Abstract
The ribulose monophosphate (RuMP) pathway, involving 3-hexulose-6-phosphate synthase (HPS) and 6-phospho-3-hexuloisomerase (PHI), is now recognized as a widespread prokaryotic pathway for formaldehyde fixation and detoxification. Interestingly, HPS and PHI homologs are also found in a variety of archaeal strains, and recent biochemical and genome analyses have raised the possibility that the reverse reaction of formaldehyde fixation, i.e., ribulose 5-phosphate (Ru5P) synthesis from fructose 6-phosphate, may function in the biosynthesis of Ru5P in some archaeal strains whose pentose phosphate pathways are imperfect. In this study, we have taken a genetic approach to address this possibility by using the hyperthermophilic archaeon Thermococcus kodakaraensis KOD1. This strain possesses a single open reading frame (TK0475) encoding an HPS- and PHI-fused protein. The recombinant HPS-PHI-fused enzyme exhibited the expected HPS and PHI activities in both directions (formaldehyde fixing and Ru5P synthesizing). The TK0475 deletion mutant Δhps-phi-7A did not exhibit any growth in minimal medium, while growth of the mutant strain could be recovered by the addition of nucleosides to the medium. This auxotrophic phenotype together with the catalytic properties of the HPS-PHI-fused enzyme reveal that HPS and PHI are essential for the biosynthesis of Ru5P, the precursor of nucleotides, showing that the RuMP pathway is the only relevant pathway for Ru5P biosynthesis substituting for the classical pentose phosphate pathway missing in this archaeon.
The ribulose monophosphate (RuMP) pathway was originally found in methylotrophic bacteria, which can utilize C1 compounds as a sole source of carbon and energy (8, 15, 24, 27). In this pathway, formaldehyde is fixed with ribulose 5-phosphate (Ru5P) to form d-arabino-3-hexulose-6-phosphate (Hu6P) by 3-hexulose-6-phosphate synthase (HPS) and then isomerized to fructose 6-phosphate (F6P) by 6-phospho-3-hexuloisomerase (PHI). In bacteria characterized to date, the expression of genes encoding these two enzymes, hps and phi, respectively, is inducible by formaldehyde (9, 25, 26). Therefore, the RuMP pathway has been thought to be responsible exclusively for formaldehyde assimilation and detoxification. However, recent genome sequence analyses of various microorganisms have revealed that the hps and phi orthologs coding for these two key enzymes are widely distributed among bacterial and archaeal genomes (8, 9, 12, 21, 25-27). In order to understand the physiological roles of HPS and PHI in archaea, we have characterized the HPS and PHI from a hyperthermophilic and anaerobic archaeon, Pyrococcus horikoshii (12), in which the hps and phi genes are encoded in a single open reading frame (PH1938). Our results demonstrated that the product of PH1938 is a bifunctional fused enzyme harboring both HPS and PHI activities. Interestingly, P. horikoshii produced HPS-PHI-fused protein constitutively, i.e., with or without the presence of formaldehyde, suggesting that the RuMP pathway in this organism is involved in a more general metabolic activity.
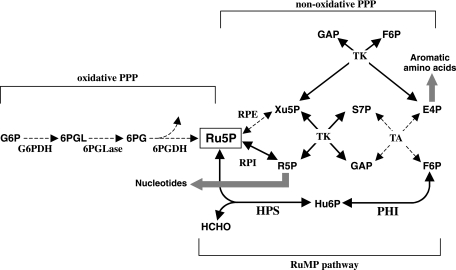
Both biochemical and genome analyses of archaea have raised the possibility that the RuMP pathway is involved in the synthesis of Ru5P from F6P through the reverse reaction of formaldehyde fixation by HPS and PHI (Fig. 1) (6-8, 19, 27). Genome information suggests that the conventional pentose phosphate pathway (PPP) is incomplete or lacking in several archaea (19). For Thermococcus zilligii, the cleavage of hexose phosphate was suggested to be ascribed to the reverse reaction of HPS (20, 23). Subsequently, although the methanogen Methanocaldococcus jannaschii possesses a complete set of genes coding for the nonoxidative PPP, Grochowski et al. reported that intermediates of the PPP, erythrose 4-phosphate (E4P), xylulose 5-phosphate (Xu5P), and sedoheptulose 7-phosphate (S7P), could not be detected in this organism (7). Instead, they could detect Hu6P, an intermediate of the RuMP pathway. These studies suggest at least an involvement of the RuMP pathway in Ru5P biosynthesis. However, the physiological significance of the RuMP pathway and the extent of its contribution towards Ru5P synthesis in these archaea are not clear, due to the lack of genetic studies.
FIG. 1.
Proposed pentose phosphate synthesis through HPS and PHI in T. kodakaraensis. Arrows with broken lines display the reactions catalyzed by enzymes whose orthologs are not apparent on the T. kodakaraensis genome. Intermediates: G6P, glucose 6-phosphate; 6PGL, 6-phosphoglucono-δ-lactone; 6PG, 6-phosphogluconate; Ru5P, ribulose 5-phosphate; Xu5P, xylulose 5-phosphate; R5P, ribose 5-phosphate; S7P, sedoheptulose 7-phosphate; GAP, glyceraldehyde 3-phosphate; E4P, erythrose 4-phosphate; F6P, fructose 6-phosphate. Enzymes: G6PDH, glucose-6-phosphate dehydrogenase; 6PGLase, 6-phosphoglucono-δ-lactonase; 6PGDH, 6-phosphogluconate dehydrogenase; RPE, ribulose-5-phosphate-3-epimerase; RPI, ribose 5-phosphate isomerase; TK, transketolase; TA, transaldolase.
In this study, we have used a hyperthermophilic archaeon, Thermococcus kodakaraensis KOD1 (2, 10), in order to gain insight into the physiological role of the RuMP pathway in Archaea. There are several reasons why this strain was chosen. A gene disruption system based on homologous recombination has been developed for this archaeon (16, 17), a technology possible only with this strain and Sulfolobus solfataricus (22) among hyperthermophiles. The complete genome sequence has also been determined and annotated (GenBank accession no. AP006878) (5). The T. kodakaraensis genome contains only one putative gene for HPS and PHI, which are fused and comprise a single open reading frame (hps-phi) encoding both enzymes on a single polypeptide (TK0475), as in the case of P. horikoshii (12). Another major factor was that in addition to the fact that T. kodakaraensis did not have putative genes for the oxidative PPP, we could not detect genes coding for two key enzymes in the nonoxidative PPP, i.e., ribulose-5-phosphate-3-epimerase and transaldolase (Fig. 1). Therefore, T. kodakaraensis seemed to lack the PPP, which thereby allows a straightforward evaluation of the importance of the RuMP pathway in Ru5P synthesis. We herein provide genetic evidence, by constructing and examining an hps-phi deletion mutant of T. kodakaraensis, that clearly demonstrates that the hps-phi gene indeed functions in the Ru5P biosynthesis in this archaeon. Our present results also enable us to propose an outline of the biosynthesis of pentoses and metabolically related compounds in this archaeon, which can also be expected to apply to other members of Archaea that exhibit phylogenetic patterns of the RuMP pathway and PPP genes that are similar to those for T. kodakaraensis.
MATERIALS AND METHODS
Strains, vectors, and culture conditions.
The strains and plasmids used in this study are listed in Table 1. T. kodakaraensis KOD1 and its derivatives were cultivated under strictly anaerobic conditions at 85°C in a rich growth medium (ASW-YT) or a synthetic medium (ASW-AA) (17). ASW-YT was composed of 0.8× artificial seawater (ASW), 0.5% of yeast extract, 0.5% of tryptone, and 0.2% of elemental sulfur. ASW-AA was composed of 0.8× ASW, 20 amino acids, trace vitamins and minerals, and 0.2% of elemental sulfur. The preparation of the plate medium and cultivation of the cells on it were performed as described previously (17). Individual modifications of the medium for investigation of the auxotrophy of mutant strains and for selection of transformants are described in the respective sections of the text below.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s) | Source or reference |
|---|---|---|
| Strains | ||
| Escherichia coli | ||
| DH5α | F− φ80dlacZΔM15 Δ(lacZYA-argF)U169 deoR recA1 endA1 hsdR17(rK− mK+) phoA supE44 λ−thi-1 gyrA96 relA1 F−ompT hsdSB(rB− mB−) gal dcm lacY1 (DE3) | Takara Bio |
| Rosetta(DE3) | F−ompT hsdSB(rB− mB−) gal dcm lacY1 (DE3) pRARE6 (Cmr) | Novagen |
| Rosetta(DE3)(pEThps-phi) | Rosetta(DE3) harboring pEThps-phi | This study |
| Thermococcus kodakaraensis | ||
| KOD1 | Wild type | 2, 10 |
| KW128 | ΔpyrF ΔtrpE::pyrF | 16 |
| Δhps-phi-7A | Δhps-phi::trpE | This study |
| Plasmids | ||
| pUC118 | Ampr general cloning vector | Takara Bio |
| pET23a(+) | Expression vector | Novagen |
| pEThps-phi | pET23a derivative; hps-phi | This study |
| pUMT2 | pUC118 derivative; trpE marker cassette (PpyrF::trpE) | 18 |
| pUDhps-phi | pUC118 derivative; Δhps-phi::trpE | This study |
Escherichia coli strain DH5α, used for general DNA manipulation, was routinely cultivated at 37°C in Luria-Bertani (LB) medium and supplemented with 50 mg/liter ampicillin when needed. The TK0475 gene was expressed in E. coli Rosetta(DE3) (Novagen, Madison, WI) grown in LB medium containing ampicillin (50 mg/liter) and chloramphenicol (34 mg/liter). E. coli transformants were grown in LB medium at 37°C, to which 0.5 mM IPTG (isopropyl-β-d-thiogalactopyranoside) was added at mid-log phase, followed by an additional 3 h of growth.
Protein preparation from T. kodakaraensis.
T. kodakaraensis cells were suspended in 20 mM Tris-HCl (pH 7.5) containing 10% glycerol and 1 mM dithiothreitol (buffer A) and were disrupted using a French press (Thermo Spectronic, Rochester, NY). Unbroken cells and cell debris were removed by centrifugation at 5,000 × g for 30 min at 4°C, and the resulting supernatants were used as cell extracts. For Western blotting, the cell extracts were further centrifuged at 150,000 × g for 60 min at 20°C. The resulting supernatant and precipitate were used as the soluble and particulate fractions, respectively.
Enzyme assays.
Activity of the combined overall HPS and PHI reaction (HPS/PHI) in the forward direction was determined by measuring the rates of F6P synthesis as described previously (1, 12). Conversion of ribose 5-phosphate (R5P) to Ru5P by ribose 5-phosphate isomerase (RPI) from spinach (Sigma-Aldrich, St. Louis, MO) was performed at 30°C for 5 min, and the following HPS/PHI reaction was conducted at 80°C. HPS/PHI activity for the reverse reaction was determined by measuring the rates of formaldehyde production. The reaction mixture (1 ml) contained 50 μl of 1 M potassium phosphate buffer (pH 7.5), 50 μl of 50 mM MgCl2, 50 μl of 200 mM F6P, and an appropriate amount of enzyme. HPS/PHI reaction was performed at 80°C for 5 min and was stopped by transfer onto ice. Released formaldehyde was measured using Nash reagent (11). One unit of activity for the reverse reaction was defined as the amount of enzyme that produced 1 μmol of formaldehyde per minute. The Km and Vmax for the forward reaction were obtained from double reciprocal plots of HPS/PHI activity versus formaldehyde concentration by using a fixed concentration of Ru5P (5 mM) (condition I) and versus Ru5P concentration by using a fixed concentration of formaldehyde (10 mM) (condition II). The Km and Vmax for the reverse reaction were obtained from double reciprocal plots of HPS/PHI activities versus F6P concentration (condition III).
Cloning and expression of TK0475.
The entire TK0475 gene was amplified by PCR using chromosomal DNA of T. kodakaraensis KOD1 as the template with the primers 5′-GGAATTCCATATGATACTCCAGGTCGCTCT-3′ and 5′-GGAATTCTCACTCAAGGGTCGCGTGCTTCC-3′ (additional NdeI and EcoRI sites are underlined). PCR was performed using Pyrobest DNA polymerase (Takara Bio, Otsu, Japan). The PCR product was digested with NdeI and EcoRI and then ligated into the NdeI/EcoRI site of the T7 expression vector pET23a(+) (Novagen). The resulting plasmid harboring TK0475 was designated pEThps-phi and introduced into E. coli Rosetta(DE3) for overexpression. The gene product encoded by TK0475 was designated rHps-Phi (recombinant HPS-PHI-fused enzyme).
Purification of rHps-Phi.
For purification of rHps-Phi, E. coli Rosetta(DE3)(pEThps-phi) cells were suspended in buffer A containing 5 mM MgCl2 and were disrupted by sonication for 10 min (19 kHz, Insonator model 201 M; Kubota, Tokyo, Japan). Unbroken cells and cell debris were removed by centrifugation at 5,000 × g for 30 min at 4°C, and the resulting supernatant was used as the cell extract. Cell extracts were further centrifuged at 100,000 × g for 60 min at 20°C. Most of the HPS/PHI activity was found in the resulting pellet. The pellet was washed with buffer A containing 5 mM EDTA and then resuspended in buffer A containing 50 mM EDTA and stirred for 12 h at 20°C. The supernatant obtained by centrifugation at 150,000 × g for 60 min at 20°C was incubated at 80°C for 30 min and centrifuged at 15,000 × g for 15 min at 20°C to remove heat-labile proteins from the E. coli strain.
Protein methods.
Protein concentration was measured using a Bio-Rad protein assay kit (Bio-Rad Laboratories, Tokyo, Japan), with bovine serum albumin as the standard. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed in a 12% gel. Western blot analysis was performed using rabbit anti-Hps antibody raised against purified rHps from P. horikoshii (12) and Western Lightning chemiluminescent reagent Plus (Perkin Elmer, Boston, MA).
Construction of the hps-phi disruption vector.
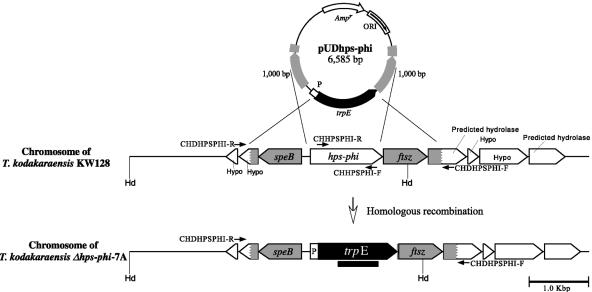
A vector for targeted disruption of the hps-phi gene in T. kodakaraensis through double-crossover homologous recombination was constructed as follows. A DNA fragment containing the target gene together with its flanking regions (1,000 bp each) was amplified from T. kodakaraensis KOD1 genomic DNA using the primer set PHPSPHI-R/PHPSPHI-F (5′-GAAAACCCTACCGTCCTTCTCAAAGAGGGGCGAAC-3′/5′-AAGGTCTTCATACCGCATATTGAGGCCAAAAAGCTC-3′) and KOD Plus (Toyobo, Osaka, Japan) as a DNA polymerase. The amplified DNA fragment was subcloned into pUC118 at the HincII site. An inverse PCR was then carried out to amplify the flanking regions and the pUC118 backbone, thereby removing the coding region of the hps-phi gene by using primers PDHPSPHI-R/PDHPSPHI-F (5′-GACACGACCTCCGGAGATTTTTCAGTTGAAGGTTT-3′/5′-TGAGTGA GGGTCGCGATGTTCTCCTTTACCCATATT-3′). The amplified DNA fragment was ligated with the trpE marker cassette (1,423 bp) that was excised from pUMT2 (18) by PvuII. The marker cassette was oriented so that it was transcribed in the same direction as that of the target gene. The homologous regions for homologous recombination were sequenced to confirm the absence of unintended mutations. The resulting disruption plasmid was designated pUDhps-phi (6,585 bp, trpE marker) (Fig. 2).
FIG. 2.
Schematic diagram of targeted disruption of hps-phi in T. kodakaraensis KW128 by use of pUDhps-phi. Relevant regions of the chromosome are illustrated for strains KW128 and Δhps-phi-7A. The gray regions display the homologous regions for recombination. The positions of primer sets used for the analyses of targeted disruption of hps-phi (CHDHPSPHI-R/CHDHPSPHI-F and CHHPSPHI-R/CHHPSPHI-F) are indicated by arrows. The thick black bar indicates the region spanned by the trpE probe used in Southern blot analyses. Abbreviations: Hd, restriction site of HindIII; Hypo, hypothetical gene; P, putative pyrF promoter region.
Transformation of T. kodakaraensis.
Transformation of T. kodakaraensis with the trpE marker was performed as described previously (18). The cells transformed with pUDhps-phi were spread on a selective synthetic Trp− ASW-AA plate medium (ASW-AAW−) supplemented with 5 nucleosides, adenosine (A), thymidine (dT), guanosine (G), cytidine (C), and uridine (U) (0.1% each) (ASW-AAW−+5NS). After cultivation for 5 to 8 days at 85°C, the transformants grown on the plate medium, tryptophan prototrophs, were isolated and cultivated in ASW-YT medium. A further round of selection on ASW-AAW−+5NS medium was performed, and one of the isolated transformants was cultivated in ASW-YT medium and designated the Δhps-phi-7A strain.
The genotype of the Δhps-phi-7A strain was analyzed by PCR using primer sets that anneal outside the homologous regions (CHDHPSPHI-R/CHDHPSPHI-F [5′-TCAGCTTCTCCCTCAGCCTCTCTTCGACGTCTTTGACCTC-3′/5′-TC GGAATGGTGTCCGGATAAACGTGGAGCTTGTGTGGGTA-3′]) and inside the target gene (CHHPSPHI-R/CHHPSPHI-F [5′-CCCCTCATCAAGAAAGAAGGAATGAGGGCAGTAGAGCTCA-3′/5′-TCCTCGAAAAGAGTTCCCATAGGGGCTATCCATTTGTACT-3′]). The genotype was also confirmed by sequencing of the targeted regions and by Southern hybridization. Southern blot analysis was carried out with 5.0 μg of genomic DNA digested with HindIII, and the overall procedures were performed as described previously (18). The probe specific to the trpE gene was prepared as described previously (18).
Investigation of growth properties of the hps-phi disruptant with various substrates.
We investigated what substrates could support the growth of the hps-phi disruptant. We examined three combinations of substrates, A+dT+G+C+U (0.1% each), A+U (0.25% each), 2′-deoxyadenosine (dA)+2′-deoxyuridine (dU) (0.2% each), and nine individual substrates, A (0.25%), dT (0.5%), G (0.25%), C (0.5%), U (0.5%), inosine (I) (0.5%), GMP (0.5%), R5P (0.1%), and Ru5P (0.1%). Furthermore, 0.5% of sodium pyruvate (a gluconeogenic substrate) and 0.5% of maltodextrin (Nippon starch chemical; Osaka, Japan) (a glycolytic substrate) were also examined as control experiments. The pHs of all media were adjusted within 6.5 to 7.0.
RESULTS
Expression of the TK0475 gene in Escherichia coli and kinetic properties of the produced protein.
To clarify whether the TK0475 gene actually encodes an HPS-PHI-fused protein, we heterologously expressed this gene in E. coli and characterized the biochemical properties of the purified protein (rHps-Phi). Cell extracts from E. coli Rosetta(DE3)(pEThps-phi) exhibited activity of the combined overall HPS and PHI reaction (HPS/PHI) in both forward (formaldehyde-fixing) and reverse (Ru5P-synthesizing) directions, and a major band whose molecular mass (44 kDa) was consistent with the molecular mass of the TK0475 gene product was detected by SDS-PAGE (data not shown). The rHps-Phi was purified 15.1-fold from the cell extract as described in Materials and Methods (Table 2) to apparent homogeneity judged by SDS-PAGE (data not shown) and was found to exhibit both forward and reverse HPS/PHI activities. These results indicated that the TK0475 gene indeed encoded an HPS-PHI-fused bifunctional enzyme.
TABLE 2.
Purification of rHps-Phi from E. coli Rosetta(DE3)(pEThps-phi)
| Step | Activitya (U) | Protein (mg) | Sp act (U/mg protein) | Recovery (%) | Purification (fold) |
|---|---|---|---|---|---|
| Cell-free extract | 145 | 296 | 9.80 | 100 | 1.0 |
| Membrane fraction | 77.0 | 125 | 15.4 | 66.4 | 1.57 |
| Washed membrane | 49.6 | 42.0 | 29.5 | 42.8 | 3.01 |
| Solubilization | 34.0 | 9.50 | 89.5 | 29.3 | 9.13 |
| Heat treatment at 80°C | 35.4 | 6.0 | 148 | 30.5 | 15.1 |
Activities were HPS/PHI forward activities assayed as described in Materials and Methods.
The kinetic parameters of purified rHps-Phi protein were determined (Table 3) . The apparent Vmax of the forward reaction was 134 U/mg under condition I or 132 U/mg under condition II, and that of the reverse reaction was 20.9 U/mg; these results were comparable to results for the purified protein of rHps-Phi from P. horikoshii (12). The catalytic efficiency (kcat/Km) of the formaldehyde-fixing reaction was 99.1 or 193 mM−1 s−1 for formaldehyde or Ru5P, respectively, while that of the Ru5P-synthesizing reaction was 21.2 mM−1 s−1. Although the catalytic efficiency of the former reaction was higher than that of the latter reaction in vitro, our results clearly indicate that rHps-Phi can catalyze the synthesis of Ru5P from F6P.
TABLE 3.
Kinetic parameters of purified rHps-Phi at 80°C
| Condition | Apparent Km (mM)a | Vmax (U/mg)a | kcat (s−1) | kcat/Km (mM−1 s−1) |
|---|---|---|---|---|
| I | 0.993 ± 0.285 | 134 ± 26.1 | 98.4 | 99.1 |
| II | 0.502 ± 0.004 | 132 ± 5.77 | 96.9 | 193 |
| III | 0.725 ± 0.181 | 20.9 ± 3.97 | 15.3 | 21.2 |
Km and Vmax were obtained by double reciprocal plots of HPS/PHI activity versus concentrations of formaldehyde (condition I), Ru5P (condition II), and F6P (condition III), as described in Materials and Methods. The means ± standard deviations of three independent experiments are presented.
HPS/PHI activity in T. kodakaraensis KOD1.
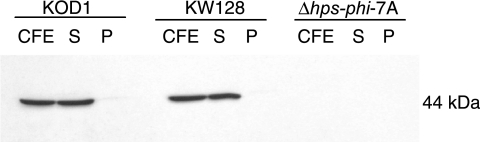
We examined HPS/PHI activity for the forward and reverse reactions in cell extract from T. kodakaraensis KOD1 cultivated under standard conditions without formaldehyde. The specific activities for the HPS/PHI forward reaction and reverse reaction were found to be 0.160 and 0.043 U per mg protein, respectively. This level of activity for the HPS/PHI forward reaction was comparable to the constitutive activity levels observed for P. horikoshii OT3 (0.250 U per mg protein) and the induced level for Bacillus subtilis (0.310 U per mg protein) (12, 25). Western blot analysis using anti-HPS antibodies revealed a positive signal corresponding to 44 kDa, thereby confirming the production of this protein in T. kodakaraensis (Fig. 3). As the HPS-PHI-fused enzyme is produced even in the absence of formaldehyde, the protein is most likely constitutive, as in the case of the enzyme in P. horikoshii (12), supporting a general metabolic role of the enzyme.
FIG. 3.
Western blot analyses of cell extracts of T. kodakaraensis strains KOD1, KW128, and Δhps-phi-7A. Abbreviations: CFE, cell extract; S, soluble fraction; P, particulate fraction.
Construction of hps-phi deletion mutant.
A gene disruption system for T. kodakaraensis has been developed and utilized for the analysis of gene function (3, 16-18). Using this system, we aimed to investigate the in vivo function of the hps-phi gene, focusing on whether this gene participates in the biosynthesis of Ru5P, the precursor of nucleotides. We constructed the disruption vector of the hps-phi gene, pUDhps-phi (Fig. 2), and transformed the KW128 strain (ΔpyrF trpE::pyrF) with the vector as described in Materials and Methods. As the hps-phi deletion mutant was expected to require exogenous nucleotides for growth, the selection medium ASW-AAW− was supplemented with five nucleosides, A, dT, G, C, and U. We applied nucleosides instead of nucleotides, since the phosphate group may have a negative effect on membrane permeability. Tryptophan prototrophs were obtained with a transformation efficiency of approximately 2 × 101/μg DNA, while a control experiment without the exogenous DNA gave no tryptophan prototrophs. One of the isolates was designated strain Δhps-phi-7A and examined further.
Confirmation of hps-phi disruption in the isolated mutant strain.
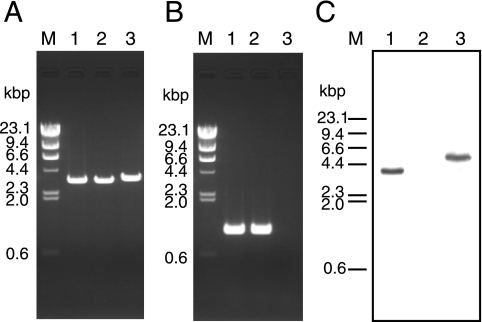
The genotype of the Δhps-phi-7A strain was confirmed by PCR, sequencing, and Southern blot analyses. Amplification of a DNA fragment with a length corresponding to that of a Δhps-phi::trpE locus formed by double-crossover recombination was observed with the Δhps-phi-7A strain (3.6 kbp against 3.4 kbp in KOD1 and KW128) (Fig. 4A). No amplification was observed with the Δhps-phi-7A strain by PCR using primer sets that anneal within the target gene (Fig. 4B), indicating complete deletion of the target gene. The replacement of hps-phi by the trpE marker was also confirmed by sequencing analysis of the targeted regions and Southern blot analysis using the probe specific to the trpE gene (Fig. 2). As shown in Fig. 4C, wild-type KOD1 showed single signals derived from endogenous trpE in the trp operon (3.7 kbp), while no positive signal could be detected in the host strain KW128, with a ΔtrpE::pyrF genotype. In the Δhps-phi-7A strain, the signals could be detected, with the expected mobility corresponding to a trpE insertion within the target locus (4.9 kbp) (Fig. 4C). We also confirmed the absence of HPS-PHI proteins in the Δhps-phi-7A strain by Western blot analysis (Fig. 3).
FIG. 4.
PCR and Southern blot analyses of T. kodakaraensis Δhps-phi-7A (ΔpyrF ΔtrpE::pyrF Δhps-phi::trpE). (A) Amplification of the locus containing hps-phi and its homologous regions in strains KOD1 (lane 1), KW128 (lane 2), and Δhps-phi-7A (lane 3), using CHDHPSPHI-R/CHDHPSPHI-F as primers. M represents the DNA size marker, HindIII-digested λ DNA. (B) Amplification of the region within the hps-phi gene in T. kodakaraensis KOD1 (lane 1), KW128 (lane 2), and Δhps-phi-7A (lane 3), using CHHPSPHI-R/CHHPSPHI-F as primers. Primers used for these analyses are displayed in Fig. 2. (C) Southern blot analysis of T. kodakaraensis Δhps-phi-7A. The probe specific to the trpE gene was used against genomic DNA of KOD1 (lane 1), KW128 (lane 2), and Δhps-phi-7A (lane 3) digested with HindIII. The bars on the left side of the panels indicate the lengths of fragments in the DNA size marker, HindIII-digested λ DNA. The region spanned by the probe used for this analysis is indicated in Fig. 2.
Nucleoside requirement for growth of the hps-phi deletion mutant.
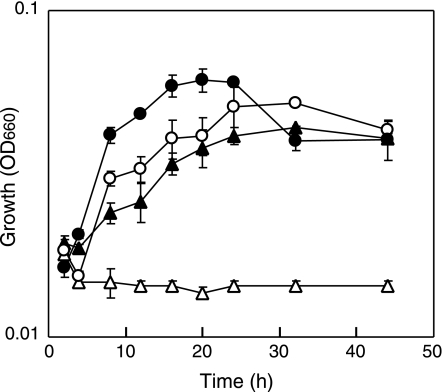
We investigated whether the Δhps-phi-7A strain showed auxotrophy for nucleosides, the precursor of nucleotides. The host strain KW128 and the mutant Δhps-phi-7A were cultivated in ASW-AA medium with or without supplementation of five nucleosides, A, dT, G, C, and U. As shown in Fig. 5, the Δhps-phi-7A strain did not exhibit any growth in ASW-AA medium, while growth of the same strain could be recovered in a medium supplemented with the five nucleosides (ASW-AA+5NS medium). The host strain could grow in both ASW-AA and ASW-AA+5NS media. This indicates that the disruption of the hps-phi gene resulted in a nucleoside-auxotrophic phenotype in T. kodakaraensis. Considering the catalytic function of the rHps-Phi mentioned above and the distribution of these genes (19), we can presume that Ru5P, the precursor of nucleotides and eventually deoxynucleotides, is produced from F6P via the hps-phi gene product in T. kodakaraensis. The growth defect of the deletion mutant also indicates that the metabolic pathway in which the hps-phi gene functions is the sole pathway leading to the production of Ru5P and nucleotides.
FIG. 5.
Growth properties of T. kodakaraensis strains KW128 and Δhps-phi-7A in minimal medium, ASW-AA (open symbols), or a medium supplemented with five nucleosides (filled symbols). The cells were cultured at 85°C in ASW-AA media without or supplemented with five nucleosides, A, dT, G, C, and U. Symbols: open circles, KW128 without nucleosides; filled circles, KW128 with nucleosides; open triangles, Δhps-phi-7A without nucleosides; filled triangles, Δhps-phi-7A with nucleosides. Error bars represent the standard deviations from three independent experiments. OD660, optical density at 660 nm.
Growth properties of the hps-phi deletion mutant with various substrates.
We examined which substrates could complement the growth defect of the Δhps-phi-7A strain. The substrates tested were A+U, dA+dU, A, dT, G, C, U, I, and the nucleoside phosphate GMP. Pyruvate (a gluconeogenic substrate) and maltodextrin (a glycolytic substrate) were also examined as controls to exclude the possibility that the disruptant did not grow in the ASW-AA medium due to shortage of the carbon source. The results are summarized in Table 4. The Δhps-phi-7A strain could grow with A+U, A, G, C, U, I, or GMP in addition to the growth with A+dT+G+C+U as shown in Fig. 5. On the other hand, the disruptant could not grow with dA+dU, dT, pyruvate, or maltodextrin. These results indicate that nucleosides could complement the defect of the hps-phi gene in T. kodakaraensis, whereas deoxynucleosides could not. We also found that the nucleotide GMP could support the growth of the disruptant, suggesting that nucleotide uptake occurs in T. kodakaraensis cells. We tested the effect of adding 0.1% of Ru5P, the product of hps-phi, or 0.1% of R5P, the metabolite downstream of Ru5P in the usual PPP. However, the disruptant could not grow with either of these substrates, probably due to insufficient uptake of the pentose phosphates. T. kodakaraensis, along with many other hyperthermophilic archaea, is known to grow on oligohexosaccharides but is not able to utilize monomeric glucose (2).
TABLE 4.
Growth properties of T. kodakaraensis Δhps-phi-7A
| Medium | % Concn of substrate (total concn [%]) | Result fora:
|
|
|---|---|---|---|
| KW128 (host strain) | Δhps-phi-7A | ||
| ASW-AA | + | − | |
| ASW-AA + A+dT+G+C+U | 0.1 each (0.5) | + | + |
| ASW-AA + A+U | 0.25 each (0.5) | + | + |
| ASW-AA + dA+dU | 0.2 each (0.4) | + | − |
| ASW-AA + A | 0.25 | + | + |
| ASW-AA + dT | 0.5 | + | − |
| ASW-AA + G | 0.25 | + | + |
| ASW-AA + C | 0.5 | + | + |
| ASW-AA + U | 0.5 | + | + |
| ASW-AA + I | 0.5 | + | + |
| ASW-AA + GMP | 0.5 | + | + |
| ASW-AA + pyruvate | 0.5 | + | − |
| ASW-AA + maltodextrin | 0.5 | + | − |
+, growth; −, no growth.
DISCUSSION
In this report we demonstrate that the RuMP pathway is the only relevant pathway for the biosynthesis of Ru5P, which is used to build the pentose moiety of nucleotides in T. kodakaraensis. T. kodakaraensis KOD1 produced a bifunctional enzyme encoded by the TK0475 gene, which exhibited both HPS and PHI activities in both directions (formaldehyde fixing and Ru5P synthesizing). The nucleoside auxotrophy of the hps-phi deletion mutant together with the catalytic properties of the HPS-PHI-fused protein strongly imply that HPS-PHI is essential for growth and functions in the biosynthesis of nucleotides and eventually deoxynucleotides in T. kodakaraensis by generating Ru5P from F6P. Kinetic properties of rHps-Phi have shown that the catalytic efficiency for the formaldehyde-fixing reaction was higher than that for the Ru5P-synthesizing reaction. However, as the RuMP pathway is most likely the sole pathway for Ru5P synthesis, the continuous consumption of Ru5P for nucleotide biosynthesis should drive the reaction in the direction of Ru5P synthesis. The activity level of HPS/PHI for the Ru5P-synthesizing reaction in the cell extracts (0.043 U per mg protein) was much higher than the estimated specific rate for R5P synthesis in archaea (0.0003 U per mg protein) (6), and it is therefore feasible that the HPS-PHI-fused protein is responsible for R5P biosynthesis in this organism.
From the genome sequence data, Ru5P can be expected to be isomerized into R5P by RPI (TK1426) and subsequently converted into phosphoribosyl pyrophosphate (PRPP) by PRPP synthase (TK2235). PRPP can then be utilized for nucleotide biosynthesis via conventional pyrimidine and purine nucleotide biosynthetic pathways, of which members are apparently present in T. kodakaraensis. These findings suggest that the wide distribution of HPS and PHI orthologs, coinciding with the absence of pentose phosphate enzyme genes, raises the possibility that this pathway is utilized in a majority of Archaea. Furthermore, all Archaea whose genomes have been sequenced possess both RPI and PRPP synthase orthologs, except for the archaeal parasite Nanoarchaeum equitans. Therefore, it can be presumed that the pathways for nucleotide biosynthesis downstream of Ru5P are generally shared in all three domains of life, Archaea, Bacteria, and Eucarya. T. kodakaraensis harbors orthologs of transketolase (N-terminal subunit, TK0270; C-terminal subunit, TK0269) (Fig. 1). The most important function of transketolase in this organism can be expected to be supplying E4P, the precursor of chorismate and eventually aromatic amino acids. However, several archaeal species possess an alternate pathway for aromatic amino acid biosynthesis that does not involve E4P as a precursor. Therefore, transketolase may well have another function. These hypotheses have been described in a previous report (19).
We were surprised to find that single nucleosides (A, G, C, U, and I) could complement the hps-phi disruption in T. kodakaraensis, indicating that all nucleotides can be generated from any one of these nucleosides. Considering the genome sequence data, PRPP production in media including the individual nucleosides A, G, I, C, and U can be explained as follows: adenosine, guanosine, and inosine can be converted to ribose 1-phosphate (R1P) by purine nucleoside phosphorylase. An enzyme from Pyrococcus furiosus (PF0016) has been shown to exhibit activity towards all three purine nucleosides (4), and a closely related homolog (81% identical) is present in T. kodakaraensis (TK1895). Cytidine can be converted into uridine by cytidine deaminase (TK1414). Therefore, both cytidine and uridine can provide R1P via uridine phosphorylase (TK1479). The R1P can then be converted into R5P by phosphopentomutase (13) and then flow into the general pathway for nucleotide synthesis.
As HPS and PHI have been found to function in Ru5P synthesis, this also results in the generation of toxic formaldehyde. One of the mechanisms known for formaldehyde detoxification is the system involving formaldehyde-activating enzyme (Fae) (21). Fae catalyzes the condensation of formaldehyde and tetrahydromethanopterin to methylene-tetrahydromethanopterin. The methanogenic archaea Methanosarcina barkeri and Methanosarcina mazei possess a gene encoding an Fae-HPS-fused enzyme, and Fae might be involved in the detoxification of formaldehyde generated during Ru5P biosynthesis (6). T. kodakaraensis does not have an Fae homolog but does harbor a formaldehyde ferredoxin oxidoreductase homolog (14). Further analyses will be necessary to clarify the involvement of ferredoxin oxidoreductase or other enzymes in the detoxification of formaldehyde in archaeal strains that do not contain Fae.
Acknowledgments
This research was supported in part by a Grant-in-Aid for Scientific Research (S) 13854008 to Y.S. This paper is supported in part by COE for Microbial-Process Development Pioneering Future Production System (COE program of the Ministry of Education, Culture, Sports, Science and Technology, Japan). A Grant-in-Aid for Scientific Research to T.I. (14103011) and a Grant-in-Aid for JSPS Fellows to T.S. (15005649) are also acknowledged.
REFERENCES
- 1.Arfman, N., L. Bystrykh, N. I. Govorukhina, and L. Dijkhuizen. 1990. 3-Hexulose-6-phosphate synthase from thermotolerant methylotroph Bacillus C1. Methods Enzymol. 188:391-397. [DOI] [PubMed] [Google Scholar]
- 2.Atomi, H., T. Fukui, T. Kanai, M. Morikawa, and T. Imanaka. 2004. Description of Thermococcus kodakaraensis sp. nov., a well studied hyperthermophilic archaeon previously reported as Pyrococcus sp. KOD1. Archaea 1:263-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atomi, H., R. Matsumi, and T. Imanaka. 2004. Reverse gyrase is not a prerequisite for hyperthermophilic life. J. Bacteriol. 186:4829-4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cacciapuoti, G., C. Bertoldo, A. Brio, V. Zappia, and M. Porcelli. 2003. Purification and characterization of 5′-methylthioadenosine phosphorylase from the hyperthermophilic archaeon Pyrococcus furiosus: substrate specificity and primary structure analysis. Extremophiles 7:159-168. [DOI] [PubMed] [Google Scholar]
- 5.Fukui, T., H. Atomi, T. Kanai, R. Matsumi, S. Fujiwara, and T. Imanaka. 2005. Complete genome sequence of the hyperthermophilic archaeon Thermococcus kodakaraensis KOD1 and comparison with Pyrococcus genomes. Genome Res. 15:352-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goenrich, M., R. K. Thauer, H. Yurimoto, and N. Kato. 2005. Formaldehyde activating enzyme (Fae) and hexulose-6-phosphate synthase (Hps) in Methanosarcina barkeri: a possible function in ribose-5-phosphate biosynthesis. Arch. Microbiol. 184:41-48. [DOI] [PubMed] [Google Scholar]
- 7.Grochowski, L. L., H. Xu, and R. H. White. 2005. Ribose-5-phosphate biosynthesis in Methanocaldococcus jannaschii occurs in the absence of a pentose-phosphate pathway. J. Bacteriol. 187:7382-7389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kato, N., H. Yurimoto, and R. K. Thauer. 2006. The physiological role of the ribulose monophosphate pathway in bacteria and archaea. Biosci. Biotechnol. Biochem. 70:10-21. [DOI] [PubMed] [Google Scholar]
- 9.Mitsui, R., Y. Sakai, H. Yasueda, and N. Kato. 2000. A novel operon encoding formaldehyde fixation: the ribulose monophosphate pathway in the gram-positive facultative methylotrophic bacterium Mycobacterium gastri MB19. J. Bacteriol. 182:944-948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morikawa, M., Y. Izawa, N. Rashid, T. Hoaki, and T. Imanaka. 1994. Purification and characterization of a thermostable thiol protease from a newly isolated hyperthermophilic Pyrococcus sp. Appl. Environ. Microbiol. 60:4559-4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nash, T. 1953. The colorimetric estimation of formaldehyde by means of the Hantzsch reaction. Biochem. J. 55:416-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orita, I., H. Yurimoto, R. Hirai, Y. Kawarabayasi, Y. Sakai, and N. Kato. 2005. The archaeon Pyrococcus horikoshii possesses a bifunctional enzyme for formaldehyde fixation via the ribulose monophosphate pathway. J. Bacteriol. 187:3636-3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rashid, N., H. Imanaka, T. Fukui, H. Atomi, and T. Imanaka. 2004. Presence of a novel phosphopentomutase and a 2-deoxyribose 5-phosphate aldolase reveals a metabolic link between pentoses and central carbon metabolism in the hyperthermophilic archaeon Thermococcus kodakaraensis. J. Bacteriol. 186:4185-4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roy, R., S. Mukund, G. J. Schut, D. M. Dunnn, R. Weiss, and M. W. W. Adams. 1999. Purification and molecular characterization of the tungsten-containing formaldehyde ferredoxin oxidoreductase from the hyperthermophilic archaeon Pyrococcus furiosus: the third of a putative five-member tungstoenzyme family. J. Bacteriol. 181:1171-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakai, Y., R. Mitsui, Y. Katayama, H. Yanase, and N. Kato. 1999. Organization of the genes involved in the ribulose monophosphate pathway in an obligate methylotrophic bacterium, Methylomonas aminofaciens 77a. FEMS Microbiol. Lett. 176:125-130. [DOI] [PubMed] [Google Scholar]
- 16.Sato, T., T. Fukui, H. Atomi, and T. Imanaka. 2005. Improved and versatile transformation system allowing multiple genetic manipulations of the hyperthermophilic archaeon Thermococcus kodakaraensis. Appl. Environ. Microbiol. 71:3889-3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sato, T., T. Fukui, H. Atomi, and T. Imanaka. 2003. Targeted gene disruption by homologous recombination in the hyperthermophilic archaeon Thermococcus kodakaraensis KOD1. J. Bacteriol. 185:210-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sato, T., H. Imanaka, N. Rashid, T. Fukui, H. Atomi, and T. Imanaka. 2004. Genetic evidence identifying the true gluconeogenic fructose-1,6-bisphosphatase in Thermococcus kodakaraensis and other hyperthermophiles. J. Bacteriol. 186:5799-5807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soderberg, T. 2005. Biosynthesis of ribose-5-phosphate and erythrose-4-phosphate in archaea: a phylogenetic analysis of archaeal genomes. Archaea 1:347-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verhees, C. H., S. W. M. Kengen, J. E. Tuininga, G. J. Schut, M. W. W. Adams, W. M. de Vos, and J. van der Oost. 2003. The unique features of glycolytic pathways in Archaea. Biochem. J. 375:231-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vorholt, J. A., C. J. Marx, M. E. Lidstrom, and R. K. Thauer. 2000. Novel formaldehyde-activating enzyme in Methylobacterium extorquens AM1 required for growth on methanol. J. Bacteriol. 182:6645-6650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Worthington, P., V. Hoang, F. Perez-Pomares, and P. Blum. 2003. Targeted disruption of the α-amylase gene in the hyperthermophilic archaeon Sulfolobus solfataricus. J. Bacteriol. 185:482-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xavier, K. B., M. S. Da Costa, and H. Santos. 2000. Demonstration of a novel glycolytic pathway in the hyperthermophilic archaeon Thermococcus zilligii by 13C-labeling experiments and nuclear magnetic resonance analysis. J. Bacteriol. 182:4632-4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yanase, H., K. Ikeyama, R. Mitsui, R. Sonmin, K. Kita, Y. Sakai, and N. Kato. 1996. Cloning and sequence analysis of the gene encoding 3-hexulose-6-phosphate synthase from the methylotrophic bacterium, Methylomonas aminofaciens 77a, and its expression in Escherichia coli. FEMS Microbiol. Lett. 135:201-205. [DOI] [PubMed] [Google Scholar]
- 25.Yasueda, H., Y. Kawahara, and S. Sugimoto. 1999. Bacillus subtilis yckG and yckF encode two key enzymes of the ribulose monophosphate pathway used by methylotrophs, and yckH is required for their expression. J. Bacteriol. 181:7154-7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yurimoto, H., R. Hirai, H. Yasueda, R. Mitsui, Y. Sakai, and N. Kato. 2002. The ribulose monophosphate pathway operon encoding formaldehyde fixation in a thermotolerant methylotroph, Bacillus brevis S1. FEMS Microbiol. Lett. 214:189-193. [DOI] [PubMed] [Google Scholar]
- 27.Yurimoto, H., N. Kato, and Y. Sakai. 2005. Assimilation, dissimilation, and detoxification of formaldehyde, a central metabolic intermediate of methylotrophic metabolism. Chem. Rec. 5:367-375. [DOI] [PubMed] [Google Scholar]