Abstract
The N-acylhomoserine lactone (AHL) signaling system comprises a producing system that includes acylhomoserine synthase (AhlI, a LuxI homolog) and a receptor, generally a LuxR homolog. AHL controls exoprotein production in Erwinia carotovora and consequently the virulence for plants. In previous studies we showed that ExpR, a LuxR homolog, is an AHL receptor and that it activates transcription of rsmA, the gene encoding an RNA binding protein which is a global negative regulator of exoproteins and secondary metabolites. An unusual finding was that the transcriptional activity of ExpR was neutralized by AHL. We subsequently determined that the genomes of most strains of E. carotovora subspecies tested possess two copies of the expR gene: expR1, which was previously studied, and expR2, which was the focus of this study. Comparative analysis of the two ExpR variants of E. carotovora subsp. carotovora showed that while both variants activated rsmA transcription, there were significant differences in the patterns of their AHL interactions, the rsmA sequences to which they bound, and their relative efficiencies of activation of rsmA transcription. An ExpR2− mutant produced high levels of exoproteins and reduced levels of RsmA in the absence of AHL. This contrasts with the almost complete inhibition of exoprotein production and the high levels of RsmA production in an AhlI− mutant that was ExpR1−. Our results suggest that ExpR2 activity is responsible for regulating exoprotein production primarily by modulating the levels of an RNA binding protein.
Erwinia carotovora subsp. carotovora strains produce an array of extracellular enzymes and proteins, including an assortment of pectinases that are primarily responsible for plant cell wall degradation (2, 9, 10, 36, 45). The ability of these bacteria to cause soft rot disease in many plants and plant products depends mostly on the quantity and quality of the exoenzymes produced. Indeed, recent studies have disclosed that exoenzyme production is elaborately regulated in these bacteria by various transcriptional factors, posttranscriptional regulators, plant signals, and quorum-sensing (QS) signals (N-acylhomoserine lactones [AHLs]) (1, 8, 11, 15, 17, 18, 19, 21, 28, 29, 33, 34, 38, 44). Two of these factors, regulatory components designated the RsmA-RsmB RNA pair (see below) and AHL, play a critical central role in exoenzyme production.
RsmA (regulation of secondary metabolites) has an RNA binding domain and acts as a posttranscriptional regulator. It promotes RNA decay and thus generally behaves like a negative regulator (8, 13). The nontranslatable regulatory RsmB RNA is a positive regulator of an array of genes for secondary metabolites and pathogenicity. It binds RsmA and neutralizes the negative regulatory effect of this RNA (28).
Since the seminal discovery of the role of N-acylhomoserine lactone in the exoenzymes and pathogenicity of E. carotovora by Jones et al. (21) and Pirhonen et al. (38), the QS systems of the soft rot bacteria have been extensively studied (see references 30, 37, 48, and 49 and references therein). It is now known that AHL is required for production not only of the exoenzymes and antibiotics but also of harpin, a protein required for elicitation of the hypersensitivity reaction in nonhost plants, as well as for the type III secretion machinery (14, 32). In E. carotovora subsp. carotovora, the AHL synthase gene, expI (ahlI), and an AHL receptor gene, expR1 (ahlR), overlap and are convergently transcribed (1, 39, 48). However, ExpR1 was found not to control ahlI expression or affect AHL production.
Early studies provided few clues regarding a connection between RsmA and AHL-mediated regulation of exoenzymes. For example, most RsmA− mutants were independent of AHL in that they produced high levels of exoproteins in the absence of AHL (8). In addition, later studies revealed that AHL-deficient mutants produced much higher levels of RsmA than their Ahl+ parents produced (6, 23), suggesting that (i) AHLs have a regulatory effect on rsmA expression and (ii) excess RsmA produced in the absence of AHL inhibits exoenzyme production by promoting decay of the cognate mRNA species. The connection between AHL and RsmA was subsequently established by the discovery that ExpR1 activates transcription of rsmA (7, 12). In contrast to the commonly held LuxR-LuxI paradigm, the ExpR1-RsmA system turned out to be different since ExpR1, and not the ExpR1-AHL complex, activates rsmA transcription. Thus, in the absence of AHL, rsmA expression is stimulated, whereas in the presence of AHL rsmA expression is suppressed.
Our previous data (6, 12) revealed that (i) a mutation in the expR1 gene in the AHL+ background of E. carotovora subsp. carotovora strain Ecc71 had little or no effect on the levels of exoenzymes and RsmA; (ii) the level of exoenzyme production in an ExpR1− AhlI− mutant was higher than the level in an ExpR1+ AhlI− strain but still lower than the level in Ecc71; and (iii) the levels of rsmA transcript and RsmA in an ExpR1− AhlI− mutant were higher than the levels in Ecc71 but lower than the levels in an ExpR1+ AhlI− mutant. These observations suggested that there is more than one functional expR gene in Ecc71. Support for this suggestion came from an analysis of the whole genome sequence of E. carotovora subsp. atroseptica strain SCRI1043 (3), which revealed two open reading frames (accession numbers YP_048234 and YP_049663) corresponding to AHL binding proteins (putative ExpR homologs), one located adjacent to the ExpI gene (AhlI; accession number YP_048233) and the other located quite a distance away from the ExpI gene.
In this study we found that (i) most E. carotovora strains tested have two expR homologs, designated expR1 and expR2; (ii) like ExpR1, ExpR2 binds rsmA DNA and activates its transcription; (iii) in addition to the expR box, ExpR2 requires 62 base sequences upstream of rsmA for efficient binding; (iv) both 3-oxo-C6-HL and 3-oxo-C8-HL prevent ExpR2-rsmA binding and ExpR2-mediated activation of rsmA transcription, whereas only 3-oxo-C6-HL prevents ExpR1-rsmA binding and ExpR1-mediated activation of rsmA transcription; (v) the DNA sequences upstream of rsmA genes which are required for ExpR2-rsmA binding are highly conserved in all E. carotovora strains; (vi) in Escherichia coli, expR2+ DNAs of all the E. carotovora strains tested activate the rsmA-lacZ fusions from different E. carotovora subspecies; and (vii) compared to ExpR1, ExpR2 is a more effective regulator of rsmA and exoprotein production.
While this paper was in preparation, Burr et al. (5) reported that a virR gene is one of the components of the E. carotovora QS system that controls virulence. Based on genetic homology, we concluded that virR is genetically identical or very similar to expR2 described here. Our findings with ExpR2 provide significant new information by explaining the mechanism by which VirR (ExpR2) might regulate the exoprotein production and virulence reported by Burr et al. (5).
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
The bacterial strains and plasmids used are described in Table 1. All wild-type E. carotovora strains were maintained on LB agar. The strains carrying antibiotic markers were maintained on LB agar containing appropriate antibiotics.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Reference or source |
|---|---|---|
| Erwinia carotovora subsp. atroseptica strains | ||
| Eca5 | Wild type | 50 |
| Eca12 | Wild type | 50 |
| Eca15 | Wild type | 50 |
| Eca20 | Wild type | 50 |
| Eca31 | Wild type | 50 |
| Erwinia carotovora subsp. betavasculorum Ecb168 | Wild type | J. E. Loper |
| Erwinia carotovora subsp. carotovora strains | ||
| Ecc71 | Wild type | 50 |
| AC5006 | Lac− derivative of Ecc71 | 35 |
| AC5091 | AhlI− derivative of AC5006 | 8 |
| AC5099 | AhlI− ExpR1− derivative of AC5006 | 12 |
| AC5115 | ExpR2− derivative of AC5006 | This study |
| AC5117 | AhlI− ExpR2− derivative of AC5006 | This study |
| AC5118 | AhlI− ExpR1− ExpR2− derivative of AC5006 | This study |
| Ecc193 | Wild type | 50 |
| AH2 | Wild type | A. K. Handa |
| SCC3193 | Wild type | 38 |
| SCRI193 | Wild type | 40 |
| EC153 | Wild type | 20 |
| Escherichia coli strains | ||
| DH5α | φ80lacZΔM15 Δ(lacZYA-argF)U169 hsdR17 recA1 endA1 thi-1 | Gibco BRL |
| MC4100 | araD139 Δ(lacIPOZYA)U169 recA1 thi-1 Strr | 26 |
| Plasmids | ||
| p34S-Gm | Gmr, source of Gmr cassette | 16 |
| pBluescript SK(+) | Apr | Stratagene |
| pCL1920 | Spr Smr | 25 |
| pLARF5 | Tcr | 22 |
| pMAL-c2g | Apr, protein expression vector | New England Biolabs |
| pMP220 | Tcr, promoter-probe vector | 43 |
| pRK415 | Tcr | 22 |
| pAKC781 | Apr, peh-1+ DNA in pBluescript SK(+) | 25 |
| pAKC783 | Apr, pel-1+ DNA in pBluescript SK(+) | 25 |
| pAKC882 | Apr, rsmA coding region in pT7-7 | 32 |
| pAKC936 | Spr, expR1+ in pCL1920 | 12 |
| pAKC938 | Spr, expR271+ in pCL1920 | This study |
| pAKC1034 | Apr, 200-bp celV fragment in pGEM-T Easy | 29 |
| pAKC1100 | Tcr, rsmA71-lacZ containing transcriptional start sites T1 and T2 in pMP220 | 6 |
| pAKC1102 | Tcr, rsmA71-lacZ containing transcriptional start site T1 in pMP220 | 7 |
| pAKC1103 | Tcr, rsmA71-lacZ containing transcriptional start site T2 in pMP220 | 7 |
| pAKC1106 | Tcr, rsmA153-lacZ, 0.4-kb rsmA153 upstream DNA in pMP220 | 7 |
| pAKC1223 | Tcr, expR2+ in pLARF5 from Ecc71 genomic library | This study |
| pAKC1224 | Apr, expR2 coding region in pMAL-c2g | This study |
| pAKC1225 | Apr, 2.0-kb BamHI-HindIII fragment containing expR2 from pAKC1223 cloned into pBluescript SK(+) | This study |
| pAKC1226 | Tcr, 2.0-kb BamHI-HindIII fragment containing expR2 from pAKC1223 cloned into pRK415 | This study |
| pAKC1227 | Tcr Gmr, expR2 derivative of pAKC1226 | This study |
| pAKC1228 | Spr, expR2153+ in pCL1920 | This study |
| pAKC1229 | Spr, expR2Eca15+ in pCL1920 | This study |
| pAKC1230 | Spr, expR2Eca20+ in pCL1920 | This study |
| pAKC1231 | Spr, expR2Ecb+ in pCL1920 | This study |
| pAKC1232 | Tcr, rsmAEca20-lacZ in pMP220 | This study |
| pAKC1233 | Tcr, rsmAEcb-lacZ in pMP220 | This study |
The compositions of LB and minimal salts media have been described previously (8, 35). When required, antibiotics were added at the following concentrations: ampicillin, 100 μg/ml; gentamicin, 10 μg/ml; kanamycin, 50 μg/ml; spectinomycin, 50 μg/ml; and tetracycline, 10 μg/ml. Media were solidified using 1.5% (wt/vol) agar. The 3-oxo-C6-HL and 3-oxo-C8-HL used in media were purified from cultural supernatants of Ecc71 and EC153 as previously described (7).
The composition of the medium used for agarose plate assays of enzymatic activities was described previously by Chatterjee et al. (8).
Extracellular enzyme assays.
The extracellular pectate lyase (Pel), polygalacturonase (Peh), protease (Prt), and cellulase (Cel) activities in the culture supernatants were determined by using previously described procedures (8).
Determination of nucleotide sequences of Ecc71 expR2 and rsmA upstream regions for several E. carotovora strains and sequence alignment.
Based on the information obtained from Southern blot analysis, a ca. 2.0-kb BamHI-HindIII fragment of pAKC1223 containing expR2 was subcloned into pBluescript SK(+) to obtain pAKC1225. The nucleotide sequence of expR2 was determined using pAKC1225. To amplify the upstream regions of rsmA genes of E. carotovora subsp. atroseptica strains Eca5, Eca12, Eca15, Eca20, and Eca31, E. carotovora subsp. betavasculorum strain Ecb168, and E. carotovora subsp. carotovora strains AH2, EC153, Ecc193, SCRI193, and SCC3193, primers 5′-GCTGGATCCGGCAAGCAGGATAGAA-3′ (corresponding to nucleotides −199 to −178 of transcriptional start site T2 of strain Ecc71 rsmA) and 5′-GCTGAATTCGGGTTTCGCCAACTCGACGAGT-3′ (corresponding to nucleotides 80 to 59 of transcriptional start site T2 of strain Ecc71 rsmA) were used for PCR. The PCR-amplified fragments were sequenced. Nucleotide sequencing was performed at the DNA Core Facility of University of Missouri-Columbia. The corresponding upstream DNA sequence of rsmA of E. carotovora subsp. atroseptica strain SCRI1043 was obtained from GenBank (accession number NC_004547). Sequence alignment was performed using ClustalW at www.expasy.ch, and default parameters were used. A domain search was performed using rpsblast at www.ncbi.nlm.nlh.gov/Structure/ccd/wrpsb.cgi.
DNA techniques.
Standard procedures were used for isolation of plasmids and chromosomal DNA, gel electrophoresis, and DNA ligation (41). Restriction and modification enzymes were obtained from Promega Biotec (Madison, WI). The Prime-a-Gene DNA labeling system (Promega Biotec) was used for labeling DNA probes. Southern blot analysis was carried out under high-stringency conditions, which included hybridization at 65°C in a solution containing 6× SSC, 5× Denhardt's solution, 0.5% (wt/vol) sodium dodecyl sulfate (SDS), and 100 μg/ml denatured salmon sperm DNA and washing at 65°C with 2× SSC for 30 min, with 1× SSC-0.1% (wt/vol) SDS for 30 min, and then with 0.1× SSC-0.1% (wt/vol) SDS for 30 min (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate).
Construction of ExpR2 mutants.
The ca. 2.0-kb BamHI-HindIII fragment containing expR2 from pAKC1223 was subcloned into pRK415 to obtain pAKC1226. To inactivate expR2, an internal ClaI fragment (positions 198 to 373, corresponding to the putative translational start site of expR2) was deleted, and a Gmr cassette obtained from p34S-Gm was inserted into a ClaI site to obtain pAKC1227. AC5115 (an AhlI+ ExpR1+ ExpR2− derivative of AC5006), AC5117 (an AhlI− ExpR1+ ExpR2− derivative of AC5006), and AC5118 (AhlI− ExpR1− ExpR2−) were constructed by marker exchange of AC5006, AC5091, and AC5099 with pAKC1227, respectively. The procedures used for marker exchange have been described previously (8). Inactivation of expR2 in mutants was confirmed by Northern blot analysis.
Construction of expR271+, expR2153+, expR2Eca15+, expR2Eca20+, and expR2Ecb+ plasmids, as well as rsmAEca20-lacZ and rsmAEcb-lacZ fusions.
DNA fragments containing expR2 of E. carotovora strains Ecc71, EC153, Eca15, Eca20, and Ecb168 were PCR amplified from chromosomal DNAs of these strains using primers 5′-TGAGGATCCTACCACACACCCTAAATTCAGTAG-3′ (corresponding to nucleotides −257 to −234 of the putative translational start site of strain Ecc71 expR2) and 5′-TGAAAGCTTTTATAAAGGCTCAGGCTTGATGAG-3′ (corresponding to nucleotides 749 to 726 of the putative translational start site of strain Ecc71 expR2). The amplified DNA fragments containing the expR2 genes from Ecc71, EC153, Eca15, Eca20, and Ecb168 were cloned into pCL1920 to obtain pAKC938, pAKC1228, pAKC1229, pAKC1230, and pAKC1231, respectively. To construct rsmAEca20-lacZ and rsmAEcb-lacZ fusions, PCR-amplified DNA fragments containing rsmA upstream DNAs of strains Eca20 and Ecb168 used for sequencing were cloned into pMP220 to obtain pAKC1232 and pAKC1233, respectively.
Northern blot and Western blot analyses.
Bacterial cultures were grown at 28°C in minimal salts medium supplemented with sucrose (0.5%, wt/vol) and other components, as indicated below. Cells were collected when the cultures reached a concentration of ca. 150 Klett units unless indicated otherwise. RNA isolation and Northern blot analysis were performed as described by Liu et al. (26). The probes used were the 183-bp NdeI-SalI fragment of rsmA from pAKC882, the 314-bp EcoRV-KpnI fragment of pel-1 from pAKC783, the 743-bp HindIII fragment of peh-1 from pAKC781, and the 200-bp EcoRI fragment of celV from pAKC1034. For Western blot analysis, bacterial cells were collected, suspended in 1× SDS-polyacrylamide gel electrophoresis sample buffer (41), and boiled. The protein concentrations were determined by using a CB-X protein assay kit (Geno Technology, Inc., St. Louis, MO) according to the manufacturer's specifications. Western blot analysis of the total bacterial protein was performed as described by Mukherjee et al. (32). Antisera raised against RsmA of Ecc71 (15) were used as probes.
Expression and purification of MBP-ExpR2 protein.
A DNA segment containing the coding region of expR2 was PCR amplified from pAKC1223 using primers 5′-TGAGGATCCATGTCTGTATTTTGCTCTGACAAT-3′ (corresponding to nucleotides 1 to 24 from the putative translational start site) and 5′-TGAAGCTTATAAAGGCTCAGGCTTGATGAG-3′ (corresponding to nucleotides 749 to 726 from the putative translational start site), digested with BamHI and HindIII, and cloned into the pMAL-c2g vector (New England Biolabs, Beverly, MA) to obtain pAKC1224.
E. coli strain DH5α carrying pAKC1224 was grown in LB medium supplemented with glucose (0.2%, wt/vol) and ampicillin at 37°C. Isopropyl-β-d-thiogalactopyranoside (IPTG) was added to a final concentration of 1 mM when the culture reached an A600 of 0.7. Bacterial cells were harvested 3 h after IPTG induction. A maltose binding protein (MBP)-ExpR2 fusion protein was purified by amylose resin (New England Biolabs, Beverly, MA) affinity chromatography, using the protocol provided by the company. The protein concentration was determined by using a CB-X protein assay kit (Geno Technology, Inc., St. Louis, MO). Crude extracts and purified MBP-ExpR2 were analyzed by SDS-polyacrylamide gel electrophoresis in a 10% (wt/vol) polyacrylamide gel.
Gel mobility shift assays.
A 62-mer DNA fragment containing the expR box and the second binding site sequence was PCR amplified using primers 5′-TGTAGATCTAACTAACAAGTAGTGACAAACCGG-3′ and 5′-GCTGAATTCCGTAAACTTAGACGATGGTAT-3′ (corresponding to nucleotides −91 to −30 from transcriptional start site T2). The DNA fragment was purified using the Wizard SV gel and PCR clean-up system (Promega Biotec, Madison, WI) and was end labeled with [α-32P]dATP and the Klenow fragment. A double-stranded DNA fragment containing the expR box was generated by annealing oligonucleotides (5′-ATGGTGTGGTTATACCATCGTCTA-3′ and 5′-TACCTAGACGATGGTATAACCACA-3′) and was end labeled with [α-32P]dATP and the Klenow fragment. Protein-DNA interaction assays were performed in 20 μl of binding buffer (50 mM Tris-HCl [pH 7.5], 10 mM MgCl2, 50 mM KCl, 1 mM dithiothreitol, 0.1 mM EDTA, 5% [wt/vol] glycerol) containing 1 μg of salmon sperm DNA, 2 μg of bovine serum albumin, and purified MBP-ExpR1 or MBP-ExpR2 protein with or without competitors or synthetic 3-oxo-C6-HL (purchased from Sigma, St. Louis, MO) or 3-oxo-C8-HL (kindly provided by Paul Williams, University of Nottingham, United Kingdom). The reaction mixtures were incubated at room temperature for 20 min and subjected to electrophoresis in 5% (wt/vol) polyacrylamide gels. The gels were dried and exposed to X-ray film.
DNase I protection analysis.
A 111-bp DNA probe was PCR amplified using 10 pmol of end-labeled primer 5′-GCTGAATTCCGTAAACTTAGACGATGGTAT-3′ (corresponding to nucleotides −30 to −50 from transcriptional start site T2 of rsmA) and 10 pmol of unlabeled primer 5′-TGTAGATCTCAGTTCTGTTGTTGTGATAAC-3′ (corresponding to nucleotides −140 to −119 from transcriptional start site T2). Labeling of the primer, the PCR, and the DNase I protection assays were carried out by using previously described procedures (27). The dried gel was developed using the FLA5000 phosphorimager system (Fuji Photo Film Co., Ltd., Kanagawa, Japan).
β-Galactosidase assays.
Bacterial constructs were grown at 28°C in LB medium supplemented with appropriate antibiotics and AHLs as described below. The β-galactosidase assays were performed as described by Miller (31).
The experiments were performed at least two or three times, and the results were reproducible.
RESULTS
E. carotovora subsp. carotovora strain Ecc71 has two copies of functional expR.
As described above, several lines of indirect evidence indicate that E. carotovora subspecies may possess more than one copy of a functional expR gene. To test this hypothesis, two primers, based on the nucleotide sequence of the expR homolog of E. carotovora subsp. atroseptica strain SCRI1043 (accession number YP_049663), were used for PCR with chromosomal DNAs of E. carotovora subsp. atroseptica strains Eca12 and Eca31, E. carotovora subsp. betavasculorum strain Ecb168, and E. carotovora subsp. carotovora strain Ecc71. A DNA fragment was amplified from Eca31 but not from any other strain. As expected, the nucleotide sequence of this fragment exhibited a high level of homology with the expR homolog of SCRI1043, the source of the PCR primers. This fragment and the expR1 DNA of E. carotovora subsp. carotovora strain Ecc71 (12) were used as probes to hybridize EcoRI-digested Ecc71 chromosomal DNA. Southern blot analysis results revealed a ca. 2.1-kb hybridization band with the probe amplified from Eca31 and a ca. 6.1-kb hybridization band with the Ecc71 expR1 probe. We concluded that (i) the 2.1-kb fragment contained a homolog of expR of SCRI1043 (designated expR2) and (ii) the expR genes in the 2.1-kb fragment and the 6.1-kb fragment were genetically distinct.
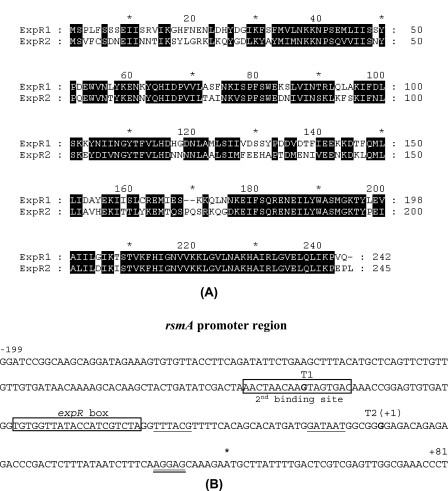
To confirm the presence of a functional expR homolog (expR2), we cloned the cognate DNA segment of Ecc71. To do this, the DNA fragment containing expR2, amplified by PCR from Eca31 chromosomal DNA, was used as the probe for colony hybridization of the Ecc71 genomic library. The expR2+ plasmid pAKC1223, obtained by colony hybridization, was digested with different restriction enzymes and subjected to Southern blot analysis. A ca. 2.0-kb BamHI-HindIII fragment containing expR2 was subcloned, and the nucleotide sequence of this fragment was determined. The nucleotide sequences of this Ecc71 expR2 fragment and the flanking DNA exhibited very high levels of homology with the sequences of E. carotovora subsp. atroseptica strain SCRI1043. Comparative analysis of ExpR2 with previously characterized ExpR1 (Fig. 1A) revealed that they have 62% identity and 81% similarity. Both ExpR variants contain autoinducer binding domains at the N terminus (residues 11 to 160) and helix-turn-helix (HTH) motifs at the C terminus (residues 175 to 231 for ExpR1 and residues 177 to 233 for ExpR2). The amino acid sequences of the HTH motifs of the two ExpR variants are very similar; 53 of 56 residues are identical or similar. By contrast, 104 of 150 residues of the autoinducer binding domains of these two proteins are identical or similar.
FIG. 1.
(A) Alignment of the deduced amino acid sequences of ExpR1 and ExpR2 of E. carotovora subsp. carotovora strain Ecc71. The numbers on the right indicate the positions of amino acid residues. (B) Nucleotide sequence of the upstream region of Ecc71 rsmA. Two transcriptional start sites are indicated by T1 and T2(+1), respectively. The expR box and the second binding site for ExpR2 are indicated. The putative −10 and −35 sequences of T2 are underlined, the Shine-Dalgarno sequence is double underlined, and the translational start site is indicated by an asterisk.
Effects of expR1+ and expR2+ DNAs on expression of rsmA71-lacZ fusions in E. coli.
To examine if ExpR2, like ExpR1, functions as a transcriptional activator in the absence of AHL, pCL1920 (cloning vector), pAKC936 (expR1+), and pAKC938 (expR2+) were transferred into E. coli strain MC4100 carrying a transcriptional rsmA71-lacZ fusion, pAKC1100. β-Galactosidase assay data (Table 2) revealed that both the expR1 and expR2 plasmids activated expression of the rsmA71-lacZ fusion and that expR2 was more effective than expR1. We reported previously that two transcription start sites, T1 and T2, occur upstream of rsmA and that ExpR1-activated rsmA transcription is initiated from the T2 start site (12) (Fig. 1B). To identify the start site for ExpR2-activated rsmA transcription, transcriptional rsmA71-lacZ fusions pAKC1102 (which contained only start site T1) and pAKC1103 (which contained only start site T2) were transferred into MC4100 carrying pCL1920, pAKC936, or pAKC938. β-Galactosidase assay results (Table 2) showed that both the expR1 and expR2 plasmids failed to stimulate the expression of lacZ in pAKC1102. By contrast, expression of rsmA71-lacZ in pAKC1103 was stimulated by both expR1+ and expR2+ DNAs. These data demonstrate that rsmA transcription activated by the two ExpR variants is initiated from the T2 site.
TABLE 2.
Expression of rsmA71-lacZ fusions in E. coli in the presence of expR1 or expR2
| Straina | Relevant characteristicsb | β-Galactosidase activity (Miller units)c |
|---|---|---|
| MC4100(pCL1920/pAKC1100) | Vector + rsmA71(T1T2)-lacZd | 658 ± 17 |
| MC4100(pAKC936/pAKC1100) | expR1 + rsmA71(T1T2)-lacZd | 1,896 ± 22 |
| MC4100(pAKC938/pAKC1100) | expR2 + rsmA71(T1T2)-lacZd | 3,119 ± 75 |
| MC4100(pCL1920/pAKC1102) | Vector + rsmA71(T1)-lacZe | 646 ± 28 |
| MC4100(pAKC936/pAKC1102) | expR1 + rsmA71(T1)-lacZe | 642 ± 20 |
| MC4100(pAKC938/pAKC1102) | expR2 + rsmA71(T1)-lacZe | 631 ± 39 |
| MC4100(pCL1920/pAKC1103) | Vector + rsmA71(T2)-lacZf | 373 ± 18 |
| MC4100(pAKC936/pAKC1103) | expR1 + rsmA71(T2)-lacZf | 1,307 ± 39 |
| MC4100(pAKC938/pAKC1103) | expR2 + rsmA71(T2)-lacZf | 2,550 ± 62 |
Bacteria were grown at 28°C in LB medium supplemented with spectinomycin and tetracycline to a concentration of ca. 250 Klett units, and whole cultures were used for the assays.
Relevant genes carried by the bacteria.
The values are means ± standard deviations for three repetitions.
The rsmA-lacZ fusion contained both of the transcriptional start sites (T1 and T2) (for the locations of T1 and T2, see Fig. 1B).
The rsmA-lacZ fusion contained transcriptional start site T1.
The rsmA-lacZ fusion contained transcriptional start site T2.
Overexpression and purification of MBP-ExpR2.
To overexpress and purify MBP-ExpR2 for DNase I protection and DNA binding studies, we amplified the coding region of expR2 by PCR and cloned it into the pMAL-c2g vector behind the ptac promoter to obtain pAKC1224. The apparent molecular mass (ca. 79 kDa) of the overexpressed protein band from E. coli DH5α carrying pAKC1224 after IPTG induction corresponded well to theoretical molecular mass of the polypeptide deduced from the expR2 sequence (28.36 kDa) plus the molecular mass of the MBP2-β-galactosidase α fragment made from the vector (50.84 kDa). MBP-ExpR2 was purified by amylose resin affinity chromatography for use in DNase I protection and DNA binding assays.
Identification of sequences upstream of rsmA to which ExpR2 binds.
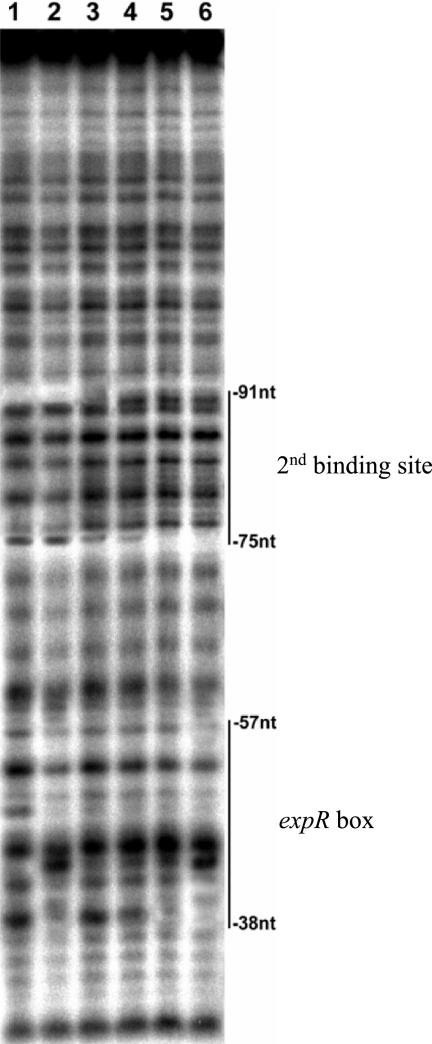
We previously identified a 20-mer expR box sequence and determined that ExpR1 binds to this 20-mer DNA (7, 12). A gel shift assay of 32P-labeled expR box DNA with ExpR2 (Fig. 2A) revealed that ExpR2 binds the expR box DNA, and the extent of the MBP-ExpR2-rsmA band shift was proportional to the concentration of MBP-ExpR2 added. However, the binding affinity of the expR box DNA with ExpR2 was very low compared to its binding affinity with ExpR1 (Fig. 2A). This observation raised the possibility that the ExpR2-rsmA binding site may be different from the binding site of ExpR1-rsmA. In order to identify the ExpR2-rsmA binding site, a DNase I protection assay was carried out with purified MBP-ExpR2. We detected clear protection regions located between nucleotides −57 and −38 (expR box) with both ExpR1 and ExpR2. In addition, we observed another protection region at nucleotides −91 to −75 with ExpR2 but not with ExpR1 (Fig. 3). These results strongly suggested that in addition to the expR box DNA, ExpR2 requires another upstream DNA region of rsmA to completely bind. As described below, these observations were confirmed by gel shift assays with various DNA fragments.
FIG. 2.
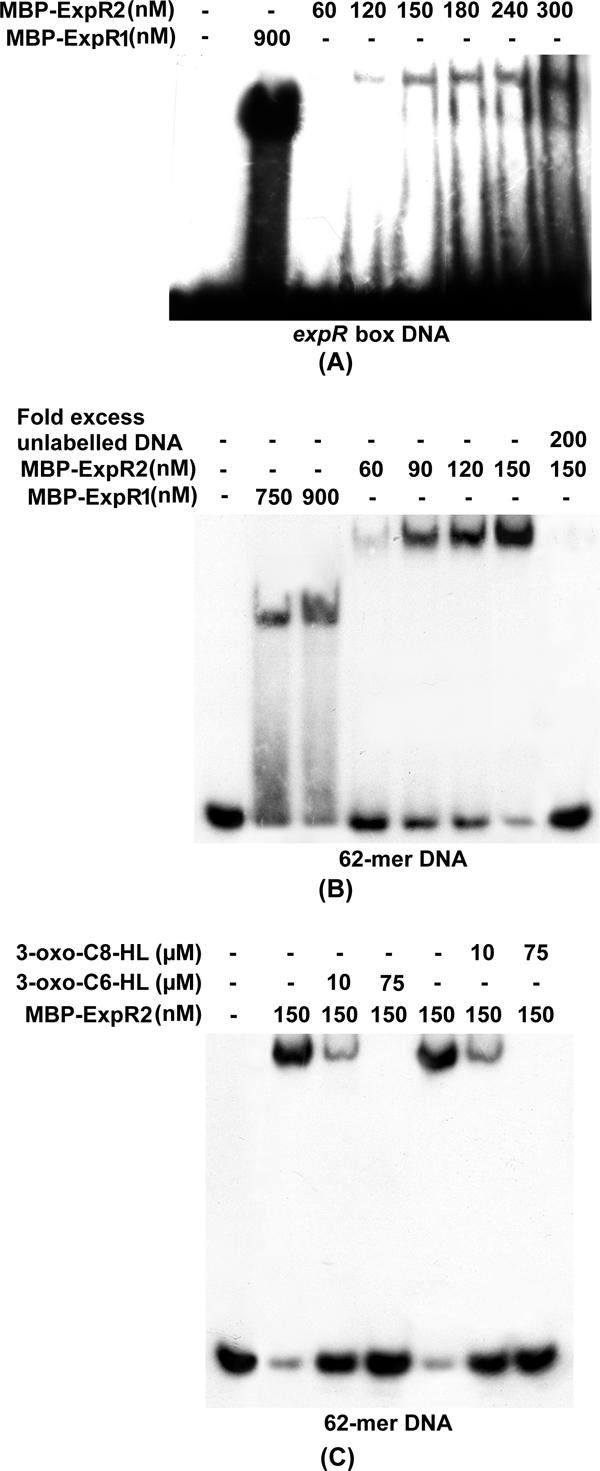
Interactions of purified MBP-ExpR1 and MBP-ExpR2 proteins with expR box DNA (A), an expR box with the second binding site (62-mer DNA fragment) (B), and a 62-mer DNA fragment (C) in the absence and presence of AHLs. DNA fragments were end labeled with [α-32P]dATP. Two nanograms of probe DNA was added to each reaction mixture. The amounts of proteins, unlabeled DNAs, 3-oxo-C6-HL, and 3-oxo-C8-HL used in each reaction are indicated above the gels.
FIG. 3.
Analysis of protection of the rsmA promoter DNA fragment from DNase I by MBP-ExpR1 and MBP-ExpR2. The lower strand of an rsmA upstream DNA fragment containing nucleotides −140 to −29 corresponding to transcriptional start site T2 was labeled with [γ-32P]ATP and used as a probe. The lines on the right indicate the nucleotide positions related to transcriptional start site T2 which are protected from DNase I digestion by MBP-ExpR1 or MBP-ExpR2. Lane 1, no protein added; lane 2, 1.8 μg of MBP-ExpR1; lanes 3 to 6, 0.3 μg, 0.4 μg, 0.8 μg, and 1.2 μg of MBP-ExpR2, respectively. nt, nucleotide.
A 62-bp DNA segment (nucleotides −91 to −30) containing both the protected regions and eight bases 3′ of the expR box (Fig. 1B) was PCR amplified and labeled with [α-32P]dATP for gel shift assays. The results (Fig. 2B) revealed that (i) both MBP-ExpR1 and MBP-ExpR2 bound to the 62-bp DNA segment; (ii) the shifted band with MBP-ExpR2 was bigger than the shifted band with MBP-ExpR1; (iii) the extent of the MBP-ExpR2-rsmA band shift was proportional to the concentration of protein added; and (iv) excess cold 62-bp DNA eliminated the shifted band, indicating that the binding was specific. By contrast, a DNA fragment containing only 10 bases of the expR box and the second protected region (nucleotides −91 to −45) did not bind MBP-ExpR2, indicating that the expR box DNA was required for MBP-ExpR2-rsmA binding. In addition, a DNA segment in which three bases were deleted from the 5′ end of the 62-bp DNA (i.e., nucleotides −88 to −30) poorly bound MBP-ExpR2 (data not shown), just like the 20-mer expR box DNA. Thus, both the protected regions, including the expR box, are essential for ExpR2 binding.
Effects of expR1 and expR2 on rsmA expression in E. carotovora subsp. carotovora.
To study the effects of expR1 and expR2 on rsmA expression in the absence of AHL, we constructed AhlI− ExpR1+ ExpR2− and AhlI− ExpR1− ExpR2− mutants of AC5006, a Lac− derivative of Ecc71. In addition, we used AhlI− ExpR1+ ExpR2+ and AhlI− ExpR1− ExpR2+ derivatives of AC5006 that were constructed previously (12). AC5006 and its mutants were grown in minimal salts medium containing sucrose for extraction of total RNA and protein. The Northern blot and Western blot analysis results (Fig. 4A) revealed that (i) the AhlI− ExpR1+ ExpR2+ strain overproduced rsmA RNA and RsmA (Fig. 4A, lane 2) compared to the parent, AC5006 (lane 1); (ii) the AhlI− ExpR1− ExpR2+ (lane 3) strain produced reduced levels of the rsmA transcript and RsmA the AhlI− ExpR1+ ExpR2+ strain (lane 2); and (iii) the levels of the rsmA transcript and RsmA protein produced by the AhlI− ExpR1+ ExpR2− and AhlI− ExpR1− ExpR2− strains (lanes 4 and 5) were similar to the levels produced by AC5006 (lane 1).
FIG. 4.
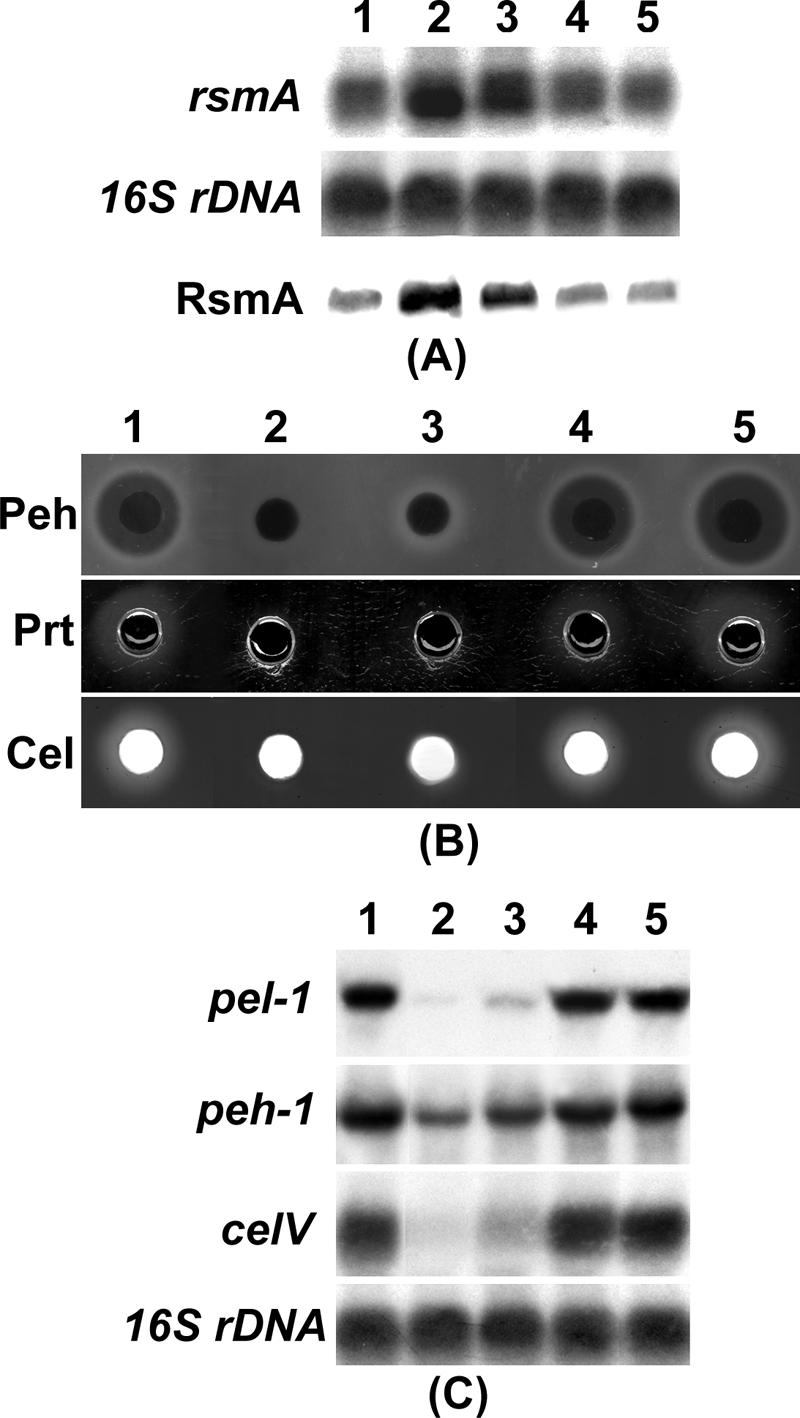
(A) Northern blot analysis of rsmA and Western blot analysis of RsmA. (B) Agarose plate assays for analysis of Peh, Prt, and Cel activities. (C) Northern blot analysis of exoenzyme genes. For the Northern blot analysis, each lane contained 10 μg of total RNA, and for the Western blot analysis each lane contained 10 μg of total protein. Equal loading of RNA was checked by hybridization of the blot with a probe corresponding to the 16S rRNA gene (rDNA). For exoenzyme assays, 50 μl of culture supernatant was loaded in each well. Enzymatic activities are indicated by halos around the wells on the assay plates. Column 1, AC5006 (AhlI+ ExpR1+ ExpR2+); column 2, AC5091 (AhlI− ExpR1+ ExpR2+); column 3, AC5099 (AhlI− ExpR1− ExpR2+); column 4, AC5117 (AhlI− ExpR1+ ExpR2−); column 5, AC5118 (AhlI− ExpR1− ExpR2−).
expR2 deficiency restores exoenzyme production in AhlI− mutant of Ecc71.
Since exoenzyme production was negatively controlled by RsmA and the levels of RsmA were reduced by inactivation of expR2 in the AhlI− mutant of Ecc71, we predicted that an expR2 deficiency would restore exoenzyme production in the AhlI− mutant. The samples from the cultures that were used for the rsmA transcript and RsmA protein assay (see above) were assayed for extracellular Pel (Table 3), Peh, Prt, and Cel production (Fig. 4B), as well as for transcripts of several exoenzyme genes (Fig. 4C). The results revealed that the AhlI− ExpR1+ ExpR2− strain (Fig. 4C, column 4) produced levels of exoenzymes and pel-1, peh-1,and celV transcripts very similar to the levels produced by the AhlI+ ExpR1+ ExpR2+ parent, AC5006 (column 1). By contrast, the levels produced by the AhlI− ExpR1− ExpR2+ strain (column 3) were only slightly higher than the levels produced by the AhlI− ExpR1+ ExpR2+ strain (column 2). These observations indicated that ExpR2, but not ExpR1, plays a major role in controlling exoenzyme production under the growth conditions used in these experiments. As expected, the levels of exoenzymes and the levels of the pel-1, peh-1, and celV transcripts in the AhlI− ExpR1− ExpR2− strain (column 5) were slightly higher than or similar to the levels in the parent strain.
TABLE 3.
Levels of pectate lyase activity in E. carotovora subsp. carotovora strainsa
| Strain | Relevant characteristics | Pel activity |
|---|---|---|
| AC5006 | AhlI+ ExpR1+ ExpR2+ | 0.44 ± 0.02 |
| AC5091 | AhlI− ExpR1+ ExpR2+ | 0.01 ± 0.01 |
| AC5099 | AhlI− ExpR1− ExpR2+ | 0.05 ± 0.01 |
| AC5117 | AhlI− ExpR1+ ExpR2− | 0.47 ± 0.03 |
| AC5118 | AhlI− ExpR1− ExpR2− | 0.56 ± 0.06 |
Bacterial cultures were grown at 28°C in minimal salts medium containing sucrose (0.5%, wt/vol) to a concentration of ca. 200 Klett units. Cultural supernatants were used for enzyme assays. Pel activities are expressed in A235/A600 per min. The values are means ± standard deviations for three repetitions.
Effects of 3-oxo-C6-HL and 3-oxo-C8-HL on expression of the rsmA-lacZ fusion in E. coli in the presence of expR1 and expR2.
The data presented above demonstrated that both expR1+ and expR2+ DNAs stimulate the expression of rsmA-lacZ in E. coli (i.e., in the absence of AHL). We found previously that the activation of transcription of rsmA by ExpR1 of Ecc71 is neutralized by 3-oxo-C6-HL but not by 3-oxo-C8-HL (7). Therefore, it was of interest to examine the effects of these two AHLs on the expression of the rsmA-lacZ fusion in the presence of expR2. The experiments were done with E. coli carrying the appropriate plasmids (see below). β-Galactosidase assay results (Table 4) revealed that (i) in the presence of 3-oxo-C6-HL, the expression of rsmA-lacZ in MC4100 carrying pAKC1100 (rsmA-lacZ fusion) with pAKC936 (expR1+) or pAKC938 (expR2+) was reduced; and (ii) in the presence of 3-oxo-C8-HL, the expression of rsmA-lacZ driven by ExpR2 was reduced, whereas the expression of rsmA-lacZ with ExpR1 remained the same as the expression in the absence of AHL. These results demonstrated that in E. coli both ExpR1 and ExpR2 stimulated rsmA-lacZ expression in the absence of AHL and that the effects of ExpR2 were neutralized by 3-oxo-C6-HL or 3-oxo-C8-HL, whereas the effects of ExpR1 were neutralized only by 3-oxo-C6-HL.
TABLE 4.
Effects of expR1 or expR2 plasmids on expression of the rsmA71-lacZ fusion in E. coli in the presence of 3-oxo-C6-HL or 3-oxo-C8-HL
| Straina | Relevant characteristicsb | AHL | β-Galactosidase activity (Miller units)c |
|---|---|---|---|
| MC4100(pCL1920/pAKC1100) | Vector + rsmA71-lacZ | 715 ± 23 | |
| MC4100(pCL1920/pAKC1100) | Vector + rsmA71-lacZ | 3-oxo-C6-HL | 727 ± 15 |
| MC4100(pCL1920/pAKC1100) | Vector + rsmA71-lacZ | 3-oxo-C8-HL | 740 ± 19 |
| MC4100(pAKC936/pAKC1100) | expR1 + rsmA71-lacZ | 2,033 ± 32 | |
| MC4100(pAKC936/pAKC1100) | expR1 + rsmA71-lacZ | 3-oxo-C6-HL | 732 ± 21 |
| MC4100(pAKC936/pAKC1100) | expR1 + rsmA71-lacZ | 3-oxo-C8-HL | 2,014 ± 25 |
| MC4100(pAKC938/pAKC1100) | expR2 + rsmA71-lacZ | 3,045 ± 43 | |
| MC4100(pAKC938/pAKC1100) | expR2 + rsmA71-lacZ | 3-oxo-C6-HL | 1,201 ± 30 |
| MC4100(pAKC938/pAKC1100) | expR2 + rsmA71-lacZ | 3-oxo-C8-HL | 1,122 ± 22 |
Bacterial cultures were grown at 28°C in LB medium supplemented with spectinomycin and tetracycline to a concentration of ca. 100 Klett units and then were divided and placed into three flasks. 3-oxo-C6-HL (final concentration, 50 μM) was added to one flask, 3-oxo-C8-HL (final concentration, 50 μM) was added to one flask, and water was added to the third flask as a control. After 3 h of incubation at 28°C, the cultures were assayed.
Relevant characteristics of the genes carried by bacteria.
The values are means ± standard deviations for three repetitions.
Effects of 3-oxo-C6-HL and 3-oxo-C8-HL on MBP-ExpR2-rsmA binding.
To examine the effects of 3-oxo-C6-HL and 3-oxo-C8-HL on MBP-ExpR2-rsmA binding, synthetic 3-oxo-C6-HL or 3-oxo-C8-HL was added to MBP-ExpR2-rsmA binding mixtures and incubated. Gel shift assay results (Fig. 2C) revealed that both 3-oxo-C6-HL and 3-oxo-C8-HL partially prevented MBP-ExpR2-rsmA binding at a lower concentration (10 μM) and eliminated binding at a higher concentration (75 μM). This contrasts with our previous observation that 3-oxo-C6-HL, but not 3-oxo-C8-HL, prevents MBP-ExpR1-rsmA binding (7).
Effects of 3-oxo-C6-HL and 3-oxo-C8-HL on rsmA expression and exoenzyme production in an AhlI− ExpR1− ExpR2+ derivative of Ecc71.
To examine the effects of 3-oxo-C6-HL or 3-oxo-C8-HL on expression of rsmA and production of Pel in the presence of only ExpR2, we used an AhlI− ExpR1− ExpR2+ derivative of Ecc71. Bacteria were grown in minimal salts medium containing sucrose without AHL and with 3-oxo-C6-HL or 3-oxo-C8-HL. Figure 5 shows that the presence of 3-oxo-C6-HL or 3-oxo-C8-HL in the medium reduced the levels of the rsmA transcript and RsmA and consequently enhanced Pel production in this mutant.
FIG. 5.
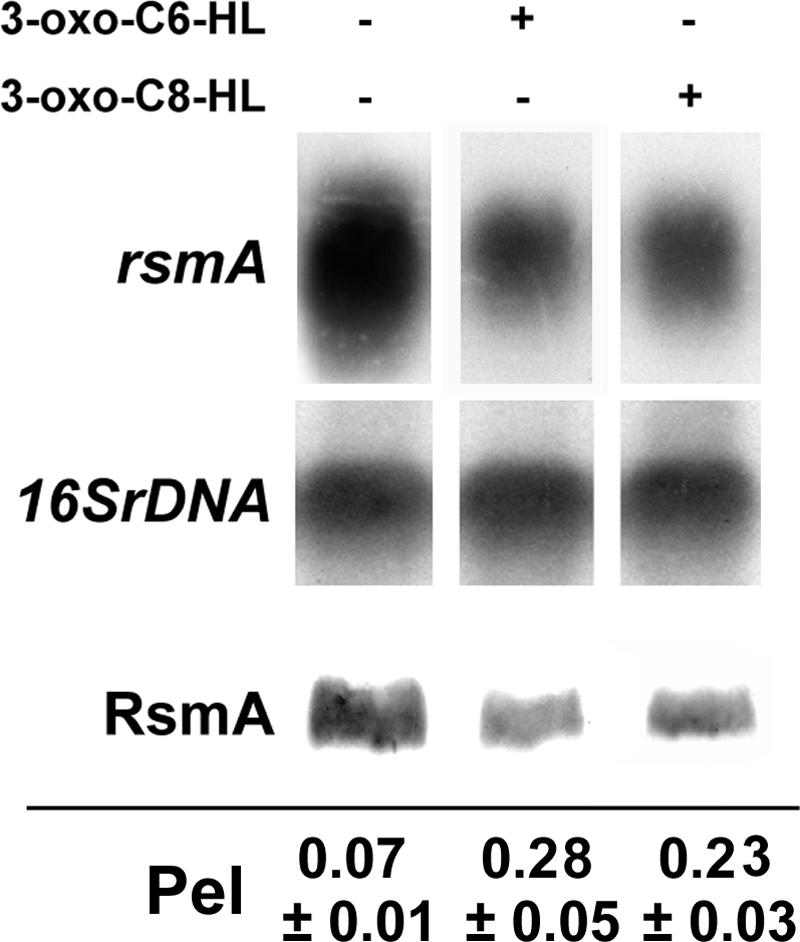
Northern blot analysis of rsmA and Western blot analysis of RsmA. Cultures of AC5099 (AhlI− ExpR1− ExpR2+) were started at a concentration of ca. 25 Klett units in 3 ml of minimal salts medium containing sucrose (0.5%, wt/vol) with or without AHLs and were grown at 28°C for 5 h. and then total RNAs or proteins were extracted. Culture supernatants were used for Pel assays. AHLs were added at a final concentration of 50 μM. For the Northern blot analysis, each lane contained 10 μg of total RNA, and for the Western blot analysis each lane contained 10 μg of total protein. Pel activities were expressed in A235/A600 per 30 min. 16SrDNA, 16S rRNA gene.
Occurrence of expR1 and expR2 in E. carotovora subspecies.
To examine if expR1 and expR2 are present in other strains of E. carotovora subspecies, EcoRI-digested chromosomal DNAs of E. carotovora subsp. atroseptica strains Eca5, Eca12, Eca15, Eca20, and Eca31, E. carotovora subsp. betavasculorum strain Ecb168, and E. carotovora subsp. carotovora strains Ecc71, Ecc193, AH2, EC153, SCRI193, and SCC3193 were subjected to Southern blot analysis under high-stringency conditions. Since there are two classes of expR1 (7), we used probes representative of each class, expR1 DNA from Ecc71 for Ecc71, SCRI193, AH2, Eca5, and Ecb168 and expR1 DNA from EC153 for EC153, Ecc193, SCC3193, Eca15, Eca12, Eca20, and Eca31. The expR2 DNA from Ecc71 was used as another probe. The results revealed that expR1 was present in all strains tested and that expR2 was present in all strains except E. carotovora subsp. carotovora strain SCRI193. Since the sizes of the hybridization bands of expR1 and expR2 for each strain are different, we considered cross hybridization with the probes highly unlikely.
Occurrence of ExpR2 binding sites upstream of rsmA genes of E. carotovora subspecies.
Sequence alignment of the rsmA upstream regions containing both the binding sites for ExpR2 revealed that the expR box and the second binding site are highly conserved in 13 E. carotovora strains tested. The 5′ end of the expR box in all E. carotovora subsp. atroseptica and E. carotovora subsp. betavasculorum strains and in one E. carotovora subsp. carotovora strain, EC153, differs by one base from the 5′ end in the other E. carotovora subsp. carotovora strains. By contrast, the 16-mer second binding site sequences are identical in all strains tested. The expR box and the second binding site are separated by 13 bases in E. carotovora subsp. atroseptica strains, whereas 17 bases separate the two binding sites in E. carotovora subsp. betavasculorum and E. carotovora subsp. carotovora strains.
expR2+ DNAs of E. carotovora subsp. atroseptica, E. carotovora subsp. betavasculorum, and E. carotovora subsp. carotovora strains activate rsmA-lacZ fusions in E. coli.
E. coli strain MC4100 variants carrying a cloning vector or a plasmid containing expR2+ DNA of E. carotovora subsp. atroseptica strain Eca15 or Eca20, E. carotovora subsp. betavasculorum strain Ecb168, or E. carotovora subsp. carotovora strain Ecc71 or EC153 and rsmA71-lacZ, rsmA153-lacZ, rsmAEca20-lacZ, and rsmAEcb-lacZ transcriptional fusions were grown, and their β-galactosidase activities were determined. The results (Table 5) revealed that expR2+ DNAs from the E. carotovora strains activated expression of the rsmA71-lacZ, rsmA153-lacZ, rsmAEca20-lacZ, and rsmAEcb-lacZ fusions.
TABLE 5.
Effects of expR2 genes of several E. carotovora strains on expression of rsmA71-lacZ, rsmA153-lacZ, rsmAEca20-lacZ, and rsmAEcb-lacZ fusions in E. coli
| Straina | Relevant characteristicsb | β-Galactosidase activity (Miller units)c |
|---|---|---|
| MC4100(pCL1920/pAKC1100) | Vector + rsmA71-lacZ | 557 ± 18 |
| MC4100(pAKC938/pAKC1100) | expR271 + rsmA71-lacZ | 3,394 ± 27 |
| MC4100(pAKC1228/pAKC1100) | expR2153 + rsmA71-lacZ | 2,942 ± 22 |
| MC4100(pAKC1229/pAKC1100) | expR2Eca15 + rsmA71-lacZ | 3,388 ± 26 |
| MC4100(pAKC1230/pAKC1100) | expR2Eca20 + rsmA71-lacZ | 3,279 ± 27 |
| MC4100(pAKC1231/pAKC1100) | expR2Ecb + rsmA71-lacZ | 3,092 ± 42 |
| MC4100(pCL1920/pAKC1106) | Vector + rsmA153-lacZ | 773 ± 29 |
| MC4100(pAKC938/pAKC1106) | expR271 + rsmA153-lacZ | 3,476 ± 91 |
| MC4100(pAKC1228/pAKC1106) | expR2153 + rsmA153-lacZ | 3,408 ± 42 |
| MC4100(pAKC1229/pAKC1106) | expR2Eca15 + rsmA153-lacZ | 3,810 ± 18 |
| MC4100(pAKC1230/pAKC1106) | expR2Eca20 + rsmA153-lacZ | 3,760 ± 208 |
| MC4100(pAKC1231/pAKC1106) | expR2Ecb + rsmA153-lacZ | 3,346 ± 111 |
| MC4100(pCL1920/pAKC1232) | Vector + rsmAEca20-lacZ | 498 ± 4 |
| MC4100(pAKC938/pAKC1232) | expR271 + rsmAEca20-lacZ | 2,954 ± 128 |
| MC4100(pAKC1228/pAKC1232) | expR2153 + rsmAEca20-lacZ | 2,957 ± 118 |
| MC4100(pAKC1229/pAKC1232) | expR2Eca15 + rsmAEca20-lacZ | 3,051 ± 58 |
| MC4100(pAKC1230/pAKC1232) | expR2Eca20 + rsmAEca20-lacZ | 3,231 ± 103 |
| MC4100(pAKC1231/pAKC1232) | expR2Ecb + rsmAEca20-lacZ | 2,758 ± 48 |
| MC4100(pCL1920/pAKC1233) | Vector + rsmAEcb-lacZ | 576 ± 29 |
| MC4100(pAKC938/pAKC1233) | expR271 + rsmAEcb-lacZ | 3,526 ± 248 |
| MC4100(pAKC1228/pAKC1233) | expR2153 + rsmAEcb-lacZ | 3,171 ± 39 |
| MC4100(pAKC1229/pAKC1233) | expR2Eca15 + rsmAEcb-lacZ | 3,780 ± 94 |
| MC4100(pAKC1230/pAKC1233) | expR2Eca20 + rsmAEcb-lacZ | 3,467 ± 50 |
| MC4100(pAKC1231/pAKC1233) | expR2Ecb + rsmAEcb-lacZ | 3,284 ± 33 |
Bacteria were grown at 28°C in LB medium supplemented with spectinomycin and tetracycline to a concentration of ca. 250 Klett units, and whole cultures were used for the assays.
Relevant genes carried by bacteria.
The values are means ± standard deviations for three repetitions.
DISCUSSION
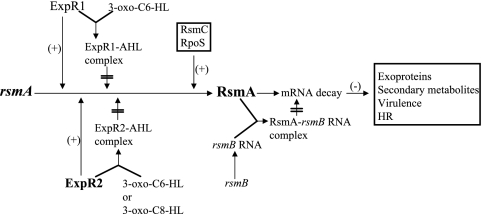
The quorum-sensing system of E. carotovora is unusual in that in the absence of AHL, the AHL receptor, ExpR1, activates transcription of rsmA, the gene encoding a global regulatory RNA binding protein. In the presence of AHL, ExpR1 is converted to an ExpR1-AHL complex which does not have DNA binding ability and, consequently, an activator function. Thus, AHL controls exoprotein production by modulating the level of RsmA (7, 12). This model predicts that an ExpR1− mutant does not require AHL for exoprotein production. However, an AhlI− ExpR1− mutant still responded to AHL (Fig. 5). In this study we demonstrated that there is redundancy in ExpR function due to the presence of duplicate copies of the expR gene, which explains the basis for our previous observation. Our findings establish that most strains of E. carotovora subspecies possess another functional expR homolog, expR2, which is genetically distinct from expR1. Recently, Burr et al. (5) reported that VirR, a LuxR homolog, negatively regulates the production of exoenzymes and virulence in the absence of AHL in E. carotovora subsp. atroseptica strain SCRI1043. Sequence analysis revealed that expR2 of E. carotovora subsp. carotovora strain Ecc71 exhibits a high level of homology (93% identity) with virR of SCRI1043. Burr et al. (5) demonstrated that a virR mutation restored the exoenzyme and virulence in an AhlI− mutant of SCRI1043; thus, these phenotypes closely resembled those resulting from inactivation of expR2 in Ecc71. Based on genetic and functional homologies, we propose that VirR of SCRI1043 is same as ExpR2 of Ecc71 and most likely other strains of E. carotovora subspecies.
ExpR2 activates transcription of rsmA in the absence of AHL. This was established by the elevated levels of expression of transcriptional rsmA-lacZ fusions in E. coli in the presence expR2+ DNAs from Ecc71, EC153, Eca15, Eca20, and Ecb168, as well as by DNA binding studies. In addition, studies with AhlI− ExpR1− ExpR2+ and AhlI− ExpR1+ ExpR2− mutants of Ecc71 also confirmed that both ExpR1 and ExpR2 activate rsmA transcription (Fig. 4A). However, ExpR1 and ExpR2 differ in their relative efficiencies of activation. The β-galactosidase level (Table 2) in E. coli MC4100 carrying the rsmA71-lacZ fusion and expR2+ DNA is higher (ca. 1.7-fold) than the level in MC4100 carrying the rsmA71-lacZ fusion and expR1+ DNA. Moreover, the levels of rsmA transcripts and RsmA are higher in the AhlI− ExpR1− ExpR2+ mutant (Fig. 4A, lane 3) than in the AhlI− ExpR1+ ExpR2− strain (Fig. 4A, lane 4).
Gel mobility shift data demonstrated that both ExpR1 and ExpR2 bind rsmA. However, the DNA binding properties of these two ExpR variants are different. MBP-ExpR2 binds poorly to the 20-mer expR box upstream of rsmA, which is sufficient for MBP-ExpR1-rsmA binding. Thus, the expR box DNA is not enough for MBP-ExpR2-rsmA binding. Several lines of evidence showed that a 16-mer DNA (second binding site) is required for the MBP-ExpR2-rsmA interaction in addition to the expR box DNA. Sequence analysis revealed that the two ExpR variants both contain HTH motifs for DNA binding at the C termini of the proteins and differ at 3 of 56 amino acid residues (Leu196Pro, Gly203Asp, and Thr206Ile). The high level of homology of the HTH motifs of ExpR1 and ExpR2 probably explains the utilization of an overlapping binding sequence (i.e., the expR box). The three-residue difference may be responsible for the requirement for the second binding site of ExpR2.
It is now well established that the interaction of AHL with the LuxR family of proteins modifies the DNA binding properties of these proteins (46). The results of our gel mobility shift assays clearly show that MBP-ExpR2-rsmA binding is prevented by 3-oxo-C6-HL or 3-oxo-C8-HL. Moreover, both AHL analogs markedly reduced the expression of the rsmA71-lacZ fusion in the presence of expR2+ DNA in E. coli MC4100. We attributed this reduction to the formation of an ExpR2-AHL complex with concomitant loss of rsmA binding ability. There is precedence for this behavior of AHL receptors. For example, in Serratia sp. strain ATCC 39006, N-butanoyl-l-homoserine lactone neutralized SmaR (a LuxR homolog)-mediated repression of the carA-H operon by inhibiting the binding ability of SmaR (42). von Bodman et al. (47) reported that an interaction of 3-oxo-C6-HL with a LuxR homolog, EsaR in Pantoea stewartii subsp. stewartii, induced structural changes that may have inhibited its DNA binding ability. The fact that 3-oxo-C6-HL prevented EsaR-lux box binding was indicated by in vivo β-galactosidase activation and repression assays performed with an artificial 35LB10-lacZ promoter fusion containing lux box sequences.
Our previous data (7, 12) established that 3-oxo-C6-HL, but not 3-oxo-C8-HL, prevented ExpR1-rsmA binding in Ecc71. In this study we found that both 3-oxo-C6-HL and 3-oxo-C8-HL prevent MBP-ExpR2-rsmA binding and neutralize the activator function of ExpR2. Examination of the sequences of ExpR1 and ExpR2 revealed significant differences (46 of 150 residues) between the autoinducer binding domains of the two ExpR variants located in the N-terminal regions. Thus, there is a strong possibility these differences could account for significant differences in the binding affinities of the ExpR variants for AHL analogs.
An AhlI+ ExpR1+ ExpR2− mutant (AC5115) produced levels of RsmA and exoenzymes similar to the levels produced by the wild-type parent. Previous studies demonstrated that mutation of ExpR1 in an AhlI+ background had no effect on the levels of RsmA and exoenzymes (1, 12). We attribute the lack of effects of ExpR1 or ExpR2 in the AhlI+ strain to neutralization of ExpR variants by activities of AHL analogs. Similar results have been reported by Burr et al. (5) since a mutation in virR in the AhlI+ background had no effect on exoenzyme production and virulence.
Our observations establish that ExpR2 is the major regulator of exoproteins and that ExpR1 plays an ancillary role under the experimental conditions which we used. In an AhlI− background, mutation of ExpR1 (i.e., AhlI− ExpR1− ExpR2+) reduced the levels of the rsmA transcript and RsmA and partially restored the exoenzymes and the transcript levels of pel-1, peh-1, and celV (Fig. 4 and Table 3) (12). Introduction of an ExpR2 mutation in an AhlI− derivative of Ecc71 resulted in dramatically reduced levels of the rsmA transcript and RsmA and increased levels of exoenzymes and transcripts of pel-1, peh-1, and celV. Moreover, inactivation of both ExpR1 and ExpR2 had no effect on the levels of the rsmA transcript, RsmA, and exoenzymes in addition to the effects observed in the Ahl− ExpR1+ ExpR2− strain.
Southern blot analysis under high-stringency conditions demonstrated that expR1 and expR2 were present in all strains of E. carotovora subsp. atroseptica, E. carotovora subsp. betavasculorum, and E. carotovora subsp. carotovora tested except E. carotovora subsp. carotovora strain SCRI193. Signals were not detected for strain SCRI193 with the expR2 probe of Ecc71. Burr et al. (5) reported that virR (=expR2) hybridization bands were observed under low-stringency conditions for several E. carotovora subsp. atroseptica, E. carotovora subsp. betavasculorum, and E. carotovora subsp. carotovora strains, including SCRI193. The absence of a signal with SCRI193 chromosomal DNA in our assay could have been due to a low level of homology between expR2 of Ecc71 and the corresponding gene in SCRI193. We note that expR1 has been identified previously (1, 7) in some of the strains used by Burr et al. After taking into consideration all of these observations, we concluded that the QS system of E. carotovora subspecies consists of ExpR1, ExpR2, and AhlI, the AHL synthase. As shown in Fig. 6, free ExpR1 and ExpR2 stimulate the expression of rsmA, which in turn controls an array of genes involved in production of exoproteins and secondary metabolites, as well as in virulence and elicitation of the hypersensitivity reaction. The ExpR1-AHL and ExpR2-AHL complexes do not have activator functions. Thus, ExpR1 and ExpR2 activate rsmA expression in the absence of AHL, and AHLs modulate this activation. The expression of rsmA is also regulated by RsmC (15) and RpoS (34). Regulatory rsmB RNA binds RsmA and neutralizes its negative regulatory effects (28). This QS signaling system in E. carotovora is novel since two or more homologs of autoinducer receptors that act on the same target are rather uncommon. Recently, Lee et al. (24) showed that in Pseudomonas aeruginosa, in addition to lasRI and rhlRI (luxRI homologs), there is another lasR and rhlR homolog, qscR, which has no corresponding lasI or rhlI homolog. QscR is capable of interacting with multiple AHL species. However, QscR does not bind the same target genes as LasR. In the presence of cognate AHLs, QscR binds the promoters of PA1897 and PA5351 and activates the expression of PA1897. We also found two autoinducer binding luxR homologs in strains of Pseudomonas syringae pathovars, including P. syringae pv. tomato strain DC3000. One of these homologs (psyR, PSPTO3863) is located adjacent to ahlI, and the other (PSPTO4539) is located away from the ahlI (4). It would be very interesting to identify the target(s) of the autoinducer binding proteins and to elucidate their roles in host-pathogen interactions.
FIG. 6.
Model showing the regulatory factors known to control rsmA expression in E. carotovora. ExpR1, ExpR2, the ExpR1-AHL complex, and the ExpR2-AHL complex modulate the levels of RsmA, an RNA binding protein that controls the expression of an array of genes involved in the production of exoproteins and secondary metabolites, as well as pathogenicity and the hypersensitivity reaction (HR). RsmC and RpoS positively regulate the expression of rsmA (15, 35). rsmB RNA, a regulatory RNA, binds to RsmA and neutralizes its effects (28).
β-Galactosidase assay data (Table 5) revealed that expR2+ DNAs cloned from E. carotovora subsp. atroseptica strains Eca15 and Eca20, E. carotovora subsp. betavasculorum strain Ecb168, and E. carotovora subsp. carotovora strains Ecc71 and EC153 activate the expression of rsmA-lacZ fusions containing promoter regions of rsmA genes from these three E. carotovora subspecies. Moreover, sequence analysis of rsmA upstream regions of 13 E. carotovora subsp. atroseptica, E. carotovora subsp. betavasculorum, and E. carotovora subsp. carotovora strains revealed the presence of highly conserved binding site sequences for ExpR1 and ExpR2. Our observations allow three general conclusions: (i) most if not all soft rot E. carotovora subspecies possess two AHL receptors; (ii) both receptors activate rsmA transcription in the absence of AHL; and (iii) the degree of specificity in an AHL-receptor interaction could vary depending on the ExpR species. The significance of the occurrence of two receptors, both of which apparently act on the same target (i.e., rsmA), is not clear. Clarification of this situation would require careful analyses of the regulation of the receptors, the spectrum of AHL analogs produced under different physiological conditions, the specificity of AHL-receptor interactions, and the cooperative interactions between various transcriptional regulators of rsmA.
Acknowledgments
This work was supported by National Science Foundation grant MCB-9728505 and by the Food for the 21st Century program of the University of Missouri.
We thank J. E. Schoelz for reviewing the manuscript, P. Williams for providing 3-oxo-C8-HL, J. E. Loper for providing strain Ecb168, T. Palva for providing strain SCC3193, and G. Salmond for providing strain SCRI193.
REFERENCES
- 1.Andersson, R. A., A. R. B. Eriksson, R. Heikinheimo, A. Mäe, M. Pirhonen, V. Kõiv, H. Hyytiäinen, A. Tuikkala, and E. T. Palva. 2000. Quorum sensing in the plant pathogen Erwinia carotovora subsp. carotovora: the role of expR (Ecc). Mol. Plant-Microbe Interact. 13:384-393. [DOI] [PubMed] [Google Scholar]
- 2.Barras, F., F. Van Gijsegem, and A. K. Chatterjee. 1994. Extracellular enzymes and pathogenesis of soft-rot Erwinia. Annu. Rev. Phytopathol. 32:201-234. [Google Scholar]
- 3.Bell, K. S., M. Sebaihia, L. Pritchard, M. T. Holden, L. J. Hyman, M. C. Holeva, N. R. Thomson, S. D. Bentley, L. J. Churcher, K. Mungall, R. Atkin, N. Bason, K. Brooks, T. Chillingworth, K. Clark, J. Doggett, A. Fraser, Z. Hance, H. Hauser, K. Jagels, S. Moule, H. Norbertczak, D. Ormond, C. Price, M. A. Quail, M. Sanders, D. Walker, S. Whitehead, G. P. C. Salmond, P. R. Birch, J. Parkhill, and I. K. Toth. 2004. Genome sequence of the enterobacterial phytopathogen Erwinia carotovora subsp. atroseptica and characterization of virulence factors. Proc. Natl. Acad. Sci. USA 101:11105-11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buell, C. R., V. Joardar, M. Lindeberg, J. Selengut, I. T. Paulsen, M. L. Gwinn, R. J. Dodson, R. T. Deboy, A. S. Durkin, J. F. Kolonay, R. Madupu, S. Daugherty, L. Brinkac, M. J. Beanan, D. H. Haft, W. C. Nelson, T. Davidsen, N. Zafar, L. Zhou, J. Liu, Q. Yuan, H. Khouri, N. Fedorova, B. Tran, D. Russell, K. Berry, T. Utterback, S. E. Van Aken, T. V. Feldblyum, M. D'Ascenzo, W. L. Deng, A. R. Ramos, J. R. Alfano, S. Cartinhour, A. K. Chatterjee, T. P. Delaney, S. G. Lazarowitz, G. B. Martin, D. J. Schneider, X. Tang, C. L. Bender, O. White, C. M. Fraser, and A. Collmer. 2003. The complete genome sequence of the Arabidopsis and tomato pathogen Pseudomonas syringae pv. tomato DC3000. Proc. Natl. Acad. Sci. USA 100:10181-10186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burr, T., A. M. L. Barnard, M. J. Corbett, C. L. Pemberton, N. J. L. Simpson, and G. P. C. Salmond. 2006. Identification of the central quorum sensing regulator of virulence in the enteric phytopathogen, Erwinia carotovora: the virR repressor. Mol. Microbiol. 59:113-125. [DOI] [PubMed] [Google Scholar]
- 6.Chatterjee, A., Y. Cui, and A. K. Chatterjee. 2002. RsmA and the quorum-sensing signal, N-[3-oxohexanoyl]-l-homoserine lactone, control the levels of rsmB RNA in Erwinia carotovora subsp. carotovora by affecting its stability. J. Bacteriol. 184:4089-4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chatterjee, A., Y. Cui, H. Hasegawa, N. Leigh, V. Dixit, and A. K. Chatterjee. 2005. Comparative analysis of two classes of quorum-sensing signaling systems that control production of extracellular proteins and secondary metabolites in Erwinia carotovora subspecies. J. Bacteriol. 187:8026-8038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chatterjee, A., Y. Cui, Y. Liu, C. K. Dumenyo, and A. K. Chatterjee. 1995. Inactivation of rsmA leads to overproduction of extracellular pectinases, cellulases, and proteases in Erwinia carotovora subsp. carotovora in the absence of the starvation/cell density sensing signal, N-(3-oxohexanoyl)-l-homoserine lactone. Appl. Environ. Microbiol. 61:1959-1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chatterjee, A. K., C. K. Dumenyo, Y. Liu, and A. Chatterjee. 2000. Erwinia: genetics of pathogenicity factors, p. 236-260. In J. Lederberg (ed.), Encyclopedia of microbiology, 2nd ed., vol. 2. Academic Press, New York, N.Y. [Google Scholar]
- 10.Collmer, A., and N. T. Keen. 1986. The role of pectic enzymes in plant pathogenesis. Annu. Rev. Phytopathol. 24:383-409. [Google Scholar]
- 11.Cui, Y., A. Chatterjee, and A. K. Chatterjee. 2001. Effects of the two-component system comprising GacA and GacS of Erwinia carotovora subsp. carotovora on the production of global regulatory rsmB RNA, extracellular enzymes, and harpinEcc. Mol. Plant-Microbe Interact. 14:516-526. [DOI] [PubMed] [Google Scholar]
- 12.Cui, Y., A. Chatterjee, H. Hasegawa, V. Dixit, N. Leigh, and A. K. Chatterjee. 2005. ExpR, a LuxR homolog of Erwinia carotovora subsp. carotovora, activates transcription of rsmA, which specifies a global regulatory RNA-binding protein. J. Bacteriol. 187:4792-4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cui, Y., A. Chatterjee, Y. Liu, C. K. Dumenyo, and A. K. Chatterjee. 1995. Identification of a global repressor gene, rsmA, of Erwinia carotovora subsp. carotovora that controls extracellular enzymes, N-(3-oxohexanoyl)-l-homoserine lactone, and pathogenicity in soft-rotting Erwinia spp. J. Bacteriol. 177:5108-5115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cui, Y., L. Madi, A. Mukherjee, C. K. Dumenyo, and A. K. Chatterjee. 1996. The RsmA− mutants of Erwinia carotovora subsp. carotovora strain Ecc71 overexpress hrpNEcc and elicit a hypersensitive reaction-like response in tobacco leaves. Mol. Plant-Microbe Interact. 9:565-573. [DOI] [PubMed] [Google Scholar]
- 15.Cui, Y., A. Mukherjee, C. K. Dumenyo, Y. Liu, and A. K. Chatterjee. 1999. rsmC of the soft-rotting bacterium Erwinia carotovora subsp. carotovora negatively controls extracellular enzyme and harpinEcc production and virulence by modulating the levels of regulatory RNA (rsmB) and RNA binding protein (RsmA). J. Bacteriol. 181:6042-6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dennis, J. J., and G. J. Zylstra. 1998. Plasposons: modular self-cloning minitransposon derivatives for rapid genetic analysis of gram-negative bacterial genomes. Appl. Environ. Microbiol. 64:2710-2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eriksson, A. R., R. A. Andersson, M. Pirhonen, and E. T. Palva. 1998. Two-component regulators involved in the global control of virulence in Erwinia carotovora subsp. carotovora. Mol. Plant-Microbe Interact. 11:743-752. [DOI] [PubMed] [Google Scholar]
- 18.Frederick, R. D., J. Chiu, J. L. Bennetzen, and A. K. Handa. 1997. Identification of a pathogenicity locus, rpfA, in Erwinia carotovora subsp. carotovora that encodes a two-component sensor-regulator protein. Mol. Plant-Microbe Interact. 10:407-415. [DOI] [PubMed] [Google Scholar]
- 19.Harris, S. J., Y. L. Shih, S. D. Bentley, and G. P. C. Salmond. 1998. The hexA gene of Erwinia carotovora encodes a LysR homologue and regulates motility and the expression of multiple virulence determinants. Mol. Microbiol. 28:705-717. [DOI] [PubMed] [Google Scholar]
- 20.Hasegawa, H., A. Chatterjee, Y. Cui, and A. K. Chatterjee. 2005. Elevated temperature enhances virulence of Erwinia carotovora subsp. carotovora strain EC153 to plants and stimulates production of the quorum sensing signal, N-acyl homoserine lactone, and extracellular proteins. Appl. Environ. Microbiol. 71:4655-4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones, S., B. Yu, N. J. Bainton, M. Birdsall, B. W. Bycroft, S. R. Chhabra, A. J. R. Cox, P. Golby, P. J. Reeves, S. Stephens, M. K. Winson, G. P. C. Salmond, G. S. A. B. Stewart, and P. Williams. 1993. The lux autoinducer regulates the production of exoenzyme virulence determinants in Erwinia carotovora and Pseudomonas aeruginosa. EMBO J. 12:2477-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keen, N. T., S. Tamaki, D. Kobayashi, and D. Trollinger. 1988. Improved broad-host-range plasmids for DNA cloning in Gram-negative bacteria. Gene 70:191-197. [DOI] [PubMed] [Google Scholar]
- 23.Kõiv, V., and A. Mäe. 2001. Quorum sensing controls the synthesis of virulence factors by modulating rsmA gene expression in Erwinia carotovora subsp. carotovora. Mol. Genet. Genomics 265:287-292. [DOI] [PubMed] [Google Scholar]
- 24.Lee, J.-H., Y. Lequette, and E. P. Greenberg. 2006. Activity of purified QscR, a Pseudomonas aeruginosa orphan quorum-sensing transcription factor. Mol. Microbiol. 59:602-609. [DOI] [PubMed] [Google Scholar]
- 25.Lerner, C. G., and M. Inouye. 1990. Low copy number plasmids for regulated low-level expression of cloned genes in Escherichia coli with blue/white insert screening capability. Nucleic Acids Res. 18:4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu, Y., A. Chatterjee, and A. K. Chatterjee. 1994. Nucleotide sequence and expression of a novel pectate lyase gene (pel-3) and a closely linked endopolygalacturonase gene (peh-1) of Erwinia carotovora subsp. carotovora 71. Appl. Environ. Microbiol. 60:2545-2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu, Y., Y. Cui, A. Mukherjee, and A. K. Chatterjee. 1997. Activation of the Erwinia carotovora subsp. carotovora pectin lyase structural gene pnlA: a role for rdgB. Microbiolgy 143:705-712. [DOI] [PubMed] [Google Scholar]
- 28.Liu, Y., Y. Cui, A. Mukherjee, and A. K. Chatterjee. 1998. Characterization of a novel RNA regulator of Erwinia carotovora ssp. carotovora that controls production of extracellular enzymes and secondary metabolites. Mol. Microbiol. 29:219-234. [DOI] [PubMed] [Google Scholar]
- 29.Liu, Y., G.-Q. Jiang, Y. Cui, A. Mukherjee, W.-L. Ma, and A. K. Chatterjee. 1999. kdgREcc negatively regulates genes for pectinases, cellulase, protease, harpinEcc, and a global RNA regulator in Erwinia carotovora subsp. carotovora. J. Bacteriol. 181:2411-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loh, J., E. A. Pierson, L. S. Pierson III, G. Stacey, and A. K. Chatterjee. 2002. Quorum sensing in plant associated bacteria. Curr. Opin. Plant Biol. 5:285-290. [DOI] [PubMed] [Google Scholar]
- 31.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 32.Mukherjee, A., Y. Cui, Y. Liu, and A. K. Chatterjee. 1997. Molecular characterization and expression of the Erwinia carotovora hrpNEcc gene, which encodes an elicitor of the hypersensitive reaction. Mol. Plant-Microbe Interact. 10:462-471. [DOI] [PubMed] [Google Scholar]
- 33.Mukherjee, A., Y. Cui, W. Ma, Y. Liu, and A. K. Chatterjee. 2000. hexA of Erwinia carotovora ssp. carotovora strain Ecc71 negatively regulates production of RpoS and rsmB RNA, a global regulator of extracellular proteins, plant virulence and the quorum-sensing signal, N-(3-oxohexanoyl)-l-homoserine lactone. Environ. Microbiol. 2:203-215. [DOI] [PubMed] [Google Scholar]
- 34.Mukherjee, A., Y. Cui, W.-L. Ma, Y. Liu, A. Ishihama, A. Eisenstark, and A. K. Chatterjee. 1998. RpoS (sigma-S) controls expression of rsmA, a global regulator of secondary metabolites, harpin, and extracellular proteins in Erwinia carotovora. J. Bacteriol. 180:3629-3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murata, H., J. L. McEvoy, A. Chatterjee, A. Collmer, and A. K. Chatterjee. 1991. Molecular cloning of an aepA gene that activates production of extracellular pectolytic, cellulolytic, and proteolytic enzymes in Erwinia carotovora subsp. carotovora. Mol. Plant-Microbe Interact. 4:239-246. [Google Scholar]
- 36.Pérombelon, M. C. M. 2002. Potato diseases caused by soft-rot erwinias: an overview of pathogenesis. Plant Pathol. 51:1-12. [Google Scholar]
- 37.Pierson, L. S., III, D. W. Wood, and S. B. von Bodman. 1999. Quorum sensing in plant-associated bacteria, p. 101-116. In G. M. Dunney and S. C. Winans (ed.), Cell-cell signaling. American Society for Microbiology Press, Washington, D.C.
- 38.Pirhonen, M., D. Flego, R. Heikinheimo, and E. T. Palva. 1993. A small diffusible signal molecule is responsible for the global control of virulence and exoenzyme production in the plant pathogen Erwinia carotovora. EMBO J. 12:2467-2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salmond, G. P. C., B. W. Bycroft, G. S. A. B. Stewart, and P. Williams. 1995. The bacterial ‘enigma’: cracking the code of cell-cell communication. Mol. Microbiol. 16:615-624. [DOI] [PubMed] [Google Scholar]
- 40.Salmond, G. P. C., J. C. D. Hinton, D. R. Gill, and M. C. M. Perombelon. 1986. Transposon mutagenesis of Erwinia using phage λ vectors. Mol. Gen. Genet. 203:524-528. [Google Scholar]
- 41.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 42.Slater, H., M. Crow, L. Everson, and G. P. C. Salmond. 2003. Phosphate availability regulates biosynthesis of two antibiotics, prodigiosin and carbapenem, in Serratia via both quorum-sensing-dependent and -independent pathways. Mol. Microbiol. 47:303-320. [DOI] [PubMed] [Google Scholar]
- 43.Spaink, H. P., R. J. H. Okker, C. A. Wijffelman, E. Pees, and B. J. J. Lugtenberg. 1987. Promoters in the nodulation region of the Rhizobium leguminosarum Sym plasmid pRL1JI. Plant Mol. Biol. 9:27-39. [DOI] [PubMed] [Google Scholar]
- 44.Thomson, N. R., A. Cox, B. W. Bycroft, G. S. Stewart, P. Williams, and G. P. C. Salmond. 1997. The rap and hor proteins of Erwinia, Serratia and Yersinia: a novel subgroup in a growing superfamily of proteins regulating diverse physiological processes in bacterial pathogens. Mol. Microbiol. 26:531-544. [DOI] [PubMed] [Google Scholar]
- 45.Thomson, N. R., J. D. Thomas, and G. P. C. Salmond. 1999. Virulence determinants in the bacterial phytopathogen Erwinia. Methods Microbiol. 29:347-426. [Google Scholar]
- 46.Urbanowski, M. L., C. P. Lostroh, and E. P. Greenberg. 2004. Reversible acyl-homoserine lactone binding to purified Vibrio fischeri LuxR protein. J. Bacteriol. 186:631-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.von Bodman, S. B., J. K. Ball, M. A. Faini, C. M. Herrera, T. D. Minogue, M. L. Urbanowski, and A. M. Stevens. 2003. The quorum sensing negative regulators EsaR and ExpR(Ecc), homologues within the LuxR family, retain the ability to function as activators of transcription. J. Bacteriol. 185:7001-7007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.von Bodman, S. B., W. D. Bauer, and D. L. Coplin. 2003. Quorum sensing in plant-pathogenic bacteria. Annu. Rev. Phytopathol. 41:455-482. [DOI] [PubMed] [Google Scholar]
- 49.Whitehead, N. A., J. T. Byers, P. Commander, M. J. Corbett, S. J. Coulthurst, L. Everson, A. K. P. Harris, C. L. Pemberton, N. J. L. Simpson, H. Slater, D. S. Smith, M. Welch, N. Williamson, and G. P. C. Salmond. 2002. The regulation of virulence in phytopathogenic Erwinia species: quorum sensing, antibiotics and ecological considerations. Antonie Leeuwenhoek Int. J. Gen. Mol. Microbiol. 81:223-231. [DOI] [PubMed] [Google Scholar]
- 50.Zink, R. T., R. J. Kemble, and A. K. Chatterjee. 1984. Transposon Tn5 mutagenesis in Erwinia carotovora subsp. carotovora and Erwinia carotovora subsp. atroseptica. J. Bacteriol. 157:809-814. [DOI] [PMC free article] [PubMed] [Google Scholar]