic conditions (▴), coculture in anaerobic conditions (⧫), coculture with periodic addition of catalase (▵), or plain BHI (•). (B) In sterile supernatant from S. pneumoniae cultures (▪), in supernatant with catalase supplementation (□), in plain BHI (•), or in BHI supplemented with catalase (○).
S. pneumoniae variants lacking the major autolytic enzyme LytA exhibited bactericidal activities similar to those of their parent strains (Table 2). This finding excludes a potential accessory role for this toxic enzyme, while also serving as a control to demonstrate that the significantly reduced bactericidal activity of ΔspxB strains was a direct result of deficient SpxB activity and not by the genetic manipulation itself.
S. aureus Newman was more susceptible to S. pneumoniae's bactericidal effect than strain ALR. CNS strains showed undetectable inhibition by S. pneumoniae (Pn-20) in cocultures (IK6 < 2). These results correlated (Table 3) with the susceptibilities of the staphylococcal strains to H2O2 added to the medium (1 μmol every 90 min) as well as to the catalase activities of these strains measured with the Amplex Red catalase assay kit (Molecular Probes, Eugene, OR). External H2O2 added periodically reduced CFU/ml of S. aureus strains by 1.5 to 2.5 log10, while the neutralization of this external H2O2 by exogenous catalase permitted an increase of 1.5 log10 CFU/ml. In contrast, under the same conditions (with no addition of catalase), the numbers of CFU/ml for CNS strains increased slightly (0.5 log10) (Table 3).
It is striking that S. aureus, a catalase-positive species, is so susceptible to a H2O2-producing bacteria. The two different S. aureus strains tested were highly susceptible to S. pneumoniae, while CNS species were highly resistant to S. pneumoniae. All staphylococci elaborate catalase, with variation between species in the number and expression of catalase enzymes (1, 3). Indeed the staphylococcal species that were resistant to S. pneumoniae secreted higher concentrations of catalase than S. aureus; however, the concentrations detected may not linearly reflect differences in intracellular catalase, and the greater antioxidant capacity of these species might be due to additional factors not measured in this study.
Our impetus for this study was the epidemiologic data showing an inverse association between S. aureus and S. pneumoniae colonization (4, 17). H2O2-mediated, unidirectional antagonism as observed here in vitro provides one possible mechanism for this inverse association. If the association is causal and acquisition of S. pneumoniae eradicates S. aureus carriage, then use of pneumococcal vaccines may eliminate the “protective” effect of S. pneumoniae against S. aureus carriage and an increase in S. aureus carriage will follow. Increased S. aureus otitis media has been observed among vaccinees in a pneumococcal conjugate vaccine randomized trial (23). Whether the current increase in severe community-acquired S. aureus infections, including methicillin-resistant S. aureus (6), is partially caused by the recent introduction of the pneumococcal conjugate vaccine is yet to be determined.
This study provides only the first step towards elucidating the mechanism of S. pneumoniae-S. aureus interference in vivo. In vivo interference could be caused by a direct bactericidal effect similar to that observed here. Alternatively, or in addition, it could occur via a host cell signaling pathway. Interestingly, such an indirect pathway might also involve H2O2, which plays key roles in cell signaling, particularly in regulation of the production of cytokines (7, 25). Future in vivo studies are required to address these important remaining questions.
Acknowledgments
We thank Michael Wessels for providing Streptococcus group A, Jean Lee for providing the staphylococcus strains used in the study and for critical review of the manuscript, and Amit Srivastava for thoughtful advice.
This study was supported by NIH grant 1R01AI48935 to M.L. and 1R01AI067737-01 and the Pamela and Jack Egan Fund to R.M.
REFERENCES
- 1.Barriere, C., R. Bruckner, D. Centeno, and R. Talon. 2002. Characterisation of the katA gene encoding a catalase and evidence for at least a second catalase activity in Staphylococcus xylosus, bacteria used in food fermentation. FEMS Microbiol. Lett. 216:277-283. [DOI] [PubMed] [Google Scholar]
- 2.Bermpohl, D., A. Halle, D. Freyer, E. Dagand, J. S. Braun, I. Bechmann, N. W. Schroder, and J. R. Weber. 2005. Bacterial programmed cell death of cerebral endothelial cells involves dual death pathways. J. Clin. Investig. 115:1607-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bisaillon, J. G., G. Dubois, R. Beaudet, M. Sylvestre, R. Charbonneau, and M. Gagnon. 1985. Quantitative determination of catalase activity produced by Neisseria gonorrhoeae, Staphylococcus epidermidis, Neisseria meningitidis and other bacterial strains using the Catalasemeter. Exp. Biol. 43:225-230. [PubMed] [Google Scholar]
- 4.Bogaert, D., A. van Belkum, M. Sluijter, A. Luijendijk, R. de Groot, H. C. Rumke, H. A. Verbrugh, and P. W. Hermans. 2004. Colonisation by Streptococcus pneumoniae and Staphylococcus aureus in healthy children. Lancet 363:1871-1872. [DOI] [PubMed] [Google Scholar]
- 5.Braun, J. S., J. E. Sublett, D. Freyer, T. J. Mitchell, J. L. Cleveland, E. I. Tuomanen, and J. R. Weber. 2002. Pneumococcal pneumolysin and H(2)O(2) mediate brain cell apoptosis during meningitis. J. Clin. Investig. 109:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buescher, E. S. 2005. Community-acquired methicillin-resistant Staphylococcus aureus in pediatrics. Curr. Opin. Pediatr. 17:67-70. [DOI] [PubMed] [Google Scholar]
- 7.DeYulia, G. J., Jr., J. M. Carcamo, O. Borquez-Ojeda, C. C. Shelton, and D. W. Golde. 2005. Hydrogen peroxide generated extracellularly by receptor-ligand interaction facilitates cell signaling. Proc. Natl. Acad. Sci. USA 102:5044-5049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duane, P. G., J. B. Rubins, H. R. Weisel, and E. N. Janoff. 1993. Identification of hydrogen peroxide as a Streptococcus pneumoniae toxin for rat alveolar epithelial cells. Infect. Immun. 61:4392-4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feldman, C., R. Anderson, R. Cockeran, T. Mitchell, P. Cole, and R. Wilson. 2002. The effects of pneumolysin and hydrogen peroxide, alone and in combination, on human ciliated epithelium in vitro. Respir. Med. 96:580-585. [DOI] [PubMed] [Google Scholar]
- 10.Hoskins, J., W. E. Alborn, Jr., J. Arnold, L. C. Blaszczak, S. Burgett, B. S. DeHoff, S. T. Estrem, L. Fritz, D. J. Fu, W. Fuller, C. Geringer, R. Gilmour, J. S. Glass, H. Khoja, A. R. Kraft, R. E. Lagace, D. J. LeBlanc, L. N. Lee, E. J. Lefkowitz, J. Lu, P. Matsushima, S. M. McAhren, M. McHenney, K. McLeaster, C. W. Mundy, T. I. Nicas, F. H. Norris, M. O'Gara, R. B. Peery, G. T. Robertson, P. Rockey, P. M. Sun, M. E. Winkler, Y. Yang, M. Young-Bellido, G. Zhao, C. A. Zook, R. H. Baltz, S. R. Jaskunas, P. R. Rosteck, Jr., P. L. Skatrud, and J. I. Glass. 2001. Genome of the bacterium Streptococcus pneumoniae strain R6. J. Bacteriol. 183:5709-5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McLeod, J. W., and J. Gordon. 1922. Production of hydrogen peroxide by bacteria. Biochem. J. 16:499-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Obaro, S., and R. Adegbola. 2002. The pneumococcus: carriage, disease and conjugate vaccines. J. Med. Microbiol. 51:98-104. [DOI] [PubMed] [Google Scholar]
- 13.Oggioni, M. R., and G. Pozzi. 1996. A host-vector system for heterologous gene expression in Streptococcus gordonii. Gene 169:85-90. [DOI] [PubMed] [Google Scholar]
- 14.Pericone, C. D., D. Bae, M. Shchepetov, T. McCool, and J. N. Weiser. 2002. Short-sequence tandem and nontandem DNA repeats and endogenous hydrogen peroxide production contribute to genetic instability of Streptococcus pneumoniae. J. Bacteriol. 184:4392-4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pericone, C. D., K. Overweg, P. W. Hermans, and J. N. Weiser. 2000. Inhibitory and bactericidal effects of hydrogen peroxide production by Streptococcus pneumoniae on other inhabitants of the upper respiratory tract. Infect. Immun. 68:3990-3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ravin, A. W. 1959. Reciprocal capsular transformations of pneumococci. J. Bacteriol. 77:296-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Regev-Yochay, G., R. Dagan, M. Raz, Y. Carmeli, B. Shainberg, E. Derazne, G. Rahav, and E. Rubinstein. 2004. Association between carriage of Streptococcus pneumoniae and Staphylococcus aureus in children. JAMA 292:716-720. [DOI] [PubMed] [Google Scholar]
- 18.Shoemaker, N. B., and W. R. Guild. 1974. Destruction of low efficiency markers is a slow process occurring at a heteroduplex stage of transformation. Mol. Gen. Genet. 128:283-290. [DOI] [PubMed] [Google Scholar]
- 19.Spellerberg, B., D. R. Cundell, J. Sandros, B. J. Pearce, I. Idanpaan-Heikkila, C. Rosenow, and H. R. Masure. 1996. Pyruvate oxidase, as a determinant of virulence in Streptococcus pneumoniae. Mol. Microbiol. 19:803-813. [DOI] [PubMed] [Google Scholar]
- 20.Sung, C. K., H. Li, J. P. Claverys, and D. A. Morrison. 2001. An rpsL cassette, Janus, for gene replacement through negative selection in Streptococcus pneumoniae. Appl. Environ. Microbiol. 67:5190-5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tettelin, H., K. E. Nelson, I. T. Paulsen, J. A. Eisen, T. D. Read, S. Peterson, J. Heidelberg, R. T. DeBoy, D. H. Haft, R. J. Dodson, A. S. Durkin, M. Gwinn, J. F. Kolonay, W. C. Nelson, J. D. Peterson, L. A. Umayam, O. White, S. L. Salzberg, M. R. Lewis, D. Radune, E. Holtzapple, H. Khouri, A. M. Wolf, T. R. Utterback, C. L. Hansen, L. A. McDonald, T. V. Feldblyum, S. Angiuoli, T. Dickinson, E. K. Hickey, I. E. Holt, B. J. Loftus, F. Yang, H. O. Smith, J. C. Venter, B. A. Dougherty, D. A. Morrison, S. K. Hollingshead, and C. M. Fraser. 2001. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science 293:498-506. [DOI] [PubMed] [Google Scholar]
- 22.Trzcinski, K., C. M. Thompson, and M. Lipsitch. 2003. Construction of otherwise isogenic serotype 6B, 7F, 14, and 19F capsular variants of Streptococcus pneumoniae strain TIGR4. Appl. Environ. Microbiol. 69:7364-7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Veenhoven, R., D. Bogaert, C. Uiterwaal, C. Brouwer, H. Kiezebrink, J. Bruin, E. IJzerman, P. Hermans, R. de Groot, B. Zegers, W. Kuis, G. Rijkers, A. Schilder, and E. Sanders. 2003. Effect of conjugate pneumococcal vaccine followed by polysaccharide pneumococcal vaccine on recurrent acute otitis media: a randomised study. Lancet 361:2189-2195. [DOI] [PubMed] [Google Scholar]
- 24.Wertheim, H. F., D. C. Melles, M. C. Vos, W. van Leeuwen, A. van Belkum, H. A. Verbrugh, and J. L. Nouwen. 2005. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect. Dis. 5:751-762. [DOI] [PubMed] [Google Scholar]
- 25.Xue, X., J. H. Piao, A. Nakajima, S. Sakon-Komazawa, Y. Kojima, K. Mori, H. Yagita, K. Okumura, H. Harding, and H. Nakano. 2005. Tumor necrosis factor alpha (TNFalpha) induces the unfolded protein response (UPR) in a reactive oxygen species (ROS)-dependent fashion, and the UPR counteracts ROS accumulation by TNFalpha. J. Biol. Chem. 280:33917-33925. [DOI] [PubMed] [Google Scholar]