Abstract
We examined changes in weight for 10 captive adult male cotton-top tamarins (Saguinus oedipus) from before the birth of infants through the first 16 weeks of infant life. Compared to before birth, males weighed significantly less in Weeks 1–4, 5–8, and 9–12 following the birth. Weights in Weeks 13–16 did not differ significantly from prebirth weights. Maximum weight loss for individual males ranged from 1.3 to 10.8% of prebirth body weight. Males in groups with fewer helpers lost significantly more weight than ones in groups with more helpers. For the 3 males that had no helper other than their mates, weight loss was particularly striking, ranging from 10.0 to 10.8% of their prebirth body weight. These results suggest that caring for infants is energetically costly, and that in this cooperatively breeding species, the presence of more individuals to share the burden of infant carrying reduces the cost to individual caregivers.
Keywords: cotton-top tamarin, Saguinus, cooperative breeding, weight loss, infant care
INTRODUCTION
Reproduction in the cooperatively breeding callitrichids is characterized by frequent twinning, a high infant-to-maternal weight ratio, and short interbirth intervals due to the female’s ability to conceive during a postpartum ovulation. Infant care is intensive, involving constant carrying of infants through an arboreal habitat, sharing food with infants during weaning, vigilance, predator defense, and assistance with thermoregulation (Snowdon, 1996). Reproduction is usually limited to one female in a social group, and in some species, the contribution of other group members appears to be vitally important for infant survival.
Numerous field and captive studies have documented relationships between infant survival and availability of caregivers. In wild moustached tamarins (Saguinus mystax), infant survival increased as the number of males in the group increased (Garber et al., 1984), and in wild cotton-top tamarins (Saguinus oedipus), the number of infants surviving increased as the number of helpers in the group increased and approached 100% at a group size of 5 (Savage et al., 1996b). In wild saddleback tamarins (Saguinus fuscicollis), groups smaller than 3 were never observed to reproduce (Goldizen, 1988). Koenig’s analysis of data from published field studies on common marmosets (Callithrix jacchus) revealed that groups with >2 adult males had more surviving infants than those with only 1 or 2 males, and that there was no surviving infant in groups with <4 adults (Koenig, 1995). In captive cotton-top tamarins. Snowdon (1996) reported that infant survival increased with group size and approached 100% at a group size of 5. Johnson et al. 1991 found that infants born into groups with siblings present had higher rates of survival than those born into groups containing only the parents. Rothe and Darms (1993), however, found no effect of the number of helpers for infant survival in their colony of captive common marmosets, Geoffroy’s tufted-eared marmosets (Callithrix geoffroyi) and black-tufted-eared marmosets (Callithrix penicillata).
Infant care significantly alters the behavior of caregivers. Goldizen (1987) found that in wild saddleback tamarins infant carriers spent less lime feeding. Price (1992) found that, while carrying infants, captive cotton-top tamarins fed less, moved about less, engaged in less social and affiliative behavior, performed less vigilance behavior, and spent more time concealed than when not carrying infants. Similarly, Sanchez et al. (1999) found that cotton-top tamarins carrying infants had reduced food intake.
In addition to reducing time available for other activities, infant care may be energetically expensive for caregivers. Using field data on travel patterns and the relative weight of infants compared to adults. Tardif (1997) estimated that an adult callitrichid carrying two 30-day-old infants would experience a minimum 21% increase in the caloric cost of traveling per minute compared to traveling without infants. However, few researchers have measured directly the energetic effects of care-giving on caregivers. Sanchez et al. (1999) found that following the birth, the infants’ fathers weighed significantly less than on the day of the birth, and the effect in other male helpers was nearly significant, though female helpers did not appear to be affected. For both fathers and male helpers, the individual’s contribution to the group’s total carrying effort was positively correlated with weight lost. In contrast, Nievergelt and Martin (1999) found that in pairs of common marmosets, fathers neither changed their energy intake nor lost weight following the birth of infants.
We examined changes in weight from before the birth of infants through the first 16 weeks of infant life for 10 captive adult male cotton-top tamarins. We investigated whether the amount of weight lost was related to the time an individual spent carrying infants. Although infant carrying is the most frequently used measure of care-giving, individuals may also engage in other behaviors related to infant care, including food sharing, thermoregulation, maintaining proximity to infants, and vigilance. Thus, we looked more generally at whether weight lost by caregivers was associated with the number of other potential caregivers in a group among which duties might be shared.
METHODS
Subjects
The 10 adult male cotton-top tamarins lived in 8 captive family groups housed at the Psychology Department at the University of Wisconsin, Madison. Not including infants, family groups ranged in size from 2 (one breeding male and female; n = 3 groups) to 6 individuals (a breeding pair and 4 offspring).
All subjects were ≥24 mo old when the infants were born (mean age 49 mo, range 24–107 mo). Female and male cotton-top tamarins reach sexual maturity at by 18 mo (Ginther et al., in press: Ziegler et al., 1987). Nonetheless, individuals of both sexes may continue to gain weight beyond 18 mo (Ginther et al., in press). To reduce complications introduced by individuals that were still growing, we limited analyses to subjects that were ≥24 mo old when the infants were born. By this time scrotal growth has reached asymptote (Ginther et al., 2000). Although individuals may continue to gain weight beyond 24 mo, such a pattern would be inconsistent with the weight loss expected if care-giving is an energetically costly activity. Furthermore, studies on caregiving indicate that older group members are more involved in infant care than younger group members are (Cleveland and Snowdon, 1984; Tardif et al., 1992).
Although mothers provided significant amounts of care, we excluded them from the analyses because many became pregnant again soon after birth. Nonreproductive females were not intentionally excluded; however, none of the focal groups contained nonproductive females that were ≥24 mo old. Six of the males were fathers and 4 were brothers of the infants. For 4 males (3 fathers and 1 brother) we obtained weights following the birth of 2 litters. Thus, the sample size for analyses is 14. For males that were sampled over 2 litters, group composition and the number of surviving infants differed between the 2 litters. In the case of 4 males only 1 infant of the litter survived, but for all others, litters consisted of 2 surviving infants.
Housing and Husbandry
Family groups lived in cages that were either 160 × 93 × 220 cm, 186 ×160 × 220 cm, or 210 × 173 × 220 cm with larger families in the larger cages. The cages contain a variety of ropes and branches, a nest box, and one or two 20 × 25 cm stainless steel food platforms mounted on the side of the cage 165 cm above the floor. Groups lived either in separate rooms or in rooms containing multiple cages with screens hung between the cages to provide visual isolation from other groups though auditory and olfactory communication were possible.
Tamarins received three feedings each day. The first occurred between 0800 and 0900 and consisted of a yogurt, applesauce, and vitamin mixture. The main feeding occurred between 1200 and 1300 and consisted of Zupreem marmoset diet. Purina New World Primate Chow, fresh fruits and vegetables and bread. The last feeding occurred between 1500 and 1700 and consisted of a high protein food such as peanuts, hard-boiled egg, mealworms, cooked hamburger, cottage cheese, or canned tuna. Enough food was provided to ensure that some food was available at all times, and water was available ad libitum (Snowdon et al., 1985).
Behavioral Measures
We obtained data during a study designed to measure care-giving behavior directed toward infants. We observed infants from 1 to 2 days after birth until 16 weeks of age. By 16 weeks, infants were no longer carried or nursed, though they may still have received some food through transfers from other individuals. We observed each infant for 4–5 20-min sessions per week between 0900 and 1200 and between 1300 and 1700. Sessions began ≥30 min after the morning or main feeds and always occurred before the late afternoon feeding. Within each week, sessions were distributed evenly between morning and afternoon hours. An observer to whom the tamarins were habituated sat in front of the cage and recorded behavior on a Tandy TRS80 Model 1200 laptop computer using the behavioral scoring system KB+ (Cook, 1988). Behavioral observations were conducted by 2 observers.
During each session, the identities of subjects that were carrying infants (as well as several other measures not relevant to these analyses) were recorded using 30 s scan samples. Interobserver reliability for the 2 observers was 97% for behaviors recorded during instantaneous scan samples (index of concordance, Martin and Bateson, 1996). From these data it was possible to calculate the amount of time that individual males spent carrying infants.
Weighing
We weighed the tamarins via a Sartorius LC 16000 S platform balance equipped with an Animal Weighing program. The scale had a stainless steel platform 30 × 40 cm, which was large enough for an adult tamarin to sit on easily. The scale was mounted on the top shelf of a 110 cm high cart, and could be wheeled into the cage by the experimenter. Following habituation to the scale, most monkeys would readily approach and sit on the platform in return for a small food reward.
We collected caregiver weights during a study designed to determine infant growth. We weighed infants weekly, and the weights of caregivers were frequently obtained opportunistically. As caregiver weights were not the original focus of the study, weights for each male are not available each week. Thus, we examined the pattern of weight changes for four 4-week time periods following the birth (Weeks 1–4, 5–8, 9–12, and 13–16 postbirth). We calculated a mean for each time period based on the average of all weights collected during that period. For all males we have at least one weight during each postbirth period.
In addition, we calculated a prebirth weight using weights obtained during the 2 m preceding the birth. All males but one were weighed at least once before the birth. To replace the missing data point, we calculated the mean change between prebirth weight and weight during the first 4 weeks following the birth for the 13 cases for which complete data were available. We added the mean change for the rest of the sample to the subject’s weight during the first 4-week period after the birth to provide an estimate of his prebirth weight. This male was one of the two for which we obtained two sets of data, and the estimate of his prebirth weight (699.8 g) is very close to his actual prebirth weight (693.5 g) for the other set of data.
RESULTS
A one-way repeated measures ANOVA revealed significant changes in weight over the 5 time periods, F(4,52) = 5.00, p = 0.002. Weights in weeks 1–4, 5–8, and 9–12 are significantly lower than before birth, and weights in weeks 5–8 are significantly lower than during Weeks 13–16 (dependent samples t tests, p < 0.05. Fig. 1). The period in which males lost weight corresponded to the period during which infants were carried the most. Infants were carried an average of 99% of the time in Weeks 1–4, 58% during Weeks 5–8, and 16% during weeks 9–12. The time infants were carried declined to <5% during Weeks 13–16, and by Weeks 13–16 average male weight had increased and no longer differed significantly from average prebirth weight.
Fig. 1.
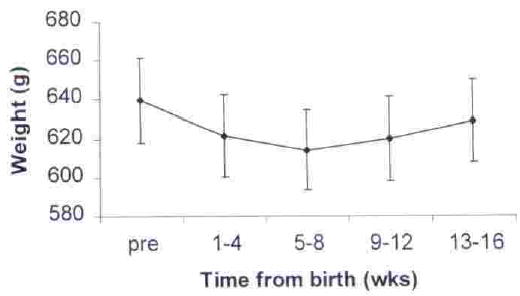
Mean weight (±SE) of males before and after the birth of infants.
There was variability in the pattern of weight loss exhibited by individual males with some males reaching their lowest weighs during each of the 4 postbirth periods. To better depict the magnitude of weight loss experienced by individual males, we calculated the maximum weight loss for each male by comparing his prebirth weight to his lowest postbirth weight, regardless of when it occurred. The mean maximum weight loss is 37.5 ± 6.0 g or 5.7 ± 0.9% of body weight. Maximum weight loss ranged from 9.1 (1.3% of body weight) to 75.5 g (10.8% of body weight).
In an attempt to explain some of the variation in weight loss between individuals, we calculated correlations between the maximum body weight lost and the maximum percentage of time a male carried infants in either Weeks 1–8 or 9–16(in 13 of 14 cases the maximum carrying occurred during the first 8 weeks, but one male carried more during Weeks 9–16). This correlation is not significant (r = 0.38, df = 12, p = 0.178). However, the percent of body weight that an individual male lost is negatively correlated with the number of potential caregivers in a group (the number of non-infant group members) (r = −0.71, df = 12, p = 0.004, Fig. 2). We found no difference in patterns of weight loss between reproductive males and subadult males or between those males with 1 versus 2 surviving infants.
Fig. 2.
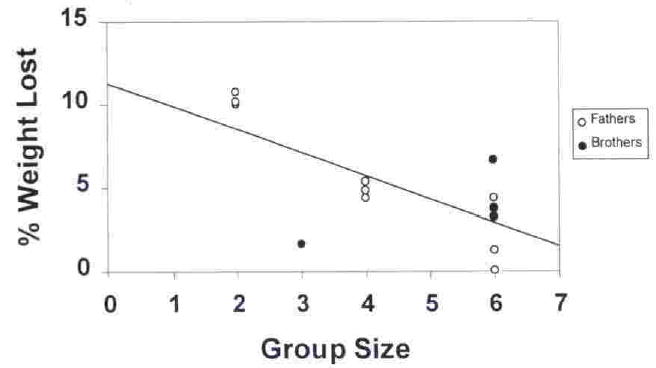
The relationship between weight loss and group size (excluding infants) for males. Open circles indicate breeding males: dot indicate subadult males.
Three males were first time fathers with no helper other than their mates. Their weight loss was particularly striking, with all 3 showing maximum declines of ≥10% (10.0, 10.2. and 10.8%) from their prebirth weights. In addition, although average male weight in weeks 13–16 did not differ significantly from their prebirth weight, the weights of males without helpers remained substantially below their prebirth weights during weeks 13–16.
DISCUSSION
Following infant births, captive adult male tamarins that participated in infant care weighed significantly less than before the births. Males in groups with fewer helpers lost more weight than those with more helpers, though the percent of weight lost is not correlated with the time individual males carryied infants. The loss of weight in male caregivers is particularly striking given that the tamarins were housed with continuously available food, minimal travel requirements, no predators, and little fluctuation in ambient temperature.
Males in smaller groups lost more weight than males in larger groups did, implying that with more group members to share infant care, the cost to individual caregivers may be reduced. The weight loss was most dramatic in first time fathers, which also had the fewest helpers. Increased stress might lead to elevated cortisol, which would mobilize energy and lead to greater weight loss. However, there is no difference in cortisol levels between first time and experienced fathers (Ziegler and Snowdon, 2000).
The absence of a significant relationship between weight loss and the amount each male was observed to carry infants suggests that additional types of care-giving behavior might contribute to male weight loss. Price (1992) reported that caregivers fed less during infant carrying. Two other behaviors that might place energetic demands on caregivers include sharing food with infants and vigilance.
Vigilance in the form of scanning is a frequent behavior of callitrichids even in captivity (Caine, 1984; 1993), and vigilant individuals may position themselves in a vantage somewhat separate from the rest of the group (Savage et al., 1996b). Indeed, Savage et al. 1996b included infant protection and predator detection along with carrying as major functions that helpers perform. Caine (1993) proposed that the need for vigilance was a driving force in the evolution of cooperative and flexible social systems in callitrichids. There are two ways in which vigilance requirements might be more demanding for carriers in smaller groups. First, although both Price (1992) for captive tamarins and Savage et al. 1996b for wild tamarins report that individuals were less vigilant while carrying infants. Savage et al. 1996b also found that individuals in small groups were more likely than those in large groups to be vigilant while carrying. Thus, in smaller groups, caregivers might be forced lo engage in vigilance and carrying simultaneously, rather than adopting the more typical cryptic strategy proposed by Price (1992). Second, caregivers in smaller groups might be more vigilant when they are not carrying at the expense of other activities such as foraging and feeding.
Sanchez et al. (1999) similarly found that cotton-top tamarin fathers lost weight following the birth of infants. In that study weight loss in fathers and male helpers was associated with an indirect measure of carrying effort: the individual’s contribution to the group's total carrying effort. Nievergelt and Martin (1999) however, found no effect of infant care in weight in common marmosets. A possible explanation for this discrepancy is that marmoset infants are less costly to rear than tamarin infants are. Compared to tamarins, marmosets exhibit somewhat less paternal involvement in infant care: paternal experience appears to be less important to infant survival: and infants are independent of carriers earlier (Tardif et al., 1984, 1986, 1993). Marmosets provision infants for shorter spans than tamarins do, and in the wild, marmosets exhibit shorter daily path lengths than those of tamarins (Tardif et al., 1993). In marmosets, the presence of additional caregivers may not increase infant survival (Rothe and Darms, 1993: captivity: but see Koenig, 1995: wild marmosets).
To avoid complications introduced by caregiver growth, we excluded individuals <24 m old from the analysis. The lower level of care observed in younger group members has been attributed by Tardif et al. 1992 to lack of interest or to inexperience and their inability to compete successfully for access to infants by Achenbach and Snowdon (1998) and Price (1991). Additionally, if care-giving has physiological effects that could interfere with growth, then caring for infants could be more costly for an immature individual than for a mature adult.
No nonreproductive females were available for inclusion in our study. Future studies should establish whether nonreproductive females show patterns of weight loss similar to those observed in males. In reproductive females, nursing patterns are associated with the time to the first postpartum ovulation (Ziegler et al., 1990). Similarly infant care and the availability of helpers might affect patterns of weight change in reproductive females.
Individual variation in the severity and timing of caregivers’ weight loss might be further investigated through more detailed analyses of carrying that consider factors such as whether carriers tends to transport one or two infants. Variation in other caregiver behavior such as food-sharing with infants, time devoted to vigilance, and the caregiver’s own food intake might also contribute to differences in patterns of weight loss.
The finding that adult male cotton-top tamarins lost weight following the birth of infants and that the percent of weight loss is greater in groups containing fewer potential caregivers suggests that even in captivity, care-giving is an energetically costly behavior for tamarins. It is difficult, however, to determine whether the immediate costs of care-giving have long-term effects on reproductive fitness. Nevertheless, it seems plausible that in the wild, the energetic costs associated with care-giving would be more severe than in captivity and could reduce an individual’s fitness by compromising his or her health, survival, or competitive ability.
Wild cotton-top tamarins do not have an immediate postpartum estrous but instead exhibit a 5–6-mo period with no ovulation (Savage et al., 1997). Thus the birth spacing in wild cotton-top tamarins might allow for physiological recovery from caring for infants for both males and females, and annual reproductive rate is lower for wild cotton-top tamarins than captive ones.
All of the males in our study were closely related to the infants they cared for. However, field studies indicate that callitrichid groups often contain unrelated animals, and that females may mate with more than one male (Goldizen, 1988; Savage et al., 1996 a; Sussman and Garber, 1987). Several studies indicate that caregivers will care for unrelated infants (Baker et al., 1993; Cleveland and Snowdon. 1984; Wamboldt et al., 1988). Since caregivers incur significant costs through weight loss, reduced feeding, and vigilance, it is important to document the benefits that caregivers gain whether through direct fitness, inclusive fitness, group membership, acquisition of infant care skills, or future mating opportunities.
Acknowledgments
We thank Carla Boe and Rebecca Sellin for their help with data collection and two anonymous reviewers for constructive improvements of the paper. The research was supported by an NSF Predoctoral Fellowship to GGA and by NIH grants MH 35215 and MH 00177 to CTS.
References
- Achenbach GG, Snowdon CT. Responses to sibling birth in juvenile cotton-top tamarins (Saguinus oedipus) Behaviour. 1998;135:845–862. [Google Scholar]
- Baker AJ, Dietz JM, Kleiman DG. Behavioural evidence for monopolization of paternity in multi-male groups of golden lion tamarins. Anim Behav. 1993;46:1091–1103. [Google Scholar]
- Caine NG. Visual scanning by tamarins. Folia Primatol. 1984;43:59–67. [Google Scholar]
- Caine, N. G. (1993). Flexibility and co-operation as unifying themes in Saguinus social organization and behaviour: The role of predation pressures. In Rylands, A. B. (ed.). Marmosets and Tarmarins: Systematics. Behaviour and Ecology Oxford University Press. Oxford, pp. 200–219
- Cleveland J, Snowdon CT. Social development during the first twenty weeks in the cotton-top tamarin (Saguinus oedipus) Anim Behav. 1984;32:432–444. [Google Scholar]
- Cook, M. B. (1988). KB+ Data Entry Software University of Wisconsin, Madison.
- Garber PA, Moya L, Malaga C. A preliminary field study if the moustached tamarin monkey (Saguinus mystax) in Northeastern Peru: Questions concerned with the evolution of a communal breeding system. Folia Primatol. 1984;42:17–32. [Google Scholar]
- Ginther AJ, Washabaugh KF, Snowdon CT. Measurement of scrotum, and testis size of unrestrained captive cotton-top tamarins (Saguinus oedipus oedipus) Am J Primatol. 2000;51:187–195. doi: 10.1002/1098-2345(200007)51:3<187::AID-AJP3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Ginther, A. J., Carison, A. A., Ziegler, T. E., and Snowdon, C. T. (in press). Neonatal and pubertal development in males of a cooperatively-breeding primate, the cotton-top tamarin (Saguinus oedipus oedipus). Biol. Reprod. [DOI] [PMC free article] [PubMed]
- Goldizen AW. Facultative polyandry and the role of infant-carrying in wild saddleback tamarins (Saguinus fuscicollis) Behav Ecol Sociobiol. 1987;20:99–109. [Google Scholar]
- Goldizen AW. Tamarin and marmoset mating systems: Unusual flexibility. Trends Ecol Evol. 1988;35:36–40. doi: 10.1016/0169-5347(88)90045-6. [DOI] [PubMed] [Google Scholar]
- Johnson LD, Petto AJ, Sehgal PK. Factors in the rejection and survival of captive cotton-top tamarins (Saguinus oedipus) Am J Primatol. 1991;25:91–102. doi: 10.1002/ajp.1350250203. [DOI] [PubMed] [Google Scholar]
- Koenig A. Group size, composition and reproductive success in wild common marmosets (Callithrix jaccchus) Am J Primatol. 1995;35:311–317. doi: 10.1002/ajp.1350350407. [DOI] [PubMed] [Google Scholar]
- Martin, P., and Bateson, P. (1996). Measuring Behaviour: An Introductory Guide 2nd, edn. Cambridge University Press, Cambridge.
- Nievergelt CM, Martin RD. Energy intake during reproduction in captive common marmosets (Callithrix jacchus) Physiol Behav. 1999;65:849–854. doi: 10.1016/s0031-9384(98)00249-2. [DOI] [PubMed] [Google Scholar]
- Price EC. Competition to carry infants in captive families of cotton-top tamarins (Saguinus oedipus) Behaviour. 1991;118:66–88. [Google Scholar]
- Price EC. The costs of infant carrying in captive cotton-top tamarins. Am J Primatol. 1992;26:23–33. doi: 10.1002/ajp.1350260106. [DOI] [PubMed] [Google Scholar]
- Rothe, H., and Darms, K. (1993). The social organization of marmosets: A critical evaluation of recent concepts. In Rylands, A. B. (ed). Marmosets and Tamarins: Systematics, Behaviour and Ecology, Oxford University Press, Oxford. pp. 176–199.
- Sanchez S, Pelaez F, Gil-Burmann C, Kaumanns W. Costs of infant-carrying in the cotton-top tamarin (Saguinus oedipus) Am J Primatol. 1999;48:99–111. doi: 10.1002/(SICI)1098-2345(1999)48:2<99::AID-AJP2>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Savage A, Giraldo LH, Soto LH, Snowdon CT. Demography, group composition and dispersal in wild cotton-top tamarins (Saguinus oedipus) groups. Am J Primatol. 1996a;38:85–100. doi: 10.1002/(SICI)1098-2345(1996)38:1<85::AID-AJP7>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Savage, A., Snowdon, C. T., Giraldo, L. H., and Soto, L. H. (1996b). Parental care patterns and vigilance in wild cotton-top tamarins (Saguinus oedipus). In Norconk, M., Rosenberger, A. L., and Garber, P. A. (eds). Adaptive Radiations of Neotropical Primates Plenum, New York. pp. 187–199.
- Savage A, Shideler SE, Soto LH, Causado J, Giraldo LH, Lasley BL, Snowdon CT. Reproductive events of wild cotton-top tamarins (Saguinus oedipus) in Colombia. Am J Primatol. 1997;43:329–337. doi: 10.1002/(SICI)1098-2345(1997)43:4<329::AID-AJP4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Snowdon, C. T. (1996). Infant care in cooperatively breeding species. In Rosenblatt, J. S., and Snowdon, C. T. (eds). Parental Care: Evolution, Mechanisms and Adaptive Significance, Academic Press, San Diego, pp. 643–689.
- Snowdon CT, Savage A, McConnell PB. A breeding colony of cotton-top tamarins. Lab Anim Sci. 1985;35:477–480. [PubMed] [Google Scholar]
- Sussman RW, Garber PA. A new interpretation of the social organization and mating system of the Callitrichidae. Int J Primatol. 1987;8:73–92. [Google Scholar]
- Tardif, S. D. (1997). The bioenergetics of parental behavior and the evolution of alloparental care in marmosets and tamarins. In Solomon. N. G., and French J. A. (eds). Cooperative Breeding in Mammals Cambridge University Press. Cambridge, pp. 11–33.
- Tardif SD, Carson RL, Gangaware BL. Comparison of infant-care in family groups of the common marmoset (Callithrix jacchus) and the cotton-top tamarin (Saguinus oedipus) Am J Primatol. 1986;11:103–110. doi: 10.1002/ajp.1350110202. [DOI] [PubMed] [Google Scholar]
- Tardif SD, Carson RL, Gangaware BL. Infant-care behavior of non-reproductive helpers in a communal-care primate, the cotton-top tamarin (Saguinus oedipus) Ethology. 1992;92:155–167. doi: 10.1002/ajp.1350220202. [DOI] [PubMed] [Google Scholar]
- Tardif, S. D., Harrison, M. L., and Simek, M. A. (1993). Communal infant care in marmosets and tamarins: Relation to energetics ecology and social organization. In Rylands, A. B. (ed.). Marmosets and Tamarins: Systematics, Behaviour and Ecology Oxford University Press, Oxford, pp. 220–234.
- Tardif SD, Richter CB, Carson RL. Effects of sibling rearing experience on future reproductive success in two species of Callitrichidae. Am J Primatol. 1984;6:377–380. doi: 10.1002/ajp.1350060408. [DOI] [PubMed] [Google Scholar]
- Wamboldt MZ, Gelhard RE, Insel TR. Gender differences in caring for infant Cebuella pygmaea: The role of infant age and relatedness. Dev Psychobiol. 1988;21:187–202. doi: 10.1002/dev.420210207. [DOI] [PubMed] [Google Scholar]
- Ziegler TE, Savage A, Scheffler G, Snowdon CT. The endocrinology of puberty and reproductive functioning in female cotton-top tamarins (Saguinus oedipus) under varying social conditions. Biol Reprod. 1987;37:618–627. doi: 10.1095/biolreprod37.3.618. [DOI] [PubMed] [Google Scholar]
- Ziegler TE, Snowdon CT. Preparental hormone levels and parenting experience in male cotton-top tamarins. Saguinus oedipus Horm Behav. 2000;38:159–167. doi: 10.1006/hbeh.2000.1617. [DOI] [PubMed] [Google Scholar]
- Ziegler TE, Widowski TM, Larson ML, Snowdon CT. Nursing does affect the duration of the postpartum to ovulation interval in cotton-top tamarins (Saguinus oedipus) J Reprod Fertil. 1990;90:563–570. doi: 10.1530/jrf.0.0900563. [DOI] [PubMed] [Google Scholar]


