Abstract
Background
The use of methylprednisolone (MP) and other corticosteroids for the treatment of acute liver allograft rejection is associated with severe toxicities in non-target tissues. Therefore, selective delivery of MP to the liver may improve its efficacy and alleviate its side effects. We investigated the effects of a novel liver-targeted dextran prodrug of MP (DMP) in an orthotopic rat liver transplantation (OLT) model.
Methods
The model consisted of a high responder rejection strain combination (Dark Agouti donors and Lewis recipients). Liver recipients were intravenously administered saline or a single subtherapeutic dose of MP (5 mg/kg) as the parent drug (MP) or its prodrug (DMP). Different groups were then monitored for graft survival or euthanized 5 or 9 days post-transplantation. Plasma chemistry, including alkaline phosphatase and bilirubin, allograft histology, and survival duration were determined.
Results
Untreated recipients exhibited elevated plasma levels of liver injury markers, progressive portal and venous inflammation and cellular infiltration in liver allografts, and a mean graft survival time (MST) of 10.5 days. MP treatment did not alter any of these parameters. In contrast, a single dose of DMP resulted in a decrease in plasma levels of liver injury markers, a decrease in histological grade of rejection on day 5, and a substantial increase in MST (27.5 days).
Conclusions
These results demonstrate attenuation of acute rejection following local (allograft) immunosuppression with a single subtherapeutic dose of MP delivered as a liver-targeted prodrug. Dextran prodrugs may be useful for selective delivery of immunosuppressants to the liver following liver transplantation.
Keywords: Dextran prodrugs, Methylprednisolone, Local immunosuppression, Targeted delivery
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; DMP, dextran methylprednisolone succinate; IL-2, interleukin-2; MP, methylprednisolone; OLT, orthotopic rat liver transplantation; RAI, rejection activity index; TNF-α, tumor necrosis factor-α
INTRODUCTION
Liver transplantation is currently the treatment of choice for many types of acute or chronic liver diseases (1). Advances in immunosuppressive therapy have played a major role in the success of liver transplantation (2). However, currently available drugs cause indiscriminate systemic immunosuppression and prevent graft rejection at the expense of various side effects, resulting in considerable morbidity and mortality (3). Delivery of these drugs locally to the allograft, thereby decreasing systemic exposure, has been proposed as a potential approach to overcome some of these problems (4, 5). Moreover, selective immunosuppression in the graft necessitates lower drug doses, potentially reducing side effects (6).
The strategy of local immunosuppression at the graft site has been shown by several investigators to be superior to non-specific systemic immunosuppression (6–11). Initial attempts at local immunosuppression focused on local application of drugs via intra-arterial infusions using delivery systems like catheters and mini-pumps (6, 7). However, these procedures are invasive, causing infections and thrombotic complications, and rarely are applicable clinically (10). Moreover, the local immunosuppressive efficacy in these instances is dependent on the pharmacokinetics of these drugs (4, 6), and therefore not generally applicable to all immunosuppressive agents (5). To overcome these problems, we proposed (12, 13) the use of dextrans as macromolecular carriers for selective delivery of immunosuppressive drugs to the liver for liver transplantation.
Dextrans are glucose polymers that have long been used as plasma volume expanders (14, 15). We coupled methylprednisolone (MP), as a model immunosuppressive drug, to a 70 kDa dextran via a succinate linker to produce a dextran-MP prodrug (DMP) (16). We further demonstrated that DMP accumulates selectively in the liver and spleen, where it slowly regenerates the active drug MP (17). This selective accumulation in the liver and spleen is reflective of the property of the macromolecular/polymeric carrier dextran (18). Furthermore, DMP was demonstrated (19, 20) to have a more intense and sustained systemic (spleen) and local (liver) immunosuppressive effect, compared with equimolar doses of MP. Given these superior pharmacokinetic and pharmacodynamic properties, we hypothesized that DMP treatment decreases liver allograft rejection and, consequently, improves survival even at sub-therapeutic doses of MP. Therefore, an orthotopic rat liver transplantation (OLT) model in a high responder rejection strain combination (Dark Agouti liver donors and Lewis recipients) was performed to study the effects of MP and DMP treatment on acute allograft rejection.
MATERIALS AND METHODS
All procedures involved animals were approved by the Texas Tech University Health Sciences Center Animal Care and Use Committee and were in accordance with the guidelines set by the National Institutes of Health (publication no. 85–23, revised 1985, Bethesda, MD).
Orthotopic Rat Liver Transplantation (OLT)
Inbred male Dark Agouti (DA, RT1av1) and Lewis (LEW, RT1) rats served as liver donors and recipients, respectively. All surgical procedures were performed under aseptic conditions using isoflurane anesthesia delivered in an oxygen:nitrous oxide mixture. A non-arterialized OLT was performed according to Kamada’s technique (21), as modified by Tsuchimoto et al. (22). In the donor rats (225–250 g), the right adrenal, right renal, gastro-duodenal, left phrenic, and splenic veins were ligated. The liver was perfused via the portal vein with 10 mL of cold University of Wisconsin (UW) solution (Viaspan, Dupont Pharma Wilmington, DE) prior to liver isolation. The excised liver was then immersed in cold UW solution, and cuffs were mounted on the portal vein (PV), infrahepatic vena cava (IHVC), and the suprahepatic vena cava (SHVC). The recipient rats (250–275 g) were anesthetized, and a long midline incision was made on the abdomen. The hepatic artery was doubly ligated and divided. The bile duct was severed at the junction of the hepatic ducts. The recipient liver was mobilized, the PV and IHVC were cross clamped, and the SHVC, including a part of the diaphragm, was clamped with a pediatric Satinsky’s clamp. The liver was resected, and the donor liver placed orthotopically. Prior to implantation, the donor liver was flushed with 12 mL of cold lactated Ringers solution. The SHVC, PV, and IHVC anastomoses were performed using the cuff technique. The bile duct was reconstructed with an indwelling Teflon stent (I.D. 0.6 mm). Recipients were administered a total of 7 mL of saline (0.45%): glucose (2.5%) mixture during the surgery to compensate for fluid loss. Following transplantation, the animals were allowed to recover under a heating lamp and had free access to food and water. There were no significant differences in duration of cold preservation (<40 min) and portal venous clamping time (<18 min) among groups.
Experimental Design
The effects of MP (sodium succinate), its liver-targeted dextran 70 kD prodrug (DMP) (16), or vehicle (saline) on recipient survival, allograft rejection, and splenocyte proliferation were evaluated in three separate experiments. In all experiments, recipients were administered a single 5-mg/kg (MP equivalent) dose of either MP or DMP or an equal volume of saline via penile vein.
In the first transplanted set, drugs or vehicle were administered immediately following the transplantation, and the recipients were monitored every day for survival. In the second experiments, the transplanted rats were injected with drugs or vehicle, and five and nine days following transplantation, a subset of recipients from each group was euthanized. Blood (~ 500 μL) was collected from the tail vein on days 2, 4, and 5 in the recipients euthanized on day 5, and on days 3, 6, and 9 in the recipients euthanized on day 9. Plasma was immediately separated and stored at −20°C until further analysis. A portion of the liver grafts from recipients euthanized on days 5 or 9 was immersed in 10% (v/v) neutral-buffered formalin for subsequent histological analysis. A second portion of the liver was frozen immediately at −20°C for biochemical analysis. Finally, in the third set of experiments, nine days after the injection of vehicle, MP, or DMP, naïve Lewis rats were euthanized and spleen was removed for splenocyte proliferation assay.
Analysis of Plasma Liver Function Markers
Plasma levels of ALT, AST, ALP, and bilirubin were estimated spectrophotometrically using commercially available kits (Teco Diagnostics, Anaheim, CA). Blood glucose was measured with an automated analyzer (Onetouch Ultra®, Lifescan, Inc, Milpitas, CA).
Allograft Histology
Formalin-fixed samples were embedded in paraffin and subsequently stained with hematoxylin and eosin. A pathologist (D.L.M.), who was blinded to the treatment groups, graded rejection using the Banff Rejection Activity Index (RAI) (23).
Cytokine Analysis in Plasma and Liver
The concentrations of TNF-α and IL-2 were measured in both plasma and liver extracts. The liver extracts were prepared in a protease inhibitor cocktail according to the manufacturer’s (Sigma Chemical Co., St. Louis, MO) instructions. The concentrations of TNF-α and IL-2 in the plasma and those of IL-2 in the liver extracts were quantified using ELISA (Biosource International, Inc., Camarillo, CA). The concentrations of TNF-α in the liver extracts were measured using the established cytotoxicity of TNF-α against murine L929 cells (NCTC clone 929; American Type Culture Collection, Manassas, VA). Briefly, after overnight incubation of the cells (2 x 104/well) in 96-well microplates, standards containing rat TNF-α (PeproTech, Rocky Hill, NJ) or diluted liver extracts were added to the wells in a media containing 4 μg/ml actinomycin D. After an overnight incubation and subsequent decanting of samples and rinsing the wells with the media, the viability of the cells was determined using the CellTiter 96 Aqueous Non-Radioactive Proliferation Assay (Promega Corporations, Madison, WI).
Alloantibody Response
The donor-specific IgM alloantibody response in recipient rats was determined using sera from 9-day transplanted rats by flow cytometry. Peripheral blood mononuclear cells (PBMCs) obtained from donor DA blood by cardiac puncture were isolated on a Lympholyte R density gradient (Cedarlane Laboratories, Ontario, Canada). Diluted (1:10) serum from Control-, MP-, and DMP-treated rats was incubated with the donor PBMCs (1 x 106 cells). After washing, the cells were stained with FITC conjugated mouse anti-rat IgM monoclonal antibody (clone: HIS40; eBioscience, San Diego, CA) and analyzed using a FACScan (Beckton-Dickinson, CA).
Splenocyte Proliferation
Spleen lymphocytes were prepared as described before (24). Briefly, the cells (2 x 105) were incubated in the presence of bacterial lipoplysaccharide (LPS, 10 μg/ml) (Sigma Chemical Co., St. Louis, MO) for 66 hr, and during the last 18 hr, 0.5 μCi of tritiated thymidine was added. After harvesting, the cells were counted using a Beckman LS 6500 Counter.
Statistical Analysis
The statistical evaluation of differences in recipient survival was performed using the log rank test applied to Kaplan-Meier plots. All other statistical comparisons among groups were conducted using ANOVA with subsequent Dunnett’s post-hoc analysis. All tests were conducted at significance level of 0.05. Data are presented as mean ± S.D.
RESULTS
Effects of MP and DMP on Recipient Survival
The plot of percent survival as a function of post-transplantation time for Control, MP, and DMP groups is shown in Fig. 1. All untreated Control recipients died on or within day 12, with a mean survival time (MST) of 10.5 days (range, 9–12 days). As expected, a single subtherapeutic dose (5 mg/kg) of MP did not significantly prolong allograft survival, as recipients in this group died within day 12, with a MST of 11 days (range, 10–12 days). However, a single dose (5 mg/kg, MP equivalent) DMP treatment significantly (P<0.05) prolonged recipient survival with a MST of 27.8 days (range, 14–60 days).
FIGURE 1.
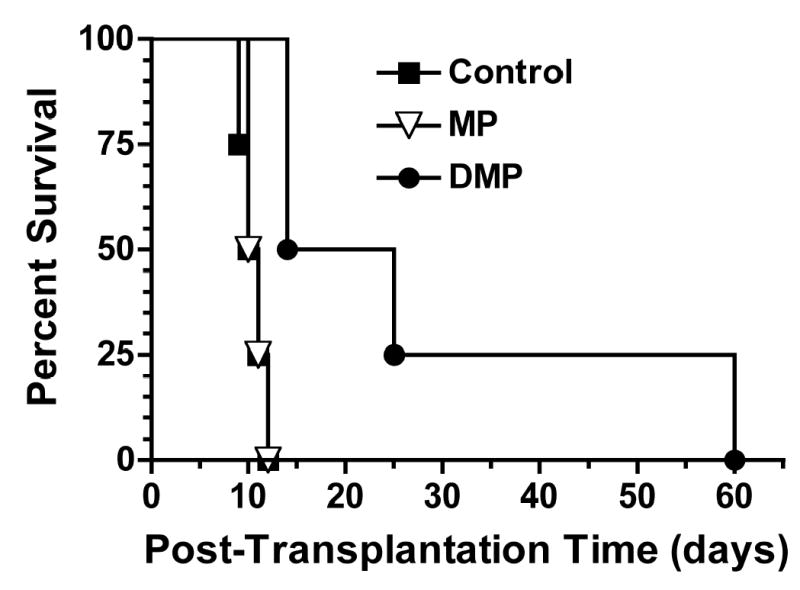
Percent survival curve of LEW recipients of DA liver allografts. Recipients were treated with a single 5-mg/kg i.v. dose of MP or DMP or with saline (Control) on day 0 (n=4/group). Mean survival times (MST) in Control, MP, and DMP groups are 10.5, 11, and 27.5 days, respectively (P<0.05 for DMP vs. Control or MP).
Effects of MP and DMP on Acute Allograft Rejection
Plasma liver function markers
The plasma bilirubin levels as a function of post-transplantation time for Control, MP, and DMP groups are shown in Fig. 2 (top panel). In general, plasma bilirubin increased with post-transplantation time, with maximum levels observed on day 9. Whereas DMP treatment significantly (P<0.05) decreased (range, 50–80% inhibition compared with Control) the acute rejection-mediated elevation of plasma bilirubin from day 3 to 9, the effects of MP on bilirubin levels were significant only on day 3 (Fig. 2, top).
FIGURE 2.
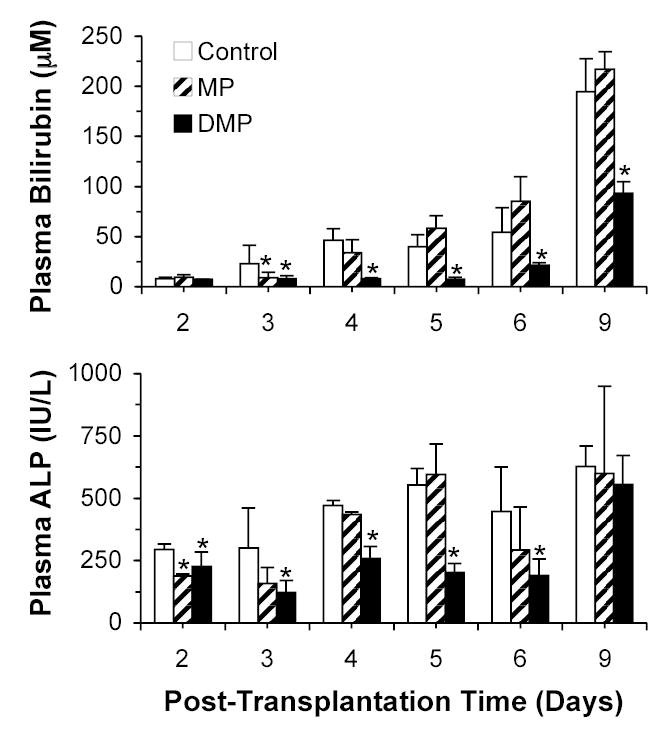
Plasma bilirubin (top) and ALP (bottom) concentrations in LEW recipients of DA liver allografts. Recipients were treated with a single 5-mg/kg i.v. dose of MP or DMP or with saline (Control) on day 0. Columns and bars represent mean and SD values, respectively (n=4/group). *, Significantly different (P<0.05) from Control.
The plasma ALP levels as a function of post-transplantation time and treatment are also shown in Fig. 2 (bottom panel). Similar to the bilirubin levels (Fig. 2, top), plasma ALP levels tended to increase with post-transplantation time, with maximum levels observed on day 9. DMP treatment significantly (P<0.05) decreased (range, 40–60% inhibition compared with Control) the rejection-mediated increase in the plasma ALP levels from day 2 to 6 post-transplantation. However, a subsequent lack of inhibition was observed on day 9 (Fig. 2, bottom). As for MP, the ALP levels were reduced only on day 2 with this treatment (Fig. 2, bottom).
The plasma concentrations of AST and ALT in different treatment groups on days 5 and 9 post-transplantation are shown in Fig. 3. Only on day 9, both MP and DMP significantly (P<0.05) decreased (~52 and 58%, respectively) the plasma ALT levels, compared with Control group (Fig. 2, bottom). Plasma AST levels were, however, significantly (P<0.05) decreased (~53%) only by DMP treatment on day 9 (Fig. 3, top).
FIGURE 3.
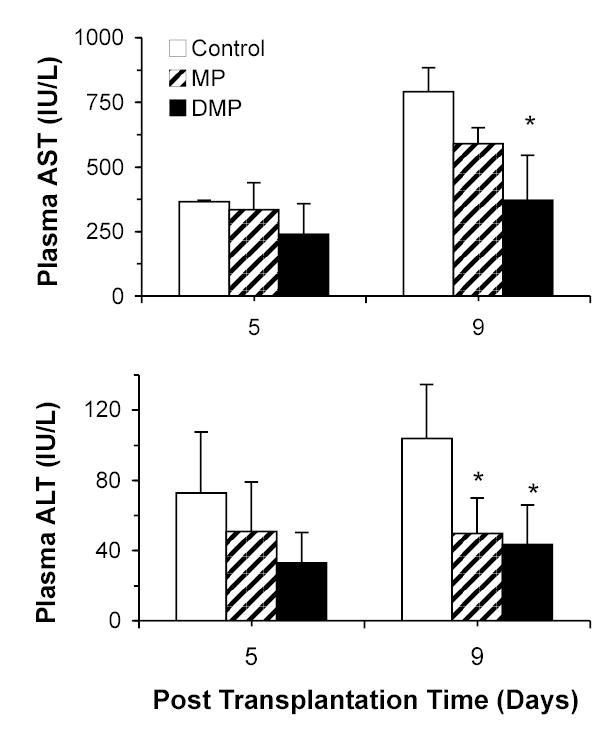
Plasma AST (top) and ALT (bottom) concentrations on the 5th and 9th post-transplantation days in LEW recipients of DA liver allografts. Recipients were treated with a single 5-mg/kg i.v. dose of MP or DMP or with saline (Control) on day 0. Columns and bars represent mean and SD values, respectively (n=4/group). *, Significantly different (P<0.05) from Control.
Allograft histology
Representative images of liver allografts on days 5 and 9 in Control, MP, and DMP groups are shown in Fig. 4. In untreated allografts on day 5 (Fig 4, A), marked expansion of most of the portal triads by a mixed leukocytic infiltrate, characteristic of rejection, was prominent, with inflammatory spillover into periportal parenchyma. Bile ducts contained a variable leukocytic infiltrate and were lined by disorganized epithelium having a high nucleus to cytoplasm ratio. Evidence of endothelitis (subendothelia leukocytic infiltrates) and perivenous inflammation was extensive in portal as well as sublobular veins (Fig 4, A). Inflammation around sublobular veins was prominent and extended into perivenous hepatic parenchyma. In the MP group on day 5 (Fig. 4, B), the histological lesions of rejection were very similar to those in the saline-treated recipients (Fig. 4, A). In the DMP group on day 5, lesions were less severe (Fig 4, C). Some, but not the majority of portal triads, were expanded by a mixed infiltrate of lymphocytes, lymphoblasts, and few neutrophils and eosinophils. However, there was little to no extension into periportal hepatic parenchyma. Few bile ducts contained a leukocytic infiltrate, and changes in biliary epithelial cells were mild. Mild inflammation of some soblobular veins was noted, and the infiltrate did not extend into perivenous parenchyma in this group (Fig. 4, C).
FIGURE 4.
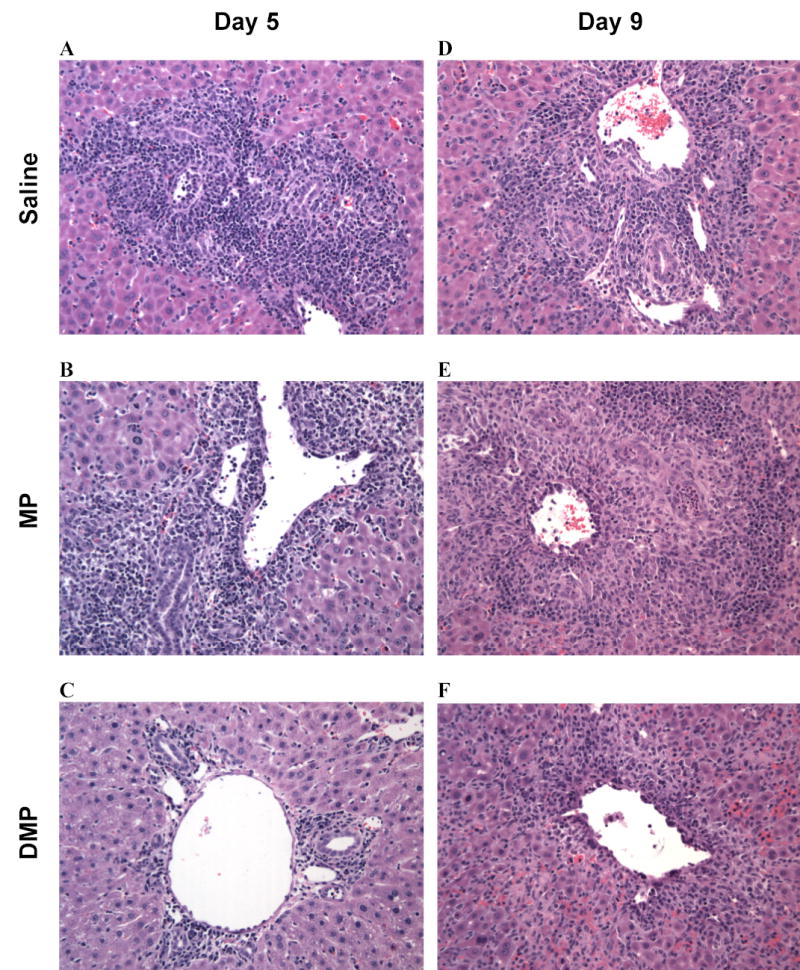
Representative hematoxylin-eosin stained liver sections on the 5th (left column) and 9th (right column) post-transplantation days in LEW recipients of DA liver allografts. Recipients were treated with saline (A and D) or a single 5-mg/kg i.v. dose of MP (B and E) or DMP (C and F) on day 0.
On day 9, the Control (Fig. 4, D) and MP (Fig. 4, E) group allografts demonstrated similar histological signs of rejection as seen on day 5 (Fig. 4, A and B, respectively). In the DMP group, the infiltration drastically increased from day 5 (Fig. 4, C) to day 9 (Fig. 4, F), with no apparent difference among the three treatment groups on day 9 (Fig. 4).
The semi-quantitative evaluation of allograft rejection for all the livers harvested on day 5 is shown in Table 1. Whereas the portal, bile duct, and venous endothelial inflammatory indices were, respectively, ranked severe, moderate, and severe for both the vehicle and MP-treated groups, the respective indices were reduced to moderate, mild, and mild after the DMP treatment (Table 1). Consequently, overall RAI in the DMP group (3.8 ± 0.5) was significantly (p<0.05) lower (~50%) than that in both the Control and MP groups (Table 1). In contrast to the data for day 5, most of the inflammatory indices on day 9 were severe and not significantly affected by the treatments (data not shown).
TABLE 1.
Semi-quantitative assessment of histological grade of acute rejection
| Category |
Treatment |
||
|---|---|---|---|
| Control | MP | DMP | |
| Portal Inflammation | 3.0 ± 0.0 (Severe)a | 3.0 ± 0.0 (Severe) | 1.8 ± 0.5b (Moderate) |
| Bile Duct Inflammation | 1.8 ± 0.5 (Moderate) | 1.8 ± 0.5 (Moderate) | 1.0 ± 0.0 (Mild) |
| Venous Endothelial Inflammation | 2.8 ± 0.5 (Severe) | 2.5 ± 0.6 (Severe) | 1.0 ± 0.0b (Mild) |
| Rejection Activity Index (RAI) | 7.5 ± 0.6 | 7.3 ± 1.0 | 3.8 ± 0.5b |
Rejection grading was based on Banff criteria (Ref (23) in liver allografts on day 5 following OLT. Recipients were treated with a single 5-mg/kg i.v. dose of MP or DMP or with saline (Control) on day 0.
Key: 3, severe; 2, moderate; 1, mild.
p<0.05 Compared to Control and MP groups.
Plasma and hepatic cytokines
Plasma and hepatic concentrations of TNF-α and IL-2 proteins on days 5 and 9 are shown in Fig. 5. Plasma TNF-α levels significantly decreased in Control recipients from day 5 to day 9. In terms of treatment effect, only DMP significantly (P<0.05) decreased plasma TNF-α protein levels (~85 % inhibition, compared with Control group) on day 5. The TNF-α profiles in the liver tissue were qualitatively similar to those in the plasma in that only DMP treatment in the 5-day group resulted in a significant (P<0.05) decline in the hepatic TNF-α levels. In contrast, treatment with MP did not have any significant effect on either plasma or hepatic TNF-α levels (Fig. 5, left panels).
FIGURE 5.
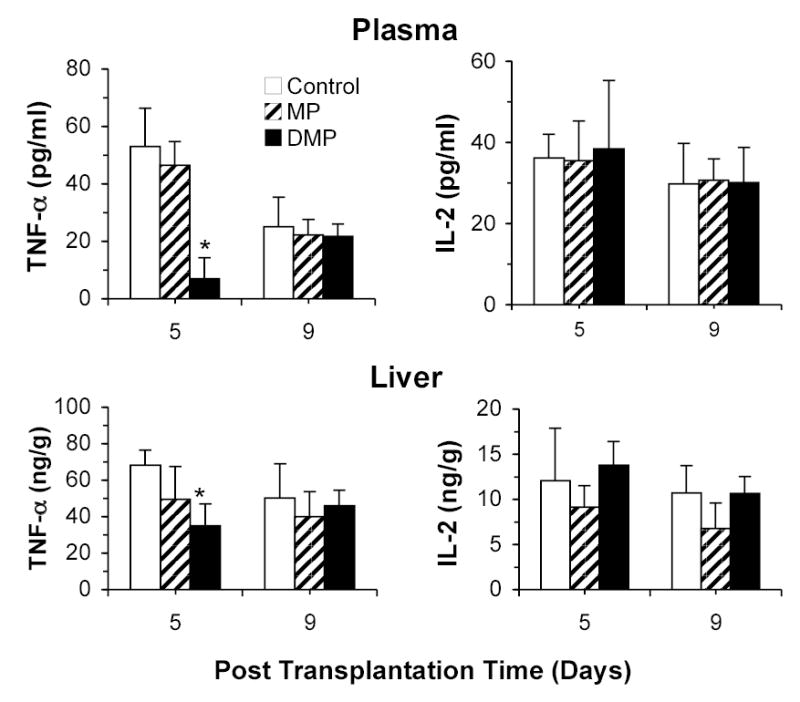
TNF-α and IL-2 protein concentrations in the plasma (top panel) and liver (bottom panel) on the 5th and 9th post-transplantation days in LEW recipients of DA liver allografts. Recipients were treated with a single 5-mg/kg i.v. dose of MP or DMP or with saline (Control) on day 0. Columns and bars represent mean and SD values, respectively (n=4/group). *, Significantly different (P<0.05) from Control.
The plasma and hepatic concentrations of IL-2 protein were unaltered by post-transplantation time or treatment (Fig. 5, right panels).
Alloantibody response
The IgM alloantibody responses on day nine after transplantation are shown in Figure 6A for vehicle (Control)-, MP-, and DMP-treated rats. Whereas MP treatment did not alter the IgM response, the DMP-treatment resulted in a 46% decline in mean channel fluorescence, compared with untreated animals.
FIGURE 6.
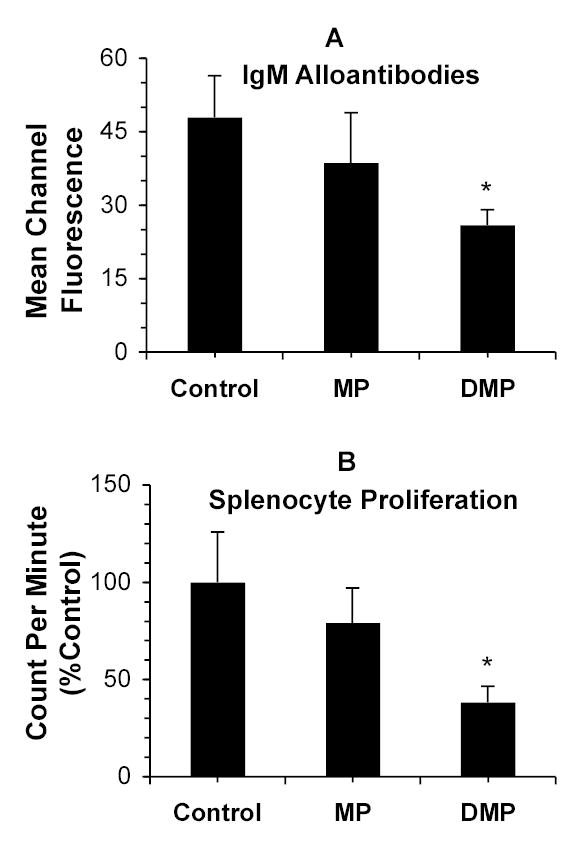
Top Panel: Donor (DA)-specific IgM alloantibody response (mean channel fluorescence) in the 9-day sera of recipient (Lewis) rats treated with a single 5-mg/kg i.v. dose of MP or DMP or with saline (Control) on day 0 (n=4/group), quantitated using flow cytometry. Bottom Panel: Splenocyte proliferation (count per minute) in naïve Lewis rats treated with vehicle (Control) or a single 5 mg/kg dose (MP equivalent) of MP or DMP 9 days before spleen harvest (n=3/group). Columns and bars represent mean and SD values, respectively. *, Significantly different (P<0.05) from Control.
Effect of MP and DMP on Splenocyte Proliferation
Methylprednisolone treatment did not change the LPS-induced proliferation of spleen lymphocytes nine days after drug injection (Fig. 6B). However, DMP treatment inhibited this parameter by ~60% (Figure 6B).
DISCUSSION
Large intravenous doses of MP (up to three 1-g pulses) are currently the first-line therapy for management of acute cellular rejection in liver transplant patients (2). Although effective in controlling acute rejection, this treatment has been fatal in a few cases (25–30) and/or associated with severe life-threatening toxicities related to cardiovascular system (e.g., cardiac arrest and arrhythmia) (27, 29–31), central nervous system (e.g., seizure and blindness) (25, 32–35), severe infections (both viral and bacterial) (36–39), and metabolic complications (e.g., hypokalemia) (40). Except for infections, these toxicities are mainly related to the extensive extra-hepatic distribution of MP to organs like heart, lung, and brain. Therefore, selective delivery of MP to the liver may increase the allograft exposure to the drug, improving graft function, while decreasing systemic drug exposure. This strategy may allow administration of lower doses of the drug, reducing toxicity without sacrificing efficacy. Indeed, our current results in an OLT model show that a single 5-mg/kg dose of the liver-targeted dextran prodrug (DMP) significantly decreases liver allograft rejection (Fig. 4 and Table 1) and enhances graft survival (Fig. 1). In contrast, treatment with equimolar doses of the parent drug MP resulted in acute allograft rejection and recipient survival similar to those in untreated animals (Figs. 1 and 4 and Table 1).
Allograft rejection, as assessed by plasma bilirubin and ALP levels (Fig. 2) and liver histology (Fig. 4 and Table 1), was ameliorated significantly only by DMP treatment. This advantage over the parent drug MP is most likely due to the prodrug’s unique pharmacokinetic and pharmacodynamic properties (17, 20). Our previous studies in rats (17) demonstrated that ~30% of the DMP dose accumulates in the liver and gradually regenerates the active drug (MP) for days. This is in contrast to a maximum of only ~4% of the dose found in the liver following MP dosing, which rapidly declines to below detection levels in few hours (17). Furthermore, this selective accumulation resulted in the prodrug having a more intense and sustained local (hepatic) immunosuppressive activity after systemic administration of the equivalent doses of DMP or MP to rats (20); the area under the effect-time curve, as measured by the inhibition of bacterial endotoxin-induced TNF-α release in ex vivo perfused livers, was ~ 4 fold greater after the injection of DMP than after MP injection (20). Therefore, the superiority of DMP over MP observed in our current studies with the OLT model is partly due to the selective accumulation of DMP in the liver, resulting in a gradual regeneration of the active drug (MP).
Besides induction of local immunosuppression in the liver, DMP also inhibits immune responses in the spleen (19). Previous studies in our laboratory (17, 19) have shown that, in addition to the liver, DMP accumulates in the spleen, where it regenerates the active drug MP. Moreover, the inhibition of concanavalin A-induced splenocyte proliferation by DMP was both more intense and sustained than that by an equimolar dose of MP (maximum inhibition of 100% at 24 hour vs. 50% inhibition at 2 hour, for DMP and MP, respectively) (19). However, the splenocyte proliferation returned to baseline values within 48 hr after drug administration. Because Concanavalin A and LPS mainly stimulate T cells and B cells (41), respectively, we used LPS in our current study to determine the effects of our conjugate on the proliferation of B cells. As demonstrated in the results (Fig. 6B), DMP treatment caused a significant reduction in the LPS-induced splenocyte proliferation nine days after transplantation. Therefore, the effect of DMP on B lymphocytes appears to be longer lasting than those for the T cells.
The LPS-induced decrease in splenocyte proliferation (Fig. 6B) in DMP-treated transplanted rats was associated with a decrease in IgM alloantibodies (Fig. 6A). In agreement with our studies (Fig. 1), previous studies (42) have also shown a correlation between the steroid-induced reduction in IgM alloantibodies and increased survival. Our data further shows a correlation between inhibition of LPS-induced splenocyte proliferation and alloantibody levels, suggesting that B cells may contribute to the liver allograft rejection. Nevertheless, the observed reduction in alloantibody levels and in the LPS-stimulated splenocytes in DMP-treated rats (Fig. 6) suggests that the systemic immunosuppressive activity of DMP contributes, at least in part, to its overall effects.
Our observation that a single 5-mg/kg dose of MP is ineffective in preventing acute rejection in an OLT model is not surprising. This dose of MP is considered subtherapeutic because even at doses much higher than 5 mg/kg, MP did not improve recipient survival in a high-responder OLT model (43). Wang et al. (43) reported the effects of a 5-day course of MP treatment on liver transplant recipient survival in a rat acute rejection model. Recipients were administered a 16-mg/kg i.v. dose of MP (~ 3 times the dose used in our study) following OLT, and the dose was tapered by half for the next 4 days post-transplantation. They reported a lack of significant difference in survival between the MP-treated and untreated recipients (43). Although a single dose local immunosuppression prolonged allograft survival in our study (Fig. 1), systemic immunosuppression with MP for a longer duration (5 days) and at a higher dose did not (43). Therefore, local immunosuppression in the liver via dextran conjugation may be considered as a method to decrease total dose of immunosuppressive agents while still preventing allograft rejection.
Although administered as a single dose, immunosuppressive activity of DMP was sustained for at least five days after the transplantation. Allograft rejection in the DMP group was attenuated at least until day 5 (Fig. 4 and Table 1), following which the lymphocytic infiltration returned to control levels by day 9 (Fig. 4). In addition, the plasma ALP (Fig. 2) and plasma and liver TNF-α (Fig. 5) concentrations also demonstrated a similar trend. Therefore, the observed improvement in survival (Fig. 1) in the DMP group may be, in part, due to the prevention of allograft rejection during the initial week. Although the single dose treatment did not provide long-term survival (i.e., >100 days) in our study, it is likely that long-term survival may be achieved by a multiple dosing regimen of the prodrug. Nevertheless, the present single dose study represents a foundational step in the design and conduct of future multiple dose investigations.
Corticosteroids exert a broad range of actions on the immune system, including alterations in the synthesis of various cytokines (36, 44). Reduction of plasma and hepatic TNF-α (Fig. 5) may be responsible, at least in part, for the improvement in allograft rejection and survival observed in our study. Plasma TNF-α levels are elevated in patients with allograft rejection (45), and anti-TNF-α antibodies reportedly (46) decrease rejection and improve survival in animal models. Attenuation of plasma TNF-α may decrease adhesion molecule expression on the sinusoidal endothelial cells (47), resulting in a decrease in allograft infiltration, observed in our study on day 5 (Fig. 4 and Table 1). Additionally, because TNF-α is cytotoxic (48), a DMP-induced decrease in its intrahepatic concentrations, observed in our studies (Fig. 5), is expected to decrease the graft injury, an observation consistent with the lower levels of the hepatic injury markers like ALT and AST in the DMP group (Fig. 2). Although corticosteroids are known (36) to decrease the production of another major cytokine interleukin (IL)-2, we did not notice any alterations in IL-2 protein levels in plasma or the graft (Fig. 5). Therefore, plasma and intrahepatic inhibition of TNF-α may explain some of the beneficial effects of DMP. However, the complete mechanism of action of DMP needs further evaluation.
Other investigators have tested carriers such as liposomes for selective delivery of immunosuppressive agents to the liver. Friese et al. (8) and Ko et al. (9) reported the use of cyclosporine and tacrolimus incorporated liposomes, respectively, in animal models of liver transplantation. Both of these studies (8, 9) demonstrated improvement in survival in the liposome-treated groups, compared with the free drug. Although liposomes are promising, they have limitations such as short stability and relatively limited drug load. The latter may require injection of more than acceptable lipid loads (49), especially in patients with acute rejection requiring high dose corticosteroids. The dextran prodrug reported here is an alternative to liposomes for local immunosuppression of the allograft following liver transplantation.
In addition to our studies on neutral dextran prodrugs of MP, synthesis and in vivo pharmacokinetics of a negatively-charged dextran-tacrolimus conjugate was reported by Yura et al. (50). These authors (50) showed that conjugation caused a significantly higher drug exposure in plasma, associated with only modest increases in the liver and spleen accumulation. The lower accumulation of tacrolimus conjugate in spleen and liver, compared with our MP conjugate, is most likely due to the negative charge of dextrans used in their study, which reportedly (51) decreases tissue accumulation. Nonetheless, our study is the first to examine the immunosuppressive efficacy of dextran conjugates in an animal allograft model.
In conclusion, selective delivery of MP to the liver as a dextran prodrug significantly improved allograft survival and decreased acute rejection in a rat OLT model. To our knowledge, this it the first study demonstrating the therapeutic applicability of macromolecular/polymer based prodrugs in an organ transplantation model. Dextran conjugation of immunosuppressive drugs may represent a potential technique to enhance efficacy of these agents in liver transplant patients.
Footnotes
This work was supported in part by an unrestricted research fund generated from research contracts to RM and by a grant from the National Institute of General Medical Sciences of NIH (R01 GM069869-01A2).
References
- 1.Keeffe EB. Liver transplantation: Current status and novel approaches to liver replacement. Gastroenterology. 2001;120 (3):749. doi: 10.1053/gast.2001.22583. [DOI] [PubMed] [Google Scholar]
- 2.Encke J, Uhl W, Stremmel W, Sauer P. Immunosuppression and modulation in liver transplantation. Nephrol Dial Transplant. 2004;19(Suppl 4):IV22. doi: 10.1093/ndt/gfh1037. [DOI] [PubMed] [Google Scholar]
- 3.Gruber SA, Canafax DM, Cipolle RJ, et al. Local immunosuppression of the vascularized graft. Surgery. 1990;107 (2):209. [PubMed] [Google Scholar]
- 4.Gruber SA. The case for local immunosuppression. Transplantation. 1992;54 (1):1. doi: 10.1097/00007890-199207000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Gruber SA. Locoregional immunosuppression of organ transplant. Immunol Rev. 1992;129:5. doi: 10.1111/j.1600-065x.1992.tb01417.x. [DOI] [PubMed] [Google Scholar]
- 6.Ruers TJM, Burrman WA, Smits JFM, Van Der Linden CJ, Van Dongen JJ, Struyker-Boudier HAJ, Kootstra G. Local treatment of renal allografts, a promising way To reduce the dosage of immunosuppressive drugs. Transplantation. 1986;41 (2):156. doi: 10.1097/00007890-198602000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Gruber SA, Hrushesky WJM, Cipolle RJ, et al. Local immunosuppression with reduced systemic toxicity in a canine renal allograft model. Transplantation. 1989;48 (6):936. doi: 10.1097/00007890-198912000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Freise CE, Liu T, Hong K, et al. The increased efficacy and decreased nephrotoxicity of a cyclosporine liposome. Transplantation. 1994;57 (6):928. doi: 10.1097/00007890-199403270-00027. [DOI] [PubMed] [Google Scholar]
- 9.Ko S, Nakajima Y, Kanehiro H, et al. The enhanced immunosuppressive efficacy of newly developed liposomal FK506 in canine liver transplantation. Transplantation. 1995;59 (10):1384. doi: 10.1097/00007890-199505270-00004. [DOI] [PubMed] [Google Scholar]
- 10.Su M, Li Z, Zhang Z, Feng G. Local immunosuppression with kidney-targeting prednisolone prodrug prolong rat renal allograft survival. Transplant Proc. 2002;34 (5):1422. doi: 10.1016/s0041-1345(02)02912-3. [DOI] [PubMed] [Google Scholar]
- 11.Weber T, Kalbhenn T, Herrmann G, Hanisch E. Local immunosuppression with budesonide after liver transplantation in the rat - A preliminary histomorphological analysis. Transplantation. 1997;64 (5):705. doi: 10.1097/00007890-199709150-00007. [DOI] [PubMed] [Google Scholar]
- 12.Mehvar R. Targeted delivery of methylprednisolone using a dextran prodrug. Pharm Res. 1997;14:S336. [Google Scholar]
- 13.Mehvar R. Dextrans for targeted and sustained delivery of therapeutic and imaging agents. J Control Release. 2000;69 (1):1. doi: 10.1016/s0168-3659(00)00302-3. [DOI] [PubMed] [Google Scholar]
- 14.Howard JM, Ebert RV, Bloom WL, Sloan MH. The present status of dextran as a plasma expander. Am J Surg. 1959;97 (5):593. doi: 10.1016/0002-9610(59)90251-x. [DOI] [PubMed] [Google Scholar]
- 15.Schortgen F, Deye N, Brochard L. Preferred plasma volume expanders for critically ill patients: results of an international survey. Intensive Care Med. 2004;30 (12):2222. doi: 10.1007/s00134-004-2415-1. [DOI] [PubMed] [Google Scholar]
- 16.Mehvar R. Simultaneous analysis of dextran-methylprednisolone succinate, methylprednisolone succinate, and methylprednisolone by size-exclusion chromatography. J Pharmaceut Biomed Anal. 1999;19 (5):785. doi: 10.1016/s0731-7085(98)00308-2. [DOI] [PubMed] [Google Scholar]
- 17.Zhang X, Mehvar R. Dextran-methylprednisolone succinate as a prodrug of methylprednisolone: plasma and tissue disposition. J Pharm Sci. 2001;90 (12):2078. doi: 10.1002/jps.1158. [DOI] [PubMed] [Google Scholar]
- 18.Mehvar R, Robinson MA, Reynolds JM. Molecular weight dependent tissue accumulation of dextrans: in vivo studies in rats. J Pharm Sci. 1994;83 (10):1495. doi: 10.1002/jps.2600831024. [DOI] [PubMed] [Google Scholar]
- 19.Mehvar R, Hoganson DA. Dextran-methylprednisolone succinate as a prodrug of methylprednisolone: immunosuppressive effects after in vivo administration to rats. Pharm Res. 2000;17 (11):1402. doi: 10.1023/a:1007555107691. [DOI] [PubMed] [Google Scholar]
- 20.Chimalakonda AP, Mehvar R. Dextran-methylprednisolone succinate as a prodrug of methylprednisolone: local immunosuppressive effects in liver after systemic administration to rats. Pharm Res. 2003;20 (2):198. doi: 10.1023/a:1022358702643. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi E, Kamada N, Goto S, Miyata M. Protocol for the technique of orthotopic liver transplantation in the rat. Microsurgery. 1993;14 (8):541. doi: 10.1002/micr.1920140812. [DOI] [PubMed] [Google Scholar]
- 22.Tsuchimoto S, Kusumoto K, Nakajima Y, et al. Orthotopic liver transplantation in the rat. A simplified technique using the cuff method for suprahepatic vena cava anastomosis. Transplantation. 1988;45 (6):1153. [PubMed] [Google Scholar]
- 23.Banff schema for grading liver allograft rejection: an international consensus document. Hepatology. 1997;25 (3):658. doi: 10.1002/hep.510250328. [DOI] [PubMed] [Google Scholar]
- 24.Coligan J, Kruisbeek A, Margulies D, Shevach E, Strober W. Current Protocols in Immunology: John Wiley & Sons, 1994.
- 25.Stubbs S, Morrell R. Intravenous methylprednisolone sodium succinate: adverse reactions reported in association with immunosuppressive therapy. Transplant Proc. 1973;5 (2):1145. [PubMed] [Google Scholar]
- 26.McDougal BA, Whittier FC, Cross DE. Sudden death after bolus steroid therapy for acute rejection. Transplant Proc. 1976;8 (3):493. [PubMed] [Google Scholar]
- 27.Bocanegra TS, Castaneda MO, Espinoza LR, Vasey FB, Germain BF. Sudden death after methylprednisolone pulse therapy. Ann Intern Med. 1981;95 (1):122. doi: 10.7326/0003-4819-95-1-122_1. [DOI] [PubMed] [Google Scholar]
- 28.Gardiner PV, Griffiths ID. Sudden death after treatment with pulsed methylprednisolone. Br Med J. 1990;300 (6717):125. doi: 10.1136/bmj.300.6717.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moses RE, McCormick A, Nickey W. Fatal arrhythmia after pulse methylprednisolone therapy. Ann Intern Med. 1981;95 (6):781. doi: 10.7326/0003-4819-95-6-781_3. [DOI] [PubMed] [Google Scholar]
- 30.Guillen EL, Ruiz AM, Bugallo JB. Hypotension, bradycardia, and asystole after high-dose intravenous methylprednisolone in a monitored patient. Am J Kidney Dis. 1998;32 (2):E4. doi: 10.1053/ajkd.1998.v32.pm10074612. [DOI] [PubMed] [Google Scholar]
- 31.McLuckie AE, Savage RW. Atrial fibrillation following pulse methylprednisolone therapy in an adult. Chest. 1993;104 (2):622. doi: 10.1378/chest.104.2.622. [DOI] [PubMed] [Google Scholar]
- 32.Ayoub WT, Torretti D, Harrington TM. Central nervous system manifestations after pulse therapy for systemic lupus erythematosus. Arthritis Rheum. 1983;26 (6):809. doi: 10.1002/art.1780260621. [DOI] [PubMed] [Google Scholar]
- 33.Suchman AL, Condemi JJ, Leddy JP. Seizure after pulse therapy with methyl prednisolone. Arthritis Rheum. 1983;26 (1):117. doi: 10.1002/art.1780260123. [DOI] [PubMed] [Google Scholar]
- 34.Cerilli J, Miller JA. The effect of massive pulse steroid therapy on the water content of the rat brain. Transplantation. 1972;14 (3):403. [PubMed] [Google Scholar]
- 35.El-Dahr S, Chevalier RL, Gomez RA, Campbell FG. Seizures and blindness following intravenous pulse methylprednisolone in a renal transplant patient. Int J Pediatr Nephrol. 1987;8 (2):87. [PubMed] [Google Scholar]
- 36.Volpin R, Angeli P, Galioto A, et al. Comparison between two high-dose methylprednisolone schedules in the treatment of acute hepatic cellular rejection in liver transplant recipients: a controlled clinical trial. Liver Transpl. 2002;8 (6):527. doi: 10.1053/jlts.2002.33456. [DOI] [PubMed] [Google Scholar]
- 37.Wiesner R, Ludwig J, Krom R, et al. Treatment of early cellular rejection following liver transplantation with intravenous methylprednisolone. The effect of dose on response. Transplantation. 1994;58 (9):1053. doi: 10.1097/00007890-199411150-00015. [DOI] [PubMed] [Google Scholar]
- 38.Kozeny GA, Quinn JP, Bansal VK, Vertuno LL, Hano E. Pneumocystis carinii pneumonia: a lethal complication of "pulse" methylprednisolone therapy. Int J Artif Organs. 1987;10 (5):304. [PubMed] [Google Scholar]
- 39.Adams D, Neuberger JM. Treatment of acute rejection. Semin Liver Dis. 1992;12 (1):80. doi: 10.1055/s-2007-1007379. [DOI] [PubMed] [Google Scholar]
- 40.Bonnotte B, Chauffert B, Martin F, Lorcerie B. Side-effects of high-dose intravenous (pulse) methylprednisolone therapy cured by potassium infusion. Br J Rheumatol. 1998;37 (1):109. doi: 10.1093/rheumatology/37.1.109a. [DOI] [PubMed] [Google Scholar]
- 41.Omara FO, Fournier M, Vincent R, Blakley BR. Suppression of rat and mouse lymphocyte function by urban air particulates (Ottawa dust) is reversed by N-acetylcysteine. J Toxicol Environ Health A. 2000;59 (2):67. doi: 10.1080/009841000156989. [DOI] [PubMed] [Google Scholar]
- 42.Mishina EV, Binder J, Kupiec-Weglinski JW, Jusko WJ. Effect of liposomal methylprednisolone on heart allograft survival and immune function in rats. J Pharmacol Exp Ther. 1994;271:868. [PubMed] [Google Scholar]
- 43.Wang CM, Sun JH, Sheil AGR, McCaughan GW, Bishop GA. A short course of methylprednisolone immunosuppression inhibits both rejection and spontaneous acceptance of rat liver allografts. Transplantation. 2001;72 (1):44. doi: 10.1097/00007890-200107150-00011. [DOI] [PubMed] [Google Scholar]
- 44.Cattral MS, Lilly LB, Levy GA. Immunosuppression in liver transplantation. Semin Liver Dis. 2000;20 (4):523. doi: 10.1055/s-2000-13160. [DOI] [PubMed] [Google Scholar]
- 45.Imagawa DK, Millis JM, Olthoff KM, et al. The role of tumor necrosis factor in allograft rejection. I. Evidence that elevated levels of tumor necrosis factor-alpha predict rejection following orthotopic liver transplantation. Transplantation. 1990;50 (2):219. doi: 10.1097/00007890-199008000-00009. [DOI] [PubMed] [Google Scholar]
- 46.Imagawa DK, Millis JM, Olthoff KM, et al. The role of tumor necrosis factor in allograft rejection. II. Evidence that antibody therapy against tumor necrosis factor-alpha and lymphotoxin enhances cardiac allograft survival in rats. Transplantation. 1990;50 (2):189. doi: 10.1097/00007890-199008000-00003. [DOI] [PubMed] [Google Scholar]
- 47.Bumgardner GL, Orosz CG. Transplantation and cytokines. Semin Liver Dis. 1999;19 (2):189. doi: 10.1055/s-2007-1007109. [DOI] [PubMed] [Google Scholar]
- 48.Smyth MJ, Johnstone RW. Role of TNF in lymphocyte-mediated cytotoxicity. Microsc Res Tech. 2000;50 (3):196. doi: 10.1002/1097-0029(20000801)50:3<196::AID-JEMT3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 49.Storm G, Oussoren C, Peeters PAM, Barenholz Y. Tolerability of liposomes in vivo In: Gregoriadis G, ed. Liposome Technology: Interaction of Liposomes with the Biological Milieu, vol III. Ann Arbor: CRC, 1993: 345.
- 50.Yura H, Yoshimura N, Hamashima T, et al. Synthesis and pharmacokinetics of a novel macromolecular prodrug of tacrolimus (FK506), FK506-dextran conjugate. J Control Release. 1999;57 (1):87. doi: 10.1016/s0168-3659(98)00150-3. [DOI] [PubMed] [Google Scholar]
- 51.Takakura Y, Fujita T, Hashida M, Sezaki H. Disposition characteristics of macromolecules in tumor-bearing mice. Pharm Res. 1990;7 (4):339. doi: 10.1023/a:1015807119753. [DOI] [PubMed] [Google Scholar]


