Abstract
The analysis of the P-selectin/PSGL-1 catch-slip bond that is periodically driven by a detaching force predicts that in the frequency range on the order of 1 s−1 the bond lifetime undergoes significant changes with respect to both frequency and amplitude of the force. The result indicates how variations in the heart rate could have a substantial effect on leukocyte and lymphoid cell transport and adhesion to endothelial cells and platelets during inflammatory processes.
Noncovalent interactions among biological macromolecules underlie the structure and function of living organisms. Proteins of the selectin family facilitate leukocyte adhesion to endothelial cells and platelets, creating a particularly interesting and important example of such interactions. The adhesion between neutrophils and P-selectin on inflamed endothelium is of great physiological importance in the recruitment of flowing cells to the surrounding tissues (1). The highest affinity interaction with P-selectin is provided by the mucin-like transmembrane P-selectin glycoprotein ligand (PSGL-1) found on leukocytes and lymphoid cells.
The recent experiments (2,3) on PSGL-1 binding to P- and L-selectin receptors demonstrate highly nontrivial behavior, whereby the lifetime of the bond is increased by a detaching force. This catch-binding phenomenon was predicted theoretically a number of years ago (4). As the force grows above a certain threshold, the catch-bond transforms to the much more common slip-bond, in which a detaching force promotes bond dissociation. The enhancement of the bond lifetime at the catch-slip transition point can be >100-fold relative to the bond lifetime in the absence of the force (2,5,6).
One expects (1) that the catch-bond between P-, L-selectins, and PSGL-1 has evolved in response to the biological conditions in which it functions. The bond operates in blood flowing with velocities ranging from 0.03cm/s in capillaries to 30cm/s in aorta. The bond helps leukocytes attach and penetrate through blood vessel walls and combat inflammations. It is much easier for leukocytes to penetrate thin capillary walls than thick aorta walls, and, therefore, fewer leukocytes need to attach to the capillary walls. Multiple immobile leukocytes can block narrow capillaries, but not the wide aorta. The catch mechanism in which the binding increases with higher blood velocities may have developed to resolve these issues.
This report addresses a new and biologically very relevant aspect of the selectin:PSGL-1 bond. To date, the properties of the catch-slip complex have been investigated either under the action of a constant force (2,3) or a force that linearly grows with time (7,8). In contrast, the bond functions in vivo under blood flow that oscillates with the heartbeat's periodicity. It is, therefore, essential to establish whether the bond lifetime is sensitive to the frequency and amplitude of the periodic perturbation.
Both experimental, e.g., (9), and theoretical (10) results indicate that periodic perturbations can create unusual conditions in biological complexes. Hsiai et al. (9) determined how the oscillatory shear stress affects the ability of endothelial cells to induce adhesion and migration of flowing neutrophils. The theoretical study (10) of an ensemble of receptor-ligand bonds with two states subjected to a weak periodic perturbation found stochastic resonance, in which the bond distribution oscillates at a specific low frequency against an equilibrium background.
The biological catch-slip bond is well described by the two-pathway model (5,6) comprising a single bound state and two separate catch and slip barriers leading to the free state. The model assumes that the return to the bound state upon dissociation has low probability, which is the case in the atomic force microscopy (AFM) experiments (2,3,7,8) and can be expected in vivo, where leukocytes roll after the bond is broken. In contrast to Braun and Seifert (10), the periodic perturbation is not assumed to be small.
The probability P(t) that the complex remains in the bound state at time t obeys the equation
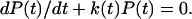 |
(1) |
The bond exists initially, i.e., 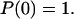 The bond dissociation rate in the two-pathway model equals
The bond dissociation rate in the two-pathway model equals 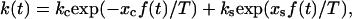 where
where 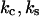 are the rates of dissociation through the catch and slip pathways in the absence of the force;
are the rates of dissociation through the catch and slip pathways in the absence of the force; 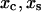 are the distances from the bound state potential energy minimum to the catch and slip barrier maxima; and
are the distances from the bound state potential energy minimum to the catch and slip barrier maxima; and 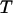 is temperature. The force oscillation
is temperature. The force oscillation 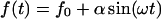 is characterized by amplitude
is characterized by amplitude 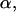 frequency
frequency 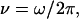 and force
and force 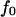 that describes the average effect of blood flow (11). A nonzero
that describes the average effect of blood flow (11). A nonzero 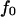 is often used in jump/ramp AFM experiments (7).
is often used in jump/ramp AFM experiments (7).
Although constant force and jump/ramp AFM data are typically represented as functions of force, it is more convenient to represent the current results versus time, because time and force cannot be given a one-to-one correspondence for periodic forces. The equation for the bond dissociation probability density 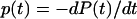 follows from Eq. 1:
follows from Eq. 1:
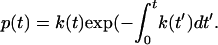 |
(2) |
The bond lifetime is defined by
 |
(3) |
and depends on force amplitude 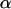 and frequency
and frequency 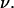
The parameters of the two-pathway model of the P-selectin:PSGL-1 bond have been determined (5) based on the dynamic force experimental data (7): 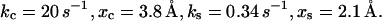
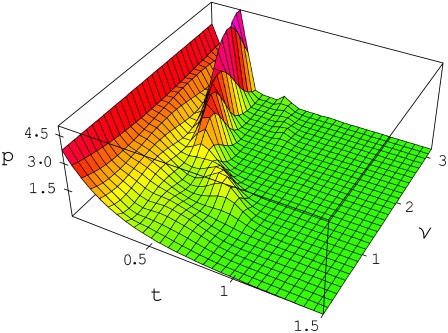
Fig. 1 shows the bond dissociation probability density, Eq. 2, versus time and frequency for  At zero frequency the probability density is monotonically decreasing with time. As the frequency grows, additional maxima appear starting around
At zero frequency the probability density is monotonically decreasing with time. As the frequency grows, additional maxima appear starting around 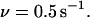 The new regions of increased dissociation probability density have a profound effect on the bond lifetime; Figs. 2–4.
The new regions of increased dissociation probability density have a profound effect on the bond lifetime; Figs. 2–4.
FIGURE 1 .
Bond dissociation probability density p defined by Eq. 2 as a function of time t, s and frequency ν, s−1of the applied force.
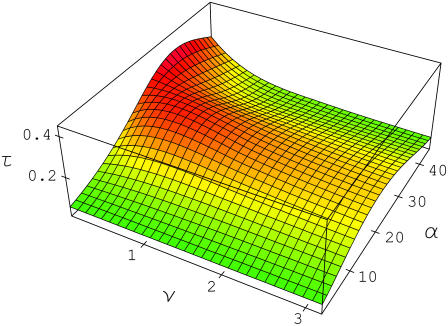
FIGURE 2 .
Bond lifetime 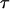 defined by Eq. 3 as a function of frequency ν, s−1 and amplitude
defined by Eq. 3 as a function of frequency ν, s−1 and amplitude 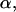 pN of the applied force; τ is maximized at ν = 0.5 s−1 and α = 21.5 pN. The solid red curves in Figs. 3 and 4 show sections of τ in this point.
pN of the applied force; τ is maximized at ν = 0.5 s−1 and α = 21.5 pN. The solid red curves in Figs. 3 and 4 show sections of τ in this point.
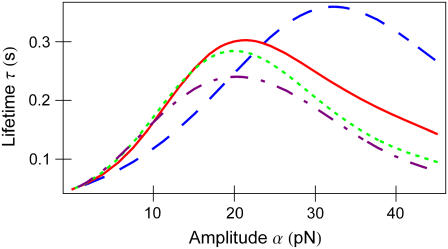
FIGURE 3 .
Bond lifetime 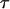 versus force amplitude
versus force amplitude 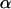 for frequencies ν = 0 s−1 (blue dash), 0.5 s−1 (red solid), 1 s−1 (green dot), and 1.6 s−1 (purple dash-dot).
for frequencies ν = 0 s−1 (blue dash), 0.5 s−1 (red solid), 1 s−1 (green dot), and 1.6 s−1 (purple dash-dot).
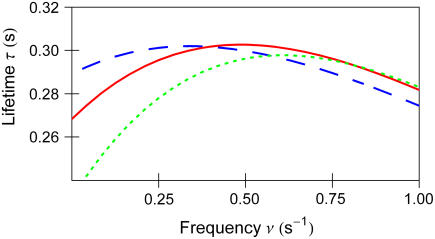
FIGURE 4 .
Bond lifetime 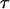 versus force frequency ν for force amplitude α = f0 = 23pN (blue dash), 21.5 pN (red solid), 19 pN (green dot).
versus force frequency ν for force amplitude α = f0 = 23pN (blue dash), 21.5 pN (red solid), 19 pN (green dot).
In the stationary force regime 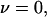 the bond lifetime, Eq. 3, is largest at
the bond lifetime, Eq. 3, is largest at 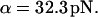 As the force begins to oscillate, the maximum lifetime rapidly shifts to lower forces. Already at
As the force begins to oscillate, the maximum lifetime rapidly shifts to lower forces. Already at 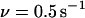 the bond lifetime is maximized with the force amplitude of
the bond lifetime is maximized with the force amplitude of 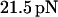 that is two-thirds of its zero frequency value. Further frequency increase bears little effect on the optimal force value, as emphasized in Fig. 3. Considering the bond lifetime as a function of frequency for amplitudes fixed around
that is two-thirds of its zero frequency value. Further frequency increase bears little effect on the optimal force value, as emphasized in Fig. 3. Considering the bond lifetime as a function of frequency for amplitudes fixed around 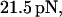 one observes a well-pronounced maximum that appears at the frequency of about
one observes a well-pronounced maximum that appears at the frequency of about 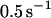 ; Fig. 4.
; Fig. 4.
In summary, the presented theoretical analysis indicates that a periodically driven P-selectin:PSGL-1 catch-slip bond is unusually sensitive to the driving frequency within the physiologically relevant range. Two types of stochastic resonance conditions have been established. First, the force required to maximize the bond lifetime rapidly decreases as the frequency grows from 0 to 30 beats per minute. Second, for oscillating force, the lifetime is maximized at the frequency close to 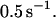 Incidentally, the lowest human heart-beat rate known with long-distance athletes at rest is around this value. The frequency effects predicted for a single P-selectin:PSGL-1 bond will be significantly accentuated with multiple bonds that occur in vivo between flowing cells and surrounding tissues. Provided that the nature of the shear flows near blood vessel walls is properly described by the AFM data used in present, the reported results indicate that changes in the heart rate should have a major effect on leukocyte and lymphoid cell transport and adhesion to endothelial cells and platelets during inflammatory processes.
Incidentally, the lowest human heart-beat rate known with long-distance athletes at rest is around this value. The frequency effects predicted for a single P-selectin:PSGL-1 bond will be significantly accentuated with multiple bonds that occur in vivo between flowing cells and surrounding tissues. Provided that the nature of the shear flows near blood vessel walls is properly described by the AFM data used in present, the reported results indicate that changes in the heart rate should have a major effect on leukocyte and lymphoid cell transport and adhesion to endothelial cells and platelets during inflammatory processes.
Acknowledgments
The authors are thankful to Evgeni Sokurenko, Wendy Thomas, and Anatoly Kolomeisky for fruitful discussions.
Financial support from the National Science Foundation (grant No. CHE-0094012), Petroleum Research Fund of the American Chemical Society (grant No. 150393), and National Institutes of Health Bioengineering Research Partnership (grant No. R01 AI050940) is gratefully acknowledged.
References
- 1.Zhu, C., J. Lou, and R. P. McEver. 2005. Catch bonds: physical models, structural bases, biological function and rheological relevance. Biorheology. 42:443–462. [PubMed] [Google Scholar]
- 2.Marshall, B. T., M. Long, J. W. Piper, T. Yago, R. P. McEver, and C. Zhu. 2003. Direct observation of catch bonds involving cell-adhesion molecules. Nature. 423:190–193. [DOI] [PubMed] [Google Scholar]
- 3.Sarangapani, K. K., T. Yago, A. G. Klopocki, M. B. Lawrence, C. B. Fieger, S. D. Rosen, R. P. McEver, and C. Zhu. 2004. Low force decelerates L-selectin dissociation from P-selectin glycoprotein ligand-1 and endoglycan. J. Biol. Chem. 279:2291–2298. [DOI] [PubMed] [Google Scholar]
- 4.Dembo, M., D. C. Torney, K. Saxman, and D. Hammer. 1988. The reaction-limited kinetics of membrane-to-surface adhesion and detachment. Proc. R. Soc. Lond. B. Biol. Sci. 234:55–83. [DOI] [PubMed] [Google Scholar]
- 5.Pereverzev, Y. V., O. V. Prezhdo, M. Forero, E. Sokurenko, and W. Thomas. 2005. The two-pathway model for the catch-slip transition in biological adhesion. Biophys. J. 89:1446–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pereverzev, Y. V., O. V. Prezhdo, W. E. Thomas, and E. V. Sokurenko. 2005. Distinctive features of the biological catch bond in the jump-ramp force regime predicted by the two-pathway model. Phys. Rev. E. 72:010903. [DOI] [PubMed] [Google Scholar]
- 7.Evans, E., A. Leung, V. Heinrich, and C. Zhu. 2004. Mechanical switching and coupling between two dissociation pathways in a P-selectin adhesion bond. Proc. Natl. Acad. Sci. USA. 101:11281–11286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marshall, B. T., K. K. Sarangapani, J. Lou, R. P. McEver, and C. Zhu. 2005. Force history dependence of receptor-ligand dissociation. Biophys. J. 88:1458–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsiai, T. K., S. K. Cho, P. K. Wong, M. Ing, A. Salazar, A. Sevanian, M. Navab, L. L. Demer, and C. M. Ho. 2003. Monocyte recruitment to endothelial cells in response to oscillatory shear stress. FASEB J. 17:1648–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braun, O., and U. Seifert. 2004. Periodically driven stochastic un- and refolding transitions of biopolymers. Europhys. Lett. 68:746–752. [Google Scholar]
- 11.Jadhav, S., C. D. Eggleton, and K. Konstantopoulos. 2005. A 3-D computational model predicts that cell deformation affects selectin-mediated leukocyte rolling. Biophys. J. 88:96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]