Abstract
Using mixed-species cultures, we have undertaken a study of interactions between two common spore-forming soil bacteria, Bacillus subtilis and Streptomyces coelicolor. Our experiments demonstrate that the development of aerial hyphae and spores by S. coelicolor is inhibited by surfactin, a lipopeptide surfactant produced by B. subtilis. Current models of aerial development by sporulating bacteria and fungi postulate a role for surfactants in reducing surface tension at air-liquid interfaces, thereby removing the major barrier to aerial growth. S. coelicolor produces SapB, an amphipathic peptide that is surface active and required for aerial growth on certain media. Loss of aerial hyphae in developmental mutants can be rescued by addition of purified SapB. While a surfactant from a fungus can substitute for SapB in a mutant that lacks aerial hyphae, not all surfactants have this effect. We show that surfactin is required for formation of aerial structures on the surface of B. subtilis colonies. However, in contrast to this positive role, our experiments reveal that surfactin acts antagonistically by arresting S. coelicolor aerial development and causing altered expression of developmental genes. Our observations support the idea that surfactants function specifically for a given organism regardless of their shared ability to reduce surface tension. Production of surfactants with antagonistic activity could provide a powerful competitive advantage during surface colonization and in competition for resources.
Microbial populations in natural settings almost invariably consist of complex mixtures of species, yet most laboratory studies of bacterial physiology have focused on the artificial setting of single-species cultures. Relatively little is known, at the molecular level, about how bacteria of different species interact with each other when cocultivated. Nonetheless, what little we know about interspecies interactions makes it clear that individual cellular physiology and intercellular signaling can be greatly affected when two species are cocultured (12, 13, 31). As part of our ongoing interest in interspecies interactions, we have begun to analyze the effects of cocultivating two well-characterized soil bacteria that, in all likelihood, share habitats in the wild: Bacillus subtilis and Streptomyces coelicolor (1). Our rationale is that by studying cocultures of genetically tractable microorganisms that coexist in nature, we might be able to uncover some of the molecular mechanisms underlying their interactions.
Our interest in studying possible interactions between B. subtilis and S. coelicolor is partly driven by the fact that both of these species enter elaborate developmental pathways as a consequence of nutrient limitation (3). These developmental pathways have been extensively studied and are characterized by the production of numerous secreted secondary metabolites and the formation of spores (4). Up to now, our knowledge of the molecular mechanisms governing secondary metabolite production and sporulation by these two bacteria has been derived from studies utilizing exclusively pure cultures. We were thus interested in determining if the developmental pathways of B. subtilis and S. coelicolor were in any way affected during cocultivation.
A widespread feature of sporulating bacteria and fungi is that aerial structures give rise to spores. Clear examples of this are aerial hypha formation by streptomycetes (9) and fruiting-body formation by myxobacteria (7). The notion is that by erecting aerial structures and having spores preferentially develop in those structures, microbes greatly increase their spore dispersal capacity (1). While the formation of spore-bearing aerial hyphae by S. coelicolor has been recognized for many years, it was not until recently that B. subtilis sporulation was shown to involve preferential development of spores at the tips of aerial structures. The B. subtilis fruiting-body-like structures that serve as preferential sites for sporulation were recognized in the context of biofilms formed by wild isolates of this bacterium and appear to be a developmental feature that has been lost in many laboratory strains (2).
The process of erecting an aerial structure probably requires that a growing microorganism first reduce the water surface tension of the water-air interface. It appears that a common solution to this challenge is the production of surfactants, amphiphilic molecules with the intrinsic capacity to reduce surface tension (9). For S. coelicolor, the primary surfactant involved in erecting aerial hyphae is the secreted peptide SapB (16). Additionally, a set of eight surface-active peptides collectively called the chaplins act during development of aerial hyphae (6, 8). Wild-type S. coelicolor colonies have a hairy morphology due to the presence of aerial hyphae, while developmental mutants that are unable to produce aerial hyphae are referred to as bald (bld) mutants (34). The bld mutants have been ordered into a developmental pathway that culminates with the synthesis of SapB. Addition of purified SapB to bald mutant colonies restores their hairy phenotype (33). Quite importantly, a protein from the basidiomycete Schizophyllum commune (SC3) known for its role in aerial structure formation in that fungus (32) can supplant SapB in promoting aerial hyphal development in S. coelicolor bald mutants (28).
The fact that exogenously applied SapB and SC3 can recover aerial hypha formation but not spore formation on S. coelicolor bld mutants has lent support to the idea that surfactants play a mechanical role in this process by reducing surface tension (37). On the basis of this model, one might predict that, in general, surfactants of comparable activity from other species could replace the loss of SapB activity in a bld mutant. However, this was previously shown not to be the case by using surfactin and fengycin from B. subtilis and viscosin from Pseudomonas (24). In this report, however, we show that the most striking effect on S. coelicolor physiology when it is cocultivated with B. subtilis is that development of aerial hyphae in a wild-type strain is blocked. Quite surprisingly and contrary to expectation, we discovered that the blockage is due to B. subtilis' ability to produce the surfactant lipopeptide surfactin. In addition, we show that surfactin is required for raising the aerial structures that B. subtilis produces. The fact that a surfactant involved in raising aerial structures in B. subtilis not only does not supplant SapB in S. coelicolor (24) but actually antagonizes its activity suggests that surfactants may be playing additional roles in raising aerial structures over and beyond lowering surface tension.
MATERIALS AND METHODS
Bacterial strains and media.
B. subtilis strain 3610 is a prototrophic undomesticated strain related to B. subtilis168 (2). B. subtilis 168 and PY79 were used for genetic manipulation and as domesticated strain controls. Media used were LB (1% Bacto Tryptone, 0.5% yeast extract [Difco], 0.5% NaCl [Mallinckrodt]), YEME-pH 7 (1% Bacto Malt Extract, 0.4% yeast extract, 0.4% d-glucose [pH 7.0], buffered with 100 mM morpholinepropanesulfonic acid [MOPS] and 5 mM potassium phosphate), and MSgg (5 mM potassium phosphate [pH 7], 100 mM MOPS [pH 7], 2 mM MgCl2, 700 μM CaCl2, 50 μM MnCl2, 50 μM FeCl3, 1 μM ZnCl2, 2 μM thiamine, 0.5% glycerol, 0.5% glutamate, 50 μg of tryptophan/ml, 50 μg of phenylalanine/ml) (adapted from reference 2). Solid media contained 1.5% Bacto Agar. Antibiotics were used at the following concentrations: spectinomycin, 100 μg/ml; erythromycin, 0.4 μg/ml.
Transposon mutagenesis and mutant screening.
Strain 3610 was transposon mutagenized with a mini-Tn10 transposon (27). Cells containing the plasmid pIC333 were grown from freezer stocks in 5 ml of LB with erythromycin at room temperature overnight. Overnight cultures were diluted 1:100 into LB with spectinomycin and incubated for 3 h at room temperature. Cultures were shifted to 37°C and incubated for 4.5 h in LB-spectinomycin. Samples were removed from culture, serially diluted, and plated on the following prewarmed (37°C) media: 1 × 10−4 and 1 × 10−5 (100 μl) on LB-spectinomycin and 1 × 10−6 (100 μl) on LB. Efficiency of transformation was calculated as the ratio of the number of spectinomycin-resistant CFU on LB-spectinomycin to the number of CFU on LB. In general, a transposition efficiency of 0.2 to 1.0% was observed. Plates were replica plated to LB-erythromycin to determine the frequency of plasmid integration (expected, 0.01%; observed, 0.01%). Colonies were picked from LB-spectinomycin plates and transferred to 96-well plates containing 200 μl of LB-spectinomycin per well. Cultures were grown overnight at 37°C. To carry out the screen, a lawn of S. coelicolor spores (0.5 × 106 to 1.5 × 106 spores per plate) was plated on YEME-pH 7 plates (predried for 20 min). Aliquots (2.5 μl) from an overnight culture of each transposon mutant were spotted onto lawns of S. coelicolor at a density of 24 spots per plate, allowed to dry, and incubated at 30°C for 3 days.
Plating assays and surfactant treatment.
Spore suspensions of S. coelicolor were generated with a sterile inoculating loop to scrape spores from an agar surface. Spores were placed in sterile water, vortexed, and dissociated in a sonicating water bath twice for 15 s to produce a uniform suspension. For plating assays, the spore suspensions (∼1 × 107 spores/ml) were diluted 1:10 and 100 μl was spread onto the agar surface of a petri dish. Following this procedure, a confluent lawn of S. coelicolor vegetative hyphae developed within 2 days at 30°C and aerial development was visible after 3 days at 30°C.
To perform coculture assays, we plated a lawn of S. coelicolor spores as already described and then spotted 2 μl of B. subtilis from a 5-ml overnight culture in LB medium. Purified surfactin (catalog no. S-3523; Sigma-Aldrich, St. Louis, MO) was applied as 50 μl of a 10-μg/μl solution in 100% ethanol to a sterile Whatman filter disk (no. 1, 0.25-in. diameter) on a lawn of S. coelicolor spores.
Extraction of chaplins from cell walls.
To generate a suspension of purified chaplin peptides, isolated cell wall fragments from a rodlin-deficient strain of Streptomyces lividans, M145ΔrdlAB, were extracted with trifluoroacetic acid (TFA) as described by Claessen et al. (6). Desiccated cell wall material was resuspended in 500 μl of TFA and vortexed for 5 min. After the samples were briefly centrifuged to remove solid material, the TFA fraction was transferred to a clean tube and the TFA was evaporated under a stream of air. The extracted chaplin material was gently resuspended in 500 μl of sterile water and used immediately.
Chaplin extract (in water) and SapB (1 mg/ml in dimethyl sulfoxide [DMSO]) were applied to plates of surfactin-treated S. coelicolor by soaking a strip of nylon filter (10 mm by 2 mm) with chaplins or SapB and then placing the middle of the filter at a distance of 1.5 cm from the edge of a surfactin-treated Whatman filter disk on a lawn of S. coelicolor spores. Plates were incubated at 30°C for 3 days.
Quantitative real-time reverse transcriptase PCR (RT-PCR) analysis of S. coelicolor surfactant gene expression.
Real-time RT-PCR experiments were performed with triplicate reaction mixtures. The experiments were carried out three times with RNAs from three separate isolations, each isolation utilizing two plates of surfactin-treated or untreated S. coelicolor. RNA isolation was performed by growing S. coelicolor on YEME-pH 7 plates with a sheet of sterile cellophane (catalog no. 500PU; UCB, Smyrna, GA) placed on top and a thin (3-ml) layer of YEME-pH 7-1.5% agar poured on top of the cellophane. Cells were harvested following approximately 3 days of incubation at 30°C, when the onset of aerial development was visible outside the bald-halo zone of surfactin treatment. The cellophane layer was removed from the plate, and patches of cells from the surfactin-treated and untreated areas of the plate were excised with a scalpel. The sheet of cells was immersed in ice-cold 100% methanol for 30 min. Cell material was then pelleted by centrifugation, and total RNA was extracted by the hot phenol-sodium dodecyl sulfate method (10).
Real-time RT-PCR amplification was performed with the QIAGEN QuantiTect SYBR green RT-PCR kit according to the manufacturer's instructions. RNA was pretreated with the Promega RQ1 RNase-free DNase set (Promega) prior to experiments. Controls consisted of reaction mixtures without RT. For each sample RNA, standard curves were generated with twofold serial dilutions of template RNA following measurement of the total RNA concentration. Cycle threshold (CT) values were converted to relative RNA concentrations, and each sample value was normalized to the rpoB controls for a given experiment. Values reported are averages of triplicate samples from a single representative experiment. Primers used for real-time RT-PCR analysis were as follows (5′ to 3′): chpA-Forward, CTCGTCCTCGTCCTCGACTT; chpA-Reverse, GTCGTTCTCGCACTTGTTGC; chpH-Forward, CACCGGTGGTCTGGTTCTC; chpH-Reverse, ATCACGGAGATCGTGTTGC; rpoB-Forward, GAGTTCGGCGAGTACGAGTC; rpoB-Reverse, CGCTTCGGGTTGAAGTAGAG; ramC-Forward, CGGACCCGTACTTCTACGAC; ramC-Reverse, CGGAGACATGGATCTTCCAC. Real-time RT-PCR was carried out with an ABI 7700 cycler, and data were analyzed with ABI software. Amplification cycles consisted of 50°C for 30 min and 95°C for 15 min, followed by 40 cycles of 95°C for 15 s, 55°C for 30 s, and 72°C for 30 s. Analysis of data derived from CT values for each sample was performed as described in the ABI quantitative PCR protocol pamphlet (http://docs.appliedbiosystems.com, ABI 7700 user bulletin no. 2). We used CT values from twofold serial dilutions to generate standard curves for each sample. For all samples, CT values were converted to log nanogram values and then replicate experiments were averaged and a standard deviation was calculated. Average values for each sample were compared to the standard curve for that sample to generate the relative mRNA concentration. The concentration of each sample was normalized to the calculated concentration of rpoB mRNA from treated and untreated samples. Surfactin-treated and untreated samples were compared to determine the relative change in expression values reported.
Purification of SapB and Western blot analysis.
Extracellular protein was extracted from S. coelicolor M145, and Western blot assays were performed as previously described (33). S. coelicolor cell material was scraped from the surface of plates following incubation at 30°C for 2.5 days. At the first sign of visible aerial development on control plates, cells were scraped from the area surrounding filters treated with either surfactin or ethanol as described above. Protein was extracted with alkaline buffer, and the extracted material was TCA precipitated for use in Western blot analysis.
RESULTS
B. subtilis inhibits aerial hyphal development of S. coelicolor.
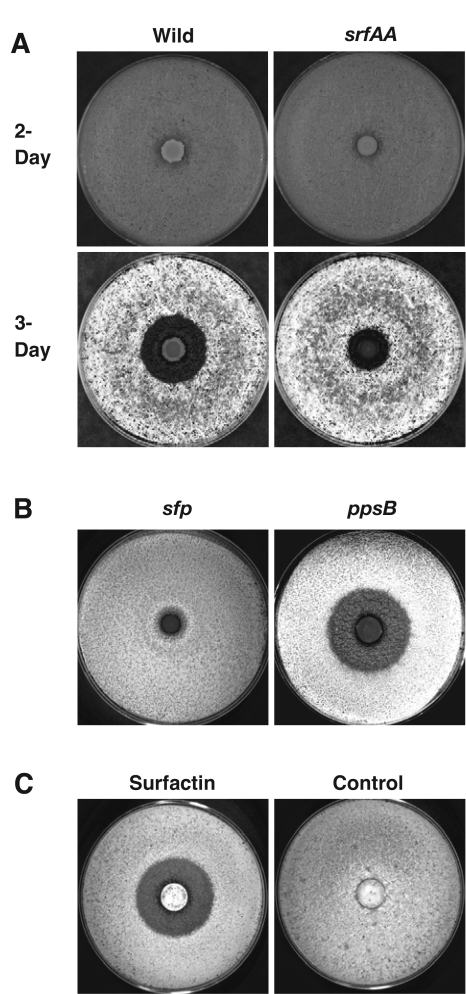
To examine the effect of diffusible compounds on interactions between B. subtilis and S. coelicolor, we spread 0.5 × 106 to 1 × 106 spores of S. coelicolor M145 on YEME-pH 7 solid medium and spotted a 2.5-μl aliquot of a B. subtilis NCIB3610 overnight liquid culture in the center of the plate. Following 2 days of coincubation at 30°C, robust growth was visible for both B. subtilis at the site of inoculation and the substrate mycelium of S. coelicolor throughout the plate, even in the area adjacent to the growth of B. subtilis (Fig. 1A, Wild, 2-Day). After 1 more day of coincubation, a dramatic interspecies effect was apparent. S. coelicolor had transitioned from substrate mycelium growth to the formation of aerial hyphae and spores. By that time, S. coelicolor clearly failed to produce aerial hyphae in the vicinity of B. subtilis (Fig. 1A, Wild, 3-Day). It is important to note that, adjacent to B. subtilis, the growth of S. coelicolor substrate hyphae was normal; there simply were no aerial hyphae. This indicates that this strain of B. subtilis does not produce any agents displaying strong antibiotic activity against this strain of S. coelicolor. Close examination of the cells in the region of aerial growth inhibition revealed a block to spore formation, consistent with a developmental block (not shown). The effect that B. subtilis had on the growth of S. coelicolor phenocopies the effect of bld mutations, which disrupt signaling leading to aerial development (34). Therefore, we describe the interaction between B. subtilis and S. coelicolor as interference with S. coelicolor aerial hyphal development resulting in a bald halo.
FIG. 1.
B. subtilis inhibits aerial hyphal development by S. coelicolor through the action of surfactin. Agar petri dishes covered with a lawn of S. coelicolor spores were spotted with 2.5 μl of an overnight culture of B. subtilis. B. subtilis colonies are visible as discreet spots in the center of the plate. Plates were incubated for 2 to 3 days at 30°C. (A) After 2 days at 30°C, comparison of a wild-type strain (left) with an srfAA mutant strain (right) reveals no inhibition of substrate mycelial growth (2-day). After 3 days, aerial hyphae appear as a hairy, white surface on the agar plate. Aerial hyphae do not form surrounding the wild-type B. subtilis colony (3-day). Surrounding the srfAA mutant colony, aerial growth is largely unimpeded. (B) Inhibition of aerial growth requires Sfp (left), an enzyme needed for the synthesis of surfactin and plipastatin lipopeptides. A ppsB (plipastatin) mutant strain inhibits aerial development to the same degree as the wild-type strain. (C) Purified surfactin (10 μg/μl in ethanol) placed on a Whatman filter circle is sufficient for inhibition of aerial development. The control filter contains only ethanol.
We were interested in surveying the generality of this inhibitory effect of B. subtilis on Streptomyces development. To this end, we tested a number of B. subtilis strains with S. coelicolor, as well as other species of Streptomyces. We found that multiple bacilli share the ability to induce a similar effect on aerial growth of multiple streptomycetes (Table 1). Furthermore, we tested nine strains of wild Bacillus species, identified by 16S rRNA gene sequence, that were isolated from a soil sample. Five of the nine strains induced a bald halo on S. coelicolor. Thus, this phenomenon is not limited to the particular strain (3610) that we utilized for our experiments.
TABLE 1.
Bacillus and Streptomyces strains tested in coculture
| Name | Strain | Bald haloa |
|---|---|---|
| B. subtilis 3610 | PSK0207 | + |
| B. subtilis PY79 | PSK0001 | − |
| B. subtilis srfAA::Mlsr | PSK0060 | − |
| B. subtilis ppsB ΩTn10 Specr | PSK0156 | + |
| B. subtilis sfp::Mlsr | EG220-1 | − |
| B. subtilis RO-NN-I | NRRL B-14823 | + |
| B. mojavensis RO-H-I | NRRL B-14698 | + |
| B. mojavensis RO-C-2 | NRRL B-14699 | − |
| B. amyloliquefaciens | FZB42 | + |
| B. parabrevis | ATCC 8185 | − |
| B. licheniformis | ATCC 10716 | − |
| B. subtilis subsp. spizizenii | NRRL B-14472 | + |
| B. subtilis subsp. spizizenii | NRRL B-23049 | ±b |
| S. coelicolor | M145 | + |
| S. avermitilis | ATCC 31267 | + |
| S. griseoverticillatum | ATCC 31499 | + |
| Streptomyces sp. strain Mg1 | PSK0296 | + |
For B. subtilis, the presence (+) or absence (−) of a bald halo on strain M145 is shown. For Streptomyces strains, the presence or absence of a halo with strain 3610 is shown.
±, strain has a transient balding effect on S. coelicolor. Early bald halo formation is eventually overcome by aerial hyphae.
B. subtilis mutants unable to produce surfactin fail to block the development of S. coelicolor aerial hyphae.
In order to identify a factor or factors from B. subtilis involved in causing the bald phenotype on S. coelicolor, we screened a B. subtilis 3610 library of random mini-Tn10 transposon insertion mutants. After testing 2,000 insertion mutants, we obtained one clone that caused a reduction in the diameter of the bald halo on S. coelicolor. Nucleotide sequencing of the site of transposon insertion in these mutants revealed that the transposon insertion was located in the srfAB gene. This gene is a component of the surfactin operon including srfAA, srfAB, srfAC, and srfAD. The operon encodes a large synthetase enzyme required for production of the lipopeptide surfactin (23). To confirm that surfactin was involved in the inhibition of aerial hyphal development, we directly tested a B. subtilis strain with an engineered deletion of the first gene in the operon, srfAA. After 2 days of incubation, there was no observable difference in the phenotype of a surfactin mutant and a wild-type strain when grown on S. coelicolor (Fig. 1A, srfAA, 2-Day). However, after 3 days of coincubation we observed that the surfactin mutants were unable to cause a bald halo, indicating that surfactin is involved in the inhibition of S. coelicolor aerial hyphal development (Fig. 1A, srfAA, 3-Day).
The presence of a small residual bald halo surrounding the B. subtilis srfAA mutant prompted us to investigate this matter further (Fig. 1A, srfAA, 3-Day). In addition to the srfAA gene, the synthesis of surfactin requires the activity of a phosphopantetheinyl transferase, the product of the sfp gene (18). When we tested an sfp mutant for its effect on S. coelicolor development, we found that it completely abolished the bald-halo phenotype (Fig. 1B, sfp). The complete loss of a balding effect of the B. subtilis 3610 strain carrying a mutant allele of sfp resembles the phenotype of laboratory strains such as 168 and PY79, which are known to harbor a mutant sfp gene (19) (Table 1). The Sfp enzyme is also required for the synthesis of another lipopeptide, plipastatin, produced by B. subtilis (30). The products of genes ppsA to ppsE are dedicated enzymes responsible for plipastatin biosynthesis (29). To test whether plipastatin is also involved in the bald-halo phenotype, we introduced a ppsB mutation into our B. subtilis wild-type strain. Surprisingly, loss of plipastatin production had no effect on the diameter of the bald halo (Fig. 1B, ppsB). Furthermore, the bald-halo phenotype of a ppsB srfAA double mutant was indistinguishable from the phenotype of the srfAA single mutant (results not shown). These results suggest that surfactin is the metabolite primarily responsible for inhibiting S. coelicolor aerial development and that another Sfp-dependent metabolite, but not plipastatin, contributes slightly to the effect. We have not pursued the identification of this other metabolite further.
Surfactin is both necessary and sufficient for inhibition of S. coelicolor aerial hyphae.
To determine if surfactin by itself could inhibit aerial hyphal development, we treated lawns of S. coelicolor with purified surfactin (Fig. 1C). Fifty microliters of a 10-μg/μl surfactin solution in 100% ethanol was spotted onto a filter disk placed on a lawn of S. coelicolor spores. Following 3 days of incubation at 30°C, a bald halo was visible around the filter disk. No bald halo was observed on a filter disk in which 50 μl of 100% ethanol was placed as a control. Application of surfactin to S. coelicolor did not affect substrate mycelium formation. To determine if the observed effect was dose dependent, we tested serial dilutions of surfactin and found that balding was detected even when 50 μl of a 0.1-μg/μl surfactin solution was applied to the filter disk (results not shown). The effect of surfactin on S. coelicolor, as observed with this filter disk assay, appeared to saturate when 50 μl of a 2-μg/μl solution was applied to the filter. At that and all of the higher concentrations tested, the edge of the bald halo extended 1.5 cm beyond the edge of the filter circle. Yet, even at the highest concentration tested (50 μl of a 10-μg/μl solution), there were no signs of antibiotic activity against the substrate mycelium. These results demonstrate that the lipopeptide surfactin produced by B. subtilis is able to specifically inhibit the development of aerial hyphae in S. coelicolor without any killing effect.
Surfactin is required for B. subtilis colonies to build aerial structures.
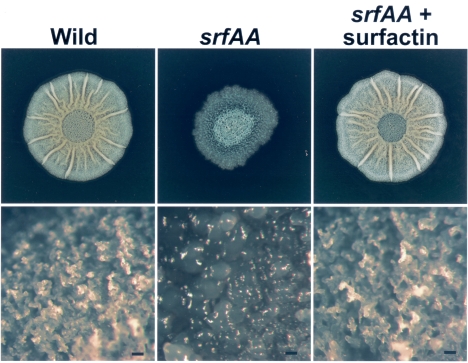
With the same B. subtilis wild-type strain used here (3610), Branda et al. demonstrated that aerial projections, whose tips serve as preferential sites of sporulation, develop when the strain is grown in MSgg medium (2). We tested a mutant specifically blocked in surfactin production (srfAA) and found that it failed to form aerial projections (Fig. 2, srfAA). The srfAA mutation results in colonies smaller than those produced by the wild-type strain and whose edges appear smooth and wet. Low-magnification microscopy revealed a lack of aerial projections in the srfAA mutant. We tested the effect of growing the srfAA mutant in the presence of purified surfactin and found that surfactin was able to restore aerial projection formation by the mutant (Fig. 2, srfAA + surfactin). We conclude from these experiments that surfactin is directly involved in the production of aerial projections by B. subtilis 3610. The requirement of surfactin for formation of aerial structures in B. subtilis contrasts with the balding effect of surfactin when applied to S. coelicolor but is consistent with the notion that surfactant activity is needed to raise aerial structures in the producing microorganism.
FIG. 2.
Development of fruiting-body-like aerial projections on the surface of a B. subtilis colony requires surfactin. (Top) Colonies of wild-type and srfAA mutant strains were spotted onto MSgg plates that were pretreated by spotting directly on the plate either 50 μl of 100% ethanol (Wild and srfAA) or 50 μl of a 10-μg/μl surfactin solution in 100% ethanol (srfAA + surfactin). Wild-type morphology is restored to the srfAA mutant strain upon addition of purified surfactin. (Bottom) Higher magnification of the colony surfaces from the top panels shows that surfactin is sufficient to restore fruiting bodies to a srfAA mutant. The wild-type strain of B. subtilis (left) produces aerial projections. The surfactin-deficient srfAA mutant strain (center) has a smooth colony surface lacking aerial projections. When 50 μl of a 10-μg/μl solution of purified surfactin is added to the plate, aerial projections are formed by the srfAA mutant strain. Bars, 100 μm.
S. coelicolor surfactants restore aerial growth when applied to surfactin-treated hyphae.
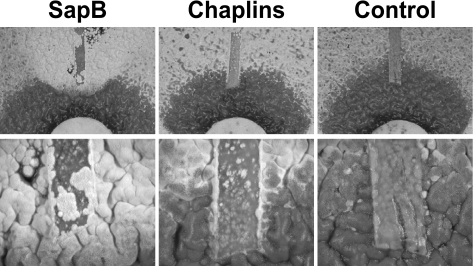
S. coelicolor mutations that lead to a bald phenotype (bld) generally disrupt the production of the lantibiotic-like peptide SapB or a set of eight small polypeptides called the chaplins (8, 34). In order to determine if surfactin interferes with SapB and chaplin function during aerial hypha formation, we examined the capacity of exogenously applied SapB and chaplins to overcome the surfactin balding effect. Lawns of wild-type S. coelicolor spores were plated, and a filter containing 50 μl of a 10-μg/μl surfactin solution was placed in the center of the plate. Subsequently, strips of nylon filter (1 cm by 2 mm) were placed on the plate, 1 cm from the edge of the surfactin-containing filter disk. The strips of nylon were soaked in either 1 μg/μl SapB in DMSO, TFA-extracted chaplins in water, or DMSO alone (Fig. 3). Following 3 days of incubation at 30°C, aerial hyphae were visible outside the 1-cm bald halo caused by the presence of surfactin. In the regions containing SapB or chaplins, restoration of aerial growth was observed. The effect of SapB on development was clearly visible as a ring of aerial hyphal growth. As expected, addition of SapB triggered early onset of aerial hypha formation where the peptide was applied (results not shown). Application of a suspension of chaplin proteins stimulated some aerial hyphal growth close to the filter. However, the degree of the effect of chaplin addition is moderate in contrast to that of SapB, likely because of the lower concentration and limited diffusion of mixed-chaplin peptides extracted by TFA from cell walls (6). Nonetheless, comparison of the DMSO control with the chaplin-treated sample shows a clear indication of aerial hypha induction by the addition of chaplins. From these experiments, we conclude that the effect of surfactin on aerial hyphae arises as a result of interference with the normal expression, function, or assembly of SapB and chaplins that are required for the erection of S. coelicolor aerial hyphae. We examined the extent of spore formation in treated cells by excising a fragment of the mycelium and examining the cells by phase-contrast microscopy (not shown). Exogenously added SapB and chaplins restored both aerial growth and spore formation to surfactin-treated cells.
FIG. 3.
The balding effect of surfactin is overcome by addition of SapB and chaplin protein extract. SapB (left) and TFA-extracted chaplins (center) were added to nylon filters placed at the edge of the surfactin-inhibited bald zone. DMSO (right) was added as a control. Treatment with SapB restores aerial growth to an area within the surfactin-treated zone surrounding the filter strip. Treatment with the chaplin extract is weakly but detectably effective in overcoming surfactin-induced balding. The control strip (right) shows no effect on inhibition of aerial growth from surfactin treatment. Top, plate photographs; bottom, high magnification (×80).
Expression of S. coelicolor genes involved in surfactant production reveals a surfactin-induced developmental block to aerial growth.
Development of aerial hyphae and spore production in S. coelicolor requires proper timing of gene expression (5). To investigate the timing of surfactin interference with aerial development, we examined the expression of S. coelicolor genes involved in the production of surfactants. We used a quantitative real-time RT-PCR approach to study gene expression in surfactin-treated samples versus untreated controls. We chose to examine the expression of genes involved in the production of SapB (ramC), ChpA (chpA), and ChpH (chpH). As a control for changes in mRNA concentration, we monitored the expression of rpoB, which encodes the β subunit of RNA polymerase and whose expression remains relatively constant throughout development (14) (http://sncohenlab.stanford.edu/streptomyces/data.html). Previous analyses have shown that the ramCSAB operon is expressed in the substrate mycelium prior to the onset of aerial growth (15, 22). The ramS gene encodes the precursor peptide for SapB, while the ramC gene encodes an enzyme that is predicted to process pre-SapB to mature SapB (16). The ramS gene is small (129 bp), and two attempts to generate primer sets useful for real-time RT-PCR analysis were unsuccessful. Therefore, we chose to analyze the expression of the first gene in the operon, ramC. The eight chaplin peptides are expressed differentially throughout the growth of S. coelicolor substrate and aerial hyphae. The chpH gene is expressed early during the course of development, while expression of chpA is induced at the onset of aerial growth and peaks during sporulation (6, 8). We thus chose chpH and chpA as representative genes for early and late expression of chaplin peptides, respectively.
Plates containing lawns of S. coelicolor spores were treated with 50 μl of a 10-μg/μl surfactin solution, and samples were taken from plates at the onset of aerial development. Cells were harvested from the bald (treated) and hairy (untreated) regions of the plate, and RNA was extracted. Analysis of mRNA by real-time RT-PCR demonstrates that surfactin treatment results in altered expression of genes encoding chaplins and SapB (Table 2). Expression of ramR, which activates the expression of the ramCSAB cluster, is normally induced in advance of aerial growth and repressed during spore formation (21). The effect of surfactin on S. coelicolor is a prolonged and abnormal presence of the ramC mRNA transcript. Because enhanced expression of the ramCSAB gene cluster results in overproduction of SapB, it is possible that production of SapB is enhanced when the Streptomyces mycelium is treated with surfactin (21). However, with an antibody to the mature SapB peptide, we were unable to detect SapB by Western blotting of extracts from surfactin-treated cells but not from the mock-treated control (results not shown). Assuming that the ramS transcript is properly translated, this result suggests that, in the presence of surfactin, SapB is not posttranslationally modified to its mature form. In this case, the pre-SapB would either accumulate intracellularly or be secreted as an immature form not recognized by the anti-SapB antibody. In contrast to ramC, the level of the late chaplin transcript, chpA, is dramatically reduced upon surfactin treatment. Whereas ramC levels increased ∼15-fold, the abundance of chpA transcript was reduced at least 50-fold. Similar to that of the ramC gene, expression of chpH is normally reduced during late stages of aerial development. However, the chpH mRNA level was unchanged or slightly increased (about twofold) in response to treatment with surfactin, consistent with an arrest in development.
TABLE 2.
Real-time RT-PCR analysis of SapB and chaplin gene expression in surfactin-treated S. coelicolor
| Gene | Untreateda | Surfactin treatedb | Relative changec (fold) |
|---|---|---|---|
| ramC | 0.33 ± 0.03d | 4.87 ± 3.23 | Up ∼15 |
| chpA | 0.51 ± 0.22 | 0.01 ± 0.01 | Down >50 |
| chpH | 0.88 ± 0.10 | 1.75 ± 0.55 | Up ∼2 |
| rpoB | 0.69 ± 0.07 | 0.69 ± 0.07 | None |
RNA extracted from mycelia outside the bald-halo zone.
RNA extracted from mycelia within bald halo.
Change in mRNA abundance from surfactin-treated samples relative to untreated control.
Values are average relative nanograms of mRNA, normalized to rpoB, ± the standard deviation from triplicate samples. Value for rpoB is from combined untreated and surfactin-treated cultures.
DISCUSSION
Many bacteria synthesize surfactants that are thought to provide a wide range of benefits to the producer organism (25). Surfactants can aid in the colonization of surfaces and acquisition of nutrients through their surface-wetting and detergent properties (20). More germane to the present study, surfactants have been shown to be required for the development of aerial structures in numerous microbes (36). Prior work had shown that the lanthionine-containing peptide SapB from S. coelicolor is required for raising aerial hyphae. In this report, we present direct evidence that the lipopeptide surfactin from B. subtilis is similarly required for the development of aerial structures by this bacterium. By coculturing these two bacteria on an agar surface, we discovered an unexpected surfactant-dependent interspecies interaction. Surfactin produced by B. subtilis inhibited aerial growth by S. coelicolor. However, surfactin did not inhibit the growth of substrate hyphae, as would be expected if it were functioning as an antibiotic. Instead, surfactin specifically inhibited the development of aerial hyphae. Recent studies with yeast and Bacillus have shown similar effects. Saccharomyces cerevisiae produces an invertase that affects aerial development of Streptomyces lividans (26). Also, the biocontrol strain B. subtilis subsp. sunhua produces iturin A, a lipopeptide structurally similar to surfactin that inhibits the development of Streptomyces scabies (11).
The surfactant-mediated antagonistic interaction between B. subtilis and S. coelicolor would not have been predicted by current models of surfactant function on the raising of aerial structures during microbial development (37). These models posit that the role of surfactants in raising aerial structures is solely to reduce surface tension at interfaces between air and hydrated surfaces. The mechanism of function for these surfactants is not known, but recent structural studies of SapB and a related surfactant peptide, SapT, will aid in understanding how these molecules assemble to carry out their function (16, 17). If simply reducing surface tension were the only requirement for these surfactants, then surfactin from B. subtilis should have promoted aerial development of S. coelicolor hyphae because of its comparable surfactant activity. Previous experiments showed that surfactin does not restore aerial hyphal growth when applied to a bld mutant (24). Surprisingly, the results of our coculture experiments demonstrate that surfactin has the opposite effect: surfactin specifically antagonized aerial hyphal development by wild-type S. coelicolor. This observation, coupled with the fact that surfactin is itself required for raising aerial structures by its producer B. subtilis, strengthens the hypothesis that there is specificity in the mechanisms through which surfactants aid in raising aerial structures (35). Furthermore, the observation that a compound with surface activity comparable to that of SapB acts antagonistically on aerial development highlights the important role that the structure of a given surfactant may play in the aerial growth of its producer organism.
While we do not know the molecular mechanism underlying the surfactin-mediated inhibition of aerial hypha formation, we offer three speculative possibilities. First, surfactin might interfere directly with the processing and secretion of mature SapB, which is required for aerial development. The inability of S. coelicolor to produce SapB would result in a bald phenotype and could trigger a developmental arrest even though the ramCSAB gene cluster is fully expressed. One possibility is that surfactin blocks the function of the RamAB proteins, predicted to encode an ABC transporter involved in SapB secretion (35). How SapB processing and secretion by RamAB occur is unknown, but surfactin could exert its effect directly on these proteins. Alternatively, surfactin could interfere directly with SapB during maturation and folding, such that the peptide is secreted but no longer able to function as a surfactant or to be recognized by the anti-SapB antibody. Second, surfactin might alter the S. coelicolor membrane in a way that prevents normal processing and secretion of proteins or metabolites required for aerial growth, leading to an arrest in developmental progression. Third, surfactin could act directly as a signaling molecule that interacts with a specific receptor, most likely at the surface of the S. coelicolor cell. Regardless of the molecular mechanism, through examining the surfactin effect on aerial development we have recognized that production of mature SapB is regulated not only at the transcriptional level but posttranscriptionally as well. Furthermore, surfactin appears to block aerial development at a late stage during development, characterized by increased ramC expression and repressed chpA expression. By exploiting surfactin as a probe, it should be possible to explore further how aerial growth is attuned to developmental regulatory circuits and uncover the mechanism by which development is arrested.
Here we have applied a different approach to our ongoing interest in studying interspecies interactions. We have specifically investigated the effects of the presence of a second bacterial species on a well-characterized pathway of bacterial development. The results clearly demonstrate that a secondary metabolite produced by one species can affect the developmental pathway of a bacterium without having detrimental effects on vegetative growth. In this particular case, we have revealed a possible ecological role and novel function of a previously well-characterized molecule. These results validate this as an approach that can be applied to many different systems with potentially informative results. In addition, if this approach is applied to many systems it could lead to the discovery of additional molecules involved in chemical communication among different species of bacteria.
Acknowledgments
We thank Steve Branda, Eduardo Gonzales-Pastor, and Dan Kearns for advice and strains. We thank Dennis Claessen for provision of material to extract chaplins and Francois Bleus for technical assistance.
This work was supported by NIH grants to R.K. (GM58213) and J.M.W. (GM069398-01). P.D.S. was supported by an NSF postdoctoral fellowship in microbial biology (DBI-0200307).
REFERENCES
- 1.Atlas, R., and R. Bartha. 1998. Microbial ecology: fundamentals and application. Benjamin/Cummings Publishing, San Francisco, Calif.
- 2.Branda, S. S., J. E. Gonzalez-Pastor, S. Ben-Yehuda, R. Losick, and R. Kolter. 2001. Fruiting body formation by Bacillus subtilis. Proc. Natl. Acad. Sci. USA 98:11621-11626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chater, K. F. 1993. Genetics of differentiation in Streptomyces. Annu. Rev. Microbiol. 47:685-713. [DOI] [PubMed] [Google Scholar]
- 4.Chater, K. F. 1989. Multilevel regulation of Streptomyces differentiation. Trends Genet. 5:372-377. [DOI] [PubMed] [Google Scholar]
- 5.Chater, K. F. 2001. Regulation of sporulation in Streptomyces coelicolor A32: a checkpoint multiplex? Curr. Opin. Microbiol. 4:667-673. [DOI] [PubMed] [Google Scholar]
- 6.Claessen, D., R. Rink, W. de Jong, J. Siebring, P. de Vreugd, F. G. Boersma, L. Dijkhuizen, and H. A. Wosten. 2003. A novel class of secreted hydrophobic proteins is involved in aerial hyphae formation in Streptomyces coelicolor by forming amyloid-like fibrils. Genes Dev. 17:1714-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dawid, W. 2000. Biology and global distribution of myxobacteria in soils. FEMS Microbiol. Rev. 24:403-427. [DOI] [PubMed] [Google Scholar]
- 8.Elliot, M. A., N. Karoonuthaisiri, J. Huang, M. J. Bibb, S. N. Cohen, C. M. Kao, and M. J. Buttner. 2003. The chaplins: a family of hydrophobic cell-surface proteins involved in aerial mycelium formation in Streptomyces coelicolor. Genes Dev. 17:1727-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elliot, M. A., and N. J. Talbot. 2004. Building filaments in the air: aerial morphogenesis in bacteria and fungi. Curr. Opin. Microbiol. 7:594-601. [DOI] [PubMed] [Google Scholar]
- 10.Fawcett, P., P. Eichenberger, R. Losick, and P. Youngman. 2000. The transcriptional profile of early to middle sporulation in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 97:8063-8068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han, J. S., J. H. Cheng, T. M. Yoon, J. Song, A. Rajkarnikar, W. G. Kim, I. D. Yoo, Y. Y. Yang, and J. W. Suh. 2005. Biological control agent of common scab disease by antagonistic strain Bacillus sp. sunhua. J. Appl. Microbiol. 99:213-221. [DOI] [PubMed] [Google Scholar]
- 12.Hogan, D. A., and R. Kolter. 2002. Pseudomonas-Candida interactions: an ecological role for virulence factors. Science 296:2229-2232. [DOI] [PubMed] [Google Scholar]
- 13.Hogan, D. A., A. Vik, and R. Kolter. 2004. A Pseudomonas aeruginosa quorum-sensing molecule influences Candida albicans morphology. Mol. Microbiol. 54:1212-1223. [DOI] [PubMed] [Google Scholar]
- 14.Huang, J., C. J. Lih, K. H. Pan, and S. N. Cohen. 2001. Global analysis of growth phase responsive gene expression and regulation of antibiotic biosynthetic pathways in Streptomyces coelicolor using DNA microarrays. Genes Dev. 15:3183-3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keijser, B. J., G. P. van Wezel, G. W. Canters, and E. Vijgenboom. 2002. Developmental regulation of the Streptomyces lividans ram genes: involvement of RamR in regulation of the ramCSAB operon. J. Bacteriol. 184:4420-4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kodani, S., M. E. Hudson, M. C. Durrant, M. J. Buttner, J. R. Nodwell, and J. M. Willey. 2004. The SapB morphogen is a lantibiotic-like peptide derived from the product of the developmental gene ramS in Streptomyces coelicolor. Proc. Natl. Acad. Sci. USA 101:11448-11453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kodani, S., M. A. Lodato, M. C. Durrant, F. Picart, and J. M. Willey. 2005. SapT, a lanthionine-containing peptide involved in aerial hyphae formation in the streptomycetes. Mol. Microbiol. 58:1368-1380. [DOI] [PubMed] [Google Scholar]
- 18.Nakano, M. M., N. Corbell, J. Besson, and P. Zuber. 1992. Isolation and characterization of sfp: a gene that functions in the production of the lipopeptide biosurfactant, surfactin, in Bacillus subtilis. Mol. Gen. Genet. 232:313-321. [DOI] [PubMed] [Google Scholar]
- 19.Nakano, M. M., M. A. Marahiel, and P. Zuber. 1988. Identification of a genetic locus required for biosynthesis of the lipopeptide antibiotic surfactin in Bacillus subtilis. J. Bacteriol. 170:5662-5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neu, T. R. 1996. Significance of bacterial surface-active compounds in interaction of bacteria with interfaces. Microbiol. Rev. 60:151-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen, K. T., J. M. Willey, L. D. Nguyen, L. T. Nguyen, P. H. Viollier, and C. J. Thompson. 2002. A central regulator of morphological differentiation in the multicellular bacterium Streptomyces coelicolor. Mol. Microbiol. 46:1223-1238. [DOI] [PubMed] [Google Scholar]
- 22.O'Connor, T. J., P. Kanellis, and J. R. Nodwell. 2002. The ramC gene is required for morphogenesis in Streptomyces coelicolor and expressed in a cell type-specific manner under the direct control of RamR. Mol. Microbiol. 45:45-57. [DOI] [PubMed] [Google Scholar]
- 23.Peypoux, F., J. M. Bonmatin, and J. Wallach. 1999. Recent trends in the biochemistry of surfactin. Appl. Microbiol. Biotechnol. 51:553-563. [DOI] [PubMed] [Google Scholar]
- 24.Richter, M., J. Willey, R. Susmuth, G. Jung, and H.-P. Fiedler. 1998. Streptofactin, a novel biosurfactant with aerial mycelium inducing activity from Streptomyces tendae Tu 901/8c. FEMS Microbiol. Lett. 163:165-171. [Google Scholar]
- 25.Ron, E. Z., and E. Rosenberg. 2001. Natural roles of biosurfactants. Environ. Microbiol. 3:229-236. [DOI] [PubMed] [Google Scholar]
- 26.Santamaria, R. I., F. Leal, M. Diaz, and J. M. Fernandez-Abalos. 2002. Morphological and physiological changes in Streptomyces lividans induced by different yeasts. Arch. Microbiol. 177:259-266. [DOI] [PubMed] [Google Scholar]
- 27.Steinmetz, M., and R. Richter. 1994. Easy cloning of mini-Tn10 insertions from the Bacillus subtilis chromosome. J. Bacteriol. 176:1761-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tillotson, R. D., H. A. Wosten, M. Richter, and J. M. Willey. 1998. A surface active protein involved in aerial hyphae formation in the filamentous fungus Schizophillum commune restores the capacity of a bald mutant of the filamentous bacterium Streptomyces coelicolor to erect aerial structures. Mol. Microbiol. 30:595-602. [DOI] [PubMed] [Google Scholar]
- 29.Tosato, V., A. M. Albertini, M. Zotti, S. Sonda, and C. V. Bruschi. 1997. Sequence completion, identification and definition of the fengycin operon in Bacillus subtilis 168. Microbiology 143:3443-3450. [DOI] [PubMed] [Google Scholar]
- 30.Tsuge, K., T. Ano, M. Hirai, Y. Nakamura, and M. Shoda. 1999. The genes degQ, pps, and lpa-8 (sfp) are responsible for conversion of Bacillus subtilis 168 to plipastatin production. Antimicrob. Agents Chemother. 43:2183-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weaver, V. B., and R. Kolter. 2004. Burkholderia spp. alter Pseudomonas aeruginosa physiology through iron sequestration. J. Bacteriol. 186:2376-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wessels, J., O. De Vries, S. A. Asgeirsdottir, and F. Schuren. 1991. Hydrophobin genes involved in formation of aerial hyphae and fruit bodies in Schizophyllum. Plant Cell 3:793-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Willey, J., R. Santamaria, J. Guijarro, M. Geistlich, and R. Losick. 1991. Extracellular complementation of a developmental mutation implicates a small sporulation protein in aerial mycelium formation by S. coelicolor. Cell 65:641-650. [DOI] [PubMed] [Google Scholar]
- 34.Willey, J., J. Schwedock, and R. Losick. 1993. Multiple extracellular signals govern the production of a morphogenetic protein involved in aerial mycelium formation by Streptomyces coelicolor. Genes Dev. 7:895-903. [DOI] [PubMed] [Google Scholar]
- 35.Willey, J. M., A. Willems, S. Kodani, and J. R. Nodwell. 2006. Morphogenetic surfactants and their role in the formation of aerial hyphae in Streptomyces coelicolor. Mol. Microbiol. 59:731-742. [DOI] [PubMed] [Google Scholar]
- 36.Wosten, H. A., M. Richter, and J. M. Willey. 1999. Structural proteins involved in emergence of microbial aerial hyphae. Fungal Genet. Biol. 27:153-160. [DOI] [PubMed] [Google Scholar]
- 37.Wosten, H. A., and J. M. Willey. 2000. Surface-active proteins enable microbial aerial hyphae to grow into the air. Microbiology 146:767-773. [DOI] [PubMed] [Google Scholar]