Abstract
Cyclodextrin is a water-soluble circular oligosaccharide with a cylinder shape characterized by exterior hydrophilic rims and an interior hydrophobic cavity, which makes it an ideal additive to prevent proteins from aggregating during refolding. In this research, three hydroxypropyl cyclodextrins (HPCDs), HP-α-, β-, and γ-CD, were used to investigate the molecular mechanism of their effects on assisting aminoacylase refolding. The aggregation and reactivation experiments suggested that at moderate concentrations, HPCDs could suppress aggregation and assist aminoacylase refolding in a concentration-dependent manner, and HP-β-CD was the most efficient of the three HPCDs. Low concentrations of HP-α-CD and high concentrations of HP-γ-CD promoted off-pathway aggregation. Spectroscopic studies indicated that the hydrophobic exposure of the unstructured species in the refolded solutions was gradually reduced by the three HPCDs with the efficiency HP-β-CD > HP-γ-CD > HP-α-CD. Furthermore, the fast phase of aminoacylase reactivation was slowed down by the addition of 75 mM HP-β- and γ-CD, but no significant effect was observed for HP-α-CD. The dissimilarity in the effects of the three HPCDs suggested that the internal cavity size played a crucial role in their antiaggregation ability. Further analysis suggested that the observations might be much more complicated than expected because of the various types of interactions between cyclodextrins and proteins in addition to their ability to bind to protein aromatic residues.
INTRODUCTION
Refolding of recombinant proteins from solubilized inclusion bodies is often frustrated by off-pathway aggregation leading to low yields of soluble native proteins. In general, aggregation is caused by the nonnative association of hydrophobic surfaces that are exposed during the refolding process (1). Preventing protein aggregation has attracted numerous researchers' attention for many years, and various strategies have been developed to facilitate the refolding of the denatured states to functional proteins (2,3). Improved protein-refolding yields have been obtained by using various dilution protocols and the addition of buffer additives or folding aids (4–7). Particularly, the method known as the “dilution additive technique” (4,8) has been found to be able to assist protein refolding by the addition of low molecular weight folding aids in the buffer, which is used to dilute the chemically denatured protein. Although these methods have been found to work for many proteins, the efficiency of various methods is usually different from case to case (9–12). Thus the development of new strategies is still a challenge in enhancing protein-refolding yields.
Cyclodextrin is a water-soluble, nontoxic, stable, circular oligosaccharide composed of α-(1,4)-linked α-D-glucosyl units. The structure of cyclodextrin is cylinder shaped with hydrophilic rims outside where OH-6 is on the narrow rim and OH-2 and OH-3 are on the wide rim. The interior, where H-3, H-5, and H-6 hydrogens and O-4 ether-like oxygens are located, forms a hydrophobic cavity (13). The exterior polar groups help cyclodextrin to dissolve in water, whereas the central nonpolar cavity enables it to interact with the hydrophobic residues of proteins or drugs. The most common types of cyclodextrin are α-, β-, and γ-cyclodextrin, which consist of six, seven, and eight glucosyl units. The more glucose units in the cyclodextrin circle, the larger the cavity (13). Natural cyclodextrins have rather limited solubility in water (14), and various derivatives have been synthesized to improve its solubility (15). Cyclodextrins as well as their chemically modified variants have been widely used in pharmaceuticals as drug carriers (14,16). The properties of cyclodextrin also make it an ideal additive to prevent proteins from sticking together during refolding (17–21).
Aminoacylase (N-acyl-L-amino acid amido hydrolase, EC 3.5.1.14), which exists in mammalian kidneys and in many microorganisms, is an important enzyme participating in amino acid metabolism in organisms (22–25). The unfolding of aminoacylase has been thoroughly studied (26–30), whereas the refolding and reactivation of aminoacylase has always failed due to serious aggregation (26,27,30). The refolding of aminoacylase was thought to be irreversible for a long time, until recently, when it was shown that the reactivation of aminoacylase could be successful under low concentrations and low temperature conditions (7). Thus aminoacylase is a good model to test the effect of various refolding-enhancing strategies including the addition of cyclodextrins. In this work, the effects of hydroxypropyl cyclodextrins (HPCDs) on the inhibition of aggregation and the promotion of refolding were studied by using guanidine hydrochloride (GdHCl)-denatured aminoacylase as a model system. It was found that aminoacylase refolding could be promoted significantly by the addition of HPCDs in the dilution buffer. Moreover, it was also of interest to find that the hydroxypropyl α-, β-, and γ-cyclodextrins (HP-α-CD, HP-β-CD, HP-γ-CD, respectively) have quite different abilities in assisting the refolding of aminoacylase in vitro. The main difference among these three HPCDs is the size of the cavity, and thus the different behavior of the three HPCDs helps us to understand the structure-activity relationship of cyclodextrins. The results herein also provide clues in designing improved cyclodextrin-based antiaggregation agents.
MATERIALS AND METHODS
Materials
Aminoacylase was prepared from pig kidney according to the procedure of Birnbaum (23) to the step of acetone fraction. The crude preparation was then purified first by gel filtration though Sephadex G-150 and then by ion exchange with diethylaminoethyl cellulose as described by Kordel and Schneider (24,25). The final preparation was homogeneous on polyacrylamide gel electrophoresis in the presence and absence of sodium dodecyl sulfate (SDS). Sephadex G-150 was purchased from Pharmacia (Piscataway, NJ). Hydroxypropyl-α-cyclodextrin (HP-α-CD) was a Fluka (Milwaukee, WI) product, and hydroxypropyl-β-cyclodextrin (HP-β-CD) and hydroxypropyl-γ-cyclodextrin (HP-γ-CD) were Acros (Somerville, NJ) products. Ultrapure grade GdHCl, chloroacetyl-L-leucine, SDS, and 8-anilino-1-naphthalenesulfonate (ANS) were purchased from Sigma (St. Louis, MO). All other reagents were local products of analytical grade.
Binding of HPCDs to free Trp
The binding ability of the three HPCDs to free Trp was monitored by intrinsic fluorescence on an F-2500 fluorescence spectrophotometer using 1-cm path-length cuvettes with an excitation wavelength of 280 nm at 25°C. The fluorescence spectra over a wavelength range of 300–600 nm were measured for 0.25 mM Trp in 30 mM Tris-HCl buffer with or without the addition of 10 mM HPCDs. The concentration-dependent effect of HP-β-CD on the fluorescence spectra of Trp was studied by ranging the concentration of HP-β-CD from 0.5 to 75 mM with a Trp concentration of 0.1 mM. The fitting of the fluorescence spectra was carried out using the discrete states model as described previously (31,32).
Aminoacylase activity assay
The enzyme concentration was determined by measuring the absorbance at 280 nm and using the absorption coefficient  (24). Enzyme activity was determined at 25°C by measuring the absorbance at 238 nm accompanied with hydrolysis of the substrate and using the molar absorption coefficient ɛ238 = 185 M−1cm−1 as reported by Kordel and Schneider (24,25) except that chloroacetyl-L-leucine was used instead of pure L-enantionmorph. The reaction system was prepared by mixing 10 μl of 1 μM enzyme to 0.5 ml of substrate medium.
(24). Enzyme activity was determined at 25°C by measuring the absorbance at 238 nm accompanied with hydrolysis of the substrate and using the molar absorption coefficient ɛ238 = 185 M−1cm−1 as reported by Kordel and Schneider (24,25) except that chloroacetyl-L-leucine was used instead of pure L-enantionmorph. The reaction system was prepared by mixing 10 μl of 1 μM enzyme to 0.5 ml of substrate medium.
Spectroscopy measurements
Aminoacylase was denatured in 30 mM Tris-HCl buffer, pH 7.5, containing 4 M GdHCl. The solution was incubated overnight at 25°C before refolding experiments were carried out. The denatured aminoacylase (60 μM) was diluted 60-fold into the refolding buffer (30 mM Tris-HCl, pH 7.5) containing various concentrations of HPCDs and refolded at 4°C for 24 h. Then the samples were used for all biospectroscopy studies except for aggregation experiments. All experiments were repeated three to four times to ensure the reproducibility of the data.
Emission fluorescence spectra were recorded with an F-2500 fluorescence spectrophotometer using 1-cm path-length cuvettes at 4°C. An excitation wavelength of 295 nm was used to measure the intrinsic fluorescence of aminoacylase Trp. Far-ultraviolet circular dichroism (CD) spectra were measured on a Jasco (Tokyo, Japan) 715 spectropolarimeter with a 1-mm path-length cell over a wavelength range of 200–250 nm. The final enzyme concentration was 1 μM for fluorescence and CD experiments.
For aggregation measurements, the denatured aminoacylase (60 or 120 μM) was diluted 60-fold into refolding buffer (30 mM Tris-HCl, pH 7.5) containing various concentrations of HPCDs. The final enzyme concentration was 1 or 2 μM. Aggregation was monitored by measuring the turbidity at 400 nm using a Perkin-Elmer Lambda Bio units/V spectrophotometer (Norwalk, CT) at 25°C.
RESULTS
Binding of hydroxypropyl cyclodextrins to free Trp
It has been proposed that the ability of cyclodextrins to bind with protein aromatic residues contributes to their effects on protein refolding (19,20,33). It is impossible to monitor the interactions between HPCDs and aminaocylase during refolding due to serious aggregation; thus the binding ability of the three HPCDs to aromatic residues was studied using free Trp as a model system. As presented in Fig. 1 A, it is clear that the intrinsic fluorescence of free Trp was affected by the addition of 10 mM HP-β-CD but was not affected by the other two HPCDs. The addition of HP-β-CD to free Trp solutions not only increased the emission intensity but also blue shifted the emission maximum from 351 nm to 348.5 nm. This result suggested that the binding of free Trp with HP-β-CD led to a microenvironmental change of the aromatic side chains. However, when the HP-β-CD concentration exceeded 50 mM, the fluorescence intensity gradually decreased (Fig. 1 B, solid squares), which might have been caused by the viscosity change of the solutions. To more precisely characterize the effect of HP-β-CD, curve fitting was carried out according to the discrete states model of Trp as described previously (31,32). In general, the appearance of the Class III component centered around 350 nm indicates the existence of fully water-exposed fluorophores, whereas the Class II component centered around 340 nm reflects the existence of fluorophores exposed to bonded waters (32). The fluorescence spectrum of free Trp was found to have only the Class III component centered at 353 nm, which is consistent with the fact that free Trp are fully exposed to water in solutions. With the addition of HP-β-CD, a Class II component centered at 342 nm appeared and increased with the increase of the concentration of HP-β-CD. Thus the binding of Trp with HP-β-CD resulted in a partial shielding of the Trp aromatic ring from water.
FIGURE 1.
Binding ability of HPCDs to free Trp by intrinsic fluorescence. (A) Fluorescence spectra of 0.25 mM free Trp in 30 mM Tris-HCl buffer, pH 7.5, without (solid line) or with 10 mM HP-α-CD (dashed line), HP-β-CD (dashed dotted line), HP-γ-CD (dotted line). (B) The intensity changes of the Trp fluorescence (solid squares), the class II (open squares), and class III (open circles) components as a function of HP-β-CD concentration. The concentration of Trp was 0.1 mM. The fitting of the fluorescence spectra was carried out using the discrete states model as described previously (31,32). Class III component centered at 353 nm reflects the existence of fluorophores that are fully exposed to water in solutions, whereas Class II component centered at 342 nm indicates the existence of fluorophores exposed to bonded waters (32).
Effects of HPCDs on aminoacylase aggregation during refolding
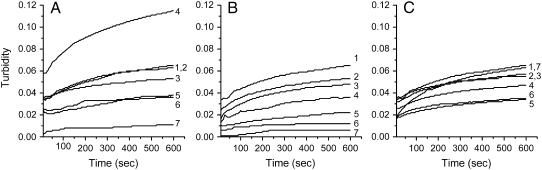
When 1 μM GdnHCl-denatured aminoacylase was refolded in the absence of HPCDs, aggregation appeared immediately, whereas in the presence of HPCDs, aggregation was inhibited by the three HPCDs in different manners (Fig. 2). In the case of HP-α-CD, the aggregation was slightly inhibited at low concentrations, greatly increased at the 25 mM concentration, and then gradually inhibited at concentrations above 25 mM. For HP-β-CD, as the concentration was increased, the aggregation of aminoacylase was significantly prevented in a concentration-dependent manner. HP-γ-CD inhibited the aminoacylase aggregation with the highest efficiency achieved when the concentration of HP-γ-CD reached 50 mM. Surprisingly, at a high concentration (150 mM) although the aggregation rate was gradually reduced by the addition of HP-γ-CD, the degree of aggregation reached a level similar to that of the control after 600-s incubation. Among the three HPCDs, HP-β-CD reduced the turbidity of refolding aminoacylase most effectively, and 75–150 mM HP-β-CD could almost completely inhibit protein aggregation. When aminoacylase concentration in the refolding buffer increased from 1 to 2 μM, the efficiency of the three HPCDs to suppress aggregation was much smaller than the conditions with 1 μM enzyme (Fig. 3). However, the concentration-dependent manners of the three HPCDs were similar to those in Fig. 2. The results in Fig. 3 suggested that at low concentrations, the HPCDs were aggregation promoters and HP-α-CD was the most effective one. These results from aggregation experiments revealed that the three HPCDs might work by a much more complex mechanism than that proposed previously (17–21).
FIGURE 2.
Aggregation of aminoacylase in refolding buffers with the addition of HPCDs. Aminoacylase denatured in 4 M GdHCl was diluted into the standard buffer (30 mM Tris-HCl, pH 7.5) and preequilibrated at 25°C in the absence (1) or presence of 5 (2), 10 (3), 25 (4), 50 (5), 75 (6), or 150 (7) mM HP-α-CD (A), HP-β-CD (B), and HP-γ-CD (C). Aggregation was monitored by measuring the turbidity at 400 nm. All reactions occurred at 25°C. The final concentration of aminoacylase was 1 μM.
FIGURE 3.
Aggregation of 2 μM aminoacylase in the refolding buffers with the addition of various concentrations of HP-α-CD (circles), HP-β-CD (squares), and HP-γ-CD (triangles). The final concentration of aminoacylase in the refolding buffers was 2 μM. The turbidity was measured 600 s after the refolding was initiated. The data were normalized by the turbidity of the refolded sample in absence of HPCDs (approximately fivefold of the 1 μM enzyme). All other conditions were the same as those in Fig. 2.
Effects of HPCDs on the activity recovery of aminoacylase
The activity of aminoacylase was measured 2 h after dilution, and the relative activity was obtained by normalizing the data by taking the activity of the native enzyme at the same concentration as 100%. The activity of the enzyme without the addition of HPCDs could be recovered to ∼36% of the native enzyme, which is quite consistent with results reported in the literature (7). Like the results from aggregation studies, the three HPCDs behaved quite differently in assisting the reactivation of aminoacylase (Fig. 4 A). In the case of HP-β-CD, as the concentration was increased from 10 to 75 mM, the activity of refolded aminoacylase gradually increased, and the activity recovered to ∼66% of that of native enzyme when the concentration of HP-β-CD was 75 mM. The effect of HP-γ-CD was similar to HP-β-CD at concentrations below 50 mM. However, the reactivation reached a maximum at ∼25 mM HP-γ-CD, and the activity of aminoacylase recovered to ∼52% of that of the native enzyme in the presence of 75 mM HP-γ-CD. For HP-α-CD, similar to its effect on protein aggregation, a significant decrease of the reactivity of aminoacylase was observed in the presence of 25 mM HP-α-CD, and then the activity of the enzyme was slightly enhanced to ∼44% with the addition of 75 mM HP-α-CD.
FIGURE 4.
Reactivation of aminoacylase in the absence or presence of various HPCDs. (A) The recovered activity of aminoacylase in the presence of various concentrations of HPCDs. (B) Change of aminoacylase activity on the time course in the presence of 75 mM HPCDs. The data were fitted by a biphasic model. Enzymatic activity was measured 2 h after the addition of the denatured aminoacylase to the standard buffer in the absence (solid circles) and presence (5–75 mM) of HP-α-CD (open circles), HP-β-CD (open squares), and HP-γ-CD (open triangles). The activity of native enzyme at the same concentration was taken as 100%. The final aminoacylase concentration was 1 μM. The enzymatic activity was monitored by absorption at 238 nm. All reactions occurred at 25°C.
Fig. 4 B shows the change of aminoacylase activity on the time course in the presence of 75 mM HPCDs. The reactivation of GdnHCl-denatured aminoacylase shows a typical biphasic process. To characterize whether the reactivation rates were affected by the addition of HPCDs, the kinetic parameters were obtained by fitting the data using a biphasic model, and the rate constants are presented in Table 1. It was interesting to find that the three HPCDs had differential effects on the reactivation kinetics. Both HP-β- and γ-CD decreased the fast phase rate constant ∼23-fold but had no significant effect on the slow phase rate constant. In contrast, the addition of HP-α-CD did not affect the fast phase rate constant but slightly increased the slow phase rate constant (approximately twofold). As a control, we also performed reactivation kinetic studies by using osmolytes as dilution additives as those described in our previous work (34). No significant effects on the reactivation kinetics were found for conditions using 30% dimethylsulfoxide (DMSO), 1 M proline, or 1 M sucrose as folding aids (Table 1).
TABLE 1.
Reactivation rate constants of aminoacylase in the presence of HPCDs or osmolytes
| kf (× 102 s−1) | ks (× 103 s−1) | |
|---|---|---|
| Without HPCDs | 35 ± 7 | 1.2 ± 0.4 |
| 75 mM HP-α-CD | 35 ± 7 | 2.8 ± 0.4 |
| 75 mM HP-β-CD | 1.5 ± 0.3 | 0.8 ± 0.1 |
| 75 mM HP-γ-CD | 1.9 ± 0.4 | 1.0 ± 0.2 |
| Without osmolytes* | 33 ± 6 | 2.7 ± 0.9 |
| 30% DMSO* | 37 ± 7 | 2.2 ± 0.7 |
| 1 M proline* | 40 ± 8 | 2.3 ± 1.0 |
| 1 M sucrose* | 47 ± 9 | 1.9 ± 0.5 |
| 30% glycerol*† | – | – |
The data were from Kim et al. (34).
The kinetic parameters could not be obtained due to the failure of fitting the data into a biphasic model.
Effects of HPCDs on the secondary and tertiary structure recovery of aminoacylase
CD, intrinsic, and extrinsic fluorescence spectra were measured to characterize the effects of HPCDs on the structural recovery of aminoacylase. In the control experiment, no significant effect of the 75 mM HPCDs on the spectra of native enzyme was found (data not shown). Fig. 5 shows the typical CD spectra of the aminoacylase refolded in buffer without or with 25 and 75 mM HPCDs. The concentration-dependent effects of HPCDs on aminoacylase refolding were monitored by the CD ellipticity at 222 nm ([θ]222). As shown in the inset of Fig. 5, the results indicated that as concentration increased, the three HPCDs had different behavior in the secondary structure recovery of aminoacylase. For HP-β-CD, [θ]222 decreased very quickly as the concentration of HP-β-CD increased to 10 mM and then decreased slowly as HP-β-CD concentration increased. For HP-γ-CD, [θ]222 did not change significantly at low concentrations of HP-γ-CD (<10 mM) but then decreased continuously as the concentration increased. The behavior of HP-α-CD was more complicated. With increasing HP-α-CD concentration, [θ]222 slightly decreased at first, then increased gradually at a concentration of 25 mM, and finally decreased slowly. Consistent with the aggregation and reactivation studies, HP-β-CD was the most efficient in assisting aminoacylase to recover its native secondary structures, and the ellipticity could be recovered to ∼60% of the native enzyme in the presence of 75 mM HP-β-CD.
FIGURE 5.
CD spectra of refolded aminoacylase in different concentrations of HPCDs. Denatured aminoacylase was added into the standard buffer in the absence (+) and presence of 25 (open symbols) or 75 (solid symbols) mM HP-α-CD (circles), HP-β-CD (squares), and HP-γ-CD (triangles). The final aminoacylase concentration was 1 μM. The inset shows the HPCD concentration dependence of the molar ellipticity at 222 nm ([θ]222, expressed in [103 · degrees · cm2 · dmol−1]). The native aminoacylase (×) is also presented.
The effects of HPCDs on the recovery of the tertiary structures of aminoacylase were studied by intrinsic fluorescence to monitor the microenvironment of Trp residues. The change of the fluorescence emission maximum (Emax) is presented in Fig. 6 A. Since high concentrations of HP-β-CD had profound effects on the fluorescence of the protein, only samples with HP-β-CD concentrations lower than 25 mM were measured. Emax of native aminoacylase was at ∼333.5 nm, whereas that of the denatured enzyme was at ∼349 nm. In the absence of HPCDs, Emax could be recovered to 338 nm. A slight blue shift from 338 to 336.5 nm was observed when the concentration of HP-β-CD increased to 25 mM. With increasing HP-γ-CD concentrations up to 75 mM, Emax blue shifted to 335.5 nm. For HP-α-CD, Emax red shifted to 338.5 nm at a concentration of 25 mM and then blue shifted to 337 nm, proving that HP-α-CD has little effect on the recovery of aminoacylase's tertiary structures. It might be worth noting that HP-β-CD red shifted the emission maximum of free Trp (Fig. 1), whereas HP-α- and γ-CD did not. Thus the results of HP-β-CD might be composed of both protein conformational changes and the effects of HP-β-CD. However, considering that HP-β-CD had little effect on native enzyme (data not shown), it is safe to conclude that the results in Fig. 6 A were dominated by the conformational changes of the enzyme.
FIGURE 6.
The changes of the emission maximum of intrinsic fluorescence (A) and the maximum intensity of ANS fluorescence (B) of the refolded aminoacylase in various concentrations of HP-α-CD (circles), HP-β-CD (squares), and HP-γ-CD (triangles). All samples were incubated at 4°C for 24 h to be refolded completely. For ANS fluorescence experiments, ANS was added to each refolded sample and equilibrated for 30 min. The final aminoacylase and ANS concentrations were 1 and 40 μM, respectively. The excitation wavelength was 380 nm, and the emission wavelength was 400–600 nm.
The effects of HPCDs on the recovery of the tertiary structure of aminoacylase were also studied by ANS fluorescence to investigate the exposure of the hydrophobic groups. ANS was added to the refolded samples and the ANS fluorescence spectra were measured after a further 30-min equilibration. Since HPCDs are potential competitors of ANS to access the hydrophobic region of the protein, the results shown in Fig. 6 B are the apparent results of conformational changes and the shielding effects of HPCDs. It is difficult to distinguish the two effects directly from Fig. 6 B. However, a comparison of Fig. 6, A and B, suggested that the shielding effects of HPCDs on aminoacylase dominated the apparent results shown in Fig. 6 B. The different concentration-dependent behavior suggested that the three HPCDs had different binding ability to the enzyme with decreasing order HP-β-CD > HP-γ-CD > HP-α-CD. This result was quite consistent with the studies using free Trp as shown in Fig. 1. Furthermore, this result also indicated that although no significant effects were observed for HP-γ-CD and HP-α-CD on free Trp, these two HPCDs also had the ability to bind with the hydrophobic regions of proteins. The results in Figs. 5 and 6 A also suggested that the refolded solutions contained considerable amounts of nonnative structures. The presence of all three HPCDs could successfully reduce the hydrophobic exposure of these nonnative species, as indicated by Fig. 6 B, and thus protect them against aggregation.
DISCUSSION
The efficiency of protein refolding is largely determined by the rates of refolding and aggregation (35–37). To improve the recovery yield of active proteins, various methods have been developed to block off-pathway aggregation (2–6,38–40). Particularly, the use of low molecular weight chemical additives in the dilution buffer has been found to have the ability to improve the refolding yields of many proteins (8–11,34,41–43). These additives have been found to work by different mechanisms: osmolytes stabilize the native states via “preferential hydration” (39) or “solvophobic thermodynamic force” (40); poly-(ethylene glycol) as well as other surfactants block aggregation by specifically binding with aggregation-prone species (5,9); whereas cyclodextrin assists protein refolding by binding with the aromatic residues (17–21,41,42).
The ability of various cyclodextrin derivatives to prevent protein aggregation varied significantly in previous investigations (17,19,33,44,45). It is believed that the binding of the “host” cyclodextrin derivatives with the aromatic side chains of the “guest” proteins is crucial for its ability to assist protein refolding. Thus the numbers of aromatic residues and their locations in proteins might play a crucial role in investigating the effects of cyclodextrins. Table 2 summarizes the effects of cyclodextrins on different proteins in literature and the numbers of aromatic residues of these proteins. Although only limited numbers of proteins have been studied, a preliminary hypothesis could be proposed. That is, β-cyclodextrins were the most efficient of the cyclodextrin derivatives when the proteins contained high content of aromatic residues (>0.1), whereas α-cyclodextrins were the best when the proteins contained low content of aromatic residues (<0.1). Moreover, the effects of HPCDs were also found to be dependent on both cyclodextrin and protein concentrations. This might be one of the reasons different effects of cyclodextrins were observed for different proteins.
TABLE 2.
Summary of effects of different cyclodextrins on protein aggregation
| Proteins | Effects of cyclodextrins | Residues (n) | Aromatic residues (m) | m/n | Reference |
|---|---|---|---|---|---|
| Carbonic anhydrase | α > β > γ | 252 | 10F + 8Y+ 4W | 0.087 | (17) |
| Phosphofructokinase-1 | α; γ = 0 | 322 | 8F + 5Y | 0.04 | (44) |
| Phosphatidylinositol phosphate kinase | β > α, γ | 202 | 12F + 8Y + 2W | 0.109 | (45) |
| Amyloid β-peptide | β; α, γ = 0 | 17 | 2F | 0.118 | (33) |
| Human growth hormone | β > α, γ | 192 | 1F + 8Y + 13W | 0.115 | (19) |
| Aminoacylase | β > γ > α | 782 | 46F + 18Y + 16W | 0.102 | – |
The molecular mechanism of the aggregation-suppressing effect of cyclodextrins has been attributed to the binding of cyclodextrins with protein aromatic side chains (19,20,33). The interactions between cyclodextrins and the aromatic residues might have several different effects on protein refolding. First, globular proteins are characterized by a hydrophobic interior core and a hydrophilic surface. The hydrophobic packing and exclusion of water from the interior are crucial for protein folding (36,46). If the aromatic residues crucial for protein refolding are masked by cyclodextrin, the correct hydrophobic interactions of proteins may be retarded by the hydrophilic cyclodextrin rims. As a result, the refolding rate may be slowed and the extent of slowing depends on the dissociation rate. Second, if the aromatic residues responsible for protein aggregation are protected by cyclodextrin, the aggregation can be blocked. Third, if the aromatic residues are located on the surface of the proteins, no significant effects may be observed. In this research, it was found that HP-β- and γ-CD could alter the reactivation kinetics of aminoacylase (Table 1). This property seems to be specific to cyclodextrins, which was supported by the observation that no similar effect was found for osmolytes (Table 1) and surfactants (5,9).
Based on the results in previous studies (7,26), the refolding pathway of aminoacylase was proposed to be a multistate process, as shown in Scheme 1. For such a multistate process, the observation might be complicated by the effects of cyclodextrins on the transition from the unfolded state (U) to the intermediate (I), from I to the native state (N), and from I to the aggregates (A). It might be worth noting that most of the aromatic residues might be exposed to water in the unfolded state and the intermediate state has limited numbers of exposed aromatic residues, whereas the native state contains only the exposed residues located on the surface. Thus in general, cyclodextrin has few effects on the native state of proteins. At low concentrations of cyclodextrin, only parts of the aromatic side chains of proteins in the unfolded state could be masked by cyclodextrin molecules, which may not significantly affect k1. Since the number of exposed aromatic residues in the intermediate state is much smaller than that of the unfolded state, the addition of low concentrations of cyclodextrin may lead to decrease of k2 and kA. The decrease of k2 and kA further results in an accumulation of the aggregation-prone intermediate state. Thus cyclodextrin may act as an aggregation promoter at low concentrations, which was observed in Figs. 2 and 3. At high concentrations, cyclodextrin could affect all the transitions, and the observation was dependent on the nature of the refolding kinetics and the binding constant of cyclodextrin. If the transition rates of U to I and I to A are slowed down, the aggregation may be gradually suppressed. This may explain the suppression effects of HPCDs under moderate concentrations. If the transition rate of I to N* was affected the most, an accumulation of the aggregation-prone intermediate state I would result in more off-pathway aggregates. This may explain the opposite effects of 75 and 150 mM HP-γ-CD shown in Figs. 2 and 3. These theoretical analyses could be further supported if refolding kinetics could be obtained. Unfortunately, no refolding kinetic data could be obtained for the refolding of aminoacylase due to severe aggregation. However, the reactivation kinetics clearly showed that cyclodextrin could slow down the reactivation rate.
SCHEME 1.
A proposed refolding pathway of GdHCl-denatured aminoacylase. U, I, N*, N, and A denotes the unfolded state, intermediate, native-like state with partial activity, native state, and aggregates.
As analyzed above, the different binding constants with the aromatic residues of proteins may contribute to the different concentration-dependent manners of the three HPCDs used in this research. This could explain the good ability of HP-β-CD to assist aminoacylase reactivation. As for HP-α and γ-CD, the binding is rather weak compared to HP-β-CD (see Fig. 1 and 19,20,35). However, HP-α and γ-CD could also prevent aggregation and assist aminoacylase reactivation. This suggested that besides the interactions between the cyclodextrin cavity and the aromatic residues, there are other interactions including the incorporation of linear hydrophobic side chains into the cyclodextrin cavity, the transient interaction between the aromatic side chains and the cavity edge, and the transient interaction between the surface of the protein and the hydrophilic exterior rims of the cyclodextrin (its oligosaccharide nature). This conclusion is supported by the results from ANS spectra (see Fig. 6 B), which suggested that the hydrophobic exposure in the refolded solutions was significantly protected by the addition of all of the three HPCDs, whereas the corresponding CD and intrinsic fluorescence spectra (Figs. 5 and 6 A) indicated that considerable amounts of unstructured species were present. Although it is difficult to distinguish the different types of interactions in this research, the fact that HP-α-CD did not affect the reactivation kinetics but also gradually increased the refolding yield indicated that these interactions also contributed to the ability to assist protein refolding. Moreover, the similar effects of HP-β- and γ-CD on the reactivation kinetics (Table 1) suggested that the ability to bind with the aromatic side chains only partially contributed to the effect of HPCDs. This may also explain why the substituents on the cyclodextrin ring were very important in determining its ability to assist the refolding of proteins (18).
In conclusion, among the various cyclodextrin derivatives, the relatively strong interaction between aromatic residues and the cavity of β-cyclodextrins may present a very suitable system to suppress aggregation during refolding. Besides this specific interaction, other transient interactions may also contribute to the efficiency of cyclodextrins. Although the different effects of cyclodextrins on the rate of the different transition stages during protein refolding may complicate the prediction of the final results, the addition of moderate concentrations of cyclodextrins is expected to enhance the refolding yield of proteins. The results herein also suggested that cyclodextrins might work by a quite different mechanism from the well-established ones of osmolytes, surfactants, and chaperones.
Acknowledgments
This investigation was supported by funds from the National Natural Science Foundation of China (30500084 and 30221003) and the Fok Ying Tong Education Foundation (101023).
References
- 1.Horowitz, P., and N. L. Criscimagna. 1986. Low concentrations of guanidinium chloride expose apolar surfaces and cause differential perturbation in catalytic intermediates of rhodanese. J. Biol. Chem. 261:15652–15658. [PubMed] [Google Scholar]
- 2.Middelberg, A. P. J. 2002. Preparative protein refolding. Trends Biotechnol. 20:437–443. [DOI] [PubMed] [Google Scholar]
- 3.Tsumoto, K., D. Ejima, I. Kumagai, and T. Arakawa. 2003. Practical considerations in refolding proteins from inclusion bodies. Protein Expr. Purif. 28:1–8. [DOI] [PubMed] [Google Scholar]
- 4.Tandon, S., and P. Horowitz. 1986. Detergent-assisted refolding of guanidinium chloride-denatured rhodanese. The effect of lauryl maltoside. J. Biol. Chem. 261:15615–15618. [PubMed] [Google Scholar]
- 5.Cleland, J. L., and T. W. Randolph. 1992. Mechanism of polyethylene glycol interaction with the molten globule folding intermediate of bovine carbonic anhydrase B. J. Biol. Chem. 267:3147–3153. [PubMed] [Google Scholar]
- 6.Gething, M. J., and J. Sambrook. 1992. Protein folding in the cell. Nature. 355:33–45. [DOI] [PubMed] [Google Scholar]
- 7.Xie, Q., and H. M. Zhou. 2004. Refolding intermediate of guanidine hydrochloride denatured aminoacylase. Int. J. Biochem. Cell Biol. 36:1332–1340. [DOI] [PubMed] [Google Scholar]
- 8.Tandon, S., and P. Horowitz. 1988. The effects of lauryl maltoside on the reactivation of several enzymes after treatment with guanidinium chloride. Biochim. Biophys. Acta. 955:19–25. [DOI] [PubMed] [Google Scholar]
- 9.Wetlaufer, D. B., and Y. Xie. 1995. Control of aggregation in protein refolding: a variety of surfactants promote renaturation of carbonic anhydrase II. Protein Sci. 4:1535–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meng, F. G., Y. K. Hong, H. W. He, A. E. Lyubarev, B. I. Kurganov, Y. B. Yan, and H. M. Zhou. 2004. Osmophobic effect of glycerol on irreversible thermal denaturation of rabbit creatine kinase. Biophys. J. 87:2247–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ou, W. B., Y. D. Park, and H. M. Zhou. 2002. Effect of osmolytes as folding aids on creatine kinase refolding pathway. Int. J. Biochem. Cell Biol. 34:136–147. [DOI] [PubMed] [Google Scholar]
- 12.Singh, R., I. Haque, and F. Ahmad. 2005. Counteracting osmolyte trimethylamine N-oxide destabilizes proteins at pH below its pK(a). Measurements of thermodynamic parameters of proteins in the presence and absence of trimethylamine N-oxide. J. Biol. Chem. 280:11035–11042. [DOI] [PubMed] [Google Scholar]
- 13.Szejtli, J. 1998. Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 98:1743–1753. [DOI] [PubMed] [Google Scholar]
- 14.Uekama, K., F. Hirayama, and T. Irie. 1998. Cyclodextrin drug carrier systems. Chem. Rev. 98:2045–2076. [DOI] [PubMed] [Google Scholar]
- 15.Khan, A. R., P. Forgo, K. J. Stine, and V. T. D'Souza. 1998. Methods for selective modifications of cyclodextrins. Chem. Rev. 98:1977–1996. [DOI] [PubMed] [Google Scholar]
- 16.Davis, M. E., and M. E. Brewster. 2004. Cyclodextrin-based pharmaceutics: past, present and future. Nat. Rev. Drug Discov. 3:1023–1035. [DOI] [PubMed] [Google Scholar]
- 17.Karuppiah, N., and A. Sharma. 1995. Cyclodextrins as protein folding aids. Biochem. Biophys. Res. Commun. 211:60–66. [DOI] [PubMed] [Google Scholar]
- 18.Sharma, L., and A. Sharma. 2001. Influence of cyclodextrin ring substituents on folding-related aggregation of bovine carbonic anhydrase. Eur. J. Biochem. 268:2456–2463. [DOI] [PubMed] [Google Scholar]
- 19.Otzen, D. R., B. R. Knudsen, F. Aachmann, K. L. Larsen, and R. Wimmer. 2002. Structural basis for cyclodextrins' suppression of human growth hormone aggregation. Protein Sci. 11:1779–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aachmann, F. L., D. E. Otzen, K. L. Larsen, and R. Wimmer. 2003. Structural background of cyclodextrin-protein interactions. Protein Eng. 16:905–912. [DOI] [PubMed] [Google Scholar]
- 21.Khajehpour, M., T. Troxler, V. Nanda, and J. M. Vanderkooi. 2004. Melittin as model system for probing interactions between proteins and cyclodextrins. Proteins. 55:275–287. [DOI] [PubMed] [Google Scholar]
- 22.Anders, M. W., and W. Dekant. 1994. Aminoacylases. Adv. Pharmacol. 27:431–448. [DOI] [PubMed] [Google Scholar]
- 23.Birnbaum, S. M. 1955. Aminoacylase, amino acid acylase I and II from hog kidney. Methods Enzymol. 2:115–119. [Google Scholar]
- 24.Kordel, W., and F. Schneider. 1976. Chemical investigation on pig kidney aminoacylase. Biochim. Biophys. Acta. 445:446–457. [DOI] [PubMed] [Google Scholar]
- 25.Kordel, W., and F. Schneider. 1976. Chemical modification of two tryptophan residues abolishes the catalytic activity of aminoacylase. Hoppe Seylers Z. Physiol. Chem. 357:1109–1115. [DOI] [PubMed] [Google Scholar]
- 26.Wang, H. R., T. Zhang, and H. M. Zhou. 1995. Comparison of inactivation and conformational changes of aminoacylase during guanidinium chloride denaturation. Biochim. Biophys. Acta. 1248:97–106. [DOI] [PubMed] [Google Scholar]
- 27.Wang, H., X. Wang, T. Zhang, and H. M. Zhou. 1995. Comparison between conformational change and inactivation rates of aminoacylase during denaturation in urea solutions. Sci. China B. 38:328–335. [PubMed] [Google Scholar]
- 28.He, B., M. Yan, T. Zhang, and H. M. Zhou. 1994. Unfolding and inactivation during thermal denaturation of aminoacylase. Chin. Sci. Bull. 39:1122–1127. [Google Scholar]
- 29.He, B., Y. Zhang, T. Zhang, H. R. Wang, and H. M. Zhou. 1995. Inactivation and unfolding of aminoacylase during denaturation in sodium dodecyl sulfate solutions. J. Protein Chem. 14:349–357. [DOI] [PubMed] [Google Scholar]
- 30.He, B., T. Zhang, and H. M. Zhou. 1997. Comparison of inactivation and conformational changes of aminoacylase during denaturation in lithium dodecylsulphate solutions. Int. J. Biol. Macromol. 20:53–62. [DOI] [PubMed] [Google Scholar]
- 31.He, H. W., J. Zhang, H. M. Zhou, and Y. B. Yan. 2005. Conformational change in the C-terminal domain is responsible for the initiation of creatine kinase thermal aggregation. Biophys. J. 89:2650–2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burstein, E. A., S. M. Abornev, and Y. K. Reshetnyak. 2001. Decomposition of protein tryptophan fluorescence spectra into log-normal components. I. Decomposition algorithms. Biophys. J. 81:1699–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qin, X. R., H. Abe, and H. Nakanishi. 2002. NMR and CD studies on the interaction of Alzheimer β-amyloid peptide (12–28) with β-cyclodextrin. Biochem. Biophys. Res. Commun. 297:1011–1015. [DOI] [PubMed] [Google Scholar]
- 34.Kim, S. H., Y. B. Yan, and H. M. Zhou. 2006. Role of osmolytes as chemical chaperones during the refolding of aminoacylase. Biochem. Cell Biol. 84:30–38. [DOI] [PubMed] [Google Scholar]
- 35.Marston, F. A. 1986. The purification of eukaryotic polypeptides synthesized in Escherichia coli. Biochem. J. 240:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dill, K. A. 1990. Dominant forces in protein folding. Biochemistry. 31:12345–12352. [DOI] [PubMed] [Google Scholar]
- 37.Chi, E. Y., S. Krishnan, T. W. Randolph, and J. F. Carpenter. 2003. Physical stability of proteins in aqueous solutions: mechanism and driving forces in nonnative protein aggregation. Pharm. Res. 20:1325–1336. [DOI] [PubMed] [Google Scholar]
- 38.Rozema, D., and S. H. Gellman. 1995. Artificial chaperones: protein refolding via sequential use of detergent and cyclodextrin. J. Am. Chem. Soc. 117:2373–2374. [Google Scholar]
- 39.Timasheff, S. N. 1998. Control of protein stability and reactions by weakly interacting cosolvents: the simplicity of the complicated. Adv. Protein Chem. 51:355–432. [DOI] [PubMed] [Google Scholar]
- 40.Bolen, D. W. 2001. Protein stabilization by naturally occurring osmolytes. Methods Mol. Biol. 168:17–36. [DOI] [PubMed] [Google Scholar]
- 41.Khodarahmi, R., and R. Yazdanparast. 2004. Refolding of chemically denatured alpha-amylase in dilution additive mode. Biochim. Biophys. Acta. 1674:175–181. [DOI] [PubMed] [Google Scholar]
- 42.Tavornvipas, S., S. Tajiri, F. Hirayama, H. Arima, and K. Uekama. 2004. Effects of hydrophilic cyclodextrins on aggregation of recombinant human growth hormone. Pharm. Res. 21:2369–2376. [DOI] [PubMed] [Google Scholar]
- 43.Mishra, R., R. Seckler, and R. Bhat. 2005. Efficient refolding of aggregation prone citrate synthase by polyol osmolytes: how well are protein folding and stability aspects coupled? J. Biol. Chem. 280:15553–15560. [DOI] [PubMed] [Google Scholar]
- 44.Bär, J., R. Golbik, G. Hübner, and G. Kopperschläger. 2000. Denaturation of phosphofructokinase-1 from Saccharomyces cerevisiae by guanidinium chloride and reconstitution of the unfolded subunits to their catalytically active form. Biochemistry. 39:6960–6968. [DOI] [PubMed] [Google Scholar]
- 45.Davis, A. J., I. Y. Perera, and W. F. Boss. 2004. Cyclodextrins enhance recombinant phosphatidylinositol phosphate kinase activity. J. Lipid Res. 45:1783–1789. [DOI] [PubMed] [Google Scholar]
- 46.ten Wolde, P. R., and D. Chandler. 2002. Drying-induced hydrophobic polymer collapse. Proc. Natl. Acad. Sci. USA. 99:6539–6543. [DOI] [PMC free article] [PubMed] [Google Scholar]