Abstract
Clostridium perfringens causes fatal human infections, such as gas gangrene, as well as gastrointestinal diseases in both humans and animals. Detailed molecular analysis of the tetracycline resistance plasmid pCW3 from C. perfringens has shown that it represents the prototype of a unique family of conjugative antibiotic resistance and virulence plasmids. We have identified the pCW3 replication region by deletion and transposon mutagenesis and showed that the essential rep gene encoded a basic protein with no similarity to any known plasmid replication proteins. An 11-gene conjugation locus containing 5 genes that encoded putative proteins with similarity to proteins from the conjugative transposon Tn916 was identified, although the genes’ genetic arrangements were different. Functional genetic studies demonstrated that two of the genes in this transfer clostridial plasmid (tcp) locus, tcpF and tcpH, were essential for the conjugative transfer of pCW3, and comparative analysis confirmed that the tcp locus was not confined to pCW3. The conjugation region was present on all known conjugative plasmids from C. perfringens, including an enterotoxin plasmid and other toxin plasmids. These results have significant implications for plasmid evolution, as they provide evidence that a nonreplicating Tn916-like element can evolve to become the conjugation locus of replicating plasmids that carry major virulence genes or antibiotic resistance determinants.
Conjugative plasmids are self-replicating molecules that encode their own transfer to recipient strains, usually by a type IV secretion apparatus. Conjugative plasmids from gram-positive bacteria, such as Enterococcus, Staphylococcus, and Bacillus spp., employ similar mechanisms (23); however, the absence of an outer membrane and the presence of a much thicker peptidoglycan layer necessitates a requirement for different conjugation complexes. Integrative conjugative elements (ICEs), or conjugative transposons, are also capable of encoding their own conjugative transfer, but these elements are not self replicating and require genomic integration for their stable maintenance. The best known ICEs are Tn916 from Enterococcus faecalis and Tn1545 from Streptococcus pneumoniae, but little is known about their mechanisms of conjugation (45). ICEs have been identified from many gram-positive bacteria, including the pathogenic clostridia. These elements include the Tn916-like conjugative element Tn5397 from Clostridium difficile and the defective element CW459tet(M) from Clostridium perfringens (46).
The 47-kb tetracycline resistance plasmid pCW3 (49) carries a novel tetracycline resistance operon (56) and is the paradigm conjugative plasmid from C. perfringens. It is closely related to pIP401, which carries the same tetracycline resistance genes, and also carries Tn4451, an integrative mobilizable element that confers chloramphenicol resistance (5). Deletion of Tn4451 from pIP401 (3) results in a plasmid that has a restriction profile identical to that of pCW3. Comparative restriction and hybridization analysis has shown that all conjugative tetracycline resistance plasmids from C. perfringens are either indistinguishable from pCW3 or have a large common region (3). In addition, there are large regions of similarity between pCW3 and the plasmids that carry the enterotoxin gene, cpe (12, 36).
The objective of this study was to determine if the region that was common between these plasmids was involved in conjugative transfer. We have identified the replication and conjugation regions of pCW3 and carried out comparative analyses. In this study, we report that pCW3 carries a unique conjugation region that is common to all conjugative C. perfringens plasmids and that several of the conjugation genes have putative products with similarity to conjugation proteins from Tn916.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The C. perfringens strains used in this study were wild-type tetracycline-resistant isolates from diverse sources. The recipients in conjugation experiments were either JIR325, a rifampin- and nalidixic acid-resistant derivative of strain 13 (33), or JIR4394, a streptomycin- and chlorate-resistant derivative of strain 13. C. perfringens strains were cultured at 37°C in TPG broth (48), brain heart infusion broth (Oxoid), FTG medium (Difco), or nutrient agar (47) supplemented with tetracycline (10 μg/ml), rifampin (20 μg/ml), nalidixic acid (20 μg/ml), chloramphenicol (10 μg/ml), thiamphenicol (10 μg/ml), or streptomycin (1 mg/ml). When required, 1% (vol/vol) saturated potassium chlorate was included. C. perfringens agar cultures were incubated in an atmosphere of 10% H2-10% CO2-80% N2. The Escherichia coli host strain used was DH5α (Life Technologies) or EC300 (Epicenter), which was grown at 37°C in 2× YT medium (50) supplemented with ampicillin (100 μg/ml), erythromycin (150 μg/ml), or kanamycin (50 μg/ml). Plasmids are listed in Table 1.
TABLE 1.
Origin and source of plasmids
| Plasmid | Description | Source or reference |
|---|---|---|
| pCW3 | Confers conjugative tetracycline resistance | 49 |
| pJIR15 | pBR322 ΩpCW3(ClaI 9.8 kb) | 1 |
| pJIR16 | pBR322 ΩpCW3(ClaI 12.2 kb) | 1 |
| pJIR17 | pBR322 ΩpCW3(ClaI 10.4 kb) | 1 |
| pJIR18 | pBR322 ΩpCW3(ClaI 9.9 kb) | 1 |
| pJIR26 | pJIR27 ΔTn4452 | 3 |
| pJIR32 | pBR322 ΩpCW3(ClaI 5.0 kb) | 1 |
| pJIR936 | pBR322 ΩpCW3(ClaI 9,744 bp) Ω1.2 kb BamHI, erm(B) | K. Koutsis and J. I. Rood, unpublished |
| pJIR1909 | pCW3 10,358-bp ClaI (bp 41533-4628) + pCW3 9,744-bp ClaI (bp 4628-14372) | P. Johanesen and J. I. Rood, unpublished |
| pJIR2765 | pJIR936 Δ5.2 kb HpaI | This study |
| pJIR2766 | pJIR936 Δ8.8 kb EcoRV | This study |
| pJIR2767 | pJIR936 Δ3.1 kb XbaI | This study |
| pJIR2768 | pJIR2675 Δ1.2 kb NheI/SpeI | This study |
| pJIR2715 | Base C. perfringens suicide vector, erm(Q)+catP+ (inactivating Knr) oriT+ | C. Hennequin and J. I. Rood, unpublished |
| pJIR2898 | pJIR2715 (Asp718/BamHI) ΩJRP1997/JRP1998 PCR product (Asp718/BamHI; 1,900 bp) (5′ tcpH fragment) | This study |
| pJIR2899 | pJIR2898 (XhoI/SacI) ΩJRP1999/JRP2000 PCR product (XhoI/SacI; 1,996 bp) (tcpH suicide vector) | This study |
| pJIR2901 | pJIR750 (Asp718/BamHI) ΩJRP2119/JRP2120 PCR product (Asp718/BamHI; 3,030 bp) (ptcpH+ complementation vector) | This study |
| pJIR3023 | pJIR2715 (XhoI/SacI) ΩJRP1995/JRP1996 PCR product (XhoI/SacI; 2,070 bp) (3′ tcpF fragment) | This study |
| pJIR3024 | pJIR3023 (Asp718/BamHI) ΩJRP1993/JRP1994 PCR product (Asp718/BamHI; 1,981 bp) (tcpF suicide vector) | This study |
| pJIR3025 | pJIR750 (Asp718/BamHI) ΩJRP2347/JRP2348 PCR product (Asp718/BamHI; 2,869 bp) (ptcpF+ complementation vector) | This study |
| pMRS4969 | pCPF4969 cpe ΩcatP | 12 |
Molecular techniques.
E. coli plasmid DNA was isolated using alkaline lysis (QIAGEN). Crude C. perfringens DNA was extracted by resuspending cells from an agar culture in 100 μl of lysis buffer, boiling for 10 min, and centrifuging for 10 min at 11,000 × g at room temperature. The DNA in the supernatant was phenol:chloroform and chloroform extracted, isopropanol precipitated, washed with ethanol, and resuspended in 10 μl of H2O. Purified C. perfringens DNA was obtained as described previously (48). PCR amplification used Taq DNA polymerase (Roche) and a 0.5 μM concentration of each primer. Denaturation (94°C for 1 min), annealing (50°C for 2 min), and extension (72°C for 3 to 5 min) steps were carried out for 30 cycles. PCR products were purified using the QIAquick PCR purification kit before sequencing on an Applied Biosystems 3730S capillary sequencer.
The complete pCW3 sequence was determined on both strands, with the exception of the previously sequenced tetracycline resistance determinant (56). Templates for plasmid sequencing were existing (1) and newly constructed subclones of pCW3. Sequencing of PCR products was used to cross all restriction sites used in cloning. Sequence data were analyzed using Sequencher version 3.0 (Gene Codes Corporation), and potential genes were identified using GeneMarkS (11), in conjunction with the Sanger Institute freeware Artemis, release 6. Putative gene products were analyzed using PSI-BLAST (6, 7), TopPred (14), and PSORT Prediction (38). Sequences were aligned using ClustalW (28).
Construction of C. perfringens mutants by allelic exchange.
To increase the frequency of double crossovers, the suicide plasmids contained ca. 2 kb of sequence upstream and downstream of the gene to be mutated. These regions were generated by PCR and then cloned sequentially into the E. coli vector pJIR2715, which contains genes encoding thiamphenicol and erythromycin resistance (C. Hennequin and J. Rood, unpublished data). The base pair (bp) 28,500 to 30,481 pCW3 region was cloned upstream of erm(Q), and the bp 32,648 to 34,718 region was cloned downstream of erm(Q) to generate the tcpF suicide vector pJIR3024. Similarly for tcpH, the regions bp 32,084 to 33,984 and bp 36,275 to 38,271 of pCW3 were cloned upstream and downstream, respectively, of erm(Q) to form pJIR2899.
The suicide vectors were independently introduced into JIR325(pCW3) by electroporation (52). DNA preparations of potential erythromycin-resistant/thiamphenicol-sensitive recombinants were tested by PCR and sequence analysis to confirm the replacement of the target gene with the erm(Q) cassette and loss of the suicide plasmid. For complementation studies, PCR products carrying the wild-type tcpF and tcpH genes were generated and cloned into the C. perfringens-E. coli shuttle vector pJIR750 to generate pJIR3025 and pJIR2901, respectively.
Conjugation.
Matings on solid media were carried out as described previously (47, 48). Nutrient agar supplemented with tetracycline, streptomycin, and potassium chlorate was used to select for transconjugants when C. perfringens strain JIR4394 was used as the recipient. The efficiency of conjugative transfer is reported as the number of transconjugants/donor cell.
Transposon mutagenesis.
Transposon mutagenesis with the EZ::TN In-Frame Linker Insertion kit (EPICENTRE), performed per the manufacturer's instructions, was used to analyze the target plasmid, pJIR2768. The in vitro reaction mixture was introduced into EC300 cells by electroporation, and kanamycin-resistant cells were selected. EZ::TN insertions were mapped and sequenced.
Nucleotide sequence accession numbers.
GenBank accession numbers for pCW3 and the tcp regions of pJIR26 and pMRS4969 are DQ366035, DQ338471, and DQ338472, respectively.
RESULTS
Sequence analysis of pCW3.
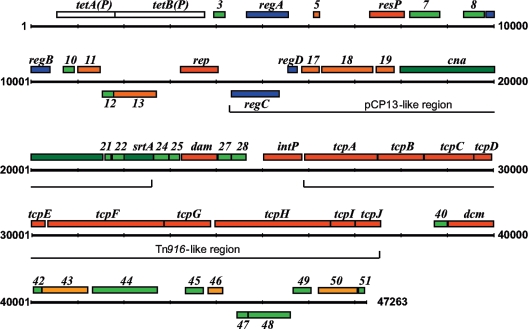
The complete nucleotide sequence of pCW3 was determined by a combination of sequence analysis of subclones and PCR fragments as well as primer walking. Where PCR fragments were used, the sequence was confirmed by sequencing at least two separate PCR products on both strands. pCW3 was shown to comprise 47,263 bp, with a G+C content of 27.6%, which is similar to that of the C. perfringens chromosome. Our analysis identified 51 open reading frames (ORFs) (Fig. 1 and Table 2).
FIG. 1.
Genetic organization of pCW3. The first T of the −35 box of the promoter for the tetAB(P) operon (24), which encodes tetracycline resistance, was designated nucleotide number 1. ORFs on the sense and complementary strands are indicated above and below the plasmid line, respectively. Genes with unknown functions are indicated by their designated pCW3 gene number, putative conjugation genes as tcp genes, and putative regulatory genes as reg genes. The labeled bars indicate regions with either similarity to a locus present on pCP13 or genes whose products have low-level similarity to Tn916 conjugation proteins. Colors indicate the gene product's function: red, DNA metabolism (replication, recombination, DNA transfer, and modification); dark green, membrane and surface associated; yellow, miscellaneous metabolism; orange, conserved hypothetical; light green, unknown; white, antibiotic resistance; and blue, regulation.
TABLE 2.
Predicted pCW3 genes
| pCW3 gene | Function of closest relative of gene product, source, and/or similarity | Size (aa) | Coding sequence position |
|---|---|---|---|
| tetA(P) | TetA(P) tetracycline efflux resistance protein | 420 | 559-1821 |
| tetB(P) | TetB(P) tetracycline resistance protein | 652 | 1805-3763 |
| pcw303 | Hypothetical protein | 92 | 3955-4233 |
| regA | Transcriptional regulator A, AraC related, Bacillus anthracis AraC, 150/292 (51%), AraC-type DNA binding domain COG2207 | 289 | 4684-5553 |
| pcw305 | Conserved hypothetical protein, C. perfringens CPE2599, 48/50 (96%) | 50 | 6101-6253 |
| resP | Site-specific serine recombinase-resolvase family, Bacillus thuringiensis plasmid pBtoxis, pBt142, 91/210 (43%); C. perfringens pIP404, ORF8, 54/150 (36%); C. perfringens pCP13, PCP15, 50/150 (33%), resolvase pfam00239\ | 247 | 7333-8076 |
| pcw307 | Hypothetical protein | 218 | 8195-8851 |
| pcw308 | Hypothetical protein | 156 | 9351-9821 |
| regB | Transcriptional regulator B, LexA-like SOS response transcriptional repressor COG1974, C. perfringens CPE1161, 68/200 (34%) | 176 | 9888-10418 |
| pcw310 | Hypothetical protein | 75 | 10704-10931 |
| pcw311 | Conserved hypothetical protein, C. perfringens pCP13, PCP12, 76/168 (45%); C. perfringens pIP404, ORF6, 49/163 (30%) | 165 | 10997-11494 |
| pcw312 | Hypothetical protein | 87 | 11552-11815c |
| pcw313 | Possible plasmid partitioning protein; MreB-like, actin-like ATPase COG1077 | 308 | 11794-12720c |
| rep | Rep, replication protein | 276 | 13239-14069 |
| regC | Transcriptional regulator C, LexA-like SOS response transcriptional repressor COG1974, C. perfringens pCP13 PCP61, 135/378 (35%) | 344 | 14336-15370c |
| regD | Putative transcriptional regulator, Enterococcus faecalis EF0306, 19/55 (34%), HTH_XRE cd00093 | 77 | 15531-15764 |
| pcw317 | Conserved hypothetical protein, C. perfringens pCP13 PCP60, 32/119 (26%) | 124 | 15855-16229 |
| pcw318 | Conserved hypothetical protein, C. perfringens pCP13 PCP59, 184/397 (46%) | 366 | 16290-17390 |
| pcw319 | Probable PemK-like plasmid postsegregational toxin, C. perfringens, pCP13 PCP58, PemK pfam02452, 73/121 (60%) | 124 | 17458-17832 |
| cna | Probable surface-anchored protein (LPXTG motif), collagen adhesin-like protein, C. perfringens pCP13 PCP57, 778/1150 (67%), predicted outer membrane protein COG4932 | 1,187 | 17972-21535 |
| pcw321 | Hypothetical protein | 42 | 21606-21731 |
| pcw322 | Hypothetical protein | 85 | 21750-22007 |
| srtA | Sortase, surface protein transpeptidase, C. perfringens pCP13 PCP56, 109/204 (53%), sortase cd0004 | 214 | 22017-22661 |
| pcw324 | Hypothetical protein | 100 | 22672-22974 |
| pcw325 | Hypothetical protein | 72 | 22978-23196 |
| dam | Site-specific DNA methyltransferase (adenine specific), COG0338 | 254 | 23260-24024 |
| pcw327 | Hypothetical protein | 94 | 24051-24335 |
| pcw328 | Hypothetical protein | 102 | 24336-24644 |
| intP | Potential tyrosine recombinase (integrase), pfam00589 | 266 | 25036-25836 |
| tcpA | Probable coupling protein with similarity to DNA segregation ATPases FTsK/SpoIIIE and related proteins, COG1674 | 530 | 25890-27482 |
| tcpB | Possible DNA segregation ATPase FtsK/SpoIIIE-like ATPase, COG1674, COG0630 | 327 | 27497-28480 |
| tcpC | Tn916 ORF13-like protein, Enterococcus faecium Efae2512, 75/305 (24%) | 359 | 28473-29552 |
| tcpD | Hypothetical protein | 115 | 29564-29911 |
| tcpE | Tn916 ORF17-like protein, Listeria monocytogenes lmo1107, 26/93 (27%) | 122 | 29923-30291 |
| tcpF | Tn916 ORF16-like conjugation protein with predicted ATPase, Listeria monocytogenes lmo1106, 226/845 (26%) | 841 | 30349-32874 |
| tcpG | Peptidoglycan hydrolase, C. perfringens PCP44, 121/161 (75%), Tn916 ORF14-like protein, COG1705, FlgJ, muramidase, amidase_4, pfam01832 | 334 | 32874-33878 |
| tcpH | Tn916 ORF15-like membrane protein | 832 | 33981-36479 |
| tcpI | Membrane-bound metal-dependant hydrolase, Bacillus anthracis pX02-79, 63/162 (38%) | 164 | 36482-36976 |
| tcpJ | Hypothetical protein | 193 | 36985-37566 |
| pcw340 | Hypothetical protein | 101 | 38691-38996 |
| dcm | Site-specific DNA methyltransferase (cytosine specific), pfam00145, C. perfringens strain 297442, 153/155 (98%) | 339 | 39011-40030 |
| pcw342 | Hypothetical protein | 57 | 40047-40220 |
| pcw343 | Conserved hypothetical protein C. perfringens strain 297442, 325/340 (95%) | 340 | 40217-41239 |
| pcw344 | Hypothetical protein | 468 | 41334-42740 |
| pcw345 | Hypothetical protein | 133 | 43328-43729 |
| pcw346 | Conserved hypothetical protein C. perfringens PCP33, 37/68 (54%) | 103 | 43827-44138 |
| pcw347 | Hypothetical protein | 77 | 44450-44683c |
| pcw348 | Hypothetical protein | 300 | 44708-45610c |
| pcw349 | Hypothetical protein | 135 | 45740-46147 |
| pcw350 | Conserved hypothetical protein, C. perfringens CPE0425, 250/284 (88%) | 285 | 46211-47068 |
| pcw351 | Hypothetical protein | 29 | 47085-47174 |
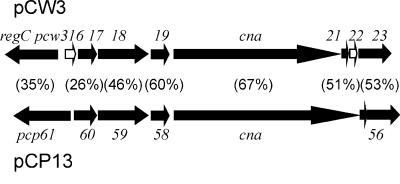
Comparative analysis showed that the bp 16332 to 22432 region of pCW3 had 70% nucleotide sequence identity to a similar-size region (bp 51011 to 44615) of the 53-kb plasmid pCP13 from C. perfringens strain 13 (55). pCP13 carries a defective β2 toxin gene, cbp2, that is not present on pCW3. This region of pCW3 carried nine putative genes, with the seven largest genes having distinct homologues within pCP13 (Fig. 2). The region included the cna gene, whose product had similarity to a collagen adhesin from Staphylococcus aureus (39, 40, 44, 57) (Fig. 1). Two potential methyltransferase genes (dam and dcm) were identified on pCW3, both of which have been identified on the conjugative cpe plasmid pCPF4969 and on another cpe plasmid, pCPF5603 (35, 36). The dcm gene is located in close proximity to the cpe gene on these plasmids and is present in other cpe+ and non-cpe-carrying C. perfringens strains (35, 36).
FIG. 2.
Genetic comparison of the cna locus. Depicted are the arrangement and coding orientation of the genes surrounding the cna gene within both pCW3 and pCP13. The percentages of amino acid sequence identities between the encoded proteins are shown in parentheses. Genes present only on pCW3 are filled in with white.
Tn4451 is inserted into the cna gene of pIP401.
The well-studied element Tn4451 (4, 9, 29, 30, 32) was originally identified on the pCW3-like plasmid pIP401 (34). It was postulated based on comparative restriction endonuclease analysis that pIP401 consisted of a pCW3 plasmid into which Tn4451 had inserted (2). By comparing the nucleotide sequence of the left and right ends of the insertion site of Tn4451 to the sequence of pCW3, the corresponding site within pCW3 to the insertion site of the element on pIP401 has now been identified at bp 21,329 in pCW3. This site is located within the cna gene, 67 codons from the stop codon. As pIP401 is still able to encode its own conjugative transfer, this result indicates that the terminal 67 amino acids of the putative Cna protein are not required for conjugation.
Replication of pCW3 involves a unique Rep protein.
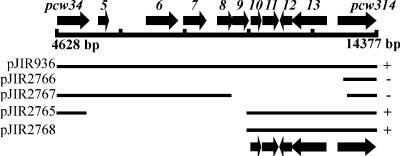
Annotation of the pCW3 sequence did not reveal any proteins with similarity to plasmid replication proteins. A functional genetic approach, therefore, was used to identify the replication region. It was initially shown that pJIR1909, a deletion derivative of pCW3 that encompassed a 20.1-kb (bp 41533 to 14372) region, could support its own independent replication in C. perfringens (P. Johanesen, D. Lyras, and J. Rood, unpublished data). To further delineate the pCW3 replication region, a plasmid containing the 9,744-bp ClaI fragment (bp 4628 to 14372) and the erm(B) erythromycin resistance gene was constructed. This plasmid, pJIR936, could replicate in C. perfringens (K. Koutsis and J. Rood, unpublished data). Subsequently, deletion derivatives of pJIR936 were isolated and tested for their abilities to replicate in C. perfringens. The smallest derivative that still supported plasmid replication in this organism was pJIR2768, which carried the pcw310 to pcw314 genes (Fig. 3).
FIG. 3.
Localization of the pCW3 plasmid replication region. Shown at the top is the genetic organization of the relevant 9.8-kb ClaI fragment of pCW3. At the bottom, the line diagrams denote the region of pCW3 contained within each of the depicted plasmids. A plus sign indicates that the plasmid replicates independently in C. perfringens; a minus sign indicates that the plasmid does not replicate independently in C. perfringens.
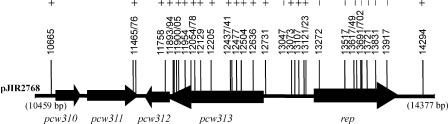
To determine which of these genes was essential for plasmid replication, a series of transposon mutants of pJIR2768 was generated by EZ-TN mutagenesis in Escherichia coli and mapped. We identified 34 plasmids that had insertions in the pCW3-derived region and independently introduced them into C. perfringens strain JIR325. The ability of the plasmid derivatives to replicate was determined by the isolation of erythromycin-resistant transformants and confirmed by plasmid isolation and restriction analysis. None of the derivatives containing an insert within the pcw314 gene replicated in C. perfringens (Fig. 4). These results demonstrated that pcw314 was essential for plasmid replication, and it was therefore designated the rep gene.
FIG. 4.
Identification of the rep gene. The arrows indicate the pCW3-derived genes within the cloned insert of pJIR2768. Vertical lines with numbers represent independent EZ::TN insertion mutants within pJIR2768. Insertion derivatives are named by corresponding base pairs within the pCW3 sequence. A plus sign indicates that the EZ::TN-containing pJIR2768 derivative plasmid replicates independently in C. perfringens; a minus sign indicates that the EZ::TN-containing pJIR2768 derivative plasmid does not replicate independently in C. perfringens.
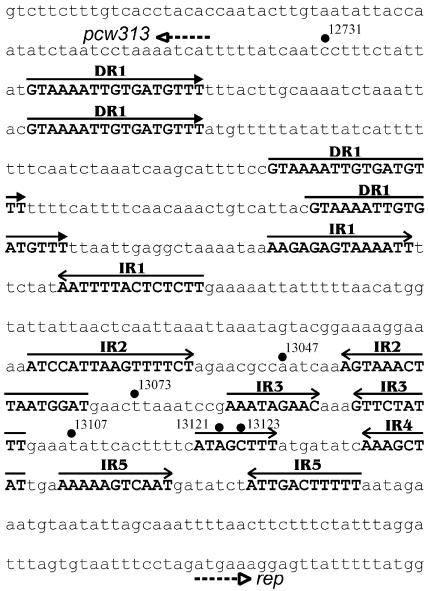
The region between pcw313 and the rep gene (Fig. 5) contained five distinct pairs of inverted repeats and four 17-bp direct repeats that could act as iteron-like sequences in the initiation of pCW3 replication. These repeats may represent either iteron-like Rep binding sites or the binding sites for plasmid stability proteins encoded elsewhere in the rep region. Several of the EZ-TN inserts were located within this region (Fig. 5), one of which (at bp 13047) was nonfunctional, suggesting that the integrity of at least part of this region is important for either Rep expression or function.
FIG. 5.
Repeated elements in the intergenic region between the pcw313 and rep genes. Depicted is the pCW3 nucleotide sequence from bp 12661 to 13260. Closed arrowheads denote the four copies of the identical direct repeats (DR1). The open-headed arrows depict the five pairs of perfect inverted repeats (IR1 to IR5). Shaded circles highlight insertion sites of EZ::TN derivatives in this region. The dotted line shows the location of the pcw313 and rep genes.
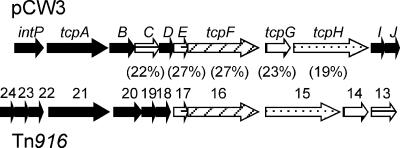
pCW3 encodes functional homologues of Tn916-encoded proteins.
Several putative proteins encoded within the pcw329-pcw339 region had varying but low levels of amino acid sequence identity to conjugation proteins encoded by the conjugative transposon Tn916 (15), although the genetic arrangement was different (Fig. 6). The Tn916 proteins are involved in conjugative transfer, although their exact functions are not known. Therefore, we postulated that the pCW3 genes were involved in conjugation and designated them the tcp genes, for “transfer clostridial plasmid.” Bioinformatic analysis suggested that the intP-tcpJ genes comprised an operon.
FIG. 6.
Genetic comparison of the transfer-related regions of pCW3 and Tn916. Numbers in parentheses denote the percentages of amino acid sequence identity between the encoded proteins. Related genes are shaded in a similar manner. Genes shaded black encode proteins with no similarity.
The first gene of the putative intP-tcpJ operon encoded a potential site-specific tyrosine recombinase, IntP. Although this protein was somewhat smaller than most tyrosine recombinases, it was similar in size to XerC- and XerD-like recombinases, which are known to be involved in the resolution of chromosome dimers (54). The second gene, tcpA, encoded a predicted integral inner membrane protein with two putative N-terminal transmembrane domains and a C-terminal cytoplasmic region that contained consensus Walker A and B boxes. These features are commonly found within coupling proteins such as TraG (RP4), TraD (F plasmid), and TrwB (R388) (22). TcpA also had a conserved domain (COG1674) found within DNA transport proteins, such as FtsK, a DNA segregation ATPase from E. coli (43), and SpoIIIE, a protein involved in chromosomal segregation during sporulation of Bacillus subtilis (53). Members of the FtsK/SpoIIIE protein family are generally larger than TcpA, and their transmembrane region consists of five membrane-spanning segments (20). Coupling proteins from conjugative plasmids often share functional and structural features with members of the FtsK/SpoIIIE protein family. TcpA and ORF21 from Tn916 had very little amino acid sequence identity but may have similar functions, since they both have similarity to members of this family. The next pCW3 gene, tcpB, encoded a predicted cytoplasmic protein that had 28% amino acid sequence identity to the central section of TcpA and a less well-conserved FtsK/SpoIIIE domain.
tcpC was one of five genes whose products had low-level sequence identity to Tn916 proteins, having similarity to ORF13 homologues from Tn916, Tn5397, and CW459tet(M). The other putative pCW3 products that had similarity to Tn916 homologues included TcpE (ORF17), the potential ATPase TcpF (ORF16), a putative peptidoglycan hydrolase, TcpG (ORF14), and TcpH (ORF15). Very little is known about the function of these proteins in Tn916.
TcpH was predicted to be an integral membrane protein, with eight potential transmembrane domains in the first half of the protein. Within this section of TcpH and ORF15 was a region that had similarity to a conserved TrbL/VirB6 domain. VirB6 from Agrobacterium tumefaciens is an integral membrane protein that is involved in mating pair formation (Mpf), in particular in the stabilization of other Mpf-related proteins (25). It is possible that TcpH may play a role similar to that of VirB6, but it may have additional functions, since it has a cytoplasmic region of 402 amino acids that is not found within VirB6.
TcpG appeared to have an N-terminal signal peptide as well as both the N-terminal catalytic domain and the C-terminal cell wall binding domains common to peptidoglycan hydrolases, in particular to N-acetylmuramoyl-l-alanine amidases. TcpG was similar to PCP44 from pCP13, with similarity limited to the predicted cell wall binding region of the protein. TcpG did not contain the same putative amidase domain as PCP44. TcpI was another potential inner membrane protein, with three predicted transmembrane domains. It was most similar to pX02-79, a conserved hypothetical protein encoded by the plasmid pX02 from Bacillus anthracis, and also contained a predicted membrane-bound metal-dependent hydrolase domain. We suggest that TcpG and TcpI may work synergistically to digest the cell wall so that conjugative transfer can occur.
All known conjugative plasmids encode a relaxase protein that contains a highly conserved motif. However, we were unable to locate a pCW3-encoded protein that contains this motif. Similarly, we were not able to identify a potential origin of transfer within the pCW3 sequence.
Allelic exchange was used to determine if Tn916 homologues located within the tcp region were required for conjugative transfer. The tcpF and tcpH genes were chosen as targets, because TcpF was the protein most similar to its Tn916 homologue and because TcpH was a putative inner membrane Mpf protein. Gene regions (ca. 2 kb) flanking either tcpF or tcpH were cloned on either side of the erm(Q) erythromycin resistance gene on the suicide vector pJIR2715 (C. Hennequin, K. Farrow, and J. Rood, unpublished data). Elsewhere on this vector, there is a chloramphenicol resistance gene (catP) that enables screening for double crossovers by selecting colonies that are susceptible to chloramphenicol or thiamphenicol. The suicide plasmids pJIR3024 (tcpF) and pJIR2899 (tcpH) were constructed and used to transform C. perfringens strain JIR325(pCW3) to erythromycin resistance. Two independently derived mutants of each of the tcpF and tcpH genes were isolated, and PCR analysis was used to confirm that they were derived from double crossovers onto pCW3 (data not shown).
The resultant pCW3ΔtcpF::erm(Q) or pCW3ΔtcpH::erm(Q) mutant was unable to encode conjugative transfer (Table 3). To establish that the loss of conjugative transfer was the result of the specific mutation, complementation analysis was performed. The wild-type tcpF and tcpH genes were cloned independently into the C. perfringens-E. coli shuttle vector pJIR750, and the resultant plasmids were introduced into strains harboring the mutated pCW3 plasmids. Conjugation experiments showed that complementation in trans restored conjugative ability to both the tcpF and tcpH mutants (Table 3), although not to wild-type levels. This result may be due to polar effects on the genes located downstream of tcpF and tcpH on the mutated plasmids or different expression levels of the genes located on the shuttle plasmids. Nonetheless, it is clear that both mutants can be complemented in trans and therefore that these genes are essential for conjugative transfer of pCW3, which confirms experimentally that genes within the tcp region are involved in conjugative transfer.
TABLE 3.
Conjugation frequencies of tcpF and tcpH mutants and their complemented derivatives
| Plasmid | Conjugation frequency (transconjugants/donor cell) |
|---|---|
| pCW3 | (1.4 ± 0.1) × 10−1 |
| pCW3 ΔtcpH1::erm(Q) | <(1.1 ± 0.2) × 10−8 |
| pCW3 Δ tcpH2::erm(Q) | <(1.3 ± 0.2) × 10−8 |
| pCW3 ΔtcpH1::erm(Q)(ptcpH+) | (1.2 ± 0.2) × 10−4 |
| pCW3 ΔtcpH2::erm(Q)(ptcpH+) | (4.8 ± 2.2) × 10−5 |
| pCW3 ΔtcpF1::erm(Q) | <(8.1 ± 1.1) × 10−9 |
| pCW3 ΔtcpF2::erm(Q) | <(1.8 ± 0.2) × 10−8 |
| pCW3 ΔtcpF1::erm(Q)(ptcpF+) | (1.3 ± 0.6) × 10−6 |
| pCW3 ΔtcpF2::erm(Q)(ptcpF+) | (2.7 ± 0.6) × 10−6 |
All known conjugative plasmids from C. perfringens carry the tcp genes.
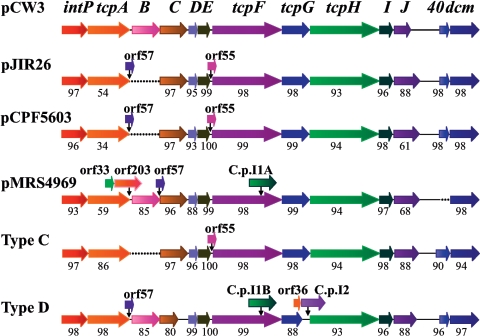
Since the area of identity between pCW3 and other conjugative C. perfringens plasmids appeared to encompass the tcp region, we used PCR analysis with 10 overlapping primer pairs to show that 11 diverse strains that harbored conjugative tetracycline resistance plasmids had the tcpEFGH gene region. More extensive analysis was carried out on the conjugative tetracycline resistance plasmid, pJIR26 (the plasmid most different from pCW3), and pMRS4969, a genetically marked derivative of the conjugative cpe plasmid pCPF4969 (12). PCR amplification across the entire intP-dcm region was performed, and the resultant products were completely sequenced. The tcp regions of all three conjugative plasmids were very similar (Fig. 7), with the homologous proteins having 88% to 99.4% amino acid sequence identity. The exceptions were the TcpA proteins, which all had the same FtsK/SpoIIIE-like domains but only had 54% to 61% identity, and TcpJ, which in pCW3 was truncated by approximately 70 amino acids. In addition, there was no tcpB gene on pJIR26. Upstream of tcpC in pJIR26, there was an ORF also found in pMRS4969. pJIR26 also had an additional ORF between tcpE and tcpF.
FIG. 7.
Comparative analysis of the intP to dcm region. The genetic organization of this region within pCW3 is depicted at the top of the diagram. The genetic maps of the tcp regions of the tetracycline resistance plasmid pJIR26 and the CPE-derived plasmid pMRS4969 were determined as part of this study, and that of pCPF5603 is from Miyamoto et al. (36). The comparative maps of the equivalent regions from type C and type D toxin plasmids were derived from data from The Institute for Genomic Research (see the text). Insertions with respect to the pCW3 sequence are indicated by arrows above the linear maps. Deletions with respect to the pCW3 sequence are depicted by dotted lines. Related genes are shaded in a similar manner. Percentages indicate amino acid sequence identity to the equivalent pCW3 homologue.
Compared to pCW3, pMRS4969 contained two large insertions and a deletion. The first insertion introduced three additional ORFs between tcpA and tcpB, the largest encoding an additional FtsK-like protein, ORF204, that had 31 to 39% identity to the TcpA and TcpB proteins of pCW3. The other ORFs encoded two small hypothetical proteins. The second insertion consisted of a group II intron, C.p.In1A, that was located within the 3′ end of tcpF. The putative reverse transcriptase of C.p.In1A had 55 to 60% similarity to enzymes from group II introns of Enterococcus and Bacillus spp. and 43% identity to the reverse transcriptase of the group II intron located on Tn5397. The deletion within pMRS4969 removed the gene encoding the small hypothetical protein PCW340, located upstream of the dcm gene.
Finally, the genes for many of the major lethal toxins produced by C. perfringens are known to be carried on large plasmids (42). Sequence analysis of some of these plasmids is under way as part of a collaborative project involving several laboratories. Analysis of data made available from this project (G. Myers, I. Paulsen, J. Songer, B. McClane, R. Titball, J. Rood, and S. Melville, personal communication) indicated that a plasmid carrying the β-toxin gene from a C. perfringens type C strain and a separate plasmid carrying the ɛ-toxin gene from a C. perfringens type D strain contained transfer regions to almost identical those found within pCW3 (Fig. 7). The type C plasmid was missing tcpB and had a small ORF also present in pJIR26. The type D plasmid had tcpB and two group II introns, one of which (C.p.In1B) had a homologue on pMRS4969. Also included in this comparison was the tcp region from another cpe plasmid, pCPF5603, which has very recently been reported (36). The tcp region of this plasmid, which has not yet been shown to be conjugative, has the same genetic organization as the conjugative tetracycline resistance plasmid pJIR26 (Fig. 7).
DISCUSSION
In this study, we have shown that the tcp conjugation region from pCW3 is present in all known conjugative plasmids from C. perfringens, including a plasmid that encodes the enterotoxin. It is also present on plasmids encoding β-toxin or ɛ-toxin, which are major lethal toxins produced by C. perfringens. Based on our functional data, we suggest that the β-toxin and ɛ-toxin plasmids may also be conjugative. This finding has significant implications for the epidemiology of diseases caused by these strains. As previously discussed in relation to the enterotoxin plasmid (12), it may no longer be necessary for an invading C. perfringens isolate to have the ability to colonize the gastrointestinal tract. By conjugative transfer of a β-toxin and ɛ-toxin plasmid to an already adherent C. perfringens cell, the resident bacterium could acquire the potential to produce the relevant toxin and thereby cause disease.
Very recently the complete sequences of the cpe plasmids pCPF5603 and pCPF4969 from C. perfringens were reported (36). Both plasmids had the tcp region, and PCR analysis indicated that this region was present in other cpe plasmids and plasmids from type B to E strains of C. perfringens. The sequence analysis of the tcp region from pCPF4969 is in agreement with the sequence of its derivative, pMRS4969, reported here. The demonstration in the current study that genes located in the common tcp region were essential for conjugative transfer provides experimental evidence to support the hypothesis that some or all of the toxin plasmids from C. perfringens are conjugative.
Comparative genomic analysis indicated that the products of the tcp transfer region had limited but significant similarity to conjugation proteins from Tn916. Transposon mutagenesis of Tn916 has identified a region carrying 12 genes (orf24-orf13) that is involved in the conjugative transfer process, including the respective Tn916 homologues of TcpF and TcpH, ORF15 and ORF16 (13, 16). These genes are present in all functional members of the Tn916 family, including Tn5397 from C. difficile, although to our knowledge no studies confirming the functional role of individual proteins within these elements have been reported. Since only five of these genes (orf13 to orf17) encode products with similarity to the pCW3 conjugation proteins, it is clear that the conjugative transfer mechanisms of pCW3 and Tn916, although related, must involve distinct processes.
Many of the conjugative plasmids identified from gram-positive bacteria have significant similarity (16). These plasmids include the staphylococcal plasmids pG01 and pSK41 (37) (10), the lactococcal plasmid pMRC01 (18), the enterococcal plasmid pRE25 (51), and the streptococcal plasmid pIP501 (27). The proteins encoded by the shared 11-kb transfer region have 80% to 100% identity, suggesting that these plasmids utilize a common conjugation mechanism. However, the conjugation proteins from these plasmids have very little primary sequence similarity to the proteins encoded by conjugative transposons or pCW3.
A second group of conjugative plasmids from gram positives includes the closely related enterococcal plasmids pAD1 and pAM373. Transposon mutagenesis of pAD1 was used to identify a contiguous region of 31 kb that contained genes involved in conjugation (19), and more recently its complete sequence was determined (21). Although the pAD1 conjugation machinery appears to be more complex in terms of the number of proteins involved, it does include homologs of the ORF15 and ORF16 proteins of Tn916 (17). Genes similar to the transfer genes of pAD1, including orf15 and orf16, are also present on the conjugative plasmid pAM373 and on pTEF1, a plasmid from E. faecalis strain V583 (41). However, none of these genes have been reported to be functional conjugation genes.
Deletion and mutagenesis studies have led to the identification of the pCW3 rep gene, which was shown to be essential for plasmid replication. Note that the putative Rep protein had a basic pI of 10, as expected for a DNA binding protein, but it had no significant similarity to any known Rep initiator proteins from other plasmids. However, a protein with 98% identity to Rep is encoded by both pCPF5603 (pCPF5603_16) and pCPF4969 (pCPF4969_01), although its function was not previously recognized (36). We suggest that these toxin plasmids replicate by a mechanism similar to that of pCW3 and that Rep is a very C. perfringens-specific protein, which would explain why homologues of pCW3 or the toxin plasmids have not been found in any other bacterial species.
In conclusion, the derivation of the complete sequence of pCW3 from C. perfringens and the functional identification of the conjugation and replication regions, coupled with the comparative analysis of the equivalent regions of several other conjugative plasmids from C. perfringens, have identified a unique family of conjugative plasmids that to date are restricted to this species. These closely related plasmids carry either a novel tetracycline resistance operon that has not been found in any other genus or toxin genes that are restricted to C. perfringens. These plasmids presumably evolved in C. perfringens from a common progenitor. The presence of an integrase gene, the first gene in the putative tcp operon, and the fact that the products of the conjugation genes within this novel plasmid family have similarity to conjugation proteins encoded by Tn916 support the hypothesis that the tcp region evolved from an exogenous precursor, presumably from the conjugative transfer of a Tn916-like ICE into C. perfringens, followed by its insertion into a nonconjugative native plasmid. Subsequently, evolution of this now-conjugative plasmid has led to the loss of the ability of the ICE to excise independently, the rearrangement and divergent development of the conjugation genes, and the acquisition of different genes that confer a selective advantage to the host, such as toxin and antibiotic resistance genes. This hypothesis is supported by previous studies that showed that Tn916 is able to integrate into the C. perfringens genome after conjugative transfer (8, 26, 33), evidence that Tn916-like tet(M) genes are relatively common in C. perfringens (31), and the fact that a defective element closely related to Tn916 is present in at least one nonconjugative isolate of C. perfringens (46). Finally, we propose that pCW3 may represent an evolutionary intermediate between conjugative plasmids that replicate independently and nonreplicating ICEs such as Tn916. However, the various recombination events that led to the evolution of pCW3 do not appear to have been recent, since this plasmid is only distantly related to Tn916.
Acknowledgments
We thank Torsten Seemann from the Victorian Bioinformatics Consortium for his most valuable assistance.
W.L.T. was the recipient of a Monash Postgraduate Scholarship. This research was supported by grants from the Australian Research Council to the ARC Centre of Excellence in Structural and Functional Microbial Genomics and grant AI056177-03 from the United States National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Abraham, L. J., and J. I. Rood. 1985. Cloning and analysis of the Clostridium perfringens tetracycline resistance plasmid, pCW3. Plasmid 13:155-162. [DOI] [PubMed] [Google Scholar]
- 2.Abraham, L. J., and J. I. Rood. 1987. Identification of Tn4451 and Tn4452, chloramphenicol resistance transposons from Clostridium perfringens. J. Bacteriol. 169:1579-1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abraham, L. J., A. J. Wales, and J. I. Rood. 1985. Worldwide distribution of the conjugative Clostridium perfringens tetracycline resistance plasmid, pCW3. Plasmid 14:37-46. [DOI] [PubMed] [Google Scholar]
- 4.Adams, V., I. S. Lucet, D. Lyras, and J. I. Rood. 2004. DNA binding properties of TnpX indicate that different synapses are formed in the excision and integration of the Tn4451 family. Mol. Microbiol. 53:1195-1207. [DOI] [PubMed] [Google Scholar]
- 5.Adams, V., D. Lyras, K. A. Farrow, and J. I. Rood. 2002. The clostridial mobilisable transposons. Cell. Mol. Life Sci. 59:2033-2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Altschul, S. F., and E. V. Koonin. 1998. Iterated profile searches with PSI-BLAST-a tool for discovery in protein databases. Trends Biochem. Sci. 23:444-447. [DOI] [PubMed] [Google Scholar]
- 7.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Awad, M. M., and J. I. Rood. 1997. Isolation of alpha-toxin, theta-toxin and kappa-toxin mutants of Clostridium perfringens by Tn916 mutagenesis. Microb. Pathog. 22:275-284. [DOI] [PubMed] [Google Scholar]
- 9.Bannam, T. L., P. K. Crellin, and J. I. Rood. 1995. Molecular genetics of the chloramphenicol-resistance transposon Tn4451 from Clostridium perfringens: the TnpX site-specific recombinase excises a circular transposon molecule. Mol. Microbiol. 16:535-551. [DOI] [PubMed] [Google Scholar]
- 10.Berg, T., N. Firth, S. Apisiridej, A. Hettiaratchi, A. Leelaporn, and R. A. Skurray. 1998. Complete nucleotide sequence of pSK41: evolution of staphylococcal conjugative multiresistance plasmids. J. Bacteriol. 180:4350-4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Besemer, J., A. Lomsadze, and M. Borodovsky. 2001. GeneMarkS: a self-training method for prediction of gene starts in microbial genomes. Implications for finding sequence motifs in regulatory regions. Nucleic Acids Res. 29:2607-2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brynestad, S., M. R. Sarker, B. A. McClane, P. E. Granum, and J. I. Rood. 2001. Enterotoxin plasmid from Clostridium perfringens is conjugative. Infect. Immun. 69:3483-3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Churchward, G. 2002. Conjugative transposons and related mobile elements, p. 177-191. In N. L. Craig, R. Craigie, M. Gellert, and A. M. Lambowitz (ed.), Mobile DNA II. ASM Press, Washington, D.C.
- 14.Claros, M. G., and G. von Heijne. 1994. TopPred II: an improved software for membrane protein structure predictions. Comput. Appl. Biosci. 10:685-686. [DOI] [PubMed] [Google Scholar]
- 15.Clewell, D. B., S. E. Flannagan, and D. D. Jaworski. 1995. Unconstrained bacterial promiscuity: the Tn916-Tn1545 family of conjugative transposons. Trends Microbiol. 3:229-236. [DOI] [PubMed] [Google Scholar]
- 16.Clewell, D. B., and M. V. Francia. 2004. Conjugation in gram-positive bacteria, p. 227-256. In B. E. Funnell and G. J. Phillips (ed.), Plasmid biology. ASM Press, Washington, D.C.
- 17.Clewell, D. B., M. V. Francia, S. E. Flannagan, and F. Y. An. 2002. Enterococcal plasmid transfer: sex pheromones, transfer origins, relaxases, and the Staphylococcus aureus issue. Plasmid 48:193-201. [DOI] [PubMed] [Google Scholar]
- 18.Dougherty, B. A., C. Hill, J. F. Weidman, D. R. Richardson, J. C. Venter, and R. P. Ross. 1998. Sequence and analysis of the 60 kb conjugative, bacteriocin-producing plasmid pMRC01 from Lactococcus lactis DPC3147. Mol. Microbiol. 29:1029-1038. [DOI] [PubMed] [Google Scholar]
- 19.Ehrenfeld, E. E., and D. B. Clewell. 1987. Transfer functions of the Streptococcus faecalis plasmid pAD1: organization of plasmid DNA encoding response to sex pheromone. J. Bacteriol. 169:3473-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Errington, J., J. Bath, and L. J. Wu. 2001. DNA transport in bacteria. Nat. Rev. Mol. Cell Biol. 2:538-545. [DOI] [PubMed] [Google Scholar]
- 21.Francia, M. V., W. Haas, R. Wirth, E. Samberger, A. Muscholl-Silberhorn, M. S. Gilmore, Y. Ike, K. E. Weaver, F. Y. An, and D. B. Clewell. 2001. Completion of the nucleotide sequence of the Enterococcus faecalis conjugative virulence plasmid pAD1 and identification of a second transfer origin. Plasmid 46:117-127. [DOI] [PubMed] [Google Scholar]
- 22.Gomis-Ruth, F. X., M. Sola, F. de la Cruz, and M. Coll. 2004. Coupling factors in macromolecular type-IV secretion machineries. Curr. Pharm. Des. 10:1551-1565. [DOI] [PubMed] [Google Scholar]
- 23.Grohmann, E., G. Muth, and M. Espinosa. 2003. Conjugative plasmid transfer in gram-positive bacteria. Microbiol. Mol. Biol. Rev. 67:277-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johanesen, P. A., D. Lyras, T. L. Bannam, and J. I. Rood. 2001. Transcriptional analysis of the tet(P) operon from Clostridium perfringens. J. Bacteriol. 183:7110-7119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Judd, P. K., R. B. Kumar, and A. Das. 2005. The type IV secretion apparatus protein VirB6 of Agrobacterium tumefaciens localizes to a cell pole. Mol. Microbiol. 55:115-124. [DOI] [PubMed] [Google Scholar]
- 26.Kaufmann, P., Y. Lehmann, and L. Meile. 1996. Conjugative transfer of Tn916 from Enterococcus faecalis and Escherichia coli into Clostridium perfringens. Syst. Appl. Microbiol. 19:35-39. [Google Scholar]
- 27.Kurenbach, B., C. Bohn, J. Prabhu, M. Abudukerim, U. Szewzyk, and E. Grohmann. 2003. Intergeneric transfer of the Enterococcus faecalis plasmid pIP501 to Escherichia coli and Streptomyces lividans and sequence analysis of its tra region. Plasmid 50:86-93. [DOI] [PubMed] [Google Scholar]
- 28.Li, K. B. 2003. ClustalW-MPI: ClustalW analysis using distributed and parallel computing. Bioinformatics 19:1585-1586. [DOI] [PubMed] [Google Scholar]
- 29.Lucet, I. S., F. E. Tynan, V. Adams, J. Rossjohn, D. Lyras, and J. I. Rood. 2005. Identification of the structural and functional domains of the large serine recombinase TnpX from Clostridium perfringens. J. Biol. Chem. 280:2503-2511. [DOI] [PubMed] [Google Scholar]
- 30.Lyras, D., V. Adams, I. Lucet, and J. I. Rood. 2004. The large resolvase TnpX is the only transposon-encoded protein required for transposition of the Tn4451/3 family of integrative mobilizable elements. Mol. Microbiol. 51:1787-1800. [DOI] [PubMed] [Google Scholar]
- 31.Lyras, D., and J. I. Rood. 1996. Genetic organization and distribution of tetracycline resistance determinants in Clostridium perfringens. Antimicrob. Agents Chemother. 40:2500-2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lyras, D., and J. I. Rood. 2000. Transposition of Tn4451 and Tn4453 involves a circular intermediate that forms a promoter for the large resolvase, TnpX. Mol. Microbiol. 38:588-601. [DOI] [PubMed] [Google Scholar]
- 33.Lyristis, M., A. E. Bryant, J. Sloan, M. M. Awad, I. T. Nisbet, D. L. Stevens, and J. I. Rood. 1994. Identification and molecular analysis of a locus that regulates extracellular toxin production in Clostridium perfringens. Mol. Microbiol. 12:761-777. [DOI] [PubMed] [Google Scholar]
- 34.Magot, M. 1984. Physical characterization of the Clostridium perfringens tetracycline-chloramphenicol resistance plasmid pIP401. Ann. Microbiol. (Paris) 135B:269-282. [DOI] [PubMed] [Google Scholar]
- 35.Miyamoto, K., G. Chakrabarti, Y. Morino, and B. A. McClane. 2002. Organization of the plasmid cpe locus in Clostridium perfringens type A isolates. Infect. Immun. 70:4261-4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miyamoto, K., D. J. Fisher, J. Li, S. Sayeed, S. Akimoto, and B. A. McClane. 2006. Complete sequencing and diversity analysis of the enterotoxin-encoding plasmids in Clostridium perfringens type A non-food-borne human gastrointestinal disease isolates. J. Bacteriol. 188:1585-1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morton, T. M., D. M. Eaton, J. L. Johnston, and G. L. Archer. 1993. DNA sequence and units of transcription of the conjugative transfer gene complex (trs) of Staphylococcus aureus plasmid pGO1. J. Bacteriol. 175:4436-4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakai, K., and P. Horton. 1999. PSORT: a program for detecting sorting signals in proteins and predicting their subcellular localization. Trends Biochem. Sci. 24:34-36. [DOI] [PubMed] [Google Scholar]
- 39.Patti, J. M., K. House-Pompeo, J. O. Boles, N. Garza, S. Gurusiddappa, and M. Hook. 1995. Critical residues in the ligand-binding site of the Staphylococcus aureus collagen-binding adhesin (MSCRAMM). J. Biol. Chem. 270:12005-12011. [DOI] [PubMed] [Google Scholar]
- 40.Patti, J. M., H. Jonsson, B. Guss, L. M. Switalski, K. Wiberg, M. Lindberg, and M. Hook. 1992. Molecular characterization and expression of a gene encoding a Staphylococcus aureus collagen adhesin. J. Biol. Chem. 267:4766-4772. [PubMed] [Google Scholar]
- 41.Paulsen, I. T., L. Banerjei, G. S. Myers, K. E. Nelson, R. Seshadri, T. D. Read, D. E. Fouts, J. A. Eisen, S. R. Gill, J. F. Heidelberg, H. Tettelin, R. J. Dodson, L. Umayam, L. Brinkac, M. Beanan, S. Daugherty, R. T. DeBoy, S. Durkin, J. Kolonay, R. Madupu, W. Nelson, J. Vamathevan, B. Tran, J. Upton, T. Hansen, J. Shetty, H. Khouri, T. Utterback, D. Radune, K. A. Ketchum, B. A. Dougherty, and C. M. Fraser. 2003. Role of mobile DNA in the evolution of vancomycin-resistant Enterococcus faecalis. Science 299:2071-2074. [DOI] [PubMed] [Google Scholar]
- 42.Petit, L., M. Gibert, and M. R. Popoff. 1999. Clostridium perfringens: toxinotype and genotype. Trends Microbiol. 7:104-110. [DOI] [PubMed] [Google Scholar]
- 43.Recchia, G. D., M. Aroyo, D. Wolf, G. Blakely, and D. J. Sherratt. 1999. FtsK-dependent and -independent pathways of Xer site-specific recombination. EMBO J. 18:5724-5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rhem, M. N., E. M. Lech, J. M. Patti, D. McDevitt, M. Hook, D. B. Jones, and K. R. Wilhelmus. 2000. The collagen-binding adhesin is a virulence factor in Staphylococcus aureus keratitis. Infect. Immun. 68:3776-3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rice, L. B. 1998. Tn916 family conjugative transposons and dissemination of antimicrobial resistance determinants. Antimicrob. Agents Chemother. 42:1871-1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roberts, A. P., P. A. Johanesen, D. Lyras, P. Mullany, and J. I. Rood. 2001. Comparison of Tn5397 from Clostridium difficile, Tn916 from Enterococcus faecalis and the CW459tet(M) element from Clostridium perfringens shows that they have similar conjugation regions but different insertion and excision modules. Microbiology 147:1243-1251. [DOI] [PubMed] [Google Scholar]
- 47.Rood, J. I. 1983. Transferable tetracycline resistance in Clostridium perfringens strains of porcine origin. Can. J. Microbiol. 29:1241-1246. [DOI] [PubMed] [Google Scholar]
- 48.Rood, J. I., E. A. Maher, E. B. Somers, E. Campos, and C. L. Duncan. 1978. Isolation and characterization of multiply antibiotic-resistant Clostridium perfringens strains from porcine feces. Antimicrob. Agents Chemother. 13:871-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rood, J. I., V. N. Scott, and C. L. Duncan. 1978. Identification of a transferable tetracycline resistance plasmid (pCW3) from Clostridium perfringens. Plasmid 1:563-570. [DOI] [PubMed] [Google Scholar]
- 50.Sambrook, J., E. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 51.Schwarz, F. V., V. Perreten, and M. Teuber. 2001. Sequence of the 50-kb conjugative multiresistance plasmid pRE25 from Enterococcus faecalis RE25. Plasmid 46:170-187. [DOI] [PubMed] [Google Scholar]
- 52.Scott, P. T., and J. I. Rood. 1989. Electroporation-mediated transformation of lysostaphin-treated Clostridium perfringens. Gene 82:327-333. [DOI] [PubMed] [Google Scholar]
- 53.Sharp, M. D., and K. Pogliano. 2002. Role of cell-specific SpoIIIE assembly in polarity of DNA transfer. Science 295:137-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sherratt, D. J., B. Soballe, F. X. Barre, S. Filipe, I. Lau, T. Massey, and J. Yates. 2004. Recombination and chromosome segregation. Philos. Trans. R. Soc. Lond. B 359:61-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shimizu, T., K. Ohtani, H. Hirakawa, K. Ohshima, A. Yamashita, T. Shiba, N. Ogasawara, M. Hattori, S. Kuhara, and H. Hayashi. 2002. Complete genome sequence of Clostridium perfringens, an anaerobic flesh-eater. Proc. Natl. Acad. Sci. USA 99:996-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sloan, J., L. M. McMurry, D. Lyras, S. B. Levy, and J. I. Rood. 1994. The Clostridium perfringens Tet P determinant comprises two overlapping genes: tetA(P), which mediates active tetracycline efflux, and tetB(P), which is related to the ribosomal protection family of tetracycline-resistance determinants. Mol. Microbiol. 11:403-415. [DOI] [PubMed] [Google Scholar]
- 57.Symersky, J., J. M. Patti, M. Carson, K. House-Pompeo, M. Teale, D. Moore, L. Jin, A. Schneider, L. J. DeLucas, M. Hook, and S. V. Narayana. 1997. Structure of the collagen-binding domain from a Staphylococcus aureus adhesin. Nat. Struct. Biol. 4:833-838. [DOI] [PubMed] [Google Scholar]