Abstract
Molluscan catch muscle can maintain tension for a long time with little energy consumption. This unique phenomenon is regulated by phosphorylation and dephosphorylation of twitchin, a member of the titin/connectin family. The catch state is induced by a decrease of intracellular Ca2+ after the active contraction and is terminated by the phosphorylation of twitchin by the cAMP-dependent protein kinase (PKA). Twitchin, from the well-known catch muscle, the anterior byssus retractor muscle (ABRM) of the mollusc Mytilus, incorporates three phosphates into two major sites D1 and D2, and some minor sites. Dephosphorylation is required for re-entering the catch state. Myosin, actin and twitchin are essential players in the mechanism responsible for catch during which force is maintained while myosin cross-bridge cycling is very slow. Dephosphorylation of twitchin allows it to bind to Factin, whereas phosphorylation decreases the affinity of the two proteins. Twitchin has been also been shown to be a thick filament-binding protein. These findings raise the possibility that twitchin regulates the myosin cross-bridge cycle and force output by interacting with both actin and myosin resulting in a structure that connects thick and thin filaments in a phosphorylation-dependent manner.
Introduction
Molluscan smooth muscles such as bivalve adductor and mussel anterior byssus retractor muscles exhibit a unique contraction referred to as ‘catch’, which uses little energy during maintenance of tension. Since the discovery of catch almost a century ago (reviewed in Bayliss, 1927), many studies have been performed to investigate the mechanism responsible for tension maintenance.
The schematic representation of the catch contraction is shown in Fig. 1. Molluscan catch muscle is controlled by cholinergic and serotonergic nerves. The stimulation of the former brings about an increase of the intracellular Ca2+ concentration, initiating phasic contraction, and the subsequent decrease of Ca2+ shifts it to the catch state. Catch tension is maintained until serotonin is released causing an accumulation of cAMP in the muscle cell. It has been thought that a cAMP-dependent protein kinase (PKA) activated by cAMP phosphorylates a myofibrillar protein which is the key component in regulation of catch (Cole and Twarog, 1972; Achazi et al., 1974).
Fig. 1.
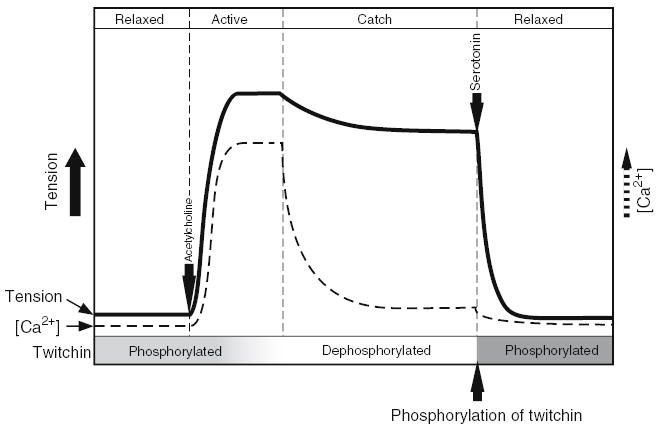
Relationship between tension, intracellular Ca2+ concentration and phosphorylation of twitchin in catch muscles. Solid and dotted lines represent muscle tension and intracellular Ca2+ concentration, respectively. In the relaxed muscle twitchin is phosphorylated. Contraction is initiated by release of acetylcholine which induces an increase in intracellular Ca2+ and muscle contraction. Dephosphorylation of twitchin by a Ca2+-dependent phosphatase occurs while intracellular Ca2+ is high. When Ca2+ concentration falls, catch force is maintained and energy consumption is very low. The catch state is terminated by release of serotonin which causes an increase of intracellular cAMP and a cAMP-dependent protein kinase-mediated phosphorylation of twitchin.
Components of molluscan muscle seem similar to those of vertebrate muscle, but the structure of the thick filament is extremely different. Paramyosin is a dimer of a 100 kDa subunit, and it makes up the core of thick filament which is wrapped by a monolayer of myosin (Kendrick-Jones et al., 1969; Szent-Györgyi et al., 1971; Epstein et al., 1975; Cohen, 1982). Paramyosin is widely distributed in all invertebrate muscles. Its content varies depending on species and tissues, and correlates with the tension which the muscle can develop (Lowy et al., 1964). Paramyosin is most abundant in catch muscles (Winkelman, 1976). Therefore, it has been suggested that paramyosin plays a role in the catch contraction (Watabe and Hartshorne, 1990). Paramyosin is thought to be necessary for the thick filament to bear very high tension (up to 15 kg/cm2; Millman, 1964), but the function of paramyosin is not yet clearly identified. Another unique protein is myosin rod-like protein called myorod (Shelud’ko et al., 1999), of which an homologous protein exists in the insect flight muscle (Standiford et al., 1997). The inert protein, which shares the rod region with myosin heavy chain, is expressed by alternative splicing from the myosin heavy chain gene (Yamada et al., 2000). Yamada et al. 2000 named the protein ‘catchin’ because they thought that it might be a regulator of the catch contraction, but as is discussed later, this does not seem to be the case. The name myorod, which emphasizes the relationship to myosin thus seems to be more appropriate. Myorod has unique N-terminal sequences, depending on species (Yamada et al., 2000). At present, its function in the muscle cell is unknown. Molluscan myosin is quite similar to vertebrate myosin, except for the regulatory system. Molluscan myosin is activated by direct binding of Ca2+ to myosin (Kendrick-Jones et al., 1970; Szent-Györgyi and Chantler, 1994). Unbinding of Ca2+ from myosin inactivates the molecule. Thus, molluscan catch muscle has a myosin-linked regulatory system somewhat similar to vertebrate smooth muscle, but differs from vertebrate skeletal muscle, which employs the actin-linked regulatory system.
It is difficult to explain the mechanism of catch contraction using only the contractile proteins myosin and actin, and it has been thought that the most likely third player was the protein phosphorylated by PKA when the muscle relaxes from catch. The target protein of PKA had been explored for a long time. Myosin heavy chain (Castellani and Cohen, 1987), myosin light chain (Sohma et al., 1985, 1988a, Sohma et al., b), paramyosin (Achazi, 1979a,b; Watabe et al., 1989) and myorod (Yamada et al., 2000) were all reported to be candidates for the regulator of catch because they are phosphorylated by PKA in vitro. However, these proteins were rejected because their phosphorylation state did not change when catch tension was released in skinned muscles.
Phosphorylation of twitchin associated with the release of catch
A study using ABRM skinned fibers, 32P-ATP and caged 32P-ATP with a photoflash technique, has identified the protein phosphorylated by PKA when catch is released (Siegman et al., 1997). The protein of ~600 kDa specifically incorporated phosphate, whereas the phosphorylation level of other ABRM components, myosin heavy chain, myosin light chain, paramyosin and myorod remained unchanged. A PKA inhibitor completely inhibited this phosphorylation as well as the relaxation of catch tension. Partial protein and cDNA sequencing illustrated that the phosphorylated protein is twitchin, a member of the titin/connectin family, well-known as a myosin-binding protein (Siegman et al., 1998). In ABRM skinned fibers, twitchin is irreversibly thiophosphorylated in the presence of cAMP and ATPγS. This causes the muscle to lose the ability to enter the catch state, but has no effect on force output in a high calcium-containing solution. These results showed that twitchin is the regulator of the catch state.
Twitchin was first reported as the unc-22 gene product of Caenorhabditis elegans (Moreman et al., 1988; Benian et al., 1989), and mutation of it caused abnormal twitching movements (Waterston et al., 1980). The collapse of the sarcomere structure of body wall muscle in the unc-22 mutant raises the possibility that twitchin functions as does titin/connectin in vertebrate skeletal muscle, namely, a ruler of thick filaments. However, it is unlikely that twitchin in catch muscle plays a role equivalent to titin/connectin in skeletal muscle because molluscan smooth muscle has no distinct sarcomere structure. The discovery of the new function of twitchin as the regulator of catch has facilitated elucidation of the regulatory system of the unique energy-saving contraction.
Structure of Mytilus twitchin
The full sequence of Mytilus twitchin was determined by cDNA cloning with 3′ and 5′ RACE methods (Funabara et al., 2003). Mytilus twitchin is composed of a single polypeptide of 530 kDa consisting of 4736 amino acids, which is a substantially smaller than that of C. elegans (~754 kDa) (Benian et al., 1989, 1993). Mytilus twitchin has 24 immunoglobulin (Ig) and 15 fibronectin type 3 (Fn) motifs as well as one kinase domain, making it a member of the titin/connectin family. The alignments of the Ig and Fn motifs are very similar to those of other invertebrate twitchin related proteins, C. elegans twitchin and Crayfish projectin (Oshino et al., 2003) (Fig. 2).
Fig. 2.
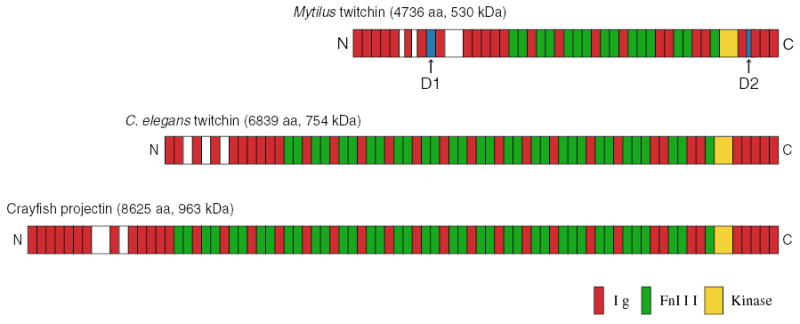
Schematic representation of Mytilus twitchin and related proteins. The phosphorylation sites D1 and D2 in Mytilus twitchin are shown.
The proteins of the titin/connectin family usually have a PEVK domain whose length depends on species and/or tissue source (Labeit and Kolmerer, 1995). The PEVK domain is a unique region rich in the amino acid residues P, E, V and K, and it is thought to contribute to muscle elasticity. Mytilus twitchin has a short PEVK domain in the sequence between the sixth and seventh Ig domain, composed of only 79 amino acids, and the content of PEVK is lower than those of other vertebrate proteins (Funabara et al., 2003). The function of the short PEVK in Mytilus twitchin is unknown.
Phosphorylation of twitchin by PKA
Twitchin purified from Mytilus ABRM is a good in vitro substrate for the catalytic subunit of PKA (Funabara et al., 2001a). Phosphorylation occurs very rapidly, and 3 mol phosphate are incorporated into 1 mol twitchin within a few minutes. This indicates that there are at least three phosphorylation sites in the twitchin molecule. The rapid phosphorylation of twitchin corresponds to the rapid release of catch caused by the addition of cAMP in vivo. So far, two of the phosphorylation sites, referred to as D1 and D2, have been identified (Funabara et al., 2003). D1 and D2 exist in the linker regions connecting the 7th and 8th Ig motifs from the N-terminus and the 3rd and 4th Ig motifs from the C-terminus (Fig. 2). D1 and D2 contain the typical recognition sequence for PKA, RRXS. Three additional RRXS sites are located in the region N-terminal to the D1 site raising the possibility that additional phosphorylation occurs in the same linker region as D1.
The expressed protein including the 7th and 8th Ig domains was phosphorylated in vitro even though the D1 serine was replaced with an alanine residue (D. Funabara, unpublished observation). Two-dimensional phosphopeptide mapping of tryptic digests of the phosphorylated twitchin purified from ABRM showed several small spots in addition to major ones representing D1 and D2. In vivo experiments using skinned ABRM muscles and phosphorylation dependent antibodies for the D1 and D2 sites showed that relaxation of catch occurs only when both sites are phosphorylated. The endogenous PKA that phosphorylates twitchin has not been identified, but it is known that a soluble fraction of the ABRM homogenate phosphorylates twitchin in vitro.
Kinase activity of twitchin
Twitchin and its related proteins have near the C-terminus a kinase domain that is highly homologous to the catalytic domain of myosin light chain kinase. This suggests that twitchin should act as a kinase in vivo. Indeed, it has been reported that native twitchin, its kinase domain and projectin (the arthropod equivalent of twitchin) phosphorylate myosin light chains and are capable of autophosphorylation in vitro (Maroto et al., 1992; Heierhorst et al., 1994, 1995; Lei et al., 1994; Weitkamp et al., 1998). It has been reported that twitchin is activated by binding of Ca2+/S100 to the kinase domain (Heierhorst et al., 1996). On the other hand, in twitchin from molluscan catch muscle, phosphorylation assays showed no autophosphorylation (Funabara et al., 2001a). Furthermore, SDS-PAGE and autoradiography of proteins from permeabilized ABRM treated with cAMP showed no incorporation of phosphate into myofibrillar proteins other than twitchin. Thus, twitchin does not seem to function as a kinase during relaxation of catch (Siegman et al., 1997). It is possible that the kinase activity of twitchin differs in catch and non-catch muscles, or that it phosphorylates a yet unknown substrate that plays a role other than regulation of catch.
Dephosphorylation of twitchin
Phosphorylated twitchin must be dephosphorylated before the muscle can enter the catch state. The phosphatase that acts on twitchin is active when the intracellular Ca2+ concentration is high during activation of the muscle (Funabara et al., 2003). The most likely candidate for the phosphatase is calcinuerin, also called protein phosphatase IIB, which is activated by Ca2+/CaM (Castellani and Cohen, 1988; Yamada et al., 2004). It is well-known that calcinuerin dephosphorylates sites phosphorylated by PKA. In addition, in an in vitro system, it was shown that bovine calcinuerin dephosphorylated twitchin and initiated the catch state (Yamada et al., 2004).
Interaction of twitchin with myofibrillar proteins
There is some evidence concerning the interaction of twitchin with other myofibrillar proteins. Since the identification of twitchin in C. elegans, it has been thought that it associates with myosin because the loss of twitchin causes alteration of the thick filaments. Recently, the interaction between Mytilus twitchin and myosin was shown by a co-sedimentation assay in vitro (Yamada et al., 2001). However, how twitchin binds to myosin is unknown. Our study on the nucleotide sequence of Mytilus twitchin raised the possibility that twitchin binds to myosin in the same ways as does myosin binding protein C (MyBP-C) in striated muscle, because the twitchin structure including the phosphorylation site D1 shows some resemblance to C-protein (Funabara et al., 2003). Furthermore, there is a common sequence for actin-binding near D1, suggesting that twitchin has the ability to interact with actin. Shelud’ko et al. 2004 recently showed that twitchin binds to F-actin in a phosphorylation-dependent manner. However, Yamada et al. 2001 concluded from co-sedimentation assays that twitchin does not bind to actin. Our preliminary studies showed that Mytilus twitchin has the ability to bind to actin in a phosphorylationdependent manner (D. Funabara, unpublished). The data thus suggest that twitchin may bind to both myosin and actin, and may, in doing so, regulate the actin-myosin cross-bridge cycle in a phosphorylation dependent manner.
As discussed above, the most abundant protein in catch muscle is paramyosin. Projectin, a counterpart of twitchin in arthropods, affects paramyosin organization in synthetic filaments in vitro (Kolsch et al., 1995) and has a dramatic effect on formation of thick filament in vitro (Fährmann et al., 2002). In catch muscle, purification of twitchin involves treatment with a high-salt buffer, which is usually used to extract the salt-soluble proteins such as myosin and paramyosin (Funabara et al., 2001). This is consistent with the idea that twitchin is on the thick filament and interacts with myosin and/or paramyosin.
How is twitchin involved in catch contraction?
The mechanism of maintenance of the catch state is unknown. In an in vitro assembly system it has been shown that the essential components to form the catch state are myosin, actin and twitchin (Yamada et al., 2001). Myosin crossbridge cycling in catch must be very slow since the energy consumption is very low (Lowy et al., 1964; Lowy and Poulsen, 1982). Molluscan actin-activated myosin ATPase is activated by direct binding of Ca2+, and it has a very low ATPase in the absence of Ca2+ binding (see, Szent-Györgyi and Chantler, 1994). The low intracellular Ca2+ concentration during catch is undoubtedly responsible for the low ATPase in catch, but the tension maintenance in the catch state means that the interaction between myosin and actin persists despite the low Ca2+. Phosphorylation of twitchin removes the interaction between actin and myosin at low Ca2+ and allows relaxation of force. Since twitchin interacts with actin in a phosphorylation-dependent manner, it is possible that binding of dephosphorylated twitchin to actin would strengthen the cross-bridge interaction, leading to the catch state. The details of how phosphorylation of twitchin modifies the actin-myosin interaction to give rise to relaxation of force at low Ca2+concentration remain to be elucidated.
‘Latch’ in vertebrate smooth muscle
Vertebrate smooth muscles are capable of exhibiting catch-like contraction named ‘latch’ (Dillon et al., 1981), which is induced when the intracellular Ca2+ concentration decreases after an active contraction, as in the case of molluscan catch muscle (Remboldt and Murphy, 1986). It is assumed that during tension maintenance the cross-bridge interaction remains or cycles very slowly. Two proteins have been proposed as regulators of latch contraction so far. Caldesmon well-known as a thin-filament binding protein, first isolated as a Ca2+/calmodulin binding protein (Sobue et al., 1981), is thought to regulate vertebrate smooth muscle contractility by binding to thin filament. It has been shown that the protein has effects on the latch state of skinned vertebrate smooth muscles (Katsuyama et al., 1992; Albrecht et al., 1997). Calponin, also known as a thin-filament binding protein, may be another regulator of the latch contraction (Szymanski, 2004). Calponin inhibits actomyosin ATPase (Winder and Walsh, 1990) and the sliding of actin filament in in vitro motility assay (Shirinsky et al., 1992; Haeberle, 1994). Thus in vertebrate smooth muscles latch is possibly regulated by the thin-filament associated proteins. Is there the same regulatory system in molluscan catch muscle? Interestingly, calponin also expresses in molluscan catch muscle and inhibits actomyosin ATPase in vitro (Funabara et al., 2001b). However there is no available information whether molluscan calponin is involved in the catch contraction at present. Further studies are needed to find if there is a common mechanism between the catch and latch contractions.
Acknowledgments
This work was supported in part by NIH AR 042758 (to MJS and TMB), a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to DF) and a Joint Project of Japan–U.S. Cooperative Science Program of the Japan Society for the Promotion of Science (to SW).
References
- Achazi RK, Dolling B, Haakshorst R. 5-HT-induced relaxation and cyclic AMP in a molluscan smooth muscle. Pflügers Arch. 1974;349:19–27. doi: 10.1007/BF00587913. [DOI] [PubMed] [Google Scholar]
- Achazi RK. 5–HT induced accumulation of 3′,5′-AMP and the phosphorylation of paramyosin in the ABRM of Mytilus edulis. Malacologia. 1979a;18:465–468. [PubMed] [Google Scholar]
- Achazi RK. Phosphorylation of molluscan paramyosin. Pflügers Arch. 1979b;379:197–201. doi: 10.1007/BF00586948. [DOI] [PubMed] [Google Scholar]
- Albrecht K, Schneider A, Liebetrau C, Ruegg JC, Pfitzer G. Exogenous caldesmon promotes relaxation of guinea-pig skinned taenia coli smooth muscles: inhibition of cooperative reattachment of latch bridges? Pflügers Arch. 1997;434:534–542. doi: 10.1007/s004240050433. [DOI] [PubMed] [Google Scholar]
- Bayliss WM (1927) Principles of General Physilogy Longmans, Green and Co, New York.
- Benian GM, Ki JE, Neckelmann N, Moerman DG, Waterston RH. Sequence of an unusually large protein implicated in regulation of myosin activity in C. elegans. Nature. 1989;342:45–50. doi: 10.1038/342045a0. [DOI] [PubMed] [Google Scholar]
- Benian GM, L’Hernault SW, Morris ME. Additional sequence complexity in the muscle gene, unc-22, and its encoded protein, twitchin, of Caenorhabditis elegans. Genetics. 1993;134:1097– 1104. doi: 10.1093/genetics/134.4.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellani L, Cohen C. Myosin rod phosphorylation and the catch state of molluscan muscles. Science. 1987;235:334–337. doi: 10.1126/science.3026049. [DOI] [PubMed] [Google Scholar]
- Castellani L, Cohen C. A calcineurin-like phosphatase is required for catch contraction. FEBS Lett. 1988;309:321–326. doi: 10.1016/0014-5793(92)80798-l. [DOI] [PubMed] [Google Scholar]
- Cohen C. Matching molecules in the catch mechanism. Proc Natl Acad Sci USA. 1982;79:3176–3178. doi: 10.1073/pnas.79.10.3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole RA, Twarog BM. Relaxation of catch in a molluscan smooth muscle. I Effects of drugs which act on the adenyl cyclase system. Comp Biochem Physiol. 1972;43A:321–330. doi: 10.1016/0300-9629(72)90191-0. [DOI] [PubMed] [Google Scholar]
- Dillon PF, Aksoy MO, Driska SP, Murphy RA. Myosin phosphorylation and the cross-bridge cycle in arterial smooth muscle. Science. 1981;211:495–497. doi: 10.1126/science.6893872. [DOI] [PubMed] [Google Scholar]
- Epstein HF, Aronow BJ, Harris HE. Interaction of myosin and paramyosin. J Supramol Struct. 1975;3:354–360. doi: 10.1002/jss.400030407. [DOI] [PubMed] [Google Scholar]
- Funabara D, Kinoshita S, Watabe S, Siegman MJ, Butler TM, Hartshorne DJ. Phosphorylation of molluscan twitchin by the cAMP-dependent protein kinase. Biochemistry. 2001a;40:2087– 2095. doi: 10.1021/bi0022691. [DOI] [PubMed] [Google Scholar]
- Funabara D, Nakaya M, Watabe S. Isolation and characterization of a novel 45 kDa calponin-like protein from anterior byssus retractor muscle of the mussel Mytilus galloprovincialis. Fish Sci. 2001b;67:511–517. [Google Scholar]
- Funabara D, Watabe S, Mooers SU, Narayan S, Dudas C, Hartshorne DJ, Siegman MJ, Butler TM. Twitchin from molluscan catch muscle: primary structure and relationship between sitespecific phosphorylation and mechanical function. J Biol Chem. 2003;278:29308–29316. doi: 10.1074/jbc.M303272200. [DOI] [PubMed] [Google Scholar]
- Fährmann M, Fonk I, Beinbrech G. The kinase activity of the giant protein projectin of the flight muscle of Locusta migratoria. Insect Biochem Mol Biol. 2002;32:1401–1407. doi: 10.1016/s0965-1748(02)00060-7. [DOI] [PubMed] [Google Scholar]
- Haeberle JR. Calponin decreases the rate of cross-bridge cycling and increases maximum force production by smooth muscle myosin in an in vitro motility assay. J Biol Chem. 1994;269:12424–12431. [PubMed] [Google Scholar]
- Heierhorst J, Probst WC, Vilim FS, Buku A, Weiss KR. Autophosphorylation of molluscan twitchin and interaction of its kinase domain with calcium/calmodulin. J Biol Chem. 1994;269:21086– 21093. [PubMed] [Google Scholar]
- Heierhorst J, Probst WC, Kohanski RA, Buku A, Weiss KR. Phosphorylation of myosin regulatory light chains by the molluscan twitchin kinase. Eur J Biochem. 1995;233:426–431. doi: 10.1111/j.1432-1033.1995.426_2.x. [DOI] [PubMed] [Google Scholar]
- Heierhorst J, Kobe B, Feil SC, Parker MW, Benian GM, Weiss KR, Kemp BE. Ca2+/S100 regulation of giant protein kinases. Nature. 1996;380:636–639. doi: 10.1038/380636a0. [DOI] [PubMed] [Google Scholar]
- Katsuyama H, Wang CL, Morgan KG. Regulation of vascular smooth muscle tone by caldesmon. J Biol Chem. 1992;267:14555–14558. [PubMed] [Google Scholar]
- Kendrick-Jones J, Cohen C, Szent-Györgyi AG, Longley W. Paramyosin: molecular length and assembly. Science. 1969;163:1196– 1198. doi: 10.1126/science.163.3872.1196. [DOI] [PubMed] [Google Scholar]
- Kendrick-Jones J, Lehman W, Szent-Györgyi AG. Regulation in molluscan muscles. J Mol Biol. 1970;54:313–326. doi: 10.1016/0022-2836(70)90432-8. [DOI] [PubMed] [Google Scholar]
- Kolsch B, Ziegler C, Beinbrech G. Length determination of synthetic thick filaments by cooperation of two myosin-associated proteins, paramyosin and projectin. Naturwissenschaften. 1995;82:239–241. doi: 10.1007/BF01133600. [DOI] [PubMed] [Google Scholar]
- Labeit S, Kolmerer B. Titins: giant proteins in charge of muscle ultrastructure and elasticity. Science. 1995;270:293–296. doi: 10.1126/science.270.5234.293. [DOI] [PubMed] [Google Scholar]
- Lei J, Tang X, Chambers TC, Pohl J, Benian GM. Protein kinase domain of twitchin has protein kinase activity and an autoinhibitory region. J Biol Chem. 1994;269:21078–21085. [PubMed] [Google Scholar]
- Lowy J, Millman B, Manson J. Structure and function in smooth tonic muscles of lammellibranch molluscs. Proc Roy Soc Lond B. 1964;160:525–536. doi: 10.1098/rspb.1964.0068. [DOI] [PubMed] [Google Scholar]
- Lowy J, Poulsen FR. Time-resolved X-ray diffraction studies of the structural behavior of myosin heads in a living contracting unstriated muscle. Nature. 1982;299:308–312. doi: 10.1038/299308a0. [DOI] [PubMed] [Google Scholar]
- Maroto M, Vinos J, Marco R, Cervera M. Autophosphorylating protein kinase activity in titin-like arthropod projectin. J Mol Biol. 1992;224:287–291. doi: 10.1016/0022-2836(92)90994-u. [DOI] [PubMed] [Google Scholar]
- Millman BM. Contraction of the opaque part of the adductor muscle of the oyster (Crassostrea angulata) J Physiol. 1964;173:239– 262. doi: 10.1113/jphysiol.1964.sp007455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moerman DG, Benian GM, Barstead RJ, Schriefer LA, Waterston RH. Identification and intracellular localization of the unc-22 gene product of Caenorhabditis elegans. Genes Dev. 1988;2:93–105. doi: 10.1101/gad.2.1.93. [DOI] [PubMed] [Google Scholar]
- Oshino T, Shimamura J, Fukuzawa A, Maruyama K, Kimura S. The entire cDNA sequences of projectin isoforms of crayfish claw closer and flexor muscles and their localization. J Muscle Res Cell Motil. 2003;24:431–438. doi: 10.1023/a:1027313204786. [DOI] [PubMed] [Google Scholar]
- Rembold CM, Murphy RA. Myoplasmic calcium, myosin phosphorylation, and regulation of the crossbridge cycle in swine arterial smooth muscle. Circ Res. 1986;58:803–815. doi: 10.1161/01.res.58.6.803. [DOI] [PubMed] [Google Scholar]
- Shelud’ko NS, Tuturova KF, Permyakova TV, Plotnikov SV, Orlova AA. A novel thick filament protein in smooth muscles of bivalve molluscs. Comp Biochem Physiol. 1999;122B:277–285. [Google Scholar]
- Shelud’ko NS, Matusovskaya GG, Permyakova TV, Matusovsky OS. Twitchin, a thick-filament protein from molluscan catch muscle, interacts with F-actin in a phosphorylation-dependent way. Arch Biochem Biophys. 2004;432:269–277. doi: 10.1016/j.abb.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Shirinsky VP, Biryukov KG, Hettasch JM, Sellers JR. Inhibition of the relative movement of actin and myosin by caldesmon and calponin. J Biol Chem. 1992;267:15886–15892. [PubMed] [Google Scholar]
- Siegman MJ, Mooers SU, Li C, Narayan S, Trinkle-Mulcahy L, Watabe S, Hartshorne DJ, Butler TM. Phosphorylation of a high molecular weight (~600 kDa) protein regulates catch in invertebrate smooth muscle. J Muscle Res Cell Motil. 1997;18:655–670. doi: 10.1023/a:1018683823020. [DOI] [PubMed] [Google Scholar]
- Siegman MJ, Funabara D, Kinoshita S, Watabe S, Hartshorne DJ, Butler TM. Phosphorylation of a twitchin-related protein controls catch and calcium sensitivity of force production in invertebrate smooth muscle. Proc Natl Acad Sci USA. 1998;95:5383–5388. doi: 10.1073/pnas.95.9.5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobue K, Muramoto Y, Fujita M, Kakiuchi S. Purification of a calmodulin-binding protein from chicken gizzard that interacts with F-actin. Proc Natl Acad Sci USA. 1981;78:5652–5655. doi: 10.1073/pnas.78.9.5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohma H, Yazawa M, Morita F. Phosphorylation of regulatory light chain a (RLC-a) in smooth muscle myosin of scallop, Patinopecten yessoensis. J Biochem. 1985;98:569–572. doi: 10.1093/oxfordjournals.jbchem.a135311. [DOI] [PubMed] [Google Scholar]
- Sohma H, Inoue K, Morita F. A cAMP-dependent regulatory protein for RLC-a myosin kinase catalyzing the phosphorylation of scallop smooth muscle myosin light chain. J Biochem. 1988a;103:431–435. doi: 10.1093/oxfordjournals.jbchem.a122287. [DOI] [PubMed] [Google Scholar]
- Sohma H, Sasada H, Inoue K, Morita F. Regulatory light chain-a myosin kinase (aMK) catalyzes phosphorylation of smooth muscle myosin heavy chains of scallop, Patinopecten yessoensis. J Biochem. 1988b;104:889–893. doi: 10.1093/oxfordjournals.jbchem.a122578. [DOI] [PubMed] [Google Scholar]
- Standiford DM, Davis MB, Miedema K, Franzini-Armstrong C, Emerson CP. Myosin rod protein: a novel thick filament component of Drosophila muscle. J Mol Biol. 1997;265:40–55. doi: 10.1006/jmbi.1996.0710. [DOI] [PubMed] [Google Scholar]
- Szent-Györgyi AG, Cohen C, Kendrick-Jones J. Paramyosin and the filaments of molluscan ‘‘catch’’ muscles. II. Native filaments: isolation and characterization. J Mol Biol. 1971;56:239–258. doi: 10.1016/0022-2836(71)90462-1. [DOI] [PubMed] [Google Scholar]
- Szent-Györgyi AG and Chantler PD (1994) Control of contraction by calcium binding to myosin In: Engel AG,, Franzini-Armstrong C, et al. (eds.) Mycology. (pp. 506–528). McGraw Hill, New York.
- Szymanski PT. Calponin (CaP) as a latch-bridge protein-a new concept in regulation of contractility in smooth muscles. J Muscle Res Cell Motil. 2004;25:7–19. doi: 10.1023/b:jure.0000021349.47697.bf. [DOI] [PubMed] [Google Scholar]
- Watabe S, Tsuchiya T, Hartshorne DJ. Phosphorylation of paramyosin. Comp Biochem Physiol. 1989;94B:813–821. doi: 10.1016/0305-0491(89)90171-5. [DOI] [PubMed] [Google Scholar]
- Watabe S, Hartshorne DJ. Paramyosin and the catch mechanism. Comp Biochem Physiol. 1990;96B:639–646. doi: 10.1016/0305-0491(90)90207-a. [DOI] [PubMed] [Google Scholar]
- Waterston RH, Thomson JN, Brenner S. Mutants with altered muscle structure of Caenorhabditis elegans. Dev Biol. 1980;77:271–302. doi: 10.1016/0012-1606(80)90475-3. [DOI] [PubMed] [Google Scholar]
- Weitkamp B, Jurk K, Beinbrech G. Projectin-thin filament interactions and modulation of the sensitivity of the actomyosin ATPase to calcium by projectin kinase. J Biol Chem. 1998;273:19802– 19808. doi: 10.1074/jbc.273.31.19802. [DOI] [PubMed] [Google Scholar]
- Winder SJ, Walsh MP. Smooth muscle calponin. Inhibition of actomyosin MgATPase and regulation by phosphorylation. J Biol Chem. 1990;265:10148–10155. [PubMed] [Google Scholar]
- Winkelman L. Comparative studies of paramyosins. Comp Biochem Physiol. 1976;55B:391–397. doi: 10.1016/0305-0491(76)90310-2. [DOI] [PubMed] [Google Scholar]
- Yamada A, Yoshio M, Oiwa K, Nyitray L. Catchin, a novel protein in molluscan catch muscles, is produced by alternative splicing from the myosin heavy chain gene. J Mol Biol. 2000;295:169–178. doi: 10.1006/jmbi.1999.3349. [DOI] [PubMed] [Google Scholar]
- Yamada A, Yoshio M, Kojima H, Oiwa K. An in vitro assay reveals essential protein components for the ‘‘catch’’ state of invertebrate smooth muscle. Proc Natl Acad Sci USA. 2001;98:6635– 6640. doi: 10.1073/pnas.111585098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada A, Yoshio M, Nakamura A, Kohama K, Oiwa K. Protein phosphatase 2B dephosphorylates twitchin, initiating the catch state of invertebrate smooth muscle. J Biol Chem. 2004;279:40762– 40768. doi: 10.1074/jbc.M405191200. [DOI] [PubMed] [Google Scholar]


