Abstract
Muc4 serves as an intramembrane ligand for the receptor tyrosine kinase ErbB2. The time to complex formation and the stoichiometry of the complex were determined to be <15 min and 1:1 by analyses of Muc4 and ErbB2 coexpressed in insect cells and A375 tumor cells. In polarized CACO-2 cells, Muc4 expression causes relocalization of ErbB2, but not its heterodimerization partner ErbB3, to the apical cell surface, effectively segregating the two receptors. The apically located ErbB2 is phosphorylated on tyrosines 1139 and 1248. The phosphorylated ErbB2 in CACO-2 cells recruits the cytoplasmic adaptor protein Grb2, consistent with previous studies showing phosphotyrosine 1139 to be a Grb2 binding site. To address the issue of downstream signaling from apical ErbB2, we analyzed the three MAPK pathways of mammalian cells, Erk, p38, and JNK. Consistent with the more differentiated phenotype of the CACO-2 cells, p38 phosphorylation was robustly increased by Muc4 expression, with a consequent activation of Akt. In contrast, Erk and JNK phosphorylation was not changed. The ability of Muc4 to segregate ErbB2 and other ErbB receptors and to alter downstream signaling cascades in polarized epithelial cells suggests that it has a role in regulating ErbB2 in differentiated epithelia.
INTRODUCTION
ErbB2 is a 185-kDa class I receptor tyrosine kinase that is structurally related to the epidermal growth factor receptor EGFR. The ErbB family of receptors includes four members: epidermal growth factor receptor (EGFR, HER1, or c-ErbB1), c-ErbB2 (HER2, p185neu), c-ErbB3 (HER3), and c-ErbB4 (HER4) (Riese and Stern, 1998), which share 40–45% sequence identity (Stein and Staros, 2000). The ErbB receptor extracellular domains are composed of four subdomains, which in order from the N-terminus are known as I (L1), II (CR1), III (L2), and IV (CR2) (Bajaj et al., 1987; Lax et al., 1988; Ward et al., 1995); subdomains I and III and subdomains II and IV are homologous. ErbB ligand binding is mediated primarily by subdomain III, and to a lesser extent by subdomain I (Lax et al., 1989; Khoda et al., 1993). Subdomains II and IV mediate the formation of specific ErbB homo and heterodimers (Garrett et al., 2002; Ogiso et al., 2002). The extracellular domain is followed by a transmembrane domain, a tyrosine kinase domain, and a segment of variable length of ∼200 amino acids containing several tyrosine phosphorylation sites. On activation, these phosphotyrosines become docking sites for cytoplasmic signaling proteins that, in turn, initiate characteristic downstream signaling events (Klapper et al., 2000). The pathways activated may lead the cell to proliferation, differentiation, or apoptosis (Alroy and Yarden, 1997; Riese and Stern, 1998). All ErbB family members, with the exception of ErbB2, have high-affinity soluble ligands that induce receptor homo- or heterodimer formation (Carraway and Cantley, 1994) and phosphorylation and trigger downstream signaling. The preferred ErbB heterodimer partner is ErbB2, and the ErbB2–ErbB3 pair generates the strongest proliferative signal, even though they are unable to generate such signals individually. Though dimer formation is necessary for signaling, it is not sufficient to initiate it, as indicated by mutation studies for the VEG domain (Val663-Glu664-Gly665). Instead, it is the adoption of a specific conformation that triggers signaling in receptor tyrosine kinases (Burke et al., 1997; Garrett et al., 2002; Ogiso et al., 2002).
Recent high-resolution structural studies have provided data that are beginning to elucidate this mechanism. Structures of unliganded ErbB3 and EGFR revealed a β-hairpin loop that extends from domain II to domain IV, preventing close contact between domains I and III. Binding of EGF or TGFα to surfaces on domains I and III of the EGFR bring these two domains close together, and the domain II loop is no longer in contact with domain IV, but rather mediates interreceptor dimerization (Heldin, 1995). Site-directed mutagenesis studies have demonstrated the relevance of the domain II loop in signaling (Schlessinger, 2000). The uniqueness of ErbB2 is confirmed in its structure. The domain II loop is not in contact with domain IV; instead, domains I and III are in contact with each other. This conformation is very similar to the conformation adopted by ErbB1 bound to EGF or TGFα, suggesting that ErbB2 does not have an unliganded conformation. Instead, ErbB2 presents a structure that prevents canonical ligand binding, one that is suitable for the formation of dimers, with preference toward heterodimers because of ionic and steric constraints (Cho et al., 2003; Garrett et al., 2003; Franklin et al., 2004). This constitutively active structure, which does not require different binding surfaces for each ErbB receptor, offers an explanation for the status of ErbB2 as the preferred heterodimer partner. These interactions have been implicated in numerous developmental processes in normal tissues, such as those of the heart, brain, and mammary gland (Lee et al., 1995; Morris et al., 1999) as well as in cancers of the breast, ovary, colon, kidney, gall bladder, stomach, pancreas, and salivary gland, where ErbB2 is aberrantly expressed in some tumors and is regarded as a major contributor to tumor progression (Lee et al., 1995; Alroy and Yarden, 1997; Klapper et al., 2000).
Although ErbB2 has no high-affinity soluble ligand, it has been shown to form a ligand-receptor-like complex with the membrane mucin Muc4. Thus, there is a potential discrepancy between the structural studies and the observations with Muc4. In the present report we provide a resolution to this discrepancy by showing that the interaction of Muc4 with ErbB2 occurs very early after synthesis of the proteins, most likely before ErbB2 adopts its active structure, which precludes the possibility of a ligand interaction. The Muc4–ErbB2 complex is observed to be localized at the cell surface. This complex is involved in a novel mechanism for activation and modulation of ErbB2 phosphorylation and signaling. Muc4 is composed of two noncovalently associated subunits, ASGP-1 and ASGP-2 (Sherblom and Carraway, 1980), which arise from proteolytic processing of a single gene product (Sheng et al., 1990). The mucin subunit ASGP-1 (∼600 kDa; Sherblom et al., 1980) endows the molecule with antiadhesive properties (Komatsu et al., 1997) and contributes to the ability of cells to evade immune recognition (Komatsu et al., 1999). Subunit ASGP-2 (∼120 kDa) (Hull et al., 1990) tethers the complex to the membrane and serves as an intramembrane ligand for ErbB2 via an EGF-like domain (Carraway et al., 1999b). This interaction induces phosphorylation of ErbB2 in the absence of a soluble ligand and potentiates the phosphorylation of the ErbB2–ErbB3 heterodimer in the presence of the ErbB3-soluble ligand neuregulin. The Muc4–ErbB2 complex was first observed in highly metastatic rat ascites 13762 mammary adenocarcinoma cells, where the receptor and several of its associated intracellular signaling proteins appeared constitutively tyrosine phosphorylated (Carraway et al., 1999a). The Muc4–ErbB2 interaction has also been demonstrated in several cell and tissue systems, including normal lactating mammary gland, ascites tumors, isolated rat mammary epithelial cells, and Muc4-transfected MCF-7 breast cancer cells (Carraway et al., 2002). Muc4 is constitutively expressed in many epithelial tissues, where it is apically located and serves mainly a protective function (Carraway et al., 2002). Its expression is tightly regulated in the mammary gland and the female reproductive tract (Price-Schiavi et al., 1998; Idris and Carraway, 2000), and its expression at specific times during epithelial differentiation in certain organs suggests a role in developmental processes (Carraway et al., 2002). In some carcinomas, the regulatory mechanisms controlling Muc4 expression have been suppressed, and Muc4 is highly overexpressed (Price-Schiavi et al., 1998; Singh et al., 2004).
In polarized cells, upon induction of Muc4 overexpression, ErbB2 is translocated from its lateral localization to the apical surface where it is observed in an activated state in complex with Muc4 (Ramsauer et al., 2003). The examination of a number of Muc4-expressing epithelia indicate that ErbB2 localization at the apical surface is not an unusual situation. In general, ErbB2 is apically localized, although not exclusively, in simple epithelia in which Muc4/SMC is present in its membrane form and is apical. Examples include the mammary gland (Price-Schiavi et al., 2005) and uterus and oviduct (Idris et al., 2001). An exception is the lacrimal gland, in which the membrane Muc4/SMC is not predominantly apical, as it is in other simple epithelia. However, in the lacrimal gland ErbB2 is colocalized with the membrane form of Muc4/SMC but not with a soluble form of Muc4/SMC present in secretory granules (Arango et al., 2001). A second exception is the colon, in which Muc4/SMC is predominantly in a soluble form in secretion granules in goblet cells (Rossi et al., 1996). Even though the mechanism by which ErbB2 localization in the presence of Muc4 is changed to the apical surface is not known, this event has crucial implications in cell behavior by positioning the receptor in a cellular location with altered signaling potential. The central issue in this work was the nature of the signaling from the Muc4–ErbB2 complex in the polarized epithelial cells. The ErbB2 carboxy-terminal region contains five tyrosine residues that upon phosphorylation provide potential binding sites for cytoplasmic signaling molecules (Kavanaugh et al., 1995; van der Geer et al., 1995; Dankort et al., 1997) containing Src homology 2 (SH2) (Dankort and Muller, 2000) and/or protein tyrosine binding (PTB) domains (Schlessinger 1994; Riese et al., 1995; Graus-Porta et al., 1997; Dankort et al., 2001). These proteins interact in a sequence-specific manner, thereby initiating signaling cascades conducive to proliferation, transformation, or differentiation. In the present work we show that in the Muc4–ErbB2 complex, the receptor is activated at tyrosines 1139 and 1248 in both polarized and nonpolarized cells. Muc4 in the polarized CACO2 cells activates p38 MAPK, a downstream signaling kinase associated with cell differentiation and stability (Laprise et al., 2002), rather than proliferation, as is the case in myogenesis (Cuenda and Cohen, 1999; Zetser et al., 1999) and neuronal differentiation (Morooka and Nishida, 1998; Iwasaki. et al., 1999). Interestingly, phosphorylation of p38 activates Akt at serine 473, and not at threonine 308, which is associated with cell survival (Horowitz et al., 2004).
MATERIALS AND METHODS
Antibodies and Reagents
To study ErbB2, we used primary antibodies from several sources: five monoclonal antibodies from Lab Vision/Invitrogen (Freemont, CA), namely Neomarkers 2 (clone 9G6.10), 8 (clone E2–4001), 10 (clone L87 + e2-4001), 17 (clone e2-4001 + 3b5), and 18 (clone PN2A). The Neomarkers 18 reacts with the phosphorylated tyrosine at position 1248. Additional antibodies against phosphorylated tyrosines included the following: pY1248 from Upstate (Charlottesville, VA) and pY1139 from Biosource (Camarillo, CA). Antibodies against ErbB2 from DakoCytomation (Carpinteria, CA) and from Calbiochem (San Diego, CA) (anti-c-neu5) were also used. To probe the histidine-tagged ErbB2, we used a monoclonal antibody (mAb) against His-tag from Qiagen (Valencia, CA). The mAb 4F12 against Muc4 used for immunoblots, and the polyclonal antibodies against Muc4 used for immunoprecipitations (anti-ASGP-2 and anti-c-pep) were described previously (Rossi et al., 1996). The inhibitor studies were carried out using AG825 at a final concentration of 3.5 μM and SB203580 at a final concentration of 7 μM, both from Calbiochem. AG825 is a selective inhibitor of ErbB2 autophosphorylation and SB203580 is a highly specific inhibitor of p38 MAPK. ErbB3 was studied with the following antibodies from Lab Vision: Neomarkers 4, 5, 7, and 10, and antibodies G-4 and G16 (Santa Cruz Biotechnology, Santa Cruz, CA). Grb2 was detected with monoclonal antibodies from Transduction Labs/BD Biosciences (Rockville, MD), and from Cell Signaling (Danvers, MA). Erk1/2, p38, JNK, and their phosphorylated forms were analyzed using antibodies from Cell Signaling. All secondary antibodies were affinity-purified and did not cross-react with immunoglobulins of species other than their specific target. Absence of cross-reactivity was determined by agar diffusion assay before colocalization experiments. Peroxidase-conjugated secondary antibodies were obtained from Pierce Biotechnology (Rockford, IL) and Promega (Madison, WI). Alexa Fluor 488 and Texas Red–conjugated secondary antibodies (Molecular Probes, Eugene, OR), as well as FITC and CY3-conjugated secondary antibodies (Jackson ImmunoResearch, West Grove, PA) were used as specified by the manufacturers. Biotinylation of cell surfaces was accomplished using EZ-Link Sulfo-NHSBiotin from Pierce Biotechnology.
Plasmids
An RT-PCR amplimer that contains the coding sequence for the entire extracellular domain of subunit ASGP-2 of Muc4 was made by oligo dT-primed reverse transcription of MAT-C1 13762 ascites cell RNA. A BamHI restriction site was synthesized into the 5′ PCR primer, and an in-frame stop codon was engineered into the 3′ end. The resulting amplimer was cloned into the PCRII vector with the TA cloning kit (Invitrogen). The ASGP-2 coding sequence was excised from the PCRII plasmid with the restriction endonucleases BamHI (from the PCR primer) and NotI (vector) and directionally cloned into the Acgp67 baculovirus transfer vector (PharMingen, San Diego, CA) to generate the ASGP-2-ECD-gp67 clone. The insert was cloned in frame with the sequence for the viral gp67 signal peptide to allow secretion of the extracellular domain of ASGP-2 (ASGP-2 ECD). The ErbB2 ECD plasmid was a kind gift from Dr. Kermit Carraway III (University of California at Davis). The Muc4 plasmid composed of subunit ASGP-2 and five repeats of subunit ASGP-1 was generated from cloning the five repeats of subunit ASGP-1 into the pcDNAIII vector containing the ASGP-2 subunit.
Insect Cell Cultures and the Isolation and Characterization of Muc4–ErbB2 Complex
For the Baculovirus expression vector system BVES, High-5 insect cells were obtained from PharMingen/BD Biosciences (San Diego, CA). They were grown in serum-free media at a density of 2 × 106 cells/ml and seeded in 75-cm2 flasks from Corning (Acton, MA). The High-5 cells were infected with high titer viral stocks (MOI of 5) of the extracellular domain of HIS-tagged ErbB2 (soluble), the extracellular domain of Muc4 (subunit ASGP-2, also soluble), or coinfected with both together. After a 1-h incubation with the viral stocks, the medium was changed, and the cells were incubated at 27°C until time of maximal expression. Medium containing the expressed Muc4–ErbB2HIS complex from the coinfected cells was collected and mixed 1:1 with 2× RIPA buffer (50 mM Tris-HCl, pH 7.4, 1% Nonidet P-40, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM EDTA, 1 mM phenylmethyl sulfonyl fluoride, 1 μg/ml each aprotinin, leupeptin, and pepstatin, 1 mM Na3VO4, and 1 mM NaF), and clarified by centrifugation. The complex, present in the clarified medium, was purified by His-Tag affinity chromatography (Invitrogen ProBond Purification system). The Muc4–ErbB2HIS complex was eluted by an imidazole step gradient (50, 200, 350, and 500 mM). The fractions obtained were analyzed by SDS-PAGE and immunoblotting, and those containing the Muc4–ErbB2HIS complex were immunoprecipitated with an antibody against Muc4 to eliminate the uncomplexed ErbB2HIS. The immunoprecipitates were biotinylated using EZ-Link Sulfo-NHS-Biotin from Pierce Biotechnology and analyzed via SDS-PAGE, and subsequent probing with streptavidin conjugated to horseradish peroxidase (Pierce Biotechnology).
CACO-2 and A375 Cell Cultures
Colon adenocarcinoma CACO-2 cells were obtained from the American Type Culture Collection (Manassas, VA). They were maintained in DMEM-F12 supplemented with 10% fetal bovine serum (FBS) and 1 mM sodium pyruvate (Invitrogen) at 5% CO2 and 37°C. The cell stocks were kept in 25-cm2 tissue culture flasks and were collected after dissociation with 0.25% trypsin, 2 mM EDTA for 15 min. For immunofluorescence experiments, the cells were plated on 12-mm round coverslips (Fisher Scientific, Pittsburgh, PA) or on 6-mm Transwell-ClearTM filters (Corning, Costar, Cambridge, MA) at high density (≈5 × 104 cells/cm2) in order to obtain confluence in 2–3 d. Forty-eight hours before immnuofluorescence or immunoblotting experiments, CACO-2 cells at 70% confluence were transiently transfected with Muc4 or with the empty vector tagged with GFP as a control, using X-Gene (Fermentas, Hanover, MD) according to the manufacturer’s instructions. Cells for inhibition assays were supplemented with 3.5 μM AG825 or 7 μM SB 203580 before transfection. Osmotic stress control experiments were carried out by treating the cells with 400 mM sorbitol for 10 min before cell lysis (Schafer et al., 1998). For biotinylation experiments the cells were plated on 24-mm Transwell-ClearTM filters (Corning, Costar). Muc4 stably transfected A375 cells under the tetracycline inducible on/off expression system were maintained in DMEM with 4 mM l-glutamine adjusted to contain 90% 1.5 g/l sodium bicarbonate and 4.5 g/l glucose and 10% FBS at 5% CO2 and 37°C. The A375 cells were cultured in the same manner as the CACO-2 cells.
Pulse Chase Analyses
High-5 cells, 2.5 × 106, were seeded on 60-mm plates, infected with high-titer viral stocks (MOI 5–10), and incubated for 24–48 h at 27°C. The medium was changed to cysteine/methionine free, and the cells were incubated for 30 min. EXPRE35S35S (50 μCi; Perkin-Elmer Cetus, Norwalk, CT) was added to each plate for 10 min, then the medium was changed to remove label, and the cells and/or the medium was collected at different time points in RIPA buffer (50 mM Tris-HCl, pH 7.4, 1% Nonidet P-40, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM EDTA, 1 mM phenylmethyl sulfonyl fluoride, 1 μg/ml each aprotinin, leupeptin, and pepstatin, 1 mM Na3VO4, and 1 mM NaF). The complex was isolated as described above via affinity chromatography and immunoprecipitation and analyzed by SDS-PAGE and fluorography. Pulse labeling was performed on A375 cells in the same manner as described above, after turning on Muc4 expression by the removal of tetracycline from the medium.
Immunofluorescence
CACO-2 cells grown to confluence on 12-mm round coverslips (Fisher Scientific), or on 6-mm Transwell-ClearTM filters (Corning, Costar), were processed for immunofluorescence studies 48 h after transient transfection with Muc4. The cells were fixed with 4% paraformaldehyde for 20 min at room temperature. After rinsing, the cells were permeabilized with 0.2% Triton X-100 for 5 min or 0.1% saponin throughout the procedure. Permeabilization was used in all cases, except with anti-ErbB2 Neomarkers 2 (Labvision). The permeabilization was followed by rinsing and quenching of the aldehyde groups in 50 mM NH4Cl, after which the cells were incubated with primary antibody for 1 h at room temperature. The primary antibody was diluted in 1% BSA; in colocalization experiments, 0.1% immunoglobulin (Ig) G of the same species as the secondary antibody was used instead of 1% BSA for rinsing steps and dilution of the primary antibody. Once this first incubation was completed, the cells were rinsed and then incubated with the secondary antibody conjugated to the fluorescent dye (Alexa Fluor 488 and Texas Red from Molecular Probes, or FITC, CY3, and CY5 from Jackson ImmunoResearch Laboratories) for 1 h at room temperature in the dark. After this, the cells were mounted in 10% polyvinyl alcohol, 30% glycerol, 1% n-propyl gallate, and Slow FadeTM (Molecular Probes) at a dilution of 5:1. The preparations were first observed in a Leitz DM RB microscope (Leica Instruments, GmbH, Wetzlar, Germany) equipped with a Leica Orthomat E microphotography system using a 63× (1.4 NA) infinity-corrected objective. Laser confocal microscopy was performed with a LSM 510 microscope from Zeiss (Carl Zeiss, GmbH, Oberkochen, Germany) equipped with two laser sources and the option of up to three channels. Cell monolayers stained with FITC, Alexa Fluor 488, and Texas Red were analyzed using a 63× oil immersion objective. The images were collected using the LSM 510 software (Carl Zeiss, GmbH), and each confocal section was obtained as the average of four frames.
Laser Scanning Cytometry
The laser scanning cytometer provides the quantification capabilities of flow cytometry to specimens on a solid substrate; it records each cell in space and time so it can be viewed and reanalyzed. Laser scanning cytometry (LSC) was performed in a LSC 2 from Compucyte (Cambridge, MA) equipped with three lasers. Cell monolayers were stained in the same manner as for immunofluorescence, and the secondary antibodies were conjugated with FITC, CY3, CY5 (Jackson Laboratory, Bar Harbor, ME) and DAPI (Vector Laboratories, Burlingame, California). Monolayers were analyzed using a 40× objective; the images were collected using the LSC 2 software. Muc4-transfected CACO-2 cells were seeded as stated above on 12-mm glass coverslips (Fisher), and the complete circular area was scanned for fluorescent cells; as a control, CACO-2 cells were mock-transfected with the empty vector tagged with GFP.
Polarity Assays
The method followed was previously described by Salas et al., (1997). Briefly, cell monolayers grown to confluence on 24-mm Transwell-ClearTM filters (Corning, Costar) were biotinylated on the apical or on the basolateral surface 48 h after transient transfection with Muc4. After rinsing, the surface proteins of the cells were biotinylated at 4°C using a cell membrane–impermeable biotin derivative, sulfo-NHS biotin (Pierce Biotechnology). For proteins on the apical surface, the monolayer was exposed to the biotinylation agent for 15 min, whereas for the basolateral surface it was exposed for 40 min. After standard rinsing and quenching of the aldehyde groups in 50 mM NH4Cl, the cells were lysed with RIPA Buffer (50 mM Tris-HCl, pH 7.4, 1% NP-40, 0.25% Na-deoxycholate, 150 mM NaCl, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 1 μg/ml each of aprotinin, leupeptin, and pepstatin, 1 mM Na3VO4, and 1 mM NaF). The cells were gently scraped from the filter with a rubber policeman, sonicated on ice for 30 s, and centrifuged at 15,000 × g for 10 min at 4°C. Biotinylated proteins were affinity-purified in batch mode overnight at 4°C with streptavidin-conjugated agarose beads (Pierce). The biotinylated proteins were eluted from the beads by 1 mM Tris, 2% SDS and subsequently were subjected to TCA precipitation. After rinsing the pellet with acetone, it was resuspended in 1 mM Tris buffer, pH 7.
SDS-PAGE and Immunoblots
The preparations of biotinylated proteins were obtained as described above. Unbiotinylated cell preparations used as negative controls or as positive controls for primary antibodies were processed in the same way minus the biotinylation steps. The samples were run in SDS-PAGE and then blotted onto nitrocellulose sheets (Ramsauer et al., 2003). The signal of primary monoclonal or polyclonal antibodies was detected using secondary affinity-purified goat anti-mouse or anti-rabbit immunoglobulins coupled to peroxidase and a chemiluminescent system (Pierce) and exposed on x-ray film (Eastman Kodak, Rochester, NY). The intensity of the bands was estimated by digitizing the image (Scion Image, Frederick, MD) from x-ray film. After subtracting the background, all band intensities were compared against a control.
RESULTS
Expression, Isolation, and Stoichiometry of Muc4–ErbB2 Complex from Insect Cells
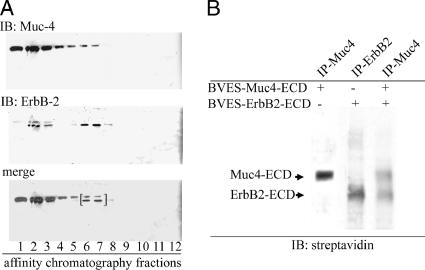
ErbB2 is frequently stated to be an “orphan” or “ligandless” receptor (Alroy and Yarden, 1997). In fact, crystallographic analyses indicate that the putative ligand-binding site for ErbB2 is in a “locked” conformation, which would exclude ligand binding (Cho et al., 2003; Garrett et al., 2003; Franklin et al., 2004). Despite these observations, we have shown that the membrane mucin Muc4 forms tight complexes with ErbB2 in multiple cell types (Carraway et al., 2002) and presented strong evidence that the complex formation requires the EGF-like domain 1 of the Muc4 (Carraway et al., 1999b), consistent with a ligand-receptor complex. To facilitate isolation of complex, we expressed a His-tagged extracellular domain of ErbB2 in the insect cells with the extracellular domain of Muc4. As previously reported, these cells secrete a soluble complex of Muc4 and ErbB2. The complex was isolated by binding to the His-tag affinity column and elution with an imidazole gradient, under nondenaturing conditions, indicating that the interaction between the two molecules was stable enough for copurification. The complex was released at an imidazole concentration of 250 μM; these fractions are bracketed in Figure 1A. Fractions containing complex were then immunoprecipitated with anti-Muc4 to remove unbound ErbB2. To confirm the interaction of Muc4 and ErbB2, complex was biotinylated with EZLink Sulfo-NHS-Biotin and detected with streptavidin. Extracellular domains of ErbB2 and Muc4 were also expressed separately in insect cells and biotinylated to provide standards for comparison (Figure 1B). The identity of the bands was confirmed by using antibodies against Muc4 and ErbB2.
Figure 1.
Isolation of soluble Muc4–ErbB2 complex from insect cells and confirmation of complex formation and determination of its stoichiometry by biotinylation. The extracellular domains of Muc4 (≈120 kDa) and histidine-tagged ErbB2 (≈90 kDa) were coexpressed in High-5 insect cells as described in Materials and Methods. Insect cell lysates were fractionated by affinity chromatography to isolate His-tagged proteins. (A) Immunoblot analysis to locate the fractions containing the Muc4–ErbB2HIS complex using a mAb 4f12 against Muc4 (Rossi et al., 1996) and anti-ErbB2 Neomarkers 10. Fractions 6 and 7 containing complex were combined, immunoprecipitated with anti-Muc4, and biotinylated with EZ-Link Sulfo-NHS-Biotin. Bracket indicates fractions used for anti-Muc4 immunoprecipitation and biotinylation. (B) Blot analyses of biotinylated, soluble Muc4–ErbB2 complex from the BEVS, using horseradish peroxidase–conjugated streptavidin. Soluble Muc4 and ErbB2 were expressed separately and analyzed in parallel to the complex.
Timing of Formation and Stoichiometry by Labeling of Muc4–ErbB2 Complex
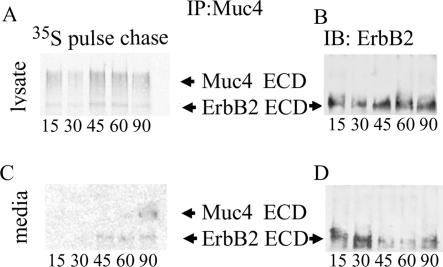
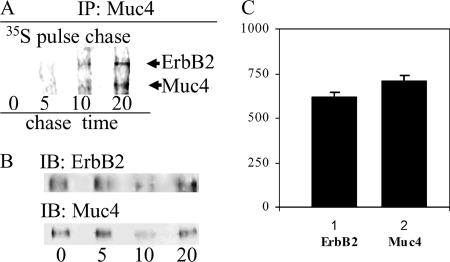
Because the Muc4 and ErbB2 only form complex when the two are made in the same cell (Carraway et al., 1999b), one resolution to the question of how Muc4 forms a complex with ErbB2 is that the Muc4–ErbB2 complex forms before ErbB2 assumes its mature, “locked” conformation. To determine when the Muc4–ErbB2 complex is formed in the cell, we analyzed the timing of complex formation in High-5 insect cells that were coinfected to coexpress the extracellular domains of histidine-tagged ErbB2 and Muc4 by pulse-chase analysis. Cells were labeled with 35S-methionine/cysteine for timing the expression of the proteins. Complex was isolated at timed intervals after labeling by binding to and eluting from the His-tag affinity column, as shown in Figure 1A. The Muc4–ErbB2 complex was isolated from eluted material by immunoprecipitation using a polyclonal antibody against Muc4. The immunoprecipitated samples were then analyzed by SDS PAGE and fluorography (Figure 2, A and C) or anti-ErbB2 immunoblotting (Figure 2, B and D). The 35S fluorography showing the appearance of labeled ErbB2 revealed that the complex was formed within the first 15 min after synthesis of the molecules (Figure 2A). Analyses of the secretion of the two components indicated that the time required for these molecules to reach the cell surface is 45–60 min (Figure 2C). The presence of the ErbB2 in the Muc4-immunoprecipitated complex was confirmed by immunoblotting with antibodies against ErbB2 (Figure 2D) and Muc4, and an antihistidine antibody to confirm purification of the complex after affinity chromatography (unpublished data). Complex formation in most cells involves the membrane forms of Muc4 and ErbB2 (Carraway et al., 2002). To confirm the early formation of the membrane complex, the same experiment was performed in A375 human melanoma cells, which express membrane ErbB2 and have been transfected with membrane Muc4 (Komatsu et al., 1997). The complex forms within the first 5 to 10 min after synthesis of the molecules (Figure 3A). The identity of the bands was confirmed using antibodies separately against Muc4 and ErbB2 (Figure 3B). The intensity of the bands was estimated by digitizing the image (Scion Image) from x-ray film. After subtracting the background, all band intensities were normalized against a control, the ratio of the content of methionines and cysteines in Muc4 and ErbB2. This analysis indicates that the stoichiometry of the complex is 1:1 (Figure 3C). These results are consistent with crystallographic studies that show that ErbB ligands bind to a single receptor subunit rather than cross-linking two subunits (Ogiso et al., 2002). These combined results indicate that the Muc4–ErbB2 complex forms intracellularly and are consistent with a mechanism by which Muc4 associates with ErbB2 in a ligand-receptor complex before ErbB2 has assumed its mature, “locked” conformation.
Figure 2.
Timing of formation and secretion of soluble Muc4–ErbB2 complex in insect cells by 35S labeling. Muc4- and His-tagged ErbB2 extracellular domains were coexpressed in High-5 insect cells, which were pulse-labeled 10 min with 35S amino acids and chased for periods of 15–120 min. Muc4–ErbB2 complex was isolated from cell lysates by His-tag affinity chromatography followed by Muc4 immunoprecipitation and subjected to SDS-PAGE and fluorography (A). Complex formation is observed within 15 min. by label in the ErbB2 band of the Muc4 immunoprecipitate. The presence and location of the ErbB2 on the fluorograph were confirmed by immunoblot (B), using mAb Neomarkers 10. To determine the time for secretion of complex, complex was isolated from High-5 media using the same affinity chromatography immunoprecipitation method, followed by SDS-PAGE and fluorography. Labeled ErbB2 was detected in the media after 45–60 min (C). The presence and location of the ErbB2 on the fluorograph were confirmed by immunoblot (D).
Figure 3.
Time of formation and stoichiometry of the Muc4–ErbB2 complex in A375 cells by 35S labeling. (A) Tetracycline-controlled expression of Muc4 in A375 cells was initiated by the removal of tetracycline, and the cells were pulse-labeled 10 min with 35S amino acids and chased for periods of 0–20 min. (B) Muc4–ErbB2 complex was immunoprecipitated with anti-Muc4. The presence and location of ErbB2 on the fluorography were confirmed by immunoblot using mAb Neomarkers 10. The presence and location of Muc4 were confirmed by immunoblot (bottom left panel) after stripping of the membrane. (C) Graph of the relative intensities of the bands detected by the 35S fluorography after subtraction of the background and normalization by the ratio of the content of cysteines and methionines in both molecules. This graph indicates a stoichiometry of 1:1 for the Muc4–ErbB2 complex.
Segregation of ErbB2 and ErbB3 in Polarized CACO-2 Cells
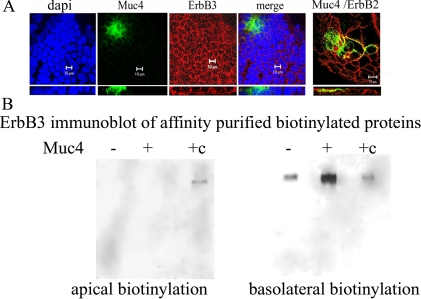
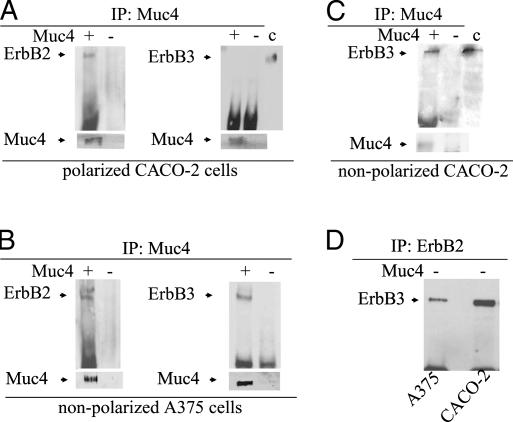
Our previous studies have indicated that the Muc4–ErbB2 complex can interact with ErbB3 at the cell surfaces of nonpolarized tumor cells when the cells are stimulated with the ErbB3 ligand neuregulin. In polarized CACO-2 epithelial cells, the Muc4–ErbB2 complex is found at the apical surfaces of the cells. These results raise the question of whether Muc4 can regulate ErbB signaling in polarized epithelial cells by segregating the ErbB2 and ErbB3 receptors. To address this question, we first examined the localization of ErbB3 in the polarized CACO-2 epithelial cells. By confocal immunofluorescence ErbB3 is found mainly in the lateral surfaces of the epithelial cells (Figure 4A), in a location similar to that of ErbB2 and cadherin in these cells, which we observed previously (Ramsauer et al., 2003); it is also found in the cytoplasm. In contrast to ErbB2, the location of ErbB3 is not changed in cells expressing Muc4 (Figure 4A). These observations were confirmed by biotinylation experiments. CACO-2 cells, whether expressing Muc4 or not expressing Muc4, exhibited ErbB3 in a basolateral location (Figure 4B). In contrast, we previously showed that expression of Muc4 in these cells results in a relocation of ErbB2 from the lateral to the apical surface (Ramsauer et al., 2003).
Figure 4.
Basolateral localization of ErbB3 in Muc4-transfected and untransfected, polarized CACO-2 cells. (A) X-y and x-z localization of ErbB3 by confocal immunofluorescence in a confluent monolayer of CACO-2 cells. First panel, nuclear staining with DAPI; second panel, Muc4-expressing cells (green) showing apical localization; third panel, staining with monoclonal anti-ErbB3 from Labvision/Invitrogen (red) indicates mainly a basolateral and some cytoplasmic staining for all cells; fourth panel, a merged image of the previous three panels; and fifth panel, an x-y and x-z merge of Muc4-expressing cells (green) and ErbB2 (red) detected with polyclonal anti ErbB2 from DAKO (Carpinteria, CA). In Muc4-expressing cells, ErbB2 is colocalized at the apical surface with Muc4, as we had demonstrated previously (Ramsauer et al., 2003). Confocal sections are shown in the x-y plane with the apical side up, and in the x-z plane; bar, 10 μm. (B) ErbB3 localization in Muc4-transfected and untransfected polarized CACO-2 cells by directed biotinylation. CACO-2 cells were biotinylated from the apical or basolateral side and lysed; biotinylated proteins were precipitated with streptavidin. ErbB3 was detected in the precipitates by SDS-PAGE and immunoblotting with mAb Neo markers 7 against ErbB3. c, control for locating the ErbB3 band.
To determine the effect of ErbB3 localization on complex formation between the Muc4–ErbB2 complex and ErbB3, we immunoprecipitated Muc4 from cell lysates of Muc4-expressing, polarized CACO-2 cells with two different antibodies against Muc4, and analyzed the immunoprecipitates by immunoblotting for ErbB2 and ErbB3. ErbB2, but not ErbB3, was found in the Muc4 immunoprecipitates (Figure 5A). Neither ErbB2 nor ErbB3 was found in Muc4 immunoprecipitates in CACO-2 cells not transfected with Muc4 (Figure 5). In contrast, both ErbB2 and ErbB3 were immunoprecipitated with anti-Muc4 from a nonpolarized cell, A375 melanoma cells, expressing Muc4 (Figure 5B).This Muc4–ErbB2–ErbB3 complex was also observed in nonpolarized CACO-2 cells (Figure 5C), which were lysed for immunoprecipitation before they attained a polarized status. They were plated at high density, transfected the next day, and lysed 48 h later. These results indicate that Muc4 can form a complex with ErbB2 and ErbB3 even without the addition of the ErbB3 ligand neuregulin, but that this complex formation is possible only in nonpolarized cells. In polarized cells, the Muc4–ErbB2 complex is segregated from ErbB3. Muc4 is not required for ErbB2–ErbB3 complex formation, which can be observed in both CACO-2 and A375 cells that do not express Muc4 (Figure 5D).
Figure 5.
Coimmunoprecipitation of ErbB3 with the Muc4–ErbB2 complex in nonpolarized, but not polarized, cells. (A and B) Lysates from Muc4- and mock-transfected polarized CACO-2 cells and nonpolarized A375 tumor cells were immunoprecipitated with anti-Muc4. The immunoprecipitates were immunoblotted with anti-ErbB2 or anti-ErbB3. The results indicate that ErbB3 is associated with the Muc4–ErbB2 complex in the nonpolarized cells, but not the polarized cells. (C) Lysates from Muc4- and mock-transfected recently plated, nonpolarized CACO-2 cells were immunoprecipitated with anti-Muc4. The immunoprecipitates were analyzed by SDS-PAGE and immunoblotted with anti-ErbB3. The results indicate that ErbB3 is associated with the Muc4–ErbB2 complex in the nonpolarized CACO-2 cells. (D) Lysates from CACO-2 and A-375 cells not expressing Muc4 were immunoprecipitated with anti-ErbB2. The immunoprecipitates were immunoblotted with anti-ErbB3, indicating an interaction between ErbB2 and ErbB3 in these cells in the absence of Muc4.
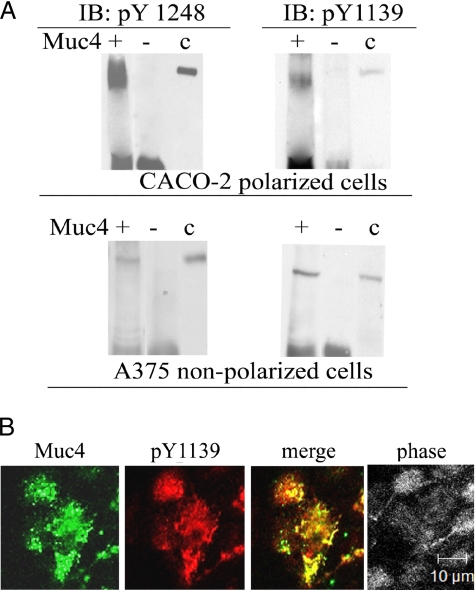
ErbB2 Present in the Muc4–ErbB2 Complex Is Phosphorylated on Tyrosines 1139 and 1248 in CACO-2 and A375 Cells
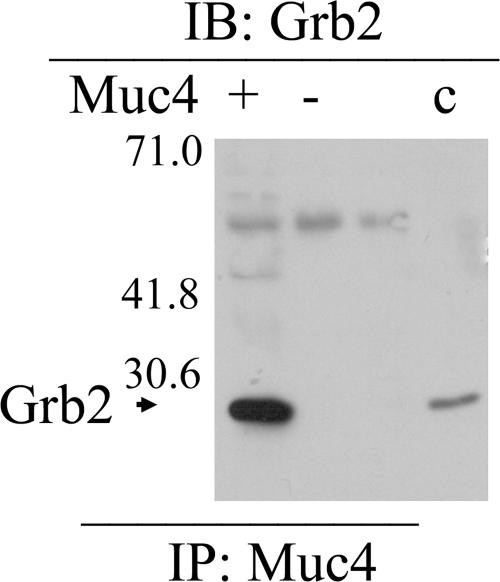
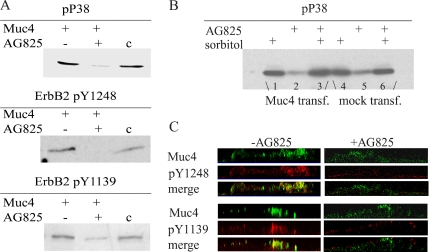
ErbB2 has five tyrosines in its C-terminal regulatory region, which are important to cell signaling. To determine whether any of these sites is phosphorylated in the CACO-2 polarized epithelial cells, we used commercial antibodies against the phosphorylated tyrosines. A375 cells were also examined, because we had shown previously that phosphorylation of one of the sites (Tyr 1248) is induced by Muc4 transfection in these cells (Jepson et al., 2002). As shown in Figure 6A, immunoblot analyses of Muc4-transfected CACO-2 and A375 cells indicated Muc4 induction of phosphorylation of two of the sites, tyrosines 1139 and 1248, in both cell types. No phosphorylation of either tyrosine was observed in nontransfected cells. The role of Muc4 in phosphorylation of Tyr 1139 was supported by the apical colocalization of ErbB2 phospho-Tyr 1139 with Muc4, demonstrated by confocal immunofluorescence, as shown in Figure 6B. Previous studies have indicated that the Tyr 1139 phosphorylation site binds Grb2, the adaptor protein linked to downstream signaling from receptor tyrosine kinases. As shown in Figure 7, immunoblots of anti-Muc4 immunoprecipitates from Muc4-transfected CACO-2 were strongly positive for Grb2, supporting the data for phosphorylation of Tyr 1139.
Figure 6.
Phosphorylation of ErbB2 in the Muc4–ErbB2 complex in CACO-2 and A375 cells. (A) Lysates from Muc4-transfected CACO-2 and A375 cells were immunoprecipitated with anti-Muc4. The precipitates were then immunoblotted using antibodies against activated ErbB2, phosphorylated on tyrosines 1248 and 1139, showing that ErbB2 from both cell types was phosphorylated on both sites. (B) Confocal immunofluorescence showing colocalization of ErbB2 activated at tyrosine 1139 with polyclonal anti-ErbB2 pY1139 and Muc4 in a confluent monolayer of CACO-2 cells. Confocal sections are shown with the apical side up, x-y plane; bar, 10 μm.
Figure 7.
Grb2 associated with Muc4–ErbB2 complex in polarized cells. Immunoblot analyses were performed on Muc4–ErbB2 complex precipitated with anti-Muc4 from polarized CACO-2 using an mAb against Grb2. Note the Ig band in both + and − lanes.
Activation of p38 MAPK in Polarized Cells with Muc4–ErbB2 Complex
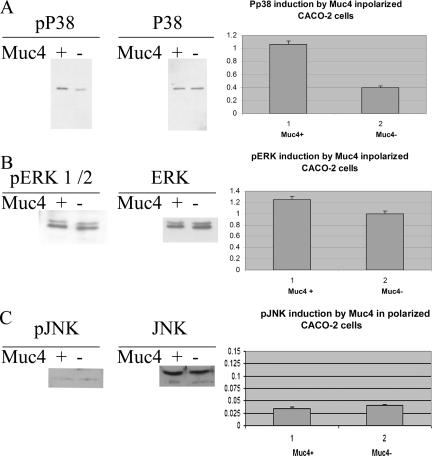
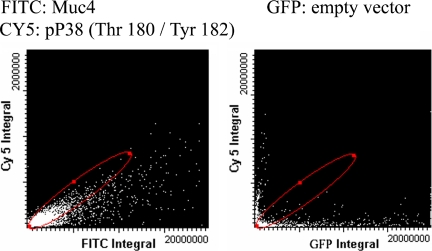
Among the primary contributors to ErbB receptor signaling are the MAPKs. There are three main MAPK cascades in mammalian cells: the extracellular signal-regulated kinases (Erk1/2), the stress-activated protein kinases/c-Jun N-terminal kinases (JNK also known as SAPK), and a second stress-activated protein kinase, the p38 group. To determine whether any of these are activated by Muc4 expression in the polarized CACO-2 cells, we analyzed cell lysates of Muc4-transfected and mock-transfected cells with specific anti-MAPK antibodies for each of the three classes. Immunoblots with the anti phospho-MAPK antibodies indicated a robust phosphorylation of p38 MAPK and no increased phosphorylation of Erk or JNK (Figure 8). Because Muc4 was expressed in these cells by transient transfection, only a fraction of the cells is transfected. This approach raises the question whether the increased MAPK phosphorylation results from the Muc4 expression in the transfected cells. This question was addressed by LSC. Figure 9 shows an LSC scan of a fixed monolayer of polarized CACO-2 cells that had been transfected with Muc4 or with an empty vector tagged with GFP before being treated with immunofluorescent probes against activated p38 (Pp38) and total p38. The cells were analyzed using 405-, 488-, and 633-nm laser light on the LSC. The scans were gated using DAPI, to limit the fluorescent events to those observed in cells only; fluorescent data displayed was determined by cell radius to be single events. The scan displays a positive r of 0.95 between Muc4 expression and p38 activation.
Figure 8.
Analysis of phosphorylation of p38, ERK, and JNK induced by Muc4 expression in polarized CACO-2 cells. (A) Immunoblot analyses of the phosphorylated and total forms of p38 of CACO-2 cell lysates of Muc4-transfected cells. Plot of the induction of phosphorylated p38 was based on the immunoblot data. Control cells were transfected with empty vector and analyzed in the same manner. (B) Immunoblot analyses of the phosphorylated and total forms of ERK 1/2 of CACO-2 cell lysates of Muc4-transfected cells. Plot of the increase in activated ERK levels was based on the immunoblot data. Control cells were transfected with empty vector and analyzed in the same manner. (C) Immunoblot analyses of the phosphorylated and total forms of JNK of CACO-2 cell lysates of Muc4-transfected cells. Plot of the activation of JNK was based on the immunoblot data. Control cells were transfected with empty vector and analyzed in the same manner.
Figure 9.
Analysis of the induction of p38 MAPK phosphorylation by Muc4 expression in polarized CACO-2 cells by LSC. Bivariate analysis of the phosphorylated and total forms of p38 and Muc4 in CACO-2 cells by LSC was performed using FITC and Cy3. CACO-2 cell monolayers were fixed and processed for immunofluorescence and probed with antibodies against Muc4 and the activated and total forms of p38. Control cells were transfected with the empty vector tagged with GFP and analyzed in the same manner.
P38 Activation via ErbB2 Phosphorylation
AG825 is a potent selective inhibitor of ErbB2 autophosphorylation (IC50 = 0.35 μM relative to ErbB1 IC50 = 19 μM). To determine whether the robust Muc4-dependent activation of MAPK p38 is mediated by the activation of ErbB2 in the polarized CACO-2 cells, we analyzed cell lysates of Muc4-transfected cells with a specific antibody against the phosphorylated form of p38 in cells treated with or without AG825. As shown in Figure 10A, immunoblots with the anti-phospho p38 MAPK antibody indicate a pronounced decrease in the levels of phosphorylation of p38 MAPK in the presence of AG825. These results indicate that the activation of this MAPK is induced by the phosphorylation of ErbB2 initiated by complex formation with Muc4. As a control, CACO-2 cells were subjected to osmotic stress as indicated above and analyzed by immunoblot of activated p38. Muc4 or mock-transfected cells were treated with ErbB2 phosphorylation inhibitor AG825 and or sorbitol.
Figure 10.
Analysis of phosphorylation of p38 and ErbB2 at tyrosines 1248 and 1139, in the presence of the ErbB2 phosphorylation inhibitor AG825. (A) Immunoblot analyses of the phosphorylated forms of p38, ErbB2 pY1248, and pY1139 of CACO-2 cell lysates of Muc4-transfected cells treated with or without ErbB2 inhibitor AG825. A375 cell lysates were used as control. (B) Immunoblot of the activated form of p38 of CACO-2 lysates of Muc4 (lanes 1–3) and mock-transfected cells (lanes 4–6), under osmotic stress by treatment with 400 mM sorbitol. (C) X-z plane by confocal immunofluorescence of ErbB2 pY1248 and pY1139 in a confluent monolayer of CACO-2 cells in the presence and in the absence of AG825. First panel, Muc4 (green); second panel, staining with polyclonal anti-pY1248 or anti-pY1139; and third panel, merge of panels one and two.
As shown in Figure 10B, immunoblots indicate that the robust activation of p38 observed in Muc4-transfected cells is not decreased by AG825 in the presence of sorbitol. In the mock-transfected cells, the basal level of activated p38 remains unchanged in the presence of AG825 and is not affected by the presence of sorbitol. To confirm the inhibition of phosphorylation of ErbB2 by AG825, treated and untreated cell monolayers were analyzed by immunofluorescence with antibodies against tyrosines 1248 and 1139 (Figure 10C).
Akt Is Activated by p38 in Cells Expressing Muc4
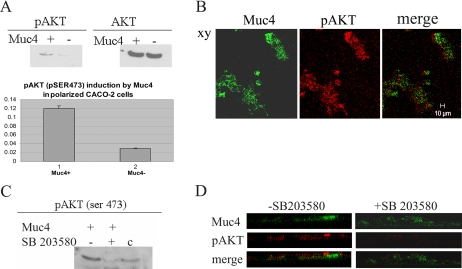
Recent studies have shown that the kinase Akt can be activated via phosphorylated p38 (Laprise et al., 2002; Cabane et al., 2004). To determine whether Akt is activated by Muc4 expression in the polarized CACO-2 cells, we analyzed cell lysates of Muc4- and mock-transfected cells with antibodies against the activated and total forms of Akt. As shown in Figure 11A, immunoblots with the anti-phospho-Akt indicate a strong activation of Akt at serine 473. Apical localization of activated Akt was observed by immunofluorescence, as shown in Figure 11B, supporting a role for Muc4 in the Akt activation. SB203580 is a highly specific inhibitor of p38MAPK. To determine whether the activation of AKT at serine 473 was a consequence of the activation of p38 in the polarized CACO-2 cells, lysates of Muc4-transfected cells were analyzed with antibodies against Akt phosphoserine 473 in cells treated with or without SB203580. In the presence of this p38 inhibitor, the levels of activated AKT are greatly decreased, as indicated on the immunoblot shown in Figure 11C. This result is confirmed via immunofluorescence with an antibody against Akt activated at serine 473 in the absence and in the presence of the inhibitor SB203580, as shown in Figure 11D.
Figure 11.
Analysis of the induction of Akt phosphorylation at serine 473 in Muc4-expressing CACO-2 cells via p38 activation. (A) Immunoblot analyses of the phosphorylated and total forms of Akt of CACO-2 cell lysates in Muc4-transfected cells. Plot of the induction of phosphorylated Akt at serine 473 was based on the immunoblot data. Control cells were transfected with empty vector and analyzed in the same manner. (B) Confocal immunofluorescence localization of pAkt in a confluent monolayer of CACO-2 cells. First panel, Muc4-expressing cells (green); second panel, staining with monoclonal anti-pAkt on serine 473 shows apical staining for cells (red). Note that only cells expressing Muc4 have activated Akt. Confocal sections are shown with the apical side up, x-y plane; bar 10 μm. (C) Immunoblot analyses of the phosphorylated Akt at serine 473 of CACO-2 cell lysates of Muc4-transfected cells treated with or without p38 inhibitor SB203580. A375 cell lysates were used as control. (D) X-z plane by confocal immunofluorescence of pAkt in a confluent monolayer of CACO-2 cells in the presence and in the absence of SB203580. First panel, Muc4 (green); second panel, staining with polyclonal anti-pAkt; and third panel, merge of panels one and two.
DISCUSSION
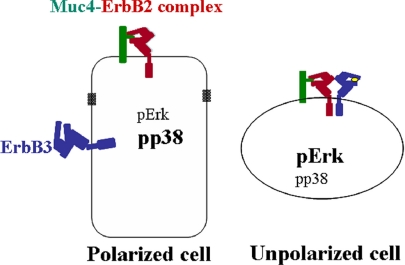
The functions of ErbB receptors are determined by their activation by ligands and locations within cells. The heterodimerization mechanism of activating the ErbBs is particularly important, because ErbB2 has no soluble ligand, and ErbB3 has no kinase activity (Guy et al., 1994). Recent crystallographic studies have explained the failure to find an ErbB2 ligand by showing that the receptor is in a “locked” conformation that prevents ligand binding. An interesting facet of ErbB2 biology is its implication in both differentiated and proliferative cells. Such behaviors suggest a mechanism for regulating ErbB2 that is dependent on cell context. The current studies indicate that Muc4 may provide such a mechanism by acting as a ligand for the ErbB2 in polarized epithelial cells. First, Muc4 forms a specific complex with ErbB2 shortly after synthesis of the two proteins. We have previously shown that the complex formation requires the EGF1 domain of the Muc4 (Carraway et al., 1999b). Moreover, as shown in this study and previous studies (Jepson et al., 2002; Ramsauer et al., 2003), the complex formation leads to specific phosphorylation of the ErbB2. We suggest that these observations provide the basis for a mechanism by which Muc4 can act as an unorthodox, intramembrane ligand for the receptor. Second, formation of the Muc4–ErbB2 complex in CACO-2–polarized epithelial cells results in localization of the ErbB2 to the apical surface of these cells without altering their polarization (Ramsauer et al., 2003). In contrast, ErbB2 is found in lateral surfaces, colocalized with cadherin junctions, in polarized cells not expressing Muc4. Moreover, ErbB3 is not relocalized by Muc4 from its lateral location in the polarized cells. Thus, Muc4 is able to segregate ErbB2 from ErbB3 (Figure 12) and prevent it from acting as a coreceptor in the polarized cells. In this case Muc4 is acting as an unorthodox ligand. Third, formation of the apical Muc4–ErbB2 complex in CACO-2 cells promotes phosphorylation of p38 MAPK and subsequent activation of Akt. In nonpolarized A375 cells, p38 is not activated, and Akt is phosphorylated via ErbB3 in the presence of neuregulin (Jepson et al., 2002),indicating a different pathway of Akt activation in the polarized versus the nonpolarized cell. Previous studies have indicated the p38 activation is associated with a differentiated cell phenotype in some cell types (Houde et al., 2001).
Figure 12.
Model for localization of Muc4, ErbB2, and ErbB3 in polarized and nonpolarized cells and their effects on MAPK signaling. The unpolarized cell shows the “quadcomplex” formed by neuregulin activation of ErbB3 to promote its association with the Muc4–ErbB2 complex (Carraway et al., 1999b, 2002). Note differences in MAPK phosphorylated forms between the polarized and unpolarized cells.
This kinase pathway, originally associated with stress response and apoptosis, has recently been implicated in the regulation of differentiation mechanisms and cell cycle control. Its pathways of activation are not exclusive to environmental stress and proinflammatory cytokines, but also include other activator proteins, such as insulin and ErbB2. Extensive studies on osmotic stress in CACO-2 cells indicate that this is indeed a proinflammatory event that involves the increase of production of cytokines (Nemeth et al., 2002, Hubert et al., 2004) via the canonical p38 MAPK stress pathway. This is not the case of growth factor receptor–induced cell differentiation or proliferation via p38, where the evidence presented so far indicates that these events are mediated through a different signaling cascade although its components have not been completely elucidated (Cuenda and Cohen, 1999; Maher, 1999; Zetser et al., 1999). Specifically in CACO-2 cells, activation of p38 is required for differentiation mechanisms (Houde et al., 2001; Daniel et al., 2004), with subsequent activation of Akt on serine 473 (Laprise et al., 2002). Growth factor–induced activation of Akt via p38 has been recognized as a prosurvival pathway in lung cells (Horowitz et al., 2004). Our results indicate that in polarized CACO-2 cells expressing Muc4, p38 is activated through ErbB2 and is involved in functions of cell survival and maintenance via the activation of Akt on serine 473. Further studies are necessary to determine how the activated Akt influences the behavior of the polarized cells.
These studies suggest that Muc4 is associated with the differentiated phenotype of polarized epithelia through its ability to influence the localization and signaling of ErbB2, i.e., its function as an unothordox ligand for ErbB2. Our hypothesis is that Muc4 acts as part of the protection mechanism for epithelia by serving as a sensor of damage (Ramsauer et al., 2003). Damage to the epithelium results in loss of polarization of the cells and elimination of the barrier to segregation of ErbB2 and ErbB3 that was imposed by Muc4 in the polarized cells. Formation of the Muc4–ErbB2–ErbB3 complex resulting from depolarization can change downstream signaling from p38 MAPK associated with differentiation to Erk MAPK associated with proliferation (Figure 12). Similar behavior is expected in transformed, neoplastic cells, which have also lost their polarization.
The key to this mechanism is the ability of Muc4 to act as an unorthodox ligand for ErbB2, moving it to an apical location. Thus, Muc4 must be able to override ErbB2 signals that localize it to the lateral junctions in polarized cells not expressing Muc4. Presumably, Muc4 accomplishes this via a strong signal for apical localization. The nature of this signal is yet unknown. Multiple mechanisms have been proposed to explain apical localization of glycoproteins in polarized epithelial cells, including N- glycosylation, O-glycosylation, and incorporation into lipid rafts facilitated by acylation (Milligan et al., 1995; Gut et al., 1998; Resh, 2004). Perhaps all of these mechanisms can contribute to apical Muc4 localization, because it is highly N- and O-glycosylated and contains cytoplasmic juxtamembrane cysteines that are appropriately located for palmitoylation (Sheng et al., 1992). Further studies are underway to decipher the potential mechanisms for Muc4 apical localization.
ACKNOWLEDGMENTS
We thank Ms. Brigitte Shaw from the Imaging Core at the Diabetes Research Institute, University of Miami School of Medicine, for her assistance with the LSC. This research was supported in part by Grants CA 74072 (K.L.C.) and CA 102248 (V.P.R.) from the National Institutes of Health and by the Sylvester Comprehensive Cancer Center of the University of Miami.
Abbreviations used:
- EGFR
epidermal growth factor receptor
- SMC
sialomucin complex
- ASGP
ascites sialoglycoprotein
- BEVS
baculovirus expression vector system
- LSC
laser scanning cytometer
- RIPA
radioimmune precipitation assay.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05-09-0895) on April 19, 2006.
REFERENCES
- Alroy I., Yarden Y. The ErbB signaling network in embryogenesis and oncogenesis: signal diversification through combinatorial ligand-receptor interactions. FEBS Lett. 1997;410:83–86. doi: 10.1016/s0014-5793(97)00412-2. [DOI] [PubMed] [Google Scholar]
- Arango M. E., Li P., Komatsu M., Montes C., Carraway C.A.C., Carraway K. L. Production and localization of Muc4/sialomucin complex and its receptor tyrosine kinase ErbB2 in the rat lacrimal gland. Invest. Ophthalmol. Vis. Sci. 2001;42:2749–2756. [PubMed] [Google Scholar]
- Bajaj M., Waterfield M. D., Schlessinger J., Taylor W. R., Blundell T. On the tertiary structure of the extracellular domains of the epidermal growth factor and insulin receptors. Biochim. Biophys. Acta. 1987;916:220–226. doi: 10.1016/0167-4838(87)90112-9. [DOI] [PubMed] [Google Scholar]
- Burke C. L., Lemmon M. A., Coren B. A., Engelman D. M., Stern D. F. Di-merization of the p185neu transmembrane domain is necessary but not sufficient for transformation. Oncogene. 1997;14:687–696. doi: 10.1038/sj.onc.1200873. [DOI] [PubMed] [Google Scholar]
- Cabane C., Coldefy A. S., Yeow K., Derijard B. The p38 pathway regulates Akt both at the protein and transcriptional activation levels during myogenesis. Cell Signal. 2004;16:1405–1415. doi: 10.1016/j.cellsig.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Carraway K. L., III, Cantley L. C. A neu acquaintance for erbB3 and erbB4, a role for receptor heterodimerization in growth signaling. Cell. 1994;78:5–8. doi: 10.1016/0092-8674(94)90564-9. [DOI] [PubMed] [Google Scholar]
- Carraway C.A.C., Carvajal M. E., Carraway K. L. Association of the Ras to mitogen-activated protein kinase signal transduction pathway with microfilaments. Evidence for a p185(neu)-containing cell surface signal transduction particle linking the mitogenic pathway to a membrane-microfilament association site. J. Biol. Chem. 1999a;274:25659–25667. doi: 10.1074/jbc.274.36.25659. [DOI] [PubMed] [Google Scholar]
- Carraway K. L., III, Rossi E. A., Komatsu M., Price-Shiavi S. A., Huang D., Carvajal M. E., Guy P. M., Fregien N., Carraway C.A.C., Carraway K. L. An intramembrane modulator of the ErbB2 receptor tyrosine kinase that potentiates neuregulin signaling. J. Biol. Chem. 1999b;274:5263–5266. doi: 10.1074/jbc.274.9.5263. [DOI] [PubMed] [Google Scholar]
- Carraway K. L., Perez A., Idris N., Jepson S., Arango M., Komatsu M., Haq B., Price-Schiavi S. A., Zhang J., Carraway C.A.C. Muc4/sialomucin complex, the intramembrane ErbB2 ligand, in cancer and epithelia: to protect and to survive. Prog. Nucleic Acids Res. Mol. Biol. 2002;71:149–185. doi: 10.1016/s0079-6603(02)71043-x. [DOI] [PubMed] [Google Scholar]
- Cho H. S., Mason K., Ramyar K. X., Stanley A. M., Gabelli S. B., Denney D. W., Jr, Leahy D. J. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature. 2003;421:756–770. doi: 10.1038/nature01392. [DOI] [PubMed] [Google Scholar]
- Cuenda A., Cohen P. Stress-activated protein kinase 2/p38 and a rapamycin sensitive pathway are required for C2C12 myogenesis. J. Biol. Chem. 1999;274:4341–4346. doi: 10.1074/jbc.274.7.4341. [DOI] [PubMed] [Google Scholar]
- Daniel C., Schroeder O., Zahn N., Gaschott T., Stein J. p38 MAPK signaling pathway is involved in butyrate-induced vitamin D receptor expression. Biochem. Biophys. Res. Commun. 2004;324:1220–1226. doi: 10.1016/j.bbrc.2004.09.191. [DOI] [PubMed] [Google Scholar]
- Dankort D. L., Wang Z., Blackmore V., Moran M. F., Muller W. J. Distinct tyrosine autophosphorylation sites negatively and positively modulate neu-mediated transformation. Mol. Cell. Biol. 1997;17:5410–5425. doi: 10.1128/mcb.17.9.5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dankort D. L., Muller W. J. Signal transduction in mammary tumorigenesis: a transgenic perspective. Oncogene. 2000;19:1038–1044. doi: 10.1038/sj.onc.1203272. [DOI] [PubMed] [Google Scholar]
- Dankort D., Maslikowski B., Warner N., Kanno N., Kim H., Wang Z., Moran M. F., Oshima R. G., Cardiff R. D., Muller W. D. Grb2 and Shc adapter proteins play distinct roles in Neu (ErbB-2)-induced mammary tumorigenesis: implications for human breast cancer. Mol. Cell. Biol. 2001;21:1540–1551. doi: 10.1128/MCB.21.5.1540-1551.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin M. C., Carey K. D., Vajdos F. F., Leahy D. J., de Vos A. M., Sliwkowski M. X. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell. 2004;5:317–328. doi: 10.1016/s1535-6108(04)00083-2. [DOI] [PubMed] [Google Scholar]
- Garrett T. P., McKern N. M., Lou M., Elleman T. C., Adams T. E., Lovrecz G. O., Zhu H. J., Walker F., Frenkel M. J., Hoyne P. A. Crystal structure of a truncated epidermal growth factor receptor extracellular domain bound to transforming growth factor alpha. Cell. 2002;110:763–773. doi: 10.1016/s0092-8674(02)00940-6. [DOI] [PubMed] [Google Scholar]
- Garrett T. P., McKern N. M., Lou M., Elleman T. C., Adams T. E., Lovrecz G. O., Kofler M., Jorissen R. N., Nice E. C., Burgess A. W., Ward C. W. The crystal structure of a truncated ErbB2 ectodomain reveals an active conformation, poised to interact with other ErbB receptors. Mol. Cell. 2003;11:495–505. doi: 10.1016/s1097-2765(03)00048-0. [DOI] [PubMed] [Google Scholar]
- Graus-Porta D., Beerli R. R., Daly J. M., Hynes N. E. ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. EMBO J. 1997;16:1647–1655. doi: 10.1093/emboj/16.7.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gut A., Kappeler F., Hyka N., Balda M., Hauri H-P., Matter K. Carbohydrate-mediated Golgi to cell surface transport and apical targeting of membrane proteins. EMBO J. 1998;17:1919–1929. doi: 10.1093/emboj/17.7.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy P. M., Platko J. V., Cantley L. C., Cerione R. A., Carraway K. L., III Insect cell-expressed p180erbB3 possesses an impaired tyrosine kinase activity. Proc. Natl. Acad. Sci. USA. 1994;91:8132–8136. doi: 10.1073/pnas.91.17.8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin C. H. Dimerization of cell surface receptors in signal transduction. Cell. 1995;80:213–223. doi: 10.1016/0092-8674(95)90404-2. [DOI] [PubMed] [Google Scholar]
- Horowitz J. C., Lee D. Y., Waghray M., Keshamouni V. G., Thomas P. E., Zhang H., Cui Z., Thannickal V. J. Activation of the pro-survival phosphatidylinositol 3-kin-ase/AKT pathway by transforming growth factor beta-1 in mesenchymal cells is mediated by p38 MAPK-dependent induction of an autocrine growth factor. J. Biol. Chem. 2004;279:1359–1367. doi: 10.1074/jbc.M306248200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houde M., Laprise P., Jean D., Blais M., Asselin C., Rivard N. Intestinal epithelial cell differentiation involves activation of p38 mitogen-activated protein kinase that regulates the homeobox transcription factor CDX2. J. Biol. Chem. 2001;276:21885–21894. doi: 10.1074/jbc.M100236200. [DOI] [PubMed] [Google Scholar]
- Hubert A., Cauliez B., Chedeville A., Husson A., Levionne A. Osmotic stress, a proinflammatory signal in Caco-2 cells. Biochimie. 2004;86:533–541. doi: 10.1016/j.biochi.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Hull S. R., Sheng Z., Vanderpuye O., David C., Carraway K. L. Isolation and partial characterization of ascites sialoglycoprotein-2 (ASGP-2) of the cell surface sialo-mucin complex of 13762 rat mammary adenocarcinoma cells. Biochem. J. 1990;265:121–129. doi: 10.1042/bj2650121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idris N., Carraway K. L. Regulation of sialomucin complex/Muc4 expression in rat uterine luminal epithelial cells by transforming growth factor-beta: implications for blastocyst implantation. J. Cell Physiol. 2000;185:310–316. doi: 10.1002/1097-4652(200011)185:2<310::AID-JCP16>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Idris N., Carothers Carraway C. A., Carraway K. L. Differential localization of ErbB2 in different tissues of the rat female reproductive tract: implications for the use of specific antibodies for ErbB2 analysis. J. Cell Physiol. 2001;189:162–170. doi: 10.1002/jcp.10012. [DOI] [PubMed] [Google Scholar]
- Iwasaki S., Iguchi M., Watanabe K., Hoshino R., Tsujimoto M., Khono M. Specific activation of the p38 mitogen-activated protein kinase signaling pathway and induction of neurite outgrowth in PC12 cells by bone morphogenetic protein-2. J. Biol. Chem. 1999;274:26503–26510. doi: 10.1074/jbc.274.37.26503. [DOI] [PubMed] [Google Scholar]
- Jepson S., Komatsu M., Haq B., Arango M. E., Huang D., Carraway C.A.C., Carraway K. L. Muc4/Sialomucin complex, the intramembrane ErbB2 ligand, induces specific phosphorylation of ErbB2 and enhances expression of p27kip, but does not activate mitogen-activated kinase or protein kinase B/Akt pathways. Oncogene. 2002;21:7524–7532. doi: 10.1038/sj.onc.1205970. [DOI] [PubMed] [Google Scholar]
- Kavanaugh W. M., Turck C. W., Williams L. T. PTB domain binding to signaling proteins through a sequence motif containing phosphotyrosine. Science. 1995;268:1177–1179. doi: 10.1126/science.7539155. [DOI] [PubMed] [Google Scholar]
- Klapper L. N., Kirschbaum M. H., Sela M., Yarden Y. Biochemical and clinical implications of the ErbB/HER signaling network of growth factor receptors. Adv. Cancer Res. 2000;77:25–79. [PubMed] [Google Scholar]
- Khoda D., Odaka M., Lax I., Kawasaki H., Suzuki K., Ullrich A., Schlessinger J., Inagaki F. A 40-kDa epidermal growth factor/transforming growth factor alpha-binding domain produced by limited proteolysis of the extracellular domain of the epidermal growth factor receptor. J. Biol. Chem. 1993;268:1976–1981. [PubMed] [Google Scholar]
- Komatsu M., Carraway C.A.C., Fregien N. L., Carraway K. L. Reversible disruption of cell-matrix and cell-cell interactions by overexpression of sialomucin complex. J. Biol. Chem. 1997;272:33245–33254. doi: 10.1074/jbc.272.52.33245. [DOI] [PubMed] [Google Scholar]
- Komatsu M., Yee L., Carraway K. L. Overexpression of sialomucin complex, a rat homologue of MUC4, inhibits tumor killing by lymphokine-activated killer cells. Cancer Res. 1999;59:2229–2236. [PubMed] [Google Scholar]
- Laprise P., Chailler P., Houde M., Beaulieu J. F., Boucher M. J., Rivard N. Phosphatidylinositol 3-kinase controls human epithelial cell differentiation by promoting adherens junctions assembly and p38 MAPK activation. J. Biol. Chem. 2002;277:8226–8234. doi: 10.1074/jbc.M110235200. [DOI] [PubMed] [Google Scholar]
- Lax I., Johnson A., Howk R., Sap J., Bellot F., Winkler M., Ullrich A., Vennstrom B., Schlessinger J., Givol D. Chicken epidermal growth factor (EGF) receptor: cDNA cloning, expression in mouse cells, and differential binding of EGF and transforming growth factor alpha. Mol. Cell. Biol. 1988;8:1970–1978. doi: 10.1128/mcb.8.5.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lax I., Bellot F., Howk R., Ullrich A., Givol D., Schlessinger J. A 40kDa epidermal growth factor/transforming growth factor alpha-binding domain produced by limited proteolysis of the extracellular domain of the epidermal growth factor receptor. EMBO J. 1989;8:421–427. [Google Scholar]
- Lee K. F., Simon H., Chen H., Bates B., Hung M. C., Hauser C. Requirement for neuregulin receptor erbB2 in neural and cardiac development. Nature. 1995;378:394–398. doi: 10.1038/378394a0. [DOI] [PubMed] [Google Scholar]
- Maher P. P38 mitogen-activated protein kinase activation is required for fibroblast growth factor-2-stimulated cell proliferation but not differentiation. J. Biol. Chem. 1999;274:17491–17498. doi: 10.1074/jbc.274.25.17491. [DOI] [PubMed] [Google Scholar]
- Milligan G., Parenti M., Magee A. I. The dynamic role of palmitoylation in signal transduction. Trends Biochem. Sci. 1995;5:181–187. doi: 10.1016/s0968-0004(00)89004-0. [DOI] [PubMed] [Google Scholar]
- Morooka T., Nishida E. Requirement of p38 mitogen-activated protein kinase for neuronal differentiation in PC12 cells. J. Biol. Chem. 1998;273:24285–24288. doi: 10.1074/jbc.273.38.24285. [DOI] [PubMed] [Google Scholar]
- Morris J. K., Lin W., Hauser C., Marchuk Y., Getman D., Lee K. F. Rescue of the cardiac defect in ErbB2 mutant mice reveals essential roles of ErbB2 in peripheral nervous system development. Neuron. 1999;23:273–283. doi: 10.1016/s0896-6273(00)80779-5. [DOI] [PubMed] [Google Scholar]
- Nemeth Z. H., Deitch E. A., Lu Q., Szabo C., Hasko G. NHE blockade inhibits chemokine production and NF-kappaB activation in immunostimulated endothelial cells. Am. J. Physiol. Cell Physiol. 2002;283:C396–C403. doi: 10.1152/ajpcell.00491.2001. [DOI] [PubMed] [Google Scholar]
- Ogiso H., et al. Crystal structure of the complex of human epidermal growth factor and receptor extracellular domains. Cell. 2002;110:775–787. doi: 10.1016/s0092-8674(02)00963-7. [DOI] [PubMed] [Google Scholar]
- Price-Schiavi S. A., Carraway C.A.C., Fregien N., Carraway K. L. Posttranscriptional regulation of a milk membrane protein, the sialomucin complex (Ascites sialo-glycoprotein, ASGP-1/ASGP-2, rat muc4), by transforming growth factor beta. J. Biol. Chem. 1998;273:35228–35237. doi: 10.1074/jbc.273.52.35228. [DOI] [PubMed] [Google Scholar]
- Price-Schiavi S. A., Andrechek E., Idris N., Li P., Ring M., Zhang J., Carraway C.A.C., Muller W. J., Carraway K. L. Expression, location, and interactions of ErbB2 and its intramembrane ligand Muc4 (sialomucin complex) in rat mammary gland during pregnancy. J. Cell Physiol. 2005;203:43–53. doi: 10.1002/jcp.20200. [DOI] [PubMed] [Google Scholar]
- Ramsauer V. P., Carraway C.A.C., Salas P.J.I., Carraway K. L. Muc4/sialo-mucin complex, the intramembrane ErbB2 ligand, translocates ErbB2 to the apical surface in polarized epithelial cells. J. Biol. Chem. 2003;278:30142–30147. doi: 10.1074/jbc.M303220200. [DOI] [PubMed] [Google Scholar]
- Resh M .D. Membrane targeting of lipid modified signal transduction proteins. Subcell. Biochem. 2004;37:217–232. doi: 10.1007/978-1-4757-5806-1_6. [DOI] [PubMed] [Google Scholar]
- Riese D. J., II, van Raaij T. M., Plowman G. D., Andrews G. C., Stern D. F. The cellular response to neuregulins is governed by complex interactions of the ErbB receptor family. Mol. Cell Biol. 1995;15:5770–5776. doi: 10.1128/mcb.15.10.5770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riese D. J., II, Stern D. F. Specificity within the EGF family/ErbB receptor family signaling network. Bioessays. 1998;20:41–48. doi: 10.1002/(SICI)1521-1878(199801)20:1<41::AID-BIES7>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Rossi E. A., McNeer R., Price-Schiavi S. A., Komatsu M., Van den Brande J.M.H., Thompson J. F., Carraway C.A.C., Fregien N. L., Carraway K. L. Sialomucin complex, a heterodimeric glycoprotein complex: expression as a soluble, secretable form in lactating mammary gland and colon. J. Biol. Chem. 1996;271:33476–33485. doi: 10.1074/jbc.271.52.33476. [DOI] [PubMed] [Google Scholar]
- Salas P.J.I., Rodriguez M. L., Viciana A. L., Vega-Salas D. E., Hauri H. P. The apical submembrane cytoskeleton participates in the organization of the apical pole in epithelial cells. J. Cell Biol. 1997;137:359–375. doi: 10.1083/jcb.137.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer C., Ross S. E., Bragado M. J., Groblewski G. E., Ernst S. A., Williams J. A. A role for the p38 mitogen-activated protein kinase/Hsp 27 pathway in cholecystokinin–induced changes in the actin cytoskeleton in rat pancreatic acini. J. Biol. Chem. 1998;273:24173–24180. doi: 10.1074/jbc.273.37.24173. [DOI] [PubMed] [Google Scholar]
- Schlessinger J. SH2/SH3 signaling proteins. Curr. Opin. Genet. Dev. 1994;4:25–30. doi: 10.1016/0959-437x(94)90087-6. [DOI] [PubMed] [Google Scholar]
- Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–225. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- Sheng Z., Hull S. R., Carraway K. L. Biosynthesis of the cell surface sialo-mucin complex of ascites 13762 rat mammary adenocarcinoma cells from a high Mr precursor. J. Biol. Chem. 1990;265:8505–8510. [PubMed] [Google Scholar]
- Sheng Z., Wu K., Carraway K., Fregien N. Molecular cloning of the transmembrane component of the 13762 mammary adenocarcinoma sialomucin complex. A new member of the epidermal growth factor superfamily. J. Biol. Chem. 1992;267:16341–16346. [PubMed] [Google Scholar]
- Sherblom A. P., Carraway K. L. A Complex of two cell surface glycoproteins from ascites mammary adenocarcinoma cells. J. Biol. Chem. 1980;255:12051–12059. [PubMed] [Google Scholar]
- Sherblom A. P., Buck R. L., Carraway K. L. Purification of the major sialoglycoproteins of 13762 MAT-B1 and MAT-C1 rat ascites mammary adenocarcinoma cells by density gradient centrifugation in cesium chloride and guanidine hydrochloride. J. Biol. Chem. 1980;255:783–790. [PubMed] [Google Scholar]
- Singh A. P., Moniaux N., Chauhan S. C., Meza J. L., Batra S. K. Inhibition of MUC4 expression suppresses pancreatic tumor cell growth and metastasis. Cancer Res. 2004;64:622–630. doi: 10.1158/0008-5472.can-03-2636. [DOI] [PubMed] [Google Scholar]
- Stein R. A., Staros J. V. Evolutionary analysis of the ErbB receptor and ligand families. J. Mol. Evol. 2000;50:397–412. doi: 10.1007/s002390010043. [DOI] [PubMed] [Google Scholar]
- van der Geer P., Wiley S., Lai V.K.-M., Olivier J. P., Gish G. D., Stephens R., Kaplan D., Shoelson S., Pawson T. A conserved amino-terminal Shc domain binds to phosphotyrosine motifs in activated receptors and phosphopeptides. Curr. Biol. 1995;5:404–412. doi: 10.1016/s0960-9822(95)00081-9. [DOI] [PubMed] [Google Scholar]
- Ward C. W., Hoyne P. A., Flegg R. H. Insulin and epidermal growth factor receptors contain the cysteine repeat motif found in the tumor necrosis factor receptor. Proteins. 1995;22:141–153. doi: 10.1002/prot.340220207. [DOI] [PubMed] [Google Scholar]
- Zetser A., Gredinger E., Bengal E. P38 mitogen activated protein kinase pathway promotes skeletal muscle differentiation. Participation of the Mef2c transcription factor. J. Biol. Chem. 1999;274:5193–5200. doi: 10.1074/jbc.274.8.5193. [DOI] [PubMed] [Google Scholar]