Abstract
Cell division in the fission yeast Schizosaccharomyces pombe requires the formation and constriction of an actomyosin ring at the division site. The actomyosin ring is assembled in metaphase and anaphase A, is maintained throughout mitosis, and constricts after completion of anaphase. Maintenance of the actomyosin ring during late stages of mitosis depends on the septation initiation network (SIN), a signaling cascade that also regulates the deposition of the division septum. However, SIN is not active in metaphase and is not required for the initial assembly of the actomyosin ring early in mitosis. The FER/CIP4-homology (FCH) domain protein Cdc15p is a component of the actomyosin ring. Mutations in cdc15 lead to failure in cytokinesis and result in the formation of elongated, multinucleate cells without a division septum. Here we present evidence that the requirement of Cdc15p for actomyosin ring formation is dependent on the stage of mitosis. Although cdc15 mutants are competent to assemble actomyosin rings in metaphase, they are unable to maintain actomyosin rings late in mitosis when SIN is active. In the absence of functional Cdc15p, ring formation upon metaphase arrest depends on the anillin-like Mid1p. Interestingly, when cytokinesis is delayed due to perturbations to the division machinery, Cdc15p is maintained in a hypophosphorylated form. The dephosphorylation of Cdc15p, which occurs transiently in unperturbed cytokinesis, is partially dependent on the phosphatase Clp1p/Flp1p. This suggests a mechanism where both SIN and Clp1p/Flp1p contribute to maintenance of the actomyosin ring in late mitosis through Cdc15p, possibly by regulating its phosphorylation status.
INTRODUCTION
The formation of a contractile actomyosin-based ring is a key feature of cytokinesis that is conserved from yeast to mammals (Balasubramanian et al., 2004). Depending on the cell type studied, the actomyosin ring has different functions including force generation and targeted membrane addition. In the fission yeast Schizosaccharomyces pombe, the actomyosin ring is assembled upon entry into mitosis when its various components are recruited to the ring in a sequential manner (Rajagopalan et al., 2003; Wu et al., 2003; Wolfe and Gould, 2005). The future division site is determined even earlier, in the G2 phase of the cell cycle, by localization of anillin-related Mid1p to a cortical band that overlays the interphase nucleus (Sohrmann et al., 1996; Wu et al., 2003). The actomyosin ring is maintained through anaphase and begins constriction only after nuclear segregation to the cell ends in order to ensure faithful separation of the genetic material (Wolfe and Gould, 2005). In S. pombe, the actomyosin ring is essential for cytokinesis. This has allowed for isolation of mutants that are defective in assembly or maintenance of the ring and hence display cell division arrest phenotypes, i.e., they fail to lay down a division septum and form elongated multinucleate cells. Such genetic screens have yielded several temperature-sensitive alleles of the cdc15 gene (Nurse et al., 1976; Chang et al., 1996; Balasubramanian et al., 1998).
Cdc15p is the founding member of a protein family characterized by a FER/CIP4-homology domain at the N-terminus followed by a coiled-coil region and an SH3-domain at the C-terminus. Homologues have been identified in various organisms including budding yeast, chicken, mouse and human (Fankhauser et al., 1995; Lippincott and Li, 2000). In S. pombe, Cdc15p itself localizes to the actomyosin ring during cell division, whereas it is found in patches concentrated at the cell tips during interphase (Fankhauser et al., 1995; Carnahan and Gould, 2003). It is hyperphosphorylated in interphase and hypophosphorylated in dividing cells (Fankhauser et al., 1995).
Several studies have examined the mutant phenotype of cdc15 with respect to the ability to form an actomyosin ring. This has led to conflicting results that may be due to differing experimental designs, e.g., use of synchronous versus asynchronous cultures, different mutant alleles, different markers for the actomyosin ring or sensitivity of the assays (Fankhauser et al., 1995; Chang et al., 1996; Balasubramanian et al., 1998; Arai and Mabuchi, 2002; Carnahan and Gould, 2003). Using rhodamin-conjugated phalloidin to visualize F-actin in cdc15-140 mutant cells, Fankhauser et al. (1995) noted infrequent formation of actin rings (ca. 5% of wild-type) that showed fainter staining than wild-type cells. Applying the same probe to another allele, cdc15-287, Chang et al. (1996) observed up to 20% of mitotic cells with faint actin rings but attributed this to incomplete penetrance. Carnahan and Gould (2003) report detection of poorly organized and incomplete actin rings in <2% of cdc15-140 mutant cells, but they did not detect any rings with Alexa 488–conjugated phalloidin in cdc15Δ cells. Although all of the above-mentioned studies used asynchronous cultures that had been shifted to the restrictive temperature for varying periods of time, Balasubramanian et al. (1998) detected actin rings in mitotic cdc15-A5 cells as well as in dividing cdc15-140 cells obtained from synchronous cultures using rhodamin-conjugated phalloidin and α-Cdc4p antibodies to detect F-actin and myosin II, respectively. Similarly, Arai and Mabuchi (2002) observed Bodipy-phallacidin–stained F-actin rings in mitotic cdc15-140 cells whose cell cycle stage was determined by costaining for a spindle pole body marker. These authors noted that although cdc15-140 mutant cells were capable of assembling distorted F-actin rings comparable to those in anaphase A wild-type cells, they failed to form fully compacted and constricting rings, as seen in wild-type cells from anaphase B onward.
However, it appears unambiguous that Cdc15p promotes medial F-actin assembly because overexpression of cdc15+ during interphase results in ectopic formation of F-actin structures in the middle of the cell (Fankhauser et al., 1995). The role of Cdc15p in the rearrangement of F-actin may be mediated by its ability to recruit F-actin nucleation pathways to the site of cell division. The activators of the Arp2/3 complex Myo1p (type I myosin), Wsp1p (Wiskott Aldrich Syndrome protein [WASP] homologue) and Vrp1p (homologue of verprolin/WASP interacting protein [WIP]) fail to localize to the medial region in a cdc15 mutant, and Myo1p has also been shown to interact directly with Cdc15p. Moreover, Cdc15p colocalizes with the formin Cdc12p in a medial spot and in the actomyosin ring, and these two proteins exhibit direct interaction (Carnahan and Gould, 2003). Cdc15p has also been shown to organize sterol-rich membrane domains (Takeda et al., 2004), although how these domains function in actomyosin ring maintenance is presently unclear.
The septation initiation network (SIN) is a signaling cascade that becomes activated in anaphase upon cyclin B degradation and is related to the mitotic exit network (MEN) in the budding yeast Saccharomyces cerevisiae. It comprises a small GTPase and several protein kinases as well as regulatory and scaffolding proteins (Krapp et al., 2004). In addition, the protein phosphatase Clp1p (also termed Flp1p) has been identified as a nonessential component of SIN under normal conditions but becomes important for cell survival upon perturbations to the cytokinetic machinery (Cueille et al., 2001; Trautmann et al., 2001; Mishra et al., 2004). SIN appears to provide a link between cell cycle progression, actomyosin ring stability upon mitotic exit, and division septum assembly. As a result, SIN mutants do not assemble division septa because of the unstable nature of the actomyosin ring as well as the requirement for SIN signaling in septum assembly. How SIN affects actomyosin ring stability and septum assembly is currently unclear. Interestingly, genetic analysis of the interactions between cdc15 and components of SIN suggests that SIN may regulate the function of Cdc15p (Marks et al., 1992).
Here we have reinvestigated Cdc15p function and present evidence that Cdc15p becomes essential for actomyosin ring assembly and maintenance only upon activation of SIN but not in early mitosis. Thus, Cdc15p may function downstream of SIN in ring formation to promote septation in unperturbed cells and may contribute to the long-term stability of the actomyosin ring in a SIN-dependent manner upon cytokinesis delay.
MATERIALS AND METHODS
Media for cell culture were as described previously (Moreno et al., 1991). Growth temperatures were 24°C (permissive) and 36°C (restrictive) for temperature-sensitive strains, 30°C (permissive) and 18°C (restrictive) for cold-sensitive strains, and 30°C for all other strains. Cells were arrested in S phase by addition of hydroxyurea (HU; Sigma, St. Louis, MO) to 12 mM final concentration to the medium followed by the same amount of HU after 4 h. For fluorescence microscopy, cells were fixed with 7% formaldehyde. We either observed GFP-epifluorescence or processed cells for immunostaining as described previously (Balasubramanian et al., 1997). We visualized DNA with DAPI (Sigma) and F-actin with Alexa 488–conjugated phalloidin (Molecular Probes, Eugene, OR). Antibodies were from Molecular Probes (α-GFP, Alexa 488–conjugated α-rabbit, Alexa 594–conjugated α-mouse), a kind gift of Dr Keith Gull (α-tubulin) or as described previously (α-Cdc4p; McCollum et al., 1995). Fluorescence microscopy was done with Leica DMLB or DMIRE2 (Deerfield, IL) or Olympus IX71 (Melville, NY) microscopes and appropriate sets of filters. Confocal microscopy was done with a Zeiss LSM 510 (Thornwood, NY). Images were captured using Photometrics CoolSNAP ES or HQ cameras (Tucson, AZ) and MetaVue or MetaMorph software (Universal Imaging, West Chester, PA). Quantitative data are based on counting of at least 200 cells per sample unless otherwise stated. Error bars represent SDs.
Cells were lysed by beating with glass beads in the presence of TNE buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 5 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, supplemented with protease inhibitors; Complete EDTA-free; Roche Diagnostics, Basel, Switzerland). Total protein lysates were prepared by addition of 2× Laemmli sample buffer and dithiothreitol (Harlow and Lane, 1988). Proteins were separated by electrophoresis in gels of appropriate polyacrylamide concentration and blotted on PVDF membrane (Millipore, Bedford, MA). Antibodies for detection were from Roche Diagnostics (α-HA), Jackson ImmunoResearch (West Grove, PA; horseradish peroxidase–conjugated α-mouse and α-rabbit) or as described previously (α-Arp3p; McCollum et al., 1996).
RESULTS
cdc15 Mutant Cells Form Actomyosin Rings during Metaphase and Anaphase
To better understand the molecular nature of Cdc15p function as well as its regulation, we set out to define the window of the cell cycle during which Cdc15p is active in actomyosin ring formation. To this end we shifted asynchronously growing temperature-sensitive cdc15-140 mutant cells to the restrictive temperature for 3 h and fixed and stained for tubulin and Cdc4p, the essential light chain of myosin II that served as a marker for the actomyosin ring (McCollum et al., 1995; Naqvi et al., 1999). We noticed that a subpopulation of cells displayed Cdc4p in medial ring structures. Counting of 50 cells bearing Cdc4p rings revealed that 8 showed short spindles indicative of metaphase or anaphase A, 38 had long spindles indicative of anaphase B, and 4 had no spindles (Figure 1; cdc15-140). This suggested that Cdc15p is not strictly required for localization of Cdc4p to the medial ring structure at early stages of division (metaphase and anaphase) and is similar to what has been observed in SIN mutants (Wu et al., 2003). Cdc7p is a protein kinase that is an essential component of the SIN pathway (Krapp et al., 2004). When we shifted asynchronously growing temperature-sensitive cdc7-24 mutant cells to the restrictive temperature for 3 h, we found that, as in the case of the cdc15-140 mutant, the majority of cells with Cdc4p rings displayed mitotic spindles (32 of 50 cells), although rings appeared to persist longer after spindle breakdown, compared with the cdc15-140 mutant (Figure 1; cdc7-24).
Figure 1.
Formation of actomyosin rings during metaphase and anaphase in cdc15 mutant cells. cdc15-140, cdc15-287, and cdc7-24 cells were grown asynchronously at the permissive temperature and shifted to the restrictive temperature for 3 h. Spores from a cdc15Δ::ura4+/cdc15+ strain were incubated for 3 h in YES medium to assist germination, washed, and shifted to selective medium lacking uracil for 24 h. Cells were fixed and stained with α-Cdc4p antibodies, α-TAT1 antibodies, and DAPI to visualize actomyosin rings, tubulin, and DNA, respectively.
We have shown that nearly all cdc15-140 mutant cells with Cdc4p rings were in various stages of nuclear separation, indicated by the presence of a mitotic spindle. We then asked whether the reverse is true, i.e., if all cdc15-140 cells with spindles would form actomyosin rings. Of 50 cdc15-140 mutant cells with anaphase spindles, 49 had assembled Cdc4p rings (Figure 1; cdc15-140). This observation supports previous studies that used centrifugal elutriation to synchronize cdc15-140 mutant cells and demonstrates their ability to form transient actomyosin rings (Balasubramanian et al., 1998). To test whether the ability to form actomyosin rings at early stages of mitosis may be specific to the cdc15-140 allele, we shifted asynchronously growing temperature-sensitive cdc15-287 mutant cells to the restrictive temperature for 3 h. Of 50 cdc15-287 mutant cells with anaphase spindles, 47 had assembled Cdc4p rings (Figure 1; cdc15-287). This suggested that actomyosin ring assembly in early mitosis may not require Cdc15p.
The cdc15-140 and cdc15-287 mutant alleles are completely defective for septum formation at the restrictive temperature (Nurse et al., 1976; Chang et al., 1996). However, it cannot be ruled out that there may be residual function associated with the mutant protein that may be sufficient for actomyosin ring assembly during early stages of mitosis but insufficient for completion of cytokinesis. To address this concern, we germinated spores from a strain in which the cdc15+ open reading frame was replaced by the ura4+-cassette in selective medium, allowing only the spores carrying the cdc15 deletion to germinate and grow (Fankhauser et al., 1995). Staining for tubulin and Cdc4p revealed that 90% (90 of 100 cells) of cells with short spindles indicative of metaphase or anaphase A displayed Cdc4p in a ring. Twenty-six of these metaphase cells had multiple short spindles, suggesting that these cells had failed in previous rounds of cytokinesis and are indeed deleted for cdc15. Likewise, 99% (198 of 200 cells) of cells with elongated spindles indicative of anaphase B exhibited Cdc4p ring localization. Fifty-nine of these anaphase cells had multiple long spindles (Figure 1; cdc15Δ). We did not observe cells displaying Cdc4p rings that did not have a spindle. Our results obtained from examination of cdc15Δ cells show that Cdc15p is not required for actomyosin ring formation in early stages of cell division and that our results obtained with the temperature-sensitive cdc15-140 and cdc15-287 mutant alleles are likely not due to residual activity of the mutant protein.
cdc15 Mutant Cells Maintain Stable Actomyosin Rings in Metaphase
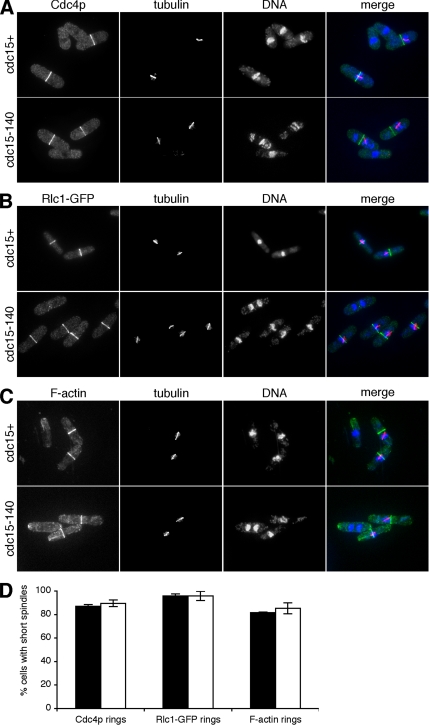
To more rigorously examine the possibility that Cdc15p was not required for actomyosin ring assembly in early mitosis, we arrested cdc15+ and cdc15-140 cells in metaphase by activation of the spindle assembly checkpoint through overexpression of mad2+ (He et al., 1997). Cells were shifted to the restrictive temperature for 3 h to ensure inactivation of mutant Cdc15p. It has been shown previously that 20–50% of cells arrest in metaphase upon overexpression of mad2+ (He et al., 1997; Guertin et al., 2000). To ensure that the observed cells were indeed arrested, we stained cells for tubulin and Cdc4p. Of all cells with short spindles indicative of metaphase arrest, 87% of wild-type and 90% of cdc15-140 cells displayed Cdc4p rings (Figure 2, A and D). We conclude that Cdc15p function is not required for Cdc4p ring assembly in metaphase.
Figure 2.
Maintenance of stable actomyosin rings in metaphase in cdc15 mutant cells. (A) pREP1-mad2+ and cdc15-140 pREP1-mad2+ cells were arrested in metaphase by growth in medium lacking thiamine for 24 h. Cells were shifted to the restrictive temperature for 3 h, fixed, and stained with α-Cdc4p antibodies, α-TAT1 antibodies, and DAPI to visualize actomyosin rings, tubulin, and DNA, respectively. (B) pREP1-mad2+ rlc1-GFP and cdc15-140 pREP1-mad2+ rlc1-GFP were grown and treated as in A except for staining with α-GFP antibodies to visualize actomyosin rings. (C) pREP1-mad2+ and cdc15-140 pREP1-mad2+ cells were grown and treated as in A except for staining with Alexa 488–conjugated phalloidin to visualize F-actin. (D) Quantitation of Cdc4p, Rlc1-GFP, and F-actin rings in cells with short (metaphase) spindles in pREP1-mad2+ [rlc1-GFP] (■) and cdc15-140 pREP1-mad2+ [rlc1-GFP] (□).
To test whether actomyosin ring components other than Cdc4p required Cdc15p function to assemble at the future division site in early mitosis, we stained cells from the same experiment with tubulin and Alexa 488–conjugated phalloidin to visualize F-actin. Furthermore, we carried out an identical experiment with strains expressing Rlc1-GFP (Rlc1p is the regulatory light chain of myosin II). Of all cells arrested in metaphase, 96% of both wild-type and cdc15-140 cells displayed Rlc1-GFP rings (Figure 2, B and D). Likewise, 82% of wild-type and 85% of cdc15-140 cells exhibited F-actin in a medial ring structure (Figure 2, C and D). Our results show that both F-actin and myosin II localize to the actomyosin ring in the absence of functional Cdc15p.
The formin Cdc12p has been implicated in actomyosin ring formation because cdc12-112 mutant cells lack any detectable ring structures (Chang et al., 1997). Cdc15p and Cdc12p directly interact and it has been proposed that Cdc15p recruits Cdc12p to the division site (Carnahan and Gould, 2003). Because our results indicated that cdc15-140 mutant cells are able to form rings containing F-actin and myosin II in metaphase, we asked whether Cdc12p was also localized to the middle of the cell at this stage of mitosis. To this end we arrested cdc15+ cells and cdc15-140 cells expressing Cdc12-GFP in metaphase as described above. When we stained cells for tubulin and Cdc12-GFP, we found that of all cells with short spindles, 86% of wild-type cells and 78% of cdc15-140 cells displayed Cdc12-GFP rings (Figure 3, A and C). However, we noted that the intensity of the Cdc12-GFP signal was reduced in cdc15 mutant cells compared with wild-type cells (Figure 3A). We conclude that S. pombe cells recruit the formin Cdc12p to the division site in metaphase even when Cdc15p function is compromised, although Cdc15p likely contributes to the maximal recruitment or retention of Cdc12p at the division site.
Figure 3.
Maintenance of stable formin rings in metaphase in cdc15 mutant cells. (A) pREP1-mad2+ cdc12-GFP and cdc15-140 pREP1-mad2+ cdc12-GFP cells were arrested in metaphase by growth in medium lacking thiamine for 24 h. Cells were shifted to the restrictive temperature for 3 h, fixed, and stained with α-GFP antibodies, α-TAT1 antibodies, and DAPI to visualize formin rings, tubulin, and DNA, respectively. (B) pREP1-mad2+ cdc15-GFP cells were grown and treated as in A except for staining with α-GFP antibodies to visualize Cdc15p rings. (C) Quantitation of Cdc12-GFP rings in cells with short (metaphase) spindles in pREP1-mad2+ cdc12-GFP (■) and cdc15-140 pREP1-mad2+ cdc12-GFP (□) and of Cdc15-GFP rings in cells with short (metaphase) spindles in pREP1-mad2+ cdc15-GFP (▩).
Because our observations suggested that in metaphase Cdc15p is dispensable for actomyosin ring formation, we asked whether Cdc15p itself is present at the division site under our experimental conditions. Thus, we arrested Cdc15-GFP cells in metaphase as described above. Staining for tubulin and Cdc15-GFP showed that 91% of all cells with short spindles displayed Cdc15-GFP in a ring (Figure 3, B and C). We therefore conclude that, although Cdc15p localizes to the ring early in mitosis and may contribute to the maximal recruitment or retention of Cdc12p to the medial ring, Cdc15p is not essential for ring assembly during early stages of mitosis.
Mid1p Is Required for Actomyosin Ring Maintenance in Metaphase in the Absence of Functional Cdc15p
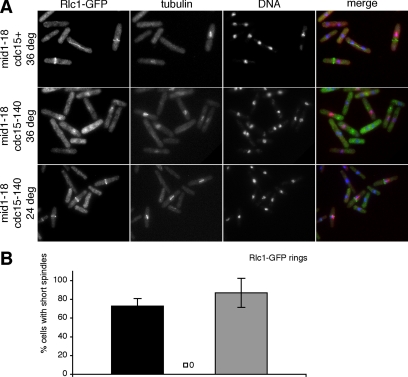
We have shown that cdc15 mutant cells form actomyosin rings in metaphase and anaphase and that recruitment of Cdc12p to the division site is reduced but not abolished by the absence of Cdc15p function. We sought to identify other gene products that may (directly or indirectly through interacting proteins) execute the functions of Cdc15p at early stages of cell division. We chose the anillin-related Mid1p as a candidate because it is the first known protein to localize to the future division site (Wu et al., 2003), it is involved in myosin II recruitment to the middle of the cell (Motegi et al., 2004), and overexpression of mid1+ leads to accumulation of actin structures in the medial region (Paoletti and Chang, 2000). Moreover, actomyosin ring assembly in cycling mid1 mutants is delayed compared with wild-type control cells (Wu et al., 2003). To test whether the formation of actomyosin rings in cdc15-140 mutant cells depended on the function of Mid1p, we arrested cdc15+ mid1-18 cells and cdc15-140 mid1-18 cells in metaphase as described above in strains expressing Rlc1-GFP. When we stained cells for tubulin and Rlc1-GFP, we found that of all cells with short spindles 73% of the mid1-18 single mutant cells but none of the cdc15-140 mid1-18 double mutant cells exhibited actomyosin rings (Figure 4, A and B). Although actomyosin rings in the mid1-18 single mutant were frequently mispositioned or aligned with the long axis of the cell, the cdc15-140 mid1-18 double mutant cells often displayed spot-like structures of Rlc1-GFP (Figure 4A) that resembled the interphase progenitor thought to be a template for the actomyosin ring (Wong et al., 2002). To ensure that the failure to form rings in the cdc15-140 mid1-18 double mutant was indeed due to the loss of function of these two genes and not the reporter gene, we examined double mutant cells arrested at metaphase that had been kept at the permissive temperature throughout the experiment. Of all cells with short spindles 87% displayed an actomyosin ring (Figure 4, A and B). Taken together with our results from metaphase arrested wild-type and cdc15-140 cells (Figure 2), we show that Mid1p has a role not only in ring positioning but also in formation of the actomyosin ring, which is only uncovered in the absence of functional Cdc15p.
Figure 4.
Requirement of Mid1p for actomyosin ring maintenance in metaphase in the absence of functional Cdc15p. (A) mid1-18 pREP1-mad2+ rlc1-GFP and cdc15-140 mid1-18 pREP1-mad2+ rlc1-GFP cells were arrested in metaphase by growth in medium lacking thiamine for 24 h. Cells were shifted to the restrictive temperature for 3 h or kept at the permissive temperature as control, fixed and stained with α-GFP antibodies, α-TAT1 antibodies, and DAPI to visualize actomyosin rings, tubulin, and DNA, respectively. (B) Quantitation of actomyosin rings in cells with short (metaphase) spindles in mid1-18 pREP1-mad2+ rlc1-GFP at the restrictive temperature (■) and cdc15-140 mid1-18 pREP1-mad2+ rlc1-GFP at the restrictive (□; y = 0) and permissive temperature (▩).
cdc15 Mutant Cells Fail to Form or Maintain Actomyosin Rings when SIN Is Active
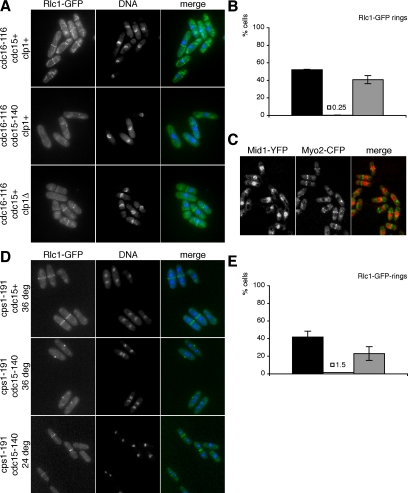
Previous studies have shown that actomyosin rings in cycling cdc15 mutant cells are maintained only transiently (Balasubramanian et al., 1998). Moreover, we did not observe cdc15Δ cells with rings in other stages than metaphase or anaphase. This suggested that Cdc15p may be required for ring formation or maintenance after completion of anaphase when the SIN pathway is activated after cyclin B proteolysis (Guertin et al., 2000). Previous studies have shown that activation of SIN leads to assembly of actomyosin rings and division septa from all cell cycle stages, even in the absence of mitosis (Fankhauser et al., 1993; Schmidt et al., 1997). Cdc16p is part of a two-component GTPase-activating protein negatively regulating the small GTPase Spg1p believed to be a key regulator of the SIN signaling cascade, depending on the nucleotide bound to it. Loss of Cdc16p function leads to ectopic SIN activation (Minet et al., 1979; Fankhauser et al., 1993). To test whether Cdc15p function is required for actomyosin ring formation in cells with active SIN, we arrested cdc15+ cdc16-116 and cdc15-140 cdc16-116 cells in S phase by addition of HU to the medium. Cells were then shifted to the restrictive temperature to inactivate the Cdc15p and Cdc16p mutant proteins leading to activation of SIN. Both strains carried Rlc1-GFP as a marker for the actomyosin ring. Eighty minutes after shift to the restrictive temperature 52% of cdc16-116 single mutant cells had formed actomyosin rings. In contrast, the cdc15-140 cdc16-116 double mutant cells did not form rings throughout the course of the experiment (Figure 5, A and B). This failure to assemble actomyosin rings was indeed due to the loss of function of these two genes, because cdc15-140 cdc16-116 double mutant control cells that were grown asynchronously at the permissive temperature exhibited no defect in actomyosin ring formation (unpublished data). We conclude that Cdc15p is essential for assembly of actomyosin rings in response to SIN activation.
Figure 5.
Failure to form or maintain actomyosin rings in cdc15 mutant cells when the septation initiation network is active. (A) cdc16-116 rlc1-GFP, cdc15-140 cdc16-116 rlc1-GFP and clp1Δ::ura4+ cdc16-116 rlc1-GFP cells were arrested in S phase by growth in the presence of 12 mM HU. After 6 h cells were shifted to the restrictive temperature (t = 0 min) to ectopically activate the septation initiation network. At t = 80 min, cells were fixed, stained with DAPI to visualize DNA, and examined for presence of actomyosin rings marked by Rlc1-GFP epifluorescence. (B) Quantitation of actomyosin rings in cdc16-116 rlc1-GFP cells (■), cdc15-140 cdc16-116 rlc1-GFP cells (□; y = 0.25) and clp1Δ::ura4+ cdc16-116 rlc1-GFP cells (▩). (C) cdc16-116 mid1-YFP CFP-myo2 cells were arrested in S phase as above. At t = 60 min, live cells were imaged by confocal microscopy. (D) cps1-191 rlc1-GFP and cdc15-140 cps1-191 rlc1-GFP cells were shifted to the restrictive temperature for 4 h or kept at the permissive temperature as control. Samples were fixed, stained with DAPI to visualize DNA, and examined for presence of actomyosin rings marked by Rlc1-GFP epifluorescence. (E) Quantitation of actomyosin rings in cps1-191 rlc1-GFP cells at the restrictive temperature (■) and cdc15-140 cps1-191 rlc1-GFP cells at the restrictive (□; y = 1.5) and permissive temperature (▩).
The rings assembled upon activation of SIN in S phase contained Rlc1p (Figure 5A), the myosin II heavy chain Myo2p (Figure 5C), and F-actin and Cdc4p (unpublished data). Interestingly, however, Mid1p was not detected in the rings assembled in interphase-arrested cells and was instead present in the nucleus in these cells. Figure 5C shows localization of Mid1p in relation to Myo2p in interphase-arrested cells with ectopic SIN activation. On the basis of these studies we conclude that rings assembled upon SIN activation do not contain Mid1p, but contain several other components of the actomyosin ring. These observations are consistent with previous findings that Mid1p is not detected in a large fraction of cells containing unconstricted rings, upon delays in septation in the cps1-191 mutant, under conditions in which SIN appears to remain active (Pardo and Nurse, 2003).
Previous studies have shown that S. pombe cells are able to remain viable and form colonies in response to minor perturbations of the division machinery. For example when the function of the catalytic subunit of 1,3-β-glucan synthase, encoded by cps1+ (Le Goff et al., 1999; Liu et al., 1999, 2000), is partially compromised, cells remain viable and in a cytokinesis competent state that is characterized by prolonged maintenance of the actomyosin ring and a G2 nuclear cycle delay (Mishra et al., 2004). These responses require the SIN and the phosphatase Clp1p (Cueille et al., 2001; Trautmann et al., 2001; Mishra et al., 2004). To test whether Cdc15p function is required for the prolonged maintenance of the actomyosin ring upon cytokinesis delay, we shifted cdc15+ cps1-191 and cdc15-140 cps1-191 cells to the restrictive temperature leading to inactivation of mutant Cdc15p and Cps1p. Both strains expressed Rlc1-GFP as a marker for the actomyosin ring. Four hours after temperature shift, 42% of cps1-191 single mutant cells but only 1.5% of cdc15-140 cps1-191 double mutant cells displayed actomyosin rings (Figure 5, D and E). This failure to maintain actomyosin rings was indeed due to the loss of function of these two genes because cdc15-140 cps1-191 double mutant control cells that were kept at the permissive temperature showed no defect in actomyosin ring formation (23% displayed rings; Figure 5, D and E). These observations indicate that Cdc15p function is required for maintenance of the actomyosin ring in response to cytokinesis delay upon perturbation of the division machinery.
Cdc15p Acts Downstream of SIN
Previous studies that examined the genetic interaction between cdc15 and SIN indicated that components of SIN may function upstream of Cdc15p (Marks et al., 1992). This idea is supported by the finding that upon cytokinesis failure, cdc15 mutants delay progression through the next nuclear cycle and maintain a binuclear configuration in a SIN- and Clp1p-dependent manner (Jianhua Liu and M. K. Balasubramanian, unpublished results; Mishra et al., 2004). To further examine the function of Cdc15p relative to SIN, we characterized the localization of the nonessential SIN component, the phosphatase Clp1p, and of a representative essential SIN molecule, the protein kinase Cdc7p, in cdc15+ cps1-191 cells and cdc15-140 cps1-191 cells.
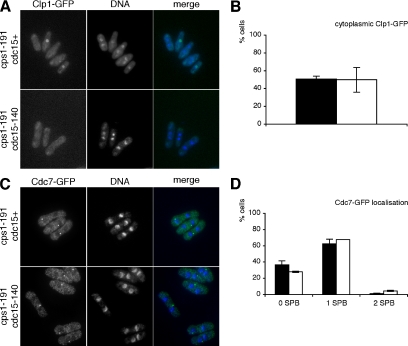
Clp1p localizes to the nucleolus in interphase and to the cytoplasm during cell division (Cueille et al., 2001; Trautmann et al., 2001). Upon cytokinesis delay, Clp1p is retained in the cytoplasm until completion of cytokinesis. This retention requires active SIN signaling and the 14-3-3 protein Rad24p (Trautmann et al., 2001; Mishra et al., 2005). When we shifted cdc15+ cps1-191 and cdc15-140 cps1-191 cells that expressed Clp1-GFP to the restrictive temperature for 4 h, 51% of cps1-191 single mutant cells and 50% of cdc15-140 cps1-191 double mutant cells displayed Clp1-GFP localization in the cytoplasm, whereas all other cells showed a visible concentration of Clp1p in the nucleolus (Figure 6, A and B). Note that cells that show nucleolar Clp1-GFP localization in Figure 6A are uninucleate cells and were not undergoing mitosis. Hence, the retention of Clp1p in the cytoplasm upon cytokinesis delay is not dependent on Cdc15p function.
Figure 6.
Cytoplasmic retention of Clp1p and spindle pole body localization of Cdc7p are maintained upon cytokinesis delay in cdc15 mutant cells. (A) cps1-191 clp1-GFP and cdc15-140 cps1-191 clp1-GFP cells were shifted to the restrictive temperature for 4 h. Samples were fixed, stained with DAPI to visualize DNA, and examined for cytoplasmic localization of Clp1-GFP (epifluorescence). (B) Quantitation of cytoplasmic Clp1-GFP localization in cps1-191 clp1-GFP cells (■) and cdc15-140 cps1-191 clp1-GFP cells (□). (C) cps1-191 cdc7-GFP and cdc15-140 cps1-191 cdc7-GFP cells were shifted to the restrictive temperature for 4 h. Samples were fixed and stained with α-GFP antibodies and DAPI to visualize Cdc7p localization and DNA, respectively. (D) Quantitation of Cdc7-GFP localization in cps1-191 cdc7-GFP cells (■) and cdc15-140 cps1-191 cdc7-GFP cells (□).
In cycling cells, Cdc7p localizes to both spindle pole bodies (SPBs) early in mitosis and to only one SPB in later stages of cell division, and Cdc7p shows no distinct localization during interphase (Sohrmann et al., 1998). On cytokinesis delay, Cdc7p is maintained on one SPB for prolonged periods (Liu et al., 1999; Mishra et al., 2004). When we shifted cdc15+ cps1-191 and cdc15-140 cps1-191 cells that expressed Cdc7-GFP to the restrictive temperature for 4 h, similar percentages of both cps1-191 single mutant cells (63%) and cdc15-140 cps1-191 double mutant cells (68%) localized Cdc7-GFP to a single SPB (Figure 6, C and D). Most other cells showed no distinct localization of Cdc7-GFP, whereas a minor fraction in both strains localized Cdc7-GFP to both SPBs (Figure 6D). Thus, the prolonged maintenance of Cdc7p on a single SPB is not dependent on Cdc15p function.
Taken together, our results further establish a pathway in which Cdc15p functions downstream of SIN. The presence of cytoplasmic Clp1p and active SIN signaling in cdc15-140 cps1-191 cells (Figure 6, A–D) also suggests that the lack of actomyosin rings in these double mutant cells (Figure 5, D and E) is not due to disruption of the signaling network that establishes the cytokinesis delay but likely due to structural problems related to actomyosin ring maintenance.
Hypophosphorylation of Cdc15p Occurs in At Least Two Distinct Steps
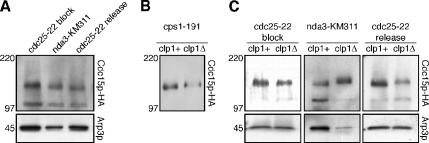
We have shown that the requirement of Cdc15p for actomyosin ring formation is dependent on the stage of the cell cycle. To gain a biochemical handle we set out to assess changes in the molecular properties of Cdc15p that may correlate with its function. Cdc15p is a phosphoprotein that is hyperphosphorylated during interphase and hypophosphorylated during cell division resulting in the detection of slow and fast migrating forms of Cdc15p by Western blotting (Fankhauser et al., 1995). To compare the mobility of Cdc15p at different stages of the cell cycle, we first obtained samples from synchronous cultures to serve as a reference. We arrested cdc25-22 cells expressing HA-tagged Cdc15p at the G2/M boundary by incubation at the restrictive temperature. Under these conditions Cdc15p is found in a slow migrating band (Figure 7A; cdc25-22 block). On shift to the permissive temperature, the fastest migrating form of Cdc15p first appears 60 min after release (Figure 7A; cdc25-22 release).
Figure 7.
Hypophosphorylation of Cdc15p in at least two distinct steps, of which one is dependent on Clp1p, and maintenance of hypophosphorylation upon cytokinesis delay. (A) cdc25-22 cdc15-HA cells were arrested at the G2/M boundary by incubation at the restrictive temperature for 4 h and synchronously released by shift to the permissive temperature (t = 0 min). nda3-KM311 cdc15-HA cells were arrested at metaphase by incubation at the restrictive temperature for 6 h. Samples were taken (cdc25-22 block is t = 0 min; cdc25-22 release is t = 60 min), and total protein lysates were prepared, separated by SDS-PAGE, blotted, and detected with α-HA antibodies. Note that the protein level of Cdc15p-HA differed among the samples; total protein per lane was adjusted accordingly to compare similar amounts of Cdc15p-HA. (B) Samples from cps1-191 cdc15-HA cells and clp1Δ::ura4+ cps1-191 cdc15-HA cells incubated at the restrictive temperature for 4 h were separated by SDS-PAGE, blotted, and detected with α-HA antibodies. Membrane preparations of these samples were used because they allowed for a slightly improved resolution of Cdc15p-HA bands. (C) clp1Δ::ura4+ cdc25-22 cdc15-HA cells were arrested at the G2/M boundary by incubation at the restrictive temperature for 4 h and synchronously released by shift to the permissive temperature (t = 0 min). clp1Δ::ura4+ nda3-KM311 cdc15-HA cells were arrested at metaphase by incubation at the restrictive temperature for 6 h. These samples and samples from experiments in A (cdc25-22 block is t = 0 min; cdc25-22 release is t = 60 min for clp1+ and t = 80 min for clp1Δ) were separated by SDS-PAGE, blotted, and detected with α-HA antibodies. Note that the protein level of Cdc15p-HA differed among the samples; total protein per lane was adjusted accordingly to compare similar amounts of Cdc15p-HA.
To study the mobility of Cdc15p in cells arrested in metaphase we utilized the β-tubulin mutant nda3-KM311 (Toda et al., 1983), which yields a tighter metaphase arrest compared with overexpression of mad2+. We observed that Cdc15p mobility at metaphase is faster than in interphase but slower than in cells undergoing cytokinesis (Figure 7A; nda3-KM311 compared with cdc25-22 block and cdc25-22 release). We conclude that hypophosphorylation of Cdc15p during cell division occurs in at least two distinct steps: in metaphase and during cytokinesis.
Hypophosphorylation of Cdc15p Is Maintained upon Cytokinesis Delay
We have shown that maintenance of actomyosin rings during cytokinesis delay upon perturbations to the division machinery depends on Cdc15p. To assess the mobility of Cdc15p under these conditions, we incubated cps1-191 mutant cells at the restrictive temperature to induce cytokinesis delay. Interestingly, we observed Cdc15p in a fast migrating band similar to cycling cells undergoing cytokinesis. The mobility was faster than in cells that did not delay cytokinesis because of deletion of the phosphatase Clp1p (Figure 7B, cps1-191 clp1+ compared with cps1-191 clp1Δ). Note that the majority of cells in both cultures are in G2 (Le Goff et al., 1999; Liu et al., 1999). However, there is no actomyosin ring and SIN is not active in the latter, whereas there is a stable actomyosin ring and active SIN signaling in the former. We conclude that in cells with cytokinesis delay, upon perturbation of the division machinery, Cdc15p is maintained in a fast migrating form indicating hypophosphorylation. This is different from cells that succeed in cell division, where Cdc15p returns to the hyperphosphorylated form (Fankhauser et al., 1995).
Hypophosphorylation of Cdc15p Is Partially Dependent on Clp1p
Maintenance of active SIN (and of a stable actomyosin ring) upon cytokinesis delay requires the Clp1p phosphatase (Mishra et al., 2004). Given that Cdc15p appeared to be hypophosphorylated upon cytokinesis delay, we asked if its dephosphorylation depended on Clp1p. To compare the mobility of Cdc15p in the presence and absence of Clp1p, we first obtained samples from synchronous cultures. We arrested clp1Δ cdc25-22 cells at the G2/M boundary by incubation at the restrictive temperature. Under these conditions Cdc15p is found in a slow migrating band (Figure 7C; cdc25-22 block clp1Δ). On shift to the permissive temperature, the fast migrating form of Cdc15p first appears 80 min after release (Figure 7C; cdc25-22 release clp1Δ). Note that although we observed the occurrence of fast migrating Cdc15p in clp1Δ cells 20 min later than in clp1+ cells, the peak of actomyosin ring formation was similarly delayed by 20 min in clp1Δ cells compared with clp1+ cells (unpublished data). This is consistent with results published in previous studies: because Clp1p has an additional role in mitotic exit by dephosphorylating the mitosis-promoting phosphatase Cdc25p, progression through mitosis after release from a G2/M block is delayed in a clp1Δ background compared with clp1+ (Wolfe and Gould, 2004). We therefore considered t = 60 min in clp1+ cells and t = 80 min in clp1Δ cells to be equivalent with respect to mitotic progression.
We also obtained samples from clp1Δ cells arrested in metaphase by incubation of the β-tubulin mutant nda3-KM311 at the restrictive temperature. Next, we compared the mobility of Cdc15p in clp1+ cells synchronized at the G2/M boundary, in metaphase and in cytokinesis, respectively, to the equivalent samples in a clp1Δ background. Although we were unable to detect any difference in Cdc15p mobility between clp1+ and clp1Δ cells arrested at G2/M (Figure 7C; cdc25-22 block), we observed a faster migration of Cdc15p in clp1+ compared with clp1Δ in both cells arrested at metaphase and cells synchronized in cytokinesis (Figure 7C; nda3-KM311 and cdc25-22 release). Thus, the Clp1p phosphatase plays a (direct or indirect) role in the hypophosphorylation of Cdc15p during cell division. However, hypophosphorylation is only partially affected because we detected a mobility shift of Cdc15p upon entry in cytokinesis even in clp1Δ cells (unpublished data). It is possible, that Clp1p contributes to only some but not all dephosphorylation events on Cdc15p during cell division. Alternatively, there may be another pathway that is only partially redundant to Clp1p with respect to Cdc15p dephosphorylation. Interestingly, when we arrested clp1Δ cdc16-116 cells in S phase by addition of HU to the medium and ectopically activated the SIN pathway by a shift to the restrictive temperature, these double mutant cells were still able to assemble actomyosin rings, albeit at somewhat reduced frequency compared with cdc16-116 single mutant cells (41% of clp1Δ cdc16-116 double mutant cells vs. 52% of cdc16-116 single mutant cells displayed Rlc1-GFP rings 80 min after shift to the restrictive temperature; Figure 5, A and B). We conclude that complete hypophosphorylation of Cdc15p to wild-type levels depends on the Clp1p phosphatase and the maximally dephosphorylated form of Cdc15p may be most potent in nucleating actomyosin rings in response to SIN and Clp1p activation.
It has been shown previously that Cdc15p expression levels are elevated in dividing cells compared with interphase cells (Fankhauser et al., 1995). We noted however that compared with interphase cells, Cdc15p levels are also increased when cells are arrested in metaphase or exhibit cytokinesis delays (Figure 7A and unpublished data). Moreover, Cdc15p levels are higher in clp1Δ cells that are arrested in metaphase or undergoing division as compared with clp1+ cells at comparable cell cycle stages (Figure 7C). Future studies should investigate the precise timing of and molecular machinery involved in cell cycle-dependent fluctuations of Cdc15p levels.
DISCUSSION
We have shown that asynchronously growing cdc15 mutant cells assemble actomyosin rings upon entry into mitosis (Figure 1). Results obtained from arrested cells indicate that cdc15 mutants maintain actomyosin rings for prolonged periods of time if progression through mitosis is halted at metaphase (Figures 2 and 3). Although our observations support previous studies (Balasubramanian et al., 1998; Arai and Mabuchi, 2002), our work seems to contradict some other studies (Fankhauser et al., 1995; Chang et al., 1996; Carnahan and Gould, 2003). Given that actomyosin rings are assembled in germinating cdc15Δ spores undergoing metaphase or anaphase (Figure 1), we think it is unlikely that the differences between our work and that of others are due to the relative strengths of the mutant alleles used. We have observed rings using four different probes (GFP-tagged Rlc1p and Cdc12p to visualize myosin II and formin, respectively; Alexa 488–conjugated phalloidin to visualize F-actin and α-Cdc4p antibodies to visualize myosin II), and we have ensured that the cells monitored are in mitosis by microtubule staining. We have also shown that cdc15-140 and cdc15Δ mutants disassemble actomyosin rings upon mitotic exit. Examination of cells at different points in the cell cycle may have contributed to the observed phenotypic differences, in particular because entry into the next round of mitosis occurs only after a significant delay after cytokinesis failure in the absence of Cdc15p and other actomyosin ring proteins (Cueille et al., 2001; Trautmann et al., 2001; Mishra et al., 2004). Thus, we conclude that Cdc15p is dispensable for actomyosin ring assembly at mitosis but is required for its maintenance and constriction upon mitotic exit.
We have found that in the absence of functional Cdc15p the anillin-related Mid1p becomes essential for assembly of the actomyosin ring (Figure 4). Although Mid1p has previously been implicated in ring positioning (Sohrmann et al., 1996), we have shown here that it has an additional role in ring formation that is only uncovered in a cdc15 mutant background. The fact that mid1 mutants assemble rings (albeit mispositioned and with a somewhat reduced frequency) and maintain them upon metaphase arrest (Figure 4) suggests a functional redundancy for actomyosin ring formation: we propose that both Mid1p- and Cdc15p-dependent pathways contribute to ring assembly early in mitosis. Thus, transient ring formation in cdc15 mutants may be mediated by Mid1p. Inversely, because Cdc15p is present at the division site in metaphase (Figure 3, B and C), it may recruit Cdc12p and other ring components, and hence actomyosin rings assemble even in the absence of functional Mid1p. In the concomitant absence of both Mid1p and Cdc15p function, actomyosin ring formation is completely aborted. Given that mid1 mutants assemble rings later in mitosis (Wu et al., 2003) compared with wild-type, the Mid1p-dependent mechanism may play a primary role in ring assembly in metaphase, although the Cdc15p-dependent mechanism may compensate for this if cells are held in metaphase.
In S. pombe, actomyosin ring assembly at metaphase is independent of the function of the SIN pathway but maintenance and constriction of the actomyosin ring upon mitotic exit requires SIN function (Wu et al., 2003; Krapp et al., 2004). SIN is also indispensable for prolonged maintenance of the actomyosin ring in response to cytokinesis delay caused by mild perturbation of the cell division and septation apparatuses (Le Goff et al., 1999; Liu et al., 2000). Finally, ectopic activation of SIN in interphase cells leads to actomyosin ring assembly and septation before entry into mitosis (Fankhauser et al., 1993; Schmidt et al., 1997). Given that both SIN mutants and cdc15 mutants assemble actomyosin rings during mitosis but are unable to maintain these rings after anaphase, it seems likely that SIN-mediated actomyosin ring assembly and maintenance may function via Cdc15p. Consistently, we find that cps1-191 mutants, which maintain actomyosin rings for prolonged periods in a SIN-dependent manner, are unable to do so in the absence of functional Cdc15p (Figure 5, D and E). Furthermore, SIN-dependent assembly of actomyosin rings and septa induced by ectopic SIN activation in interphase-arrested cells also depends on Cdc15p (Figure 5, A and B). On the basis of these studies we conclude that SIN-mediated actomyosin ring assembly and septation may depend on Cdc15p function. Interestingly, we have found that Mid1p is not detectable in rings induced by ectopic SIN activation (Figure 5C).
The phosphatase Clp1p is retained in the cytoplasm upon cytokinesis delay in a SIN-dependent manner (Trautmann et al., 2001). This cytoplasmic retention is maintained even in the absence of Cdc15p function (Figure 6, A and B). Similarly, the protein kinase Cdc7p continues to localize to a single SPB upon cytokinesis delay in a Clp1p-dependent manner, suggesting a positive feedback loop between Clp1p and SIN to sustain each other's activity as well as stability of the actomyosin ring and delay of progression of the nuclear cycle (Mishra et al., 2004). The SPB localization of Cdc7p is believed to indicate active SIN signaling (McCollum and Gould, 2001) and is maintained even in the absence of Cdc15p function (Figure 6, C and D). The cytoplasmic retention of Clp1p and localization of Cdc7p in one SPB in cdc15 mutants suggests that Cdc15p does not act upstream of or as an integral part of the feedback loop between Clp1p and SIN because in that case loss of function of Cdc15p would abrogate the maintenance of their respective localization patterns. We hence conclude that SIN signaling may regulate Cdc15p function, which in turn may induce actomyosin ring formation and maintenance.
Previous studies have shown that Cdc15p undergoes dephosphorylation upon progression through mitosis (Fankhauser et al., 1995). We have further characterized the mobility of Cdc15p at various stages of the cell cycle. We have shown that Cdc15p exists in at least three forms: a slow migrating hyperphosphorylated form in interphase, an intermediately phosphorylated form in metaphase, and a fast migrating hypophosphorylated form that appears during cytokinesis (Figure 7A). A fast migrating form of Cdc15p is also detected in cells delayed in cytokinesis although their nuclei have returned to a G2 configuration (Figure 7B). It is possible that increasing dephosphorylation of Cdc15p enhances its affinity toward the formin Cdc12p and activators of the Arp2/3 complex and may thus aid actomyosin ring assembly and maintenance. Alternatively, the stability of Cdc15p may be regulated by its phosphorylation status. Understanding the precise biochemical roles of these different phosphorylated and dephosphorylated forms depends on the identification and characterization of the phosphorylation sites. Interestingly, the Cdc15p homologue in S. cerevisiae, Hof1p, is degraded in late mitosis through a mechanism that includes the SCF-type E3 ubiquitin ligase Grr1p, the PEST-domain of Hof1p and possibly its phosphorylation (Blondel et al., 2005). Future studies should also investigate the role of ubiquitination in S. pombe Cdc15p function.
Prolonged actomyosin ring maintenance upon cytokinesis delay depends on SIN and Clp1p, a protein phosphatase of the Cdc14p family (Cueille et al., 2001; Trautmann et al., 2001). We have shown that although partial dephosphorylation of Cdc15p and the resulting shift in its mobility is detected in clp1Δ mutants progressing through mitosis, the fastest migrating form indicating maximal dephosphorylation is not detected (Figure 7C). It is currently unclear if Clp1p is directly responsible for the dephosphorylation of Cdc15p or if Clp1p activates another phosphatase that in turn dephosphorylates Cdc15p. In either case, the change in phosphorylation brought about by Clp1p is not essential for cell division in conditions of unperturbed cytokinesis, because S. pombe cells form rings and divide under standard laboratory conditions in the absence of clp1+ (Cueille et al., 2001; Trautmann et al., 2001). However, it may be important for prolonged maintenance of the actomyosin ring upon cytokinesis delay or for most efficient recruitment of other ring components to the division site.
In summary, we have shown that the FCH-domain protein Cdc15p is a key element downstream of SIN and plays an essential role in actomyosin ring assembly and maintenance after anaphase, although Cdc15p is dispensable for ring assembly early in mitosis. The activities of Cdc15p may in part be regulated by its phosphorylation status that changes upon progression through mitosis. Future studies should investigate the precise mechanism of SIN- and Cdc15p-mediated actomyosin ring maintenance upon mitotic exit as well as cytokinesis delay. Identification of the phosphorylation sites as well as characterization of the interacting protein kinases and phosphatases should help unravel the molecular function of Cdc15p in cytokinesis and its regulation through the cell cycle.
ACKNOWLEDGMENTS
We thank Dan McCollum for discussions and helpful comments on the manuscript. Strains and reagents were kindly shared by Kathy Gould, Keith Gull, Dan McCollum, Snezhana Oliferenko, Viesturs Simanis, and Jian-Qiu Wu. This work was supported by research funds from the Temasek Life Sciences Laboratory. V.W. and J.K. were supported by fellowships from the Singapore Millennium Foundation.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05-11-1086) on May 10, 2006.
REFERENCES
- Arai R., Mabuchi I. F-actin ring formation and the role of F-actin cables in the fission yeast Schizosaccharomyces pombe. J. Cell Sci. 2002;115:887–898. doi: 10.1242/jcs.115.5.887. [DOI] [PubMed] [Google Scholar]
- Balasubramanian M. K., Bi E., Glotzer M. Comparative analysis of cytokinesis in budding yeast, fission yeast and animal cells. Curr. Biol. 2004;14:R806–R818. doi: 10.1016/j.cub.2004.09.022. [DOI] [PubMed] [Google Scholar]
- Balasubramanian M. K., McCollum D., Chang L., Wong K. C., Naqvi N. I., He X., Sazer S., Gould K. L. Isolation and characterization of new fission yeast cytokinesis mutants. Genetics. 1998;149:1265–1275. doi: 10.1093/genetics/149.3.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian M. K., McCollum D., Gould K. L. Cytokinesis in fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1997;283:494–506. doi: 10.1016/s0076-6879(97)83039-x. [DOI] [PubMed] [Google Scholar]
- Blondel M., Bach S., Bamps S., Dobbelaere J., Wiget P., Longaretti C., Barral Y., Meijer L., Peter M. Degradation of Hof1 by SCF(Grr1) is important for actomyosin contraction during cytokinesis in yeast. EMBO J. 2005;24:1440–1452. doi: 10.1038/sj.emboj.7600627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnahan R. H., Gould K. L. The PCH family protein, Cdc15p, recruits two F-actin nucleation pathways to coordinate cytokinetic actin ring formation in Schizosaccharomyces pombe. J. Cell Biol. 2003;162:851–862. doi: 10.1083/jcb.200305012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang F., Drubin D., Nurse P. cdc12p, a protein required for cytokinesis in fission yeast, is a component of the cell division ring and interacts with profilin. J. Cell Biol. 1997;137:169–182. doi: 10.1083/jcb.137.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang F., Woollard A., Nurse P. Isolation and characterization of fission yeast mutants defective in the assembly and placement of the contractile actin ring. J. Cell Sci. 1996;109(Pt 1):131–142. doi: 10.1242/jcs.109.1.131. [DOI] [PubMed] [Google Scholar]
- Cueille N., Salimova E., Esteban V., Blanco M., Moreno S., Bueno A., Simanis V. Flp1, a fission yeast orthologue of the s. cerevisiae CDC14 gene, is not required for cyclin degradation or rum1p stabilisation at the end of mitosis. J. Cell Sci. 2001;114:2649–2664. doi: 10.1242/jcs.114.14.2649. [DOI] [PubMed] [Google Scholar]
- Fankhauser C., Marks J., Reymond A., Simanis V. The S. pombe cdc16 gene is required both for maintenance of p34cdc2 kinase activity and regulation of septum formation: a link between mitosis and cytokinesis? EMBO J. 1993;12:2697–2704. doi: 10.1002/j.1460-2075.1993.tb05931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fankhauser C., Reymond A., Cerutti L., Utzig S., Hofmann K., Simanis V. The S. pombe cdc15 gene is a key element in the reorganization of F-actin at mitosis. Cell. 1995;82:435–444. doi: 10.1016/0092-8674(95)90432-8. [DOI] [PubMed] [Google Scholar]
- Guertin D. A., Chang L., Irshad F., Gould K. L., McCollum D. The role of the sid1p kinase and cdc14p in regulating the onset of cytokinesis in fission yeast. EMBO J. 2000;19:1803–1815. doi: 10.1093/emboj/19.8.1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E., Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1988. [Google Scholar]
- He X., Patterson T. E., Sazer S. The Schizosaccharomyces pombe spindle checkpoint protein mad2p blocks anaphase and genetically interacts with the anaphase-promoting complex. Proc. Natl. Acad. Sci. USA. 1997;94:7965–7970. doi: 10.1073/pnas.94.15.7965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapp A., Gulli M. P., Simanis V. SIN and the art of splitting the fission yeast cell. Curr. Biol. 2004;14:R722–R730. doi: 10.1016/j.cub.2004.08.049. [DOI] [PubMed] [Google Scholar]
- Le Goff X., Woollard A., Simanis V. Analysis of the cps1 gene provides evidence for a septation checkpoint in Schizosaccharomyces pombe. Mol. Gen. Genet. 1999;262:163–172. doi: 10.1007/s004380051071. [DOI] [PubMed] [Google Scholar]
- Lippincott J., Li R. Involvement of PCH family proteins in cytokinesis and actin distribution. Microsc. Res. Tech. 2000;49:168–172. doi: 10.1002/(SICI)1097-0029(20000415)49:2<168::AID-JEMT9>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Liu J., Wang H., Balasubramanian M. K. A checkpoint that monitors cytokinesis in Schizosaccharomyces pombe. J. Cell Sci. 2000;113(Pt 7):1223–1230. doi: 10.1242/jcs.113.7.1223. [DOI] [PubMed] [Google Scholar]
- Liu J., Wang H., McCollum D., Balasubramanian M. K. Drc1p/Cps1p, a 1,3-beta-glucan synthase subunit, is essential for division septum assembly in Schizosaccharomyces pombe. Genetics. 1999;153:1193–1203. doi: 10.1093/genetics/153.3.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks J., Fankhauser C., Simanis V. Genetic interactions in the control of septation in Schizosaccharomyces pombe. J. Cell Sci. 1992;101(Pt 4):801–808. doi: 10.1242/jcs.101.4.801. [DOI] [PubMed] [Google Scholar]
- McCollum D., Balasubramanian M. K., Pelcher L. E., Hemmingsen S. M., Gould K. L. Schizosaccharomyces pombe cdc4+ gene encodes a novel EF-hand protein essential for cytokinesis. J. Cell Biol. 1995;130:651–660. doi: 10.1083/jcb.130.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCollum D., Feoktistova A., Morphew M., Balasubramanian M., Gould K. L. The Schizosaccharomyces pombe actin-related protein, Arp3, is a component of the cortical actin cytoskeleton and interacts with profilin. EMBO J. 1996;15:6438–6446. [PMC free article] [PubMed] [Google Scholar]
- McCollum D., Gould K. L. Timing is everything: regulation of mitotic exit and cytokinesis by the MEN and SIN. Trends Cell Biol. 2001;11:89–95. doi: 10.1016/s0962-8924(00)01901-2. [DOI] [PubMed] [Google Scholar]
- Minet M., Nurse P., Thuriaux P., Mitchison J. M. Uncontrolled septation in a cell division cycle mutant of the fission yeast Schizosaccharomyces pombe. J. Bacteriol. 1979;137:440–446. doi: 10.1128/jb.137.1.440-446.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra M., Karagiannis J., Sevugan M., Singh P., Balasubramanian M. K. The 14–3-3 protein rad24p modulates function of the cdc14p family phosphatase clp1p/flp1p in fission yeast. Curr. Biol. 2005;15:1376–1383. doi: 10.1016/j.cub.2005.06.070. [DOI] [PubMed] [Google Scholar]
- Mishra M., Karagiannis J., Trautmann S., Wang H., McCollum D., Balasubramanian M. K. The Clp1p/Flp1p phosphatase ensures completion of cytokinesis in response to minor perturbation of the cell division machinery in Schizosaccharomyces pombe. J. Cell Sci. 2004;117:3897–3910. doi: 10.1242/jcs.01244. [DOI] [PubMed] [Google Scholar]
- Moreno S., Klar A., Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Motegi F., Mishra M., Balasubramanian M. K., Mabuchi I. Myosin-II reorganization during mitosis is controlled temporally by its dephosphorylation and spatially by Mid1 in fission yeast. J. Cell Biol. 2004;165:685–695. doi: 10.1083/jcb.200402097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi N. I., Eng K., Gould K. L., Balasubramanian M. K. Evidence for F-actin-dependent and -independent mechanisms involved in assembly and stability of the medial actomyosin ring in fission yeast. EMBO J. 1999;18:854–862. doi: 10.1093/emboj/18.4.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurse P., Thuriaux P., Nasmyth K. Genetic control of the cell division cycle in the fission yeast Schizosaccharomyces pombe. Mol. Gen. Genet. 1976;146:167–178. doi: 10.1007/BF00268085. [DOI] [PubMed] [Google Scholar]
- Paoletti A., Chang F. Analysis of mid1p, a protein required for placement of the cell division site, reveals a link between the nucleus and the cell surface in fission yeast. Mol. Biol. Cell. 2000;11:2757–2773. doi: 10.1091/mbc.11.8.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo M., Nurse P. Equatorial retention of the contractile actin ring by microtubules during cytokinesis. Science. 2003;300:1569–1574. doi: 10.1126/science.1084671. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S., Wachtler V., Balasubramanian M. Cytokinesis in fission yeast: a story of rings, rafts and walls. Trends Genet. 2003;19:403–408. doi: 10.1016/S0168-9525(03)00149-5. [DOI] [PubMed] [Google Scholar]
- Schmidt S., Sohrmann M., Hofmann K., Woollard A., Simanis V. The Spg1p GTPase is an essential, dosage-dependent inducer of septum formation in Schizosaccharomyces pombe. Genes Dev. 1997;11:1519–1534. doi: 10.1101/gad.11.12.1519. [DOI] [PubMed] [Google Scholar]
- Sohrmann M., Fankhauser C., Brodbeck C., Simanis V. The dmf1/mid1 gene is essential for correct positioning of the division septum in fission yeast. Genes Dev. 1996;10:2707–2719. doi: 10.1101/gad.10.21.2707. [DOI] [PubMed] [Google Scholar]
- Sohrmann M., Schmidt S., Hagan I., Simanis V. Asymmetric segregation on spindle poles of the Schizosaccharomyces pombe septum-inducing protein kinase Cdc7p. Genes Dev. 1998;12:84–94. doi: 10.1101/gad.12.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda T., Kawate T., Chang F. Organization of a sterol-rich membrane domain by cdc15p during cytokinesis in fission yeast. Nat. Cell Biol. 2004;6:1142–1144. doi: 10.1038/ncb1189. [DOI] [PubMed] [Google Scholar]
- Toda T., Umesono K., Hirata A., Yanagida M. Cold-sensitive nuclear division arrest mutants of the fission yeast Schizosaccharomyces pombe. J. Mol. Biol. 1983;168:251–270. doi: 10.1016/s0022-2836(83)80017-5. [DOI] [PubMed] [Google Scholar]
- Trautmann S., Wolfe B. A., Jorgensen P., Tyers M., Gould K. L., McCollum D. Fission yeast Clp1p phosphatase regulates G2/M transition and coordination of cytokinesis with cell cycle progression. Curr. Biol. 2001;11:931–940. doi: 10.1016/s0960-9822(01)00268-8. [DOI] [PubMed] [Google Scholar]
- Wolfe B.A., Gould K. L. Fission yeast Clp1p phosphatase affects G2/M transition and mitotic exit through Cdc25p inactivation. EMBO J. 2004;23:919–929. doi: 10.1038/sj.emboj.7600103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe B. A., Gould K. L. Split decisions: coordinating cytokinesis in yeast. Trends Cell Biol. 2005;15:10–18. doi: 10.1016/j.tcb.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Wong K. C., D’Souza V. M., Naqvi N. I., Motegi F., Mabuchi I., Balasubramanian M. K. Importance of a myosin II-containing progenitor for actomyosin ring assembly in fission yeast. Curr. Biol. 2002;12:724–729. doi: 10.1016/s0960-9822(02)00790-x. [DOI] [PubMed] [Google Scholar]
- Wu J. Q., Kuhn J. R., Kovar D. R., Pollard T. D. Spatial and temporal pathway for assembly and constriction of the contractile ring in fission yeast cytokinesis. Dev. Cell. 2003;5:723–734. doi: 10.1016/s1534-5807(03)00324-1. [DOI] [PubMed] [Google Scholar]