Abstract
The transfer of protons in water wires was studied in native gramicidin A (gA), and in the SS- and RR- diastereoisomers of dioxolane-linked gA channels (SS and RR channels). These peptides were incorporated into membranes comprised of distinct combinations of phospholipid headgroups and acyl chains. Quantitative relationships between single channel conductances to H+ (gH) and [H+] were determined in distinct phospholipid membranes, and are in remarkable contrast with results previously obtained in monoglyceride membranes. In particular: 1), gH-[H+] relationships for the various gA channels in distinct phospholipid membranes are well fitted by single adsorption isotherms. A simple kinetic model assuming mono-occupancy of channels by protons fits said relationships. This does not occur with monoglyceride membranes. 2), Under nonsaturating [H+], gH is ∼1 order of magnitude larger in phospholipid than in monoglyceride membranes. 3), Differences between rates of H+ transfer in various gA channels are still present but considerably attenuated in phospholipid relative to monoglyceride membranes. 4), Charged phospholipid headgroups affect gH via changes in [H+] at the membrane/solution interfaces. 5), Phosphoethanolamine groups caused a marked attenuation of gH relative to membranes with other phospholipid headgroups. This attenuation is voltage-dependent and tends to saturate H+ currents at voltages larger than 250 mV. This effect is likely to occur by limiting the access and exit of H+ in and out of the channel due to relatively strong oriented H-bonds between waters and phosphoethanolamine groups at channel interfaces. The differential effects of phospholipids on proton transfer could be reasoned by considering solvation effects of side chain residues of gramicidin channels by double acyl chains and by the presence of polar headgroups facilitating the entrance/exit of protons through the channel mouths.
INTRODUCTION
Gramicidin A (gA) is a hydrophobic pentadecapeptide comprised mostly of an alternating sequence of D and L aminoacids (HCO-L-Val1-Gly2-L-Ala3-D-Leu4-L-Ala5-D-Val6-L-Val7-D-Val8-L-Trp9-D-Leu10-L-Trp11-D-Leu12-L-Trp13-D-Leu14-L-Trp15-NH-(CH2)2-OH). In a lipid environment gA adopts a right-hand β6.3 helix structure in which the side chain residues face the hydrophobic environment, and the backbone with the carbonyl and amide groups line up the lumen of the peptide (1–4). The association of two gA monomers located in opposite monolayers of a membrane via six interpeptide H-bonds forms a water-filled ion channel that is selective to monovalent cations (5). A single file of water molecules is present inside the pore of gA channels (6–8). The permeation of protons in gA channels does not seem to occur via hydrodynamic diffusion of protonated waters. Instead, an H+ transfer Grotthuss-type mechanism may occur in various gA channels (5,6,9–17).
Proton translocation in proteins and across membranes is an essential and general phenomenon in biology (18,19). In particular, ATP production in cells is ultimately triggered by the flow of protons in bioenergetic membrane proteins. Even though chains of water molecules (water or proton wires (20,21) have been found in bioenergetic proteins (18), the detailed mechanisms by which protons are transferred along water wires are not well known. In this regard, proton-selective channels based on gA molecules offer a simple and interesting model to study structure-function relationships of proton transfer in simple quasi-unidimensional water wires. Moreover, proton transfer can be measured in a single channel molecule. Recent work from our laboratory (10–12,16,22–24) have used the SS and RR diastereoisomers of dioxolane-linked gA channels (16,25) to measure H+ transfer in water wires, and to correlate those experimental measurements with possible molecular mechanisms (16,26). It was suggested that the chiral centers of the dioxolane linker underlie the profound quantitative and qualitative differences in the rate of H+ transfer in distinct gA channels. Because monoglyceride bilayers are electrostatically neutral in a wide range of pH values, most of our previous studies of H+ transfer in dioxolane-linked and native gA channels were performed in those membranes.
In this study, the properties of H+ transfer in various gA channels incorporated in phospholipids membranes were studied. We here demonstrate that the properties of H+ transfer in various gA channels in phospholipid membranes are markedly different from those previously studied in monoglyceride membranes. In particular, it is shown that phospholipid headgroups exert a significant influence on the rate of H+ transfer in water wires in various gA channels possibly by controlling the entrance/exit rate of H+ in and out of the channel.
MATERIALS AND METHODS
Planar lipid bilayers
Planar lipid bilayers were formed on a 0.15-mm diameter hole in a polysterene partition separating two compartments containing aqueous solutions. Bilayers were formed from solutions of phospholipid in decane (∼70 mM). The following synthetic phospholipids used in this study were obtained from Avanti Polar Lipids (Alabaster, AL): 1,2di-palmitoleoy (16:1, 9-cis-hexadecenoic)-phosphatidylcholine (PC, DiPPC), or -phosphatidylethanolamine (PE, DiPPE); 2di-oleoy l (16:1, 9-cis-octadecenoic)-PC (DiOPC) or –PE (DiOPE); 1-palmitoyl (16:0, hexadecanoic)- 2-oleoyl-PC (POPC) or –PE (POPE); 1,2 di-phytanoyl-PC (DiPhPC) or –PE (DiPhPE); 1-palmitoyl 2-oleoyl-phosphatidylglycerol (POPG).
gA channels and solutions
Native gA was obtained from Fluka (Milwaukee, WI). The SS- and RR-diastereoisomers of dioxolane-linked gA channels were synthesized as previously described (16,23,25). Symmetrical HCl solutions (concentration range of 0.01–5 M) were used in most experiments. Some experiments were also performed with 1 M CsCl (or KCl) solutions. Experiments were performed with native gA and the SS- and RR-dioxolane linked gA dimers. For the sake of conciseness only, most of the experimental results presented in this study were from native gA and SS channels only. The experimental results with the RR-channels were qualitatively similar to those obtained with native gA or SS channels.
Single channel current measurements
The experiments consisted in incorporating a single type of gA channel in the lipid membrane and measuring single channel currents. Because the SS- and RR-dioxolane linked gA dimers remain in the open state for very long times (minutes to hours), voltage ramps from 0 to usually 400 mV were applied in ∼5 s across the membrane itself and across the membrane with a single channel. Single channel currents were obtained by subtracting the membrane current recording from the (membrane + channel) recording. Single channel conductances to protons (gH) were obtained from the linear portion of current versus voltage relationships (∼0–75 mV). In other experiments, mostly with native gA channels, gH was measured from differences in current between the closed and open states of the channel at a constant DC voltage. Junction potentials between the Ag/AgCl electrodes immersed in solutions were always measured at the end of the experiment. Because junction potentials were usually small (<1 mV) the applied transmembrane potential was rarely corrected for the calculation of gH. Means and their standard error of the man (sem) for 8–50 distinct measurements of gH in each experimental condition are being reported in this study. Analysis (using pClamp software, Axon Instruments, Union City, CA), and modeling of experimental results were performed using Mathcad 11 (Cambridge, MA) and Sigmaplot (SPSS Science, Chicago, IL).
Calculation of [H+]x=0
In the experimental conditions of this study, phospholipid membranes are not neutral. Consequently the [H+] at the membrane/solution interface ([H+]x=0) is not equal to [H+]bulk. [H+]x=0 was calculated as previously described (27). It should be noted that pK values for the protonation of the phosphate oxygen depend on the terminal group present in phospholipids. In our calculations, pK values of 1 were used for the various PC and PE membranes, and a value of 3 was used for POPG membranes (28).
RESULTS
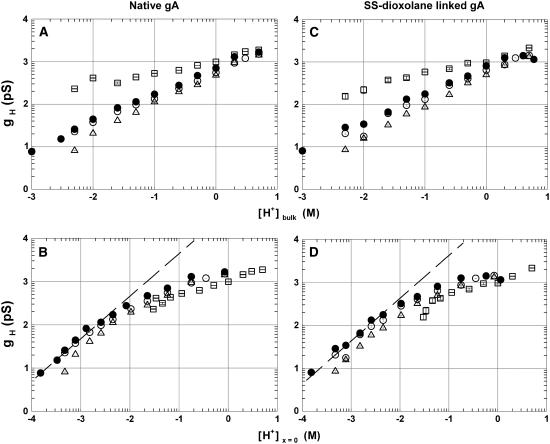
In Fig. 1 the log-log relationships between gH and either [H+]bulk (A and C) or [H+]x=0 (B and D) in native gA (A and B) and in the SS-dioxolane gA dimer (C and D) are shown. Distinct symbols correspond to channels in POPC (open circles), POPE (triangles), DiPhPC (solid circles), and POPG (squares) membranes. These plots are qualitatively similar for the various gA channels studied including the RR dioxolane-linked gA channel (see Fig. 5). In a wide range of [H+]bulk (panels A and C), the rate of H+ transfer in gA channels increases in the following order: POPE < POPC < DiPhPC ≪ POPG. The markedly larger values for gH measured in POPG at concentrations below 1 M (panels A and C) can be accounted for by the effect of surface charges at the membrane/solution interface on [H+]x=0. In being negatively charged at low [H+] and neutral at high [H+], PG bilayers have [H+]x=0 larger than [H+]bulk at low [H+]bulk. These effects were quantitatively accounted for in panels B and D in Fig. 1 for the distinct phospholipid membranes (see Materials and Methods). In fact, gH values in POPG/decane membranes are generally smaller than those in PC or PE membranes. The dashed lines in panels B and D fitting the filled circles for [H+]x=0 <5 mM had slopes of ∼1.
FIGURE 1.
Log-log plots of gH versus [H+]bulk (A and C) or [H+]x=0 (B and D) for the native gA (A and C) and the SS-dioxolane linked gA dimmer (B and D). Distinct symbols correspond to single channel conductance measurements in various phospholipid membranes (POPC (○), POPE (▵), DiPhPC (•), and POPG (□)). Points were plotted as mean ± SE. See text for the evaluation of [H+]x=0.
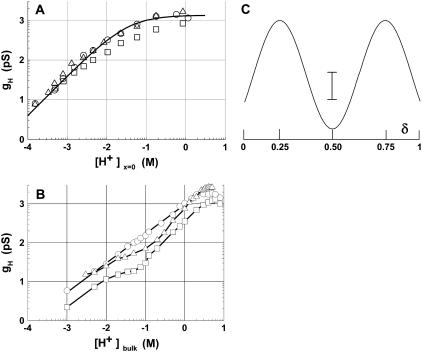
FIGURE 5.
(A) Log-log gH − [H+]x=0 relationships in the SS- (○), RR-dioxolane linked gA (□), and native gA (▵) channels in DiPhPC/decane membranes. The curve is a fit to the experimental points
(○ )
according to the energetic profile depicted in panel C (see below). As in panel B below, points were plotted as mean ± SE. (B) Same as in A but in GMO/decane bilayers. Curves are connecting the experimental points. (C) This shows a symmetrical energy (kcal/mol) profile for H+ transfer in various gA channels as a function of the fractional electrical distance (δ). The energy peaks are arbitrarily located at 0.25 and 0.5 δ and well is in the middle of the electric field. Values for the SS-, RR-dioxolane linked and native gA channels are: energy peaks, 3.66 (SS), 3.84 (RR), and 3.56 (native gA) kcal/mol; And energy wells, −2.01 (SS), −2.24 (RR), and −2.24 (native gA) kcal/mol. The vertical calibration bar is 1.4 kcal/mol. See text for discussion.
)
according to the energetic profile depicted in panel C (see below). As in panel B below, points were plotted as mean ± SE. (B) Same as in A but in GMO/decane bilayers. Curves are connecting the experimental points. (C) This shows a symmetrical energy (kcal/mol) profile for H+ transfer in various gA channels as a function of the fractional electrical distance (δ). The energy peaks are arbitrarily located at 0.25 and 0.5 δ and well is in the middle of the electric field. Values for the SS-, RR-dioxolane linked and native gA channels are: energy peaks, 3.66 (SS), 3.84 (RR), and 3.56 (native gA) kcal/mol; And energy wells, −2.01 (SS), −2.24 (RR), and −2.24 (native gA) kcal/mol. The vertical calibration bar is 1.4 kcal/mol. See text for discussion.
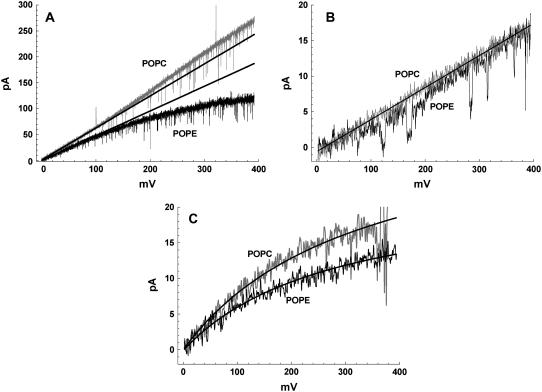
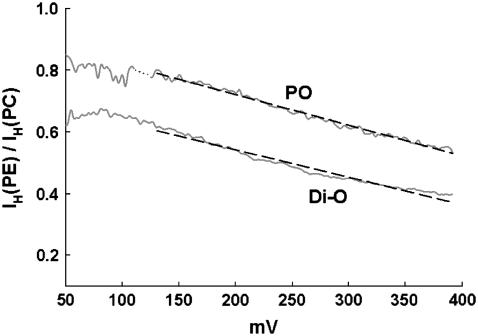
The graphs in Fig. 1 also show that gH values in gA channels in POPE (triangles) are consistently smaller than in POPC (open circles) membranes. These differences appear small in the log-log plots of Fig. 1, but they are significant and interesting as shown in Fig. 2. Panel A in Fig. 2 shows exemplary I/V recordings of single SS-dioxolane linked gA channels in POPC and POPE membranes in 1 M HCl solutions. In POPC bilayers, these channels display a linear (ohmic) relationship through ∼200 mV. A consistently slight supralinear relationship was measured at larger membrane voltages. By contrast, in POPE membranes the ohmic relationship extends to ∼140 mV only, and there is a marked saturation of H+ currents at larger voltages indicating a significant voltage-dependent effect. As the transmembrane voltage increases, so does the attenuation of H+ currents in PE membranes. This is better illustrated in Fig. 3 in which the ratio of H+ currents in PE or PC bilayers (IH(PE)/IH(PC)) with distinct fatty acid chains are shown. The straight lines in the plot of Fig. 2 A were drawn with gH values of 624 (POPC) and 477 pS (POPE). Attenuation and saturation of currents measured in POPE bilayers seem to occur only when protons are the permeating cations. In CsCl or KCl solutions those effects are not present. Fig. 2 B shows I/V recordings of SS-dioxolane linked gA channels in POPC or POPE membranes in 1 M CsCl solutions. In contrast to Fig. 2 A, these relationships are ohmic in the 0–400 mV range and there are no major differences in single channel conductances to Cs (gCs) under these experimental conditions (45 pS). Similar results were also obtained with KCl (not shown). In 50 mM [H+]bulk (Fig. 2 C), a concentration in which the magnitude of proton currents is about the same as cesium currents in 1 M CsCl, the I/V relationships are for the most part nonlinear, and IH is larger in POPC than in POPE membranes (see legend to Fig. 2) showing that attenuation of IH through gA channels in PE bilayers occurs irrespective of [H+] and the intensity of channel current. The distinct I/V profiles in 1 M vs. 50 mM HCl is not further investigated here and is consistent with the idea of diffusion limitation of H+ to or from the channel.
FIGURE 2.
Recordings of single SS-dioxolane linked gA channels in various experimental conditions. A shows superimposed recordings in POPC (gray) and POPE (black) membranes in 1 M HCl. Straight lines have slopes of 624 and 477 pS for POPC and POPE membranes, respectively. The original recordings of this figure were low-pass filtered at 2 kHz with a Bessel filter and digitized at 5 kHz. For the purpose of the illustration in the panel. the original recordings were digitally filtered at 500 Hz. B shows similar recordings as in A but in 1 M CsCl solutions. These recordings were low-pass filtered at 100 Hz; C, as in A but in 50 mM HCl with filtering the same as in B. The curves were empirically fitted according to IH = A/[(mV/B) +1], where A has values of 33.9 (POPC) and 23.0 pA (POPE), and B 327.4 (POPC) and 282.7 mV (POPE).
FIGURE 3.
Ratios between H+ currents in distinct PE and PC bilayers (IH(PE)/IH(PC)) against membrane potential. Di-O and PO are bilayers having dioleoyl and palmitoyl-oleoyl as acyl chains, respectively. Notice that proton currents were measured in distinct channels and bilayers. For these plots, original recordings were low-pass Bessel filtered at 10 Hz to remove the fast closing flickers (48). Linear regression lines are −9.93 × 10−4 × (mV) + 0.92 (PO), and −8.89×10−4 × (mV) + 0.72 (Di-O) indicating attenuation of IH by ∼20–25% per 100 mV.
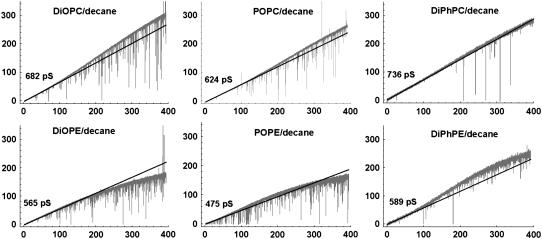
Fig. 4 shows representative I/V recordings of single SS channels in bilayers with various fatty acid compositions (1 M HCl). The recordings in the top and bottom panels in this figure were obtained in SS channels in distinct PC and PE bilayers, respectively. Fig. 4 demonstrates that the larger gH measured in PC in relation to PE bilayers (Figs. 1–3) is not dependent on the fatty acid composition of membranes (see Table 1). In both PC and PE bilayers gH values were significantly larger in bilayers with fully saturated fatty acid chains (DiPh). Also, in bilayers with distinct combinations of unsaturated fatty acid chains there were no remarkable alterations of gH (Table 1).
FIGURE 4.
Recordings of single SS-dioxolane linked gA channels in 1 M HCl in PC and PE membranes with various fatty acid chains. Recordings were originally filtered at 5 kHz and digitized at 10 kHz. For illustration purposes, the recordings in this figure were digitally filtered at 500 Hz. See text and Table 1 for further discussion and information.
TABLE 1.
gH (pS) in the linear region of I/V plots in PC and PE membranes in 1 M HCl
| PC | PE | PC/PE | |
|---|---|---|---|
| Di-palmitoleoyl | 664 ± 38 | 536 ± 22 | 1.24 |
| Di-oleoyl | 660 ± 9 | 517 ± 7 | 1.28 |
| Palmitoyl-oleoyl | 637 ± 10 | 496 ± 11 | 1.28 |
| Di-phytanoyl | 789 ± 12 | 575 ± 10 | 1.37 |
Mean ± SE; n = 5–10 SS-dioxolane linked gA channels.
In Fig. 5 A, the log-log relationships between gH and [H+]x=0 are illustrated for the SS- (circles), RR-dioxolane linked gA (squares, and native gA (triangles) channels in DiPhPC/decane membranes. The curve in this panel is the best fit to the experimental points of the SS channel, and was calculated using an energy barrier model shown in panel C (see Discussion). In panel B of the same figure gH measurements are shown in glycerolmonoleate (GMO) bilayers (data extracted from Chernyshev et al. (10) and Cukiereman (29). There are marked differences between gH values in phospholipid versus GMO membranes: 1), At nonsaturating [H+]x=0, gH values in phospholipid membranes are ∼10-fold larger than in monoglyceride bilayers. For example, gH for the SS-dioxolane linked gA channel is 309 pS (panel A) versus 28 pS (panel B) at 10 mM [H+]x=0 or [H+]bulk. 2), The shapes of the relationships in panels A and B are markedly different. 3), In phospholipid bilayers and at a given [H+], differences between gH values in the various gA channels were consistently measured. These differences however, are considerably smaller than those measured in monoglyceride membranes (10,12,29).
DISCUSSION
gH-[H+] relationships in native gA and dioxolane-linked gA dimers were investigated in distinct phospholipid membranes. The new experimental findings in this study are: 1), gH-[H+] relationships in various gA channels in phospholipid membranes are markedly different from those previously studied in monoglyceride bilayers (10,29). In particular, a), in nonsaturating [H+], gH is ∼10-fold larger in phospholipid than in monoglyceride bilayers; b), the shapes of gH-[H+] relationships are strongly dependent on the type of bilayer; and c), differences between gH values in various gA channels in phospholipid membranes are minimized but still present in relation to monoglyceride bilayers. 2), An interesting and unsuspected finding was that a marked attenuation of proton transfer in various gA channels occurs in phospholipid membranes ending with an ethanolamine group. These effects are not dependent on the fatty acid chains.
The experimental values for gH in phospholipid membranes were correlated with [H+]x=0 using Gouy-Chapman models (see 27 for details). Despite the assumptions and limitations of such model it reproduces well the electrostatic behavior of electrified interfaces (30,32). The calculation of [H+]x=0 rests on the knowledge of the dissociation constants of various protonatable phospholipid headgroups, and these measurements pose significant experimental and interpretational challenges (28). In this study, pK values for PO− groups were taken as 1 for PC and PE, and 3 for PG membranes (28).
Proton transfer in various gA channels in phospholipid versus monoglyceride membranes
In phospholipid membranes, the rates of proton transfer in various gA channels are consistent with the simplest energy barrier model shown in Fig. 5 C. In this symmetrical model based on Eyring kinetic rate theory (32–34) two energy barriers for H+ to enter or exit the channel and a “binding” (see below) well in the middle of the water wire are present (Fig. 5 C). In this case, the channel works in a single occupancy mode, i.e., at any given time the waters inside the channel can accommodate at most one H+. This energy profile differs from the 2 well 3 peak barrier model that has been extensively used in permeation studies of alkalines (5,13, 25,35,36) as well as of H+ permeation in monoglyceride membranes in gA channels (37), see below). Structural studies also showed the existence of two major binding sites for alkalines in gA dimers (38–40). Because a), it is not likely that protons bind directly to the carbonyls of gA channels, instead they are solvated by waters inside various gA channels (15,26,41), and b), the experimental points for the various gA channels studied can be well fitted by simple adsorption isotherms (see legend to Fig. 5), we opted for the simplest energy barrier model. A few additional points must be discussed regarding this model. First, the experimental results with distinct PC and PE bilayers show that access/exit of H+ to/from channel is a significant factor in determining the overall gH (Figs. 1–4; (24,37)). The entry/exit energy barriers are not explicitly represented in the model. Second, at [H+]x=0 <5 mM, gH values are well approximated by a linear relationship (slope = 1) in log-log plots (Fig. 1, B and D) suggesting that diffusion-limitation of H+ from (to) the bulk to (from) channel at these low proton concentrations are the rate limiting steps in determining gH. In our previous experiments in monoglyceride membranes this point has not been not clearly demonstrated (10,29). It is likely that part of the problem is the considerable uncertainty in measuring gH in experimental conditions where the ionic strength and [H+] are extremely low (see Fig. 5 B). In fact, gH values at those low [H+] in native gA channels in monoglyceride bilayers were not directly measured but estimated via noise analysis (13) in solutions containing Mg2+ salts which could be interfering with gH. A third issue concerns conceptual problems related to the interpretation of ion transport in channels using such rate models (42,43). In this context, we see the model above as an approximation to the overall energetic cost involved in transferring H+ across various gA channels in phospholipid membranes. This model is in reasonable agreement with experimentally determined values of the Gibbs activation energies of H+ transfer in various gA channels in DiPhPC membranes (∼5 kcal/mol, 12). Simplistic energetic profiles like the one discussed above may provide a useful frame of reference for calculations of the potential of mean force for H+ transfer across the channel.
In contrast to the apparent simplicity of gH-[H+] relationships in phospholipid membranes, in monoglyceride bilayers (10,29) these relationships are complex and not yet completely understood (37). Using the approach described above (Fig. 5, A and B) with more complex energy diagrams consisting of multiple energy peaks and wells, and multiple occupancy of the pore by H+ we could not fit our measurements in monoglyceride bilayers (results not shown). In particular, the power dependency of gH on [H+] in SS channels in GMO/decane bilayers (straight line with slope of ∼0.75 that extends throughout ∼3 orders of magnitude of [H+], Fig. 5 B; (10,29)) remains a challenge. gH-[H+] relationships of gA channels in monoglyceride bilayers have the profile of a channel that can be occupied by more than one cation (13,34,37). This contrasts with phospholipid membranes where H+ transfer in various gA channels can be easily described by models that assume mono-occupancy of the channel by protons. If this line of reasoning is followed it is possible that the lipid environment surrounding the channel somehow determines the number of protons that the water wire inside gA can accommodate. It would be of interest to study the molecular basis of this hypothesis.
H+ transfer is ∼10-fold faster in the various gA channels in phospholipid than in monoglyceride bilayers (Fig. 5). This observation is opposite to what is seen with the single channel conductances to alkalines. It has been shown by distinct investigators that permeation of alkalines is larger in monoglyceride than in phospholipid membranes for various gA channels (our unpublished observations and (35,44,45)). In itself, this adds to the peculiar nature of H+ permeation in gA channels (46).
The experimental measurements of gH and the shapes of gH-[H+] relationships does not define a precise molecular mechanism for the differences between H+ transfer in gA channels in phospholipid versus monoglyceride bilayers. However, the significant differences in activation entropies for H+ transfer in gA channels in these distinct membranes may offer some clues (12). For the native gA and RR channels, activation entropies for gH in phospholipid membranes were 50 and 83% less, respectively, than in monoglyceride bilayers. For the SS channel that difference was much less and amounted to ∼10%. The larger rate of proton transfer in gA channels in phospholipid membranes can be due to an increased organization of water molecules in the proton wire that optimizes the transfer of protons in relation to monoglyceride bilayers. This could be due to a “better” solvation of the side chain residues of gA channels by the phospholipid acyl chains resulting in a more stable (less fluctuating) structure of the complex membrane/gA channel in relation to monoglyceride membranes. This may reflect in a more organized or stable water wire in the middle of the channel. The entropic factors related to different bilayers are not quantitatively the same for the various gA channels because of significant structural differences in the middle of the channels (16,25,26).
A significant and additional consideration is that the polarity of headgroups in phospholipid membranes which are not present in monoglycerides may facilitate the entrance or exit of H+ in and out of the channel. In a preliminary publication (51) molecular dynamics calculations of H+ transfer in various gA channels were performed in GMO and DiPhPC bilayers. An interesting result in this study was that H+ entry in gA channels in DiPhPC was more likely to occur than in GMO membranes. The probable cause for this phenomenon was attributed to the significant polarization of water molecules next to the channel's entrance in DiPhPC but not in GMO membranes. In fact, some experimental results that are discussed below add significant support to this idea.
Proton transfer in gA channels in phospholipid membranes containing ethanolamine end groups
H+ transfer in various gA channels in PE membranes is peculiarly attenuated in relation to other phospholipid membranes. This effect occurs independent of the phospholipid acyl chains (Fig. 4 and Table 1), and does not seem to be present when alkalines are the permeating cations suggesting specificity for H+ (Fig. 2). Attenuation of H+ currents in PE bilayers is weekly voltage dependent being more intense at larger transmembrane potentials (see Figs. 2 and 3). Phospholipid membranes with other terminal headgroups were tested and they do not share the effects of PE.
The location of ethanolamine groups in PE bilayers suggests that the marked attenuation of gH may well be a consequence of a major decrease in the rates of entry and exit of H+ in and out of the channel. Because water wires in gA channels do not offer a significant resistance to H+ flow compared to bulk solution (28,46), the series resistances associated with channel mouths need to be considered. The weak voltage dependence of this attenuation (Fig. 3) suggests that this process is probably occurring at the membrane/solution interfaces in the vestibules of the channel's mouths. Because phospholipid membranes are significantly thicker (the thicknesses of DiPhPC/ or DiPhPE/decane bilayers are in the 44–48 Å range which is in agreement with previous measurements in 47) than the length of functional gA channels (∼25 Å), these vestibules develop as a consequence of hydrophobic shielding of side chain residues of gA by the membrane (see 48 and references therein).
The transfer of H+ in water wires should depend on a), a proper orientation of adjacent water molecules to establish a covalent bond with the H+ being transferred, and b), on the reorientation of water molecules after a H+ had been transferred (18). In general, water molecules form stronger H-bonds with (NH4)+ than with the terminal choline (CH3) groups (49). These relatively strong H-bonds in PE membranes could hamper both steps in H+ transfer at the mouths of the channel located in the vestibules thus attenuating gH. In this regard, comparative molecular dynamics simulations were performed in plain PE and PC membranes (50). Of particular interest to our observations is the result showing that in PC membranes the choline groups favor the formation of water clathrates at the membrane interface without a preferential orientation of the water molecules. By contrast, the ammonium group in PE forms H-bonds with waters favoring a given dipole orientation of the H-bond network. Even though this is one difference reported between PE and PC membranes, it suggests that these different types of water structures at the mouths of the channel could explain the distinctive H+ transfer characteristics in PE and PC membranes. In summary, interesting and unsuspected observations were shown here in a simple molecular system that is computationally treatable. By providing a frame of reference for protein-membrane interactions, these results will hopefully encourage and facilitate further studies of H+ transfer in membrane-protein interactions using molecular dynamics methods.
Dr. Chernyshev's present address is Dept. of Chemistry, University of Iowa, Iowa City, IA 52241.
References
- 1.Arseniev, A. S., I. L. Barsukov, V. F. Bystrov, A. L. Lonize, and Y. A. Ovchinikov. 1985. Proton NMR study of gramicidin A transmembrane ion channel. Head-to-head right handed, single stranded helices. FEBS Lett. 186:168–174. [DOI] [PubMed] [Google Scholar]
- 2.Ketchem, R. R., W. Hu, and T. A. Cross. 1993. High resolution of gramicidin A in a lipid bilayer by solid-state NMR. Science. 261:1457–1460. [DOI] [PubMed] [Google Scholar]
- 3.Ketchem, R. R., B. Roux, and T. A. Cross. 1997. High-resolution polypeptide structure in a lamellar phase lipid environment from solid state NMR derived constraints. Structure. 5:1655–1669. [DOI] [PubMed] [Google Scholar]
- 4.Urry, D. W. 1971. Gramicidin A transmembrane channel: a proposed π(L,D) helix. Proc. Natl. Acad. Sci. USA. 68:672–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hladky, S. B., and D. A. Haydon. 1972. Ion transfer across lipid membranes in the presence of gramicidin A. I. Studies of the unit conductance channel. Biochim. Biophys. Acta. 274:294–312. [DOI] [PubMed] [Google Scholar]
- 6.Finkelstein, A. 1987. Water Movement through Lipid Bilayers, Pores, and Plasma Membrane. Theory and Reality. John Willey, New York.
- 7.Levitt D.G. 1984. Kinetics of movement in narrow channels. Curr. Topics Membr. and Transp. 21:181–197. [Google Scholar]
- 8.Tripathi, S., and S. B. Hladky. 1998. Streaming potentials in gramicidin channels measured with ion selective electrodes. Biophys. J. 74:2912–2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akeson, M., and D. W. Deamer. 1991. Proton conductance by the gramicidin water wire. Model for proton conductance in the F0F1ATPases? Biophys. J. 60:101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chernyshev, A., K. M. Armstrong, and S. Cukierman. 2003. Proton transfer in gramicidin channels is modulated by the thickness of monoglyceride bilayers. Biophys. J. 84:238–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chernyshev, A., R. Pomès, and S. Cukierman. 2003. Kinetic isotope effects of proton transfer in aqueous and methanol containing solutions, and in gramicidin channels. Biophys. Chem. 103:179–190. [DOI] [PubMed] [Google Scholar]
- 12.Chernyshev, A., and S. Cukierman. 2002. Thermodynamic view of activation energies of proton transfer in various gramicidin A channels. Biophys. J. 82:182–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eisenman, G., B. Enos, J. Hägglund, and J. Sandblom. 1980. Gramicidin A as an example of a single filing ionic channel. Ann. N. Y. Acad. Sci. 329:8–20. [DOI] [PubMed] [Google Scholar]
- 14.Myers, V. B., and D. A. Haydon. 1972. Ion transfer across lipid membranes in the presence of gramicidin A. Biochim. Biophys. Acta. 274:313–322. [DOI] [PubMed] [Google Scholar]
- 15.Pomès, R., and B. Roux. 1996. Structure and dynamics of a proton wire: a theoretical study of H+ translocation along the single-file water chain in the gramicidin A channel. Biophys. J. 71:19–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quigley, E. P., P. Quigley, D. S. Crumrine, and S. Cukierman. 1999. The conduction of protons in different stereoisomers of dioxolane-linked gramicidin A channels. Biophys. J. 77:2479–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Voth, G. A. 2003. The computer simulation of proton transport in biomolecular systems. Front. Biosci. 8:1384–79. [DOI] [PubMed] [Google Scholar]
- 18.Cukierman, S. 2003. The transfer of protons in water wires inside proteins. Front. Biosci. 8:1118–1139. [DOI] [PubMed] [Google Scholar]
- 19.DeCoursey, T. E. 2003. Voltage-gated proton channels and other proton transfer pathways. Physiol. Rev. 83:475–579. [DOI] [PubMed] [Google Scholar]
- 20.Nagle, J. F., and S. Tristam-Nagle. 1983. Hydrogen bonded chain mechanisms for proton conduction and proton pumping. J. Membr. Biol. 74:1–14. [DOI] [PubMed] [Google Scholar]
- 21.Nagle, J. F., and H. J. Morowitz. 1978. Molecular mechanisms for proton transport in membranes. Proc. Natl. Acad. Sci. USA. 75:298–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Armstrong, K. M., E. P. Quigley, P. Quigley, D. S. Crumrine, and S. Cukierman. 2001. Covalently linked gramicidin channels: effects of linker hydrophobicity and alkaline metals on different stereoisomers. Biophys. J. 80:1810–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cukierman, S., E. P. Quigley, and D. S. Crumrine. 1997. Proton conduction in gramicidin A and in its dioxolane-linked dimer in different lipid bilayers. Biophys. J. 73:2489–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quigley, E. P., A. Emerick, D. S. Crumrine, and S. Cukierman. 1998. Proton current attenuation by methanol in a dioxolane-linked gramicidin A dimer in different lipid bilayers. Biophys. J. 5:2811–2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stankovic, C. J., S. H. Heinemann, J. M. Delfino, F. J. Sigworth, and S. L. Schreiber. 1989. Transmembrane channels based on tartaric acid-gramicidin A hybrids. Science. 244:813–817. [DOI] [PubMed] [Google Scholar]
- 26.Yu, C. H., S. Cukierman, and R. Pomès. 2003. Theoretical study of the structure and dynamic fluctuations of dioxolane-linked gramicidin channels. Biophys. J. 84:816–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Godoy, C. M. G., and S. Cukierman. 2001. Modulation of proton transfer in the water wire of dioxolane-linked gramicidin channels by lipid membranes. Biophys. J. 81:1430–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marsh, D. 1990. CRC Handbook of Lipid Bilayers. CRC Press, Boca Raton, FL. 81–85
- 29.Cukierman, S. 2000. Proton mobilities in water and in different stereoisomers of covalently linked gramicidin A channels. Biophys. J. 78:1825–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McLaughlin, S. 1977. Electrostatic potentials at membrane solution interfaces. Curr. Top. Membr. Trans. 9:71–144. [Google Scholar]
- 31.Reference deleted in proof.
- 32.Eyring, H., R. Lumry, and J. W. Woodbury. 1949. Some applications of modern rate theory to physiological systems. Rec. Chem. Prog. 10:100–114. [Google Scholar]
- 33.Zwolinski, B. J., H. Eyring, and C. E. Reese. 1949. Diffusion and membrane permeability. J. Phys. Chem. 53:1426–1453. [Google Scholar]
- 34.Hille, B., and W. Schwarz. 1978. Potassium channels as multi-ion single-file pores. J. Gen. Physiol. 72:409–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cole, C. D., A. S. Frost, N. Thompson, M. Cotton, T. A. Cross, and D. D. Busath. 2002. Noncontact dipole effects on channel permeation. VI. 5F- and 6F-Trp gramicidin channel currents. Biophys. J. 83:1974–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heinemann, S. H., and F. J. Sigworth. 1989. Estimation of Na+ dwell time in gramicidin A channel. Na+ ions as blockers of H+ currents. Biochim. Biophys. Acta. 987:8–14. [DOI] [PubMed] [Google Scholar]
- 37.Gowen, J. A., J. C. Markham, S. E. Morrison, T. A. Cross, D. D. Busath, E. J. Mapes, and M. F. Schumaker. 2002. The role of Trp side chains in tuning single proton conduction through gramicidin channels. Biophys. J. 83:880–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jing, N., K. U. Prasad, and D. W. Urry. 1995. The determination of binding constants of micellar-packaged gramicidin A by 13C- and 23Na-NMR. Biochim. Biophys. Acta. 1238:1–11. [DOI] [PubMed] [Google Scholar]
- 39.Olah, G. A., H. W. Huang, W. Liu, and Y. Wu. 1991. Location of ion-binding sites in the gramicidin channel by X-ray diffraction. J. Mol. Biol. 218:847–858. [DOI] [PubMed] [Google Scholar]
- 40.Tian, F., K. C. Lee, W. Hu, and T. A. Cross. 1996. Monovalent cation transport: lack of structural information upon cation binding. Biochemistry. 35:11959–11966. [DOI] [PubMed] [Google Scholar]
- 41.Sagnella, D. E., and G. A. Voth. 1996. Structure and dynamics of hydronium in the ion channel gramicidin A. Biophys. J. 70:2043–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jordan, P. C. 1999. Ion permeation and channel kinetics. J. Gen. Physiol. 114:601–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roux, B. 1999. Theories of ion permeation: a chaser. J. Gen. Physiol. 114:605–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Busath, D. D., C. D. Thulin, R. W. Hendershot, L. R. Phillips, P. Maughan, C. D. Cole, N. C. Bingham, S. Morrison, L. C. Baird, R. J. Hendershot, M. Cotten, and T. A. Cross. 1998. Noncontact dipole effects on channel permeation. I. Experiments with (5F-Indole) Trp13 gramicidin A channels. Biophys. J. 75:2830–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Phillips, L. R., C. D. Cole, R. J. Hendershoot, M. Cotten, T. A. Cross, and D. D. Busath. 1999. Noncontact dipole effects on channel permeation. III. Anomalous proton conductance effects in gramicidin. Biophys. J. 77:2492–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cukierman S. 1999. Flying protons in linked gramicidin A channels. Israel Journal of Chemistry. 39:419–426. [Google Scholar]
- 47.Lundbæk, J. A., and O. S. Andersen. 1994. Lysophospholipids modulate channel function by altering the mechanical properties of lipid bilayers. J. Gen. Physiol. 104:645–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Armstrong, K. M., and S. Cukierman. 2002. On the origin of closing flickers in gramicidin channels: a new hypothesis. Biophys. J. 82:1329–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jeffrey, G. A. 1997. An Introduction to Hydrogen Bonding. Oxford University Press, New York.
- 50.Damodaran, K. V., and K. M. Merz Jr. 1994. A comparison of DMPC- and DLPE-based lipid bilayers. Biophys. J. 66:1076–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qin, Z., and G. A. Voth. 2006. The effect of membrane environment on proton permeation through gramicidin A channels. Biophys. J. 90:405a. [DOI] [PubMed] [Google Scholar]