Abstract
Objective
To examine the extent to which maternal prenatal smoking is associated with adiposity, central adiposity, and blood pressure in 3-year-old children.
Research Methods and Procedures
We studied 746 mother-child pairs in Project Viva, a prospective cohort study, and categorized mothers as never, early pregnancy, or former smokers. Main outcome measures were overweight (BMI for age and sex > 85th percentile), BMI z-score, sum of subscapular (SS) and triceps (TR) skinfolds, SS:TR skinfold ratio, and systolic blood pressure (SBP).
Results
One hundred sixty-one (22%) mothers quit smoking before pregnancy, 71 (10%) smoked in early pregnancy, and 514 (69%) never smoked. At age 3 years, 204 (27%) children were overweight. On multivariable analysis, compared with children of never smokers, children of early pregnancy smokers had an elevated risk for overweight [odds ratio (OR), 2.2; 95% confidence interval (CI), 1.2, 3.9] and higher BMI z-score (0.30 units; 95% CI, 0.05, 0.55), SS + TR (2.0 mm; 95% CI, 0.9, 3.0), and SBP (2.4 mm Hg; 95% CI, −0.1, 4.9). Children of former smokers were not more overweight (BMI z-score, 0.02 units; 95% CI, −0.15, 0.19) but had higher SBP (1.5 mm Hg; 95% CI, −0.1, 3.2). We saw no relationship of smoking with central adiposity (SS:TR).
Discussion
Former and early pregnancy smokers had children with somewhat higher SBP, but only early pregnancy smokers had children who were more overweight. Mechanisms linking smoking with child adiposity and blood pressure may differ. A long-term impact of maternal smoking on offspring cardiovascular risk provides further reason to reduce smoking in women.
Keywords: pregnancy, smoking, blood pressure, central obesity
Introduction
The prevalence of obesity has continued to increase over recent decades among both children and adults, a trend that may presage reduced life expectancy in the United States (1). A new paradigm for understanding health risks such as obesity and cardiovascular disease has emerged in recent years, evolved from the notion that environmental factors in early life and even in utero can profoundly influence lifelong health. Maternal smoking during pregnancy is one such early life exposure that may have a persistent influence on offspring body size and cardiovascular health.
Several investigators have reported an increased risk of obesity in childhood or adulthood after intrauterine exposure to maternal cigarette smoking (2–8). This association seems paradoxical, given that maternal smoking during mid- to late pregnancy reduces fetal growth (9), and babies born smaller tend to have lower BMI and lower risk of overweight in childhood and adulthood (10). A smaller number of studies have also reported that maternal smoking during pregnancy is associated with child blood pressure, particularly in later childhood (11–13).
The influence of prenatal cigarette exposure on offspring health merits additional study. Recent nationally representative data suggest that approximately one-third of U.S. women smoked within a year before becoming pregnant, and 11% smoke during pregnancy (14,15). Many women may quit smoking when planning a pregnancy (15), yet the influence of preconceptional smoking on offspring outcomes is not known. Exposure to maternal smoking in early pregnancy has not been well studied, because in previous studies, the timing of smoking was not assessed, or if it was, most mothers smoked throughout pregnancy. Furthermore, to our knowledge, no studies have yet examined whether maternal prenatal smoking is associated with the offspring’s central adiposity, which itself is associated with both lower birth weight and higher attained blood pressure (10).
In this study, we used data from a prospective prenatal cohort study to examine the extent to which maternal smoking before pregnancy and during early pregnancy is associated with overall adiposity, central adiposity, and blood pressure among children at 3 years of age.
Research Methods and Procedures
Population and Study Design
Study subjects were participants in Project Viva, a prospective cohort study of pregnant women and their offspring. Research assistants recruited women attending their initial prenatal visit at one of eight urban and suburban offices of a multispecialty group practice in eastern Massachusetts (16). Eligibility criteria included fluency in English, gestational age <22 weeks, and singleton pregnancy. We enrolled 64% of eligible women (n = 2341), of whom 9% withdrew or were lost to follow-up, leaving 2128 subjects who delivered a live infant. We obtained informed consent from all mothers. Institutional review boards of participating institutions approved the study. All procedures were in accordance with the ethical standards established by the Declaration of Helsinki (17).
We periodically freeze data in Project Viva for analysis; this paper includes data available as of February 15, 2005. Of the 2128 births, 1293 children were enrolled for study continuation beyond age 6 months and reached 3 years of age, and we completed in-person visits with 812 by the time of the data freeze. Excluding those missing information on parental BMI, gestational weight gain, or outcomes, we have complete data on both maternal prenatal smoking and child BMI for 746 participants, smoking and skinfold thicknesses for 707, and smoking and blood pressure for 689. Using a “missing” category, we included participants missing information on income (6%), glucose tolerance (1%), breastfeeding (8%), introduction of solids (16%), television (6%), fast food (4%), and sugar-sweetened beverages (5%).
We performed in-person study visits with the mother after her clinic appointments after the first and second trimesters of pregnancy, with both mother and child in the hospital after delivery, and at 6 months and 3 years postpartum in a research office or at home. Participants completed mailed questionnaires at 1 and 2 years postpartum, on which they updated child feeding practices and reported child weight and length measured at the most recent clinical visit.
Assessment of Exposures and Covariates
At the first study visit, we asked mothers whether they had ever smoked, and categorized those women who had smoked >100 cigarettes in their lifetime as ever smokers. We asked ever smokers to report the number of years and average packs per day that they smoked, from which we calculated lifetime pack years. We then asked ever smokers whether they had smoked in the 3 months before learning they were pregnant. We categorized as former smokers those who had ever smoked but quit before 3 months before learning of pregnancy. We classified those who smoked during the 3 months before learning of pregnancy as early pregnancy smokers. We asked early pregnancy smokers to report in five categories the average number of cigarettes they currently smoked. We also asked about exposure to environmental cigarette smoke at home, work, and in public. At each subsequent study visit, we updated maternal smoking. We classified early pregnancy smokers who reported smoking at the second trimester as continued smokers.
Using a combination of questionnaires and interviews, we collected information about maternal race/ethnicity, age, education, parity, and household income. We obtained information from the prenatal medical record on maternal blood pressure, glucose tolerance test results, serial pregnancy weights, and infant birth weight and delivery date. Mothers reported their prepregnancy weight and height and paternal weight and height. We calculated gestational weight gain as the difference between prepregnancy weight and the last clinically recorded weight before delivery. We calculated gestational age from the last menstrual period or from the second trimester ultrasound if the two estimates differed by >10 days. We determined sex-specific birth weight for gestational age (fetal growth) percentile and z-value based on U.S. national natality data (18) and defined small for gestational age as birth weight for gestational age below the 10th percentile.
On the 6-month and 1-year questionnaires, mothers reported on infant diet including breast and formula feeding and complementary food intake. At the 3-year visit, mothers completed a semiquantitative food frequency questionnaire regarding the child’s diet during the previous month, including consumption of 91 foods and 7 beverages. We included intake of fast food and sugar-sweetened beverages as covariates in this analysis, because these factors have been most strongly associated with overweight (19,20). Mothers also reported the average number of hours the child spent watching television on both weekdays and weekends.
Assessment of 3-Year Outcomes
We measured children’s heights and weights using a calibrated stadiometer (Shorr Productions, Olney, MD) and scale (Seca model 881; Seca Corp., Hanover, MD). We calculated age- and sex-specific BMI percentiles and z-scores using U.S. national reference data (21). We defined overweight as BMI for age and sex >85th percentile. We measured subscapular (SS)1 and triceps (TR) skinfold thicknesses using Holtain calipers (Holtain, Cross-well, United Kingdom), and calculated the sum (SS + TR) and the ratio (SS:TR) of skinfolds. BMI and SS + TR represent overall adiposity, whereas SS:TR is a measure of central or truncal adiposity. Research assistants followed standardized techniques (22) and participated in biannual in-service training to ensure measurement validity (IJ Shorr; Shorr Productions). Inter- and intra-rater measurement error were within published reference ranges for all measurements (23).
Using biannually calibrated Dinamap Pro-100 oscillometric automated monitors (GE Medical Services, Tampa, FL), trained research assistants recorded child blood pressure up to five times at 1-minute intervals. We recorded conditions of measurement including order of readings, cuff size, limb, and child position and activity. Our primary outcome for blood pressure analyses was systolic blood pressure (SBP) at 3 years of age. We used systolic rather than diastolic blood pressure because of the validity of its measurement and its superior prediction of later blood pressure (24).
Statistical Analysis
We compared characteristics of early pregnancy smokers and former smokers with never smokers using Student’s t tests and χ2 analysis. We used multivariable logistic regression to examine independent associations of maternal smoking with child overweight and linear regression to examine associations of smoking with the continuous outcomes BMI z-score, SS + TR, and SS:TR. For SBP, we used mixed effect regression models, incorporating each of the up to five measurements per child as repeated outcome measures (25). Mixed effect models weight subjects based on the number of measurements and their variability and, thus, yield more appropriate SEs than linear regression. We considered as covariates parental and child factors that might be independent predictors of the exposure or outcomes or might confound associations of smoking with outcomes. We include covariates modeled in Table 1.
Table 1.
Prevalence of characteristics by maternal smoking status among 746 participants in Project Viva
| Characteristics | Never smokedN= 514 [mean (SD) or percent] | Former smoker, quit before pregnancyN= 161 [mean (SD) or percent] | Smoked in early pregnancyN= 71 [mean (SD) or percent] | Former vs. never smokers (p) | Early pregnancy vs. never smokers (p) |
|---|---|---|---|---|---|
| Maternal sociodemographics | |||||
| Age (years) | 32.7 (4.8) | 33.8 (4.1) | 29.7 (5.2) | 0.005 | <0.0001 |
| College graduate | 77% | 76% | 45% | 0.74 | <0.0001 |
| Married | 87% | 92% | 61% | 0.10 | <0.0001 |
| Household income <$40,000/yr* | 11% | 4% | 23% | 0.02 | 0.001 |
| Parous | 49% | 61% | 44% | 0.007 | 0.36 |
| White | 72% | 89% | 69% | <0.0001 | 0.55 |
| Parental characteristics | |||||
| Maternal BMI (kg/m2) | 24.5 (5.0) | 24.8 (5.2) | 25.9 (6.2) | 0.43 | 0.06 |
| Gestational weight gain (pounds) | 33.3 (11.3) | 34.9 (12.4) | 38.2 (14.5) | 0.13 | 0.007 |
| Paternal BMI (kg/m2) | 26.3 (4.1) | 26.8 (3.4) | 26.6 (3.6) | 0.13 | 0.55 |
| Maternal third trimester SBP (mm Hg) | 111 (8.8) | 111 (8.5) | 113 (7.8) | 0.94 | 0.16 |
| Abnormal glucose tolerance | 17% | 20% | 15% | 0.81 | 0.13 |
| Infant/child characteristics | |||||
| SGA (<10th percentile) | 5% | 3% | 7% | 0.23 | 0.59 |
| Birth weight (kg) | 3.46 (0.57) | 3.55 (0.53) | 3.46 (0.60) | 0.06 | 0.70 |
| Preterm (<37 weeks) | 8% | 6% | 7% | 0.28 | 0.74 |
| Solids introduced <4 months* | 10% | 14% | 30% | 0.36 | <0.0001 |
| Breastfeeding at 6 months | 0.38 | 0.0002 | |||
| Breast only | 24% | 20% | 7% | ||
| Mixed | 26% | 26% | 17% | ||
| Weaned | 36% | 34% | 46% | ||
| Formula only | 7% | 10% | 15% | ||
| ≥2 hours of television/d* | 50% | 57% | 52% | 0.37 | 0.77 |
| Fast food ≥ 1 time/mo* | 69% | 66% | 77% | 0.73 | 0.15 |
| No sugar-sweetened beverages in the past month* | 40% | 38% | 34% | 0.22 | 0.36 |
| 3-year child outcomes† | |||||
| BMI ≥ 85th percentile | 25% | 27% | 45% | 0.68 | 0.0004 |
| BMI z-score | 0.41 (0.96) | 0.49 (1.02) | 0.86 (1.31) | 0.38 | 0.007 |
| SS + TR skinfolds (mm) | 16 (4) | 17 (4) | 18 (6) | 0.13 | 0.007 |
| SS:TR skinfold ratio | 0.66 (0.15) | 0.66 (0.16) | 0.67 (0.16) | 0.33 | 0.57 |
| SBP (mm Hg) | 92 (11) | 94 (10) | 94 (10) | 0.14 | 0.18 |
SGA, small for gestational age.
Missing data for income (6%), abnormal glucose tolerance (1%), breastfeeding (8%), solids (16%), television (6%), fast food (5%), and sugar-sweetened beverages (4%).
BMI available for 746, skinfolds for 707, and blood pressure for 689 children.
We performed all analyses using SAS version 8.2 (SAS Institute, Cary, NC).
Results
Among the 746 mothers, 232 (31%) had ever smoked, of whom 161 (22%) quit before pregnancy and 71 (10%) smoked during early pregnancy. Only 13 of the 71 early pregnancy smokers reported smoking beyond the first trimester. The majority of early pregnancy smokers reported smoking 1 to 4 (37%) or 5 to 14 (34%) cigarettes per day. Ever smokers reported a mean of 4 pack-years (range, <1 to 30 pack-years), and early pregnancy smokers reported a mean of 5 pack-years (range, <1 to 25 pack-years).
Mothers who smoked in early pregnancy were younger, less educated, less likely to be married, and had lower household income compared with never smokers (Table 1). Smoking mothers were more overweight and gained more weight during pregnancy. Children of mothers who smoked in early pregnancy were not more likely to be born preterm or small for gestational age, but they were breast fed for a shorter duration and had earlier introduction of solid foods. Compared with never smokers, mothers who had previously smoked but quit before the 3 months before conception were older and more likely to be white, parous, and have higher income, but did not differ in BMI or gestational weight gain (Table 1). Child television viewing, fast food intake, and sugar-sweetened beverages did not differ by maternal smoking status.
One-half (50%) of the children were boys, and 204 (27%) children were overweight (BMI > 85th percentile) at 3 years. Compared with children of never smokers, children of early pregnancy smokers had higher mean BMI and sum of SS + TR skinfolds and higher prevalence of obesity but did not have different SS:TR (Table 1). Mean SBP was slightly higher among children of early pregnancy and former smokers than children of never smokers (Table 1).
On multivariable analysis, maternal early pregnancy smoking was strongly associated with overweight. Compared with children of never smokers, among those exposed to smoking in early pregnancy, the adjusted odds ratio (OR) for overweight was 2.2 [95% confidence interval (CI), 1.2, 3.9]. In contrast, children of mothers who had quit smoking before pregnancy were not more overweight (multivariable OR, 1.0; 95% CI, 0.7, 1.6) compared with children of never smokers.
Maternal smoking in early pregnancy was also directly associated with the continuous outcomes BMI z-score (Figure 1; Table 2) and the sum of SS + TR skinfolds. These associations were somewhat attenuated after adjustment for potential confounders (Table 2). It seemed that maternal weight characteristics accounted for most of this attenuation: for example, after adjustment for maternal prepregnancy BMI and gestational weight gain, the effect estimate for early pregnancy smoking on BMI z-score went from 0.43 (95% CI, 0.18, 0.68) to 0.30 (95% CI, 0.06, 0.55) but did not change appreciably further after adjustment for maternal race/ethnicity, education, age, paternal BMI, and fetal growth (0.30; 95% CI, 0.05, 0.55). Mothers who smoked in early pregnancy had children who were heavier at 3 years (0.7 kg; 95% CI, 0.2, 1.2) but not different in height (0.3 cm; 95% CI, −0.7, 1.2), suggesting that the influence of smoking on BMI was mediated by alterations in weight alone. Early pregnancy smoking was not associated with the ratio of SS:TR skinfolds (Table 2).
Figure 1.
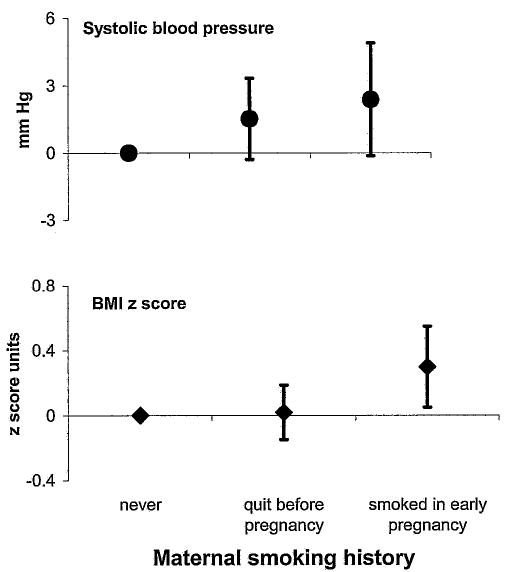
Child SBP and BMI z-score at 3 years of age, comparing children whose mothers smoked in early pregnancy and whose mothers previously smoked but quit before pregnancy with children of never smokers.
Table 2.
Associations of maternal prenatal smoking with child BMI z-score, SS + TR, SS:TR, and SBP at age 3 years
| BMI z-score | SS + TR skinfolds (mm) | SS:TR ratio | SBP (mm Hg) | |
|---|---|---|---|---|
| Smoked in early pregnancy | ||||
| Age and sex adjusted* | 0.43 (0.18, 0.68) | 2.2 (1.1, 3.2) | 0.01 (−0.03, 0.05) | 1.8 (−0.7, 4.2) |
| Multivariable† ‡ | 0.30 (0.05, 0.55) | 2.0 (0.9, 3.0) | 0.001 (−0.04, 0.04) | 2.4 (−0.1, 4.9) |
| Multivariable + age 3 BMI z-score | — | — | −0.001 (−0.04, 0.04) | 1.5 (−1.0, 3.9) |
| Quit smoking before pregnancy | ||||
| Age and sex adjusted* | 0.08 (−0.10, 0.25) | 0.5 (−0.2, 1.2) | 0.004 (−0.02, 0.04) | 1.4 (−0.2, 3.0) |
| Multivariable† ‡ | 0.02 (−0.15, 0.19) | 0.4 (−0.3, 1.1) | 0.01 (−0.02, 0.04) | 1.5 (−0.1, 3.2) |
| Multivariable + age 3 BMI z-score | — | — | 0.01 (−0.02, 0.04) | 1.4 (−0.2, 3.0) |
Values are effect estimate (95% CI).
Adjusted for child sex and age; SBP is additionally adjusted for measurement conditions.
BMI z-score, SS + TR, and SS:TR are additionally adjusted for paternal BMI; maternal prepregnancy BMI, gestational weight gain, education, income, race/ethnicity, and parity, and fetal growth and gestation length.
SBP is additionally adjusted for maternal age, third trimester SBP, education, income, race/ethnicity, and parity, fetal growth and gestation length, and child height.
We next studied factors that might serve as intermediates in the pathway between maternal early pregnancy smoking and child adiposity. Inclusion into the multivariable model of glucose tolerance, breastfeeding, early introduction of solid foods, and child behaviors at age 3 (television viewing, consumption of sugar-sweetened beverages and fast food) did not alter observed associations of early pregnancy smoking with BMI z-score (effect estimate adjusted for all factors listed previously, 0.30; 95% CI, 0.04, 0.56). We measured weight and length on 294 participants at 6 months of age. On an analysis limited to this subset of participants, inclusion of change in weight for length z-score from birth to 6 months in the multivariable analysis also did not markedly change the effect estimate for early pregnancy smoking on BMI z-score at 3 years (data not shown).
We observed evidence of a dose effect of smoking on 3-year adiposity. Among children of early pregnancy smokers, BMI z-score was 0.57 units lower among those who had smoked <1 pack-year in their lifetime compared with those who had smoked 10 or more pack-years (p = 0.02). Among former smokers, the number of pack-years smoked was not associated with age 3 BMI z-score. We saw no association of the number of daily cigarettes smoked during early pregnancy with 3-year adiposity, perhaps because of the limited range of the exposure (83% of participants smoked <10 cigarettes daily). Early pregnancy smokers were more likely to report exposure to environmental tobacco smoke in early pregnancy (42% compared with 13% for both former and never smokers). We saw no evidence for an independent effect of environmental tobacco smoke on child adiposity or blood pressure (data not shown).
The small number of women who continued smoking past the first trimester did not allow multivariable analysis of exposure to maternal smoking throughout pregnancy. However, as anticipated, both the prevalence of small for gestational age (14%) and mean unadjusted offspring age 3 BMI z-score (0.83 units) were both higher among these 13 participants than among never smokers (5% and 0.41 units, respectively). Excluding these women, the effect of early pregnancy smoking on child BMI z-score at 3 years remained (0.27 units; 95% CI, 0.003, 0.54).
We obtained SBP measurements from 689 children. After adjustment for blood pressure measurement conditions, maternal sociodemographics, mean third trimester SBP, fetal growth, gestation length, and child sex, age, and height, SBP was 2.4 mm Hg (95% CI, −0.01, 4.9) higher among children of smokers than among children of never smokers (Figure 1; Table 2). After including child BMI, the effect of smoking was reduced to 1.5 mm Hg (95% CI, −1.0, 3.9), suggesting that higher adiposity among offspring of smokers was partially but not entirely responsible for the elevation in blood pressure.
Smoking before pregnancy was not associated with overall (BMI z-score, SS + TR) or central (SS:TR) adiposity (Table 2). Among children of former smokers, estimates of age 3 SBP appeared higher than among children of never smokers (1.5 mm Hg; 95% CI, −0.1, 3.2; Figure 1), an effect minimally changed after adjustment for child BMI (1.4 mm Hg; 95% CI, −0.2, 3.0; Table 2).
Discussion
In this prospective U.S. study, maternal smoking during early pregnancy was directly associated with child adiposity, measured by both BMI and sum of skinfolds, with evidence of a dose–effect relationship. At age 3 years, children of women who smoked in early pregnancy were heavier, but not shorter, than children of women who had never smoked. Smoking before pregnancy was not associated with adiposity. We saw no evidence of an association of maternal prenatal smoking with central distribution of adiposity. Maternal smoking in early pregnancy and even before pregnancy was somewhat associated with higher SBP at age 3.
Both in this study cohort and many other populations, women who smoke in pregnancy tend to have different sociodemographic and anthropometric characteristics and to feed their children differently than non-smokers. Because many of these factors may also be associated with risk for child obesity or hypertension, concern exists that observed associations may reflect sociodemographic confounding rather than a causal relationship (7,12). However, in this study, as in others, adjustment for factors such as maternal education, race/ethnicity, income, and child diet only minimally influenced effect sizes. To our knowledge, this is the first prospective U.S. cohort to report associations of maternal smoking with child outcomes. Because the associations of prenatal smoking with child obesity and elevated blood pressure have now been shown in several populations in different countries, it becomes increasingly likely that these associations have a biological basis. Additionally, data from experimental animal studies confirm these observational human results (26).
It does not seem that smoking influences later obesity risk by means of an influence on fetal growth. Smoking in early pregnancy, as in this population, does not necessarily influence size at birth, whereas women who smoke throughout pregnancy or only in the third trimester have smaller babies (27). Even in studies in which maternal smoking has been associated with both lower birth weight and increased later size, adjustment for size at birth either strengthened or did not alter the observed association (3,8,28). In addition, lower birth weight is associated with central but not overall adiposity (10,29). In this study, we did not observe an association of smoking with central adiposity, measured by the SS:TR skinfold ratio, which has been found to be a valid proxy for intra-abdominal adipose tissue among children (30). It is possible that an association of prenatal smoking with central adiposity might appear only after age 3.
Maternal smoking may influence size by means of an influence on hypothalamic centers that direct appetite and activity. In adults, smoking is associated with lower body weight, and smoking cessation typically produces weight gain from a combination of increased intake and decreased energy expenditure, although the physiology underlying these weight effects is not yet well understood (31). Animal studies suggest that nicotine is the component of cigarette smoke that is primarily responsible for influences on body weight (26,31,32).
Also in this study, children of mothers who smoked in early pregnancy and even before pregnancy had somewhat higher SBP at age 3. Although CIs did not exclude zero, the magnitude of the effect (1.5 mm Hg for early pregnancy smokers, after adjustment for child height and BMI) was similar to previous studies with narrower CIs, in which the effect was even greater at age 6 (1.2 mm Hg) than at age 3 (1.0 mm Hg) (13). Previous investigators have reported higher SBP in children exposed to prenatal smoking, although in these studies, mothers smoked throughout pregnancy, and children were born at lower birth weight (13). Others have not found an association of smoking with child blood pressure (33). To our knowledge, this is the first report that maternal prenatal smoking might have a sustained influence on child blood pressure that did not act through restricted growth. The association of former smoking with offspring blood pressure has not previously been reported. Our results suggest that smoking may have a persistent influence on offspring blood pressure even if the mother quits months before pregnancy.
How might exposure to cigarettes or nicotine during pregnancy, and even before pregnancy as in this study, program offspring blood pressure? Nicotine may have a direct effect on the development of the fetal renal, cardiovascular, or nervous systems, as well as an indirect effect by means of changes in maternal vasculature, thereby affecting placental formation and blood flow (34). In a recent study, investigators injected pregnant rats with nicotine or saline throughout pregnancy and lactation (26). Offspring of nicotine-treated rats had elevated body and fat pad weight and altered function of the perivascular adipose tissue, which influences arterial contractility. Other investigators have shown elevated blood pressure in the offspring of spontaneously hypertensive rats prenatally exposed to nicotine (34). Further experimental studies may help elucidate pathways by which smoking or nicotine exposure before pregnancy might program later blood pressure.
Our study has many strengths. We collected longitudinal data beginning in early pregnancy. Previous studies of the relationship between smoking and child outcomes are mainly retrospective or cross-sectional, with smoking habits recalled as many as 6 to 9 years after pregnancy (4–6), although some have been prospective (3,8). We collected detailed information on a large number of potential biological, demographic, and nutritional predictors of adiposity and blood pressure. We measured child height and weight as well as skinfolds. Most previous studies used BMI as the primary outcome, although one group also had information on the sum of skinfolds (7,8); none have reported on skinfold ratio or central adiposity.
Several limitations to this study also exist. Study participants were relatively older and well educated, and all resided in eastern Massachusetts, so findings may not be able to be generalized to other populations. We assessed smoking by questionnaire and did not biochemically validate this exposure. Social desirability concerns may have caused under-reporting of smoking behavior. As with all observational studies, unmeasured systematic differences between smokers and non-smokers that are associated with development of outcomes could explain our findings.
In conclusion, using data from a prospective U.S.-based study, we observed a direct association of maternal smoking before pregnancy with offspring blood pressure and of maternal smoking in early pregnancy with both child adiposity and blood pressure. Maternal smoking during pregnancy is one of a few identified modifiable prenatal risk factors for obesity and cardiovascular risk. A long-term impact of maternal smoking on offspring cardiovascular risk provides further reason to support programs to reduce smoking in women of reproductive age.
Acknowledgments
This project was supported by grants from the NIH (HD34568, HL64925, HL68041, and HD44807), the Robert H. Ebert Fellowship, the March of Dimes Birth Defects Foundation, and Harvard Medical School and the Harvard Pilgrim Health Care Foundation. We appreciate the invaluable contributions of Sheryl Rifas-Shiman and the staff and participants of Project Viva.
Footnotes
Nonstandard abbreviations: SS, subscapular; TR, triceps; SPB, systolic blood pressure; OR, odds ratio; CI, confidence interval.
References
- 1.Olshanksy SJ, Passaro DJ, Hershow RC, et al. A potential decline in life expectancy in the United States in the 21st century. N Engl J Med. 2005;352:1138–45. doi: 10.1056/NEJMsr043743. [DOI] [PubMed] [Google Scholar]
- 2.Montgomery SM, Ekbom A. Smoking during pregnancy and diabetes mellitus in a British longitudinal birth cohort. BMJ. 2002;324:26–7. doi: 10.1136/bmj.324.7328.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Power C, Jefferis BJ. Fetal environment and subsequent obesity: a study of maternal smoking. Int J Epidemiol. 2002;31:413–9. [PubMed] [Google Scholar]
- 4.Toschke AM, Koletzko B, Slikker W, Jr, Hermann M, von Kries R. Childhood obesity is associated with maternal smoking in pregnancy. Eur J Pediatr. 2002;161:445–8. doi: 10.1007/s00431-002-0983-z. [DOI] [PubMed] [Google Scholar]
- 5.von Kries R, Toschke AM, Koletzko B, Slikker W., Jr Maternal smoking during pregnancy and childhood obesity. Am J Epidemiol. 2002;156:954–61. doi: 10.1093/aje/kwf128. [DOI] [PubMed] [Google Scholar]
- 6.Toschke AM, Montgomery SM, Pfeiffer U, von Kries R. Early intrauterine exposure to tobacco-inhaled products and obesity. Am J Epidemiol. 2003;158:1068–74. doi: 10.1093/aje/kwg258. [DOI] [PubMed] [Google Scholar]
- 7.Vik T, Jacobsen G, Vatten L, Bakketeig LS. Pre- and post-natal growth in children of women who smoked in pregnancy. Early Hum Dev. 1996;45:245–55. doi: 10.1016/0378-3782(96)01735-5. [DOI] [PubMed] [Google Scholar]
- 8.Wideroe M, Vik T, Jacobsen G, Bakketeig LS. Does maternal smoking during pregnancy cause childhood overweight? Paediatr Perinat Epidemiol. 2003;17:171–9. doi: 10.1046/j.1365-3016.2003.00481.x. [DOI] [PubMed] [Google Scholar]
- 9.Kramer MS. Intrauterine growth and gestational duration determinants. Pediatrics. 1987;80:502–11. [PubMed] [Google Scholar]
- 10.Oken E, Gillman MW. Fetal origins of obesity. Obes Res. 2003;11:496–506. doi: 10.1038/oby.2003.69. [DOI] [PubMed] [Google Scholar]
- 11.Morley R, Leeson Payne C, Lister G, Lucas A. Maternal smoking and blood pressure in 7.5 to 8 year old offspring. Arch Dis Child. 1995;72:120–4. doi: 10.1136/adc.72.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams S, Poulton R. Twins and maternal smoking: ordeals for the fetal origins hypothesis? BMJ. 1999;318:897–900. doi: 10.1136/bmj.318.7188.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blake KV, Gurrin LC, Evans SF, et al. Maternal cigarette smoking during pregnancy, low birth weight and subsequent blood pressure in early childhood. Early Hum Dev. 2000;57:137–47. doi: 10.1016/s0378-3782(99)00064-x. [DOI] [PubMed] [Google Scholar]
- 14.Hamilton BE, Martin JA, Sutton PD. Births: preliminary data for 2003. Natl Vital Stat Rep. 2004;53:1–18. [PubMed] [Google Scholar]
- 15.Kahn RS, Certain L, Whitaker RC. A reexamination of smoking before, during, and after pregnancy. Am J Public Health. 2002;92:1801–8. doi: 10.2105/ajph.92.11.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gillman MW, Rich-Edwards JW, Rifas-Shiman SL, Lieberman ES, Kleinman KP, Lipshultz SE. Maternal age and other predictors of newborn blood pressure. J Pediatr. 2004;144:240–5. doi: 10.1016/j.jpeds.2003.10.064. [DOI] [PubMed] [Google Scholar]
- 17.World Medical Association. Recommendations guiding physicians in biomedical research involving human subjects. JAMA. 1997;277:925–6. [PubMed] [Google Scholar]
- 18.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. doi: 10.1186/1471-2431-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ludwig DS, Peterson KE, Gortmaker SL. Relation between consumption of sugar-sweetened drinks and childhood obesity. Lancet. 2001;357:505–8. doi: 10.1016/S0140-6736(00)04041-1. [DOI] [PubMed] [Google Scholar]
- 20.Bowman SA, Gortmaker SL, Ebbeling CB, Pereira MA, Ludwig DS. Effects of fast-food consumption on energy intake and diet quality among children in a national household survey. Pediatrics. 2004;113:112–8. doi: 10.1542/peds.113.1.112. [DOI] [PubMed] [Google Scholar]
- 21.National Center for Health Statistics. CDC Growth Charts, United States. Available at http://www.cdc.gov/growthcharts/ Accessed July 20, 2004.
- 22.Shorr IJ.How to Weigh and Measure Children. New York: United Nations; 1986.
- 23.Mueller WH, Martorell R. Reliability and accuracy of measurement. In: Lohman TG, Roche AF, Martorell R, eds. Anthropometric Standardization Reference Manual. Champaign, IL: Human Kinetics Books; 1988.
- 24.Gillman MW, Cook NR. Blood pressure measurement in childhood epidemiological studies. Circulation. 1995;92:1049–57. doi: 10.1161/01.cir.92.4.1049. [DOI] [PubMed] [Google Scholar]
- 25.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–74. [PubMed] [Google Scholar]
- 26.Gao Y-J, Holloway AC, Zeng Z, et al. Prenatal exposure to nicotine causes postnatal obesity and altered perivascular adipose tissue function. Obes Res. 2005;13:1–6. doi: 10.1038/oby.2005.77. [DOI] [PubMed] [Google Scholar]
- 27.Lieberman E, Gremy I, Lang JM, Cohen AP. Low birth-weight at term and the timing of fetal exposure to maternal smoking. Am J Public Health. 1994;84:1127–31. doi: 10.2105/ajph.84.7.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Meulen J. Maternal smoking during pregnancy and obesity in the offspring. Int J Epidemiol. 2002;31:420–1. doi: 10.1093/ije/31.2.420. [DOI] [PubMed] [Google Scholar]
- 29.Parsons TJ, Power C, Logan S, Summerbell CD. Childhood predictors of adult obesity. Int J Obes Relat Metab Disord. 1999;23(Suppl 8):S1–107. [PubMed] [Google Scholar]
- 30.Goran MI, Kaskoun M, Shuman WP. Intra-abdominal adipose tissue in young children. Int J Obes Relat Metab Disord. 1995;19:279–83. [PubMed] [Google Scholar]
- 31.Li MD, Parker SL, Kane JK. Regulation of feeding-associated peptides and receptors by nicotine. Mol Neurobiol. 2000;22:143–65. doi: 10.1385/MN:22:1-3:143. [DOI] [PubMed] [Google Scholar]
- 32.Chen WJ, Kelly RB. Effect of prenatal or perinatal nicotine exposure on neonatal thyroid status and offspring growth in rats. Life Sci. 2005;76:1249–58. doi: 10.1016/j.lfs.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 33.Law CM, Barker DJ, Bull AR, Osmond C. Maternal and fetal influences on blood pressure. Arch Dis Child. 1991;66:1291–5. doi: 10.1136/adc.66.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pausova Z, Paus T, Sedova L, Berube J. Prenatal exposure to nicotine modifies kidney weight and blood pressure in genetically susceptible rats. Kidney Int. 2003;64:829–35. doi: 10.1046/j.1523-1755.2003.00172.x. [DOI] [PubMed] [Google Scholar]


