Abstract
Dog thyroid epithelial cells in primary culture constitute a physiologically relevant model of positive control of DNA synthesis initiation and G0-S prereplicative phase progression by cAMP as a second messenger for thyrotropin (thyroid-stimulating hormone [TSH]). As previously shown in this system, the cAMP-dependent mitogenic pathway differs from growth factor cascades as it stimulates the accumulation of p27kip1 but not cyclins D. Nevertheless, TSH induces the nuclear translocations and assembly of cyclin D3 and cdk4, which are essential in cAMP-dependent mitogenesis. Here we demonstrate that transforming growth factor β1 (TGFβ1) selectively inhibits the cAMP-dependent cell cycle in mid-G1 and various cell cycle regulatory events, but it weakly affects the stimulation of DNA synthesis by epidermal growth factor (EGF), hepatocyte growth factor, serum, and phorbol esters. EGF+serum and TSH did not interfere importantly with TGFβ receptor signaling, because they did not affect the TGFβ-induced nuclear translocation of Smad 2 and 3. TGFβ inhibited the phosphorylation of Rb, p107, and p130 induced by TSH, but it weakly affected the phosphorylation state of Rb-related proteins in EGF+serum-treated cells. TGFβ did not inhibit c-myc expression. In TSH-stimulated cells, TGFβ did not affect the expression of cyclin D3, cdk4, and p27kip1, nor the induced formation of cyclin D3–cdk4 complexes, but it prevented the TSH-induced relocalization of p27kip1 from cdk2 to cyclin D3–cdk4. It prevented the nuclear translocations of cdk4 and cyclin D3 without altering the assembly of cyclin D3–cdk4 complexes probably formed in the cytoplasm, where they were prevented from sequestering nuclear p27kip1 away from cdk2. This study dissociates the assembly of cyclin D3–cdk4 complexes from their nuclear localization and association with p27kip1. It provides a new mechanism of regulation of proliferation by TGFβ, which points out the subcellular location of cyclin d–cdk4 complexes as a crucial factor integrating mitogenic and antimitogenic regulations in an epithelial cell in primary culture.
INTRODUCTION
Transforming growth factor β1 (TGFβ1) is a multifunctional cytokine, member of a large family of growth and differentiation factors subdivided into three groups that include the TGFβs, the activins, and the bone morphogenetic proteins, plus various other distantly related members such as Müllerian-inhibiting substance. TGFβ1 exerts different, and often opposite, activities in controlling cell cycle progression, cell differentiation, cell adhesion, chemotaxy, and extracellular matrix deposition in a variety of cell lineages (Barnard et al., 1990; Lyons and Moses, 1990). However, in many cell types, including most epithelial cells, TGFβ1 is the most potent growth inhibitory polypeptide known (Roberts et al., 1985; Barnard et al., 1990). Deregulation of TGFβ function has been implicated in carcinogenesis and the pathological processes of several human diseases. The in vitro inhibitory response is lost in many neoplastically transformed epithelial cell lines (Polyak, 1996; Massague, 1998).
TGFβ elicits its biological effects by signaling through a heteromeric receptor complex consisting of the type I and type II receptors, two transmembrane serine/threonine kinases. Binding of TGFβ to type II receptor results in the recruitment, phosphorylation and activation of type I receptor within a ternary signaling complex (Derynck, 1994). The first identified substrates of type I receptor kinase are proteins of the SMAD family (Smad 2 and 3). In response to TGFβ, phosphorylated Smad 2 and 3 form a complex with Smad 4, move into the nucleus, and activate target genes by binding to specific promoter elements (Nakao et al., 1997; Massague, 1998; Zhang et al., 1998). Though a role for Smad 3 in mediating the antiproliferative effects of TGFβ has been established (Liu and Massague, 1997; Datto et al., 1999), the exact link with the inhibition of cell cycle machinery has yet to be defined. In different cell systems, TGFβ treatment induces growth arrest at various stages of the G1 phase of the cell cycle (Alexandrow and Moses, 1995; Reynisdottir et al., 1995; Sandhu et al., 1997). These effects have been attributed largely to an inhibition of the phosphorylation of the retinoblastoma susceptibility gene product Rb (Laiho et al., 1990; Herrera et al., 1996), thus preventing the release of the inhibition by Rb of E2F-dependent gene transcription. Overexpression of E2F-1 overcomes TGFβ-mediated growth suppression (Schwarz et al., 1995), and cells lacking functional Rb proteins are generally resistant to growth arrest by TGFβ (Herrera et al., 1996).
The phosphorylation of Rb is initiated by cyclin-dependent kinase (cdk4) 4 and cdk6 activated by the d-type cyclins (D1, D2, D3) synthesized in response to growth factors. It is maintained in partially different sites by cyclin E–cdk2 and then by cyclin A–cdk2 (Bartek et al., 1996; Connell-Crowley et al., 1997; Kelly et al., 1998; Lundberg and Weinberg, 1998), which also phosphorylate other substrates essential for initiation of DNA replication and/or cell cycle progression. The activation process of cdk2 kinases involves the relocalization of the cdk inhibitor p27kip1 from cyclin E/A–cdk2 complexes to cyclin d–cdk4 and –cdk6 complexes (Reynisdottir et al., 1995; Pagano et al., 1995; Poon et al., 1995; Sandhu et al., 1997; Massague, 1998) and/or the downregulation of p27kip1 (Kato et al., 1994; Nourse et al., 1994; Pagano et al., 1995; Resnitzky et al., 1995). In different epithelial cell systems, TGFβ inhibits cdk4 activity and increases the association of p27kip1 with cyclin E–cdk2 complexes (Polyak, 1996), but the primary mechanism may vary considerably. TGFβ inhibits cdk4 synthesis in mink lung epithelial cells without altering the expression of d-type cyclins (Ewen et al., 1993), whereas in rat intestinal cells, TGFβ inhibits cyclin D1 synthesis (Ko et al., 1995). In keratinocytes and mammary cells, TGFβ induces the cdk4 inhibitor p15INK4B without modifying cdk4 and d-type cyclin expression (Hannon and Beach, 1994). In mammary cells, TGFβ prevents the association of cyclin D1 with cdk4, apparently by upregulation of p15 (Sandhu et al., 1997), but overexpression of p15INK4B was claimed not to disrupt cyclin d–cdk4 complexes in mink lung cells (Reynisdottir and Massague, 1997). The TGFβ induction of p21cip1 (Datto et al., 1995) and p27kip1 (Florenes et al., 1996) are also cell-type specific. Cells lacking p15INK4B are not resistant to TGFβ inhibition (Florenes et al., 1996; Iavarone and Massague, 1997), which was then ascribed either to downregulation of the cdk-activating tyrosine phosphatase cdc25A (Iavarone and Massague, 1997) or induction of p21cip1 (Florenes et al., 1996). In many but not all cell lines that are responsive to its antiproliferative effect, TGFβ also downregulates the expression of c-myc (Pietenpol et al., 1990; Warner et al., 1999), a protooncogenic transcription factor that exerts essential but poorly understood cell cycle regulatory functions, mediated in part by a sequestration of p27kip1 through cyclin d–cdk4-dependent and -independent mechanisms (Steiner et al., 1995; Vlach et al., 1996; Bouchard et al., 1999; Perez-Roger et al., 1999).
Expression of TGFβ1 has been demonstrated in thyroid gland (see discussion and references in Roger [1996]) and might be involved in a mechanism contributing to the stabilization of thyroid-stimulating hormone (TSH)-dependent thyroid hyperplasia (goiter) (Logan et al., 1994; Contempre et al., 1996). TGFβ1 inhibits cell proliferation in the different thyroid cell culture systems (Tsushima et al., 1988; Colletta et al., 1989; Grubeck-Loebenstein et al., 1989). In normal human thyroid epithelial cells in primary culture, TGFβ1 prevents most cAMP-mediated responses to TSH, including DNA synthesis induction (Taton et al., 1993). In this article, we studied the action of TGFβ1 on dog thyroid epithelial cells in primary culture. In this physiologically relevant system, TSH through cAMP triggers cell cycling and positively controls a late G1 restriction point (Roger et al., 1987a, 1999). The cAMP-dependent mitogenic pathway differs from rapidly converging pathways of growth factors and phorbol esters, because it does not involve the activation of mitogen-activated protein (MAP) kinases (Lamy et al., 1993) and downregulates c-jun and egr1 mRNA (Reuse et al., 1991; Deleu et al., 1999) and after a short initial induction, c-myc mRNA and protein (Pirson et al., 1996). Like mitogenic stimulations by growth factors, the induction of DNA synthesis by TSH is associated with the phosphorylation of Rb, p107, and p130 (Coulonval et al., 1997) and requires the activity of cdk4 (Lukas et al., 1996; Depoortere et al., 1998). However, the cAMP-dependent mitogenic stimulation does not upregulate d-type cyclins, but it specifically requires the high expression of cyclin D3 (Depoortere et al., 1998) supported by insulin (Van Keymeulen et al., 1999), providing the first evidence of a physiological activation of cyclin D3–cdk4 through their enhanced assembly and nuclear translocation (Depoortere et al., 1998; Van Keymeulen et al., 1999). Unlike growth factors, TSH through cAMP paradoxically enhances the accumulation of p27kip1 in dog thyrocytes (Depoortere et al., 1996) (as found in cell systems where cAMP blocks G1 progression [Kato et al., 1994]), leading us to postulate that this could influence the cell sensitivity to growth inhibition by TGFβ. Here we demonstrate that TGFβ1 indeed specifically inhibits the cAMP-dependent cell cycle of dog thyrocytes but weakly affects the stimulation of DNA synthesis by growth factors, serum, and phorbol esters, and we investigate the mechanism of this inhibition.
MATERIALS AND METHODS
Primary Cultures of Dog Thyroid Follicular Cells
Dog thyrocytes, seeded as follicles (2 × 104 cells/cm2) were cultured in monolayer in the following mixture, which constitutes the control medium (Roger et al., 1987b): DMEM + Ham's F12 medium + MCDB104 medium (Life Technologies Laboratories, Paisley, United Kingdom; 2:1:1, by volume), supplemented with ascorbic acid (40 μg/ml), insulin (5 μg/ml; Sigma Chemical Co., St. Louis, MO), and antibiotics. The medium was changed every other day. At day 4, the cells were quiescent and were treated with the following stimulants in the presence of bovine serum albumin (500 μg/ml, crystallized; Serva-Boehringer, Heidelberg, Germany): bovine TSH (Sigma Chemical Co.), murine epidermal growth factor (EGF; Collaborative Research, Waltham, MA), forskolin (Calbiochem-Bering, La Jolla, CA), fetal bovine serum (Sera-Lab, Sussex, United Kingdom), dibutyryl cAMP (Sigma), recombinant human hepatocyte growth factor (HGF) (a kind gift of T. Nakamura, Osaka University Medical School) in the absence or presence of recombinant human TGFβ1 (R&D Systems, Minneapolis, MN).
Gel Electrophoresis and Immunodetection of Proteins
Cell proteins were separated by PAGE and immunodetected after Western blotting as previously described (Baptist et al., 1995). The following antibodies were used: a rabbit polyclonal antibody against recombinant bovine cyclin A, kindly provided by J. Gannon and T. Hunt (Imperial Cancer Research Fund, Herts, United Kingdom) (Baptist et al., 1996), rabbit polyclonal antibodies against Rb, p107, p130, cdk2, cdk4, and p27kip1 from Santa Cruz Biotechnology (Santa Cruz, CA), monoclonal antibodies against cyclin D3 (DCS-22) (Bartkova et al., 1996) and against cdc2 from Santa-Cruz Biotechnology. Horseradish peroxidase-labeled or 125I-labeled anti-mouse or anti-rabbit antibodies from goat (both from Amersham International, Little Chalfont, United Kingdom) were used as secondary reagents to detect monoclonal and polyclonal antibodies, respectively.
Indirect Immunofluorescence
Cells in Petri dishes (2 × 104 cells/cm2) were fixed with 2% paraformaldehyde for 90 s at 4°C and then with methanol for 10 min at −20°C and permeabilized with 0.1% Triton X-100. Indirect immunofluorescence detection of proliferating cell nuclear antigen (PCNA), cyclin D3, cdk4, cdk2, and p27kip1, and double labeling of cyclin D3, cdk4, or cdk2 with PCNA was performed exactly as described (Baptist et al., 1996; Depoortere et al., 1996, 1998). Percentages of cells in the different phases of the cell cycle were determined from the different patterns of PCNA staining by counting at least 500 cells per dish (Baptist et al., 1993, 1996). To unmask the DCS-22 epitope of cyclin D3, cells were fixed and permeabilized as above and then incubated for 10 min at room temperature with a solution of 0.01% trypsin (ICN Pharmaceuticals, Costa Mesa, CA) before normal processing for immunofluorescent detection using DCS-22, as described (Depoortere et al., 1998). For immunofluorescent detection of Smad 2 and 3 proteins, cells were fixed with 3% paraformaldehyde for 15 min at room temperature and then permeabilized with 1% Triton X-100. The following antibodies were used: PCNA, PC10 (Dakopatt, Carpinteria, CA); cyclin D3, DCS-22, or DCS-28 (Bartkova et al., 1996; Depoortere et al., 1998); cdk4, DCS-31 (Depoortere et al., 1998); cdk2, M2 from Santa Cruz Biotechnology; and Smad 2/3, N19 from Santa Cruz Biotechnology.
The nuclear immunofluorescent detection of cyclin D3 was quantitated using a photomultiplier tube attached to the Zeiss Axiovert 135 microscope (Carl Zeiss, Thornwood, NY) and a 100× oil immersion lens exactly as described previously (Baptist et al., 1995). Fluorescence was measured from 100 nuclei selected at random in each dish. All the conditions were assayed in duplicate with an excellent reproducibility. Much care was taken to avoid fluorescence fading and bleaching during measurements. All the measurements were recorded the same day, and immunofluorescence preparations were never observed before measurements.
Bromodeoxyuridine (BrdU) incorporation was assayed as described (Roger et al., 1992). The percentage of BrdU-labeled nuclei was determined by counting at least 1000 cells per dish.
Immunoprecipitation
Subconfluent cultures of dog thyrocytes in 100-mm Petri dishes that contained the same number of cells 20 h after stimulation were washed with calcium- and magnesium-free PBS and lysed in 1 ml lysis buffer containing 150 mM NaCl, 50 mM Tris-HCl (pH 7.5), 0.5% NP-40, 50 mM NaF, 1 mM sodium orthovanadate, dithiothreitol, and protease inhibitors. One milliliter of precleared cellular lysate was incubated at 4°C for 3 h with 2 μg of antibody (monoclonal antibodies against cyclin D3 [DCS-28] and cdk4 [DCS-35], polyclonal antibodies against p27kip1 or cdk2 [Santa Cruz]), linked to gamma bind plus Sepharose (Pharmacia Biotechnology, Piscataway, NJ). After three washings, the immune complexes were suspended in SDS lysis buffer, boiled for 4 min, and analyzed on 10% SDS-polyacrylamide gels. The proteins were immunodetected as described above using either the DCS-22 cyclin D3 antibody or the Santa Cruz cdk4, cdk2, and p27kip1 antibodies.
Northern Blot Analysis
Subconfluent dog thyrocytes in 100-mm Petri dishes were disrupted in 4 M guanidinium monothiocyanate and the total RNA (10 μg/lane) was separated as described previously (Pirson et al., 1996). After Northern blotting transfer, filters were hybridized with the c-myc probe (1398-bp ClaI fragment of PKH 47 human c-myc. Acridine orange staining of the gel was performed to assess that equal amounts of RNA were loaded in each lane.
RESULTS
TGFβ1 Specifically Inhibits the cAMP-dependent Cell Cycle
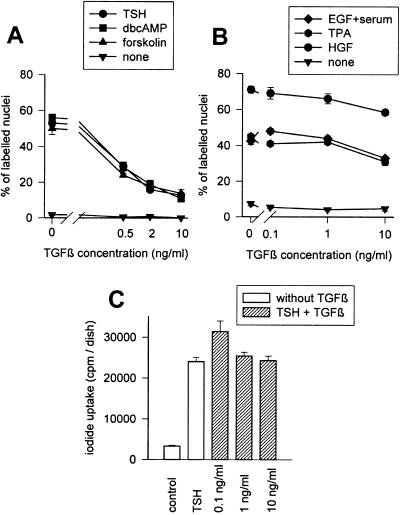
After 4 days without mitogenic agents but in the presence of insulin, dog thyrocytes were spread and quiescent. Cells were then stimulated to proliferate using TSH, forskolin, and dibutyryl cAMP, or by various cAMP-independent treatments, including EGF, HGF, and 12-O-tetradecanoylphorbol-13-acetate (TPA) in the absence or presence of TGFβ1 at different concentrations. As illustrated in Figure 1A, TGFβ1 strongly inhibited (55–93%) the induction of DNA synthesis by TSH. The fact that TGFβ1 similarly inhibited the DNA synthesis stimulated by dibutyryl cAMP or forskolin indicates that this effect occurred distal to cAMP generation. By contrast the mitogenic effects of growth factors and phorbol esters were weakly affected and only at higher concentrations (10 ng/ml) of TGFβ1 (Figure 1B). This differential sensitivity to TGFβ1, which constitutes a new major difference between cAMP-dependent and -independent mitogeneses in dog thyrocytes, was extremely reproducible in the present study. Other cAMP-mediated functions of TSH were unaffected by various TGFβ1 concentrations, including the stimulation of iodide uptake (Figure 1C) and morphological modifications characterized by a cell retraction associated with the disruption of actin stress fibers (unpublished data). The interaction of TGFβ and cAMP pathways, therefore, specifically concerned the regulation of cell cycle.
Figure 1.
Selective inhibition by TGFβ of the cAMP-dependent stimulation of DNA synthesis. Quiescent 4-day-old dog thyrocytes were stimulated for 48 h with either (A) TSH (1 mU/ml), forskolin (10−5 M), or dibutyryl cAMP (10−4 M), or (B) EGF (25 ng/ml), HGF (40 ng/ml), or TPA (10 ng/ml), or none, in combination or not with different TGFβ1 concentrations. BrdU was present for the last 24 h, and the percentage of BrdU-labeled cells was determined. (C) Iodide uptake was measured in parallel. Four-day-old dog thyrocytes were stimulated for 48 h by TSH (1 mU/ml) with or without various TGFβ1 concentrations or remained in control condition. Cells were then incubated for 2 h with 131I-labeled Na (10−6 M, 2 μCi/ml) and mercaptomethylimidazol (1 mM), and the cell radioactivity was measured as described (Taton et al., 1993).
To define more precisely the stage in G1 when TGFβ exerts its growth inhibitory effect, TGFβ1 was added to cells at different times after TSH addition (Figure 2), and the fraction of proliferating cells was assessed by continuous BrdU incorporation 48 h after TSH administration. As previously shown (Baptist et al., 1993), dog thyrocytes progressively reached G1/S transition from ∼20 h after TSH addition. A partial inhibition of S phase entry was still observed when TGFβ1 was added 16 h after TSH, but thereafter TGFβ1 gradually lost its ability to prevent DNA synthesis. Nevertheless a careful comparison of normalized curves reflecting the kinetics of S phase entry after TSH administration and the time course of the escape from TGFβ1 inhibition during G1 progression after TSH addition suggests that the cell cycle becomes insensitive to TGFβ1 block ∼10 h before S phase onset (Figure 2), i.e., at a G1 stage that precedes the late cAMP-dependent restriction point of dog thyrocytes (Roger et al., 1987a, 1999).
Figure 2.
Dog thyrocytes are sensitive to TGFβ1 inhibition during G1. (●) Quiescent 4-day-old dog thyrocytes were stimulated at 0 h with TSH (1 mU/ml) in the presence of BrdU and the cumulative BrdU-labeling index was determined at the indicated times. (○) At the indicated times after TSH administration at 0 h in the presence of BrdU, TGFβ1 (2 ng/ml) was added and the incorporation of BrdU was allowed to proceed until 48 h after TSH addition, at which time all cultures were fixed, and the BrdU-labeling index was determined. The 48-h labeling index without TGFβ was 54% (normalized to 100% on the axis; ●) and the maximal inhibition by TGFβ1 (normalized to 100% on the axis; ○) was 70% inhibition of the nuclear labeling.
In a second experimental approach, we analyzed the influence of TGFβ1 on the kinetics of G1 progression and S phase entry, as determined using the different morphologies of PCNA labeling as markers of the different cell cycle phases (Baptist et al., 1993). In response to TSH, PCNA appears as a diffuse nuclear staining during G1 phase between 6 and 12 h before G1/S transition, and this staining becomes speckled as PCNA associates with DNA replication sites at the onset of S phase (Baptist et al., 1993). As shown in Figure 3A, TGFβ1 added with TSH strongly inhibited the appearance of S phase cells, but it more partially reduced the appearance of PCNA-positive cells with a diffuse nuclear labeling. This suggests that TGFβ1 inhibits G1 phase progression both before and after the stage of PCNA appearance. Moreover, when TGFβ was added 15 h after TSH, its effect on the proportion of S phase cells was manifested only after a delay of ∼10 h (Figure 3A), in agreement with Figure 2 results. Together, kinetics of TGFβ1 action (Figures 2 and 3A) suggest that TGFβ blocks the cAMP-dependent cell cycle at various G1 stages as in other systems (Reynisdottir et al., 1995; Sandhu et al., 1997), but not at a stage close to G1/S transition as in some other systems (Alexandrow and Moses, 1995). Nevertheless, here as in previous studies (Alexandrow and Moses, 1995; Reynisdottir et al., 1995; Sandhu et al., 1997), the interpretation of such kinetics is complex because the delay of TGFβ1 action on S phase might reflect not only the position of the TGFβ1-sensitive stage of G1, but also the lag time before the appearance of a necessary intermediary in TGFβ1 cascade.
Figure 3.
Inhibition by TGFβ of TSH-induced cell cycle progression (A) and accumulation of cell cycle regulatory proteins (B). Four-day-old dog thyrocytes were stimulated at 0 h with TSH (1 mU/ml) or remained in control (C) condition. TGFβ1 (2 ng/ml) (β) was administrated at the same time or 15 h after TSH. (A) Kinetics of cell cycle progression evaluated by the determination of the percentage of PCNA-positive cells identified as described (Baptist et al., 1993) in G1 and S phases. PCNA-negative cells are either in G0 or at a G1 stage before PCNA appearance. (B) Accumulation of cdk4, cdk2, cyclin A, and cdc2 assessed by Western blotting in the same experiment.
The cell cycle inhibitory effect of TGFβ1 was confirmed in this last experiment by the analysis of the expression of several cell cycle regulatory proteins (Figure 3B). As previously shown (Baptist et al., 1996), cyclin A and cdc2 were very weakly expressed in control unstimulated dog thyrocytes. Their accumulation was commonly induced by TSH (Figure 3B) and, independently of cAMP, by EGF+serum (10%) (Baptist et al., 1996), as first detected at 26 h (cyclin A) and 32 h (cdc2). Cdk2 (a 33-kDa form and a 38-kDa form resulting from an alternative splicing [Baptist et al., 1996]) was already expressed in control cells, and its accumulation was further enhanced by TSH and EGF+serum but at late time points corresponding to late stages of cell cycle (Figure 3B). TGFβ1 strongly repressed the stimulation by TSH of the accumulation of these different proteins (Figure 3B). By contrast, it little affected the stimulation by EGF+serum (our unpublished results). As observed in the kinetics of S phase entry (Figure 3A), the addition of TGFβ1 15 h after TSH also resulted in an inhibition of cyclin A, cdk2, and cdc2 accumulation but with a delay (Figure 3B). Unlike cyclin A, cdk2, and cdc2, cdk4 was abundantly expressed in control quiescent cells and its accumulation was very weakly influenced by TSH and TGFβ1 (Figure 3B).
The activation of cdk2 is reflected by the appearance of a downward electrophoretic shift of the 33-kDa form, which corresponds to the activating Thr160 phosphorylation by the nuclear cdk-activating kinase (CAK/cdk7) (Gu et al., 1992; Tassan et al., 1994). As shown in Figure 4A, TGFβ strongly inhibited the phosphorylation of cdk2 elicited by TSH but not the effect of EGF+serum. In quiescent control cells, cdk2 was diffusely distributed in the whole cell (Figure 4B). In TSH-stimulated cells, the double-immunofluorescent staining of cdk2 and PCNA revealed an enhanced nuclear staining due at least in part to a nuclear translocation (Baptist et al., 1996), which was restricted to PCNA-positive cells in late G1 and S phases (Figure 4B). In TSH+TGFβ1-treated cells, this nuclear translocation of cdk2 was only observed in the few PCNA-positive cells that had escaped the TGFβ1 inhibition of G1 progression (Figure 4B). As recently suggested, the nuclear translocation of cdk2 likely depends on its binding to nuclear cyclin E (Moore et al., 1999). Unfortunately, no antibody was available to detect dog cyclin E.
Figure 4.
Inhibition by TGFβ of the TSH-stimulated phosphorylation (A) and nuclear translocation (B) of cdk2. Four-day-old dog thyrocytes were stimulated by TSH (1 mU/ml) or EGF (25 ng/ml) + serum (10%) (ES) alone or in combination with TGFβ1 (2 ng/ml) (β). (A) Activating Thr160 phosphorylation of cdk2 reflected by its downward electrophoretic shift (Gu et al., 1992) demonstrated by Western blotting from cells stimulated for 32 h. (B) Double immunofluorescence labeling of PCNA used as a cell cycle marker (left panels) and cdk2 (right panels) 26 h after cell stimulation. Notice the increased nuclear labeling of cdk2 in PCNA-positive cells observed in many TSH-stimulated cells but few cells treated with TSH+TGFβ.
TGFβ1-induced Translocation of Smad 2 and 3 Is Not Prevented by EGF+Serum
Recently it has been suggested that growth factors can antagonize the effects of TGFβ1 by inducing a phosphorylation of Smad 2 and 3 and preventing their nuclear translocation via the MAP kinase pathway (Kretzschmar et al., 1999). Because in dog thyrocytes MAP kinases are activated by EGF but not by TSH (Lamy et al., 1993), this might contribute to explain the differential TGFβ1 sensitivity of the cell cycle progression induced by TSH or by growth factors. As illustrated in Figure 5, the indirect immunofluorescence staining of dog thyrocytes using an antibody that recognize Smad 2 and 3 revealed that TGFβ1 induced a marked nuclear translocation of these proteins in the whole cell population. This effect was first detected 30 min after TGFβ1 addition, reached maximum at 2 h (Figure 5), and persisted for at least 24 h. The concomitant addition of TSH or EGF+serum (10%) (unpublished results), or a 20-h pretreatment of cells with TSH or EGF+serum did not influence the nuclear translocation of Smad2 and 3 induced by TGFβ1 (Figure 5). This suggests that TSH and EGF+serum did not interfere significantly with the TGFβ1 receptor signaling pathway leading to Smad translocation.
Figure 5.
Nuclear translocation of Smad 2 and 3 induced by TGFβ. Four-day-old dog thyrocytes were stimulated for 20 h with TSH (1 mU/ml) (T), EGF (25 ng/ml) + serum (10%) (ES) or remained in control (C) condition. They were then incubated for 2 h with TGFβ1 (10 ng/ml) before fixation and immunofluorescent labeling using an antibody against Smad 2 and 3 proteins.
TGFβ1 Does Not Inhibit c-myc Expression
A sustained increase of c-myc expression is considered to be required for the progression and DNA synthesis initiation. TGFβ inhibits c-myc expression in most but not all cell types (Chambard and Pouyssegur, 1988; Pietenpol et al., 1990). Very recently this c-myc downregulation was shown to be required for TGFβ-induction of p15INK4B (Warner et al., 1999). In dog thyrocytes the kinetics of c-myc mRNA and protein accumulation are very different in response to TSH or growth factors and phorbol esters (Pirson et al., 1996). C-myc mRNA levels are still enhanced over basal levels 9 h after growth factor stimulation. By contrast, after the cAMP stimulation, c-myc expression is biphasic, with an enhancement at 1 h, followed by a rapid downregulation. As shown in Figure 6, TGFβ did not inhibit the transient induction of c-myc mRNA by TSH at 1 h and the EGF+serum effect observed at 3 h.
Figure 6.
Accumulation of c-myc mRNA in dog thyrocytes analyzed by Northern blotting. Quiescent 4-day-old cells were stimulated for 1 or 3 h with TSH (T), EGF+serum (ES) with or without TGFβ or by TGFβ (β) alone or remained in control (C) condition. Northern blots were prepared with 10 μg of glyoxal denatured total RNA. Acridine orange was performed to assess that equal amount of RNA were loaded in independent lanes.
TGFβ Specifically Inhibits Rb Phosphorylation Induced by TSH
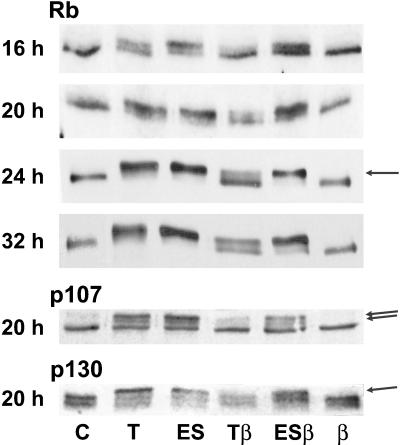
The cAMP-dependent pathway of TSH and the cAMP-independent mitogenic pathway of growth factors and phorbol esters converge before S phase initiation on the phosphorylation of Rb and related p107 and p130RB2 proteins (Coulonval et al., 1997). As observed by Western blotting, the slower migrating band corresponding to hyperphosphorylated Rb forms was almost undetectable in quiescent control cells. In response to TSH and EGF+serum, it appeared at 16 h and increased thereafter at the expense of the fast migrating hypophosphorylated form (Figure 7). As observed on DNA synthesis, TGFβ1 strongly repressed the phosphorylation of Rb induced by TSH, but it weakly affected the effect of EGF+serum (Figure 7). Similar observations were obtained for p107 and p130RB2 (Figure 7).
Figure 7.
TGFβ inhibits the TSH-stimulated phosphorylation of proteins of the Rb family, as detected by their electrophoretic shifts evidenced by Western blotting. Quiescent 4-day-old dog thyrocytes were stimulated during various times with TSH (T) (1 mU/ml), EGF (25 ng/ml) + serum (ES) with or without TGFβ, or by TGFβ alone (2 ng/ml) (β) or remained in control (C) condition. The modulated bands corresponding to the slow-migrating, hyperphosphorylated forms of Rb, p107, and p130 are indicated by arrows.
TGFβ Does Not Affect the Expression of Cyclin D3 and p27kip1
The phosphorylation of Rb, p107, and p130 is considered to be initiated by cdk4 (cdk6 is poorly expressed in dog thyrocytes) activated by d-type cyclins (Kitagawa et al., 1996; Connell-Crowley et al., 1997; Lundberg and Weinberg, 1998). Unlike growth factors that induce cyclins D1 and D2 and moderately increase cyclin D3 levels in dog thyrocytes, TSH does not induce the expression of d-type cyclins at least during the G0-S prereplicative phase. During this phase it even partially reduces the accumulation of cyclin D3, which is the most abundant cyclin d in quiescent thyrocytes cultured with insulin (Depoortere et al., 1998; Van Keymeulen et al., 1999). Nevertheless the entry into S phase of dog thyrocytes stimulated not only by EGF, but also by TSH, required the activity of cdk4 as shown by microinjection of p16INK4A (Lukas et al., 1996), and microinjections of a neutralizing cyclin D3 antibody have shown that cyclin D3 is essential in the TSH- and cAMP-dependent mitogenesis, but not in the pathway of growth factors that induce the other d-type cyclins (Depoortere et al., 1998). TGFβ1 did not inhibit cdk4 accumulation (Figure 3B). In the presence of TSH, TGFβ1 also did not reduce the concentration of cyclin D3 at times corresponding to the whole prereplicative phase (Figure 8). Nevertheless, at 26 and 32 h, TGFβ prevented the moderate increase of cyclin D3 accumulation in TSH-treated cells (Figure 8), which most likely results from the E2F-dependent transcription of cyclin D3 gene (Wang et al., 1996) in cycling cells. As previously shown (Depoortere et al., 1996), TSH unlike EGF+serum, gradually increased the accumulation of p27kip1. TGFβ1 did not influence this effect (Figure 8), nor did it increase the basal accumulation of p27 (unpublished results).
Figure 8.
Kinetics of accumulation of cyclin D3 and p27kip1 detected by Western blotting. Quiescent 4-day-old dog thyroid cells were stimulated for the indicated times using TSH (1 mU/ml) (T) with or without TGFβ (2 ng/ml) (β), EGF (25 ng/ml) + serum (ES) or remained in control (C) condition.
TGFβ1 Prevents the TSH-induced Relocalization of p27kip1 from cdk2 to cdk4, but not the Assembly of Cyclin D3–cdk4 Complexes
A crucial event in the stimulation by TSH of Rb phosphorylation and DNA replication is the assembly through a ill-defined mechanism of stable cyclin D3–cdk4 complexes (Depoortere et al., 1998). The composition of cdk4 and cdk2 immunoprecipitable complexes was analyzed 20 h after cell stimulation, i.e., when a maximum of cells were at a late G1 stage. As shown in Figure 9, TSH not only induced the assembly of cyclin D3 and cdk4, but it also strongly stimulated the association of p27kip1 with these complexes, as evidenced from the fact that cyclin D3 and cdk4 antibodies coprecipitated the same amount of p27kip1. Concomitantly the association of p27 with cdk2 was markedly reduced in both p27 and cdk2 precipitates (Figure 9). Thus, albeit TSH increased the concentration of p27kip1 (Figure 8), it induced the relocalization of p27kip1 from cdk2 to cdk4 complexes, as observed in response to growth factors in other systems (Poon et al., 1995; Reynisdottir and Massague, 1997; Sandhu et al., 1997). This was associated with an increased presence of the Thr160 activatory phosphorylation of cdk2 (Figure 9). In the presence of EGF+serum, p27kip1 was less expressed (Figure 8), and its relocalization from cdk2 to cdk4 was less prominent (Figure 9), which opens the possibility that in this condition, the role of p27kip1 is played by other related proteins, such as p21cip1.
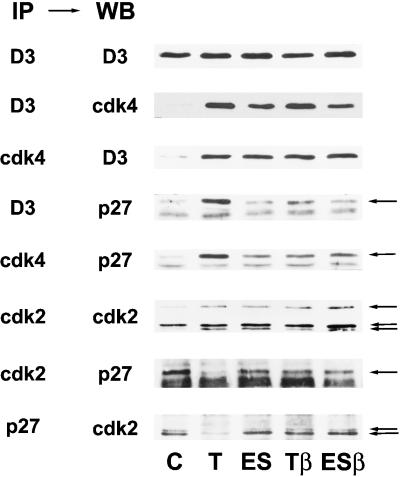
Figure 9.
TGFβ inhibits the TSH-induced relocalization of p27kip1 from cdk2 to cyclin D3–cdk4, but not the assembly of cyclin D3–cdk4 complexes. Quiescent dog thyrocytes were stimulated for 20 h with TSH (1 mU/ml) (T) or EGF (25 ng/ml) + serum (10%) (ES) with or without TGFβ (2 ng/ml) (β) or remained in control (C) condition. Immunoprecipitations (IP) were carried out from equal amounts of cell extracts with antibodies against cyclin D3, cdk4, cdk2, and p27kip1, followed by separation by SDS-PAGE. The proteins were then transferred to nitrocellulose membranes, followed by Western blotting (WB) with the indicated antibodies. The position of p27kip1 and the different forms of cdk2 are indicated by arrows in the respective panels.
In the presence of TSH, but interestingly not in the presence of EGF+serum, TGFβ prevented most of the mobilization of p27kip1 from cdk2 to cdk4 (Figure 9). Unexpectedly, however, this inhibition by TGFβ of the sequestration of p27kip1 into cyclin D3–cdk4 complexes was not associated with a reduced formation of cyclin D3–cdk4 complexes (Figure 9). As similarly observed in complexes precipitated using either cyclin D3 or cdk4 antibodies, TGFβ1 did not affect the dramatic induction by TSH of cyclin D3–cdk4 assembly, but it markedly prevented the association of p27kip1 with cyclin D3 and cdk4 (Figure 9). This striking observation raised questions as for not only the mechanism of inhibition by TGFβ of p27kip1 sequestration, but also the mechanism of TSH-induced cyclin D3–cdk4 assembly.
TGFβ1 Inhibits the Nuclear Translocation of cdk4 and Cyclin D3, and Thus Their Colocalization with Nuclear p27kip1
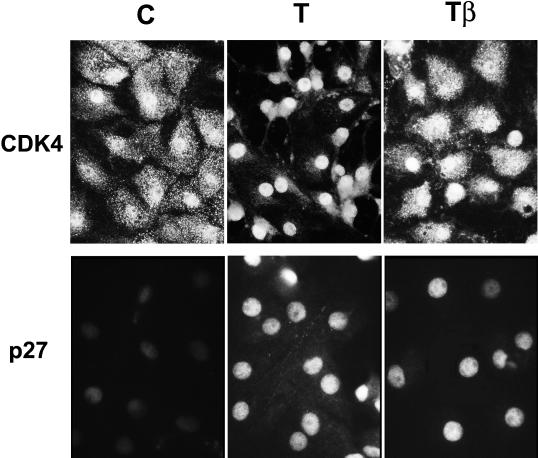
In response to TSH, the activation of cdk4 is associated with its nuclear translocation (Depoortere et al., 1998). In cells stimulated for 20 h, TGFβ prevented most of the TSH-induced nuclear translocation of cdk4, which was evidenced by the increase of its nuclear labeling at the expense of the cytoplasmic staining in many cells (Figure 10), but it did not influence the nuclear import of cdk4 in cells stimulated by EGF+serum (unpublished results).
Figure 10.
TGFβ inhibits the TSH-induced nuclear translocation of cdk4 but does not affect the nuclear location of p27kip1. Quiescent 4-day-old dog thyrocytes were stimulated for 20 h with TSH (1 mU/ml) (T) with or without TGFβ (2 ng/ml) (β) or remained in control (C) condition. Cells were then processed for cdk4 or p27kip1 immunofluorescent staining. Labeled cells were photographed using a 50× immersion lens (cdk4) or a 100× lens (p27).
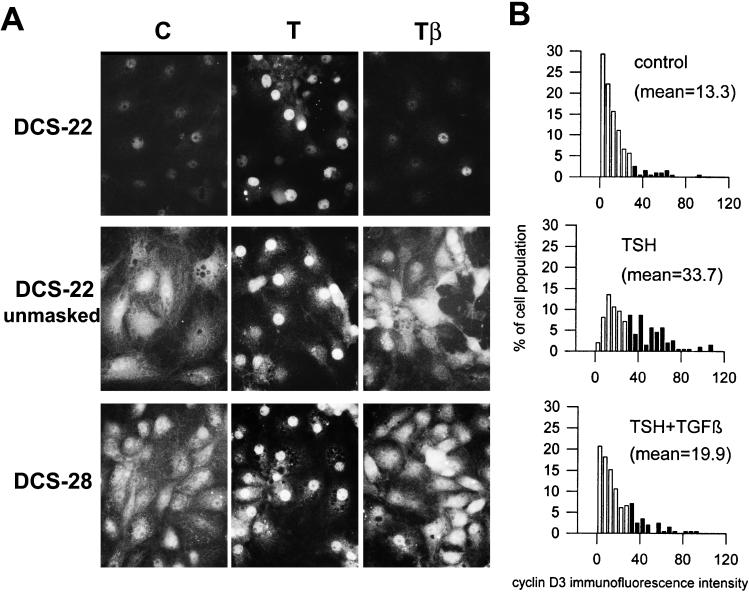
As previously observed (Depoortere et al., 1998) the nuclear translocation and activation of cdk4 induced by TSH also correlated with a marked increase of the nuclear detection of cyclin D3 using several monoclonal antibodies including DCS-22 (Figure 11, A and B). This phenomenon contrasts with a partial reduction of cyclin D3 content as evidenced by Western blotting (Figure 8) (Depoortere et al., 1998). It correlates with cell cycle progression and reflects both the nuclear translocation of cyclin D3 and the unmasking of the DCS-22 epitope, which suggests a conformational change of cyclin D3 or a modification of its interaction with other proteins (Depoortere et al., 1998). As quantitated by direct fluorescence measurements using a photomultiplier tube attached to the microscope, TGFβ markedly reduced the proportion of nuclei that display an increased reactivity to DCS-22 in the presence of TSH (from 57% in TSH-treated cells to 29% with TSH+TGFβ, compared with 10% arbitrarily fixed in control cells) (Figure 11B). A mild trypsin digestion of fixed cells (which is a classical treatment for retrieval of masked epitopes) before immunodetection using DCS-22, allows to detect the overall presence of cyclin D3 in all the conditions (Depoortere et al., 1998). This treatment allowed to us visualize an overall cellular distribution of cyclin D3, both in quiescent control cells and in the majority of TSH+TGFβ-treated cells, and its nuclear translocation in most cells stimulated by TSH (Figure 11A). The inhibition by TGFβ of the TSH-induced nuclear translocation of cyclin D3 was confirmed without unmasking treatment using the cyclin D3 COOH-terminus antibody DCS-28 (Figure 11A). TGFβ thus prevented the TSH effects on both the accessibility of cyclin D3 to DCS-22, and the nuclear translocation of cyclin D3.
Figure 11.
TGFβ inhibits the epitope unmasking and nuclear translocation of cyclin D3 induced by TSH. Quiescent 4-day-old dog thyrocytes were stimulated for 20 h with TSH (1 mU/ml) (T) with or without TGFβ (2 ng/ml) (β) or remained in control (C) condition. Cells were then processed for cyclin D3 immunofluorescent staining using the DCS-22 or DCS-28 monoclonal antibodies. For cyclin D3 detection using DCS-22, an epitope unmasking treatment by a mild trypsin digestion of fixed cells was applied (unmasked) or not. (A) Labeled cells were photographed using a 50× immersion lens. (B) Distribution of nuclear cyclin D3 immunofluorescence intensities within the cell population as measured by photometry. Cyclin D3 was detected using DCS-22 without the application of the trypsin unmasking treatment. Values of nuclear immunofluorescences exceeding the weak to moderate ones recorded in 90% of unstimulated control cells were scored positive and are shown as filled bars. Average values of immunofluorescence intensity are indicated for each treatment.
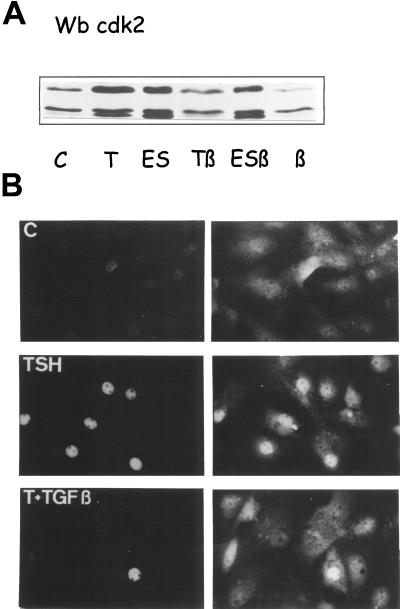
Double immunofluorescent labelings of cyclin D3 (DCS-22) or cdk4 with PCNA used as a cell cycle marker showed that the minor population of TSH+TGFβ-treated cells that escaped the cell cycle inhibition and were progressing in late G1 and S phases always displayed high nuclear detections of cyclin D3 and cdk4 (Figure 12). Conversely, in cells stimulated by TSH in the absence of TGFβ, low nuclear labelings of cyclin D3 and cdk4 were never found in PCNA-positive cycling cells (Depoortere et al., 1998; unpublished results). This indicates, at the single cell level, that the inhibition by TGFβ of the nuclear translocation of cyclin D3 and cdk4 was functionally significant with regard to the inhibition of cell cycle progression.
Figure 12.
Double immunofluorescent labeling of cyclin D3 (using DCS-22 without unmasking treatment) or cdk4, and PCNA used as a cell cycle marker. Quiescent dog thyrocytes were stimulated for 26 h with TSH (1 mU/ml) + TGFβ (2 ng/ml). Large arrows and medium size arrows show cells identified, respectively, in late G1 and S phases as described previously (Baptist et al., 1993) (the speckled appearance of PCNA-labeled S-phase nuclei was clearly seen at microscope but not in these digitalized micrographs). Small arrows show G0/G1 cells with a low PCNA staining. The microscopical fields were selected to contain cells that escape the TGFβ inhibition of proliferation. Notice that all the PCNA-positive cycling cells display intense nuclear stainings of cyclin D3 and cdk4.
Unlike cyclin D3 and cdk4, p27kip1 was detected as a purely nuclear protein both in TSH and TSH+TGFβ-treated cells (Figure 10). By inhibiting the nuclear translocation of cyclin D3 and cdk4 induced by TSH, TGFβ thus prevented their colocalization with nuclear p27kip1. Considering together observations of Figures 9–11, it is thus concluded that in the presence of TGFβ, the TSH-induced cyclin D3–cdk4 complexes were in large part formed in the cytoplasm, where they were prevented from sequestering nuclear p27kip1 away from cdk2. Moreover, by impairing their association with nuclear p27kip1, TGFβ might prevent the nuclear import of cyclin D3–cdk4 complexes, which is required for cdk4 phosphorylation by nuclear CAK and of course for access to nuclear substrates including Rb-related proteins.
DISCUSSION
TGFβ Discriminates between cAMP-dependent and -independent Mitogenic Stimulations
The present study supports the rarely considered concept that extracellular and intracellular inhibitory mechanisms may differentially target the cell cycle, depending on the specific mitogenic cascade that governs its progression. Previously, we have shown that during the growth factor–stimulated division of dog thyrocytes, a dominant intracellular repressor is generated that specifically suppresses the TSH- and cAMP-dependent mitogenic pathway, but not the growth response to EGF, nor the cAMP-dependent expression of differentiation (Roger et al., 1992). Also in rat thyroid in vivo, an irreversible desensitization mechanism specifically affects the TSH-dependent proliferation, but not the TSH-dependent thyroid function, nor the proliferation induced by tissue wounding (Wynford-Thomas et al., 1983; Smith et al., 1987). It has been suggested that TSH-dependent local expression of TGFβ1 may contribute to this mechanism (Logan et al., 1994). An escape from this growth desensitization may occur after a few months with the development of TSH-dependent tumors (Wynford-Thomas et al., 1983), and many thyroid tumor cells lose TGFβ responsiveness (Blaydes et al., 1995). Here we demonstrate that TGFβ1 very specifically inhibits the TSH- and cAMP-dependent cell cycle of dog thyrocytes, but not the TSH-dependent iodide uptake, and has little effect on the cAMP-independent proliferation elicited by EGF, HGF, phorbol esters, and serum.
The very striking similarities of these different in vivo and in vitro phenomena suggest a common mechanism to explain the high sensitivity of the cAMP-dependent mitogenic pathway and the relative resistance of the growth factor–dependent proliferation. Very recently, it has been suggested that growth factors, via the MAP kinase pathway, may antagonize the TGFβ signaling by phosphorylating Smad 2 and 3 on distinct sites and preventing their nuclear translocation (Kretzschmar et al., 1999). This seemed an attractive explanation, because in dog thyrocytes TSH, unlike growth factors and phorbol esters, does not activate the various MAP kinases (Lamy et al., 1993; Vandeput, unpublished results). However, our present data suggest that EGF+serum and TSH do not interfere importantly with TGFβ signaling, because they do not affect the nuclear translocation of Smad2 and 3 induced by TGFβ. Also in mink lung epithelial cells, HGF counteracts TGFβ-mediated growth inhibition but does not prevent TGFβ-induction of p15INK4B (Tsubari et al., 1999). A definitive assessment of the issue of possible interactions between growth factor and TGFβ signaling cascades should await the complete elucidation of the TGFβ signal transduction pathways.
As discussed below, our results are rather consistent with the hypothesis that the differential sensitivity to TGFβ growth inhibition could be a corollary of differences between cAMP-dependent and -independent regulations of cell cycle by cyclin–cdk complexes. In other words, TGFβ could selectively block the cAMP-dependent cell cycle of dog thyrocytes by inhibiting an event that is specifically rate limiting for cAMP-dependent proliferation but not for mitogenesis elicited by growth factors. In fact, the TSH–cAMP mitogenic pathway of dog thyrocytes appears very unique as it has provided the first demonstration in a normal nontransfected cell of a mitogenic stimulation targeting the nuclear import and assembly of cyclin D3 and cdk4 in the absence of any stimulation of cyclin d expression (Depoortere et al., 1998). It offers two clues that potentially could explain the high sensitivity of the cAMP-dependent stimulation to TGFβ inhibition and the relative resistance of the mitogenic stimulation by growth factors: 1) TSH, unlike EGF+serum, enhances the accumulation of p27kip1 (Depoortere et al., 1996), which plays a crucial role in cdk2 inhibition by TGFβ; and 2) exactly like TGFβ, the microinjection of a neutralizing cyclin D3 antibody blocks most of the stimulation of DNA synthesis by TSH and cAMP but has little effects on the stimulation by HGF, EGF, and EGF+serum, which, unlike TSH, induce cyclins D1 and D2 (Depoortere et al., 1998).
TGFβ Inhibits the cAMP-dependent Progression in G1 and Phosphorylation of Rb-related Proteins
In the present study, we have demonstrated that TGFβ1 blocks the TSH-stimulated cell cycle progression during G1 phase in dog thyrocytes, as often observed in other systems (Reynisdottir et al., 1995; Sandhu et al., 1997), but no longer at the very late G1 restriction point that is still positively controlled by cAMP even after cyclin D3–cdk4 complexes are stably formed (Roger et al., 1987a, 1999). This inhibition is not associated with an inhibition of c-myc expression at variance with many other systems (Pietenpol et al., 1990), but with the inhibition of the accumulation of PCNA, cyclin A, cdk2, and its activating Thr160 phosphorylation. Again the inhibition by TGFβ of these different events mostly concerns their stimulation by TSH. These events are observed at or after the restriction point and are strictly limited to cycling cells (Baptist et al., 1996), and thus their inhibition by TGFβ could be a consequence of cell cycle arrest at earlier stages of G1. We also have observed that TGFβ specifically inhibits the phosphorylation of Rb, p107, and p130 stimulated by TSH. This likely explains the cell cycle arrest and the inhibition of the aforementioned cell cycle–related events, which have been causally related with Rb phosphorylation and release of E2F-dependent gene transcription through direct or indirect mechanisms. For instance, the promoter of cyclin A contains a variant E2F site that binds to p107–E2F4 complexes containing cyclin E–cdk2 (Zerfass-Thome et al., 1997). The nuclear translocation of cdk2, which shortly precedes the initiation of DNA synthesis and cyclin A appearance (Baptist et al., 1996), probably depends on cdk2 association with cyclin E, which is imported into nuclei via a direct interaction with importin α (Moore et al., 1999). It is required for the Thr160-activating phosphorylation by the nuclear CAK (Tassan et al., 1994). Cyclin E transcription in late G1 is regulated by Rb via E2F proteins (Ohtani et al., 1995). Because cyclin E–cdk2 in addition to initiating S phase (Lukas et al., 1997) also phosphorylates and inactivates Rb (Kelly et al., 1998), this has suggested a positive feedback loop underlying the commitment to DNA synthesis at the restriction point (Weinberg, 1995; Bartek et al., 1996; Sherr, 1996).
TGFβ Inhibits the Sequestration of p27kip1 by Cyclin D3–cdk4 Complexes, but not Their cAMP-dependent Assembly
The phosphorylation of Rb by cyclin E–cdk2 requires its prior phosphorylation by cyclin d–cdk4 (Connell-Crowley et al., 1997; Lundberg and Weinberg, 1998). Moreover ectopic expression of cyclin E does not activate E2F in the absence of cdk4 and cdk6 activity (Lukas et al., 1997). On the other hand, p107 has been reported as an exclusive cdk4 substrate (Beijersbergen et al., 1995). The activation of cyclin d kinases is therefore considered as the triggering event of the phosphorylation or inactivation of Rb and related proteins and subsequent activation of E2F-dependent gene transcription. Within this conceptual context, our unexpected finding that TGFβ inhibits the cAMP-dependent phosphorylation of Rb, p107, and p130 and thus cdk4 activity, without preventing the cAMP-dependent assembly of essential cyclin D3–cdk4 complexes, is a novel and peculiarly intriguing observation. It is at variance with former studies of TGFβ inhibition of cell cycle, which all envisage a decrease of the presence of cyclin d–cdk4 or –cdk6 complexes, either due to reduced cdk4 or cdk6 expression (Ewen et al., 1993, 1995; Tsubari et al., 1999), inhibition of cyclin D1 accumulation (Ko et al., 1995) and/or induction of p15INK4B (Sandhu et al., 1997). Nevertheless, in agreement with these previous studies, we found that in dog thyrocytes TGFβ prevents the TSH-induced release of p27kip1 from cdk2 complexes and its sequestration into cyclin D3–cdk4 complexes.
TGFβ Inhibits the cAMP-dependent Nuclear Translocation of Cyclin D3 and cdk4
We explain these apparently paradoxical observations by modifications of subcellular locations of the different proteins. Unlike p27kip1, which contains a nuclear localization signal and is found as an exclusively nuclear protein (Depoortere et al., 1996) (Figure 10), cdk4 and cyclin D3 do not display obvious nuclear localization signal and are localized in large part in the cytoplasm in quiescent dog thyrocytes. In reponse to TSH, cyclin D3 and cdk4 are imported into nuclei (Depoortere et al., 1998) (Figures 10 and 11), where they have access to p27kip1 and form stable cyclin D3–cdk4-p27kip1 complexes. Whether cyclin D3 and cdk4 assemble before or after their translocation is not known. As shown here, TGFβ does not inhibit the formation of cyclin D3–cdk4 complexes induced by TSH, but prevents the nuclear import of both cdk4 and cyclin D3 without affecting the expression and nuclear location of p27kip1. This clearly implies that in the presence of TGFβ, TSH-induced cyclin D3–cdk4 complexes are largely formed in the cytoplasm where they are prevented, among other consequences, from interacting with and thus sequestering p27kip1. Cytoplasmic cyclin d–cdk complexes are expected to be inactive, as shown for cytoplasmic cyclin d–cdk6 complexes in T cells (Mahony et al., 1998), because they are not phosphorylated by nuclear CAK (Diehl and Sherr, 1997) and of course because they lack access to nuclear substrates, including Rb and related proteins. Cyclin D3–cdk4 also likely phosphorylates p107 and p130 (Dong et al., 1998b). The inhibition by TGFβ of the nuclear translocation of cyclin D3 and cdk4 observed here may thus suffice to explain the inhibition of the phosphorylation of the three Rb family members.
Cytoplasmic assembly of inactive cyclin d–cdk4 complexes recently has been found in two artificial models of transfected cells (Diehl and Sherr, 1997; Reynisdottir and Massague, 1997). In mink lung epithelial cells, ectopically expressed cytoplasmic p15INK4B, as a model recapitulating TGFβ inhibition of growth, interacts with cyclin d–cdk4 and –cdk6 complexes and prevents them from reaching the nucleus and from encountering p27kip1 (Reynisdottir and Massague, 1997). Our present observations fit well with parts of this model system. Nevertheless, it seems unlikely that the induction of p15INK4B could be the mechanism of the inhibition by TGFβ of cyclin D3–cdk4 nuclear translocation in TSH-stimulated dog thyrocytes : 1) cells lacking p15INK4B are not resistant to TGFβ inhibition of proliferation (Florenes et al., 1996; Iavarone and Massague, 1997), which is nevertheless associated with an increased association of p27kip1 with cdk2 (Florenes et al., 1996); 2) antibodies were not available to detect dog p15INK4B. Nevertheless we failed to detect p15 expression from dog thyrocytes treated with TGFβ or TSH+TGFβ by Northern blotting using 10 μg of polyA+ RNA and a murine p15INK4B probe, and we also did not detect a p15 band in cdk4 (DCS-35) immunoprecipitates from TSH+TGFβ-treated cells metabolically labeled with [35S]methionine (unpublished negative data); 3) the induction of p15INK4B has recently been found to depend on c-myc downregulation by TGFβ (Warner et al., 1999), which was not observed in the present study (Figure 6); 4) at variance with the former report by Massagué's group (Reynisdottir and Massague, 1997), p15INK4B has now been found to disrupt preexisting cyclin d–cdk4 complexes in Mv1Lu cells (Warner et al., 1999), as in mammary epithelial cells (Sandhu et al., 1997), in agreement with the general observation that INK4 proteins including p15 are found in association with cdk4 and cdk6 but not the d-type cyclins (Hall et al., 1995; Russo et al., 1998; Tsubari et al., 1999); 5) TGFβ induction of p15INK4B would not explain why the nuclear translocation of cdk4 induced by EGF+serum was resistant to TGFβ in dog thyrocytes, unless EGF+serum inhibits p15INK4B expression; and 6) TGFβ prevented the nuclear translocation of not only cdk4, but also cyclin D3, which is unlikely to interact with p15INK4B.
The second model has suggested that determinants of cyclin d location could govern the localization and activity of cdk4. A mutated cyclin D1 (T156A), which fails to enter the nucleus, competes with endogenous d-type cyclins and sequesters cdk4 as an inactive cytoplasmic complex, thus preventing its phosphorylation by CAK and DNA synthesis (Diehl and Sherr, 1997). Moreover some phosphorylations of cyclin D1 influence its subcellular localization (Diehl et al., 1998). Analogous mechanisms could be envisaged for the inhibition by TGFβ of TSH-induced cyclin D3–cdk4 nuclear translocations. Unlike nuclear cyclin D3 found in TSH-stimulated cells, which is presumably active (i.e., restricted to cycling cells [Depoortere et al., 1998; Figure 12]) and bound to cdk4 and p27kip1), the cytoplasmic cyclin D3 found both in quiescent cells and TSH+TGFβ-treated cells required a trypsin unmasking treatment for its immunofluorescent detection by DCS-22 (Figure 11A). TGFβ thus prevents both the exposition by TSH of the DCS-22 epitope (located between amino acids 241 and 260 of cyclin D3 sequence) and the nuclear translocation of cyclin D3. This suggests that a conformational change of cyclin D3 or a modification of its interaction with other proteins, which could be important for the nuclear translocation of cyclin D3 but not for cyclin D3–cdk4 assembly, is induced by TSH and repressed by TGFβ.
Role of p27kip1 in the cAMP-dependent Cell Cycle
Our previous finding that TSH stimulates the accumulation of p27kip1, including in cells progressing in G1 and S phases (Depoortere et al., 1996), was at odds with the consensual view at that time that restricted p27kip1 to the role of a general inhibitor of cdks, including cdk4, which mediates various proliferation inhibitions (Nourse et al., 1994; Polyak et al., 1994; Coats et al., 1996), including the cAMP-dependent G1 block observed in some systems (Kato et al., 1994; Ward et al., 1996; L'Allemain et al., 1997). Although there is still a consensus to consider p27kip1 as an inhibitor of cdk2 (Blain et al., 1997; Hengst and Reed, 1998), p27-related members of the cip/kip family have now turned out to be associated with an Rb-kinase activity (Soos et al., 1996; Blain et al., 1997; Dong et al., 1998a), to assemble cyclin d–cdk complexes (LaBaer et al., 1997) and target them to the nucleus (Diehl and Sherr, 1997; LaBaer et al., 1997; Reynisdottir and Massague, 1997), and even to be essential in these functions required for cdk4 activity (Cheng et al., 1999). Our present observations that TSH induces, whereas TGFβ inhibits, the association of p27kip1 with cyclin D3–cdk4 complexes also argue in favor of such a positive role. In this study, the action of TGFβ clearly dissociated the assembly of cyclin D3–cdk4 complexes from their association with p27kip1, but it reinforced the correlation between the binding to p27kip1 and the nuclear location of cyclin D3–cdk4. Therefore, the TSH-stimulated accumulation of p27kip1 is probably not instrumental in the TSH-induced cyclin D3–cdk4 assembly, but it might contribute to the activation and nuclear localization of the complex, perhaps as an anchor that stabilizes cyclin D3–cdk4 within the nuclear compartment.
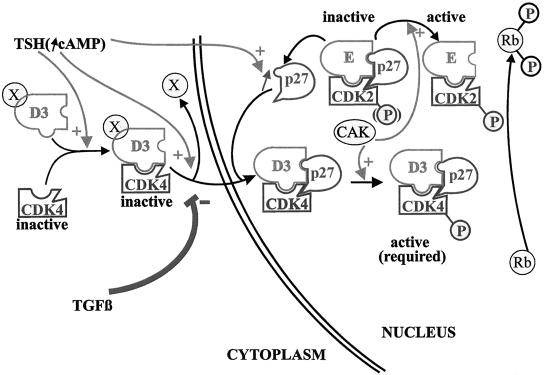
As summarized in Figure 13, the present study, investigating endogenous proteins in a physiologically relevant system of primary epithelial cells, points out the nuclear import of cyclin d–cdk4 complexes dissociated from their assembly, as a crucial factor integrating mitogenic and antimitogenic regulations.
Figure 13.
The subcellular localization of cyclin D3 and cdk4 integrates the antagonistic cell cycle effects of TSH and TGFβ. TSH does not stimulate cyclin D3 accumulation, but it assembles cyclin D3–cdk4 complexes, enhances the levels of p27kip1, and induces the nuclear translocation of cyclin D3–cdk4, which correlates with the exposition of a cyclin D3 epitope (depicted by the displacement of the X element). Cyclin D3–cdk4 complexes are stabilized in the nucleus by their binding to p27kip1, which thus might serve as a nuclear anchor. This nuclear translocation of cdk4 is assumed to be required for its phosphorylation by nuclear CAK and for access to Rb. TGFβ does not inhibit the assembly of cyclin D3–cdk4 complexes, nor p27kip1 accumulation, but it prevents the epitope unmasking of cyclin D3 and the nuclear translocation of cyclin D3–cdk4. Consequently these complexes are prevented from encountering p27kip1 and thus sequestering it away from the initial pool of nuclear cdk2 complexes. Cyclin D3–cdk4 is thus maintained in an inactive state by its cytoplasmic location, and nuclear cdk2 is inhibited by its association with p27kip1. The further import of cdk2 from the cytoplasm induced by TSH but repressed by TGFβ as a likely indirect consequence of this mechanism, is not depicted.
ACKNOWLEDGMENTS
We thank K. Coulonval for help in some experiments, T. Nakamura for the HGF, and J. Gannon and T. Hunt for the cyclin A antibody. This study was supported by the Belgium Program on University Poles of Attraction initiated by the Belgian State, Prime Minister's Office, Science Policy Programming, and by grants from the National Fund for Scientific Research (FNRS, Belgium), the Caisse Générale d'Epargne et de Retraite, the Danish Cancer Society, and the Nordic Cancer Union. F.D. is a fellow of the “Télévie” and P.R. is a Research Associate of the FNRS.
REFERENCES
- Alexandrow MG, Moses HL. Transforming growth factor beta 1 inhibits mouse keratinocytes late in G1 independent of effects on gene transcription. Cancer Res. 1995;55:3928–3932. [PubMed] [Google Scholar]
- Baptist M, Dumont JE, Roger PP. Demonstration of cell cycle kinetics in thyroid primary culture by immunostaining of proliferating cell nuclear antigen: differences in cyclic AMP-dependent and -independent mitogenic stimulations. J Cell Sci. 1993;105:69–80. doi: 10.1242/jcs.105.1.69. [DOI] [PubMed] [Google Scholar]
- Baptist M, Dumont JE, Roger PP. Intercellular heterogeneity of early mitogenic events: cAMP generalizes the EGF effect on c-Fos protein appearance but not on MAP kinase phosphorylation and nuclear translocation in dog thyroid epithelial cells. Exp Cell Res. 1995;221:160–171. doi: 10.1006/excr.1995.1363. [DOI] [PubMed] [Google Scholar]
- Baptist M, Lamy F, Gannon J, Hunt T, Dumont JE, Roger PP. Expression and subcellular localization of CDK2 and cdc2 kinases and their common partner cyclin A in thyroid epithelial cells: comparison of cyclic AMP-dependent and -independent cell cycles. J Cell Physiol. 1996;166:256–273. doi: 10.1002/(SICI)1097-4652(199602)166:2<256::AID-JCP3>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Barnard JA, Lyons RM, Moses HL. The cell biology of transforming growth factor beta. Biochim Biophys Acta. 1990;1032:79–87. doi: 10.1016/0304-419x(90)90013-q. [DOI] [PubMed] [Google Scholar]
- Bartek J, Bartkova J, Lukas J. The retinoblastoma protein pathway and the restriction point. Curr Opin Cell Biol. 1996;8:805–814. doi: 10.1016/s0955-0674(96)80081-0. [DOI] [PubMed] [Google Scholar]
- Bartkova J, Zemanova M, Bartek J. Abundance and subcellular localization of cyclin D3 in human tumors. Int J Cancer. 1996;65:323–327. doi: 10.1002/(SICI)1097-0215(19960126)65:3<323::AID-IJC8>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Beijersbergen RL, Carlee L, Kerkhoven RM, Bernards R. Regulation of the retinoblastoma protein-related p107 by G1 cyclin complexes. Genes Dev. 1995;9:1340–1353. doi: 10.1101/gad.9.11.1340. [DOI] [PubMed] [Google Scholar]
- Blain SW, Montalvo E, Massague J. Differential interaction of the cyclin-dependent kinase (Cdk) inhibitor p27Kip1 with cyclin A-Cdk2 and cyclin D2-Cdk4. J Biol Chem. 1997;272:25863–25872. doi: 10.1074/jbc.272.41.25863. [DOI] [PubMed] [Google Scholar]
- Blaydes JP, Schlumberger M, Wynford-Thomas D, Wyllie FS. Interaction between p53 and TGF beta 1 in control of epithelial cell proliferation. Oncogene. 1995;10:307–317. [PubMed] [Google Scholar]
- Bouchard C, Thieke K, Maier A, Saffrich R, Hanley-Hyde J, Ansorge W, Reed S, Sicinsky P, Bartek J, Eilers M. Direct induction of cyclin D2 by myc contributes to cell cycle progression and sequestration of p27. EMBO J. 1999;18:5321–5333. doi: 10.1093/emboj/18.19.5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambard JC, Pouyssegur J. TGF-beta inhibits growth factor-induced DNA synthesis in hamster fibroblasts without affecting the early mitogenic events. J Cell Physiol. 1988;135:101–107. doi: 10.1002/jcp.1041350114. [DOI] [PubMed] [Google Scholar]
- Cheng M, Olivier P, Diehl JA, Fero M, Roussel MF, Roberts JM, Sherr CJ. The p21(Cip1) and p27(Kip1) CDK inhibitors are essential activators of cyclin d-dependent kinases in murine fibroblasts. EMBO J. 1999;18:1571–1583. doi: 10.1093/emboj/18.6.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coats S, Flanagan WM, Nourse J, Roberts JM. Requirement of p27Kip1 for restriction point control of the fibroblast cell cycle. Science. 1996;272:877–880. doi: 10.1126/science.272.5263.877. [DOI] [PubMed] [Google Scholar]
- Colletta G, Cirafici AM, Di Carlo A. Dual effect of transforming growth factor beta on rat thyroid cells: inhibition of thyrotropin-induced proliferation and reductions of thyroid-specific differentiation markers. Cancer Res. 1989;49:3457–3462. [PubMed] [Google Scholar]
- Connell-Crowley J, Harper JW, Goodrich DW. Cyclin D1/cdk4 regulates retinoblastoma protein-mediated cell cycle arrest by site-specific phosphorylation. Mol Biol Cell. 1997;8:287–301. doi: 10.1091/mbc.8.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contempre B, Le Moine O, Dumont JE, Denef JF, Many MC. Selenium deficiency and thyroid fibrosis. A key role for macrophages and transforming growth factor beta (TGF-beta) Mol Cell Endocrinol. 1996;124:7–15. doi: 10.1016/s0303-7207(96)03921-4. [DOI] [PubMed] [Google Scholar]
- Coulonval K, Maenhaut C, Dumont JE, Lamy F. Phosphorylation of the three Rb protein family members is a common step of the cAMP-, the growth factor, and the phorbol ester-mitogenic cascades but is not necessary for the hypertrophy induced by insulin. Exp Cell Res. 1997;233:395–398. doi: 10.1006/excr.1997.3582. [DOI] [PubMed] [Google Scholar]
- Datto MB, Li Y, Panus JF, Howe DJ, Xiong Y, Wang XF. Transforming growth factor beta induces the cyclin-dependent kinase inhibitor p21 through a p53-independent mechanism. Proc Natl Acad Sci USA. 1995;92:5545–5549. doi: 10.1073/pnas.92.12.5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datto MB, Frederick JP, Pan L, Borton AJ, Zhuang Y, Wang XF. Targeted disruption of Smad3 reveals an essential role in transforming growth factor beta-mediated signal transduction. Mol Cell Biol. 1999;19:2495–2504. doi: 10.1128/mcb.19.4.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deleu S, Pirson I, Clermont F, Nakamura T, Dumont JE, Maenhaut C. Immediate early gene expression in dog thyrocytes in response to growth, proliferation and differentiation stimuli. J Cell Physiol. 1999;181:342–354. doi: 10.1002/(SICI)1097-4652(199911)181:2<342::AID-JCP16>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Depoortere F, Dumont JE, Roger PP. Paradoxical accumulation of the cyclin-dependent kinase inhibitor p27kip1 during the cAMP-dependent mitogenic stimulation of thyroid epithelial cells. J Cell Sci. 1996;109:1759–1764. doi: 10.1242/jcs.109.7.1759. [DOI] [PubMed] [Google Scholar]
- Depoortere F, Van Keymeulen A, Lukas J, Costagliola S, Bartkova J, Dumont JE, Bartek J, Roger PP, Dremier S. A requirement for cyclin D3-cyclin-dependent kinase (cdk)-4 assembly in the cyclic adenosine monophosphate-dependent proliferation of thyrocytes. J Cell Biol. 1998;140:1427–1439. doi: 10.1083/jcb.140.6.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R. TGF-beta-receptor-mediated signaling. Trends Biochem Sci. 1994;19:548–553. doi: 10.1016/0968-0004(94)90059-0. [DOI] [PubMed] [Google Scholar]
- Diehl JA, Cheng M, Roussel MF, Sherr CJ. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998;12:3499–3511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl JA, Sherr CJ. A dominant-negative cyclin D1 mutant prevents nuclear import of cyclin- dependent kinase 4 (CDK4) and its phosphorylation by CDK-activating kinase. Mol Cell Biol. 1997;17:7362–7374. doi: 10.1128/mcb.17.12.7362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong F, Agrawal D, Bagui T, Pledger WJ. Cyclin D3-associated kinase activity is regulated by p27kip1 in BALB/c 3T3 cells. Mol Biol Cell. 1998a;9:2081–2092. doi: 10.1091/mbc.9.8.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong F, Cress WDJ, Agrawal D, Pledger WJ. The role of cyclin D3-dependent kinase in the phosphorylation of p130 in mouse BALB/c 3T3 fibroblasts. J Biol Chem. 1998b;273:6190–6195. doi: 10.1074/jbc.273.11.6190. [DOI] [PubMed] [Google Scholar]
- Ewen ME, Sluss HK, Whitehouse LL, Livingston DM. TGF beta inhibition of Cdk4 synthesis is linked to cell cycle arrest. Cell. 1993;74:1009–1020. doi: 10.1016/0092-8674(93)90723-4. [DOI] [PubMed] [Google Scholar]
- Ewen ME, Oliver CJ, Sluss HK, Miller SJ, Peeper DS. p53-dependent repression of CDK4 translation in TGF-beta-induced G1 cell-cycle arrest. Genes Dev. 1995;9:204–217. doi: 10.1101/gad.9.2.204. [DOI] [PubMed] [Google Scholar]
- Florenes VA, Bhattacharya N, Bani MR, Ben-David Y, Kerbel RS, Slingerland JM. TGF-beta mediated G1 arrest in a human melanoma cell line lacking p15INK4B: evidence for cooperation between p21Cip1/WAF1 and p27Kip1. Genes Dev. 1996;11:3157–3167. [PubMed] [Google Scholar]
- Grubeck-Loebenstein B, Buchan G, Sadeghi R, Kissonerghis M, Londei M, Turner M, Pirich K, Roka R, Niederle B, Kassal H. Transforming growth factor beta regulates thyroid growth. Role in the pathogenesis of nontoxic goiter. J Clin Invest. 1989;83:764–770. doi: 10.1172/JCI113955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Rosenblatt J, Morgan DO. Cell cycle regulation of CDK2 activity by phosphorylation of Thr160 and Tyr15. EMBO J. 1992;11:3995–4005. doi: 10.1002/j.1460-2075.1992.tb05493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall M, Bates S, Peters G. Evidence for different modes of action of cyclin-dependent kinase inhibitors: p15 and p16 bind to kinases, p21 and p27 bind to cyclins. Oncogene. 1995;11:1581–1588. [PubMed] [Google Scholar]
- Hannon GJ, Beach D. p15INK4B is a potential effector of TGF-beta-induced cell cycle arrest. Nature. 1994;371:257–261. doi: 10.1038/371257a0. [DOI] [PubMed] [Google Scholar]
- Hengst L, Reed SI. Inhibitors of the Cip/Kip family. Curr Top Microbiol Immunol. 1998;227:25–41. doi: 10.1007/978-3-642-71941-7_2. [DOI] [PubMed] [Google Scholar]
- Herrera RE, Makela TP, Weinberg RA. TGF beta-induced growth inhibition in primary fibroblasts requires the retinoblastoma protein. Mol Biol Cell. 1996;7:1335–1342. doi: 10.1091/mbc.7.9.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iavarone A, Massague J. Repression of the CDK activator Cdc25A and cell-cycle arrest by cytokine TGF-beta in cells lacking the CDK inhibitor p15. Nature. 1997;387:417–422. doi: 10.1038/387417a0. [DOI] [PubMed] [Google Scholar]
- Kato JY, Matsuoka M, Polyak K, Massague J, Sherr CJ. Cyclic AMP-induced G1 phase arrest mediated by an inhibitor (p27Kip1) of cyclin-dependent kinase 4 activation. Cell. 1994;79:487–496. doi: 10.1016/0092-8674(94)90257-7. [DOI] [PubMed] [Google Scholar]
- Kelly BL, Wolfe KG, Roberts JM. Identification of a substrate-targeting domain in cyclin E necessary for phosphorylation of the retinoblastoma protein. Proc Natl Acad Sci USA. 1998;95:2535–2540. doi: 10.1073/pnas.95.5.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa M, et al. The consensus motif for phosphorylation by cyclin D1-Cdk4 is different from that for phosphorylation by cyclin A/E-Cdk2. EMBO J. 1996;15:7060–7069. [PMC free article] [PubMed] [Google Scholar]
- Ko TC, Sheng HM, Reisman D, Thompson EA, Beauchamp RD. Transforming growth factor-beta 1 inhibits cyclin D1 expression in intestinal epithelial cells. Oncogene. 1995;10:177–184. [PubMed] [Google Scholar]
- Kretzschmar M, Doody J, Timokhina I, Massague J. A mechanism of repression of TGFbeta/Smad signaling by oncogenic Ras. Genes Dev. 1999;13:804–816. doi: 10.1101/gad.13.7.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L'Allemain G, Lavoie JN, Rivard N, Baldin V, Pouyssegur J. Cyclin D1 expression is a major target of the cAMP-induced inhibition of cell cycle entry in fibroblasts. Oncogene. 1997;14:1981–1990. doi: 10.1038/sj.onc.1201038. [DOI] [PubMed] [Google Scholar]
- LaBaer J, Garrett MD, Stevenson LF, Slingerland JM, Sandhu C, Chou HS, Fattaey A, Harlow E. New functional activities for the p21 family of CDK inhibitors. Genes Dev. 1997;11:847–862. doi: 10.1101/gad.11.7.847. [DOI] [PubMed] [Google Scholar]
- Laiho M, DeCaprio JA, Ludlow JW, Livingston DM, Massague J. Growth inhibition by TGF-beta linked to suppression of retinoblastoma protein phosphorylation. Cell. 1990;62:175–185. doi: 10.1016/0092-8674(90)90251-9. [DOI] [PubMed] [Google Scholar]
- Lamy F, Wilkin F, Baptist M, Posada J, Roger PP, Dumont JE. Phosphorylation of mitogen-activated protein kinases is involved in the epidermal growth factor and phorbol ester, but not in the thyrotropin/cAMP, thyroid mitogenics pathway. J Biol Chem. 1993;268:8398–8401. [PubMed] [Google Scholar]
- Liu F, Massague J. Dual role of the Smad4/DPC4 tumor suppressor in TGFbeta-inducible transcriptional complexes. Genes Dev. 1997;11:3157–3167. doi: 10.1101/gad.11.23.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan A, Smith C, Becks GP, Gonzalez AM, Phillips ID, Hill DJ. Enhanced expression of transforming growth factor-beta 1 during thyroid hyperplasia in rats. J Endocrinol. 1994;141:45–57. doi: 10.1677/joe.0.1410045. [DOI] [PubMed] [Google Scholar]
- Lukas J, Bartkova J, Bartek J. Convergence of mitogenic signaling cascades from diverse classes of receptors at the cyclin D-cyclin-dependent kinase-pRb-controlled G1 checkpoint. Mol Cell Biol. 1996;16:6917–6925. doi: 10.1128/mcb.16.12.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas J, Herzinger T, Hansen K, Moroni MC, Resnitzky D, Helin K, Reed SI, Bartek J. Cyclin E-induced S phase without activation of the pRb/E2F pathway. Genes Dev. 1997;11:1479–1492. doi: 10.1101/gad.11.11.1479. [DOI] [PubMed] [Google Scholar]
- Lundberg AS, Weinberg RA. Functional inactivation of the retinoblastoma protein requires sequential modification by at least two distinct cyclin–cdk complexes. Mol Cell Biol. 1998;18:753–761. doi: 10.1128/mcb.18.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons RM, Moses HL. Transforming growth factors and the regulation of cell proliferation. Eur J Biochem. 1990;187:467–473. doi: 10.1111/j.1432-1033.1990.tb15327.x. [DOI] [PubMed] [Google Scholar]
- Mahony D, Parry DA, Lees E. Active cdk6 complexes are predominantly nuclear and represent only a minority of the cdk6 in T cells. Oncogene. 1998;16:603–611. doi: 10.1038/sj.onc.1201570. [DOI] [PubMed] [Google Scholar]
- Massague J. TGF-beta signal transduction. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- Moore JD, Yang J, Truant R, Kornbluth S. Nuclear import of Cdk/cyclin complexes: identification of distinct mechanisms for import of Cdk2/cyclin E and Cdc2/cyclin B1. J Cell Biol. 1999;144:213–224. doi: 10.1083/jcb.144.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao A, et al. TGF-beta receptor-mediated signaling through Smad2, Smad3 and Smad4. EMBO J. 1997;16:5353–5362. doi: 10.1093/emboj/16.17.5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nourse J, Firpo E, Flanagan WM, Coats S, Polyak K, Lee MH, Massague J, Crabtree GR, Roberts JM. Interleukin-2-mediated elimination of the p27Kip1 cyclin-dependent kinase inhibitor prevented by rapamycin. Nature. 1994;372:570–573. doi: 10.1038/372570a0. [DOI] [PubMed] [Google Scholar]
- Ohtani K, DeGregori J, Nevins JR. Regulation of the cyclin E gene by transcription factor E2F1. Proc Natl Acad Sci USA. 1995;92:12146–12150. doi: 10.1073/pnas.92.26.12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano M, Tam SW, Theodoras AM, Beer-Romero P, Del Sal G, Chau V, Yew PR, Draetta GF, Rolfe M. Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science. 1995;269:682–685. doi: 10.1126/science.7624798. [DOI] [PubMed] [Google Scholar]
- Perez-Roger I, Kim SH, Griffiths B, Sewing A, Land H. Cyclins D1 and D2 mediate myc-induced proliferation via sequestration of p27Kip1 and p21Cip1. EMBO J. 1999;18:5310–5320. doi: 10.1093/emboj/18.19.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietenpol JA, Holt JT, Stein RW, Moses HL. Transforming growth factor beta 1 suppression of c-myc gene transcription: role in inhibition of keratinocyte proliferation. Proc Natl Acad Sci USA. 1990;87:3758–3762. doi: 10.1073/pnas.87.10.3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirson I, Coulonval K, Lamy F, Dumont JE. c-Myc expression is controlled by the mitogenic cAMP-cascade in thyrocytes. J Cell Physiol. 1996;168:59–70. doi: 10.1002/(SICI)1097-4652(199607)168:1<59::AID-JCP8>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Polyak K, Kato JY, Solomon MJ, Sherr CJ, Massague J, Roberts JM, Koff A. p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-beta and contact inhibition to cell cycle arrest. Genes Dev. 1994;8:9–22. doi: 10.1101/gad.8.1.9. [DOI] [PubMed] [Google Scholar]
- Polyak K. Negative regulation of cell growth by TGF beta. Biochim Biophys Acta. 1996;1242:185–199. doi: 10.1016/0304-419x(95)00009-5. [DOI] [PubMed] [Google Scholar]
- Poon RY, Toyoshima H, Hunter T. Redistribution of the CDK inhibitor p27 between different cyclin-CDK complexes in the mouse fibroblast cell cycle and in cells arrested with lovastatin or ultraviolet irradiation. Mol Biol Cell. 1995;6:1197–1213. doi: 10.1091/mbc.6.9.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnitzky D, Hengst L, Reed SI. Cyclin A-associated kinase activity is rate limiting for entrance into S phase and is negatively regulated in G1 by p27Kip1. Mol Cell Biol. 1995;15:4347–4352. doi: 10.1128/mcb.15.8.4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuse S, Pirson I, Dumont JE. Differential regulation of protooncogenes c-jun and jun D expressions by protein tyrosine kinase, protein kinase C, and cyclic-AMP mitogenic pathways in dog primary thyrocytes: TSH and cyclic-AMP induce proliferation but downregulate C-jun expression. Exp Cell Res. 1991;196:210–215. doi: 10.1016/0014-4827(91)90253-q. [DOI] [PubMed] [Google Scholar]
- Reynisdottir I, Polyak K, Iavarone A, Massague J. Kip/Cip and Ink4 Cdk inhibitors cooperate to induce cell cycle arrest in response to TGF-beta. Genes Dev. 1995;9:1831–1845. doi: 10.1101/gad.9.15.1831. [DOI] [PubMed] [Google Scholar]
- Reynisdottir I, Massague J. The subcellular locations of p15(Ink4b) and p27(Kip1) coordinate their inhibitory interactions with cdk4 and cdk2. Genes Dev. 1997;11:492–503. doi: 10.1101/gad.11.4.492. [DOI] [PubMed] [Google Scholar]
- Roberts AB, Anzano MA, Wakefield LM, Roche NS, Stern DF, Sporn MB. Type beta transforming growth factor: a bifunctional regulator of cellular growth. Proc Natl Acad Sci USA. 1985;82:119–123. doi: 10.1073/pnas.82.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger PP, Servais P, Dumont JE. Regulation of dog thyroid epithelial cell cycle by forskolin, an adenylate cyclase activator. Exp Cell Res. 1987a;172:282–292. doi: 10.1016/0014-4827(87)90387-9. [DOI] [PubMed] [Google Scholar]
- Roger PP, Servais P, Dumont JE. Induction of DNA synthesis in dog thyrocytes in primary culture: synergistic effects of thyrotropin and cyclic AMP with epidermal growth factor and insulin. J Cell Physiol. 1987b;130:58–67. doi: 10.1002/jcp.1041300110. [DOI] [PubMed] [Google Scholar]
- Roger PP, Baptist M, Dumont JE. A mechanism generating heterogeneity in thyroid epithelial cells: suppression of the thyrotropin/cAMP-dependent mitogenic pathway after cell division induced by cAMP-independent factors. J Cell Biol. 1992;117:383–393. doi: 10.1083/jcb.117.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger PP. Thyrotropin-dependent transforming growth factor beta expression in thyroid gland [comment] Eur J Endocrinol. 1996;134:269–271. doi: 10.1530/eje.0.1340269. [DOI] [PubMed] [Google Scholar]
- Roger PP, Demartin S, Dumont JE. Nature of the critical labile event that controls RB phosphorylation in the cyclic AMP-dependent cell cycle of thyrocytes in primary culture. Exp Cell Res. 1999;252:492–498. doi: 10.1006/excr.1999.4646. [DOI] [PubMed] [Google Scholar]
- Russo AA, Tong L, Lee JO, Jeffrey PD, Pavletich NP. Structural basis for inhibition of the cyclin-dependent kinase Cdk6 by the tumor suppressor p16INK4a. Nature. 1998;395:237–243. doi: 10.1038/26155. [DOI] [PubMed] [Google Scholar]
- Sandhu C, Garbe J, Bhattacharya N, Daksis J, Pan CH, Yaswen P, Koh J, Slingerland JM, Stampfer MR. Transforming growth factor beta stabilizes p15INK4B protein, increases p15INK4B-cdk4 complexes, and inhibits cyclin D1-cdk4 association in human mammary epithelial cells. Mol Cell Biol. 1997;17:2458–2467. doi: 10.1128/mcb.17.5.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JK, Bassing CH, Kovesdi I, Datto MB, Blazing M, George S, Wang XF, Nevins JR. Expression of the E2F1 transcription factor overcomes type beta transforming growth factor-mediated growth suppression. Proc Natl Acad Sci USA. 1995;92:483–487. doi: 10.1073/pnas.92.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr CJ. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- Smith P, Williams ED, Wynford-Thomas D. In vitro demonstration of a TSH-specific growth desensitizing mechanism in rat thyroid epithelium. Mol Cell Endocrinol. 1987;51:51–58. doi: 10.1016/0303-7207(87)90118-3. [DOI] [PubMed] [Google Scholar]
- Soos TJ, Kiyokawa H, Yan JS, Rubin MS, Giordano A, DeBlasio A, Bottega S, Wong B, Mendelsohn J, Koff A. Formation of p27-CDK complexes during the human mitotic cell cycle. Cell Growth Differ. 1996;7:135–146. [PubMed] [Google Scholar]
- Steiner P, Philipp A, Lukas J, Godden-Kent D, Pagano M, Mittnacht S, Bartek J, Eilers M. Identification of a Myc-dependent step during the formation of active G1 cyclin-cdk complexes. EMBO J. 1995;14:4814–4826. doi: 10.1002/j.1460-2075.1995.tb00163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassan JP, Schultz SJ, Bartek J, Nigg EA. Cell cycle analysis of the activity, subcellular localization, and subunit composition of human CAK (CDK-activating kinase) J Cell Biol. 1994;127:467–478. doi: 10.1083/jcb.127.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taton M, Lamy F, Roger PP, Dumont JE. General inhibition by transforming growth factor beta 1 of thyrotropin and cAMP responses in human thyroid cells in primary culture. Mol Cell Endocrinol. 1993;95:13–21. doi: 10.1016/0303-7207(93)90024-e. [DOI] [PubMed] [Google Scholar]
- Tsubari M, Taipale J, Tiihonen E, Keski-Oja J, Laiho M. Hepatocyte growth factor releases mink epithelial cells from transforming growth factor beta1-induced growth arrest by restoring Cdk6 expression and cyclin E-associated Cdk2 activity. Mol Cell Biol. 1999;19:3654–3663. doi: 10.1128/mcb.19.5.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsushima T, Arai M, Saji M, Ohba Y, Murakami H, Ohmura E, Sato K, Shizume K. Effects of transforming growth factor-beta on deoxyribonucleic acid synthesis and iodine metabolism in porcine thyroid cells in culture. Endocrinology. 1988;123:1187–1194. doi: 10.1210/endo-123-2-1187. [DOI] [PubMed] [Google Scholar]
- Van Keymeulen A, Bartek J, Dumont JE, Roger PP. Cyclin D3 accumulation and activity integrate and rank the comitogenic pathways of thyrotropin and insulin in thyrocytes in primary culture. Oncogene. 1999;18:7351–7359. doi: 10.1038/sj.onc.1203164. [DOI] [PubMed] [Google Scholar]
- Vlach J, Hennecke S, Alevizopoulos K, Conti D, Amati B. Growth arrest by the cyclin-dependent kinase inhibitor p27Kip1 is abrogated by c-Myc. EMBO J. 1996;15:6595–6604. [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Sicinski P, Weinberg RA, Zhang Y, Ravid K. Characterization of the mouse cyclin D3 gene: exon/intron organization and promoter activity. Genomics. 1996;35:156–163. doi: 10.1006/geno.1996.0334. [DOI] [PubMed] [Google Scholar]
- Ward AC, Csar XF, Hoffmann BW, Hamilton JA. Cyclic AMP inhibits expression of D-type cyclins and cdk4 and induces p27Kip1 in G-CSF-treated NFS-60 cells. Biochem Biophys Res Commun. 1996;224:10–16. doi: 10.1006/bbrc.1996.0976. [DOI] [PubMed] [Google Scholar]
- Warner BJ, Blain SW, Seoane J, Massagué J. Myc downregulation by transforming growth factor beta required for activation of the p15Ink4b G1 arrest pathway. Mol Cell Biol. 1999;19:5913–5922. doi: 10.1128/mcb.19.9.5913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg RA. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- Wynford-Thomas D, Stringer BM, Harach HR, Williams ED. Control of growth in the rat thyroid—an example of specific desensitization to trophic hormone stimulation. Experientia. 1983;39:421–423. doi: 10.1007/BF01963160. [DOI] [PubMed] [Google Scholar]
- Zerfass-Thome K, Schulze A, Zwerschke W, Vogt B, Helin K, Bartek J, Henglein B, Jansen-Durr P. p27KIP1 blocks cyclin E-dependent transactivation of cyclin A gene expression. Mol Cell Biol. 1997;17:407–415. doi: 10.1128/mcb.17.1.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Feng XH, Derynck R. Smad3 and Smad4 cooperate with c-Jun/c-Fos to mediate TGF-beta-induced transcription. Nature. 1998;394:909–913. doi: 10.1038/29814. [DOI] [PubMed] [Google Scholar]