Abstract
This study tested whether chronic oral estrogen could improve memory and alter neural plasticity in the hippocampus and neocortex of middle-aged female mice. Ovariectomized C57BL/6 mice were administered 1,000, 1,500, or 2,500 nM 17β-estradiol in drinking water for 5 weeks prior to and during spatial and object memory testing. Synaptophysin, nerve growth factor (NGF), and brain-derived neurotrophic factor (BDNF) levels were then measured in hippocampus and neocortex. The medium dose impaired spatial reference memory in the radial-arm maze, whereas all doses improved object recognition. The high dose increased hippocampal synaptophysin and NGF levels, whereas the medium dose decreased these neocortical levels. The high dose decreased neocortical BDNF levels. These data suggest that chronic oral estrogen selectively affects memory and neural function in middle-aged female mice.
Menopause has been associated with memory loss and an increased risk of Alzheimer's disease (Henderson, 1997; Kawas et al., 1997; Paganini-Hill & Henderson, 1994). Although estrogen replacement reportedly improves verbal (Phillips & Sherwin, 1992; Sherwin, 1988) and spatial working memory (Duff & Hampson, 2000) in nondemented postmenopausal women, and reduces the risk of Alzheimer's disease (e.g., Henderson, 1997), many studies report little to no beneficial effect of estrogen on cognition (for a review, see Hogervorst, Williams, Budge, Riedel, & Jolles, 2000; Sherwin, 2002). Rodent models are particularly useful in examining estrogen replacement because, similar to humans (e.g., Evans, Brennan, Skorpanich, & Held, 1984; Moffat, Zonderman, & Resnick, 2001; Sharps & Gollin, 1987), both male and female rodents exhibit age-related declines in hippocampus-dependent spatial memory (e.g., Fordyce & Wehner, 1993; Frick, Baxter, Markowska, Olton, & Price, 1995; Lamberty & Gower, 1991; Markowska, 1999). The onset of mnemonic decline in middle-aged female rats (Markowska, 1999) and mice (Frick et al., 1995) is concomitant with the cessation of the estrous cycle.
Evidence suggests that various estrogen replacement regimens (e.g., short-term treatments typically ranging from 5 days to 2 weeks, and chronic treatments typically lasting from 3 weeks to several months) can ameliorate age-related memory dysfunction in aging female rodents. In middle-aged and aged rats (Foster, Sharrow, Kumar, & Masse, 2003; Gibbs, 2000; Markham, Pych, & Juraska, 2002; Markowska & Savonenko, 2002) and mice (Frick, Fernandez, & Bulinski, 2002; Miller et al., 1999; Vaucher et al., 2002), short-term and chronic estrogen treatment improves spatial reference memory in the Morris water maze (Foster et al., 2003; Frick et al., 2002; Markham et al., 2002; Markowska & Savonenko, 2002); spatial working memory in a T maze (Gibbs, 2000); and measures of nonspatial memory, including performance in an object recognition task (Miller et al., 1999; Vaucher et al., 2002).
Potentially underlying cognitive benefits are estrogen-induced alterations in the morphology and physiology of brain regions integral for learning and memory, such as the neocortex and hippocampus. In both young and aged female rats, estrogen increases hippocampal spine density (Adams, Shah, Janssen, & Morrison, 2001; Miranda, Williams, & Einstein, 1999; Woolley & McEwen, 1992, 1993), which declines with aging (Adams et al., 2001) and estrogen deprivation (Miranda et al., 1999). In young female mice, estrogen promotes spine synapse maturation in the hippocampus, which is associated with facilitated spatial memory (Li et al., 2004). Estrogen also alters levels of synaptophysin, a 38-kDa membrane-bound presynaptic vesicle protein (Jahn, Schiebler, Ouimet, & Greengard, 1985; Wiedenmann & Franke, 1985). In both aged nondemented humans (Eastwood, Burnet, McDonald, Clinton, & Harrison, 1994; X. Liu, Erikson, & Brun, 1996; Masliah, Mallory, Hansen, DeTeresa, & Terry, 1993) and Alzheimer's disease patients (X. Liu et al., 1996; Sze et al., 1997; Zhan, Beyreuther, & Schmitt, 1993), synaptophysin is reduced in the hippocampus and several cortical regions, the latter being associated with cognitive dysfunction (Terry et al., 1991). Age-related reductions are also seen in rodents (Chen, Masliah, Mallory, & Gage, 1995; Davies et al., 2003; Saito et al., 1994), although other studies have reported conserved levels with aging (Calhoun et al., 1998; Frick et al., 2002; Nicolle, Gallagher, & McKinney, 1999). Estrogen exposure increases synaptophysin expression in hippocampal slices and cultured neurons (Murphy & Segal, 1996; Pozzo-Miller, Inoue, & Murphy, 1999; Stone, Rozovsky, Morgan, Anderson, & Finch, 1998). In aged female mice, short-term estrogen treatment increases hippocampal and cortical levels of synaptophysin (Frick et al., 2002), and hippocampal increases are associated with improvements in spatial reference memory (Frick et al., 2002).
Synaptic plasticity may also be mediated by estrogen-induced alterations of the neurotrophins nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF). These related polypeptides, which are integral to neocortical and hippocampal synaptic function (Poo, 2001; Vicario-Abejon, Owens, McKay, & Segal, 2002), undergo age-related changes in expression that are associated with spatial memory deficits (Bimonte, Nelson, & Granholm, 2003; Schaaf et al., 2001; Sugaya et al., 1998; although see Croll, Ip, Lindsay, & Wiegand, 1998) and are altered in patients with Alzheimer's disease (for a review, see Siegel & Chauhan, 2000). In cultured hippocampal neurons, estrogen reduces levels of BDNF protein, giving rise to a decrease in inhibitory GABAergic transmission, which allows for increases in dendritic spine density (Murphy, Cole, & Segal, 1998). In young female rats, acute (1–2 days) estrogen treatment increases BDNF mRNA in the hippocampus and cortex (Gibbs, 1999; Sohrabji, Miranda, & Toran-Allerand, 1995), although other studies have reported decreases (Cavus & Duman, 2003) or no effects (Cavus & Duman, 2003; Gibbs, 1998, 1999) on either BDNF mRNA or protein following acute or short-term treatment. Following chronic treatment in young female rats, cortical decreases (Jezierski & Sohrabji, 2000) or no changes (Singh, Meyer, & Simpkins, 1995) in BDNF mRNA have been reported, whereas chronic treatment increases hippocampal BDNF mRNA (Berchtold, Kesslak, Pike, Adlard, & Cotman, 2001; Singh et al., 1995). Fewer studies have examined the effects of estrogen on NGF expression. In young female rats, acute and short-term estrogen administration has been reported to either decrease (Gibbs, Wu, Hersh, & Pfaff, 1994) or have no effect (Gibbs, 1998) on NGF mRNA in the hippocampus, whereas chronic treatment has no effect on cortical NGF mRNA (Jezierski & Sohrabji, 2000). Although the aforementioned studies indicate that estrogen in young females affects NGF and BDNF, it is unclear whether these changes are associated with mnemonic alterations.
In middle-aged females, the effects of estrogen on neurotrophin expression and synaptic plasticity in conjunction with memory have yet to be investigated. The effects of chronic estrogen treatment on memory and neurobiological factors in middle-aged females is of particular interest because the majority of studies reporting cognitive benefits of estrogen in postmenopausal women utilize chronic treatments ranging in duration from several months (e.g., Phillips & Sherwin, 1992) to years (e.g., Duff & Hampson, 2000). Therefore, the present study was designed to examine the effects of chronic, oral 17β-estradiol administration on spatial working and reference memory, and nonspatial object memory, as well as hippocampal and cortical levels of synaptophysin, NGF, and BDNF in ovariectomized middle-aged female mice. Oral estrogen, the most common route of administration in humans, has yet to be investigated as a cognitive enhancer in aging mice. Unlike other methods of administration (such as capsule implantation or injection), estrogen given orally in mice does not produce tonically elevated levels of estrogen, is rapidly metabolized to control levels, and does not introduce stress associated with administration (Gordon, Osterburg, May, & Finch, 1986). Ovariectomized female mice, 16–17 months of age, were tested in a water-escape motivated radial-arm maze (WRAM) and object recognition task. To examine whether estrogen-induced mnemonic alterations were associated with changes in synaptic plasticity and neurotrophin expression, levels of the presynaptic protein synaptophysin and the neurotrophins NGF and BDNF were measured in the hippocampus and frontoparietal cortex at the completion of behavioral testing.
Method
Subjects
Subjects were 35 middle-aged (16–17 months) female C57BL/6 mice obtained from the National Institutes on Aging colony at Harlan Sprague–Dawley (Indianapolis, IN). Mice were handled 5 min/day for 5 days prior to behavioral testing. Up to 5 mice per cage were housed in a room with a 12:12-hr light–dark cycle (lights on at 0700), and food and water were provided ad libitum. At least 1 week prior to the start of treatment, all mice were ovariectomized. Behavioral testing took place after 1300 to allow estrogen levels in treatment mice to return to baseline following evening elevations (Gordon et al., 1986) and avoid differences in estradiol levels between mice during testing. All procedures followed the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and were approved by the Animal Care and Use Committee of Yale University.
Ovariectomy
Mice were ovariectomized at least 1 week prior to the start of treatment (Frick & Berger-Sweeney, 2001). Mice were anesthetized with 2% isoflurane gas in 100% oxygen, and their ovaries, oviducts, and tips of the uterine horn were bilaterally removed via two dorsal incisions. For 1 week following surgery, mice were individually housed and received approximately 1% acetaminophen in their drinking water. After 1 week, mice were rehoused in groups of up to 5 mice per cage.
Estrogen Administration
17β-estradiol (E2) was dissolved at a 20 mM concentration in 100% ethyl alcohol (EtOH). This stock was then dissolved in distilled water and administered to the mice at concentrations of 1,000 nM (n = 8), 1,500 nM (n = 9), or 2,500 nM (n = 8). Doses of E2 ingested by the mice were calculated in the following manner (Gordon et al., 1986): The amount of water (in milliliters) consumed by a cage (average for 1 week) was divided by the sum of the weights of the mice in that cage to yield the number of milliliters of water consumed per kilogram body weight per day. This value was then multiplied by the estrogen concentration in the water (μg E2/ml H2O) to yield a value for micrograms of E2 per kilogram body weight per day. Mice receiving 1,000, 1,500, or 2,500 nM E2 ingested doses of about 70, 110, or 180 μg/kg per day, respectively, approximately corresponding to levels of 15, 23, and 38 pg/ml, respectively (Stone et al., 1998). Estrogen levels during the estrous cycle in young mice typically range from 10–40 pg/ml (Nelson, Karelus, Bergman, & Felicio, 1995). Thus, the doses administered likely produced a range of low to high physiological levels of estrogen. As a result of circadian patterns in drinking behavior, oral estrogen administration does not produce tonically elevated levels of circulating estrogen. Rather, elevations occur during peak nocturnal drinking periods, and levels return to baseline as soon as 15 hr later (Gordon et al., 1986). Control mice (n = 10) received an equivalent amount of EtOH in their drinking water (1%), but did not receive any estrogen. All water treatments began 5 weeks prior to behavioral testing and continued throughout an additional 3 weeks of testing until the mice were killed. Water was changed weekly.
WRAM
This task (adapted from Bimonte, Hyde, Hoplight, & Denenberg, 2000) simultaneously assesses spatial working and reference memory and began at the start of Week 6 of estrogen treatment. The apparatus and procedure have been described elsewhere (Gresack & Frick, 2003). Eight clear Plexiglas arms (38 cm × 12 cm) radiated equidistantly from the opaque center of the maze (44 cm in diameter). The maze was placed in a white circular tank (97 cm in diameter) filled with water (24 ± 2 °C) and made opaque with nontoxic white tempera paint. Various extramaze cues were present around the room. Four arms contained hidden escape platforms that were submerged 0.5 cm below the surface of the water. One of the arms was designated as the start arm and never contained a platform. Each mouse received a different sequence of semirandom platform locations (platforms were never located in more than two consecutive adjacent arms) that remained the same within a mouse throughout the duration of the task.
Prior to the first test session, mice completed a five-trial shaping procedure to habituate them to the task and teach them to use the platform to escape from the water. During shaping, one arm (the shaping arm) contained a platform that was positioned slightly above the surface of the water and made visible with tape. During the first four trials, entrances to all eight arms were blocked off and the mouse was confined to the shaping arm. During the first trial, the mouse was placed on the platform for 15 s. During Trials 2–4, the mouse was placed at progressively further distances from the platform. During the fifth trial, the entrance to the shaping arm was opened and the mouse was placed in the center of the maze. Mice were given 30 s to locate the platform, and were gently guided to it if this time was exceeded. No data were collected during shaping.
Testing began the day after shaping. Each mouse completed four consecutive trials per day for 15 consecutive days (one session a day) as previously described (Gresack & Frick, 2003). At the start of Trial 1, the mouse was placed in the start arm and given 120 s to locate a platform. The mouse was allowed to remain on the platform for 10 s and then was placed in a holding cage for a 30-s intertrial interval (ITI). If the mouse failed to find a platform, it was guided to the nearest platform. The found platform was then removed from the maze, and three more trials were conducted in the same manner until only one platform remained in Trial 4. At the completion of Trial 4, the mouse was gently dried with a towel and returned to its home cage.
Three types of errors were recorded during each trial of each session, and then totaled for each session (e.g., Bimonte et al., 2000; Gresack & Frick, 2003). Mice were considered to have entered an arm when the entire body (excluding the tail) entered at least halfway into an arm. Within each trial, initial reference memory errors were recorded the first time the mouse entered an arm that never contained a platform. Repeated reference memory errors were recorded when a mouse reentered an arm that never contained a platform. Working memory errors were recorded when the mouse reentered an arm in which a platform had been removed during a previous trial in the session. In addition, working memory errors made in each trial from Trials 2–4 (working memory errors cannot be committed in Trial 1) were also determined. This analysis assessed working memory ability as the amount of working memory information across trials (i.e., working memory load) increased (e.g., Bimonte et al., 2000; Gresack & Frick, 2003). These values were averaged for Trials 2–4 across all sessions of interest.
Object Recognition
This task (Baker & Kim, 2002; Clark, Zola, & Squire, 2000; Frick & Gresack, 2003) assesses nonspatial object memory and consisted of a habituation, sample, and choice phase conducted on separate days (as described in Frick & Gresack, 2003). The task began at the start of Week 8 of estrogen treatment. The testing apparatus consisted of a white open field box (60 cm × 60 cm × 47 cm high) viewed from above by a video camera suspended from the ceiling. The camera was connected to a video recorder, monitor, and computer located in an adjacent room. During testing, mice were observed on the monitor, and a custom-written computer program was used to record the duration (time) and frequency (visits) to the objects. Exploration was recorded when the front paws and/or nose of the mouse were in contact with the object. Mice were first habituated to the open, empty white box by allowing them to freely explore for 5 min. No data were recorded during habituation. Twenty-four hours later, each mouse was placed in the empty box for 1 min of additional habituation. The mouse was then placed in a holding cage adjacent to the testing box while two identical objects were placed in the northeast and northwest corners of the box, approximately 5 cm from the walls. The mouse was then immediately placed back in the box facing the middle of the south wall and allowed to freely investigate the objects until it accumulated a total of 30 s exploring the two objects, at which point the sample phase trial was terminated and the mouse returned to its home cage. The use of 30 s of total exploration time rather than a fixed trial duration is advantageous because it minimizes group differences in activity (Frick & Gresack, 2003). Forty-eight hours later, mice completed the choice phase of the task. This phase was run identically to the sample phase, except that a novel object was substituted for one of the identical objects used in the sample phase. The location of the novel object (northeast or northwest corner) was counter-balanced across mice. For both phases, the objects and the box were cleaned with 70% EtOH between mice.
Neurochemical Assays
At the completion of behavioral testing (Week 9), mice were briefly sedated with CO2 and decapitated (Berger-Sweeney, Berger, Sharma, & Paul, 1994). The frontoparietal cortex and hippocampus were immediately dissected bilaterally on ice. The tissue samples were weighed and stored at −70 °C until homogenization. Following brain dissections, uteri were collected from each mouse and weighed.
Tissue samples were resuspended 1:10 (wt/vol) in 0.02% Triton X-100 in 0.1 mM Tris pH 7.4 (Tris/Triton) and homogenized with a probe sonicator. For the synaptophysin assay, samples were then further diluted 1:20 with Tris/Triton. For the BDNF and NGF assays, samples were further diluted 1:20 in lysis buffer (137 mM NaCl, 20 mM Tris-HCl, 1% Tergitol NP-40, 10% glycerol, 1 mM phenylmethanesulfonyl fluoride, 10 μg/ml aprotinin, 1 μg/ml leupeptin, 0.5 mM sodium metavanadate). All samples were then centrifuged for 10 min at 10,000 G. The supernatant was collected and stored at −70 °C until the day of assay. The total protein content of the samples was measured with a Bradford protein assay (Bradford, 1976).
Synaptophysin Assay
Synaptophysin was measured with an antibody sandwich enzyme-linked immunosorbent assay (Frick, Stearns, Pan, & Berger-Sweeney, 2003) using monoclonal anti-synaptophysin Clone SY 38 and polyclonal rabbit anti-synaptophysin antibodies (DAKO Corp., Carpinteria, CA). Samples were diluted 1:32,000 from the crude extract and assayed in triplicate. Purified synaptophysin was not available for use as a standard. Therefore, sample synaptophysin levels are expressed as “equivalents” relative to synaptophysin immunoreactivity from whole mouse brain homogenates (“mouse brain standard,” or MBS), which were used as standards. Optical density was measured at a wavelength of 405 nm on a Labsystems Multiskan Plus microplate reader. The average absorbance of the triplicate wells containing no MBS was subtracted from each reading.
The relative amount of synaptophysin in each sample was determined with the equation of the line generated by plotting the average absorbances of four MBS concentrations versus the log of the total MBS protein concentration. The resulting sample values represent the concentration of MBS that would have the same absorbance value. The sample values were then normalized according to total protein content by dividing by the total protein concentration of the sample.
Neurotrophin Assays
Levels of NGF and BDNF proteins were measured with commercially available kits (NGF and BDNF Emax ImmunoAssay System; Promega, Madison, WI). To assay NGF, samples were first diluted 1:50 in Dulbecco's phosphate-buffered saline (2.7 mM KCl, 0.137 mM NaCl, 1.47 nM KH2PO4, 8.1 mM Na2HPO4, 0.5 mM MgCl2, 0.9 mM CaCl2; pH 7.4), then diluted 1:3200 using Block and Sample Buffer (provided by the kit). Anti-NGF polyclonal, anti-NGF monoclonal, and anti-rat IgG horseradish peroxidase conjugate antibodies were used, and known concentrations of NGF (0–500 pg/ml) were used as standards. Samples were assayed in triplicate according to kit instructions. To assay BDNF, samples were diluted 1:50 in Dulbecco's phosphate-buffered saline, then acidified (to < pH 3.0) by adding 2% 1N HCl per volume, vortexed, left at room temperature for 15 min, and then neutralized with 1N NaOH. Samples were then diluted 1:200 in Block and Sample Buffer. Anti-BDNF monoclonal, anti-human BDNF polyclonal, and anti-IgY horseradish peroxidase conjugate antibodies were used, and known concentrations of BDNF (0–500 pg/ml) were used as standards. Samples were assayed in triplicate according to kit instructions. The absorbances exhibited by each sample were read at a wavelength of 450 nm. The sample NGF and BDNF values were normalized according to total protein content by dividing by the total protein concentration of the sample to yield values of nanograms NGF or BDNF per milligram of total protein.
Data Analysis
In the WRAM, initial reference memory, repeated reference memory, and working memory errors were analyzed separately with a one-way (treatment) repeated-measures (sessions) analysis of variance (ANOVA; Statview, SAS Institute, Cary, NC). Working memory errors made in each trial were analyzed with a one-way (treatment) repeated-measures (trials) ANOVA. The 1st day of testing (Session 1) was considered a training day (e.g., Gresack & Frick, 2003), as this is the initial exposure to the entire maze, the locations of the platformed arms, and the concept that platforms disappear once located. Data from this session were not considered to be accurate reflections of working and reference memory and were excluded from all analyses. Therefore, working and reference memory were analyzed from Sessions 2–15. In addition, previous studies show that the most substantial amounts of learning occur during task acquisition, defined as the first half of the task (Sessions 2–8; Bimonte et al., 2000; Frick et al., 2003; Hyde, Sherman, & Denenberg, 2000; Hyde, Sherman, Hoplight, & Denenberg, 2000). Thus, separate one-way (treatment) repeated-measures (sessions) ANOVAs were conducted for Sessions 2–8 and Sessions 9–15.
In the object recognition task, separate one-sample t tests (SPSS; SPSS, Chicago, IL) were performed for each group to determine if the time spent with each object differed from 15 s (chance value for time spent with either object), indicating a preference for the novel object over the familiar object (Baker & Kim, 2002; Frick & Gresack, 2003). These analyses were used because the time spent with each object to accumulate 30 s is not independent; time spent with one object reduces the time spent with the other object. To assess group differences in number of visits to the objects, a repeated-measures (object) ANOVA was performed. Separate one-way ANOVAs without repeated measures were conducted for each brain region for the synaptophysin, NGF, and BDNF assays, and for uterine weight. Fisher's protected least significant difference post hoc tests were performed to reveal between-group differences.
Results
Subjects
All subjects appeared in good health throughout the experiment. Dermatitis was present in several mice but did not produce any discernable behavioral effects. Two 1,500 nM E2 mice were excluded from the uterine weight analysis because of missing data, resulting in the following samples sizes: control (n = 10), 1,000 nM E2 (n = 8), 1,500 nM E2 (n = 7), 2,500 nM E2 (n = 8). One control mouse was excluded from WRAM testing because of an inability to adequately perform the task. The sample sizes for the WRAM were as follows: control (n = 9), 1,000 nM E2 (n = 8), 1,500 nM E2 (n = 9), 2,500 nM E2 (n = 8). Two mice (control, 1,000 nM E2) were excluded from the object recognition data analysis as a result of their failure to explore objects in the sample phase of testing. Thus, the sample sizes included in the object recognition data analysis were as follows: control (n = 9), 1,000 nM E2 (n = 7), 1,500 nM E2 (n = 9), 2,500 nM E2 (n = 8). Two mice (control, 2,500 nM E2) were excluded from the NGF neocortex analysis, and 3 mice (control, 1,000 nM, 2,500 nM E2) were excluded from the NGF hippocampus analysis because sample values for these subjects were statistical outliers (> 2 standard deviations from the group mean). Thus, the sample sizes for the NGF assays were as follows: neocortex: control (n = 9), 1,000 nM E2 (n = 8), 1,500 nM E2 (n = 9), 2,500 nM E2 (n = 7); hippocampus: control (n = 9), 1,000 nM E2 (n = 7), 1,500 nM E2 (n = 9), 2,500 nM E2 (n = 7). All mice were included in the synaptophysin and BDNF data analyses, therefore, the samples sizes for these assays were as follows: control (n = 10), 1,000 nM E2 (n = 8), 1,500 nM E2 (n = 9), 2,500 nM E2 (n = 8).
Uterine Weights
The uterine weights differed significantly among the groups, F(3, 32) = 26.89, p < .0001. Mean (±SEM) uterine weights were as follows: control = 0.02 ± 0.004 g; 1,000 nM E2 = 0.25 ± 0.044 g; 1,500 nM E2 = 0.24 ± 0.01 g; 2,500 nM E2 = 0.24 ± 0.12 g. All doses of E2 significantly increased uterine weight relative to controls (ps < .0001), whereas the E2-treated groups did not differ from each other.
WRAM
Initial reference memory errors
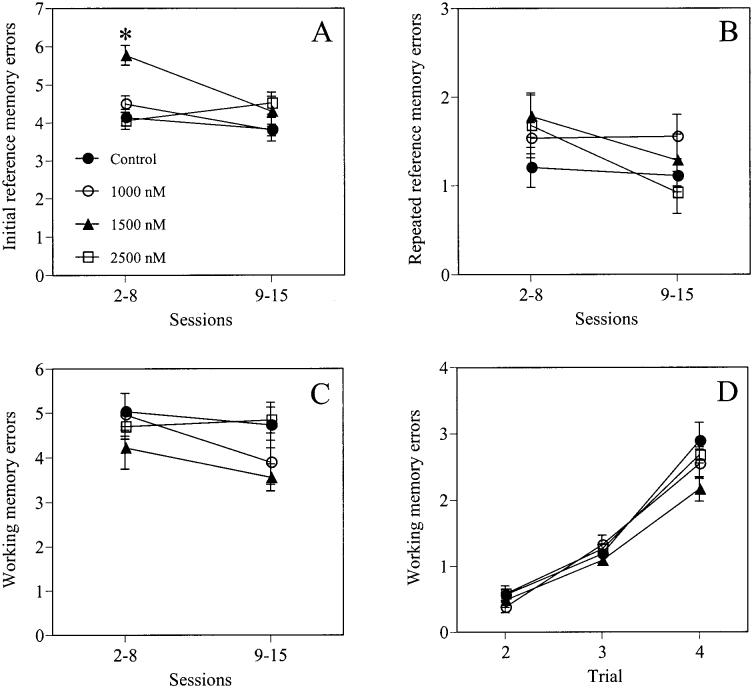
The main effect of treatment was significant for initial reference memory errors committed across Sessions 2–15, F(3, 30) = 5.52, p < .01. However, this was particularly evident during Sessions 2–8, F(3, 30) = 12.53, p < .0001, but not during Sessions 9–15, F(3, 30) = 1.30, p > .05, indicating that this effect was limited to task acquisition (see Figure 1A). Specifically, during Sessions 2–8, mice receiving 1,500 nM E2 committed more initial reference memory errors than mice in all other groups (p < .01). The main effects of session were not significant during any phase of testing: Sessions 2–15, F(13, 390) = 0.93, p > .05; Sessions 2–8, F(6, 180) = 0.35, p > .05; Sessions 9–15, F(6, 180) = 0.65, p > .05, indicating that the groups did not commit fewer initial reference memory errors across sessions in any phase of training. The Treatment × Session interactions were also not significant for any phase of testing: Sessions 2–15, F(39, 390) = 0.97, p > .05; Sessions 2–8, F(18, 180) = 0.70, p > .05; Sessions 9–15, F(18, 180) = 0.72, p > .05.
Figure 1.
The 1,500-nM dose of estrogen significantly impaired reference memory, as indicated by an increase in initial reference memory errors made across Sessions 2–15. However, this impairment was primarily evident during Sessions 2–8 (*p < .01), rather than Sessions 9–15 (A). Estrogen did not affect either repeated reference memory errors (B) or working memory errors (C), nor did it affect working memory load (errors assessed by trial for Sessions 2–15; D). Symbols represent the group mean (± SEM).
Repeated reference memory errors
The main effect of treatment was not significant for reference memory errors committed during any phase of testing: Sessions 2–15, Sessions 2–8, and Sessions 9–15, Fs(3, 30) = 1.19, 0.19, and 1.24, respectively, ps > .05 (Figure 1B). The main effects of session were significant for Sessions 2–15, F(13, 390) = 0.89, p < .01, and Sessions 2–8, F(6, 180) = 3.84, p < .01, but not for Sessions 9–15, F(6, 180) = 0.49, p > .05, indicating that the greatest reduction in repeated reference memory errors occurred during task acquisition. The Treatment × Session interactions were not significant during any phase of testing: Sessions 2–15, F(39, 390) = 1.17, p > .05; Sessions 2–8, F(18, 180) = 1.62, p > .05; Sessions 9–15, F(18, 180) = 0.73, p > .05.
Working memory errors
The main effect of treatment was not significant for working memory errors committed during any phase of testing: Sessions 2–15, Sessions 2–8, and Sessions 9–15, Fs(3, 30) = 2.30, 0.78, and 2.34, respectively, ps > .05 (Figure 1C). The main effect of Session for Sessions 2–15 approached significance, F(13, 390) = 1.74, p = .052, and was significant for Sessions 2–8, F(6, 180) = 2.22, p < .05, but not Sessions 9–15, F(6, 180) = 0.90, p > .05, indicating that the greatest improvement in working memory errors occurred during task acquisition. The Treatment × Session interactions were not significant during any phase of training: Sessions 2–15, F(39, 390) = 1.38, p > .05; Sessions 2–8, F(18, 180) = 1.48, p > .05; Sessions 9–15, F(18, 180) = 1.43, p > .05.
The main effect of treatment for working memory load was not significant (as determined by assessing working memory errors made in each trial for Trials 2–4) during any phase of testing: Sessions 2–15, Sessions 2–8, Sessions 9–15, Fs(3, 30) = 2.21, 0.79, and 2.85, respectively, ps > .05 (Figure 1D). The main effects of trial for Sessions 2–15, Sessions 2–8, and Sessions 9–15 were significant, Fs(2, 60) = 203.05, 168.90, and 45.47, respectively, ps < .05, indicating that all groups committed more working memory errors with each successive trial as the amount of working memory information to be remembered increased. The Treatment × Trial interactions were not significant during any phase of training: Sessions 2–15, Sessions 2–8, and Sessions 9–15, Fs(6, 60) = 1.41, 0.95, and 1.94, respectively, ps > .05.
Object Recognition
Sample phase
No group displayed a preference for either identical object (Figure 2A). Controls and females receiving 1,000 nM, 1,500 nM, and 2,500 nM E2 did not spend significantly more time than chance (15 s) exploring the object in the northwest (left), t(8) = −0.15, t(6) = 2.15, t(8) = 0.49, t(7) = 1.55, respectively, ps > .05, or northeast corner (right), indicating that no group was biased toward either corner of the box. There were also no group differences in the number of visits to each identical object, F(3, 29) = 0.34, p > .05. For visits, neither the main effect of object, F(1, 29) = 1.10, p > .05, nor the Treatment × Object interaction, F(3, 29) = 0.17, p > .05, were significant. See Table 1 for the mean number of object visits for each group.
Figure 2.
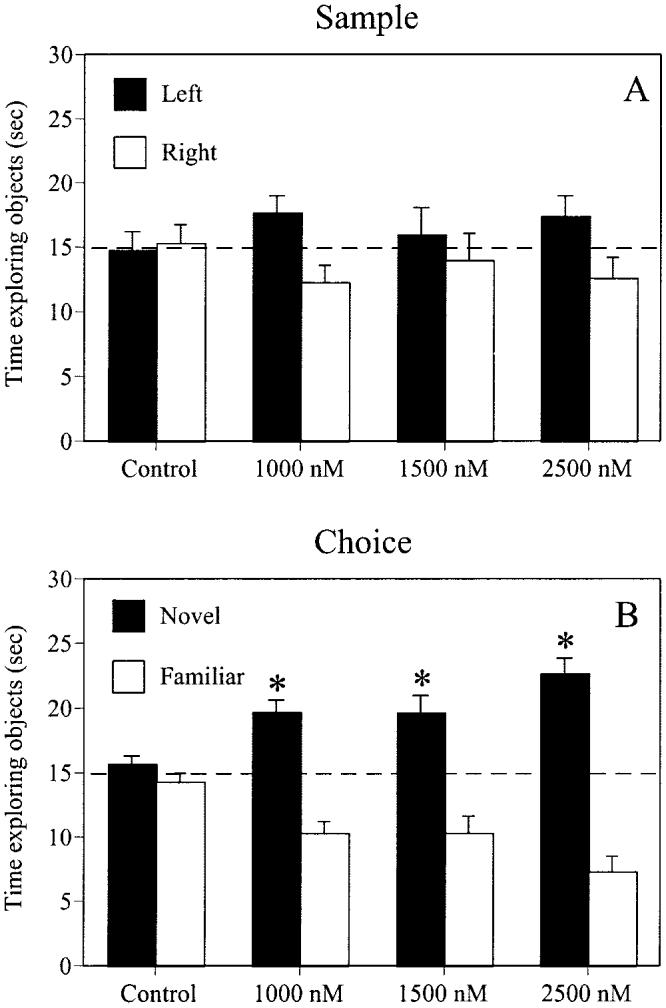
All groups performed similarly during the sample phase of testing (A), as no group showed a preference for either identical object. All doses of estrogen improved object memory in the choice phase (B), as demonstrated by a significant preference for the novel object (*p < .01) relative to chance (the dotted line at 15 s). Each bar represents the mean (± SEM) time each group spent with each object.
Table 1.
Mean (± SEM) Number of Visits to Each Object
| Group |
||||
|---|---|---|---|---|
| Phase and object | Control | 1,000 nM E2 | 1,500 nM E2 | 2,500 nM E2 |
| Sample | ||||
| Left | 14.0 ± 2.0 | 15.1 ± 2.2 | 15.0 ± 1.7 | 14.8 ± 1.3 |
| Right | 13.1 ± 0.9 | 15.6 ± 1.4 | 14.9 ± 2.2 | 12.6 ± 1.8 |
| Choice | ||||
| Novel | 18.0 ± 2.6 | 18.3 ± 2.1 | 17.8 ± 2.5 | 18.1 ± 3.1 |
| Familiar | 18.7 ± 2.0 | 12.7 ± 1.9 | 16.0 ± 2.4 | 16.1 ± 2.6 |
Note. E2 = estrogen (17β-estradiol).
Choice phase
Estrogen significantly increased the preference for the novel object, such that females receiving 1,000 nM, 1,500 nM, and 2,500 nM E2 spent significantly more time than chance (15 s) with the novel object, t(6) = 5.21, t(8) = 3.61, and t(7) = 6.58, respectively, ps < .01, and thus significantly less time with the familiar object (Figure 2B). These preferences were shown in the absence of a bias toward either corner of the testing box, both because the location of the novel object was counterbalanced across mice, and because mice did not show a corner bias in the sample phase. In contrast, controls did not spend significantly more time with the novel object, t(8) = 1.10, p > .05, and thus spent a similar amount of time with the familiar object. There were no differences in the number of visits to either object for any group, F(3, 29) = 0.68, p > .05, suggesting that E2 increased the amount of exploration time per visit to the novel object. For visits, neither the main effect of object, F(1, 29) = 1.18, p > .05, nor the Treatment × Object interaction, F(3, 29) = 0.39, p > .05, were significant. See Table 1 for mean number of object visits for each group.
Neurochemical Assays
Synaptophysin
Figure 3 illustrates estrogen-induced changes in synaptophysin levels in the hippocampus (3A) and neocortex (3B). Estrogen significantly altered levels of synaptophysin in the hippocampus, F(3, 31) = 13.17, p < .0001. Females receiving 2,500 nM E2 exhibited significantly greater synaptophysin immunoreactivity relative to all other groups (ps < .01). Estrogen also altered synaptophysin levels in the frontoparietal cortex, F(3, 31) = 11.39, p < .0001, such that females receiving 1,500 nM E2 exhibited decreased synaptophysin immunoreactivity compared to all other groups (ps < .01).
Figure 3.
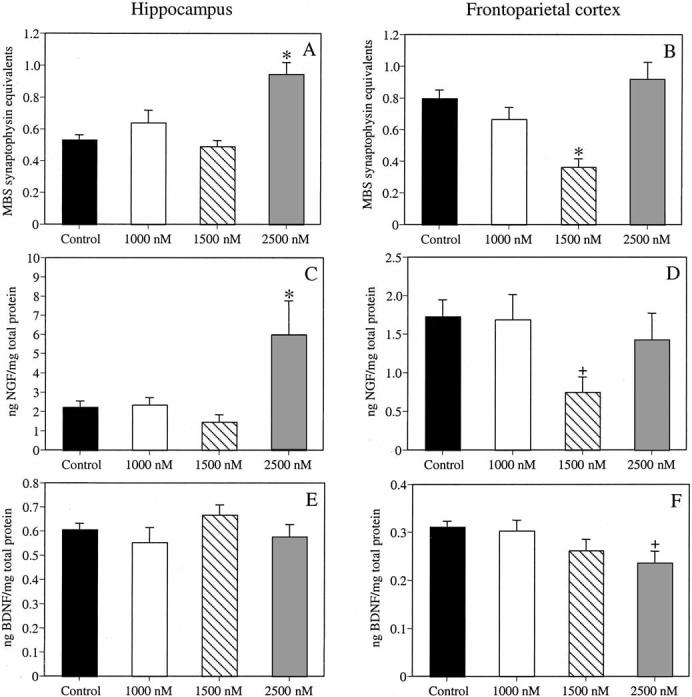
The 2,500-nM dose of estrogen (E2) significantly increased hippocampal synaptophysin levels (A), whereas the 1,500-nM dose of E2 significantly decreased neocortical levels of synaptophysin (B). The 2,500-nM dose of E2 significantly increased hippocampal levels of nerve growth factor (NGF; C), whereas the 1,500-nM dose of E2 significantly decreased neocortical NGF levels (D). Estrogen did not affect hippocampal levels of brain-derived neurotrophic factor (BDNF; E); however, the 2,500-nM dose of E2 significantly decreased neocortical BDNF levels (F). *p < .01 relative to all other groups; +p < .01 relative to controls and mice receiving 1,000 nM E2. Each bar represents the mean (± SEM) levels for each group. MBS = mouse brain standard.
Neurotrophins
Figure 3 illustrates estrogen-induced changes in hippocampal levels of NGF (3C) and BDNF (3E), and neocortical levels of NGF (3D) and BDNF (3F). Estrogen significantly altered levels of NGF in the hippocampus, F(3, 28) = 5.64, p < .01. Females receiving 2,500 nM E2 exhibited significantly greater NGF immunoreactivity compared to all other groups (ps < .01). Estrogen also significantly affected NGF levels in the frontoparietal cortex, F(3, 29) = 3.21, p < .04, such that females receiving 1,500 nM E2 exhibited decreased NGF immunoreactivity relative to controls and females receiving 1,000 nM E2 (ps < .02).
Estrogen did not affect BDNF levels in the hippocampus, F(3, 31) = 1.21, p > .05. However, estrogen significantly altered BDNF levels in the frontoparietal cortex, F(3, 31) = 2.94, p < .05, such that females receiving 2,500 nM E2 exhibited less BDNF immunoreactivity relative to controls and females receiving 1,000 nM E2 ( ps < .03).
Discussion
The present study is the first to show that chronic oral estrogen treatment can affect memory and neurochemistry in middle-aged female mice. Specifically, estrogen treatment impaired spatial reference memory and improved object memory in middle-aged female mice. In the WRAM, mice receiving 1,500 nM E2 committed more initial reference memory errors during acquisition than mice in all other groups. However, estrogen did not affect repeated reference or working memory errors. In contrast, all doses of estrogen improved nonspatial memory in the object recognition task, such that all treatment groups exhibited a significant preference for the novel object. This study is also the first to examine the effects of estrogen on synaptophysin, NGF, and BDNF in middle-aged female mice. In the frontoparietal cortex, 1,500 nM E2 decreased both synaptophysin and NGF immunoreactivity, alterations associated with an impairment in spatial reference memory. In contrast, 2,500 nM E2 increased synaptophysin and NGF immunoreactivity in the hippocampus. Estrogen also altered neocortical levels of BDNF, such that mice receiving 2,500 nM E2 exhibited decreased BDNF immunoreactivity in the frontoparietal cortex, whereas no dose of estrogen altered hippocampal levels of BDNF. It should be noted, however, that although previous work using oral estrogen (Stone et al., 1998) suggests that our treatment yielded a range of physiological estradiol levels, future studies are necessary to determine exact estradiol levels in our treatment groups. Nevertheless, the data clearly indicate that the different estrogen doses produced distinct effects on memory and aspects of neural function.
Effects of Estrogen on Memory
The finding that 6 weeks of chronic oral estrogen impaired spatial reference memory and did not affect working memory in the WRAM is somewhat surprising, given reports that chronic estrogen treatments can enhance performance in spatial memory tasks. In young female rats (Bimonte & Denenberg, 1999; Daniel, Fader, Spencer, & Dohanich, 1997; Fader, Johnson, & Dohanich, 1999; Luine, Richards, Wu, & Beck, 1998) and mice (Heikkinen, Puolivali, Liu, Rissanen, & Tanila, 2002) tested in both the WRAM and dry-land radial-arm maze (RAM) tasks, low physiological doses of estrogen administered chronically (ranging from 21 to 40 days prior to testing) improved working memory (Bimonte & Denenberg, 1999; Daniel et al., 1997; Fader et al., 1999; Luine et al., 1998) but did not affect measures of reference memory (Fader et al., 1999; Luine et al., 1998), whereas a high dose of estrogen improved both working and reference memory (Heikkinen et al., 2002). In middle-aged female rats, a low dose of chronic estrogen (administered 25 or 28 days prior to testing) improved spatial reference memory during acquisition and retention trials in a Morris water maze task (Foster et al., 2003; Markham et al., 2002). Improved retention of spatial discrimination in the Morris maze was also seen in aged female rats treated with a high dose of estrogen for 25 days prior to testing (Foster et al., 2003). In addition, female rats ovariectomized at middle age and treated with a low dose of chronic estrogen through old age exhibited improved working memory in a delayed matching-to-position task (Gibbs, 2000). The present finding of impairment is consistent with one previous report in which young female rats receiving at least 30 days of estrogen demonstrated an impairment in reference, but not working, memory on a RAM (Galea et al., 2001). However, this study utilized a high pharmacological dose of estrogen, unlike the present study, which used a range of low to high physiological doses. It is also important to note that the novel use of an oral preparation may account for differing behavioral effects because, in contrast to studies utilizing injection or capsule implantation, an oral administration allows for cyclic estrogen levels that are rapidly metabolized.
It is particularly interesting that only the 1,500-nM dose impaired reference memory in the WRAM, whereas the 1,000- and 2,500-nM doses had no effect. No prior study in aging females has examined behavioral responses to different doses of estrogen administered chronically. This dose-specific impairment may result from an increased sensitivity in aging females to differences within a range of physiological levels of estrogen, coupled with the fact that the WRAM is a particularly difficult task for aging females. The absence of a significant main effect of session for initial reference memory errors is consistent with one other report in which aged female rats tested on a similar WRAM task failed to learn across sessions in both reference and working memory (Bimonte et al., 2003). In addition, aged male rats also have difficulty acquiring the RAM (Beatty, Bierley, & Boyd, 1985; Noda, Yamada, & Nabeshima, 1997). The WRAM task is also particularly challenging because of different reference and working memory demands, perhaps accounting for the differential effects of estrogen on these types of memory. In the present study, 1,500 nM E2 may have had a deleterious effect on consolidation of long-term memory, adversely affecting spatial reference information (reflected by initial reference memory errors) to be remembered over the 24-hr interval between sessions. In contrast, because the task is run with very short (30-s) ITIs, the brief delay between trials within a session may have allowed short-term memory (encompassing both working memory and repeated reference memory errors) to remain intact. Although estrogen in young female rats can reportedly facilitate long-term spatial memory consolidation (Packard, 1998; Packard & Teather, 1997), this effect has not been examined in aging females.
In contrast to the WRAM, all doses of estrogen in middle-aged mice improved performance in the nonspatial object recognition task. Estrogen-treated females spent significantly more time with the novel object than the familiar object, whereas controls did not show a preference for the novel object. This finding is consistent with a report in which 3 weeks of a low dose of estrogen improved object recognition memory over 3- and 6-hr delays in aged female mice (Vaucher et al., 2002). In the present study, the fact that estrogen-treated mice displayed a preference for the novel object after a 48-hr delay suggests that a longer duration (8 weeks) of treatment and/or oral administration may contribute to the robust effect on object memory over a long delay period. It is unlikely that group differences in activity level contributed to object recognition performance. The limit of 30 s of total exploration time and statistical comparisons performed within each group control for group differences in activity.
The fact that all doses of estrogen improved performance on the object recognition task whereas none improved performance on the WRAM suggests several possibilities. Spatial memory tasks such as the WRAM may be more sensitive than object recognition tasks to hippocampal alterations. Discrepant studies report that hippocampal damage or inactivation either impairs (Baker & Kim, 2002; Clark et al., 2000) or no has effect on object recognition tasks (e.g., Mumby, Gaskin, Glenn, Schramek, & Lehmann, 2002; Stupien, Florian, & Roullet, 2003), although there is a clear involvement of the hippocampus in spatial memory tasks (e.g., Morris, Garrud, Rawlins, & O'Keefe, 1982; Mumby et al., 2002; Olton, Walker, & Gage, 1978; Stupien et al., 2003). Lesions to neocortical areas, however, impair performance on object recognition tasks but do not appreciably affect spatial memory, including reference and working memory tested in a RAM (e.g., Bussey, Muir, & Aggleton, 1999; Ennaceur, Neave, & Aggleton, 1996, 1997; P. Liu & Bilkey, 2001). Thus, 1,500 nM E2 may have had a deleterious effect on hippocampal function required for the spatial measures in the WRAM through neurochemical measures not examined in this study. In contrast, all doses of estrogen may have facilitated object memory via beneficial effects on the neocortex. The object recognition task is also less difficult than the WRAM, raising the possibility that the effects of estrogen in middle-aged females may be dependent on cognitive demand. Also, evidence suggests that, unlike with the WRAM, aging does not have a substantial impact on object recognition task performance (Vaucher et al., 2002). Thus, it may be possible that the mnemonic response to estrogen is dependent on the prior existence of age-related memory deficits.
The discrepancy between the tasks may also indicate that nonspatial object memory is more sensitive to estrogenic modulation than spatial memory. Thus, perhaps less estrogenic stimulation is needed to improve object memory, whereas significantly more stimulation is required to improve performance in the WRAM. However, the fact that the 1,500-nM dose of estrogen impaired reference memory in the WRAM suggests that greater estrogenic stimulation may not benefit WRAM performance. Also, perhaps the duration of chronic treatment contributed to the discrepancy between the two tasks. The present and previous data on object recognition memory in aging females suggest that either 3 (Vaucher et al., 2002) or 8 weeks (present study) of estrogen treatment can improve object memory. In contrast, 6 weeks of estrogen treatment did not improve performance on the WRAM. Perhaps the chronic nature of the treatment altered hippocampal and neocortical function in a way not measured by this study, for example, by down-regulating estrogen receptors (Brown, Scherz, Hochberg, & MacLusky, 1996) in these regions. In this case, a decrease in neuronal sensitivity to estrogen may have a lesser impact on the object recognition task than the WRAM task. Clearly, testing the effects of estrogen on a broader range of non-spatial and spatial tasks would be necessary to differentiate among these possibilities.
Effects of Estrogen on Neurochemistry
In addition to modulating memory, estrogen altered all three neurochemical measures. Synaptophysin levels were altered by estrogen depending on dose and brain region. Although the methodology utilized in the present study to determine synaptophysin levels cannot identify specific morphological changes associated with alterations in synaptophysin levels, other studies suggest that increases in synaptophysin may reflect an increase in presynaptic terminals, indicative of synaptogenesis (e.g., Murphy & Segal, 1996; Pozzo-Miller et al., 1999; Stone et al., 1998). The finding that 2,500 nM E2 increased hippocampal levels of synaptophysin is consistent with our previous report in which a high pharmacological dose (5 μg) of short-term estrogen treatment in aged female mice increased levels of synaptophysin in the whole hippocampus (Frick et al., 2002). Estrogen exposure also increased synaptophysin levels in whole hippocampal slices from young female rats (Rune et al., 2002), although one study utilizing acute estrogen in young female rats reported no change in synaptophysin (Brake et al., 2001). Estrogen exposure also elevated synaptophysin in cultured hippocampal neurons from neonatal rats (Murphy & Segal, 1996; Pozzo-Miller et al., 1999). Region-specific hippocampal increases have also been identified, as estrogen increased synaptophysin in the dentate gyrus following a lesion-induced injury (Stone et al., 1998), and elevated levels in CA1 in hippocampal slices (Rune et al., 2002). The latter finding is associated with an increase of the estrogen receptor ERα in CA3, whereas hippocampal expression of the other estrogen receptor, EBβ, was not affected (Rune et al., 2002). This finding suggests that the augmentation of synaptic plasticity (indicated by increases in synaptophysin) in CA1 is mediated presynaptically via the activation of ERα, but not ERβ (Rune et al., 2002). The finding that 1,500 nM E2 decreased levels of synaptophysin in the frontoparietal cortex is inconsistent with the only other study examining cortical alterations of synaptophysin in response to estrogen. In this report, short-term administration of a high pharmacological dose (1 μg) of estrogen increased cortical levels of synaptophysin in aged female mice, which was associated with impairments in spatial reference memory (Frick et al., 2002). In the present study, decreased cortical synaptophysin was associated with impaired spatial reference memory in mice receiving 1,500 nM E2. These discrepant findings may suggest that any alteration in cortical synaptophysin levels gives rise to mnemonic deficits.
Estrogen altered NGF expression in a pattern similar to changes in levels of synaptophysin. The finding that mice receiving 2,500 nM E2 exhibited increased NGF protein in the hippocampus is inconsistent with the few studies that have examined the effects of estrogen on NGF expression. In young female rats, acute or short-term estrogen treatment reportedly decreased hippocampal NGF mRNA in CA1 and dentate gyrus (Gibbs et al., 1994), whereas another acute treatment (single injection) did not affect hippocampal NGF mRNA (Gibbs, 1998). The finding that 1,500 nM E2 decreased NGF levels is inconsistent with a report in which 4 weeks of estrogen in young female rats treatment did not affect cortical NGF mRNA expression (Jezierski & Sohrabji, 2000). Interestingly, the present study indicates that the differential hippocampal and cortical changes in NGF levels are dose-dependent, and the decrease of NGF in the frontoparietal cortex of females receiving 1,500 nM E2 is associated with an impairment in spatial reference memory.
This study is the first to examine the effects of chronic estrogen treatment on BDNF expression in aging females. The data indicate that chronic oral estrogen has no effect on hippocampal BDNF levels. Studies utilizing acute or short-term treatments in young female rats and prairie voles have reported increases in BDNF mRNA levels in both the whole hippocampus (Gibbs, 1999), as well as in CA3 and the dentate gyrus (Y. Liu et al., 2001), although others report no alterations (Cavus & Duman, 2003; Gibbs, 1998). In contrast to BDNF levels in the hippocampus, 2,500 nM E2 decreased BDNF in the frontoparietal cortex. This finding is consistent with one report in which chronic estrogen treatment in young female rats decreased cortical BDNF mRNA (Jezierski & Sohrabji, 2000; although see Singh et al., 1995). In contrast, in the cortex of young female rats, acute or short-term treatment has been reported to increase BDNF mRNA (Gibbs, 1999; Sohrabji et al., 1995) but not alter BDNF protein (Gibbs, 1999). In the present study, inconsistencies with the current literature may result from examination of the whole hippocampus (which does not allow for the identification of regional differences in NGF and BDNF levels, and thus may mask behaviorally relevant effects that are region-specific), examination of protein levels (rather than mRNA), as well as age, species, and methodological differences. It is also important to note that differences in the metabolism of oral and injected estrogen may have altered the timing of neurotrophin alterations. Chronic estrogen treatment has also been shown to decrease expression of basal forebrain neurotrophin receptors (Gibbs & Pfaff, 1992; Gibbs et al., 1994), raising the possibility that hippocampal neurotrophin levels were influenced by neurotrophin receptor expression on afferent basal forebrain fibers.
One interesting aspect of our findings was that alterations in BDNF levels were not opposite to those of synaptophysin levels. A prior study reported that estrogen-induced reductions of BDNF protein in cultured hippocampal neurons increased dendritic spine density in cultured hippocampal neurons via the inhibition of GABAergic transmission (Murphy et al., 1998). The present study is the first to examine the effects of estrogen on both BDNF and synaptophysin levels. It is possible that BDNF does not appear to modulate synaptic plasticity in the present study because alterations of synaptophysin are not positively correlated with changes in spine density, or because chronic treatment in middle-aged females altered the relationship between BDNF levels and the augmentation of synaptic plasticity. It is also important to note that alterations in vivo (present study) may be different from changes observed in vitro.
Conclusions
In summary, the present study indicates that chronic oral estrogen treatment in middle-aged female mice differentially affects memory, synaptophysin, and neurotrophin levels. Dose-dependent impairments in spatial reference memory were associated with decreased neocortical synaptophysin and NGF levels, suggesting potential neurobiological mechanisms of this impairment. Improvements in object memory were independent of dose and not associated with any neurobiological alterations. Although additional research is needed to further evaluate types of estrogen treatments most beneficial to various types of memory in aging females, this study demonstrates that an oral estrogen preparation can influence memory and neurochemistry in middle-aged females. However, the data underscore the fact that estrogen-induced cognitive changes are not universal to all types of memory. Furthermore, because this type of treatment more closely resembles the type of hormone replacement used by menopausal women, this oral preparation should be further studied in animal models.
Footnotes
Funding was provided by a Yale Junior Faculty Fellowship, an American Federation for Aging Research/Pfizer Research Grant in Hormones and Aging, and National Institute of Mental Health Grant MH065460 to Karyn M. Frick. We thank Jeansok Kim for critical comments on this article.
Contributor Information
Stephanie M. Fernandez, Department of Psychology, Yale University
Karyn M. Frick, Department of Psychology and Interdepartmental Neuroscience Program, Yale University.
References
- Adams ,MM, Shah RA, Janssen WG, Morrison JH. Different modes of hippocampal plasticity in response to estrogen in young and aged female rats. Proceedings of the National Academy of Sciences, USA. 2001;98:8071–8076. doi: 10.1073/pnas.141215898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KB, Kim JJ. Effects of stress and hippocampal NMDA receptor antagonism on recognition memory in rats. Learning & Memory. 2002;9:58–65. doi: 10.1101/lm.46102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty WW, Bierley RA, Boyd JG. Preservation of accurate spatial memory in aged rats. Neurobiology of Aging. 1985;6:219–225. doi: 10.1016/0197-4580(85)90053-3. [DOI] [PubMed] [Google Scholar]
- Berchtold NC, Kesslak JP, Pike CJ, Adlard PA, Cotman CW. Estrogen and exercise interact to regulate brain-derived neurotrophic factor mRNA and protein expression in the hippocampus. European Journal of Neuroscience. 2001;14:1992–2002. doi: 10.1046/j.0953-816x.2001.01825.x. [DOI] [PubMed] [Google Scholar]
- Berger-Sweeney J, Berger UV, Sharma M, Paul CA. Effects of carbon dioxide-induced anesthesia on cholinergic parameters in rat brain. Laboratory Animal Science. 1994;44(4):369–371. [PubMed] [Google Scholar]
- Bimonte HA, Denenberg VH. Estradiol facilitates performance as working memory load increases. Psychoneuroendocrinology. 1999;24:161–173. doi: 10.1016/s0306-4530(98)00068-7. [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Hyde LA, Hoplight BJ, Denenberg VH. In two species, females exhibit superior working memory and inferior reference memory on the water radial-arm maze. Physiology & Behavior. 2000;70:311–317. doi: 10.1016/s0031-9384(00)00259-6. [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Nelson ME, Granholm AC. Age-related deficits as working memory load increases: Relationships with growth factors. Neurobiology of Aging. 2003;24:37–48. doi: 10.1016/s0197-4580(02)00015-5. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brake WG, Alves SE, Dunlop JC, Lee SJ, Bulloch K, Allen PB, et al. Novel target sites for estrogen action in the dorsal hippocampus: An examination of synaptic proteins. Endocrinology. 2001;142:1284–1289. doi: 10.1210/endo.142.3.8036. [DOI] [PubMed] [Google Scholar]
- Brown TJ, Scherz B, Hochberg RB, MacLusky NJ. Regulation of estrogen receptor concentrations in the rat brain: Effects of sustained androgen and estrogen exposure. Neuroendocrinology. 1996;63:53–60. doi: 10.1159/000126935. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Muir JL, Aggleton JP. Functionally dissociating aspects of event memory: The effects of combined perirhinal and postrhinal cortex lesions on object and place memory in the rat. Journal of Neuroscience. 1999;19:495–502. doi: 10.1523/JNEUROSCI.19-01-00495.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun ME, Kurth D, Phinney AL, Long JM, Hengemihle J, Mouton PR, et al. Hippocampal neuron and synaptophysin-positive bouton number in aging C57BL/6 mice. Neurobiology of Aging. 1998;19:599–606. doi: 10.1016/s0197-4580(98)00098-0. [DOI] [PubMed] [Google Scholar]
- Cavus I, Duman RS. Influence of estradiol, stress, and 5-HT2A agonist treatment on brain-derived neurotrophic factor expression in female rats. Biological Psychiatry. 2003;54:59–69. doi: 10.1016/s0006-3223(03)00236-1. [DOI] [PubMed] [Google Scholar]
- Chen KS, Masliah E, Mallory M, Gage FH. Synaptic loss in cognitively impaired aged rats is ameliorated by chronic human nerve growth factor infusion. Neuroscience. 1995;68:19–27. doi: 10.1016/0306-4522(95)00099-5. [DOI] [PubMed] [Google Scholar]
- Clark RE, Zola SM, Squire LR. Impaired recognition memory in rats after damage to the hippocampus. Journal of Neuroscience. 2000;20:8853–8860. doi: 10.1523/JNEUROSCI.20-23-08853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croll SD, Ip NY, Lindsay RM, Wiegand SJ. Expression of BDNF and trkB as a function of age and cognitive performance. Brain Research. 1998;812:200–208. doi: 10.1016/s0006-8993(98)00993-7. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Fader AJ, Spencer AL, Dohanich GP. Estrogen enhances performance of female rats during acquisition of a radial arm maze. Hormones and Behavior. 1997;32:217–225. doi: 10.1006/hbeh.1997.1433. [DOI] [PubMed] [Google Scholar]
- Davies HA, Kelly A, Dhanrajan TM, Lynch MA, Rodriguez JJ, Stewart MG. Synaptophysin ImmunoGold labelling of synapses decreases in dentate gyrus of the hippocampus of aged rats. Brain Research. 2003;986:191–195. doi: 10.1016/s0006-8993(03)03251-7. [DOI] [PubMed] [Google Scholar]
- Duff SJ, Hampson E. A beneficial effect of estrogen on working memory in postmenopausal women taking hormone replacement therapy. Hormones and Behavior. 2000;38:262–276. doi: 10.1006/hbeh.2000.1625. [DOI] [PubMed] [Google Scholar]
- Eastwood SL, Burnet PW, McDonald B, Clinton J, Harrison PJ. Synaptophysin gene expression in human brain: A quantitative in situ hybridization and immunocytochemical study. Neuroscience. 1994;59:881–892. doi: 10.1016/0306-4522(94)90292-5. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Neave N, Aggleton JP. Neurotoxic lesions of the perirhinal cortex do not mimic the behavioural effects of fornix transection in the rat. Behavioural Brain Research. 1996;80:9–25. doi: 10.1016/0166-4328(96)00006-x. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Neave N, Aggleton JP. Spontaneous object recognition and object location memory in rats: The effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum bundle and the fornix. Experimental Brain Research. 1997;113:509–519. doi: 10.1007/pl00005603. [DOI] [PubMed] [Google Scholar]
- Evans GW, Brennan PL, Skorpanich MA, Held D. Cognitive mapping and elderly adults: Verbal and location memory for urban landmarks. Journal of Gerontology. 1984;39:452–457. doi: 10.1093/geronj/39.4.452. [DOI] [PubMed] [Google Scholar]
- Fader AJ, Johnson PE, Dohanich GP. Estrogen improves working but not reference memory and prevents amnestic effects of scopolamine on a radial-arm maze. Pharmacology Biochemistry and Behavior. 1999;62:711–717. doi: 10.1016/s0091-3057(98)00219-6. [DOI] [PubMed] [Google Scholar]
- Fordyce DE, Wehner JM. Effects of aging on spatial learning and hippocampal protein kinase C in mice. Neurobiology of Aging. 1993;14:309–317. doi: 10.1016/0197-4580(93)90116-s. [DOI] [PubMed] [Google Scholar]
- Foster TC, Sharrow KM, Kumar A, Masse J. Interaction of age and chronic estradiol replacement on memory and markers of brain aging. Neurobiology of Aging. 2003;24:839–852. doi: 10.1016/s0197-4580(03)00014-9. [DOI] [PubMed] [Google Scholar]
- Frick KM, Baxter MG, Markowska AL, Olton DS, Price DL. Age-related spatial reference and working memory deficits assessed in the water maze. Neurobiology of Aging. 1995;16:149–160. doi: 10.1016/0197-4580(94)00155-3. [DOI] [PubMed] [Google Scholar]
- Frick KM, Berger-Sweeney J. Spatial reference memory and neocortical neurochemistry vary with the estrous cycle in C57BL/6 mice. Behavioral Neuroscience. 2001;115:229–237. doi: 10.1037/0735-7044.115.1.229. [DOI] [PubMed] [Google Scholar]
- Frick KM, Fernandez SM, Bulinski SC. Estrogen replacement improves spatial reference memory and increases hippocampal synaptophysin in aged female mice. Neuroscience. 2002;115:547–558. doi: 10.1016/s0306-4522(02)00377-9. [DOI] [PubMed] [Google Scholar]
- Frick KM, Gresack JE. Sex differences in the behavioral response to spatial and object novelty in adult C57BL/6 mice. Behavioral Neuroscience. 2003;117:1283–1291. doi: 10.1037/0735-7044.117.6.1283. [DOI] [PubMed] [Google Scholar]
- Frick KM, Stearns NA, Pan JY, Berger-Sweeney J. Effects of environmental enrichment on spatial memory and neurochemistry in middle-aged mice. Learning & Memory. 2003;10:187–198. doi: 10.1101/lm.50703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea LA, Wide JK, Paine TA, Holmes MM, Ormerod BK, Floresco SB. High levels of estradiol disrupt conditioned place preference learning, stimulus response learning and reference memory but have limited effects on working memory. Behavioural Brain Research. 2001;126:115–126. doi: 10.1016/s0166-4328(01)00255-8. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Levels of trkA and BDNF mRNA, but not NGF mRNA, fluctuate across the estrous cycle and increase in response to acute hormone replacement. Brain Research. 1998;787:259–268. doi: 10.1016/s0006-8993(97)01511-4. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Treatment with estrogen and progesterone affects relative levels of brain-derived neurotrophic factor mRNA and protein in different regions of the adult rat brain. Brain Research. 1999;844:20–27. doi: 10.1016/s0006-8993(99)01880-6. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Long-term treatment with estrogen and progesterone enhances acquisition of a spatial memory task by ovariectomized aged rats. Neurobiology of Aging. 2000;21:107–116. doi: 10.1016/s0197-4580(00)00103-2. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Pfaff DW. Effects of estrogen and fimbria/fornix transection on p75NGFR and ChAT expression in the medial septum and diagonal band of Broca. Experimental Neurology. 1992;116:23–39. doi: 10.1016/0014-4886(92)90173-n. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Wu D, Hersh LB, Pfaff DW. Effects of estrogen replacement on the relative levels of choline acetyltransferase, trkA, and nerve growth factor messenger RNAs in the basal forebrain and hippocampal formation of adult rats. Experimental Neurology. 1994;129:70–80. doi: 10.1006/exnr.1994.1148. [DOI] [PubMed] [Google Scholar]
- Gordon MN, Osterburg HH, May PC, Finch CE. Effective oral administration of 17 beta-estradiol to female C57BL/6J mice through the drinking water. Biology of Reproduction. 1986;35:1088–1095. doi: 10.1095/biolreprod35.5.1088. [DOI] [PubMed] [Google Scholar]
- Gresack JE, Frick KM. Male mice exhibit better spatial working and reference memory than females in a water-escape radial arm maze task. Brain Research. 2003;982:98–107. doi: 10.1016/s0006-8993(03)03000-2. [DOI] [PubMed] [Google Scholar]
- Heikkinen T, Puolivali J, Liu L, Rissanen A, Tanila H. Effects of ovariectomy and estrogen treatment on learning and hippocampal neurotransmitters in mice. Hormones and Behavior. 2002;41:22–32. doi: 10.1006/hbeh.2001.1738. [DOI] [PubMed] [Google Scholar]
- Henderson VW. Estrogen, cognition, and a woman's risk of Alzheimer's disease. The American Journal of Medicine. 1997;103(3A):11S–18S. doi: 10.1016/s0002-9343(97)00261-1. [DOI] [PubMed] [Google Scholar]
- Hogervorst E, Williams J, Budge M, Riedel W, Jolles J. The nature of the effect of female gonadal hormone replacement therapy on cognitive function in post-menopausal women: A meta-analysis. Neuroscience. 2000;101:485–512. doi: 10.1016/s0306-4522(00)00410-3. [DOI] [PubMed] [Google Scholar]
- Hyde LA, Sherman GF, Denenberg VH. Non-spatial water radial-arm maze learning in mice. Brain Research. 2000;863:151–159. doi: 10.1016/s0006-8993(00)02113-2. [DOI] [PubMed] [Google Scholar]
- Hyde LA, Sherman GF, Hoplight BJ, Denenberg VH. Working memory deficits in BXSB mice with neocortical ectopias. Physiology & Behavior. 2000;70:1–5. doi: 10.1016/s0031-9384(00)00239-0. [DOI] [PubMed] [Google Scholar]
- Jahn R, Schiebler W, Ouimet C, Greengard P. A 38,000-dalton membrane protein (p38) present in synaptic vesicles. Proceedings of the National Academy of Sciences, USA. 1985;82:4137–4141. doi: 10.1073/pnas.82.12.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jezierski MK, Sohrabji F. Region- and peptide-specific regulation of the neurotrophins by estrogen. Molecular Brain Research. 2000;85:77–84. doi: 10.1016/s0169-328x(00)00244-8. [DOI] [PubMed] [Google Scholar]
- Kawas C, Resnick S, Morrison A, Brookmeyer R, Corrada M, Zonderman A, et al. A prospective study of estrogen replacement therapy and the risk of developing Alzheimer's disease: The Baltimore Longitudinal Study of Aging. Neurology. 1997;48:1517–1521. doi: 10.1212/wnl.48.6.1517. [DOI] [PubMed] [Google Scholar]
- Lamberty Y, Gower AJ. Simplifying environmental cues in a Morris-type water maze improves place learning in old NMRI mice. Behavioral and Neural Biology. 1991;56:89–100. doi: 10.1016/0163-1047(91)90315-h. [DOI] [PubMed] [Google Scholar]
- Li C, Brake WG, Romeo RD, Dunlop JC, Gordon M, Buzescu R, et al. Estrogen alters hippocampal dendritic spine shape and enhances synaptic protein immunoreactivity and spatial memory in female mice. Proceedings of the National Academy of Sciences, USA. 2004;101:2185–2190. doi: 10.1073/pnas.0307313101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Bilkey DK. The effect of excitotoxic lesions centered on the hippocampus or perirhinal cortex in object recognition and spatial memory tasks. Behavioral Neuroscience. 2001;115:94–111. doi: 10.1037/0735-7044.115.1.94. [DOI] [PubMed] [Google Scholar]
- Liu X, Erikson C, Brun A. Cortical synaptic changes and gliosis in normal aging, Alzheimer's disease and frontal lobe degeneration. Dementia. 1996;7:128–134. doi: 10.1159/000106867. [DOI] [PubMed] [Google Scholar]
- Liu Y, Fowler CD, Young LJ, Yan Q, Insel TR, Wang Z. Expression and estrogen regulation of brain-derived neurotrophic factor gene and protein in the forebrain of female prairie voles. Journal of Comparative Neurology. 2001;433:499–514. doi: 10.1002/cne.1156. [DOI] [PubMed] [Google Scholar]
- Luine VN, Richards ST, Wu VY, Beck KD. Estradiol enhances learning and memory in a spatial memory task and affects levels of monoaminergic neurotransmitters. Hormones and Behavior. 1998;34:149–162. doi: 10.1006/hbeh.1998.1473. [DOI] [PubMed] [Google Scholar]
- Markham JA, Pych JC, Juraska JM. Ovarian hormone replacement to aged ovariectomized female rats benefits acquisition of the Morris water maze. Hormones and Behavior. 2002;42:284–293. doi: 10.1006/hbeh.2002.1819. [DOI] [PubMed] [Google Scholar]
- Markowska AL. Sex dimorphisms in the rate of age-related decline in spatial memory: Relevance to alterations in the estrous cycle. Journal of Neuroscience. 1999;19:8122–8133. doi: 10.1523/JNEUROSCI.19-18-08122.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowska AL, Savonenko AV. Effectiveness of estrogen replacement in restoration of cognitive function after long-term estrogen withdrawal in aging rats. Journal of Neuroscience. 2002;22:10985–10995. doi: 10.1523/JNEUROSCI.22-24-10985.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Mallory M, Hansen L, DeTeresa R, Terry RD. Quantitative synaptic alterations in the human neocortex during normal aging. Neurology. 1993;43:192–197. doi: 10.1212/wnl.43.1_part_1.192. [DOI] [PubMed] [Google Scholar]
- Miller MM, Hyder SM, Assayag R, Panarella SR, Tousignant P, Franklin KB. Estrogen modulates spontaneous alternation and the cholinergic phenotype in the basal forebrain. Neuroscience. 1999;91:1143–1153. doi: 10.1016/s0306-4522(98)00690-3. [DOI] [PubMed] [Google Scholar]
- Miranda P, Williams CL, Einstein G. Granule cells in aging rats are sexually dimorphic in their response to estradiol. Journal of Neuroscience. 1999;19:3316–3325. doi: 10.1523/JNEUROSCI.19-09-03316.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat SD, Zonderman AB, Resnick SM. Age differences in spatial memory in a virtual environment navigation task. Neurobiology of Aging. 2001;22(5):787–796. doi: 10.1016/s0197-4580(01)00251-2. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982 June 24;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Mumby DG, Gaskin S, Glenn MJ, Schramek TE, Lehmann H. Hippocampal damage and exploratory preferences in rats: Memory for objects, places, and contexts. Learning & Memory. 2002;9:49–57. doi: 10.1101/lm.41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DD, Cole NB, Segal M. Brain-derived neurotrophic factor mediates estradiol-induced dendritic spine formation in hippocampal neurons. Proceedings of the National Academy of Sciences, USA. 1998;95:11412–11417. doi: 10.1073/pnas.95.19.11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DD, Segal M. Regulation of dendritic spine density in cultured rat hippocampal neurons by steroid hormones. Journal of Neuroscience. 1996;16:4059–4068. doi: 10.1523/JNEUROSCI.16-13-04059.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson JF, Karelus K, Bergman MD, Felicio LS. Neuroendocrine involvement in aging: Evidence from studies of reproductive aging and caloric restriction. Neurobiology of Aging. 1995;16:837–843. doi: 10.1016/0197-4580(95)00072-m. [DOI] [PubMed] [Google Scholar]
- Nicolle MM, Gallagher M, McKinney M. No loss of synaptic proteins in the hippocampus of aged, behaviorally impaired rats. Neurobiology of Aging. 1999;20:343–348. doi: 10.1016/s0197-4580(99)00054-8. [DOI] [PubMed] [Google Scholar]
- Noda Y, Yamada K, Nabeshima T. Role of nitric oxide in the effect of aging on spatial memory in rats. Behavioural Brain Research. 1997;83:153–158. doi: 10.1016/s0166-4328(97)86060-3. [DOI] [PubMed] [Google Scholar]
- Olton DS, Walker JA, Gage FH. Hippocampal connections and spatial discrimination. Brain Research. 1978;139:295–308. doi: 10.1016/0006-8993(78)90930-7. [DOI] [PubMed] [Google Scholar]
- Packard MG. Posttraining estrogen and memory modulation. Hormones and Behavior. 1998;34:126–139. doi: 10.1006/hbeh.1998.1464. [DOI] [PubMed] [Google Scholar]
- Packard MG, Teather LA. Posttraining estradiol injections enhance memory in ovariectomized rats: Cholinergic blockade and synergism. Neurobiology of Learning and Memory. 1997;68:172–188. doi: 10.1006/nlme.1997.3785. [DOI] [PubMed] [Google Scholar]
- Paganini-Hill A, Henderson VW. Estrogen deficiency and risk of Alzheimer's disease in women. American Journal of Epidemiology. 1994;140:256–261. doi: 10.1093/oxfordjournals.aje.a117244. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Sherwin BB. Effects of estrogen on memory function in surgically menopausal women. Psychoneuroendocrinology. 1992;17:485–495. doi: 10.1016/0306-4530(92)90007-t. [DOI] [PubMed] [Google Scholar]
- Poo MM. Neurotrophins as synaptic modulators. Nature Reviews Neuroscience. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- Pozzo-Miller LD, Inoue T, Murphy DD. Estradiol increases spine density and NMDA-dependent Ca2+ transients in spines of CA1 pyramidal neurons from hippocampal slices. Journal of Neuro-physiology. 1999;81:1404–1411. doi: 10.1152/jn.1999.81.3.1404. [DOI] [PubMed] [Google Scholar]
- Rune GM, Wehrenberg U, Prange-Kiel J, Zhou L, Adelmann G, Frotscher M. Estrogen up-regulates estrogen receptor alpha and synaptophysin in slice cultures of rat hippocampus. Neuroscience. 2002;113:167–175. doi: 10.1016/s0306-4522(02)00152-5. [DOI] [PubMed] [Google Scholar]
- Saito S, Kobayashi S, Ohashi Y, Igarashi M, Komiya Y, Ando S. Decreased synaptic density in aged brains and its prevention by rearing under enriched environment as revealed by synaptophysin contents. Journal of Neuroscience Research. 1994;39:57–62. doi: 10.1002/jnr.490390108. [DOI] [PubMed] [Google Scholar]
- Schaaf MJ, Workel JO, Lesscher HM, Vreugdenhil E, Oitzl MS, de Kloet ER. Correlation between hippocampal BDNF mRNA expression and memory performance in senescent rats. Brain Research. 2001;915:227–233. doi: 10.1016/s0006-8993(01)02855-4. [DOI] [PubMed] [Google Scholar]
- Sharps MJ, Gollin ES. Memory for object locations in young and elderly adults. Journal of Gerontology. 1987;42(3):336–341. doi: 10.1093/geronj/42.3.336. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and/or androgen replacement therapy and cognitive functioning in surgically menopausal women. Psychoneuroendocrinology. 1988;13:345–357. doi: 10.1016/0306-4530(88)90060-1. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and cognitive aging in women. Trends in Pharmacological Sciences. 2002;23(11):527–534. doi: 10.1016/s0165-6147(02)02093-x. [DOI] [PubMed] [Google Scholar]
- Siegel GJ, Chauhan NB. Neurotrophic factors in Alzheimer's and Parkinson's disease brain. Brain Research Reviews. 2000;33:199–227. doi: 10.1016/s0165-0173(00)00030-8. [DOI] [PubMed] [Google Scholar]
- Singh M, Meyer EM, Simpkins JW. The effect of ovariectomy and estradiol replacement on brain-derived neurotrophic factor messenger ribonucleic acid expression in cortical and hippocampal brain regions of female Sprague-Dawley rats. Endocrinology. 1995;136:2320–2324. doi: 10.1210/endo.136.5.7720680. [DOI] [PubMed] [Google Scholar]
- Sohrabji F, Miranda RC, Toran-Allerand CD. Identification of a putative estrogen response element in the gene encoding brain-derived neurotrophic factor. Proceedings of the National Academy of Sciences, USA. 1995;92:11110–11114. doi: 10.1073/pnas.92.24.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone DJ, Rozovsky I, Morgan TE, Anderson CP, Finch CE. Increased synaptic sprouting in response to estrogen via an apolipoprotein E-dependent mechanism: Implications for Alzheimer's disease. Journal of Neuroscience. 1998;18:3180–3185. doi: 10.1523/JNEUROSCI.18-09-03180.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stupien G, Florian C, Roullet P. Involvement of the hippocampal CA3-region in acquisition and in memory consolidation of spatial but not in object information in mice. Neurobiology of Learning and Memory. 2003;80:32–41. doi: 10.1016/s1074-7427(03)00022-4. [DOI] [PubMed] [Google Scholar]
- Sugaya K, Greene R, Personett D, Robbins M, Kent C, Bryan D, et al. Septo-hippocampal cholinergic and neurotrophin markers in age-induced cognitive decline. Neurobiology of Aging. 1998;19:351–361. doi: 10.1016/s0197-4580(98)00072-4. [DOI] [PubMed] [Google Scholar]
- Sze CI, Troncoso JC, Kawas C, Mouton P, Price DL, Martin LJ. Loss of the presynaptic vesicle protein synaptophysin in hippocampus correlates with cognitive decline in Alzheimer disease. Journal of Neuropathology and Experimental Neurology. 1997;56:933–944. doi: 10.1097/00005072-199708000-00011. [DOI] [PubMed] [Google Scholar]
- Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, et al. Physical basis of cognitive alterations in Alzheimer's disease: Synapse loss is the major correlate of cognitive impairment. Annals of Neurology. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- Vaucher E, Reymond I, Najaffe R, Kar S, Quirion R, Miller MM, et al. Estrogen effects on object memory and cholinergic receptors in young and old female mice. Neurobiology of Aging. 2002;23:87–95. doi: 10.1016/s0197-4580(01)00250-0. [DOI] [PubMed] [Google Scholar]
- Vicario-Abejon C, Owens D, McKay R, Segal M. Role of neurotrophins in central synapse formation and stabilization. Nature Reviews Neuroscience. 2002;3:965–974. doi: 10.1038/nrn988. [DOI] [PubMed] [Google Scholar]
- Wiedenmann B, Franke WW. Identification and localization of synaptophysin, an integral membrane glycoprotein of Mr 38,000 characteristic of presynaptic vesicles. Cell. 1985;41:1017–1028. doi: 10.1016/s0092-8674(85)80082-9. [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. Journal of Neuroscience. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. Journal of Comparative Neurology. 1993;336:293–306. doi: 10.1002/cne.903360210. [DOI] [PubMed] [Google Scholar]
- Zhan SS, Beyreuther K, Schmitt HP. Quantitative assessment of the synaptophysin immuno-reactivity of the cortical neuropil in various neurodegenerative disorders with dementia. Dementia. 1993;4(2):66–74. doi: 10.1159/000107299. [DOI] [PubMed] [Google Scholar]