Abstract
Crk-associated substrate (Cas) is a tyrosine-phosphorylated docking protein that is indispensable for the regulation of the actin cytoskeletal organization and cell migration in fibroblasts. The function of Cas in neurons, however, is poorly understood. Here we report that Cas is dominantly enriched in the brain, especially the cerebellum, of postnatal mice. During cerebellar development, Cas is highly tyrosine phosphorylated and is concentrated in the neurites and growth cones of granule cells. Cas coimmunoprecipitates with Src family protein tyrosine kinases, Crk, and cell adhesion molecules and colocalizes with these proteins in granule cells. The axon extension of granule cells is inhibited by either RNA interference knockdown of Cas or overexpression of the Cas mutant lacking the YDxP motifs, which are tyrosine phosphorylated and thereby interact with Crk. These findings demonstrate that Cas acts as a key scaffold that links the proteins associated with tyrosine phosphorylation signaling pathways to the granule cell axon elongation.
INTRODUCTION
Crk-associated substrate (Cas) docking protein was initially identified as a major phosphotyrosine-containing protein in fibroblasts transformed by v-Src or v-Crk oncogenes (Sakai et al., 1994). It has an N-terminal Src homology 3 (SH3) domain, a substrate domain (SD) that consists of a cluster of tyrosine phosphorylation sites and a C-terminal Src-binding domain (SBD) that functions to directly bind the Src family protein tyrosine kinases (PTKs; Sakai et al., 1994; Nakamoto et al., 1996). In motile cells such as fibroblasts, Cas is tyrosine-phosphorylated by Src or Fak family PTKs after integrin stimulation, which induces Cas localization to focal adhesions. Tyrosine-phosphorylated Cas (YP-Cas) acts as a scaffold protein upstream of the Rho family small GTPase in focal adhesions (O’Neill et al., 2000). Embryonic fibroblasts lacking the Cas gene exhibit impaired actin stress fiber bundling and cell motility, indicating that Cas is indispensable for actin cytoskeleton organization and cell migration (Honda et al., 1998; Huang et al., 2002). The function of Cas in neurons, however, is unknown.
Neurons extend neurites immediately after exiting from mitotic cycles. At the tip of the extending neurites, there are motile enlargements, growth cones that build the dynamic center for signaling of the mobility and direction of extending neurites. Filopodia and lamellipodia are two dynamic structures in the growth cone that rapidly extend and retract, providing the force to advance in response to extracellular cues. Filopodia are protrusions composed of bundled F-actin fibers, whereas lamellipodia are large fanlike structures composed of a cross-linked actin meshwork (Tanaka and Sabry, 1995; Luo, 2002). The signaling pathways from the surface to the actin cytoskeletal organization in growth cones are essential for neurite outgrowth (Dickson, 2001; Dent and Gertler, 2003; Pollard and Borisy, 2003). Protein tyrosine phosphorylation is the critical factor for the signaling cascades that control growth cone motility (Korey and Van Vactor, 2000).
Cerebellar granule cells are the most abundant cell population in the cerebellar circuit. Granule cells proliferate postnatally in the external granule layer (EGL). Postmitotic granule cells move into the inner half of the EGL (iEGL) where they begin to differentiate. While migrating further down the molecular layer (ML) to the internal granule layer (IGL), granule cells develop the characteristic morphologies of their axons, parallel fibers (Ono et al., 1997). Granule cells settle in the IGL and develop their dendritic morphologies, forming synaptic glomerular rosettes with mossy fiber terminals and Golgi cell axon terminals. Src family PTKs such as Src, Fyn, Yes, Lyn, and Lck are highly expressed in the cerebellum, and their expression is developmentally regulated (Fults et al., 1985; Cartwright et al., 1988; Maness et al., 1988; Sudol et al., 1988, 1989; Zhao et al., 1991; Chen et al., 1996; Omri et al., 1996). The PTK activity of Src in the developing cerebellum is ∼6- to 10-fold that in fibroblasts (Cartwright et al., 1988). High levels of Src, Fyn, and Yes are concentrated in the growth cones of cerebellar neurons (Maness et al., 1988; Wu and Goldberg, 1993). Src family PTKs are implicated in the signaling pathways of cell adhesion molecule (CAM)-induced neurite outgrowth. Cultured cerebellar neurons prepared from mice lacking Fyn do not extend axons on neuronal cell adhesion molecule (NCAM)-coated culture matrix as rapidly as cells from wild-type littermates (Beggs et al., 1994). Similarly, cerebellar granule cells from Src-deficient mutant mice show impaired neurite outgrowth on the neural adhesion molecule L1 (Ignelzi et al., 1994). These results suggest that Src and Fyn have important roles in granule cell neurite extension. The signaling cascade from the CAMs and Src family PTKs to the cytoskeleton, however, remains to be clarified.
The present study demonstrates that among tissues of postnatally developing mice, Cas is most abundant in the brain, especially in the cerebellum. It is notable that YP-Cas peaked around the first postnatal week and was concentrated in the growth cone fractions. The Cas protein was immunocytochemically localized in the growth cones and neurites of granule cells. In the cerebellum, Cas coimmunoprecipitated with Src family PTKs, Crk, and CAM proteins N-cadherin and NCAM. Granule cell axon elongation was impaired by either RNA interference (RNAi) knockdown of Cas or overexpression of Cas mutants with deletion of the multiple tyrosine phosphorylation sites that confer the Crk-binding property. Our results suggest that YP-Cas acts as an important scaffold in the signaling of axon elongation of cerebellar neurons, linking extracellular signals to the cytoskeleton through tyrosine phosphorylation.
MATERIALS AND METHODS
Primary Dissociated Cerebellar Cell Culture
Cerebellar cells were prepared from postnatal day 0 (P0) ICR mouse cerebella as described previously (Shiraishi et al., 1999). In brief, the cerebella from P0 mice were treated with 0.1% trypsin (Sigma Chemical, St. Louis, MO) and 0.05% DNase I (Roche Diagnostics, Indianapolis, IN) in Ca2+-Mg2+-free Hanks’ balanced salt solution (HBSS), dissociated by repeated passage through a micropipette tip in Ca2+-Mg2+-free HBSS containing 0.05% DNase I and 12 mM MgSO4, and then rinsed with the culture medium. Dispersed cells (4 × 105) were plated onto poly-l-lysine (Sigma)-coated glass coverslips (12-mm diameter; Matsunami, Tokyo, Japan) in serum-free defined medium: Eagle’s medium supplemented with 1 mg/ml bovine serum albumin, 10 μg/ml insulin, 0.1 nM l-thyroxine, 0.1 mg/ml transferrin, 1 μg/ml aprotinin (all from Sigma), 30 nM selenium (Merck, Darmstadt, Germany), 0.25% (wt/vol) glucose, 2 mM glutamine, 2 mg/ml sodium bicarbonate, 0.1 mg/ml streptomycin (Meiji Seika KK, Tokyo, Japan), and 100 U/ml penicillin (Banyu Pharmaceutical, Tokyo, Japan). The cultures were maintained in a humidified atmosphere of 5% CO2 in air at 37°C.
Primary Cerebellar Neuron Transfection and Axon Length Analysis
Cas mutants produced as described previously (Huang et al., 2002) were constructed in a plasmid vector that contains the CAG promoter. Short interfering RNA (siRNA) of Cas was generated using the BLOCK-iT RNAi TOPO Transcription and BLOCK-iT Complete Dicer RNAi kits (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions (Azuma et al., 2005). siRNA of LacZ was generated using the same procedure as for Cas siRNA and was used as a negative control. Transfection of cerebellar neurons was performed soon after the neurons were dissociated using the Mouse Neuron Nucleofector kit and the Nucleofector device (Amaxa, Cologne, Germany; Liu et al., 2003; Hama et al., 2004). The transfection efficiency was 5%. The calcium phosphate method using a CellPhect Transfection Kit (Amersham Biosciences, Buckinghamshire, United Kingdom) was also used to transfect cerebellar neurons on day 1 in vitro (DIV1) in serum-free defined medium on poly-l-lysine–coated glass coverslips. The length of the longest axon was quantified in granule cells (marked by immunolabeling with antibody against Pax6) expressing the Cas mutant or siRNA. Axons were also confirmed by immunolabeling with antibody against Tau-1. Axons could be easily distinguished from dendrites because granule cells at DIV2 exhibit a representative morphology with a long single or bipolar axon with multiple short dendrites (Powell et al., 1997). The percentage of granule cells with axons longer than 200 μm in each transfection case was quantified in at least 20 fields randomly selected from three independent experiments. Student’s t test was used to compare results between the mutant and the control cells. p < 0.05 was considered significant.
RT-PCR Analysis
A series of first-strand cDNAs was produced by reverse-transcription (RT) from 20 ng of total cerebellar RNAs at the various developmental stages, using an oligo-dT primer. The cDNA sequence corresponding to the nucleotide positions 583-1182 (amino acids 175–394) of p130Cas was amplified using the primers 5′-ACATCTACCAAGTCCCTCCA-3′ (forward primer) and 5′-AGGCACGTCATACAGTGTTC-3′ (reverse primer). The cycling conditions were as follows: denaturing at 94°C for 3 min, amplification by 25 cycles of 94°C (15 s), 55°C (2 min), and 72°C (1 min), and extension at 72°C for 5 min. To analyze tissue distribution, total RNAs prepared from various tissues of P7 or P21 mice were used for RT-PCR. The RT-PCR of glyceraldehyde-3-phosphate dehydrogenase with primers 5′-GCCATCAACGACCCCTTCATTGACCTC-3′ (forward primer) and 5′-GCCATGTAGGCCATGAGGTCCACCAC-3′ (reverse primer) were used as an internal control.
In Situ Hybridization
In situ hybridization brain histochemistry was basically performed as described previously(Shiraishi et al., 1999). The cDNA sequence corresponding to nucleotide positions 583-1182 (amino acids 175–394) of the p130Cas cDNA was used as a template to prepare the digoxigenin-labeled antisense riboprobes using a digoxigenin-dUTP labeling kit (Roche Diagnostics). Paraffin sections of mouse brain (10 μm thick) were fixed in 4% paraformaldehyde for 5 min, washed twice in phosphate-buffered saline (PBS), and treated with freshly prepared 10 μg/ml proteinase K (Invitrogen) at room temperature. After acetylation, the sections were subjected to digoxigenin-based hybridization procedures. Briefly, the sections were incubated in a hybridization buffer containing 0.2 μg/ml digoxigenin-labeled riboprobes at 60°C overnight in a humid chamber. The hybridized sections were washed by successive immersion in 1× SSC (150 mM NaCl and 15 mM sodium citrate, pH 7.0, 60°C, 10 min, twice), 2× SSC (37°C, 10 min), 2× SSC containing 20 μg/ml RNase A (37°C, 30 min), 2× SSC (37°C, 10 min), and 0.2× SSC (60°C, 30 min, twice). The hybridization signals were detected using the digoxigenin detection kit (Roche Diagnostics).
Immunoprecipitation and Immunoblotting
Protein extraction and Western blotting analysis were performed as described previously (Huang et al., 2002). Briefly, mouse cerebella or cerebra were lysed in 1% Triton X-100 buffer (50 mM HEPES, 150 mM NaCl, 10% glycerol, 1% Triton X-100, 1.5 mM MgCl2, 1 mM EGTA, 100 mM NaF, 1 mM Na3VO4, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 1 mM phenylmethylsulfonyl fluoride). Aliquots of protein lysates were separated by SDS-PAGE and probed with diluted antibodies. For immunoprecipitation, protein (500 μg) was mixed with 1 μg primary antibody and incubated for 1 h on ice. The mixtures were rotated with protein A- or protein G-Sepharose (Amersham) for 1 h at 4°C. The Sepharose were washed four times with 1% Triton X-100 buffer and boiled in sample buffer before being subjected to SDS-PAGE analysis.
Antibodies
Antibodies against mouse Cas were used as described previously (Sakai et al., 1994). A phospho-specific polyclonal antibody (αCas-pYDxP) that specifically recognizes YP-Cas was generated by immunizing rabbits with a synthetic peptide, CAEDV(pY)DVP (a.a. 456–463), which is representative of the repetitive tyrosine-containing motifs in the Cas SD, after being conjugated with thyroglobulin (Miyake et al., 2005). Antibodies against Src family tyrosine kinases, N-cadherin, L1, β-integrin, NCAM140/180, hemagglutinin (HA), and Crk were from BD Bioscience (Franklin Lakes, NJ). The antibodies against Fyn (Fyn3), c-Src (N-16), NCAM120, and JNK1 were from Santa Cruz Biotechnologies (Santa Cruz, CA). The antibody anti-GAP43 was from Innogenetics (Alpharetta, GA). The antibodies against phosphotyrosine 4G10 was from Upstate Biotechnology (Waltham, MA), antibodies against Map2 (AP20) and calbindin were from Sigma, the antibody against Pax-6 was from Covance (Princeton, NJ), and the antibody against tau-1 was from Chemicon International (Temecula, CA).
Subcellular Fractionation and Isolation of Growth Cone Particles
P7 and P21 mouse cerebella were homogenized in the homogenization buffer (0.32 M sucrose, 5 mM Tris-HCl, 1 mM EGTA, 1 mM DTT, 1 mM pepstatin A, 1 mM leupeptin, and 1 mM Na3VO4). The protein homogenates were centrifuged at 1000 × g for 10 min. The pellet was lysed in 1% Triton X-100 buffer (PPt1). The supernatant was recentrifuged at 105,000 × g for 1 h. The pellet was lysed in 1% Triton X-100 buffer (PPt2 + 3), and the supernatant was used as Sup3. Growth cone particles (GCP) were prepared essentially as described previously (Pfenninger et al., 1983; Helmke et al., 1998). P7 mouse cerebella were homogenized in ∼8 volumes of 0.32 M sucrose containing 1 mM MgCl2 and 1 mM TES buffer, pH 7.3. The homogenate was centrifuged at 2000 rpm for 10 min (pellet, PPt1), and the resulting low-speed supernatant (cytosol) was loaded onto a discontinuous density gradient with steps of 0.75 and 1 M sucrose in the same buffer. The gradients were spun to equilibrium at 28,000 rpm for 1 h in a swing rotor SW-28 (Beckman Coulter, Fullerton, CA). The fraction at the 0.32/0.75 M sucrose interface containing the GCPs was collected. This fraction was diluted 3- to 4-fold with 0.32 M sucrose, and GCPs were pelleted at 40,000 rpm for 30 min (TLA-100, Beckman) and extracted with 1% Triton X-100 buffer for 30 min at 4°C (GCP).
Immunohistochemistry and Fluorescent Microscopy
The cells were fixed with 4% paraformaldehyde for 1 h, washed three times with PBS, and then permeabilized with 0.2% Triton X-100/PBS for 5 min before incubation with 5% normal goat serum in PBS (−). For native tissues, ICR mice were transcardically perfused with 4% paraformaldehyde in PBS (−), and the dissected brains were immersed for 2 h in the same fixative buffer and cryosectioned (20 μm thick). For immunoreaction, fixed cultured cells or brain sections were preincubated with 5% normal goat serum in PBS (−) for 1 h and then incubated with primary antibody (anti-Cas, 1 μg/ml), for 1 h at room temperature. After washing with PBS (−), the samples were incubated with Alexa-conjugated secondary antibody (Invitrogen). Immunofluorescence was observed using a Zeiss (Oberkochen, Germany) Meta-510 confocal laser microscope. Conventional immunostaining reaction was also performed using a diaminobenzidine and horseradish peroxidase–conjugated secondary antibody.
RESULTS
Cas mRNA Is Abundantly Expressed in Developing Mouse Cerebella
To examine the functional role of Cas in the mouse CNS, we first analyzed the expression of Cas mRNA in mice at P7 and P21 using RT-PCR technique. Of the eight tested tissues of P7 mice, the expression level of Cas mRNA was highest in the brain (Figure 1A). Cas mRNA was also expressed abundantly in P21 brain. In situ hybridization analysis revealed strong Cas mRNA labeling in the cerebellum, hippocampus, and olfactory bulb at P7 (Figure 1C) and P21 (Figure 1E). The cerebellum was the predominant region with Cas mRNA expression at P7 (Figure 1D) and P21 (Figure 1F). During postnatal cerebellar development, Cas mRNA expression was up-regulated, with the peak occurring from P7 to P12 (Figure 1B). In the P7 cerebella, Cas mRNA was primarily concentrated in the EGL, and was also present in the IGL, Purkinje cell layer (PL), and white matter (WM; Figure 1G). In P21 cerebella, on the other hand, it was predominantly localized in the PL and present in only low levels in the IGL and WM (Figure 1H). These results suggested that Cas has a functional role(s) in cerebellar development.
Figure 1.
Expression of Cas mRNA in developing mouse cerebella. (A) Semiquantitative RT-PCR analysis of Cas mRNA expression in P7 or P21 mouse tissue. (B) RT-PCR analysis of Cas mRNA expression in postnatal cerebella at six different postnatal stages. RT-PCR for GAPDH was used as an internal control. (C–H) In situ hybridization analysis of Cas distribution in the P7 (C, D, and G) and P21 (E, F, and H) mouse brains. C, P7 brain; D, P7 cerebellum; E, P21 brain; F, P21 cerebellum; G, P7 cerebellar cortex; H, P21 cerebellar cortex. EGL, external granule cell layer; ML, molecular layer; IGL, internal granule cell layer; PL, Purkinje cell layer; WM, white matter.
Cas Protein Is Highly Tyrosine-phosphorylated during Cerebellar Development
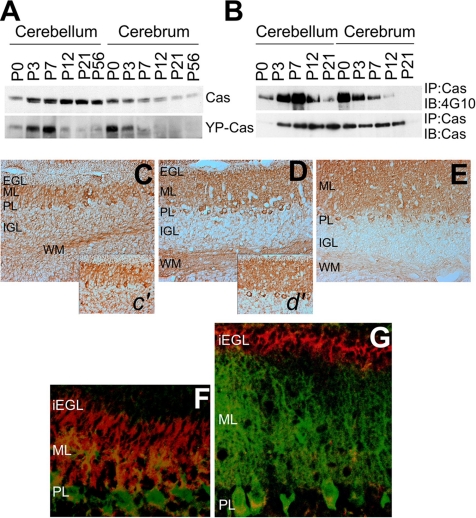
We investigated the expression and tyrosine phosphorylation of Cas protein in developing mouse brains at six different postnatal stages (P0, P3, P7, P12, P21, and P56) by immunoblotting analysis (Figure 2A). The tyrosine-phosphorylated Cas (YP-Cas) was immunodetected by an antibody that recognizes the tyrosine-phosphorylated YDxP motifs of Cas (Miyake et al., 2005). In the cerebella, Cas protein expression was up-regulated to a peak level at around P21 and then slightly down-regulated, which almost paralleled the mRNA expression profile (Figure 1B). In contrast to this developmental profile of Cas expression, YP-Cas rapidly increased after birth to reach a peak level at P7 and then sharply decreased. This steep increase of YP-Cas within the first postnatal week was also confirmed by an immunoprecipitation assay with the anti-Cas antibody followed by immunoblotting with the anti-phospho-tyrosine antibody 4G10 (Figure 2B). In the cerebra, on the other hand, both Cas and YP-Cas proteins were most abundant at P0 and then markedly decreased (Figure 2, A and B).
Figure 2.
Expression and tyrosine phosphorylation of Cas protein in developing mouse cerebella. (A) Immunoblotting of Cas and YP-Cas in the postnatal cerebella. Approximately equal amounts of protein lysates from six different postnatal stages (P0, P3, P7, P12, P21, and P56) were subjected to immunoblotting analysis by an antibody against Cas or YP-Cas. (B) Immunoprecipitation with the Cas antibody followed by immunoblotting with the phospho-tyrosine antibody 4G10. (C–E) Immunohistochemical staining of Cas in the P7 (C and c′), P12 (D and d′), and P21 (E) mouse cerebella sections. (F and G) Immunohistochemical analysis of YP-Cas detected by the specific antibody (red) in P7 (F) and P12 (G) mouse cerebella. Purkinje cells were immunostained with anti-calbindin antibody (green). iEGL, the inner half of EGL.
We then immunohistochemically analyzed the cellular distribution of Cas protein in the developing cerebella (Figure 2, C–E). In P7 cerebella, there was intense Cas immunolabeling in the ML and PL and weak immunolabeling in the EGL, IGL, and WM (Figure 2C). In P12 cerebella, there was high density labeling in the ML, PL, and WM and low density labeling in the IGL (Figure 2D). In P21 cerebella, there was strong Cas staining in the ML and PL and weak staining in the WM (Figure 2E). On the other hand, YP-Cas had characteristic cellular distribution patterns in the developing cerebella (Figure 2, F and G). Intense YP-Cas immunolabeling was distributed primarily in the iEGL and ML at P7 (F) and in the iEGL at P12 (G), and moderate labeling was observed in the WM at P7 and P12 (unpublished data). In the P12 ML, there was weak YP-Cas immunolabeling around the growing dendrites of Purkinje cells, which were coimmunostained for calbindin (a Purkinje cell marker; Figure 2G). At P21, there was very weak immunostaining in the ML and IGL (unpublished data). These data indicate that Cas is highly tyrosine-phosphorylated in postmitotic premigratory granule cells within the iEGL, in outgrowing Purkinje cell dendrites within the ML, and in the WM (probably in Purkinje cell axons), at the first and second postnatal stages.
Cas Is Enriched in the Growth Cones of Developing Cerebellar Neurons
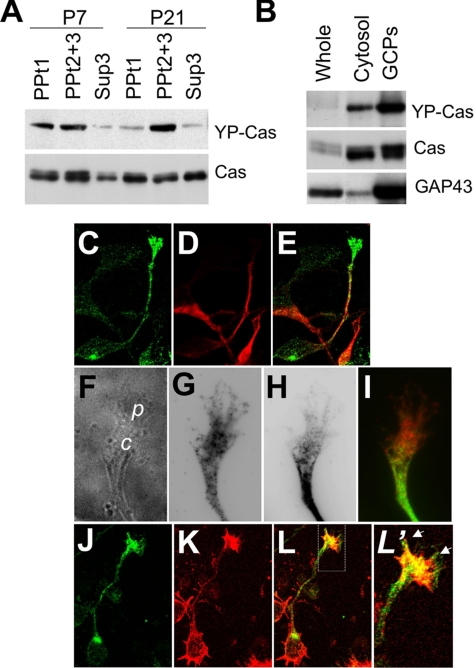
We performed subcellular fractionation of Cas protein from P7 and P21 mouse cerebella. Cas immunoreactivity was recovered in the precipitation fractions, PPt1 (nuclei and cytoskeleton fractions) and PPt2 + 3 (membrane fraction containing mitochondria and microsomes), and the supernatant fraction Sup3 (cytosolic; Figure 3A, bottom). On the other hand, YP-Cas immunoreactivity was enriched in the precipitation fractions: in the PPt1 and PPt2 + 3 at P7 and in the PPt2 + 3 at P21 (Figure 3A, top). Moreover, YP-Cas was concentrated in the GCP fraction prepared from P7 cerebella, in which a growth cone marker, growth-associated protein 43 (GAP43), was recovered (Figure 3B).
Figure 3.
Cas is enriched in the growth cones of cerebellar granule cells. (A) Immunoblotting of Cas in subcellular protein fractions from P7 and P21 mouse cerebella. (B) Immunoblotting of Cas and YP-Cas in growth cone fractions from P7 mouse cerebella. Equal amounts of proteins were loaded in each lane and immunoblotted with antibodies against YP-Cas, Cas, and GAP43 (as a control of growth cone proteins). (C–E) Confocal images of Cas (C) and Map2 (D) in the granule cells (DIV1). (E) Merged image of C and D. (F–I) Phase-contrast images (F) of Cas (G) and Map2 (H) in the granule cell growth cones. (I) Merged image of G and H. (J–L′), Immunostaining of Cas (J) and F-actin (by phalloidin staining; K) in granule cells. (L) Merged image of J and K. (L′) Magnified views of the growth cones. Arrows indicate colocalization of Cas and F-actin.
We next analyzed the subcellular localization of Cas protein in cultured cerebellar neurons (Figure 3, C–I). Cas was detected in axons of granule cells at DIV1, and was largely concentrated in their fanlike tips, growth cones (Figure 3, C–L). Although the fine structure of granule cell growth cones was difficult to observe because of their thin and tiny morphology, Cas was localized in both the central and peripheral domains (Figure 3, F–I), and colocalized with actin bundles (Figure 3, J–L′). In cultured Purkinje cells (DIV14), Cas immunoreactivity was observed in the tips of the dendritic arbors as well as in their dendrites, axons, and soma (unpublished data). These data indicate that Cas is subcellularly localized in outgrowing neurites and growth cones of cerebellar neurons. The subcellular fractionation analysis implied that Cas in growth cones is highly tyrosine-phosphorylated.
RNAi Knockdown of Cas Inhibits Axon Extension of Granule Cells
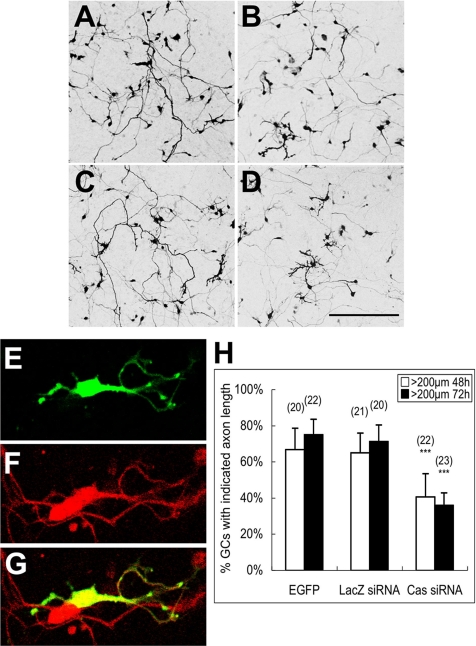
We examined whether interference of the endogenous Cas by RNAi affects granule cell axon extension (Figure 4A). The effectiveness of siRNAs was first evaluated in DLD-1 cells (human colon tumor cells), in which more than 90% of Cas protein expression was knocked down within 72 h after transfection (Supplementary Figure 1A). Then, the siRNA efficiency was confirmed by cotransfection of CasFL and Cas siRNA in cerebellar primary cultures (Supplementary Figure 1, B and C). Because of the transfection ratio and the difficulty in quantifying the Cas expression level changes in the primary culture by immunoblotting, the number of cells expressing the exogenous CasFL construct carrying the HA epitope in each observation field was quantified by HA immunostaining. HA-positive cells were decreased by cotransfection of Cas siRNA (∼3 in each field), in comparison with that of the control LacZ (Escherichia coli β-galactosidase gene) siRNA (∼10 in each field), demonstrating that Cas siRNA specifically knocked down Cas proteins in granule cells.
Figure 4.
RNAi knockdown of Cas impairs axon extension of cerebellar granule cells. (A–D) Confocal images of cerebellar granule cells transfected with Cas siRNA or LacZ siRNA. Cerebellar granule cells were cotransfected soon after cell dissociation by either Cas siRNA or LacZ siRNA with pCAG-EGFP. Cells were fixed and observed 48 or 72 h after the transfection. (A) LacZ siRNA 48 h; (B) Cas siRNA 48 h; (C) LacZ siRNA 72 h; (D) Cas siRNA 72 h. Scale bar, 200 μm. (E–G) Granule cells (stained by anti-Pax6 antibody; F) with multiple short axons observed in Cas siRNA transfection (E). (H) Percentage of cells with axons longer than 200 μm within each observation field 48 or 72 h after transfection. Data are presented as mean ± SEM. Values in the parentheses above the column indicate the number of observation fields from at least three independent experiments. ***p < 0.001, compared with the EGFP control (t test).
The knockdown effect of Cas siRNA on neurite extension of granule cells was analyzed by counting cells with an axon length of more than 200 μm cotransfected with Cas siRNA and enhanced green fluorescent protein (EGFP) in comparison with controls. The control granule cells, which were cotransfected with LacZ siRNA and EGFP, exhibited an almost normal neurite pattern with long axons at 48 and 72 h (Figure 4, A and C, respectively) after transfection. In contrast, cells cotransfected with the Cas siRNA and EGFP had an impaired axon pattern at 48 and 72 h (Figure 4, B and D, respectively) after transfection. Pax6 (a granule cell marker)-positive cells coexpressing Cas siRNA and EGFP formed a complex neurite pattern with short, branched axons (Figure 4, E–G), indicating that Cas knockdown affected the granule cells. Long axons (>200 μm) extended from 65 and 72% LacZ siRNA-expressing granule cells at 48 and 72 h after transfection, respectively (Figure 4H). These ratios were almost comparable with those in cells expressing EGFP alone. The number of long axons (>200 μm) was significantly reduced in Cas siRNA-expressing granule cells to 40 and 35% at 48 and 72 h after transfection, respectively (Figure 4H). This Cas siRNA knockdown effect was observed even in granule cells overexpressing exogenous CasFL protein (Supplementary Figure 1D). Therefore, our data suggest that Cas is related to granule cell axon elongation.
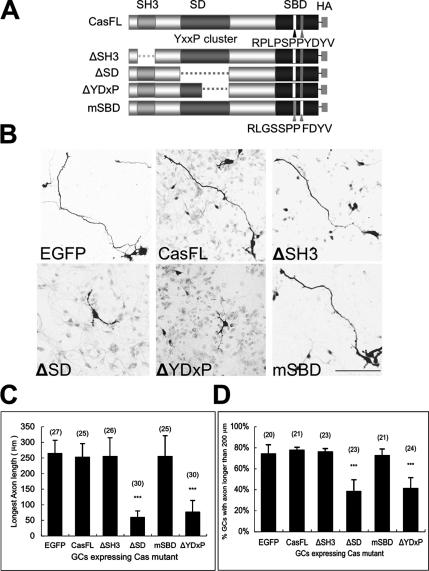
Deletions of the YDxP Motifs or the Cas Substrate Domain Impairs Axon Elongation of Granule Cells
Cas consists of three major protein-protein interaction domains (Figure 5A): the N-terminal SH3 domain (binds to the PxxP motif of Fak, Pyk2, PTP1B, etc.), the SD (consists of a cluster of YxxP motifs that are tyrosine-phosphorylated by PTKs, leading to binding to Crk, Nck, SHIP-2, etc.), and SBD (containing motifs RPLPSPP [a.a.733–739] and YDYV [a.a.762–765], which bind to Src family PTKs; Sakai et al., 1994). To assess the structure and function relationship of Cas protein in granule cell development, we constructed four Cas mutants: three deletion mutants lacking the SH3 (ΔSH3), SD (ΔSD), or only YDxP motifs within the SD (ΔYDxP), and a substitution mutant of the RPLPSPP and YDYV motifs within the SBD to RLGSSPP and FDYV, respectively (mSBD; Figure 5A). Either the full-length Cas (CasFL) or mutant Cas was coexpressed with the EGFP vector in cultured granule cells by transfection (Figure 5B). Expressed EGFP fluorescence was generally widespread over the soma and neurites of granule cells. Granule cells transfected with the CasFL, ΔSH3, or mSBD exhibited a representative morphology with single or bipolar long extending axons at this stage (DIV2; Ono et al., 1997; Powell et al., 1997), whereas cells transfected with the ΔSD or ΔYDxP tended to have significantly shorter, and sometimes branching, axons (Figure 5B), similar to that observed in Cas knockdown cells by Cas siRNA (Figure 4, E–H). These short axons were immunopositive for Tau-1 (unpublished data). The average length of the longest axon, in cells expressing CasFL, ΔSH3, or mSBD was nearly 250 μm, whereas that of cells expressing the ΔSD (∼58 μm) or ΔYDxP (∼76 μm) was very short (Figure 5C). Long axons (>200 μm) extended from more than 70% of CasFL, ΔSH3, mSBD, and EGFP-expressing cells, whereas they were observed in fewer than 40% of ΔSD or ΔYDxP-expressing cells (Figure 5D). These results suggest that the SD containing the YDxP motifs is involved in the axon elongation of granule cells and that the ΔSD and ΔYDxP act as dominant negatives to endogenous Cas in this process. The F-actins in the granule cells overexpressing Cas mutants were labeled (Supplementary Figure 4); however, there were no significant differences in the quantity of F-actins in the growth cones of cells expressing ΔYDxP and other mutants.
Figure 5.
Overexpression of the Cas mutant lacking Crk binding ability inhibits axon elongation of granule cells. (A) Cas mutants with domain deletion or mutation. ΔSH3, deletion of the SH3 domain; ΔSD deletion of a cluster of tyrosine phosphorylation sites; ΔYDxP, deletion of YDxP motifs; mSBD, double mutations RLGSSPP and FDYV at the Src binding domain. (B) Confocal images of cerebellar granule cells expressing Cas mutants. Cerebellar granule cells were double-transfected by electroporation with a Cas mutant with an EGFP vector soon after dissociation of cerebellar cells. The cells were stained with the antibody against HA 48 h after plating. Bar, 100 μm. (C) Average length of the axons in the EGFP and Cas mutants coexpressing cells. (D) Percentage of transfected cells with axon length more than 200 μm. Cerebellar granule cells (DIV1) were transfected with Cas mutants using the calcium phosphate method. Cells were fixed, stained with the antibody against HA, and observed at DIV2. Data are indicated as mean ± SEM. Values in the parentheses above the column indicate the number of the transfected cells from at least three independent experiments. ***p < 0.001, compared with the EGFP control (t test).
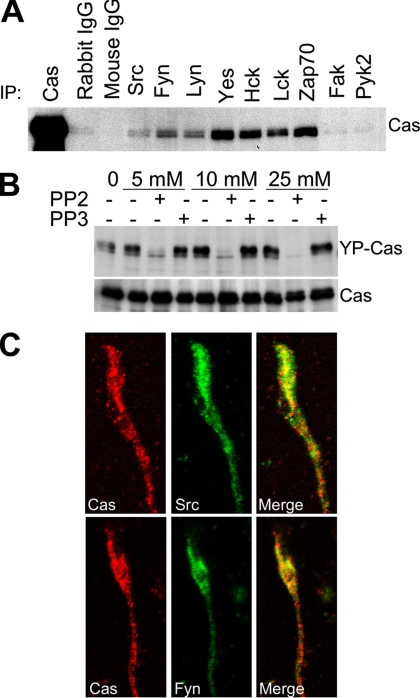
Cas Is the Substrate of Src Family Tyrosine Kinases in the Developing Mouse Cerebella
In fibroblasts, Cas binds to both Src and Fak family PTKs and is consequently phosphorylated by them (Nakamoto et al., 1996; Cary et al., 1998). To elucidate the molecular basis of Cas tyrosine phosphorylation during mouse cerebellar development, we investigated the association of Cas with nine PTKs belonging to the Src or Fak family (Figure 6). Seven Src family (Src, Fyn, Lyn, Yes, Hck, Lck, and Zap70) and the two Fak family (Fak and Pyk2) PTKs tested were expressed in the mouse cerebella (unpublished data). Only Src family PTKs, however, coimmunoprecipitated with Cas in P7 cerebellar extracts (Figure 6A), suggesting that the Src family, but not the Fak family, associates with Cas in the mouse cerebella at the early stage. This result was confirmed by in vitro experiments using a Src family PTK inhibitor PP2. In contrast to the ineffective structural analog PP3, PP2 inhibited the tyrosine phosphorylation of Cas in cultured cerebellar cells (Figure 6B). YP-Cas was reduced to an almost undetectable level by treatment with 25 μM PP2, indicating that Src family PTKs are responsible for the tyrosine phosphorylation of Cas in these cells. Src and Fyn were enriched in the GCP fraction of the P7 cerebella (Supplementary Figure 2A), and their tyrosine phosphorylation levels were high in the P3–P7 cerebella (Supplementary Figure 2B). Immunocytochemical analysis of cultured granule cells (DIV1) revealed colocalization of Cas with Src and Fyn in the growth cones (Figure 6C). In addition, Cas was colocalized with Src, Fyn, or Yes in the cerebellar cortex at P12 (Supplementary Figure 2C). Moreover, there was a punctate accumulation pattern of the mSBD along the neurites (Supplementary Figure 3A). There were similar phenotypes in cells expressing ΔSBD (unpublished data). Although the underlying mechanism of punctuate distribution on neurites is unclear, it might be related to tyrosine phosphorylation, because exogenously expressed CasFL had a similar punctate accumulation pattern when PTK activity was inhibited with PP2 for 1 h (Supplementary Figure 3B).
Figure 6.
Cas is a substrate of Src family tyrosine kinases in the developing mouse cerebella. (A) Coimmunoprecipitation of Cas with the Src family PTKs in the mouse cerebella. An equal quantity of protein lysates from P7 cerebella was first immunoprecipitated by Src, Fyn, Yes, Lyn, Hck, Lck, Zap70, Fak, or Pyk2 antibodies and then immunoblotted by the Cas antibody. (B) Src family PTK inhibitor PP2 inhibited tyrosine phosphorylation of Cas in cerebellar neurons. Primary cultured cerebellar neurons were treated with dimethyl sulfoxide, PP2, or a noninhibitory analog, PP3, at the indicated concentrations for 20 min, and the cell lysates were immunoblotted for YP-Cas. The same membrane was reblotted to indicate the quantity of Cas in each lane. (C) Cas (red) colocalizes with Src or Fyn (green) in the growth cones of cultured cerebellar granule cells. Cerebellar granule cells were fixed at DIV1, stained, and imaged using confocal microscopy.
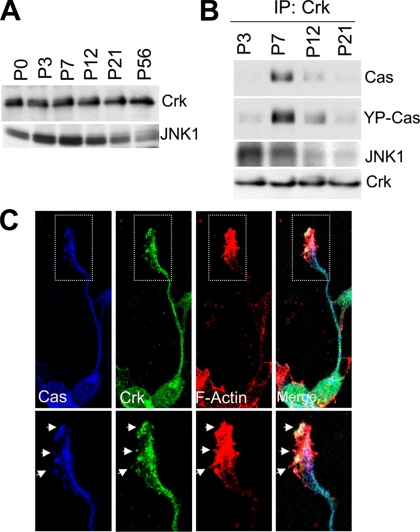
Developmental Stage-specific Interaction of the Tyrosine-phosphorylated Cas with Adaptor Protein Crk in the Mouse Cerebellum
Crk is an adaptor protein that regulates actin cytoskeleton organization and cell migration (Klemke et al., 1998). CrkII directly binds with YP-Cas in PC12 cells stimulated with nerve growth factor (Ribon and Saltiel, 1996). Our previous study indicated that YDxP motifs within the SD domain of Cas are involved in binding to CrkII in fibroblasts (Zhou et al., 1993; Huang et al., 2002). We examined whether there is an interaction between Cas and Crk in developing mouse cerebella (Figure 7, A and B). Crk protein was almost constantly expressed throughout cerebellar development (Figure 7A). Cas protein, however, was more efficiently coimmunoprecipitated with anti-Crk antibody from cerebellar extracts at P7 than from those at other stages tested (Figure 7B, top). In addition, the coimmunoprecipitated Cas was tyrosine phosphorylated (Figure 7B, middle). This P7 stage, in which there is a tight interaction between Cas and Crk, was consistent with the peak stage for tyrosine phosphorylation of Cas (Figure 2A). Moreover, both Cas and Crk immunolabels were codistributed in growth cones and neurites of cultured granule cells and were colocalized with actin bundles at the peripheral and central domains of growth cones (Figure 7C).
Figure 7.
YP-Cas forms a complex with Crk-JNK1 in P7 mouse cerebella. (A) Immunoblotting of Crk and JNK1 protein in the mouse cerebella at different development stages. (B) Coimmunoprecipitation of Crk with Cas and JNK1 in developing mouse cerebella. An equal quantity of cerebella protein lysates from P3, P7, P12, and P21 mice were immunoprecipitated with the anti-Crk antibody and then immunoblotted with the antibody against Cas, YP-Cas, or JNK1. (C) Colocalization of Cas (blue), Crk (green), and F-actin (red, phalloidin staining) in the growth cones of cultured cerebellar granule cells (DIV1). Arrows indicate the colocalized structure.
Because there is a direct interaction between CrkII and JNK1 (c-Jun N-terminal kinase 1; Girardin and Yaniv, 2001), we next investigated the Crk-JNK1 association. JNK1 expression was up-regulated with a peak at P7 and then down-regulated during mouse cerebellar development (Figure 7A). In immunoprecipitates with anti-Crk antibody, JNK1 was abundant in the early stages (P3–P7; Figure 7B). Taken together, these results suggest that the YP-Cas-Crk-JNK1 protein interaction is involved in the signaling pathway regulating actin organization of growth cones in granule cells.
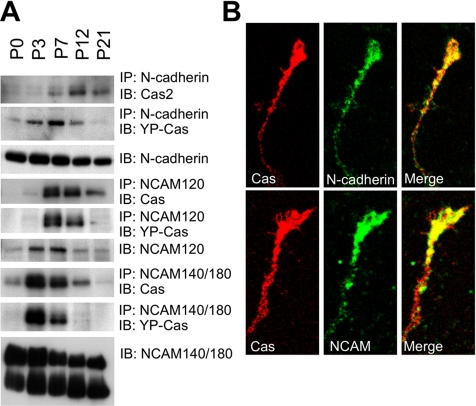
Association of Cas with N-Cadherin and NCAM in the Developing Mouse Cerebellum
There are three major classes of CAMs in the nervous system: integrin, cadherin, and the IgG superfamily of CAM (IgCAM), which serve as plasma membrane sensors for extracellular cues, leading to the activation of intracellular signaling events related to the cytoskeleton (Walsh and Doherty, 1997). Cas is tyrosine-phosphorylated by Fak or Src family PTKs after integrin stimulation (O’Neill et al., 2000). Therefore, we examined whether Cas is associated with CAMs in the mouse cerebella. Anti-Cas antibody coimmunoprecipitated with N-cadherin, NCAM, and L1, but not β-integrin, from P7 cerebellar extracts (Supplementary Figure 2D). The specific antibody for N-cadherin and NCAM coimmunoprecipitated with Cas protein from cerebellar extracts in the early developmental stage (Figure 8A). N-cadherin and NCAM140/180 mRNA were expressed in the EGL and IGL in postnatal mouse cerebella at P7 (Supplementary Figure 5), which coincides with the expression of Cas mRNA (Figure 1). Both N-cadherin and NCAM protein were enriched in the GCP fraction of P7 cerebella (Supplementary Figure 2A). Moreover, N-cadherin and NCAM colocalized with Cas in the growth cones and neurites of cultured granule cells (Figure 8B). Taken together, our data suggest that N-cadherin and NCAM interact with the Cas-mediated signaling complex and act as a cell surface signaling complex in granule cell neuritogenesis.
Figure 8.
Cas and YP-Cas are developmentally associated with N-cadherin, NCAM120, or NCAM140/180 in mouse cerebella. (A) Cas and YP-Cas were coimmunoprecipitated with N-cadherin and NCAM in postnatal developing mouse cerebella. Equal amounts of protein lysate from the P0, P3, P7, P12, and P21 mouse cerebella were immunoprecipitated by antibodies against N-cadherin, NCAM120, or NCAM140/180. The immunoprecipitants were immunoblotted by the antibody against Cas. The same immunoprecipated protein blots were reused for immunoreaction with the antibody against YP-Cas. (B) Confocal images of colocalization of Cas (Red) with N-cadherin and NCAM140/180 (green) in the growth cones of cultured cerebellar granule cells (DIV1).
DISCUSSION
Cas acts as an indispensable scaffold for the signaling proteins involved in tyrosine phosphorylation–coupled actin cytoskeleton reorganization pathways and regulates cell morphology and migration in fibroblasts (Honda et al., 1998; Huang et al., 2002). The function of Cas in the nervous system, however, is unclear. Cas-deficient mice are embryonic lethal at 11.5–12.5 d after coitum because of impaired heart development (Honda et al., 1998), making it difficult to investigate its role in the brain, which functionally develops later. The present findings demonstrate the functional importance of Cas as a signaling interface among the proteins involved in axon elongation of cerebellar granule cells during postnatal development.
The neural circuitry of the cerebellum develops through a coordinated program of neuronal migration, neurite outgrowth, and synaptic interconnections (Hatten and Heintz, 1995). To accomplish this circuit development, both granule cells and Purkinje cells undergo drastic morphological changes, in which actin cytoskeletal reorganization is very active (Ono et al., 1997). The results of the present study demonstrated that both Cas mRNA and protein predominate in the cerebellum during this postnatal period in mice. Cas is enriched in the neurites and growth cones of developing granule cells and Purkinje cells. The specific binding of Cas to Src PTKs and consequent tyrosine phosphorylation, by which Cas acquires the critical ability to bind with downstream signaling proteins, peak in the early postnatal stage. This developmental profile of YP-Cas apparently coincides with the time window during which neurite extension of the granule cells and Purkinje cells are more active. In addition, YP-Cas is largely distributed around the iEGL, where postmitotic granule cells start to differentiate by axonal extension and migration, and the ends of Purkinje cell dendrites are actively sprouting. These results indicate that tyrosine phosphorylation of Cas is closely associated with the postnatal development of cerebellar neurons.
In fibroblasts, Cas interacts with Fak family PTKs through the N-terminal SH3 domain and also directly binds to Src family PTKs via the C-terminal SBD, which contains the YDYV and RPLPSPP motifs (Sakai et al., 1994; Ruest et al., 2001), resulting in the tyrosine-phosphorylation of Cas, the production of YP-Cas. Src family PTKs are highly expressed in the cerebellum and their expression and activity is developmentally regulated (Fults et al., 1985; Cartwright et al., 1988; Maness et al., 1988; Sudol et al., 1988, 1989; Chen et al., 1996; Omri et al., 1996). A previous study indicated that Fak has important roles in axon extension and polarization of cerebellar Purkinje cells (Rico et al., 2004). There are no reports, however, of a role for Fak in cerebellar granule cell development. Our study demonstrated that Cas has no tight association with Fak and Pyk2 compared with Src family PTKs in early postnatal developing cerebella. Cas associates with Src family PTKs in developing mouse cerebella and colocalizes with Src and Fyn in the growth cones of granule cells. Moreover, the Src family PTK inhibitor PP2 almost completely blocks the tyrosine phosphorylation of Cas in in vitro cerebellar cell cultures. These findings support the idea that Src, but not Fak, family PTKs are responsible for the tyrosine phosphorylation of Cas in cerebellar neurons.
Cas is constitutively tyrosine-phosphorylated after binding to the Src family PTKs, and the major tyrosine phosphorylation sites are the SD, including four YQxP motifs and nine YDxP motifs in fibroblasts (Pellicena and Miller, 2001; Ruest et al., 2001). We previously reported that Cas null fibroblasts have defective actin stress fiber organization and that the ΔYDxP Cas mutant fails to restore the actin stress fiber organization (Huang et al., 2002). In the present study, knockdown of Cas protein expression with the Cas siRNA impaired axonal growth, and dominant-negative effects of Cas mutants, ΔSD and ΔYDxP, in axon elongation were also observed. Granule cells expressing exogenous ΔSD or ΔYDxP have abnormally truncated axonal protrusions, whereas no abnormal axon elongation was observed in cells expressing the other mutants ΔSH3 and mSBD, indicating the importance of the interaction of Cas with the SD-binding proteins for axon extension of granule cells. On the other hand, the overexpressed mSBD mutant tended to distribute in a punctate pattern in the dendrites and soma (Supplementary Figure 3), suggesting that a defect in the binding of Cas to Src family PTKs affects the subcellular distribution of Cas proteins. Loss of the dominant negative effect of mSBD on axon extension might be due to its aberrant subcellular accumulation. Taken together, these results indicate that Cas has an important role in the signaling of axon elongation through interactions with its binding partners via the tyrosine-phosphorylated YDxP motifs.
Phospho-tyrosines within the YDxP motifs are essential for binding Cas to the SH2 domain of the adaptor protein Crk (Zhou et al., 1993; Huang et al., 2002), which subsequently regulates the actin reorganization during fibroblast migration (Klemke et al., 1998). A recent study reported that Crk is recruited to the lipid rafts in growing neurites and mediates lamellipodia formation in PC12 cells (Haglund et al., 2004). Our present data indicate that the interaction between Cas and Crk occurs within the time window when Cas is highly tyrosine-phosphorylated during cerebellar development. In addition, Cas and Crk are subcellularly colocalized with F-actin bundles in the peripheral region of growth cones of cultured granule cells. These results suggest that the impaired axon elongation induced by Cas knockdown with siRNA or overexpression of the ΔYDxP mutant in granule cells might be due to a deficiency in the regulation of the actin-cytoskeletal organization through the YP-Cas-Crk interaction.
Our data demonstrate that JNK1 interacts with the YP-Cas-Crk complex in mouse cerebellum during the early postnatal stage. JNK1 interacts with the SH3 domain of CrkII (Girardin and Yaniv, 2001) and is involved in signaling of neuronal microtubule dynamics through the phosphorylation of microtubule-associated proteins (Chang et al., 2003; Bjorkblom et al., 2005). Another downstream effector of Crk is a small GTPase Rac1 that mediates the actin cytoskeletal dynamics during axonal outgrowth (Luo, 2002). Rac1 is activated by DOCK180, a Crk SH3-binding protein, leading to the lamellipodia formation by fibroblasts (Tanaka et al., 1997; Kiyokawa et al., 1998). It is notable that high Rac activity is present in early postnatal cerebellum (Arakawa et al., 2003). In our primary dissociation cultures, the growth cones of granule cells were very tiny and unstable, and they grew out very rapidly soon after plating on culture dishes. Even if there are subtle changes, it would be very difficult to observe actin dynamics within the growth cone after incubating to obtain effective cellular levels of recombinant Cas proteins, which are exogenously expressed by cDNA transfection. Therefore, we primarily analyzed the length of extending neurites after transfection experiments. Similarly, we did not focus our study on fillopodia and lamellipodia, which are more dynamic structures within the growth cones.
NCAMs actively participate in neurite elongation and dendritic and axonal arbor pathfinding (Walsh and Doherty, 1997; Rougon and Hobert, 2003). The importance of the CAMs, including NCAM, N-cadherin, and L1 for axonal growth was established by a large number of antibody perturbation experiments (Lindner et al., 1983; Hoffman et al., 1986; Walsh and Doherty, 1997; Sakurai et al., 2001; He and Meiri, 2002). A recent study demonstrated that mice deficient for both Nr-CAM and L1 exhibit severe cerebellar folial defects and reduced IGL thickness (Sakurai et al., 2001), indicating that Nr-CAM and L1 have a role in cerebellar granule cell development. Although, to our knowledge, there are no reports of NCAM or N-cadherin knockout mice with defects in cerebellar granule cell development, this might be due to a CAM redundancy. Our data indicate that the developmental expression of N-cadherin and NCAM140/180 mRNA in postnatal mouse cerebella at P7 coincides with that of Cas in the EGL and IGL at the same developmental stage. YP-Cas associates with N-cadherin and NCAMs in the early stage (P3–P12) of cerebellar development, and NCAM and N-cadherin are concentrated in the growth cones of granule cells. Integrin, however, did not coimmunoprecipitate with Cas, which seems to be consistent with a recent study in which NCAM and L1, but not β1 integrin, were detected in the detergent-resistant membranes of cerebellar granule cells (Nakai and Kamiguchi, 2002). NCAM and L1 are implicated in the underlying signaling cascades via the activation of Src and Fyn (Beggs et al., 1994; Ignelzi et al., 1994; Beggs et al., 1997). Whether Cas is tyrosine-phosphorylated after association with CAMs or Cas is tyrosine-phosphorylated before the association with CAMs remain unclear. Cell surface signals via CAMs might activate Src family PTKs, followed by Src PTK binding to and subsequent tyrosine-phosphorylation of Cas protein, leading to the axonal outgrowth of granule cells.
Although Cas mRNAs are localized in both the outer (mitotic) and inner (postmitotic) layer of the EGL, Cas proteins, including phosphorylated form, predominantly distribute in the inner EGL where postmitotic granule cells are settled and begin with their differentiation before cell migration toward the ML. Therefore, we think that Cas is mainly involved in the differentiation of granule cells rather than in the proliferation of their precursors. It is possible, however, that a small amount of Cas protein is involved in granule cell growth.
In conclusion, our data provide functional evidence that the tyrosine-phosphorylated docking protein Cas acts as a signaling interface from the protein tyrosine phosphorylation toward the axonal outgrowth in cerebellar granule cells. The present findings demonstrate that Cas is most abundant in developing mouse cerebellum and is highly tyrosine-phosphorylated in the early postnatal stage, probably by its binding partner Src PTKs. YP-Cas binds Crk, which further recruits downstream proteins such as JNK1. This sequential signaling event likely regulates granule cell axonal outgrowth.
Supplementary Material
ACKNOWLEDGMENTS
Jinhong Huang was a postdoctoral fellowship recipient of the Japan Society for the Promotion of Science (JSPS). We thank Dr. Noriyuki Morita for the cerebellar dissociation cell cultures and immunohistochemistry, Dr. Fumio Yoshikawa for subcellular fractionation of the growth cone particles, and Dr. Hiroshi Hama for DNA transfection into primary cerebellar neurons. We also thank Dr. Tamae Asawa (National Cancer Research Center) for the Cas siRNA study.
Abbreviations used:
- Cas
Crk associated substrate
- YP-Cas
tyrosine phosphorylated Cas
- SD
substrate domain
- SBD
Src binding domain
- PTK
protein tyrosine kinases
- CAM
cell adhesion molecules
- NCAM
neuronal cell adhesion molecule
- EGL
external granule cell layer
- ML
molecular layer
- IGL
internal granule cell layer
- PL
Purkinje cell layer
- WM
white matter
- EGFP
enhanced green fluorescent protein
- GCP
growth cone particle
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05-12-1122) on May 10, 2006.
 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
REFERENCES
- Arakawa Y., Bito H., Furuyashiki T., Tsuji T., Takemoto-Kimura S., Kimura K., Nozaki K., Hashimoto N., Narumiya S. Control of axon elongation via an SDF-1alpha/Rho/mDia pathway in cultured cerebellar granule neurons. J. Cell. Biol. 2003;161:381–391. doi: 10.1083/jcb.200210149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma K., Tanaka M., Uekita T., Inoue S., Yokota J., Ouchi Y., Sakai R. Tyrosine phosphorylation of paxillin affects the metastatic potential of human osteosarcoma. Oncogene. 2005;24:4754–4764. doi: 10.1038/sj.onc.1208654. [DOI] [PubMed] [Google Scholar]
- Beggs H. E., Baragona S. C., Hemperly J. J., Maness P. F. NCAM140 interacts with the focal adhesion kinase p125(fak) and the SRC-related tyrosine kinase p59(fyn) J. Biol. Chem. 1997;272:8310–8319. doi: 10.1074/jbc.272.13.8310. [DOI] [PubMed] [Google Scholar]
- Beggs H. E., Soriano P., Maness P. F. NCAM-dependent neurite outgrowth is inhibited in neurons from Fyn-minus mice. J. Cell. Biol. 1994;127:825–833. doi: 10.1083/jcb.127.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkblom B., Ostman N., Hongisto V., Komarovski V., Filen J. J., Nyman T. A., Kallunki T., Courtney M. J., Coffey E. T. Constitutively active cytoplasmic c-Jun N-terminal kinase 1 is a dominant regulator of dendritic architecture: role of microtubule-associated protein 2 as an effector. J. Neurosci. 2005;25:6350–6361. doi: 10.1523/JNEUROSCI.1517-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright C. A., Simantov R., Cowan W. M., Hunter T., Eckhart W. pp60c-src expression in the developing rat brain. Proc. Natl. Acad. Sci. USA. 1988;85:3348–3352. doi: 10.1073/pnas.85.10.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cary L. A., Han D. C., Polte T. R., Hanks S. K., Guan J. L. Identification of p130Cas as a mediator of focal adhesion kinase-promoted cell migration. J. Cell. Biol. 1998;140:211–221. doi: 10.1083/jcb.140.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L., Jones Y., Ellisman M. H., Goldstein L. S., Karin M. JNK1 is required for maintenance of neuronal microtubules and controls phosphorylation of microtubule-associated proteins. Dev. Cell. 2003;4:521–533. doi: 10.1016/s1534-5807(03)00094-7. [DOI] [PubMed] [Google Scholar]
- Chen S., Ren Y. Q., Hillman D. E. Transient expression of lyn gene in Purkinje cells during cerebellar development. Brain Res. Dev. Brain Res. 1996;92:140–146. doi: 10.1016/0165-3806(95)00208-1. [DOI] [PubMed] [Google Scholar]
- Dent E. W., Gertler F. B. Cytoskeletal dynamics and transport in growth cone motility and axon guidance. Neuron. 2003;40:209–227. doi: 10.1016/s0896-6273(03)00633-0. [DOI] [PubMed] [Google Scholar]
- Dickson B. J. Rho GTPases in growth cone guidance. Curr. Opin. Neurobiol. 2001;11:103–110. doi: 10.1016/s0959-4388(00)00180-x. [DOI] [PubMed] [Google Scholar]
- Fults D. W., Towle A. C., Lauder J. M., Maness P. F. pp60c-src in the developing cerebellum. Mol. Cell. Biol. 1985;5:27–32. doi: 10.1128/mcb.5.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardin S. E., Yaniv M. A direct interaction between JNK1 and CrkII is critical for Rac1-induced JNK activation. EMBO J. 2001;20:3437–3446. doi: 10.1093/emboj/20.13.3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haglund K., Ivankovic-Dikic I., Shimokawa N., Kruh G. D., Dikic I. Recruitment of Pyk2 and Cbl to lipid rafts mediates signals important for actin reorganization in growing neurites. J. Cell. Sci. 2004;117:2557–2568. doi: 10.1242/jcs.01148. [DOI] [PubMed] [Google Scholar]
- Hama H., Hara C., Yamaguchi K., Miyawaki A. Protein kinase C signaling mediates global enhancement of excitatory synaptogenesis in neurons triggered by local contact with astrocytes. Neuron. 2004;41:405–415. doi: 10.1016/s0896-6273(04)00007-8. [DOI] [PubMed] [Google Scholar]
- Hatten M. E., Heintz N. Mechanisms of neural patterning and specification in the developing cerebellum. Annu. Rev. Neurosci. 1995;18:385–408. doi: 10.1146/annurev.ne.18.030195.002125. [DOI] [PubMed] [Google Scholar]
- He Q., Meiri K. F. Isolation and characterization of detergent-resistant microdomains responsive to NCAM-mediated signaling from growth cones. Mol. Cell. Neurosci. 2002;19:18–31. doi: 10.1006/mcne.2001.1060. [DOI] [PubMed] [Google Scholar]
- Helmke S., Lohse K., Mikule K., Wood M. R., Pfenninger K. H. SRC binding to the cytoskeleton, triggered by growth cone attachment to laminin, is protein tyrosine phosphatase-dependent. J. Cell. Sci. 1998;111(Pt 16):465–2475. doi: 10.1242/jcs.111.16.2465. [DOI] [PubMed] [Google Scholar]
- Hoffman S., Friedlander D. R., Chuong C. M., Grumet M., Edelman G. M. Differential contributions of Ng-CAM and N-CAM to cell adhesion in different neural regions. J. Cell. Biol. 1986;103:145–158. doi: 10.1083/jcb.103.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda H., et al. Cardiovascular anomaly, impaired actin bundling and resistance to Src-induced transformation in mice lacking p130Cas. Nat. Genet. 1998;19:361–365. doi: 10.1038/1246. [DOI] [PubMed] [Google Scholar]
- Huang J., Hamasaki H., Nakamoto T., Honda H., Hirai H., Saito M., Takato T., Sakai R., et al. Differential regulation of cell migration, actin stress fiber organization, and cell transformation by functional domains of Crk-associated substrate. J. Biol. Chem. 2002;277:27265–27272. doi: 10.1074/jbc.M203063200. [DOI] [PubMed] [Google Scholar]
- Ignelzi M. A., Jr, Miller D. R., Soriano P., Maness P. F. Impaired neurite outgrowth of src-minus cerebellar neurons on the cell adhesion molecule L1. Neuron. 1994;12:873–884. doi: 10.1016/0896-6273(94)90339-5. [DOI] [PubMed] [Google Scholar]
- Kiyokawa E., Hashimoto Y., Kobayashi S., Sugimura H., Kurata T., Matsuda M. Activation of Rac1 by a Crk SH3-binding protein, DOCK180. Genes Dev. 1998;12:3331–3336. doi: 10.1101/gad.12.21.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemke R. L., Leng J., Molander R., Brooks P. C., Vuori K., Cheresh D. A. CAS/Crk coupling serves as a “molecular switch” for induction of cell migration. J. Cell Biol. 1998;140:961–972. doi: 10.1083/jcb.140.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korey C. A., Van Vactor D. From the growth cone surface to the cytoskeleton: one journey, many paths. J. Neurobiol. 2000;44:184–193. [PubMed] [Google Scholar]
- Lindner J., Rathjen F. G., Schachner M. L1 mono- and polyclonal antibodies modify cell migration in early postnatal mouse cerebellum. Nature. 1983;305:427–430. doi: 10.1038/305427a0. [DOI] [PubMed] [Google Scholar]
- Liu J. J., Ding J., Kowal A. S., Nardine T., Allen E., Delcroix J. D., Wu C., Mobley W., Fuchs E., Yang Y. BPAG1n4 is essential for retrograde axonal transport in sensory neurons. J. Cell. Biol. 2003;163:223–229. doi: 10.1083/jcb.200306075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L. Actin cytoskeleton regulation in neuronal morphogenesis and structural plasticity. Annu. Rev. Cell Dev. Biol. 2002;18:601–635. doi: 10.1146/annurev.cellbio.18.031802.150501. [DOI] [PubMed] [Google Scholar]
- Maness P. F., Aubry M., Shores C. G., Frame L., Pfenninger K. H. c-src gene product in developing rat brain is enriched in nerve growth cone membranes. Proc. Natl. Acad. Sci. USA. 1988;85:5001–5005. doi: 10.1073/pnas.85.14.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake I., Hakomori Y., Misu Y., Nakadate H., Matsuura N., Sakamoto M., Sakai R. Domain-specific function of ShcC docking protein in neuroblastoma cells. Oncogene. 2005;24:3206–3215. doi: 10.1038/sj.onc.1208523. [DOI] [PubMed] [Google Scholar]
- Nakai Y., Kamiguchi H. Migration of nerve growth cones requires detergent-resistant membranes in a spatially defined and substrate-dependent manner. J. Cell Biol. 2002;159:1097–1108. doi: 10.1083/jcb.200209077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamoto T., Sakai R., Ozawa K., Yazaki Y., Hirai H. Direct binding of C-terminal region of p130Cas to SH2 and SH3 domains of Src kinase. J. Biol. Chem. 1996;271:8959–8965. doi: 10.1074/jbc.271.15.8959. [DOI] [PubMed] [Google Scholar]
- O’Neill G. M., Fashena S. J., Golemis E. A. Integrin signalling: a new Cas. (t) of characters enters the stage. Trends Cell Biol. 2000;10:111–119. doi: 10.1016/s0962-8924(99)01714-6. [DOI] [PubMed] [Google Scholar]
- Omri B., Crisanti P., Marty M. C., Alliot F., Fagard R., Molina T., Pessac B. The Lck tyrosine kinase is expressed in brain neurons. J. Neurochem. 1996;67:1360–1364. doi: 10.1046/j.1471-4159.1996.67041360.x. [DOI] [PubMed] [Google Scholar]
- Ono K., Shokunbi T., Nagata I., Tokunaga A., Yasui Y., Nakatsuji N. Filopodia and growth cones in the vertically migrating granule cells of the postnatal mouse cerebellum. Exp. Brain Res. 1997;117:17–29. doi: 10.1007/pl00005787. [DOI] [PubMed] [Google Scholar]
- Pellicena P., Miller W. T. Processive phosphorylation of p130Cas by Src depends on SH3-polyproline interactions. J. Biol. Chem. 2001;276:28190–28196. doi: 10.1074/jbc.M100055200. [DOI] [PubMed] [Google Scholar]
- Pfenninger K. H., Ellis L., Johnson M. P., Friedman L. B., Somlo S. Nerve growth cones isolated from fetal rat brain: subcellular fractionation and characterization. Cell. 1983;35:573–584. doi: 10.1016/0092-8674(83)90191-5. [DOI] [PubMed] [Google Scholar]
- Pollard T. D., Borisy G. G. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- Powell S. K., Rivas R. J., Rodriguez-Boulan E., Hatten M. E. Development of polarity in cerebellar granule neurons. J. Neurobiol. 1997;32:223–236. doi: 10.1002/(sici)1097-4695(199702)32:2<223::aid-neu7>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Ribon V., Saltiel A. R. Nerve growth factor stimulates the tyrosine phosphorylation of endogenous Crk-II and augments its association with p130Cas in PC-12 cells. J. Biol. Chem. 1996;271:7375–7380. doi: 10.1074/jbc.271.13.7375. [DOI] [PubMed] [Google Scholar]
- Rico B., Beggs H. E., Schahin-Reed D., Kimes N., Schmidt A., Reichardt L. F. Control of axonal branching and synapse formation by focal adhesion kinase. Nat. Neurosci. 2004;7:1059–1069. doi: 10.1038/nn1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougon G., Hobert O. New insights into the diversity and function of neuronal immunoglobulin (Ig) superfamily molecules. Annu. Rev. Neurosci. 2003;26:207–238. doi: 10.1146/annurev.neuro.26.041002.131014. [DOI] [PubMed] [Google Scholar]
- Ruest P. J., Shin N. Y., Polte T. R., Zhang X., Hanks S. K. Mechanisms of CAS substrate domain tyrosine phosphorylation by FAK and Src. Mol. Cell. Biol. 2001;21:7641–7652. doi: 10.1128/MCB.21.22.7641-7652.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai R., Iwamatsu A., Hirano N., Ogawa S., Tanaka T., Mano H., Yazaki Y., Hirai H. A novel signaling molecule, p130, forms stable complexes in vivo with v-Crk and v-Src in a tyrosine phosphorylation-dependent manner. EMBO J. 1994;13:3748–3756. doi: 10.1002/j.1460-2075.1994.tb06684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T., Lustig M., Babiarz J., Furley A. J., Tait S., Brophy P. J., Brown S. A., Brown L. Y., Mason C. A., Grumet M. Overlapping functions of the cell adhesion molecules Nr-CAM and L1 in cerebellar granule cell development. J. Cell Biol. 2001;154:1259–1273. doi: 10.1083/jcb.200104122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi Y., Mizutani A., Bito H., Fujisawa K., Narumiya S., Mikoshiba K., Furuichi T. Cupidin, an isoform of Homer/Vesl, interacts with the actin cytoskeleton and activated rho family small GTPases and is expressed in developing mouse cerebellar granule cells. J. Neurosci. 1999;19:8389–8400. doi: 10.1523/JNEUROSCI.19-19-08389.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudol M., Alvarez-Buylla A., Hanafusa H. Differential developmental expression of cellular yes and cellular src proteins in cerebellum. Oncogene Res. 1988;2:345–355. [PubMed] [Google Scholar]
- Sudol M., Kuo C. F., Shigemitsu L., Alvarez-Buylla A. Expression of the yes proto-oncogene in cerebellar Purkinje cells. Mol. Cell. Biol. 1989;9:4545–4549. doi: 10.1128/mcb.9.10.4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka E., Sabry J. Making the connection: cytoskeletal rearrangements during growth cone guidance. Cell. 1995;83:171–176. doi: 10.1016/0092-8674(95)90158-2. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Ouchi T., Hanafusa H. Downstream of Crk adaptor signaling pathway: activation of Jun kinase by v-Crk through the guanine nucleotide exchange protein C3G. Proc. Natl. Acad. Sci. USA. 1997;94:2356–2361. doi: 10.1073/pnas.94.6.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh F. S., Doherty P. Neural cell adhesion molecules of the Ig superfamily: role in axon growth and guidance. Annu. Rev. Cell Dev. Biol. 1997;13:425–456. doi: 10.1146/annurev.cellbio.13.1.425. [DOI] [PubMed] [Google Scholar]
- Wu D. Y., Goldberg D. J. Regulated tyrosine phosphorylation at the tips of growth cone filopodia. J. Cell Biol. 1993;123:653–664. doi: 10.1083/jcb.123.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y. H., Baker H., Walaas S. I., Sudol M. Localization of p62c-yes protein in mammalian neural tissues. Oncogene. 1991;6:1725–1733. [PubMed] [Google Scholar]
- Zhou S., Shoelson S. E., Chaudhuri M., Gish G., Pawson T., Haser W. G., King F., Roberts T., Ratnofsky S., Lechleider R. J. SH2 domains recognize specific phosphopeptide sequences. Cell. 1993;72:767–778. doi: 10.1016/0092-8674(93)90404-e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.