Abstract
Op18/stathmin (Op18) is a phosphorylation-regulated microtubule destabilizer that is frequently overexpressed in tumors. The importance of Op18 in malignancy was recently suggested by identification of a somatic Q18→E mutation of Op18 in an adenocarcinoma. We addressed the functional consequences of aberrant Op18 expression in leukemias by analyzing the cell cycle of K562 cells either depleted of Op18 by expression of interfering hairpin RNA or induced to express wild-type or Q18E substituted Op18. We show here that although Op18 depletion increases microtubule density during interphase, the density of mitotic spindles is essentially unaltered and cells divide normally. This is consistent with phosphorylation-inactivation of Op18 during mitosis. Overexpression of wild-type Op18 results in aneugenic activities, manifest as aberrant mitosis, polyploidization, and chromosome loss. One particularly significant finding was that the aneugenic activity of Op18 was dramatically increased by the Q18→E mutation. The hyperactivity of mutant Op18 is apparent in its unphosphorylated state, and this mutation also suppresses phosphorylation-inactivation of the microtubule-destabilizing activity of Op18 without any apparent effect on its phosphorylation status. Thus, although Op18 is dispensable for mitosis, the hyperactive Q18→E mutant, or overexpressed wild-type Op18, exerts aneugenic effects that are likely to contribute to chromosomal instability in tumors.
INTRODUCTION
Cell division involves segregation of duplicated chromosomes by the bipolar spindle, which is made up of heterodimer polymers of α/β tubulin termed microtubules (MTs; reviewed in Desai and Mitchison, 1997). Spindle MTs are attached to sister chromatids by kinetochores, which are protein complexes located at the centromeres (reviewed in Biggins and Walczak, 2003). The mitotic checkpoint is activated by kinetochores that lack attached MTs, which delays the progression of mitosis until a fully functional mitotic spindle is formed. Defects in the mitotic checkpoint result in chromosomal instability and are manifested as aneuploidy (Cahill et al., 1998). Most malignant tumors show various degrees of chromosomal instability, which implies a state of balance between the negative effects on cell viability and the selective advantages that may result from changes in ploidy (reviewed in Michor et al., 2005). Instability can be caused by many different types of defects that promote spindle aberrations, such as impairment of the mitotic checkpoint response, aberrant number of spindle poles, spindle attachment defects, and defects in chromosome cohesion/disjunction (Cahill et al., 1998; Fodde et al., 2001; Jallepalli et al., 2001; Kaplan et al., 2001; Rajagopalan et al., 2004; Wang et al., 2004). In principle, any type of mutation or aberrantly expressed protein that reduces the fidelity of spindle assembly will enhance chromosomal instability and can thus be considered to be aneugenic since such defects contribute to aneuploidy.
Op18/stathmin (Op18) was initially studied either because of its complex pattern of phosphorylation in response to diverse signals or its elevated expression in a variety of human malignancies (reviewed in Lawler, 1998). Op18 was subsequently identified as an MT-destabilizing protein that promotes transitions from growing to shrinking MTs, a phenomenon that is termed catastrophe promotion (Belmont and Mitchison, 1996). Op18 binds free tubulin heterodimers and thereby also has the potential to destabilize MTs by forming a ternary tubulin-sequestering complex (Jourdain et al., 1997). This complex consists of two tubulin heterodimers arranged head-to-tail (Steinmetz et al., 2000), with each of the two tandem helical repeats of Op18 binding along one heterodimer (Gigant et al., 2000). Analysis of tubulin assembly in vitro has indicated that the N-terminal nonhelical region of Op18 is essential for catastrophe promotion, whereas the tandem helical repeats are required for tubulin-sequestering activity (Howell et al., 1999b).
Human Op18 is phosphorylated at four Ser residues (Ser-16, -25, -38, and -63) by both cell cycle–regulating and signal-transducing kinase systems, which causes various degrees of inactivation (reviewed in Cassimeris, 2002). Although Op18 is phosphorylated at multiple sites during mitosis, Op18 exists predominantly in its unphosphorylated active form during interphase (Brattsand et al., 1994; Larsson et al., 1997). Interference with the function of Op18 in newt lung cells was found to result in a reduction in catastrophe frequency and an associated 2.5-fold increase in interphase MT polymers (Howell et al., 1999a). Consistent with a role for Op18 as a major regulator of the interphase MT system, it has been shown that signal-transducing kinases can inactivate Op18 in intact cells by phosphorylation and thereby increase MT polymer content (Melander Gradin et al., 1997; Gradin et al., 1998). Thus, Op18 functions as a regulator of the interphase MT system that is suppressed by phosphorylation signals.
Analysis of Op18 phospho-isomers in metaphase-blocked human cell lines has shown complete phosphorylation of the cyclin-dependent kinase sites Ser-25 and -38 and high-stoichiometry phosphorylation at the remaining two sites, Ser-16 and -63 (Larsson et al., 1995). This implies that the activity of Op18 is inactivated by multisite phosphorylation during spindle assembly (Marklund et al., 1996; Larsson et al., 1997). This inactivation is essential for spindle assembly, as shown by the mitotic phenotype of kinase target site-deficient mutants of Op18 (Marklund et al., 1994, 1996). Op18 is not, however, phosphorylated to high stoichiometry in mitotic Xenopus egg extracts and, based on experiments using this model system, it has been proposed that local phosphorylation-inactivation of Op18 occurs in the vicinity of condensed chromosomes (Andersen et al., 1997; Tournebize et al., 1997). Moreover, although Op18 mouse knockouts are viable (Schubart et al., 1996), it has been reported that depletion of Op18 in human K562 leukemia cells using antisense RNA inhibits cell growth by causing accumulation of cells with G2/M-content DNA (Luo et al., 1994; Marklund et al., 1994). Thus, the potential role of Op18 during cell division is still poorly understood, and the extent to which conflicting results are due to species differences is still unclear.
The significance of the frequently observed up-regulated expression of the wild-type Op18 gene in tumors is elusive. However, the identification of a somatic Q18→E missense mutation of Op18 in a human esophageal adenocarcinoma (Misek et al., 2002) may have provided us with vital clues. In that study, it was shown that expression of Op18-Q18E in NIH-3T3 cells causes accumulation of cells with G2/M DNA content and an increase in the basal frequency of focus formation in soft agar. In addition, NIH-3T3 cells expressing Op18-Q18E could also form tumors in immunodeficient mice. Although intriguing, these observations did not reveal any clearcut functional difference between the wild-type and mutant Op18 protein. Here, we have addressed the significance of Op18 expression in leukemias by 1) studying the cell cycle phenotype of Op18-depleted cells, and 2) comparing MT-destabilizing activities of wild-type Op18 and the Q18E mutant protein during interphase and mitosis. We found that Op18 is not important for the growth or viability of leukemia cells. However, when strongly overexpressed, Op18 generates spindle defects, polyploidization, and generation of micronuclei, and these aneugenic effects are dramatically increased by the Q18→E mutation. It seems likely that this property of overexpressed or mutated Op18 contributes to tumor progression by exacerbating chromosomal instability.
MATERIALS AND METHODS
DNA Constructs, Transfection, and Cell Culture
The pMEP4 shuttle vector directing inducible expression of Op18 has been described (Marklund et al., 1994). To distinguish ectopic Op18 from the endogenous gene product, an 8-amino acid (aa) Flag epitope tag was introduced at the C-terminus (Marklund et al., 1994). Q18E-substituted Op18 derivatives were generated by changing the Gln codon CAG to the Glu codon GAG by PCR. The coding sequence of the PCR-generated fragment was confirmed by nucleotide sequence analysis. Protein kinase target site-deficient C-terminally truncated Op18(1-99)-tetraA, which contains Ala substitutions at all four Ser phosphorylation sites, and pseudophosphorylated Op18-TetraE, which contains negatively charged Glu residues at all four Ser phosphorylation sites, has been described previously (Holmfeldt et al., 2001). Op18 proteins were expressed in Escherichia coli strain BL21 (DE3) using pET-3d (Novagen, Madison, WI) with a six-residue His-tag at the N-terminus. The recombinant protein was purified using Ni-coupled HiTrap-chelating HP columns (Amersham Pharmacia Biotech, Piscataway, NJ) and eluted with a linear gradient of imidazole as recommended by the manufacturer.
Replicating EBV-based shuttle vectors for constitutive expression of short hairpin RNA (shRNA) have been described previously (Holmfeldt et al., 2004). The targeting sequence of Op18 (accession no.: NM-005563), which was selected according to the criteria detailed in (Ui-Tei et al., 2004), was as follows: (shRNA-Op18-443): CGT TTG CGA GAG AAG GAT A.
A BLAST search of the NCBI database was used to ensure that the sequence chosen was unique and therefore that targeting of the cognate mRNA was specific.
Transfection using pMEP4 and shRNA derivatives and subsequent selection of hygromycin-resistant cell lines were performed as described (Holmfeldt et al., 2004). Conditional expression was induced from the hMTIIa promoter, which can be suppressed by cultivation in a specifically formulated medium and then induced with 0.5 μM Cd2+ (Marklund et al., 1994).
Analysis of Op18 Phospho-isomers, Quantification of Ectopic Op18, Quantification of MT Polymers, Immunoblot, and Immunofluorescence
To analyze Flag-tagged Op18 phospho-isomers, we used a native PAGE system that separates Op18 according to the charge differences introduced by each of the four phosphorylation events identified (Marklund et al., 1993). Phospho-isomers of Op18 were revealed by immunoblotting using the M2 Flag epitope tag–specific antibody (Sigma, St. Louis, MO). Expression levels of Op18 were determined by immunoblotting or flow cytometry using affinity- purified rabbit antibodies raised against an internal peptide sequence of Op18, corresponding to residues 46–58 (Holmfeldt et al., 2003). Analysis of cellular MT content by flow cytometry (>90% of all cells were included in the acquisition gate, and >150,000 cells were collected) was performed using an FACS Calibur instrument (Becton Dickinson, Lincoln Park, NJ) with modifications allowing determination of MT content at distinct phases of the cell cycle, as detailed in Holmfeldt et al. (2003). For characterization of spindles, cells were permeabilized with saponin (0.2%) in MT-stabilizing buffer and subsequently fixed in 4% paraformaldehyde/0.5% glutaraldehyde, followed by quenching with NaBH4 (Holmfeldt et al., 2001). Epifluorescence images were acquired on an Olympus CellR imaging station (Olympus-Biosystems, Melville, NY) equipped with an inverted microscope (IX81; Olympus), a 100×, 1.4 NA Planapochromat objective, and a cooled CCD camera (Orca ER; Hamamatsu Photonics, Bridgewater, NJ).
Determination of Tubulin Heterodimer-binding Affinity by Plasmon Resonance Experiments
Plasmon resonance competition experiments were carried out on a BIAcore 3000 system (Uppsala, Sweden) with the Op18-like protein SCG10 immobilized by amine coupling on a CM5 chip according to the instructions of the manufacturer. Binding experiments were run using PEM buffer (80 mM piperazine-N,N′-bis[2-ethanesulfonic acid], 1 mM EGTA, 4 mM Mg2+) adjusted to the indicated pH with NaOH. Analyses were performed with the indicated concentrations of tubulin in PEM buffer premixed with graded concentrations of Op18 derivatives. The free tubulin concentrations were determined from the plateau levels by comparison with a tubulin-binding standard curve, as described in the BIAcore handbook. Dissociation constants refer to the binding of two tubulin heterodimers.
Cytokinesis-block Micronucleus Assay
The cytokinesis-block micronucleus assay was performed essentially as described (Fenech, 1997). Briefly, cytochalasin B was added to block cytokinesis during the last 16 h of a 24-h period of Cd2+-induced expression from the hMTIIa promoter in transfected cells. Cells were fixed with paraformaldehyde (2%) and permeabilized with saponin (0.05%), and the DNA was stained with propidium iodide. Coded slides for each duplicate culture were examined at 1000× magnification by epifluorescence. Blockage of cytokinesis generates a binucleated cell after mitosis, in which an unsegregated chromosome forms a clearly visible micronucleus. Binucleated K562 cells constitute ∼40–50% of all cells under the present conditions, and cells were scored for the presence of micronuclei using previously described scoring criteria (Fenech, 1997).
RESULTS
Op18 Is Not Required for the Cell Cycle of Human Leukemia Cells
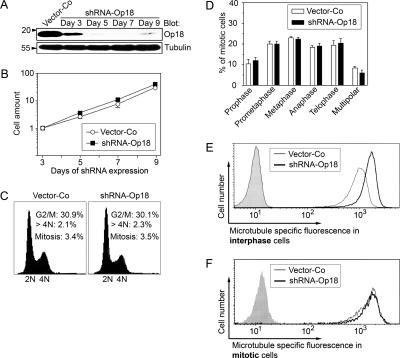
More than a decade ago, we and others reported evidence that Op18 is essential for cell division in the human K562 leukemia cell line (Luo et al., 1994; Marklund et al., 1994). However, both of these studies relied on antisense RNA techniques for Op18 depletion and lacked appropriate controls for nonspecific antisense RNA effects. In the present study, we have reevaluated the role of Op18 in the cell cycle of K562 by the more efficient RNA interference approach, using a replicating vector system that directs constitutive synthesis of shRNAs. The system allows hygromycin-dependent selection of successfully transfected cells within 3 d, and the consequences of Op18 depletion can be monitored over extended culture periods (Holmfeldt et al., 2004, 2005). A total of five distinct targeting sequences were tested, one of which was particularly efficient and depleted >96% of all Op18 after 7 d of expression (Figure 1A). By counting cells over a 9-d period, we found that Op18 depletion has no significant effect on growth rate (Figure 1B), cell cycle distribution, or frequency of mitotic cells (Figure 1C). We also analyzed the frequency of polyploid cells, which is diagnostic of mitotic slippage caused by mitotic errors (Schimke et al., 1991). Also, by this criterion we did not obtain any indication of a requirement of Op18 for proper cell division (Figure 1C, note percentage of >4N). Thus, contrary to previous reports (Luo et al., 1994; Marklund et al., 1994), we found that depletion of Op18 has no effect on K562 cell proliferation.
Figure 1.
Phenotype of K562 cells expressing Op18-specific interfering shRNA. Cells were transfected with vector-Coor shRNA-Op18-443, and nontransfected cells were counterselected with hygromycin. (A) Immunoblots of total cellular lysates using the indicated antibody for detection. Arbitrary quantification was achieved by serial dilution of cell lysates, which revealed >96% specific depletion of Op18 at day 7. (B) Viable cells grown in the presence of hygromycin were determined on the days indicated. The graph represents the mean ± SD of data from duplicate determinations. (C) Cells were transfected with either vector-Co or shRNA-Op18-443 were harvested at day 7, and DNA profiles were determined by flow cytometry. The percentage of cells with DNA content corresponding to G2/M or hyperploid genomes (>4N) is indicated in each panel, together with the mitotic index. (D) The transfected cells described in C were double-stained for DNA and MTs and analyzed by epifluorescence. Cells were categorized with respect to frequencies of specific mitotic stages and multipolar spindles, which is the predominant mitotic aberrancy in K562 cells. The data represent the means of duplicate determinations of coded samples (n = 300 mitotic cells). (E and F) Flow cytometric analysis of the distribution of MT-specific fluorescence in the specifically gated interphase population (E) or mitotic population (F) of cells described in C. Gray graphs show control staining in the absence of anti-α-tubulin but in the presence of fluorescein-conjugated rabbit anti-mouse immunoglobulin. The data are representative of three independent transfection experiments.
It has been reported previously that K562 cells depleted of Op18 by the use of antisense RNA have difficulty in completing mitosis (Iancu et al., 2001). The present study indicates that this defect cannot be attributed to Op18 deficiency, because K562 cells extensively depleted of Op18 by RNA interference show the same distribution of all the mitotic phases as control cells (Figure 1D). It is also clear that Op18 depletion has no significant influence on the frequency of multipolar spindles, which is the most common intrinsic mitotic error in K562 cells (Figure 1D, ∼70% of all obviously abnormal spindles). Thus, at the level of mitotic progression and aberrant numbers of spindle poles, we could not detect any consequences of Op18 depletion.
To determine the effect of Op18 depletion on MT polymer content during interphase and mitosis, we used a method that involves flow cytometric analysis of specifically gated interphase and mitotic cells (Holmfeldt et al., 2003). The histogram revealed that although Op18-depleted interphase cells showed a large increase in MT polymers (Figure 1E), the average MT polymer content of mitotic cells was essentially unaltered and the only effect of Op18 depletion was a slight shift in the lower part of the histogram (Figure 1F). These data are consistent with Op18 being a major destabilizer of MTs during interphase, and the destabilizing activity of Op18 appears to be substantially inactivated by phosphorylation during the mitosis of leukemia cells.
Figure 1 summarizes key data derived from results between day 3 and 9 after shRNA transfection (data from day 7 are shown in Figure 1, C–F). The results were reproduced with four distinct shRNAs, all of which were capable of depleting 70–96% of the total Op18 (unpublished data). Thus, it seems reasonable to conclude that Op18 has little, if any, importance in cell growth and fidelity of spindle assembly in K562 cells. Thus, regarding the potential role of overexpressed Op18 in malignancy, we conclude that it is not important for the malignant phenotype of leukemia cells at the level of basic growth characteristics and cell viability.
The Q18→E Mutation Increases the MT-destabilizing Activity of Phosphorylated Op18 and Causes Disruption of the Mitotic Spindle
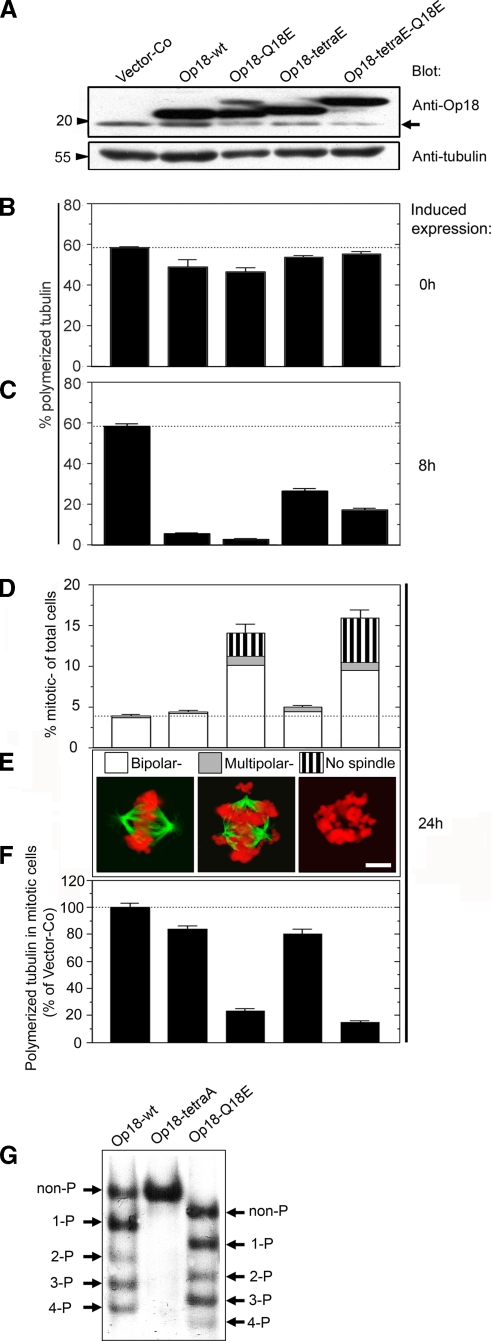
As outlined in the Introduction, functional characterization of a recently identified tumor-associated mutation may provide clues as to the significance of overexpressed Op18 in tumors. We therefore investigated whether the Q18→E mutation alters the phosphorylation-regulated activity of Op18 in K562 leukemia cells. For these studies, we used the replicating pMEP4 vector system, which allows inducible expression and confers hygromycin resistance (Marklund et al., 1994). The phenotype of the Q18→E mutation was analyzed both in the context of wild-type Op18-wt and pseudophosphorylated Op18-tetraE derivatives. To separate ectopic Op18 proteins from the endogenous gene product by SDS-PAGE, we introduced an 8-aa Flag tag at the C-terminus. As shown by immunoblotting, 8 h of Cd2+-induced expression from the hMTIIa promotor resulted in similar overexpression levels of all four Op18 derivatives (Figure 2A; the position of endogenous Op18 is indicated by an arrow). Under the present conditions, which were set to obtain intermediate levels of overexpression, serial dilutions of cell lysates revealed a 3–4-fold excess of ectopic Op18 relative to the endogenous gene product (unpublished data). In the case of Op18-tetraE-Q18E, the mutation altered the migration on SDS-PAGE, which is often observed if negative charges are introduced at the N-terminus of the Op18 protein. This effect of the Q18→E mutation was also manifested with an Op18-Q18E phospho-isomer with retarded migration on SDS-PAGE (Figure 2A).
Figure 2.
The interphase and mitotic phenotypes of the Q18→E mutation in the context of wild-type and pseudophosphorylated Op18. K562 cells were transfected with the pMEP derivative indicated (8 μg DNA mixed with 8 μg vector-Co DNA) and counterselected with hygromycin for 5 d. Cd2+ was added to induce ectopic expression from the hMTIIa-promoter, for the indicated time periods. (A) Immunoblots of total cellular lysates from Cd2+-induced cells (8 h) using the antibodies indicated for detection. Arrow indicates the migration of endogenous Op18. (B and C) The MT content of interphase cells was determined before or after 8 h of Cd2+-induced expression (B, 0 h, and C, 8 h, respectively). Data are expressed as percentage of total cellular tubulin that is polymerized. (D) After 24 h of induced expression, mitotic figures were inspected and categorized as bipolar, multipolar, or, if spindle MTs were completely absent, “no spindle.” Data are expressed as percentage of total cells (n = 300 mitotic cells) and are the average of duplicate determinations of coded samples. (E) Epifluorescence images of representative examples of a normal bipolar metaphase spindle, a multipolar spindle, or a mitotic cell lacking spindle MTs, termed no spindle. Fixed cells were stained with anti-α-tubulin (green) and propidium iodide (red). All spindles with more than three spindle poles were categorized as “multipolar.” Bar, 6 μm. (F) MT-specific fluorescence in gated mitotic populations was determined by flow cytometry as in Figure 1F. (G) Transfected cell lines were Cd2+-induced for 24 h in the presence of the mitosis-blocking drug paclitaxel (0.5 μM) and phospho-isomers of the indicated Flag epitope-tagged Op18 derivative were resolved by native PAGE and revealed using anti-Flag antibodies. The position of unphosphorylated Op18 (non-P) is defined by the migration of kinase target site-deficient Op18-tetraA, which is Ala-substituted at all four phosphorylation sites. Op18-Q18E migrates somewhat faster due to introduction of the negatively charged Glu residue. The data are representative of four independent transfection experiments.
Before induced expression, the interphase MT polymer content was only slightly decreased in Op18-transfected cell lines (Figure 2B, 0 h), which reflects the tight regulation from the hMTIIa promoter. As expected, expression of Op18-wt for 8 h caused a dramatic decrease in polymer levels (∼10% remaining polymer) and Op18-Q18E appeared even more potent (∼5% remaining polymer; Figure 2C, 8 h). The pseudophosphorylated Op18-tetraE derivative was, as expected, less active than the wild-type derivative but—also in this case of a partially inactivated derivative—the Q18→E mutation increased the MT-destabilizing activity in interphase cells.
To explore the mitotic phenotype of the Q18→E mutation, the frequency, appearance, and MT polymer content of mitotic cells were analyzed after 24 h of induced expression (Figure 2, D–F, 24 h). Consistent with both endogenous and ectopic Op18 being phosphorylation-inactivated during mitosis (Larsson et al., 1997), we found that the present overexpression level of Op18-wt caused only a slight increase in the frequency of mitotic cells despite a dramatic depolymerization of interphase MTs. Interestingly, however, introduction of the Q18→E mutation resulted in a pronounced accumulation of mitotic cells (Figure 2D), the majority of which appeared arrested in a prometaphase-like state (unpublished data). We also found severely aberrant mitotic cells in which MTs were completely absent (no spindle, Figure 2, D and E). Moreover, flow cytometric analysis of specifically gated mitotic cells showed that the Q18→E mutation results in a large decrease of the average MT polymer content within the mitotic cell population indicative of excessive MT-destabilizing activity (Figure 2F). These types of mitotic defects have also been observed in cells expressing phosphorylation site–deficient derivatives of Op18, which are resistant to phosphorylation-inactivation (Larsson et al., 1997). Thus, the phenotype of the Q18→E mutation may be explained either by interference with mitotic phosphorylation of Op18 or, alternatively, by suppression of the inactivating effect of Op18 phosphorylation.
To distinguish between these two alternatives, the Q18→E mutation was introduced in the context of the pseudophosphorylated Op18-tetraE derivative. Because of the S→E substitutions at all four phosphorylation sites, this derivative cannot be phosphorylated during mitosis but, consistent with partial inactivation by introduction of negatively charged Glu residues, the spindle-disrupting activity of Op18-tetraE is very modest. However, introduction of the Q18→E mutation into this derivative resulted in a strong mitotic phenotype that was indicative of excessive MT-destabilizing activity, namely accumulation of mitotic cells lacking spindle MTs and a large decrease of the average spindle MT polymer content (Figure 2, D–F, compare Op18-tetraE and Op18-tetraE-Q18E). This effect of the Q18→E mutation in the context of pseudophosphorylated Op18 shows that the mutation has the potential to suppress the essential phosphorylation-inactivation during mitosis.
To determine whether the Q18→E mutation alters multisite phosphorylation during mitosis, Op18 phospho-isomers were separated by charge on a native gel system. To enrich for a mitotic population, cells were induced to express ectopic Op18 in the presence of the mitosis-blocking drug paclitaxel for 20 h. It should be noted that the K562 cell line, like many other tumors, has a propensity to bypass the spindle checkpoint arrest after a few hours. This implies that only a partial enrichment for mitotic cells can be obtained. Nevertheless, a paclitaxel-treated population still consists of ∼25–30% mitotic cells, and in this cell population we found that a substantial fraction of all ectopic wild-type Op18 is phosphorylated at up to four sites. As expected, the S→A substitutions of all four mitotic kinase target sites blocked all phosphorylation (Figure 2G, compare Op18-wt and Op18-tetraA). Most importantly, we found no major differences between Op18-wt and Op18-Q18E with respect to phospho-isomer distribution (note that the Q18E substitution introduces a negative charge, and hence a small shift in migration of all phospho-isomers). Thus, the spindle-disrupting effect of the Q18→E mutation cannot be explained by defective multisite phosphorylation of Op18. This, together with the activating effect in the context of pseudophosphorylated Op18-tetraE, indicates that the mutation causes its mitotic phenotype by suppressing the inactivating effect of mitotic multisite phosphorylation.
The Q18→E Mutation Increases the MT-destabilizing Activity of Nonphosphorylated Op18
To explore the functional consequences of the Q18→E mutation further, we also analyzed the phenotype of this mutation in the context of an Op18 derivative that cannot be phosphorylated. This derivative (termed Op18-tetraA) is substituted with Ala residues at all four phosphorylation sites and is consequently constitutively active and disruptive for the mitotic spindle (Marklund et al., 1996). Given that even modest expression of Op18-tetraA causes near-complete depolymerization of both interphase and spindle MTs (Larsson et al., 1997), it was difficult to evaluate enhanced activity caused by the Q18→E mutation in the context of full-length Op18 protein (unpublished data). We therefore evaluated the effect of the mutation in a less active derivative in which the C-terminal 50 amino acids had been removed (termed Op18(1-99)-tetraA).
As shown by immunoblots, Op18(1-99)-tetraA and Op18(1-99)-tetraA-Q18E were expressed at similar levels after 8 h of Cd2+ induction of the hMTIIa promotor (Figure 3A). The level of expression of the Op18(1-99)-tetraA truncation derivative caused only a partial destabilization of interphase MTs and intermediate levels of mitotic aberrancies (Figure 3, C–E). It is significant that introduction of the Q18→E mutation strongly increased the potency by which interphase MTs and mitotic spindles were destabilized. Thus, the mitotic index was increased, a large fraction of all mitotic cells lacked MTs (no spindle), and the average MT polymer content was dramatically decreased (Figure 3, D and E, compare Op18(1-99)-tetraA and Op18(1-99)-tetraA-Q18E). It follows that in addition to the suppression of phosphorylation-inactivation described above, the Q18→E mutation also substantially increases the microtubule-destabilizing activity of nonphosphorylated Op18.
Figure 3.
The interphase and mitotic phenotype of the Q18→E mutation in the context of a phosphorylation site-deficient and C-terminally truncated Op18. K562 cells were transfected with the derivatives indicated (16 μg DNA) and expression was induced with Cd2+ as in Figure 2. (A) Immunoblots of total cellular lysates from Cd2+-induced cells (8 h) using the indicated antibody for detection. Arrow indicates the migration of endogenous Op18. (B and C) The MT content of interphase cells was determined before or after 8 h of Cd2+-induced expression (B, 0 h, and C, 8 h, respectively). Data are expressed as percentage of total cellular tubulin that is polymerized. (D) After 24 h of induced expression, mitotic figures were inspected and categorized as in Figure 2. (E) MT-specific fluorescence in gated mitotic populations was determined by flow cytometry as in Figure 1F. The data are representative of three independent transfection experiments.
The Q18→E Mutation Has Only Minor Effects on the Tubulin-binding Affinity of Op18
To evaluate whether the dramatic effect of the Q18→E mutation on Op18 activity in intact cells can be attributed to increased tubulin-sequestering activity, we performed plasmon resonance competition experiments. This technique allows determination of dissociation constants (Kds) of Op18-tubulin complexes formed in solution. Given that the calculation of affinities is based on the known stoichiometry of the Op18-tubulin heterodimer complex (1:2 M ratio), conversion of competition data to a binding curve should allow correction of the estimated protein concentrations of each Op18 derivative. This, together with the <10% interexperimental variation in Kds at the relevant affinity range, makes it feasible to detect even small differences in binding affinities. Our data show that the Q18→E mutation has only a minor effect on tubulin-binding affinity in a standard tubulin buffer at pH 6.8, namely a <25% reduction in the apparent Kd (Figure 4A, Kd values for Op18-wt and Op18-Q18E are given within parentheses). We also analyzed the effect of Q18→E substitution in the context of the truncated Op18(1-99)-tetraA derivative, which lacks the second tubulin-binding repeat and can thus be predicted to form low-affinity binary tubulin complexes. As shown by the binding curve, Op18(1-99)-tetraA binds tubulin with low affinity, and also in this context, the Q18→E mutation only has a very minor effect (Figure 4B).
Figure 4.
The Op18 Q18→E mutation has a minimal effect on tubulin heterodimer complex affinity. Plasmon resonance competition experiments at 25°C using PEM buffer, pH 6.8 (A and B) or pH 7.3 (C and D). Graded concentrations of the Op18 derivatives indicated were mixed with 5 μM tubulin heterodimers and run over a flow cell coupled with the tubulin-binding SCG10 protein to monitor the free tubulin concentration. The dotted line in A, C, and D depicts complete complex formation at an Op18–tubulin heterodimer ratio of 1:2. The dotted line in B depicts complete complex formation at a 1:1 M ratio, because Op18(1-99)-tetraA can only be expected to bind a single tubulin heterodimer due to the absence of the second tubulin heterodimer binding repeat. It should be noted that the four Ala substitutions of the Op18(1-99)-tetraA protein have no detectable effect on tubulin-binding affinity (unpublished data). The apparent Kds of each Op18 derivative, which are indicated in the figure, were derived from binding curves in which the level of complex formation was plotted against the free tubulin concentration. The estimated molar concentrations of individual preparations of Op18 derivative were adjusted to fit in with the predicted molar ratio of the cognate Op18-tubulin complex. The data are representative of two independent experiments.
The tubulin-binding affinity of Op18 is optimal at pH 6.5 (Curmi et al., 1997). We found that the Kd of Op18-tubulin binding at pH 7.3 (Figure 4C), which matches the pH of the cytosol, was about twofold higher than at pH 6.8 (Figure 4A). At pH 7.3, the differences in binding affinities between Op18-wt and Op18-Q18E were even smaller than at pH 6.8. Moreover, analysis of the pseudophosphorylated Op18-tetraE derivative at pH 7.3, which was used to confirm that Ser→Glu substitutions at phosphorylation sites reduce tubulin affinity (Curmi et al., 1997), is also consistent with the Q18→E mutation, having only a very minor effect on tubulin-binding affinity (Figure 4D). Thus, the dramatic potentiation of Op18 activity by the Q18→E mutation observed in intact cells is not associated with a corresponding effect on tubulin-binding affinity. It follows that this potentiation of MT-destabilizing activity cannot be explained in terms of a tubulin-sequestering mechanism.
Phenotype of the Q18→E Mutation Expressed at Near-physiological Levels
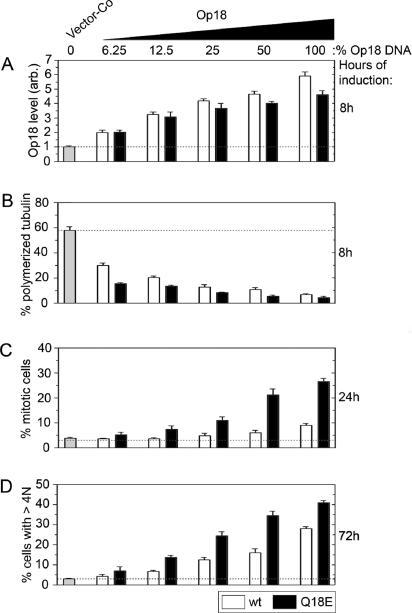
Op18 has been identified by many investigators because of its up-regulated expression in various tumor cell lines and also in primary tumor biopsies. The K562 leukemia cell line used in this study expresses Op18 at an intermediate level that is within the range found in leukemia cells (∼1% of total cellular proteins) and about fourfold lower than the levels observed in the most extreme cases (Brattsand et al., 1993). This implies that the about fourfold higher levels of ectopic Op18 over the endogenous gene product observed in transfected K562 cells represent extreme overexpression levels. It was therefore important to determine the phenotype of the Q18→E mutation when expressed at levels more commonly observed in tumor cells. Graded Op18 expression levels were achieved by premixing the specific pMEP-expression derivative with a control vector in different proportions. Because these vectors have stringent control of replication, the relative proportion of transfected DNA is maintained over several days of culture (Melander Gradin et al., 1997). Previous analysis of the expression kinetics from the hMTIIa promotor has shown close to maximal expression already after 6 h of induction, and that thereafter Op18 expression levels remain relatively constant over at least 3 d (Holmfeldt et al., 2001). The expression levels at various DNA ratios after 8 h of induction are shown in Figure 5A. The data show that the Q18E-substituted Op18 derivative is expressed at a somewhat lower level than the cognate wild-type derivative at decreasing concentrations of pMEP expression vector DNA. Analysis of interphase cells revealed that even quite modest levels of ectopic Op18 result in a pronounced decrease in MT polymers and, most importantly, that the Op18-Q18E protein has a higher specific activity than the wild-type protein, which is particularly evident at modest expression levels (Figure 5B).
Figure 5.
The interphase and mitotic phenotype of graded expression levels of wild-type Op18 and Op18-Q18E. K562 cells were transfected with a total of 18 μg DNA comprising the indicated percentage of pMEP-Op18-wt (□) or pMEP-Op18-Q18E (■), made up with vector-Co. Nontransfected cells were counterselected for 5 d with hygromycin. (A) Transfected cells were induced with Cd2+ for 8 h, fixed, and stained, and Op18 expression levels were quantitated by flow cytometric analysis using affinity-purified antibodies directed against an internal peptide sequence of the protein that does not cover the Q18E mutation. Data are expressed as fold increase relative to the endogenous gene product. (B) MT content of interphase cells was determined after 8 h of Cd2+-induced expression. (C) The mitotic index was determined after 24 h of Cd2+-induced expression. (D) The frequency of hyperploid cells was determined after 72 h by flow cytometric analysis of DNA content and gating of cells with >4N DNA content. The data are representative of three independent transfection experiments.
Determination of mitotic index showed that the Q18→E mutation increases the mitosis-blocking effect of Op18 at all expression levels (Figure 5C). It is evident, however, at least at high expression levels, that wild-type Op18 also causes accumulation of mitotic cells, but to a lesser extent. The great majority of mitotic cells overexpressing wild-type Op18 appeared normal, but the fraction of prometaphase cells was increased (unpublished data). This explains the increase in the mitotic index and suggests that excess wild-type Op18 delays MT-dependent capture of chromosomes. Most interestingly, the frequency of polyploid cells after 72 h of induced expression was found to increase dramatically in cells overexpressing either Op18-wt or Op18-Q18E (Figure 5D). Given that interference with the mitotic spindle by MT-directed drugs has been shown to cause inappropriate mitotic exit and consequent polyploidization of K562 and many other tumor cells (unpublished data; Kung et al., 1990; Roberts et al., 1990), it can be assumed that the polyploidization observed is caused by Op18-mediated interference with MT dynamics during spindle assembly. In cases of low or moderate overexpression, Op18-Q18E was clearly more potent in causing polyploidy than Op18-wt. This seems highly relevant, because moderate overexpression levels are commonly observed in different tumor cells. However, at increasing expression levels, the relative difference in this aneugenic activity between the wild-type and Op18-Q18E proteins was not that large. Thus, at sufficiently high expression levels, both Op18-Q18E and wild-type Op18 have the potential to interfere with spindle assembly and facilitate generation of polyploid cells. However, the mutated Op18 protein has these activities at much lower expression levels.
To ascertain that the mitotic block and polyploidization shown in Figure 5 is not a peculiarity of the K562 erythroleukemia, we also expressed Op18-wt and Op18-Q18E in the Burkitt B-cell lymphoma line DG75. In this tumor cell line, we also observed increased mitotic index and accumulation of polyploid cells after 48–72 h of induced expression. As with K562 cells, the specific activity of Op18-Q18E was higher than that of Op18-wt (unpublished data). Thus, the aneugenic effects of Op18 and the potentiating effect of the mutation appear to be a general phenomenon in human malignancies of hematopoetic origin.
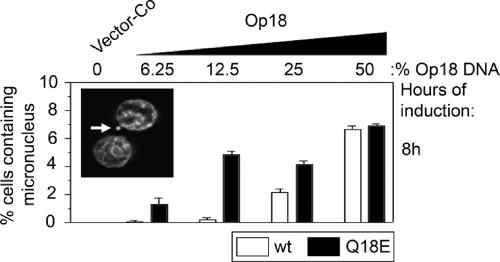
The cytokinesis-block micronucleus assay provides a readily measurable index of aneugenic activities manifested as chromosome breakage and chromosome loss, and thus provides a means of assessing chromosomal instability (Fenech, 1997). Accordingly, cells induced to express graded levels of wild-type and mutant Op18 for 24 h were scored in terms of appearance of micronuclei in binucleated cells in which cytokinesis had been blocked by cytochalasin B. We found that while micronuclei were essentially absent in cells transfected with vector-Co (<0.1%), they were prevalent in cells expressing either ectopic wild-type or mutant Op18 (Figure 6). Significantly, similar to the phenotypes manifest at the level of mitotic index and polyploidization, the mutant Op18 protein generated micronuclei at lower expression levels (see Figure 5 for expression levels with different amounts of DNA used for transfection). Because the ectopic Op18 proteins mediate their phenotype by causing excessive destabilization of MTs during spindle assembly, we interpret the appearance of micronuclei as evidence of chromosome loss and not chromosome breakage. Taken together, these results show that the Q18→E mutation increases the pre-existing potential of Op18 to interfere with spindle assembly and thereby causes chromosome loss, polyploidization, and thus chromosomal instability.
Figure 6.
Appearance of micronuclei in cells expressing graded levels of wild-type Op18 and Op18-Q18E. K562 cells were transfected with pMEP-Op18-wt (□) or pMEP-Op18-Q18E (■) as in Figure 5. The cytokinesis micronucleus assay was performed as described in Materials and Methods. Data represent duplicate determinations in which 400 binucleated cells were examined in each sample. A representative example of a micronucleus, indicated by an arrow, in a cytochalasin B–blocked binucleated cell is shown as an inset.
DISCUSSION
Op18 has been identified in many proteomic and microarray screens as a protein that is overexpressed in various types of malignancies (Mistry et al., 2005 and references therein). In accordance with its potential role in tumorigenesis, it has been shown that the Op18 gene is repressed by the tumor suppressor protein p53 (Ahn et al., 1999; Murphy et al., 1999). This may indeed explain in part why Op18 is so often overexpressed in diverse tumors and why its expression has been reported to be of prognostic significance (Roos et al., 1993; Friedrich et al., 1995; Brattsand, 2000). There has been more direct evidence for a role of Op18 in tumorigenesis from identification of the somatic Q18→E mutation in one allele from a human adenocarcinoma biopsy. This allele was shown to confer transforming properties on the mutated protein expressed in murine NIH-3T3 cells (Misek et al., 2002). Here we have addressed the significance of up-regulated expression of Op18 in human leukemia cells by 1) analyzing the cell cycle phenotype caused by Op18 depletion, 2) determining how the Q18→E mutation alters the MT-destabilizing activity of Op18 during interphase and mitosis, and 3) comparing wild-type and mutated Op18 at various expression levels with respect to aneugenic activity, manifest as polyploidization and generation of micronuclei, which are defects likely to reflect excessive MT-destabilization during spindle assembly. Our results show that Op18 has no essential role in growth and cell division of the K562 leukemia cell line. However, we have found that at high expression levels, Op18 has significant aneugenic activity and that this activity is dramatically increased by the Q18→E mutation, such that even low expression levels are sufficient for interference with spindle assembly (Figure 5).
As outlined in the Introduction, Op18 has been implicated in both assembly and disassembly of the mitotic spindle. Our current data refute two previous studies on K562 leukemia cells published in 1994 by us and others (Luo et al., 1994; Marklund et al., 1994). These previous studies used expression of antisense RNA and, in contrast to the present study, suggested that Op18 is essential for cell division. The present study used an RNA interference system that has recently been used to investigate the function of several other microtubule regulatory proteins (Holmfeldt et al., 2004, 2005) and which allows >96% depletion of Op18. This is clearly more efficient than in our previous study, in which only 60% depletion was obtained (Marklund et al., 1994). It is difficult to compare relative levels to the study of Luo et al. (1994) because these authors presented data on reduced mRNA levels rather than protein levels. Nevertheless, given the present demonstration that almost complete depletion of Op18 has no detectable consequences for growth in K562 cells, we believe that the previously reported phenotypes in this cell line were due to nonspecific effects arising from the antisense RNA approach that were not properly controlled for in either study by the use of nonspecific RNA controls. Such apparently nonspecific effects, manifested as slower growth and accumulation of cells in G2/M, may well explain why K562 cells with Op18 expression reduced with anti-sense RNA appeared to be less malignant than vector control cells (Jeha et al., 1996).
The present study confirms that Op18 is a major destabilizer of interphase MTs in K562 cells and that Op18 is phosphorylation-inactivated during mitosis (Figure 1, E and F). Op18 is an abundant protein in K562 cells (accounting for ∼1% of the total cellular protein; Brattsand et al., 1993), and it may appear surprising that depletion has no effect on the basic cell cycle and general growth properties. However, given that K562 leukemia cells are nonadherent, have no defined cell shape and grow in suspension, it still seems reasonable that regulation of interphase MTs by Op18 is not very important in this cell type. In the case of substrate-dependent cell types in which the cytoskeleton is essential for cell shape and migratory properties, it seems likely that the regulation of interphase MTs by Op18 would be more important. This has indeed been suggested by the recent demonstration that interaction of Op18 with the cyclin-dependent kinase regulator p27Kip1 influences sarcoma cell migration and invasion via an MT-dependent mechanism (Baldassarre et al., 2005). It is also consistent with the finding that reducing Op18 expression in prostate cancer cells by a ribozyme-based strategy resulted in cell detachment, growth inhibition, and a marked increase in apoptosis (Mistry et al., 2005). Because Op18 is overexpressed in many different tumor types, it may be that Op18, via its MT-regulatory properties, influences tumor progression by more than one mechanism. Thus, although overexpression of Op18 in solid tumors may have a role in promoting invasive behavior (Baldassarre et al., 2005), we propose that overexpression in leukemias results in an aneugenic activity that promotes chromosomal instability and thereby contributes to the malignant phenotype.
The somatic Q18→E mutation is the only mutation of Op18 identified so far, and it has only been observed in a single tumor. Expression of the mutant Op18-Q18E protein in NIH-3T3 cells has been found to facilitate tumor growth in immunodeficient mice as well as increasing the basal frequency of focus formation in soft agar. Together, these observations provide a strong case for the significance of this mutation in the original tumor (Misek et al., 2002). It was also reported that NIH-3T3 cells expressing Op18-Q18E grow slowly and that the fraction of cells in G2/M was increased. This is consistent with the results of present study, because we found that the Q18→E mutation suppresses phosphorylation-inactivation, i.e., confers constitutive activity that disrupts spindle assembly during mitosis and thereby causes accumulation of mitotic cells (Figure 2). On the basis of the pattern of synergistic action of the Q18E mutation and the MT-stabilizing drug paclitaxel, Misek et al. (2002) proposed that the mutant form of Op18 may enhance the stability of MTs. This proposal is refuted by the results of the present study, because we have shown that the Q18→E mutation greatly enhances the MT-destabilizing activity of Op18 during both interphase and mitosis, resulting in aneugenic activity and a partial or complete mitotic block, depending on expression levels (Figures 2 and 3). These results suggest that the aneugenic activity of Op18-Q18E promotes transformation of NIH-3T3 cells by generating chromosomal instability. Ectopic wild-type Op18 was reported to completely lack transforming activity in the NIH-3T3 cell assay (Misek et al., 2002), which may appear contradictory to our present conclusion that overexpressed wild-type Op18 also has aneugenic activity. However, ectopic wild-type and Op18-Q18E were both only expressed at ∼50% of that of the endogenous gene product in NIH-3T3 cells. At this low expression level, ectopic wild-type Op18 is efficiently inactivated by phosphorylation during mitosis and thus does not possess any aneugenic activity, whereas the mutant with its high and unregulated aneugenic activity seems likely to cause chromosomal instability even under these conditions (Figure 5).
By analysis of basal phosphorylation in exponentially growing cells, Misek et al. (2002) reported that Op18-Q18E is less phosphorylated than the wild-type protein. However, in the present analysis of populations enriched for mitotic cells, we found similar levels of multisite phosphorylated isomers of mutated and wild-type Op18. Thus, the dramatic phenotype of the Q18→E mutation we have observed in K562 cells cannot be explained by altered phosphorylation. This finding, together with the finding that the Q18→E mutation dramatically increases the activity of a pseudophosphorylated Op18 derivative (Figure 2), allows us to conclude that this mutation suppresses the inactivating effect of multisite phosphorylation of Op18. In addition, by analyzing the effect of the Q18→E mutation in the context of a kinase target site–deficient Op18 derivative, we can conclude that the mutation also dramatically increases the activity of unphosphorylated Op18 (Figure 3). Thus, suppression of phosphorylation-inactivation is associated with a general increase in the specific MT-destabilizing activity of Op18. This increase in specific activity of Op18 is particularly evident in experiments in which MT destabilization by mutated and wild-type Op18 is compared at graded expression levels (Figure 5).
Op18 has the potential to destabilize MTs by two distinct mechanisms, namely catastrophe promotion and sequestering of tubulin (Cassimeris, 2002). The latter can simply be explained by binding to free tubulin heterodimers, whereas the mechanism behind catastrophe promotion is unclear. Here, we have shown that the Q18→E mutation dramatically increases the MT-destabilizing activity of a C-terminally truncated Op18 derivative that binds tubulin with ∼10-fold reduced affinity (Figures 3 and 4). Despite its much lower tubulin-binding affinity, the activity of the truncated Op18-Q18E mutant protein is as high in interphase cells as that of intact wild-type Op18 (compare Figures 2–4). Because the Q18→E mutation has very little effect on the tubulin heterodimer affinity, it seems excluded that the phenotype of this mutation would be the result of increased tubulin-sequestering capacity. Thus, it appears likely that the Q18→E mutation will provide an important tool to help us elucidate how Op18 destabilizes MTs by a mechanism that is independent of tubulin sequestration.
ACKNOWLEDGMENTS
We thank Lynne Cassimeris and Victoria Shingler for discussions. Alistair Kidd’s editing of this manuscript is also appreciated. This work was supported by the Swedish Research Council.
Abbreviations used:
- Kd
dissociation constant
- MT
microtubule
- Op18
Oncoprotein 18/stathmin
- shRNA
short hairpin RNA.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-02-0165) on April 19, 2006.
REFERENCES
- Ahn J., Murphy M., Kratowicz S., Wang A., Levine A. J., George D. L. Down–regulation of the stathmin/Op18 and FKBP25 genes following p53 induction. Oncogene. 1999;18:5954–5958. doi: 10.1038/sj.onc.1202986. [DOI] [PubMed] [Google Scholar]
- Andersen S. S., Ashford A. J., Tournebize R., Gavet O., Sobel A., Hyman A. A., Karsenti E. Mitotic chromatin regulates phosphorylation of Stathmin/Op18. Nature. 1997;389:640–643. doi: 10.1038/39382. [DOI] [PubMed] [Google Scholar]
- Baldassarre G., Belletti B., Nicoloso M. S., Schiappacassi M., Vecchione A., Spessotto P., Morrione A., Canzonieri V., Colombatti A. p27(Kip1)-stathmin interaction influences sarcoma cell migration and invasion. Cancer Cell. 2005;7:51–63. doi: 10.1016/j.ccr.2004.11.025. [DOI] [PubMed] [Google Scholar]
- Belmont L. D., Mitchison T. J. Identification of a protein that interacts with tubulin dimers and increases the catastrophe rate of microtubules. Cell. 1996;84:623–631. doi: 10.1016/s0092-8674(00)81037-5. [DOI] [PubMed] [Google Scholar]
- Biggins S., Walczak C. E. Captivating capture: how microtubules attach to kinetochores. Curr. Biol. 2003;13:R449–R460. doi: 10.1016/s0960-9822(03)00369-5. [DOI] [PubMed] [Google Scholar]
- Brattsand G. Correlation of oncoprotein 18/stathmin expression in human breast cancer with established prognostic factors. Br. J. Cancer. 2000;83:311–318. doi: 10.1054/bjoc.2000.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brattsand G., Marklund U., Nylander K., Roos G., Gullberg M. Cell-cycle-regulated phosphorylation of oncoprotein 18 on Ser16, Ser25 and Ser38. Eur. J. Biochem. 1994;220:359–368. doi: 10.1111/j.1432-1033.1994.tb18632.x. [DOI] [PubMed] [Google Scholar]
- Brattsand G., Roos G., Marklund U., Ueda H., Landberg G., Nanberg E., Sideras P., Gullberg M. Quantitative analysis of the expression and regulation of an activation-regulated phosphoprotein (oncoprotein 18) in normal and neoplastic cells. Leukemia. 1993;7:569–579. [PubMed] [Google Scholar]
- Cahill D. P., Lengauer C., Yu J., Riggins G. J., Willson J. K., Markowitz S. D., Kinzler K. W., Vogelstein B. Mutations of mitotic checkpoint genes in human cancers. Nature. 1998;392:300–303. doi: 10.1038/32688. [DOI] [PubMed] [Google Scholar]
- Cassimeris L. The oncoprotein 18/stathmin family of microtubule destabilizers. Curr. Opin. Cell Biol. 2002;14:18–24. doi: 10.1016/s0955-0674(01)00289-7. [DOI] [PubMed] [Google Scholar]
- Curmi P. A., Andersen S. S., Lachkar S., Gavet O., Karsenti E., Knossow M., Sobel A. The stathmin/tubulin interaction in vitro. J. Biol. Chem. 1997;272:25029–25036. doi: 10.1074/jbc.272.40.25029. [DOI] [PubMed] [Google Scholar]
- Desai A., Mitchison T. J. Microtubule polymerization dynamics. Annu. Rev. Cell Dev. Biol. 1997;13:83–117. doi: 10.1146/annurev.cellbio.13.1.83. [DOI] [PubMed] [Google Scholar]
- Fenech M. The advantages and disadvantages of the cytokinesis-block micronucleus method. Mutat. Res. 1997;392:11–18. doi: 10.1016/s0165-1218(97)00041-4. [DOI] [PubMed] [Google Scholar]
- Fodde R., et al. Mutations in the APC tumour suppressor gene cause chromosomal instability. Nat. Cell Biol. 2001;3:433–438. doi: 10.1038/35070129. [DOI] [PubMed] [Google Scholar]
- Friedrich B., Gronberg H., Landstrom M., Gullberg M., Bergh A. Differentiation-stage specific expression of oncoprotein 18 in human and rat prostatic adenocarcinoma. Prostate. 1995;27:102–109. doi: 10.1002/pros.2990270207. [DOI] [PubMed] [Google Scholar]
- Gigant B., Curmi P. A., Martin-Barbey C., Charbaut E., Lachkar S., Lebeau L., Siavoshian S., Sobel A., Knossow M. The 4 A X-ray structure of a tubulin:stathmin-like domain complex. Cell. 2000;102:809–816. doi: 10.1016/s0092-8674(00)00069-6. [DOI] [PubMed] [Google Scholar]
- Gradin H. M., Larsson N., Marklund U., Gullberg M. Regulation of microtubule dynamics by extracellular signals: cAMP-dependent protein kinase switches off the activity of oncoprotein 18 in intact cells. J. Cell Biol. 1998;140:131–141. doi: 10.1083/jcb.140.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmfeldt P., Brattsand G., Gullberg M. Interphase and monoastral-mitotic phenotypes of overexpressed MAP4 are modulated by free tubulin concentrations. J. Cell Sci. 2003;116:3701–3711. doi: 10.1242/jcs.00685. [DOI] [PubMed] [Google Scholar]
- Holmfeldt P., Larsson N., Segerman B., Howell B., Morabito J., Cassimeris L., Gullberg M. The catastrophe-promoting activity of ectopic Op18/stathmin is required for disruption of mitotic spindles but not interphase microtubules. Mol. Biol. Cell. 2001;12:73–83. doi: 10.1091/mbc.12.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmfeldt P., Stenmark S., Gullberg M. Differential functional interplay of TOGp/XMAP215 and the KinI kinesin MCAK during interphase and mitosis. EMBO J. 2004;23:627–637. doi: 10.1038/sj.emboj.7600076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmfeldt P., Zhang X., Stenmark S., Walczak C. E., Gullberg M. CaMKIIgamma-mediated inactivation of the Kin I kinesin MCAK is essential for bipolar spindle formation. EMBO J. 2005;24:1256–1266. doi: 10.1038/sj.emboj.7600601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell B., Deacon H., Cassimeris L. Decreasing oncoprotein 18/stathmin levels reduces microtubule catastrophes and increases microtubule polymer in vivo. J. Cell Sci. 1999a;112:3713–3722. doi: 10.1242/jcs.112.21.3713. [DOI] [PubMed] [Google Scholar]
- Howell B., Larsson N., Gullberg M., Cassimeris L. Dissociation of the tubulin-sequestering and microtubule catastrophe-promoting activities of oncoprotein 18/stathmin. Mol. Biol. Cell. 1999b;10:105–118. doi: 10.1091/mbc.10.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iancu C., Mistry S. J., Arkin S., Wallenstein S., Atweh G. F. Effects of stathmin inhibition on the mitotic spindle. J. Cell Sci. 2001;114:909–916. doi: 10.1242/jcs.114.5.909. [DOI] [PubMed] [Google Scholar]
- Jallepalli P. V., Waizenegger I. C., Bunz F., Langer S., Speicher M. R., Peters J. M., Kinzler K. W., Vogelstein B., Lengauer C. Securin is required for chromosomal stability in human cells. Cell. 2001;105:445–457. doi: 10.1016/s0092-8674(01)00340-3. [DOI] [PubMed] [Google Scholar]
- Jeha S., Luo X. N., Beran M., Kantarjian H., Atweh G. F. Antisense RNA inhibition of phosphoprotein p18 expression abrogates the transformed phenotype of leukemic cells. Cancer Res. 1996;56:1445–1450. [PubMed] [Google Scholar]
- Jourdain L., Curmi P., Sobel A., Pantaloni D., Carlier M. F. Stathmin: a tubulin-sequestering protein which forms a ternary T2S complex with two tubulin molecules. Biochemistry. 1997;36:10817–10821. doi: 10.1021/bi971491b. [DOI] [PubMed] [Google Scholar]
- Kaplan K. B., Burds A. A., Swedlow J. R., Bekir S. S., Sorger P. K., Nathke I. S. A role for the adenomatous polyposis coli protein in chromosome segregation. Nat. Cell Biol. 2001;3:429–432. doi: 10.1038/35070123. [DOI] [PubMed] [Google Scholar]
- Kung A. L., Sherwood S. W., Schimke R. T. Cell line-specific differences in the control of cell cycle progression in the absence of mitosis. Proc. Natl. Acad. Sci. USA. 1990;87:9553–9557. doi: 10.1073/pnas.87.24.9553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson N., Marklund U., Gradin H. M., Brattsand G., Gullberg M. Control of microtubule dynamics by oncoprotein 18, dissection of the regulatory role of multisite phosphorylation during mitosis. Mol. Cell. Biol. 1997;17:5530–5539. doi: 10.1128/mcb.17.9.5530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson N., Melander H., Marklund U., Osterman O., Gullberg M. G2/M transition requires multisite phosphorylation of oncoprotein 18 by two distinct protein kinase systems. J. Biol. Chem. 1995;270:14175–14183. doi: 10.1074/jbc.270.23.14175. [DOI] [PubMed] [Google Scholar]
- Lawler S. Microtubule dynamics: if you need a shrink try stathmin/Op18. Curr. Biol. 1998;8:R212–R214. doi: 10.1016/s0960-9822(98)70128-9. [DOI] [PubMed] [Google Scholar]
- Luo X. N., Mookerjee B., Ferrari A., Mistry S., Atweh G. F. Regulation of phosphoprotein p18 in leukemic cells. Cell cycle regulated phosphorylation by p34cdc2 kinase. J. Biol. Chem. 1994;269:10312–10318. [PubMed] [Google Scholar]
- Marklund U., Brattsand G., Shingler V., Gullberg M. Serine 25 of oncoprotein 18 is a major cytosolic target for the mitogen-activated protein kinase. J. Biol. Chem. 1993;268:15039–15047. [PubMed] [Google Scholar]
- Marklund U., Larsson N., Gradin H. M., Brattsand G., Gullberg M. Oncoprotein 18 is a phosphorylation-responsive regulator of microtubule dynamics. EMBO J. 1996;15:5290–5298. [PMC free article] [PubMed] [Google Scholar]
- Marklund U., Osterman O., Melander H., Bergh A., Gullberg M. The phenotype of a “Cdc2 kinase target site-deficient” mutant of oncoprotein 18 reveals a role of this protein in cell cycle control. J. Biol. Chem. 1994;269:30626–30635. [PubMed] [Google Scholar]
- Melander Gradin H., Marklund U., Larsson N., Chatila T. A., Gullberg M. Regulation of microtubule dynamics by Ca2+/calmodulin-dependent kinase IV/Gr-dependent phosphorylation of oncoprotein 18. Mol. Cell. Biol. 1997;17:3459–3467. doi: 10.1128/mcb.17.6.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michor F., Iwasa Y., Vogelstein B., Lengauer C., Nowak M. A. Can chromosomal instability initiate tumorigenesis? Semin. Cancer Biol. 2005;15:43–49. doi: 10.1016/j.semcancer.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Misek D. E., Chang C. L., Kuick R., Hinderer R., Giordano T. J., Beer D. G., Hanash S. M. Transforming properties of a Q18→E mutation of the microtubule regulator Op18. Cancer Cell. 2002;2:217–228. doi: 10.1016/s1535-6108(02)00124-1. [DOI] [PubMed] [Google Scholar]
- Mistry S. J., Bank A., Atweh G. F. Targeting stathmin in prostate cancer. Mol. Cancer Ther. 2005;4:1821–1829. doi: 10.1158/1535-7163.MCT-05-0215. [DOI] [PubMed] [Google Scholar]
- Murphy M., Ahn J., Walker K. K., Hoffman W. H., Evans R. M., Levine A. J., George D. L. Transcriptional repression by wild-type p53 utilizes histone deacetylases, mediated by interaction with mSin3a. Genes Dev. 1999;13:2490–2501. doi: 10.1101/gad.13.19.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan H., Jallepalli P. V., Rago C., Velculescu V. E., Kinzler K. W., Vogelstein B., Lengauer C. Inactivation of hCDC4 can cause chromosomal instability. Nature. 2004;428:77–81. doi: 10.1038/nature02313. [DOI] [PubMed] [Google Scholar]
- Roberts J. R., Allison D. C., Donehower R. C., Rowinsky E. K. Development of polyploidization in taxol-resistant human leukemia cells in vitro. Cancer Res. 1990;50:710–716. [PubMed] [Google Scholar]
- Roos G., Brattsand G., Landberg G., Marklund U., Gullberg M. Expression of oncoprotein 18 in human leukemias and lymphomas. Leukemia. 1993;7:1538–1546. [PubMed] [Google Scholar]
- Schimke R. T., Kung A. L., Rush D. F., Sherwood S. W. Differences in mitotic control among mammalian cells. Cold Spring Harb. Symp. Quant. Biol. 1991;56:417–425. doi: 10.1101/sqb.1991.056.01.049. [DOI] [PubMed] [Google Scholar]
- Schubart U. K., Yu J., Amat J. A., Wang Z., Hoffmann M. K., Edelmann W. Normal development of mice lacking metablastin (P19), a phosphoprotein implicated in cell cycle regulation. J. Biol. Chem. 1996;271:14062–14066. doi: 10.1074/jbc.271.24.14062. [DOI] [PubMed] [Google Scholar]
- Steinmetz M. O., Kammerer R. A., Jahnke W., Goldie K. N., Lustig A., van Oostrum J. Op18/stathmin caps a kinked protofilament-like tubulin tetramer. EMBO J. 2000;19:572–580. doi: 10.1093/emboj/19.4.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournebize R., Andersen S. S., Verde F., Doree M., Karsenti E., Hyman A. A. Distinct roles of PP1 and PP2A-like phosphatases in control of microtubule dynamics during mitosis. EMBO J. 1997;16:5537–5549. doi: 10.1093/emboj/16.18.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ui-Tei K., Naito Y., Takahashi F., Haraguchi T., Ohki-Hamazaki H., Juni A., Ueda R., Saigo K. Guidelines for the selection of highly effective siRNA sequences for mammalian and chick RNA interference. Nucleic Acids Res. 2004;32:936–948. doi: 10.1093/nar/gkh247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., et al. Three classes of genes mutated in colorectal cancers with chromosomal instability. Cancer Res. 2004;64:2998–3001. doi: 10.1158/0008-5472.can-04-0587. [DOI] [PubMed] [Google Scholar]