Abstract
Several subclasses of type IV pili have been described according to the characteristics of the structural prepilin subunit. Whereas molecular mechanisms of type IVa pilus assembly have been well documented for Pseudomonas aeruginosa and involve the PilD prepilin peptidase, no type IVb pili have been described in this microorganism. One subclass of type IVb prepilins has been identified as the Flp prepilin subfamily. Long and bundled Flp pili involved in tight adherence have been identified in Actinobacillus actinomycetemcomitans, for which assembly was due to a dedicated machinery encoded by the tad-rcp locus. A similar flp-tad-rcp locus containing flp, tad, and rcp gene homologues was identified in the P. aeruginosa genome. The function of these genes has been investigated, which revealed their involvement in the formation of extracellular Flp appendages. We also identified a gene (designated by open reading frame PA4295) outside the flp-tad-rcp locus, that we named fppA, encoding a novel prepilin peptidase. This is the second enzyme of this kind found in P. aeruginosa; however, it appears to be truncated and is similar to the C-terminal domain of the previously characterized PilD peptidase. In this study, we show that FppA is responsible for the maturation of the Flp prepilin and belongs to the aspartic acid protease family. We also demonstrate that FppA is required for the assembly of cell surface appendages that we called Flp pili. Finally, we observed an Flp-dependent bacterial aggregation process on the epithelial cell surface and an increased biofilm phenotype linked to Flp pilus assembly.
Pseudomonas aeruginosa is a ubiquitous and opportunistic pathogen that is responsible for human infections in hospitalized and cystic fibrosis (CF) patients. Bacterial attachment is a major virulence trait required for chronic infections such as for P. aeruginosa acquiring a biofilm lifestyle in CF lungs (7). Formation and maturation of biofilm by P. aeruginosa are well documented at the molecular level and involve a large arsenal of cell surface-associated fibrils, including flagella, type IVa pili (TFP), or putative fimbrial structures called Cup (34, 51). Among these structures, the type IVa pili (TFP) are known to contribute at several stages of biofilm development, including initial attachment to surfaces, surface colonization, and development of mushroom-like structures through a mode of movement called twitching motility (25). The P. aeruginosa TFP are members of the type IVa family, which is characterized by a number of features including the size of the prepilin (9). In P. aeruginosa, the prepilin PilA is cleaved by the prepilin peptidase PilD (33), before it is assembled into a fiber structure exposed at the bacterial cell surface. The atomic resolution structures of four pilin subunits, combined with electron microscopy (EM) images of the pili, have provided information to describe the helical symmetry of the pilus fibers (8, 9, 24). Accessory components including a traffic ATPase (PilB), a secretin (PilQ), and a polytopic inner membrane protein (PilC) are required for TFP assembly (4, 32).
Another subclass of TFP, called type IVb, has also been described. Type IVb prepilins form a subgroup characterized by the 15- to 30-residue length of their leader peptide, the 190-amino-acid (aa) average length of their mature pilin, the nature of the N-terminus residue after the cleavage site, and the average length of the exposed D region in the assembled pili (9). One member of this class is the bundle-forming pilus (BFP), which is found in enteropathogenic Escherichia coli (EPEC) (11). The Bfp fibrils have a tendency to aggregate into a rope-like bundle and are responsible for bacterium-bacterium interaction and microcolony formation (6). The 14-gene bfp operon encodes proteins that are sufficient for BFP biogenesis in EPEC (39). Among these genes, bfpA encodes the bundlin, the major structural subunit of BFP (5), which is assembled with the help of typical TFP assembly components. Among these components, one can find a prepilin peptidase (BfpP), two traffic ATPases (BfpD and BfpF), BfpD involved in assembly (1), and BfpF required for retraction (3), a secretin (BfpB), a PilC-like protein (BfpE), and minor pilins (BfpI, BfpJ, and BfpK). Additional components not classically found in TFP systems are also required for BFP assembly and are a lytic trans-glycosylase (BfpH) and four proteins of unknown function, BfpG, BfpC, BfpU, and BfpL (1). The class IVb of TFP also includes the longus pilus of enterotoxigenic E. coli (14) and the toxin-coregulated pilus (TCP) of Vibrio cholerae (49).
Within the type IVb pilin family, there is a clear monophyletic Flp prepilin subfamily, which has been initially described in Actinobacillus actinomycetemcomitans, a gram-negative bacterium responsible for localized juvenile periodontitis (38, 58). The Flp prepilin subfamily shares unique features, including a long leader peptide, a short size for the mature pilin ranging from 50 to 80 aa, and a shared Flp motif of 20 hydrophobic residues at the N terminus of the mature pilin with adjacent glutamate and tyrosine residues in its center (38). In A. actinomycetemcomitans, the Flp pilin requires for its assembly a subset of components called Tad and Rcp, which includes a secretin (RcpA), a traffic ATPase (TadA) (2), and PilC-like proteins (TadB and TadC), as well as several other components that have no homologues in other type IV pilus assembly systems. The presence of thick fibrils composed of bundled thin Flp pili at the surface of A. actinomycetemcomitans is associated with the formation of extremely tenacious biofilms on a variety of solid surfaces and more particularly the teeth. Flp pili have also been described in Caulobacter crescentus (44) and Haemophilus ducreyi (31). Genome mining of the P. aeruginosa genome (45) revealed several genes encoding type IV pilins; one of these genes encodes an Flp-like protein. In this study, we further analyzed the assembly of the P. aeruginosa Flp pilin into a cell surface appendage and we characterized the role of a novel prepilin peptidase in this process.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The strains and plasmids used in this study are listed in Table 1. Strains were grown at 37°C in L broth or on LB agar. The E. coli TG1 and TOP10F′ strains were used for standard genetic manipulations. Recombinant plasmids were introduced into P. aeruginosa using E. coli S17-1 or the conjugative properties of pRK2013. Transconjugants were selected on Pseudomonas isolation agar supplemented with antibiotics. Antibiotics were used at the following concentrations (μg/ml): for E. coli, ampicillin, 50; streptomycin, 50; tetracycline, 15; and kanamycin, 50; for P. aeruginosa, carbenicillin, 500; streptomycin 2,000; tetracycline, 200; and kanamycin, 1,000.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s)a | Source |
|---|---|---|
| Strains | ||
| E. coli | ||
| TG1 | supE Δ(lac-proAB) thi hsdRΔ5 (F′ traD36 rpoA+B+lacIqZΔM15) | Lab collection |
| TOP10F′ | F′ [lacIq Tn10 (Tetr)] mrcA Δ(mrr-hsdRMS-mcrBC) φ80 lacZΔM15 ΔlacX74 recA1 | Invitrogen |
| S17-1 | Conjugative helper strain; galU galK rpsL (Strr) endA1 nupG thi pro hsdR hsdM recA (RP4-2Tc::Mu Km::Tn7)λpir | Lab collection |
| CC118(λpir) | Host strain for pKNG101 replication; Δ(ara-leu) araD ΔlacX74 galE galK phoA20 thi-1 rpsE rpoB argE(Am) recA1 Rfr (λpir) | Lab collection |
| P. aeruginosa | ||
| PAO1 | Wild type | Lab collection |
| PAO1Δflp | PAO1 deletion mutant for flp gene | This study |
| PAO1ΔtadA | PAO1 deletion mutant for tadA gene | This study |
| PAO1ΔrcpA | PAO1 deletion mutant for rcpA gene | This study |
| PAO1ΔfppA | PAO1 deletion mutant for fppA gene | This study |
| PAO1ΔpilD | PAO1 deletion mutant for pilD gene | Lab collection |
| PAO1ΔfliCΔpilA | PAO1 deletion mutant for fliC and pilA genes | Lab collection |
| Plasmids | ||
| pCR2.1 | TA cloning vector for PCR products; lacZα ColE1 f1 ori Apr Kmr | Invitrogen |
| pMMB190 | Broad-host-range vector; IncQ ptac lacZα Apr | Lab collection |
| pBBR1MCS-2 | Broad-host-range vector; Kmr | Lab collection |
| pKNG101 | Suicide vector in P. aeruginosa; sacB Strr | Lab collection |
| pMMBflp | flp gene cloned in pMMB190 at BamHI/HindIII | This study |
| pBBRfppA | fppA gene cloned in pBBR1MCS-2 at EcoRI | This study |
| pBBRfppAD17A | fppA gene with D17A substitution cloned into pBBR1MCS-2 | This study |
| pBBRfppAD78A | fppA gene with D78A substitution cloned into pBBR1MCS-2 | This study |
| pBBRfppAD17AD78A | fppA gene with D17A and D78A substitutions cloned into pBBR1MCS-2 | This study |
| pDEST14 | Destination vector of the Gateway system, T7 terminator, T7 promoter; Cmr Apr | Invitrogen |
| pLAFR3 | Cosmid, IncP, lac promoter; Tcr | Lab collection |
| pLAFRrcpA | rcpA gene cloned into pLAFR3 at BamHI/HindIII | This study |
| pLAFRtadA | tadA gene cloned into pLAFR3 at BamHI/HindIII | This study |
| pPRK2013 | ColE1 ori tra+mob+ Kmr | Lab collection |
Strr, streptomycin resistance; Apr, ampicillin resistance; Kmr, kanamycin resistance; Tcr, tetracycline resistance.
Construction of P. aeruginosa mutant strains.
Overlapping PCR was used as previously described (52) to generate a fragment that linked 500-bp-long DNA fragments corresponding to regions located upstream and downstream from the gene of interest, thus linking in frame the start and stop codons of the gene. The oligonucleotides used are listed in Table 2. The resulting DNA fragment was cloned into the suicide vector pKNG101. The recombinant plasmid was then mobilized into P. aeruginosa, and double recombination events were selected on LB plates containing 5% sucrose as previously described (22). In the mutant strain, this recombination event resulted in a nonpolar in-frame deletion of the gene of interest.
TABLE 2.
Oligonucleotides used for gene cloning and P. aeruginosa mutation engineering
| Target gene | Oligonucleotide (5′→3′)a |
|---|---|
| Cloned | |
| flp | Flp11 Up (GAGGCAGGGCTCTGTCTCGGTGAT) |
| Flp12 Down (AAGCTTCTGAACGGCAGGCTTTGTAG) | |
| fppA | FppA Up-1 (TTAATACTAGTCGTCATTCGCTCCG) |
| FppA Down-1 (GTATTAGCCATAAGCATATAGTCG) | |
| fppA mutagenesis | FppA Up-2 (CAGGAAGATCGCTGTGAATTCGATG) |
| FppA Down-2 (GTGCATTGGAATGCAGCCATACCGAAT) | |
| fppA(D17A) | FppAD17A-Up (GCGGATATCAGGCCATCAAACGTT) |
| FppAD17A-Down (ACGTTTGATGGCCTGATATCCG) | |
| fppA(D78A) | FppAD78A-Up (GGCGCTGCCGCGGTCAAGTACCT) |
| FppAD78A-Down (AGGTACTTGACCGCGGCAGCGCC) | |
| Deleted | |
| flp | Flp1 (CGCACCGAGCAGCAGCAGGC) |
| Flp2 (CTCGCGTCACATTCTTGTTTGTTTGCTC) | |
| Flp3 (ACAAGAATGTGACGCGAGCGCTTTCCCGGT) | |
| Flp4 (TGCGGCTGAAGACGGGCTGT) | |
| tadA | TadA1 (GAGTCACCACCGCCGAACTCTATC) |
| TadA2 (CGCCGTTCACATAGCAGCGCCGCCTTGGCC) | |
| TadA3 (GCTGCTATGTGAACGGCGCGCAACTGCTGG) | |
| TadA4 (GGGTGAAGATGTCGCGCAGCGGCT) | |
| rcpA | RcpA1 (CGTCCTGCCCGGCGACTATGTCGA) |
| RcpA2 (CGCCTCCTACATATGCCGGCTCCCCTCAAGG) | |
| RcpA3 (CGGCATATGTAGGAGGCGCGCATGAACCAGAACT) | |
| RcpA4 (TGCTCCTGCAACGCCTTGGCCAG) | |
| fppA | FppA1 (CTCAGCACGGGGATGATCGC) |
| FppA2 (GTTTGCTCACATCCTGTTCTTCAGCCTTTTTT) | |
| FppA3 (AACAAGATGTGAGCAAACTTGCGGAAAAC) | |
| FppA4 (GCCATGCAGCAGTTCCTGCG) |
The mutagenic codons in FppAD17A-Up and FppAD78A-Up are indicated in bold.
Cloning of P. aeruginosa genes.
The flp and fppA genes were PCR amplified using the oligonucleotide couples Flp11 Up/Flp12 Down and FppA Up-1/FppA Down-1, respectively. PCR products were cloned into the PCR2.1 vector. The flp and fppA genes were subsequently cloned into the broad-host-range plasmids pMMB190 and pBBR1MCS-2, respectively. The rcpA and tadA genes were obtained from a comprehensive P. aeruginosa gene collection (26) cloned into an entry vector of the Gateway system (Invitrogen). These genes were moved into a pDEST14 destination vector by LR recombination according to the manufacturer's instructions. The rcpA and tadA genes were further subcloned into the broad-host-range cosmid pLAFR3 at BamHI/HindIII restriction sites, yielding pLAFRrcpA and pLAFRtadA, respectively.
Site-directed mutagenesis of the fppA gene.
The mutations in the fppA gene, which resulted in the replacement of aspartate residues (at position 17 or 78) with alanine residues, were generated by overlapping PCR as previously described (13). Briefly, oligonucleotides were designed to change the aspartate codons (D17/GAC and D78/GAC) into alanine codons (A17/GCC and A78/GCG) (Table 2). The 5′ and 3′ DNA regions from these codons were amplified using oligonucleotide pairs such as FppA-Up-2 and FppAD17A-Down, FppAD17A-Up and FppA Down-2, FppA-Up-2 and FppAD78A-Down, or FppAD78A-Up and FppA-Down-2. The 5′ and 3′ DNA fragments of the fppA gene were then reassembled by mixing and reamplifying with the oligonucleotide pair FppA-Up-2 and FppA-Down-2. The double mutation (D17A and D78A) in the fppA gene was generated by using the single mutated gene fppA(D17A) as a DNA matrix.
Bioinformatic analysis.
The genome sequence of P. aeruginosa PAO1 and its annotation are available at http://www.pseudomonas.com. The flp-tad-rcp gene deduced amino acid sequences were subjected to analysis using different softwares, such as TMHMM, SignalP or PSORT, ScanProsite, BlastP or MAXHOM, PSI-BLAST, Ssearch, and T-COFFEE. These programs are available at http://embl-heidelberg.de/predictprotein/predictprotein.html.
Production of antibodies directed against Flp.
A strategy for peptide-based antibody production was developed (Eurogentec). A peptide containing the 15 amino acids (DGVGEKVGGLAPTAN) at the C-terminal end of Flp was synthesized. Preimmune sera of two rabbits were checked for absence of cross-reactivity. The rabbits were inoculated with the designed peptide at a concentration of 200 μg/ml, followed by three boosters spaced by 15 days, 1 month, and 2 months, respectively. After that period, rabbits were sacrificed. Collected sera were further purified against the initial peptide.
Tris-glycine gel electrophoresis and Western blot analysis.
Production of Flp was analyzed by 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). For enhanced resolution of the small-size Flp pilin (Flp maturation), Tris-glycine gel electrophoresis was performed with cathode and anode buffers containing 0.1 M Tris, 0.1 M Tricine, and 0.1% SDS at pH 8.25 and 0.2 M Tris at pH 8.9, respectively. Bacterial cells were grown to an A600 of 0.5, induced for at least 3 h with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG), and collected at 0.025 A600 equivalent unit per μl in SDS-PAGE loading buffer. The samples were boiled for 10 min, and the proteins were separated by electrophoresis on a 16.5% polyacrylamide gel. Proteins were blotted onto nitrocellulose membranes. Flp was immunodetected using the Flp rabbit polyclonal antibody at a dilution of 1:150. A peroxidase-conjugated goat anti-rabbit immunoglobulin G at a dilution of 1:5,000 in Tris-buffered saline containing 10% milk and 0.1% Tween was used to reveal the Flp protein.
Transmission electron microscopy.
For transmission electron microscopy (TEM), bacterial cells were scraped from LB plates containing appropriate antibiotics and 5 mM IPTG after overnight growth. Bacteria were collected in an Eppendorf tube containing 50 μl of 10 mM phosphate-buffered saline (PBS). A drop of the bacterial suspension was placed on Formvar- and carbon-coated copper grids and left for approximately 5 min. Grids were further fixed with 4% paraformaldehyde for 5 min and rinsed two times with 10 mM PBS for 5 min. Grids were then incubated with a 5% bovine serum albumin (BSA) solution in 10 mM PBS for 10 min and incubated with the Flp antiserum at a 1:150 dilution in 0.5% BSA, 10 mM PBS for 45 min. Grids were finally incubated with a 10-nm colloidal gold protein A solution for 30 min in 0.5% BSA, 10 mM PBS. After repetitive washes in 10 mM PBS and in water, grids were immersed in a drop of 1% uranyl acetate for 1 min. Grids were examined in a JEOL 1200EX transmission electron microscope operating at 80.0 kV.
Twitching motility assay.
The presence of functional, retractile pili was assessed by picking a single colony with a toothpick and stabbing it to the bottom of a 3-mm-thick 1% LB agar plate. Plates were incubated overnight at 37°C, agar was removed, and the twitching zones were stained with crystal violet for 5 min and then rinsed with tap water to remove unbound dye.
Adherence assay on inert surfaces.
The adherence assay was performed in 24-well polystyrene microtiter dishes as previously described (3). Attached bacteria were stained with 100 μl of crystal violet at 1% for 15 min and washed twice with water. Staining was extracted with treatment with 400 μl 95% ethanol. Subsequently, 600 μl water was added and A570 was measured. All quantification assays were performed in triplicate.
Adherence assay on bronchial epithelial cell surface.
Briefly, 16HBE14o- human bronchial epithelial cells were inoculated in Labteck chambers, in minimal essential medium supplemented with 10% fetal calf serum and antibiotics for 24 h at 37°C in a 5% CO2 atmosphere. Four hours before infection, the medium was exchanged with a medium free of serum and antibiotics. The epithelial cells were infected with bacteria at a multiplicity of infection of 30 for a period of 1 or 4 h at 37°C. The samples were rinsed twice with PBS, fixed with 4% formaldehyde, stained with 0.1% crystal violet for 5 min, washed twice with water, dried, sealed with Eukitt mounting medium (EMS), and observed with an Axioscop 40 microscope (Zeiss).
RESULTS
Characterization of the PAO1 flp-tad-rcp gene cluster.
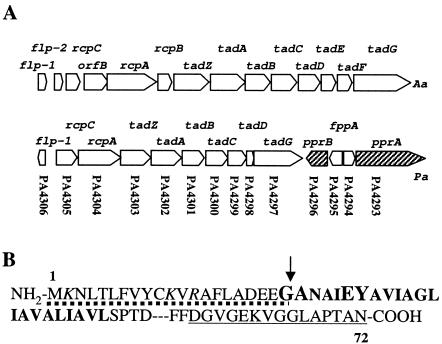
Mining the P. aeruginosa PAO1 genome identified a series of genes homologous to the flp-tad-rcp genes previously characterized in A. actinomycetemcomitans (20). The PA4306 open reading frame (ORF) was identified as an flp-like gene (21). This ORF is transcribed in a reverse orientation as compared to a cluster of nine genes, designated by locus tags PA4297 to PA4305, encoding Tad-Rcp homologous proteins (Fig. 1A and Table 3). The P. aeruginosa flp gene encodes a protein of 72 aa. The P. aeruginosa Flp precursor has a long leader peptide of 21 aa and an alanine residue instead of a phenylalanine residue at position +1 after the putative cleavage site (Fig. 1B). These features are characteristic of type IVb prepilins. The presence of a tyrosine residue at position +6 and the short C-terminal domain (smaller than 90 aa) (Fig. 1B) further supported that it belongs to the Flp subfamily (COG3847) (20). In addition, most of the contiguous genes are homologous to the flp-tad-rcp genes of A. actinomycetemcomitans (Fig. 1A). The names and characteristics attributed to the PA4297-to-PA4305 ORFs are given in Table 3. Most strikingly, PA4302 encodes a protein (421 aa) from the traffic NTPase family (37), involved in processes such as type IV pilus assembly, type II protein secretion, or T-DNA transfer from Agrobacterium tumefaciens (12, 35). The close homology with A. tumefaciens VirB11 ATPase (40) led to the annotation of PA4302 as HvbA (homolog to VirB11) at http://pseudomonas.com. However, it is now clear that the PA4302 product is homologous to the TadA ATPase (COG4962) from A. actinomycetemcomitans (2, 37). PA4304 encodes a protein (416 aa) from the secretin family, previously annotated XqhC (XcpQ homolog C) and here renamed RcpA (COG4964) (15) in accordance with the Tad-Rcp nomenclature. Type IV pilus assembly requires in all cases a traffic ATPase, a secretin, and an inner membrane protein known as PilC in P. aeruginosa. In the tad-rcp gene cluster from PAO1, it is remarkable that two ORFs encode PilC homologues, namely PA4300 (303 aa) and PA4301 (294 aa). The proteins encoded by these ORFs were renamed TadC and TadB, respectively, according to the Tad-Rcp nomenclature. The ORFs PA4297 (556 aa), PA4299 (245 aa), PA4303 (394 aa), and PA4305 (303 aa) encode homologues of TadG, TadD, TadZ, and RcpC, respectively. Finally, no obvious homology could be found between the protein encoded by PA4298 (94 aa) and a member of the Flp-Tad-Rcp system. It is notable that rcpB, tadE and tadF, which are found in the A. actinomycetemcomitans tad-rcp gene cluster, have no obvious homologues in the P. aeruginosa cluster.
FIG. 1.
The flp-tad-rcp gene cluster. (A) Genetic organization of the flp-tad-rcp (ORFs PA4297 to PA4306) gene cluster in P. aeruginosa. The P. aeruginosa (Pa) cluster is compared with the A. actinomycetemcomitans (Aa) cluster. Each arrow represents a gene (not to scale) and indicates the transcription orientation. The original nomenclature of the A. actinomycetemcomitans gene cluster is indicated above each gene and the homologous genes in the P. aeruginosa cluster have been named similarly. In the P. aeruginosa cluster, the original annotation number is also indicated underneath. The P. aeruginosa fppA gene is homologous to the A. actinomycetemcomitans orfB gene and encodes a prepilin peptidase. The PA4293 and PA4296 ORFs (hatched) encode a histidine kinase sensor, PprA, and a response regulator, PprB, respectively. (B) Part of the amino acid sequence of the P. aeruginosa Flp pilin (72 amino acids). The positively charged residues within the leader peptide (underlined with dotted line) are represented by italics. The conserved residues within the Flp family of pilins are boldace and in larger letters (G, −1; A, +1; E, +5; and Y, +6). The hydrophobic domain downstream from the FppA cleavage site (indicated by an arrow) is represented by bold letters. The peptide that was used for raising Flp antibody has been underlined.
TABLE 3.
Characteristics of the PAO1 flp-tad-rcp genes
| Gene name | PA no.a | Protein size (aa) | Predicted no. of transmembrane segments | Predicted functionb |
|---|---|---|---|---|
| flp | 4306 | 72 | 1 | Flp pilin |
| rcpC | 4305 | 303 | 1 | |
| rcpA | 4304 | 416 | 0 | Secretin |
| TadZ | 4303 | 394 | 0 | |
| TadA | 4302 | 421 | 0 | ATPase |
| TadB | 4301 | 294 | 5 | PilC-like |
| TadC | 4300 | 303 | 4 | PilC-like |
| tadD | 4299 | 245 | 0 | |
| 4298 | 94 | 1 | ||
| tadG | 4297 | 556 | 1 | |
| pprB | 4296 | 275 | 0 | Response regulator (TCS) |
| fppA | 4295 | 160 | 4 | Prepilin peptidase |
| 4294 | 168 | 1 | ||
| pprA | 4293 | 922 | 1 | Protein sensor (TCS) |
The PA number corresponds to the genome annotation (http://www.pseudomonas.com).
TCS, two-component systems.
The PAO1 tad-rcp cluster is required for Flp assembly.
The production of Flp protein from P. aeruginosa strain PAO1 was tested at different growth stages when bacteria were grown at 37°C in L broth. Analysis of whole-cell extracts by Western blotting using antibodies directed against Flp indicated that Flp production may occur at a late stage during the growth but was not strictly reproducible from one culture to the other (data not shown). The flp gene was thus cloned under the control of the IPTG-inducible tac promoter of pMMB190, yielding pMMBflp. This plasmid was mobilized into the PAO1 strain, and Flp production was analyzed by Western blotting using antibodies directed against Flp. Whole-cell extract analysis clearly showed that Flp was produced in a large amount upon induction. The export and assembly of Flp pilin subunits into filaments at the bacterial cell surface were then investigated by TEM coupled with immunogold labeling. Filaments labeled with gold particles were seen emerging from the bacterial cell surface (Fig. 2A to C and E to G), which could appear as bundled filaments (Fig. 2E). Enlargement and Fourier transformation (Fig. 2D) revealed a filament with a twisted appearance and with regularly spaced gold particles. Whether these Flp structures could be assembled in mutants affected in the tad-rcp locus was further examined. We engineered tadA or rcpA gene deletion in the PAO1 strain, as described in Materials and Methods, yielding PAO1ΔtadA and PAO1ΔrcpA, respectively. The tadA and rcpA mutants were transformed with pMMBflp and analyzed for Flp assembly. No immunogold-labeled filaments could be identified by TEM using these mutants. Introduction in trans of the tadA and rcpA genes cloned in the pLAFR3 cosmid, yielding pLAFRtadA and pLAFRrcpA, respectively, in the appropriate P. aeruginosa tadA or rcpA mutant, restored Flp pilus assembly. These data suggested that the P. aeruginosa flp gene encodes an Flp pilin whose assembly into a pilus structure required the components encoded by the tad-rcp gene cluster.
FIG. 2.
Electron micrographs of P. aeruginosa Flp pili. (A to C and E to G) TEM views at magnifications of ×50,000 (A and B), ×80,000 (C), or ×140,000 (E to G) after immunogold labeling of Flp-producing PAO1 strains (PAO1/pMMBflp). In panel E, a nonlabeled flagellar structure is clearly visible next to a labeled Flp fiber with bundled filaments (see arrows). (D) Fourier transformation and ×4 enlargement of a small region of the labeled Flp filament.
Characterization of the Flp prepilin peptidase, FppA.
Type IV prepilins are processed by prepilin peptidases which belong to a novel family of bilobed aspartic proteases called type IV prepilin peptidases (TFPP) (27). Downstream from the identified PAO1 tad-rcp gene cluster, one ORF transcribed in a reverse orientation to the tad-rcp locus encodes a homologue to CpaA from C. crescentus (44) and OrfB from A. actynomycetemcomitans (Fig. 1A and Fig. 3A). The protein encoded by PA4295 presented 45% similarity to OrfB and 42% similarity to CpaA (Fig. 3A). The PA4295-encoded protein is 160 residues long and has four predicted hydrophobic transmembrane domains (Fig. 3B). This protein corresponds to the C-terminal domain of the well-characterized PilD prepilin peptidase from P. aeruginosa (36). It thus lacks the N-terminal domain, or domain 1, that contains conserved cysteine residues. However, it still contains the two aspartate residues, which are conserved within the TFPP family (27) and which have been reported to be absolutely required for protease activity. Moreover, it clearly lacks the putative methyltransferase box, LGGKCS, which has been identified in PilD, including the critical glycine residue, G96 (36). We named the PA4295-encoded protein, FppA, for Flp prepilin peptidase A, suggesting that FppA could be the prepilin peptidase responsible for maturation of the Flp precursor and for Flp pilus biogenesis.
FIG. 3.
Characterization of the FppA prepilin peptidase. (A) T-COFFEE multiple alignment of FppA and its homologues, OrfB from A. actinomycetemcomitans and CpaA from C. crescentus. Portions inconsistent between the combined alignments are in green and blue, and consistent ones are in yellow, orange, or red. The conserved aspartate residues (catalytic site) between the three aligned prepilin peptidases are marked with an asterisk. (B) Membrane topology model of the FppA protein. Each circle represents one of the 160 FppA residues. Black circles are located within each of the four predicted transmembrane domains of FppA. Gray residues indicate the position of the two conserved aspartate residues of the TFPP active site. (C) Production and maturation of Flp prepilin from PAO1/pMMBflp (lane 1), PAO1ΔfppA/pMMBflp (lane 2), and PAO1ΔpilD/pMMBflp (lane 8). The arrows on the left indicate the precursor (p) and mature (m) forms of the Flp pilin. Flp processing was also monitored in PAO1ΔfppA/pMMBflp containing pBBR1MCS-2 (lane 4), pBBRfppA (lane 3), or one of the fppA(D17A) (lane 5), fppA(D78A) (lane 6), or fppA(D17/78A) (lane 7) mutated genes. Proteins were separated by electrophoresis on a Tris-glycine gel.
We investigated whether FppA is required for maturation of the Flp pilin subunit. For that purpose, we engineered a deletion mutant for the fppA gene, as described in Materials and Methods, yielding PAO1ΔfppA. The pMMBflp plasmid was introduced in PAO1 and PAO1ΔfppA, and whole-cell samples were analyzed by Western blotting using antibodies directed against Flp. When Flp was produced in the PAO1 parental strain, two bands were identified (Fig. 3C, lane 1). However, only the upper band was identified in the fppA mutant (Fig. 3C, lane 2), indicating that it corresponds to the precursor form, whereas in the PAO1 parental strain the lower band corresponds to the mature Flp form. Maturation of the overproduced Flp prepilin could be fully achieved by the introduction of a plasmid containing the fppA gene, namely pBBRfppA (Fig. 3C, lane 3). This observation also indicated that the level of FppA prepilin peptidase could be limiting for processing large amounts of Flp prepilin. The occurrence of the two Flp forms thus results from the FppA-dependent processing of the Flp precursor. Furthermore, similarly to the tadA and rcpA mutants, no Flp-containing appendages were assembled at the surface of an fppA mutant when assessed by TEM coupled with immunogold labeling of Flp pili, but assembly was restored when pBBRfppA was introduced in the fppA mutant (data not shown).
We further investigated whether the other TFPP encoded in the PAO1 genome, namely PilD, is able to process the Flp prepilin (Fig. 3C). No Flp processing occurred in the fppA mutant that contained an intact pilD gene (Fig. 3C, lane 2), whereas comparable Flp maturation occurred in a pilD mutant (Fig. 3C, lane 8) or the wild-type strain (Fig. 3C, lane 1), suggesting an FppA specialization in Flp maturation.
The aspartate residues are part of the FppA active site.
Since the aspartate residues identified at positions 17 and 78 were designated as critical for the activity of FppA, we mutagenized the fppA gene to substitute these aspartate residues (D) for alanine residues (A). The genes encoding FppAD17A, FppAD78A, and the double mutant FppAD17/78A were obtained by performing overlapping PCR mutagenesis as described in Materials and Methods. The level of mature Flp pilin produced from pMMBflp was subsequently analyzed in an fppA mutant complemented with the wild-type FppA or the various FppA derivatives, FppAD17A, FppAD78A, and FppAD17/78A (Fig. 3C, lanes 5 to 7). Only the production of wild-type FppA could restore maturation of Flp, whereas with any of the FppA aspartate mutants, only the Flp precursor form could be identified. These results clearly indicated that both aspartate residues are necessary for catalysis and that FppA belongs to the TFFP family of proteases.
Role of Flp pili in motility and attachment.
The twitching motility of P. aeruginosa is supported by type IV pilus assembly and retraction. A P. aeruginosa pilD mutant, which lacks type IVa pili, is thus nonmotile (Fig. 4A). Overproduction of the Flp subunit (pMMBflp) in the pilD mutant could not restore motility even though the bacteria assembled Flp pili on their surface. Overproduction of the prepilin peptidase FppA (pBBRfppA) in the pilD mutant could also not restore motility. We further investigated whether Flp pili could be associated with attachment and biofilm formation on plastic surfaces or on respiratory epithelial cell surfaces. In addition to the previously constructed fppA, tadA, and rcpA mutants, we engineered an flp mutant in the PAO1 strain and tested all strains for their biofilm formation capability in comparison to the wild-type strain. No significant alteration of the phenotype was observed (data not shown), suggesting that either the flp gene is not expressed at the chromosomal level under these growth conditions or the Flp-dependent phenotype is masked by the contribution of other appendages, such as the type IVa pili or the flagella. A strain (PAO1ΔfliCΔpilA) devoid of flagella and type IVa pili, which are major determinants of adherence in P. aeruginosa, was thus used. The plasmids allowing concomitant production of Flp and FppA, pMMBflp and pBBRfppA, were introduced into PAO1ΔfliCΔpilA. Wells of a microtiter plate were inoculated with bacteria, and after 6 to 8 h of incubation, the culture medium was removed and the remaining attached bacteria were stained with crystal violet. The strain containing both pMMBflp and pBBRfppA forms a thicker biofilm on a plastic surface (Fig. 4B), as compared to the strain carrying the cloning vectors. Quantification indicated that the PAO1ΔpilAΔfliC strain containing both pMMBflp and pBBRfppA forms a ring with a fivefold-higher efficiency than that of the PAO1ΔpilAΔfliC strain (Fig. 4C). Whether Flp assembly could also influence attachment to respiratory cell surfaces was examined. After 4 h of contact, the PAO1ΔfliCΔpilA strain containing pMMBflp binds efficiently to bronchial epithelial cells, forming substantial bacterial aggregates at the cell surface, whereas the PAO1ΔfliCΔpilA strain containing the cloning vector (pMMB190) binds poorly (Fig. 4D).
FIG. 4.
Functional analysis of P. aeruginosa Flp pili. (A) Twitching motility was tested for various P. aeruginosa strains. The PAO1 strain presents a clear zone of motility, whereas the pilD mutant is nonmotile despite overexpression of the flp (pMMBflp) or fppA (pBBRfppA) gene. (B) Attachment to polystyrene was analyzed in PAO1ΔfliCΔpilA/pMMBflp/pBBRfppA and PAO1ΔfliC ΔpilA/pMMB190/pBBR1MCS-2. Rings of attachment could be observed in strains overexpressing the flp and fppA genes, but not in strains containing the cloning vectors. (C) Quantification of crystal violet staining is presented. Error bars correspond to standard deviation from three distinct experiments. (D) Attachment to bronchial epithelial cells was examined with PAO1ΔfliCΔpilA/pMMBflp and PAO1ΔfliCΔpilA/pMMB190. Upon expression of the flp gene (pMMBflp), bacteria formed substantial aggregates at the cell surface.
DISCUSSION
Several studies have revealed that P. aeruginosa can assemble a large number of appendages on the cell surface. Those appendages are involved in processes such as motility, attachment, or both. This is the case for flagella and type IVa pili. More recently, three fimbrial gene clusters, called cupA, cupB, and cupC, have been identified as being involved in biofilm formation (51). Whereas these clusters encode components of the chaperone-usher pathway described in E. coli (50), no cup-associated fimbrial structures have been described to date in P. aeruginosa. In this study, we used functional genomic approaches to further mine the P. aeruginosa genome to identify genes involved in the assembly of cell-surface appendages that could be relevant for bacterial biofilm development. We identified a cluster whose genes encode components previously reported in A. actinomycetemcomitans as being involved in the assembly of Flp pili (17) that support tight adherence and biofilm formation (18).
The A. actinomycetemcomitans Flp pili are made following the packing of subunits encoded by the flp-1 gene (21). Next to this gene another flp homologue, flp-2, could be identified. However, neither has flp-2 been shown to be required for Flp-assembly nor could its product be found in the pilus structure. In the P. aeruginosa genome, only one flp gene (ORF PA4306) could be identified. The Flp pilins belong to the so-called type IVb family and, more precisely, to a subfamily that possesses a tyrosine at the +6 position after the putative cleavage site and that are rather short polypeptides (smaller than 90 aa) (20).
The leader peptides of the type IV prepilins are cleaved by TFPP. TFPP have been shown to process not only the type IV prepilins but also the so-called pseudopilins involved in the type II protein secretion process (12). In some cases, as in P. aeruginosa, a single TFPP is involved in type IVa pilin and pseudopilin processing, namely PilD (47). In other cases, such as in V. cholerae, one TFPP is involved in the type IV piliation process (TcpJ) (23), whereas another is required for type II protein secretion (VcpD) (30). Processing of type IVa and IVb prepilins has been shown in many cases. However, no demonstration of the functionality of a TFPP required for Flp prepilin processing has been reported, even though it has been proposed that the Flp prepilin peptidases from A. actinomycetemcomitans or C. crescentus are encoded by the orfB and cpaA genes (20, 44), respectively. In both cases, the gene product corresponds to the C-terminal domain of classical TFPP, but lacks the large cytoplasmic domain 1, which includes the four highly conserved cysteine residues (27). It was shown that replacement of these cysteine residues with alanine or serine residues in PilD of P. aeruginosa did not abolish its peptidase activity (46). In vitro and in vivo studies performed with TcpJ from V. cholerae indicated that only the conserved aspartate residues (position 125 and 189) were absolutely required for protease activity (27). We identified an ORF, PA4295, located next to the flp-tad-rcp gene cluster of P. aeruginosa, that encodes a homologue of CpaA and OrfB. The gene product is predicted to have four transmembrane segments and the conserved aspartate residues of the TFPP family are found at position 17 in the cytoplasmic N-terminal domain and at position 78 within the cytoplasmic loop (Fig. 3B). We showed in this study that FppA is able to process the P. aeruginosa Flp prepilin, revealing for the first time that naturally truncated versions of the TFPP family, such as CpaA, OrfB, and FppA, are functional peptidases. This observation confirms that cysteine residues from the cytoplasmic domain 1 are not essential. Furthermore, site-directed mutagenesis of both aspartate residues yielded an inactive FppA, as recently shown in PibD from the archaeon Sulfolobus solfataricus (48). It thus confirmed that they are part of the active site of this truncated peptidase. FppA appears to be specific for Flp prepilins, since no processing of the Flp subunit occurred in the absence of FppA, indicating that PilD cannot substitute for FppA function, and reciprocally, no functional type IVa pili were detectable in the absence of PilD. Moreover, overproduction of FppA in a pilD mutant did not allow restoration of type IVa pilus-associated phenotypes such as twitching motility. It is not clear whether particular residues or domains of FppA impart specificity for Flp or whether the additional domains found in PilD contain Flp avoidance regions. It is also not known whether FppA, just like PilD, possesses a methyltransferase activity that allows methylation of the N-terminal residue of the mature Flp pilin. However, the putative methyltransferase box LGGKCS described for PilD is clearly absent in FppA, including the glycine residue, which is essential for the N-methyltransferase activity of PilD (36). The issue of methylation of the Flp-like subunit was previously addressed with CpaA (44), and the strong homology between CpaA and FppA suggests that these peptidases have no methyltransferase activity. It is also known that N methylation of pilin, which occurs onto the phenylalanine residue of mature PilA, does not seem to be essential for type IVa pilus assembly (36).
In A. actinomycetemcomitans, Flp subunits are assembled into a pilus structure that is involved in attachment to surfaces. Upon overexpression of the flp gene of P. aeruginosa, we were able to identify by immunogold labeling cell surface structures that contain Flp. Only overexpression of the flp gene is required under our growth conditions to observe Flp pili, indicating that basal expression of the tad-rcp gene cluster is sufficient for assembly. Since the flp gene and the tad-rcp genes are divergently transcribed, their expression might be differentially controlled. One could argue that the long and thick bundle-like Flp structures that we observed do not resemble the ones that could be assembled when Flp protein is expressed at the chromosomal level; however, the structures looked quite similar to the ones observed in A. actinomycetemcomitans (21). The P. aeruginosa Flp pili appeared to be involved in surface attachment both on abiotic surfaces (polystyrene) and on biotic surfaces (human bronchial epithelial cell line 16HBE14o-). The processing and assembly into a pilus structure of the overproduced Flp subunits are limited by the amount of FppA. Upon co-overproduction of Flp and FppA, increased levels of bacteria remained attached on the surfaces. Moreover, under these conditions, bacterial aggregates could be seen, which indicates that not only are Flp pili involved in bacterium-surface or bacterium-cell attachment, but they also seem to be involved in bacterium-bacterium contact and aggregation.
Type IVa pili are assembled with the help of a complex molecular machine, the core of which consists of a traffic NTPase, a polytopic inner membrane protein, and an outer membrane channel or secretin, which are in P. aeruginosa PilB, PilC, and PilQ, respectively. Type IVb pilus assembly, such as Bfp from enteropathogenic E. coli or TCP from V. cholerae, requires a subset of additional components that are mostly cell envelope located (29, 39). In this study, we have shown that the P. aeruginosa tad-rcp gene cluster encodes a functional system that is responsible for the assembly of Flp pili at the cell surface. Indeed, no Flp pili could be seen at the surface of tadA (PA4302) and rcpA (PA4304) mutants, which have had the genes encoding the traffic NTPase and the secretin, respectively, deleted. Moreover, two ORFs encode PilC homologues, tadB (PA4301) and tadC (PA4300). PilC and other members of this family have been proposed to form a platform within the cytoplasmic membrane through which pilins and pseudopilins are driven to the periplasmic side of the cytoplasmic membrane thanks to the energy provided by the traffic NTPase (12). The extrusion of the pilus structure through the outer membrane is then mediated by the secretin (56). It is likely that PilC homologues are assembled as homomultimers, whereas in the Tad system, TadB and TadC could form heteromultimeric complexes.
The Flp assembly machinery seems to require an additional subset of components, which are conserved between C. crescentus and A. actinomycetemcomitans and have been found in P. aeruginosa. It includes rcpC (PA4305), tadZ (PA4303), tadD (PA4296), and tadG (PA4297). The rcpB, tadE, and tadF genes from A. actinomycetemcomitans could not be clearly found in the P. aeruginosa cluster. However, PA4298 encodes a protein (94 aa) with a putative signal peptide, such as RcpB, which is larger with 167 residues. Outside of the tad-rcp cluster but next to fppA, PA4294 encodes a 168-aa-long polypeptide with a putative N-terminal transmembrane domain and presenting slight homology with a domain of TadG. Whether the PA4294 protein could be related to TadE or TadF, which are both proteins presenting a predicted N-terminal transmembrane domain, is unclear. Finally, even though slight differences could be found between the tad-rcp gene clusters of A. actinomycetemcomitans and P. aeruginosa, they are conserved and are both involved in Flp pilus assembly.
Analysis of the PAO1 genome revealed how rich this organism is in terms of systems that assemble various cell surface appendages. The assembly of these different structures may respond to particular environmental conditions in order to promote the adaptation of the microorganism to a novel ecological niche. ORFs encoding a two-component system have been identified adjacent to the tad-rcp locus (Fig. 1A): PA4293 encodes a histidine kinase sensor, and PA4296 encodes a response regulator that belongs to the NarL family. This two-component system was reported as PprA and PprB (55). A mutant strain affected in the pprA gene shows an altered outer membrane profile. Whether expression of the flp-tad-rcp operon is under the control of that particular two-component system needs further investigation. Interestingly, the PprB (PA4296) response regulator has been described recently as a quorum-sensing (QS) modulator but apparently does not control any of the tad genes (10). Moreover, many studies dedicated to QS regulon showed that two or more genes of the tad cluster are QS regulated (43, 53, 54). Additional data showed that whereas flagellin down-regulation occurred in response to CF airway fluid independently of QS, flp-tad genes can be slightly up-regulated under these conditions (57; supporting table available at www.pnas.org), suggesting that the bacteria may develop alternative strategies such as Flp-mediated adherence to colonize (42) and survive in muco-purulent environments such as those encountered in CF lungs during chronic infection. Moreover, other conditions may also account for potential signals that could control the expression of the flp gene, such as exposure to hydrogen peroxide (41). Finally, it has been shown that several tad genes are VqsR regulated (19) but not AlgR regulated (28). It should be noted that we additionally tested several growth conditions, including anaerobic environment, oxidative stress, or addition of subinhibitory concentrations of aminoglycoside antibiotics (16), but none of them appeared to be relevant for a detectable level of Flp production (data not shown). Obviously, it appears that control of production and assembly of the Flp pili is highly complex and environment dependent, which supports the ubiquitous nature of P. aeruginosa, which may adapt and develop a biofilm in many distinct environments.
Acknowledgments
We thank A. Bernadac for technical assistance and A. Fox for her helpful comments.
The work of A.F. and S.D. is supported by the French cystic fibrosis foundation (VLM) and the foundation Bettencourt-Schueller. M.A. has been supported by the French research and technology ministry as well as by the VLM.
REFERENCES
- 1.Anantha, R. P., K. D. Stone, and M. S. Donnenberg. 2000. Effects of bfp mutations on biogenesis of functional enteropathogenic Escherichia coli type IV pili. J. Bacteriol. 182:2498-2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhattacharjee, M. K., S. C. Kachlany, D. H. Fine, and D. H. Figurski. 2001. Nonspecific adherence and fibril biogenesis by Actinobacillus actinomycetemcomitans: TadA protein is an ATPase. J. Bacteriol. 183:5927-5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bieber, D., S. W. Ramer, C. Y. Wu, W. J. Murray, T. Tobe, R. Fernandez, and G. K. Schoolnik. 1998. Type IV pili, transient bacterial aggregates, and virulence of enteropathogenic Escherichia coli. Science 280:2114-2118. [DOI] [PubMed] [Google Scholar]
- 4.Bitter, W. 2003. Secretins of Pseudomonas aeruginosa: large holes in the outer membrane. Arch. Microbiol. 179:307-314. [DOI] [PubMed] [Google Scholar]
- 5.Blank, T. E., H. Zhong, A. L. Bell, T. S. Whittam, and M. S. Donnenberg. 2000. Molecular variation among type IV pilin (bfpA) genes from diverse enteropathogenic Escherichia coli strains. Infect. Immun. 68:7028-7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cleary, J., L. C. Lai, R. K. Shaw, A. Straatman-Iwanowska, M. S. Donnenberg, G. Frankel, and S. Knutton. 2004. Enteropathogenic Escherichia coli (EPEC) adhesion to intestinal epithelial cells: role of bundle-forming pili (BFP), EspA filaments and intimin. Microbiology 150:527-538. [DOI] [PubMed] [Google Scholar]
- 7.Costerton, J. W. 2001. Cystic fibrosis pathogenesis and the role of biofilms in persistent infection. Trends Microbiol. 9:50-52. [DOI] [PubMed] [Google Scholar]
- 8.Craig, L., R. K. Taylor, M. E. Pique, B. D. Adair, A. S. Arvai, M. Singh, S. J. Lloyd, D. S. Shin, E. D. Getzoff, M. Yeager, K. T. Forest, and J. A. Tainer. 2003. Type IV pilin structure and assembly: X-ray and EM analyses of Vibrio cholerae toxin-coregulated pilus and Pseudomonas aeruginosa PAK pilin. Mol. Cell 11:1139-1150. [DOI] [PubMed] [Google Scholar]
- 9.Craig, L., M. E. Pique, and J. A. Tainer. 2004. Type IV pilus structure and bacterial pathogenicity. Nat. Rev. Microbiol. 2:363-378. [DOI] [PubMed] [Google Scholar]
- 10.Dong, Y. H., X. F. Zhang, H. M. Soo, E. P. Greenberg, and L. H. Zhang. 2005. The two-component response regulator PprB modulates quorum-sensing signal production and global gene expression in Pseudomonas aeruginosa. Mol. Microbiol. 56:1287-1301. [DOI] [PubMed] [Google Scholar]
- 11.Donnenberg, M. S., H. Z. Zhang, and K. D. Stone. 1997. Biogenesis of the bundle-forming pilus of enteropathogenic Escherichia coli: reconstitution of fimbriae in recombinant E. coli and role of DsbA in pilin stability—a review. Gene 192:33-38. [DOI] [PubMed] [Google Scholar]
- 12.Filloux, A. 2004. The underlying mechanisms of type II protein secretion. Biochim. Biophys. Acta 1694:163-179. [DOI] [PubMed] [Google Scholar]
- 13.Gerard-Vincent, M., V. Robert, G. Ball, S. Bleves, G. P. Michel, A. Lazdunski, and A. Filloux. 2002. Identification of XcpP domains that confer functionality and specificity to the Pseudomonas aeruginosa type II secretion apparatus. Mol. Microbiol. 44:1651-1665. [DOI] [PubMed] [Google Scholar]
- 14.Giron, J. A., O. G. Gomez-Duarte, K. G. Jarvis, and J. B. Kaper. 1997. Longus pilus of enterotoxigenic Escherichia coli and its relatedness to other type-4 pili—a minireview. Gene 192:39-43. [DOI] [PubMed] [Google Scholar]
- 15.Haase, E. M., J. L. Zmuda, and F. A. Scannapieco. 1999. Identification and molecular analysis of rough-colony-specific outer membrane proteins of Actinobacillus actinomycetemcomitans. Infect. Immun. 67:2901-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffman, L. R., D. A. D'Argenio, M. J. MacCoss, Z. Zhang, R. A. Jones, and S. I. Miller. 2005. Aminoglycoside antibiotics induce bacterial biofilm formation. Nature 436:1171-1175. [DOI] [PubMed] [Google Scholar]
- 17.Inoue, T., I. Tanimoto, H. Ohta, K. Kato, Y. Murayama, and K. Fukui. 1998. Molecular characterization of low-molecular-weight component protein, Flp, in Actinobacillus actinomycetemcomitans fimbriae. Microbiol. Immunol. 42:253-258. [DOI] [PubMed] [Google Scholar]
- 18.Inoue, T., R. Shingaki, N. Sogawa, C. A. Sogawa, J. Asaumi, S. Kokeguchi, and K. Fukui. 2003. Biofilm formation by a fimbriae-deficient mutant of Actinobacillus actinomycetemcomitans. Microbiol. Immunol. 47:877-881. [DOI] [PubMed] [Google Scholar]
- 19.Juhas, M., L. Wiehlmann, P. Salunkhe, J. Lauber, J. Buer, and B. Tummler. 2005. GeneChip expression analysis of the VqsR regulon of Pseudomonas aeruginosa TB. FEMS Microbiol. Lett. 242:287-295. [DOI] [PubMed] [Google Scholar]
- 20.Kachlany, S. C., P. J. Planet, R. DeSalle, D. H. Fine, and D. H. Figurski. 2001. Genes for tight adherence of Actinobacillus actinomycetemcomitans: from plaque to plague to pond scum. Trends Microbiol. 9:429-437. [DOI] [PubMed] [Google Scholar]
- 21.Kachlany, S. C., P. J. Planet, R. Desalle, D. H. Fine, D. H. Figurski, and J. B. Kaplan. 2001. flp-1, the first representative of a new pilin gene subfamily, is required for non-specific adherence of Actinobacillus actinomycetemcomitans. Mol. Microbiol. 40:542-554. [DOI] [PubMed] [Google Scholar]
- 22.Kaniga, K., L. Delor, and G. R. Cornelis. 1991. A wide-host-range suicide vector for improving reverse genetics in gram negative bacteria: inactivation of the bla gene of Yersinia enterocolitica. Gene 109:137-141. [DOI] [PubMed] [Google Scholar]
- 23.Kaufman, M. R., J. M. Seyer, and R. K. Taylor. 1991. Processing of TCP pilin by TcpJ typifies a common step intrinsic to a newly recognized pathway of extracellular protein secretion by gram-negative bacteria. Genes Dev. 5:1834-1846. [DOI] [PubMed] [Google Scholar]
- 24.Keizer, D. W., C. M. Slupsky, M. Kalisiak, A. P. Campbell, M. P. Crump, P. A. Sastry, B. Hazes, R. T. Irvin, and B. D. Sykes. 2001. Structure of a pilin monomer from Pseudomonas aeruginosa: implications for the assembly of pili. J. Biol. Chem. 276:24186-24193. [DOI] [PubMed] [Google Scholar]
- 25.Klausen, M., A. Heydorn, P. Ragas, L. Lambertsen, A. Aaes-Jorgensen, S. Molin, and T. Tolker-Nielsen. 2003. Biofilm formation by Pseudomonas aeruginosa wild type, flagella and type IV pili mutants. Mol. Microbiol. 48:1511-1524. [DOI] [PubMed] [Google Scholar]
- 26.Labaer, J., Q. Qiu, A. Anumanthan, W. Mar, D. Zuo, T. V. Murthy, H. Taycher, A. Halleck, E. Hainsworth, S. Lory, and L. Brizuela. 2004. The Pseudomonas aeruginosa PA01 gene collection. Genome Res. 14:2190-2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.LaPointe, C. F., and R. K. Taylor. 2000. The type 4 prepilin peptidases comprise a novel family of aspartic acid proteases. J. Biol. Chem. 275:1502-1510. [DOI] [PubMed] [Google Scholar]
- 28.Lizewski, S. E., J. R. Schurr, D. W. Jackson, A. Frisk, A. J. Carterson, and M. J. Schurr. 2004. Identification of AlgR-regulated genes in Pseudomonas aeruginosa by use of microarray analysis. J. Bacteriol. 186:5672-5684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manning, P. A. 1997. The tcp gene cluster of Vibrio cholerae. Gene 192:63-70. [DOI] [PubMed] [Google Scholar]
- 30.Marsh, J. W., and R. K. Taylor. 1998. Identification of the Vibrio cholerae type 4 prepilin peptidase required for cholera toxin secretion and pilus formation. Mol. Microbiol. 29:1481-1492. [DOI] [PubMed] [Google Scholar]
- 31.Nika, J. R., J. L. Latimer, C. K. Ward, R. J. Blick, N. J. Wagner, L. D. Cope, G. G. Mahairas, R. S. Munson, Jr., and E. J. Hansen. 2002. Haemophilus ducreyi requires the flp gene cluster for microcolony formation in vitro. Infect. Immun. 70:2965-2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nunn, D., S. Bergman, and S. Lory. 1990. Products of three accessory genes, pilB, pilC, and pilD, are required for biogenesis of Pseudomonas aeruginosa pili. J. Bacteriol. 172:2911-2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nunn, D. N., and S. Lory. 1991. Product of the Pseudomonas aeruginosa gene pilD is a prepilin leader peptidase. Proc. Natl. Acad. Sci. USA 88:3281-3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Toole, G. A., and R. Kolter. 1998. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 30:295-304. [DOI] [PubMed] [Google Scholar]
- 35.Peabody, C. R., Y. J. Chung, M. R. Yen, D. Vidal-Ingigliardi, A. P. Pugsley, and M. H. Saier, Jr. 2003. Type II protein secretion and its relationship to bacterial type IV pili and archaeal flagella. Microbiology 149:3051-3072. [DOI] [PubMed] [Google Scholar]
- 36.Pepe, J. C., and S. Lory. 1998. Amino acid substitutions in PilD, a bifunctional enzyme of Pseudomonas aeruginosa. Effect on leader peptidase and N-methyltransferase activities in vitro and in vivo. J. Biol. Chem. 273:19120-19129. [DOI] [PubMed] [Google Scholar]
- 37.Planet, P. J., S. C. Kachlany, R. DeSalle, and D. H. Figurski. 2001. Phylogeny of genes for secretion NTPases: identification of the widespread tadA subfamily and development of a diagnostic key for gene classification. Proc. Natl. Acad. Sci. USA 98:2503-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Planet, P. J., S. C. Kachlany, D. H. Fine, R. DeSalle, and D. H. Figurski. 2003. The widespread colonization island of Actinobacillus actinomycetemcomitans. Nat. Genet. 34:193-198. [DOI] [PubMed] [Google Scholar]
- 39.Ramer, S. W., G. K. Schoolnik, C.-Y. Wu, J. Hwang, S. A. Schmidt, and D. Bieber. 2002. The type IV pilus assembly complex: biogenic interactions among the bundle-forming pilus proteins of enteropathogenic Escherichia coli. J. Bacteriol. 184:3457-3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rashkova, S., X.-R. Zhou, J. Chen, and P. J. Christie. 2000. Self-assembly of the Agrobacterium tumefaciens VirB11 traffic ATPase. J. Bacteriol. 182:4137-4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salunkhe, P., T. Töpfer, J. Buer, and B. Tümmler. 2005. Genome-wide transcriptional profiling of the steady-state response of Pseudomonas aeruginosa to hydrogen peroxide. J. Bacteriol. 187:2565-2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schreiner, H. C., K. Sinatra, J. B. Kaplan, D. Furgang, S. C. Kachlany, P. J. Planet, B. A. Perez, D. H. Figurski, and D. H. Fine. 2003. Tight-adherence genes of Actinobacillus actinomycetemcomitans are required for virulence in a rat model. Proc. Natl. Acad. Sci. USA 100:7295-7300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schuster, M., C. P. Lostroh, T. Ogi, and E. P. Greenberg. 2003. Identification, timing, and signal specificity of Pseudomonas aeruginosa quorum-controlled genes: a transcriptome analysis. J. Bacteriol. 185:2066-2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Skerker, J. M., and L. Shapiro. 2000. Identification and cell cycle control of a novel pilus system in Caulobacter crescentus. EMBO J. 19:3223-3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stover, C. K., X. Q. Pham, A. L. Erwin, S. D. Mizoguchi, P. Warrener, M. J. Hickey, F. S. Brinkman, W. O. Hufnagle, D. J. Kowalik, M. Lagrou, R. L. Garber, L. Goltry, E. Tolentino, S. Westbrock-Wadman, Y. Yuan, L. L. Brody, S. N. Coulter, K. R. Folger, A. K. Kas, K. Larbig, R. Lim, K. Smith, D. Spencer, G. K. Wong, Z. Wu, I. T. Paulsen, J. Reizer, M. H. Saier, R. E. Hancock, S. Lory, and M. V. Olson. 2000. Complete genome sequence of Pseudomonas aeruginosa PA01, an opportunistic pathogen. Nature 406:959-964. [DOI] [PubMed] [Google Scholar]
- 46.Strom, M. S., P. Bergman, and S. Lory. 1993. Identification of active-site cysteines in the conserved domain of PilD, the bifunctional type IV pilin leader peptidase/N-methyltransferase of Pseudomonas aeruginosa. J. Biol. Chem. 268:15788-15794. [PubMed] [Google Scholar]
- 47.Strom, M. S., D. N. Nunn, and S. Lory. 1994. Posttranslational processing of type IV prepilin and homologs by PilD of Pseudomonas aeruginosa. Methods Enzymol. 235:527-540. [DOI] [PubMed] [Google Scholar]
- 48.Szabó, Z., S.-V. Albers, and A. J. M. Driessen. 2006. Active-site residues in the type IV prepilin peptidase homologue PibD from the archaeon Sulfolobus solfataricus. J. Bacteriol. 188:1437-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taylor, R. K., T. J. Kirn, M. D. Meeks, T. K. Wade, and W. F. Wade. 2004. A Vibrio cholerae classical TcpA amino acid sequence induces protective antibody that binds an area hypothesized to be important for toxin-coregulated pilus structure. Infect. Immun. 72:6050-6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thanassi, D. G., and S. J. Hultgren. 2000. Assembly of complex organelles: pilus biogenesis in gram-negative bacteria as a model system. Methods 20:111-126. [DOI] [PubMed] [Google Scholar]
- 51.Vallet, I., J. W. Olson, S. Lory, A. Lazdunski, and A. Filloux. 2001. The chaperone/usher pathway of Pseudomonas aeruginosa: identification of fimbrial gene clusters (cup) and their involvement in biofilm formation. Proc. Natl. Acad. Sci. USA 98:6911-6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vasseur, P., I. Vallet-Gely, C. Soscia, S. Genin, and A. Filloux. 2005. The pel genes of the Pseudomonas aeruginosa PAK strain are involved at early and late stages of biofilm formation. Microbiology 151:985-997. [DOI] [PubMed] [Google Scholar]
- 53.Wagner, V. E., D. Bushnell, L. Passador, A. I. Brooks, and B. H. Iglewski. 2003. Microarray analysis of Pseudomonas aeruginosa quorum-sensing regulons: effects of growth phase and environment. J. Bacteriol. 185:2080-2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wagner, V. E., R. J. Gillis, and B. H. Iglewski. 2004. Transcriptome analysis of quorum-sensing regulation and virulence factor expression in Pseudomonas aeruginosa. Vaccine 22:15-20. [DOI] [PubMed] [Google Scholar]
- 55.Wang, Y., U. Ha, L. Zeng, and S. Jin. 2003. Regulation of membrane permeability by a two-component regulatory system in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 47:95-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wolfgang, M., J. P. van Putten, S. F. Hayes, D. Dorward, and M. Koomey. 2000. Components and dynamics of fiber formation define a ubiquitous biogenesis pathway for bacterial pili. EMBO J. 19:6408-6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wolfgang, M. C., J. Jyot, A. L. Goodman, R. Ramphal, and S. Lory. 2004. Pseudomonas aeruginosa regulates flagellin expression as part of a global response to airway fluid from cystic fibrosis patients. Proc. Natl. Acad. Sci. USA 101:6664-6668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zambon, J. J. 1985. Actinobacillus actinomycetemcomitans in human periodontal disease: microbial factors. J. Clin. Periodontol. 12:1-20. [DOI] [PubMed] [Google Scholar]