Abstract
The end9-1 (arc35-1) mutant was identified as an endocytosis mutant and is a mutant allele of ARC35 that encodes a subunit of the Arp2/3 complex. As for other mutants in the Arp2/3 complex, arc35-1 is defective for endocytosis and organization of the actin cytoskeleton. Both defects can be suppressed by overexpression of calmodulin. Analysis of a collection of temperature-sensitive cmd1 mutants for their ability to suppress either the endocytic defect and/or the actin defect indicates that the two defects are tightly coupled. We demonstrate that Arc35p and Cmd1p interact and that Arc35p is required for cortical localization of calmodulin. This is the first report linking Arp2/3 complex function with calmodulin through which it exercises at least one of its endocytic functions.
INTRODUCTION
Endocytosis is the process whereby cells internalize part of their own plasma membrane along with extracellular material facilitating uptake of nutrients, down-regulation of receptors, and removal of damaged or surplus proteins. Endocytosis is mediated by vesicles budding from the cell surface and the delivery of endocytosed material to the lysosome via at least two intermediate compartments (Singer-Krüger et al., 1993; Mukherjee et al., 1997; Clague, 1998). Work in yeast (Riezman et al., 1996; Wendland et al., 1998) indicates that the actin cytoskeleton plays a fundamental role in endocytosis. Recent evidence, obtained mainly by using the inhibitors cytochalasin D and latrunculin A (latA), suggests an involvement of the actin cytoskeleton in the uptake step of endocytosis in animal cells (Gottlieb et al., 1993; Jackman et al., 1994; Durrbach et al., 1996; Shurety et al., 1996; Lamaze et al., 1997; Freedman et al., 1999; Valentijn et al., 1999).
The development of quantitative assays for receptor-mediated endocytosis in the yeast Saccharomyces cerevisiae (Dulic et al., 1991) combined with genetics has led to the identification of numerous genes involved in the internalization step of endocytosis (Munn and Riezman, 1994). Genes regulating lipid metabolism and various genes involved in building or regulating the actin cytoskeleton were isolated in the screen. In addition, calmodulin (Kübler et al., 1994) and the actin-related protein Arp2 (Moreau et al., 1997), which regulate the actin cytoskeleton, were shown to be involved in the uptake step of endocytosis. Other screens have also led to the isolation of endocytosis mutants affecting actin dynamics (Wendland et al., 1996; Singer-Krüger and Ferro-Novick, 1997).
Calmodulin is a major calcium sensor conserved from yeast to human. One single essential gene (CMD1) encodes calmodulin in Saccharomyces cerevisiae (Davis et al., 1986). Calmodulin plays a fundamental role in endocytosis in yeast (Kübler et al., 1994; Geli et al., 1998). The calmodulin requirement may be calcium-independent because the mutant cmd1-3, impaired in calcium binding in vitro (Geiser et al., 1991), allowed virtually wild-type internalization kinetics (Kübler et al., 1994). Calmodulin has been purified from clathrin-coated vesicles and was shown to interact with clathrin light-chain (Salisbury et al., 1980; Pley et al., 1995). Calmodulin antagonists were shown to stimulate endocytosis of ricin in Madin–Darby canine kidney (MDCK) cells (Llorente et al., 1996) and sequestration of serotonin 5-HT1A receptor in CHO cells (Della Rocca et al., 1999). On the other hand, calmodulin antagonists inhibited transcytosis of IgA in MDCK cells (Enrich et al., 1996) and sequestration of histamine H1 receptors in human astrocytoma cells (Hishinuma et al., 1998).
The Arp2/3 complex has been purified from Acanthamoeba (Machesky et al., 1994), human platelets (Welch et al., 1997b), human neutrophils (Machesky et al., 1997), and S. cerevisiae (Winter et al., 1997). It has been shown to consist of seven subunits in Acanthamoeba and human and of six subunits in yeast. Two of the subunits are the highly conserved actin-related proteins, Arp2 and Arp3. Components of the Arp2/3 complex have been localized to the cortical actin cytoskeleton in Acanthamoeba (Kelleher et al., 1995), in human (Welch et al., 1997a), in S. cerevisiae (Moreau et al., 1996; Winter et al., 1997), and in Schizosaccharomyces pombe (McCollum et al., 1996). Activities demonstrated for the Arp2/3 complex include actin (Mullins et al., 1997) and profilin binding (Machesky et al., 1994), nucleation of actin polymerization and induction of Listeria movement (Welch et al., 1998), pointed end capping and branching of actin filaments (Mullins et al., 1998), and actin patch movement and integrity (Winter et al., 1997). Mutations in the S. cerevisiae Arp2 gene have been found to cause a defect in the internalization step of endocytosis (Moreau et al., 1997). The above findings lead to the exciting possibility that the Arp2/3 complex may organize actin filaments at the sites of membrane dynamics (Machesky, 1997).
In this study, we demonstrate that mutations in the ARC35 gene lead to defects both in the internalization step of endocytosis and in the organization of the actin cytoskeleton. Both the endocytic and actin defects can be suppressed by overexpression of calmodulin. Analysis of a series of temperature-sensitive (ts) cmd1 mutant alleles for their ability to suppress the endocytic defect, the actin defect, and/or ts growth indicates that the endocytic and actin defects are tightly coupled. Two-hybrid and coimmunoprecipitation data indicate that Arc35p and Cmd1p interact. The involvement of Arc35p in endocytosis is likely to involve localization of Cmd1p to cortical sites for interaction with an essential endocytic target. Taken together, the data presented provide a novel function of the Arp2/3 complex in endocytosis via calmodulin.
MATERIALS AND METHODS
Yeast Media, Strains, and Genetic Techniques
Unless mentioned otherwise, strains without plasmids were grown in complete medium YPUADT (2% glucose, 2% peptone, 1% yeast extract, 40 μg/ml uracil [Ura], 40 μg/ml adenine, and 40 μg/ml tryptophan [Trp], 2% agar for solid medium). Strains bearing plasmids were selected on SD minimal medium containing the required nutritional supplements (Dulic et al., 1991). Sporulation, tetrad dissection, and scoring of genetic markers were performed as described (Sherman et al., 1974). Recombinant lyticase was purified from Escherichia coli as described (Hicke et al., 1997). Transformation of yeast cells was accomplished by the lithium acetate method (Gietz et al., 1992).
All yeast strains used in this study are listed in Table 1. RH3431 was generated by crossing RH2877 and RH2616. RH3429 was generated from RH3431 by inducing sporulation on minimal medium, by tetrad dissection, and by scoring of adequate markers. For the construction of RH4161, CEN.PK2 was transformed with a green fluorescent protein (GFP)-kanMX4 cassette for C-terminal tagging of ARC35 (Wach et al., 1997) amplified with the following oligos using pFA6a-GFP-kanMX4 as a template: ARC35GFPUS (5′-CAA CAG GCA AGA AGA ACC TTC ACC GGT AGA AAG ATT GTC TAC GGT CGA CGG ATC CCC GGG) and ARC35GFPDS (5′-TAT AAC CCT TTT TAC GGA TTC TTA CGT ACT TAT TTA ATC CAT CGA TGA ATT CGA GCT CG). Homology to ARC35 is shown in bold, and homology to pFA6a-GFP-kanMX4 is shown in italic. The resulting strain was sporulated, the tetrads were dissected, and the adequate markers were selected. Strain RH4164 was constructed by transforming strain B81 with the LYS2-cassette for disrupting BAR1 (Kübler and Riezman, 1993).
Table 1.
Yeast strains
| Strain | Relevant markers | Reference |
|---|---|---|
| B81 | Mata arp2-1∷URA3 ade2 ura3 leu2 trp1 his3 lys2 | B. Winsor |
| CEN.PK2 | Mata/Matα his3/his3 leu2/leu2 ura3/ura3 trp1/trp1 | K.D. Entian |
| DBY7443 | Mata cmd1-231 his3 leu2 ura3 lys2Δ trp1 bar1 | D. Botstein |
| DBY7445 | Mata cmd1-228 his3 leu2 ura3 lys2Δ trp1 bar1 | D. Botstein |
| DBY7446 | Mata cmd1-226 his3 leu2 ura3 lys2Δ trp1 bar1 | D. Botstein |
| DBY7449 | Mata cmd1-239 his3 leu2 ura3 lys2Δ trp1 bar1 | D. Botstein |
| EGY191 | Mata his3 leu2∷pLEU2-lexAop2 ura3 trp1 | Estojak et al., 1995 |
| RH2584 | Mata cmd1-3 his4 leu2 ura3 bar1 | Kübler et al., 1994 |
| RH2616 | Matα arc35-1 his4 leu2 ura3 bar1 | Riezman collection |
| RH2877 | Mata leu2 ura3 lys2 ade2 trp1 bar1 | Riezman collection |
| RH2962 | Mata/α arc35∷URA3/ARC35 his4/his4 leu2/leu2 ura3/ura3 lys2/lys2 bar1/bar1 | Riezman collection |
| RH3429 | Mata arc35-1 his4 leu2 ura3 lys2 ade2 bar1 | This study |
| RH3431 | Mata/α arc35-1/ARC35 his4/HIS4 leu2/leu2 ura3/ura3 lys2/LYS2 ade2/ADE2 trp1/TRP1 bar1/bar1 | This study |
| RH4161 | Mata ARC35-GFP∷kanMX4 his3 leu2 ura3 trp1 | This study |
| RH4164 | Mata arp2-1∷URA3 ade2 ura3 leu2 trp1 his3 lys2 bar1∷LYS2 | This study |
| Y90 | Matα arp3-1∷LEU2 ade2 ura3 leu2 trp1 his3 lys2 | B. Winsor |
DNA Techniques and Plasmid Constructions
All DNA manipulations were performed according to standard techniques (Sambrook et al., 1989) unless specified otherwise. Restriction enzymes, Klenow, T4 DNA polymerase, calf intestine phosphatase (CIP), and T4 DNA ligase were obtained from Boehringer Mannheim (Indianapolis, IN), New England Biolabs (Beverly, MA), United States Biochemical (Buckinghamshire, United Kingdom), or Stratagene Cloning Systems (La Jolla, CA). All DNA fragments were purified with the Geneclean II Kit (Bio 101, Vista, CA); all plasmids were purified with the Qiagen plasmid purification kit (Qiagen, Hilden, Germany), and transformation of E. coli was performed by electroporation (Dower et al., 1988). All PCRs for cloning purposes were performed with a DNA polymerase with proof-reading activity (Pfu, Stratagene Cloning Systems) on a TRIO-thermoblock (Biometra GmbH, Goettingen, Germany). Oligonucleotides were synthesized by Microsynth GmbH (Balgach, Switzerland). All constructs were sequenced using a Big Dye Terminator Cycle Sequencing Ready Reaction Kit (Applied Biosystems, Foster City, CA) on an ABI 377 automated sequencer (Applied Biosystems).
All plasmids used in this study are listed in Table 2. Plasmid pASZ12::ARC35 was constructed by removing the PstI/XbaI fragment spanning the complete ARC35 gene and 125 bp upstream, 2000 bp downstream sequence approximately, respectively, from a genomic library in plasmid YCplac111 (Gietz and Sugino, 1988) followed by Klenow and T4 DNA polymerase treatment and ligation into pASZ12 (Stotz and Linder, 1990), cut open at SmaI, and treated with CIP. The arc35-1 allele was amplified from genomic DNA from the strain RH3429 with the primers ARC352HYB-1 (5′-GGC CGA CTC GAG CTA CAC TTA CAA CCA) and ARC352HYB-2 (5′-GGC CGA CTC GAG CGT ACT TAT TTA ATC), both of which include an XhoI site for in-frame ligation to the lexA DNA binding domain of pEG202 (Ruden et al., 1991), cut with XhoI, and treated with CIP. The cmd1 mutant alleles for cloning into pASZ12 were amplified from genomic DNA of the strains RH2584, DBY7443, DBY7449, DBY7446, and DBY7445 or from plasmid pYOC547 with the primers CMDASZ-1 (5′-ACG CGT CGA CAT GTA TTT ATA TTT TCG TGT A) and CMDASZ-2 (5′-ACG CGT CGA CAG AAT GGT AAG GGT AAG ATA G) for RH2584 or primers CMDASZ-3 (5′-CGC GGA TCC ATG TAT TTA TAT TTT CGT GTA) and CMDASZ-4 (5′-TGA CAT GCA TGC AGA ATG GTA AGG GTA AGA TAG) for all other alleles. Primers CMDASZ-1 and CMDASZ-2 include a SalI restriction site for cloning into pASZ12 (Stotz and Linder, 1990), cut open at SalI, and treated with CIP. Primers CMDASZ-3 and CMDASZ-4 bear a BamHI or SphI site for cloning into pASZ12 (Stotz and Linder, 1990), cut open with BamHI and SphI, and treated with CIP. The cmd1 mutant alleles for the construction of the pJG4–5 series were amplified as described using the primers CMD2H-1 (5′-CCG CTC GAG TCC TCC AAT CTT ACC GAA GAA) and CMD2H-2 (5′-CCG CTC GAG CTA TTT AGA TAA CAA AGC AGC). Both primers bear an XhoI restriction site for in-frame cloning to the acid blob domain B42 of pJG4-5 (Gyuris et al., 1993), cut with XhoI, and treated with CIP.
Table 2.
Plasmids
| Plasmid | Yeast ori | Marker | Insert | Reference |
|---|---|---|---|---|
| pASZ12 | 2 μ | ADE2 | A. Stotz and P. Linder, 1990 | |
| pASZ12∷ARC35 | 2 μ | ADE2 | ARC35 | This study |
| pCMD1 | 2 μ | ADE2 | CMD1 | M.I. Geli |
| pASZ12∷cmd1-3 | 2 μ | ADE2 | cmd1-3 | This study |
| pASZ12∷cmd1-226 | 2 μ | ADE2 | cmd1-226 | This study |
| pASZ12∷cmd1-228 | 2 μ | ADE2 | cmd1-228 | This study |
| pASZ12∷cmd1-239 | 2 μ | ADE2 | cmd1-239 | This study |
| pASZ12∷cmd1-231 | 2 μ | ADE2 | cmd1-231 | This study |
| pASZ12∷cmd1-247 | 2 μ | ADE2 | cmd1-247 | This study |
| pYOC547 | CEN | TRP1 | GAL1∷cmd1-247 | Y. Ohya and D. Botstein, 1994b |
| pSH18-34 | 2 μ | URA3 | 8 lexA op. lacZ | Gyuris et al., 1993 |
| pRFHM-1 | 2 μ | HIS3 | lexAbicoid | Gyuris et al., 1993 |
| pEG202∷ARC35 | 2 μ | HIS3 | lexAARC35 | R. Lombardi and H. Riezman, 1997 |
| pEG202∷arc35-1 | 2 μ | HIS3 | lexAarc35-1 | This study |
| pJG4-5∷CMD1 | 2 μ | TRP1 | B42CMD1 | R. Lombardi and H. Riezman, 1997 |
| pJG4-5∷cmd1-3 | 2 μ | TRP1 | B42cmd1-3 | This study |
| pJG4-5∷cmd1-226 | 2 μ | TRP1 | B42cmd1-226 | This study |
| pJG4-5∷cmd1-228 | 2 μ | TRP1 | B42cmd1-228 | This study |
| pJG4-5∷cmd1-239 | 2 μ | TRP1 | B42cmd1-239 | This study |
| pJG4-5∷cmd1-231 | 2 μ | TRP1 | B42cmd1-231 | This study |
| pJG4-5∷cmd1-247 | 2 μ | TRP1 | B42cmd1-247 | This study |
Protein Techniques and Antibodies
SDS-PAGE for protein separation was performed as described (Laemmli, 1970) using a Minigel System (Hoefer Scientific Instruments, San Francisco, CA). Acrylamide gels (16.5%) were used to separate Cmd1p, Arc35p, and Act1p. Low-range SDS-PAGE molecular weight standards (Bio-Rad Laboratories, Hercules, CA) were used for determination of apparent molecular weight. Total yeast protein extractions were performed as described (Horvath and Riezman, 1994). Western blotting was performed as described (Horvath and Riezman, 1994), except for the use of 10% (wt/vol) milk and the addition of 0.1% (vol/vol) Nonidet P-40 in the solutions for blocking and subsequent incubations. Incubation with the primary antibodies, α-Cmd1p and α-Arc35p-N at a dilution of 1:1000 and α-actin (Roche Diagnostics, Mannheim, Germany) at 10 μg/ml, was followed by incubation with the secondary antibody conjugated to horseradish peroxidase (Sigma, St. Louis, MO). Membranes (Schleicher and Schuell, Dassel, Germany) were developed using an ECL immunoblotting detection reagent kit (Amersham, Arlington Heights, IL).
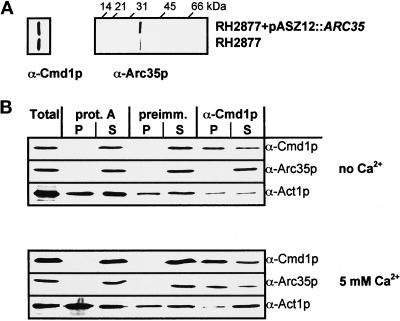
The antibody against Cmd1p has been described (Geli et al., 1998). The α-Arc35pN antibody was raised in rabbits against the Arc35p peptide LHLQPQNLLIQTK synthesized by Neosystem Laboratoire (Strasbourg, France). Arc35p antibody specificity was assessed on immunoblots by comparing extracts from cells expressing ARC35 and ARC35 on a 2-μ plasmid (see Figure 8A).
Figure 8.
Coimmunoprecipitation of Arc35p and Cmd1p. (A) To assess the specificity of the Arc35p antibody, Wt cells expressing either endogenous Arc35p or overexpressing Arc35p (RH2877+pASZ12::ARC35) were grown in selective medium and harvested, and proteins were extracted. The extracts were immunoblotted for Arc35p and Cmd1p as a loading control. (B) A Wt strain (RH2877) was grown to logarithmic phase and harvested. Total and native protein extracts were prepared. Protein A-Sepharose (prot. A), protein A-Sepharose bound to preimmune serum (preimm.), or protein A-Sepharose bound to α-Cmd1p serum (α-Cmd1p) was used for immunoprecipitation of native cell extracts in the presence of no calcium or 5 mM calcium. Immunoprecipitates (P) and supernatants (S) were immunoblotted for Arc35p, Cmd1p, or Act1p with α-Arc35p-N, α-Cmd1p, or α-actin antibodies.
Microscopy
The procedure used for immunolocalization of calmodulin was adapted from Sohrmann et al., 1996. Fixation was performed during a period of 90 min by adding formaldehyde directly to the culture to a final concentration of 3.7%. Incubation with the primary antibody or the secondary antibody coupled to CY3 was performed for 2 h or 30 min, respectively, at room temperature.
The calmodulin antibody was depleted of antibodies against chitin by passage over a chitin affinity column for use in immunofluorescence experiments. Briefly, 15 ml packed chitin particles were washed with 100 ml CMF-PBS (137 mM NaCl, 2.7 mM KCl, 1.5 mM Na2HPO4, 8.1 mM KH2PO4) followed by 100 ml CMF-PBS + 0.4 M NaCl, 100 ml 0.1 M HCl, and 400 ml CMF-PBS. The calmodulin antibody was loaded onto the column followed by washing with CMF-PBS. A 15-ml fraction corresponding to the void volume of the column was collected. Flow-through fractions in CMF-PBS were collected and used for immunolocalization of calmodulin in a dilution of 1:100 in PEMBAL. For the background control, the purified calmodulin antibody was preabsorbed with 60 μg recombinant GST-calmodulin from E. coli.
Labeling of actin with rhodamine–phalloidin was performed as described (Bénédetti et al., 1994). To preserve the GFP signal, sorbitol was added to a final concentration of 1 M, and the pH was adjusted to 7.5 during the prefixation and fixation processes.
For localizing GFP chimeras, strains were grown in SD minimal medium supplied with required nutrients. Cells were collected by centrifugation, resuspended in 1 × PBS (137 mM NaCl, 2.7 mM KCl, 3.19 mM Na2HPO4, 2.67 mM KH2PO4), and mounted onto poly-l-lysine–treated slides. Cells were observed using a Zeiss Axiophot microscope with a 100× objective. Images were taken with a Zeiss camera coupled to a video system (MWG Biotech, Ebersburg, Germany). Exposure times and other processing parameters were adjusted to the signal strength of the sample.
Latrunculin A Sensitivity
Halo assays were used for determining latrunculin A sensitivity (Ayscough et al., 1997). Relative apparent sensitivities were calculated according to Reneke et al. (1988).
α-Factor Uptake Assay
35S α-factor uptake assays were performed as described (Dulic et al., 1991). The samples were processed as described above. The internalization rates were calculated as the percentage of counts internalized per minute between 2.5 and 15 min. All uptake assays were performed at least twice.
Two-Hybrid Techniques
The interaction trap two-hybrid system was used (Gyuris et al., 1993). The assay was performed as described (Geli et al., 1998). The X-Gal plates (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) were supplied with the indicated concentration of calcium in the form of CaCl2. Pictures were taken after 1 d of growth at 37°C. Expression of all lexA-Arc35p fusion proteins was confirmed by immunoblotting using the α-Arc35p-N antibody. Expression of the B42-Cmd1p, B42-Cmd1–226p, B42-Cmd1–228p, B42-Cmd1–239p, B42-Cmd1–231p, and B42-Cmd1–247p fusion proteins was confirmed on total protein extractions of cultures grown in SD/Gal/Raff-Ura-His-Trp by immunoblotting using the calmodulin antibody. All lexA fusions and B42 fusions were tested for their ability to activate transcription of the lacZ gene without the prey or bait, respectively. None of them was found to induce transcription significantly.
Native Protein Extracts and Coimmunoprecipitations
Cells (2 × 109) were grown to a density of 1 × 107 cells/ml in YPUADT. Cells were collected by centrifugation, resuspended in 100 ml 0.1 M Tris-SO4, pH 9.4, containing 28 mM 2-mercaptoethanol, and incubated at 24°C for 30 min. Cells were collected by centrifugation, resuspended in 20 ml spheroplasting buffer (2% yeast extract, 1% peptone, 0.7 M sorbitol, 10 mM Tris-HCl, pH 8.0, 0.6 U lyticase), and incubated for 90 min at 24°C. Spheroplasts were collected, washed in 20 ml washing buffer (0.7 M sorbitol, 0.1 M NaCl, 10 mM Tris-HCl, pH 8.0, 5 mM MgCl2), resuspended in 1 ml lysis buffer (20 mM MES, pH 6.7, 0.1 M NaCl, 5 mM MgCl2, 0.5 mM PMSF, 1 μg/ml each leupeptin, pepstatin, antipain) supplemented with the indicated concentration of CaCl2, and dounce-lysed on ice with 40 strokes. Addition of a nonionic detergent such as Nonidet-P-40 to the lysis buffer did not affect the results. The lysate was spun to remove cellular debris. The lysate (500 μl) was added to the antibodies preabsorbed on protein A-Sepharose beads and incubated for 2 h at 4°C. The beads were subjected to three washes. The pellet was resuspended in 300 μl 125 mM Tris-HCl 6.8, 2% SDS, 0.1 M DTT, 30% glycerol, 5% β-mercaptoethanol, and bromophenol blue, boiled for 5 min, centrifuged for 5 min, and analyzed by SDS-PAGE. The supernatant from the antibody incubation was precipitated with trichloroacetic acid and resuspended in 300 μl as described for the pellet. Fifty microliters of each pellet and supernatant were loaded onto the gel.
RESULTS
Arc35p Is Required for Endocytosis and Organization of the Actin Cytoskeleton
The END9 gene has been identified in a screen for endocytosis-deficient mutants (Munn and Riezman, 1994). END9 was found to be identical to ARC35, which encodes the 35-kDa subunit of the Arp2/3 complex (Winter et al., 1997). Genetic analysis has revealed that the ARC35 gene is essential for cell viability. Dissection of a diploid strain in which one allele of ARC35 was replaced with the URA3 marker (RH2962) resulted in a 2:2 (viable/dead) spore segregation. Eighteen tetrads gave two viable spores, and two tetrads gave only one viable spore. All of the viable spores were ura3, indicating that deletion of ARC35 in our strain background is lethal (our unpublished results). A ts strain carrying the arc35-1 mutation is viable at 24°C but undergoes growth arrest after temperature shift to 37°C (see Figure 4B, sector A). Sequencing of the genomic copy of arc35-1 revealed two mutations. Nucleotide 1023 was changed from C to T, resulting in a silent mutation in Val341, and nucleotide 1024 was changed from T to G, resulting in replacement of Tyr342 by Asp.
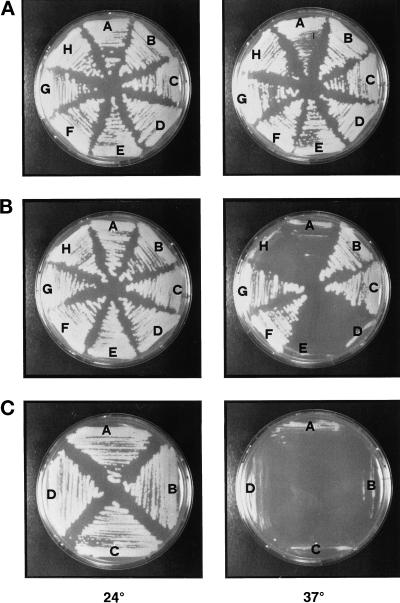
Figure 4.
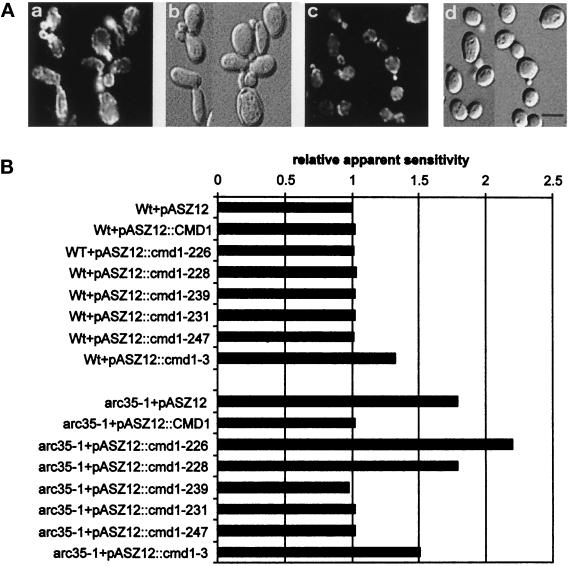
Suppression of arc35-1 ts growth by calmodulin. Wt (RH2877) (A), arc35-1 (RH3429) (B), and arp2-1 (RH4164) (C) strains were transformed with the following plasmids: (A) pASZ12, (B) pCMD1, (C) pASZ12::cmd1-226 (actin), (D) pASZ12::cmd1-228 (calmodulin mislocalized), (E) pASZ12::cmd1-239 (nuclear defect), (F) pASZ12::cmd1-231 (bud emergence), (G) pASZ12::cmd1-247 (endocytosis), (H) pASZ12::cmd1-3 (calcium binding). The strains were grown on glucose-selective medium for 3 d at 24°C and for 2 d at 37°C.
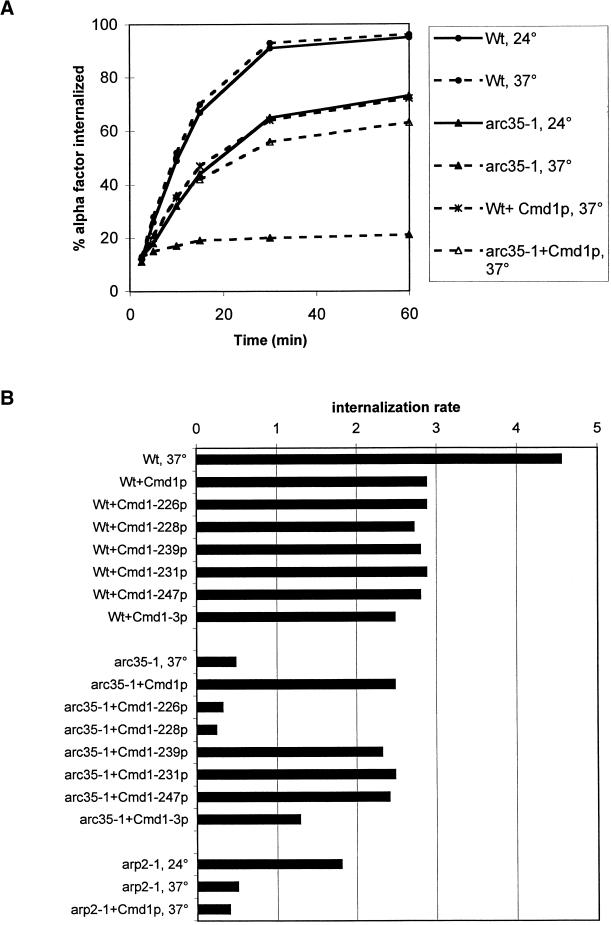
The arc35-1 mutant was shown to be impaired in the internalization of radiolabeled α-factor after a preincubation of 15 min at 37°C (Munn and Riezman, 1994). We found that the mutant was already significantly slowed down for endocytosis at the permissive temperature of 24°C when compared with an isogenic wild-type (Wt) strain (Figure 1A, Wt, 24°C and arc35-1, 24°C). While initial internalization of α-factor still occurred in the mutant upon a 15-min preincubation at 37°C (Munn and Riezman, 1994), a preincubation of 20 min abolished the internalization of α-factor almost completely (Figure 1A, arc35-1, 37°C). Under the same conditions, the isogenic Wt strain internalized α-factor with identical kinetics as at 24°C (Figure 1A, Wt, 37°C).
Figure 1.
Overexpression of calmodulin and distinct calmodulin mutant alleles restores endocytosis in arc35-1. (A) Wt (RH2877) and arc35-1 (RH3429) strains without plasmid or bearing plasmid pCMD1 (+Cmd1p) were assayed for their ability to internalize radioactive α-factor at 24 and 37°C after a preincubation of 20 min at the indicated temperature. (B) Wt (RH2877), arc35-1 (RH3429), and arp2-1 (RH4164) strains without plasmid or overexpressing the indicated mutant calmodulin protein from pASZ12 were assayed for the internalization of radioactive α-factor at 24 or 37°C after a preincubation of 20 min. The results are presented as the percentage of radioactive α-factor internalized per minute during the linear range of the uptake reaction.
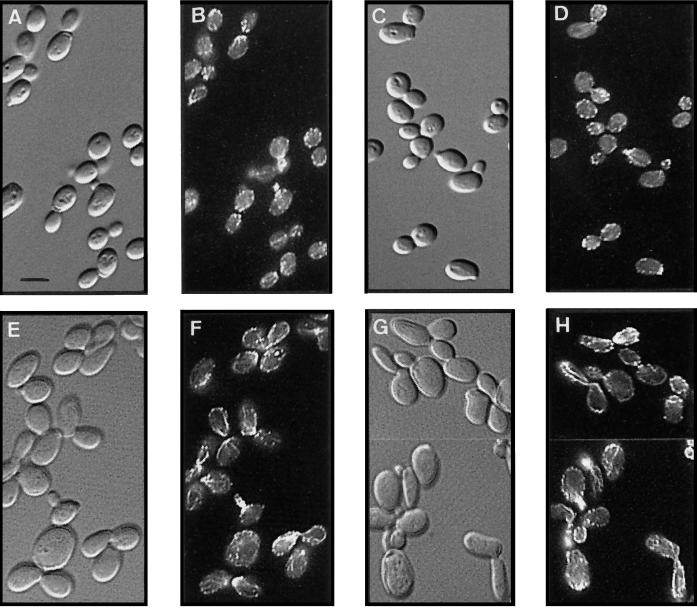
Because actin is required for endocytosis (Kübler and Riezman, 1993) and mutations in the Arp2/3 complex lead to actin defects (Moreau et al., 1996; Winter et al., 1997, 1999), we examined the organization of the actin cytoskeleton in an arc35-1 strain. Wt and arc35-1 cells were stained for filamentous actin (F-actin) at 24°C and after a preincubation of 3 h at 37°C (Figure 2). Microscopic inspection of the arc35-1 cultures at both 24°C (Figure 2E) and 37°C (Figure 2G) revealed that the mutant cells were considerably larger and more elongated than the Wt cells (Figure 2, A and C). Wt cells showed the expected actin staining at both temperatures (Figure 2, B and D), with F-actin localizing predominantly to the sites of active cell growth (Adams and Pringle, 1984). The arc35-1 mutant displayed mild defects in the organization of the actin cytoskeleton at both 24°C (Figure 2F) and 37°C (Figure 2H). The most obvious defect was the presence of highly staining actin cables that followed the cell cortex in both mother and daughter cells. Actin patches were often mislocalized to the bud neck and were apparently clustered into aggregates as described for an arc35Δ strain (Winter et al., 1999). The actin defects for other mutants in the Arp2/3 complex contrast with that of arc35-1 in that no increased staining of the actin cables was observed (Moreau et al., 1996; Winter et al., 1997, 1999; our own observation). This finding emphasizes the importance of the Arp2/3 complex in organization of the actin cytoskeleton and points to possibly different or multiple functions of the subunits in the process.
Figure 2.
Defective actin organization in arc35-1 cells. Wt (RH2877) and arc35-1 (RH3429) strains were preincubated for 3 h at either 24 or 37°C, fixed, stained with rhodamine–phalloidin, and visualized either by Nomarski optics or by epifluorescence. Shown are the Wt strain at 24°C (A, B) and 37°C (C, D) and the arc35-1 strain at 24°C (E, F) and 37°C (G, H), respectively. Bar, 5 μm.
Arc35-GFP Colocalizes with Filamentous Actin
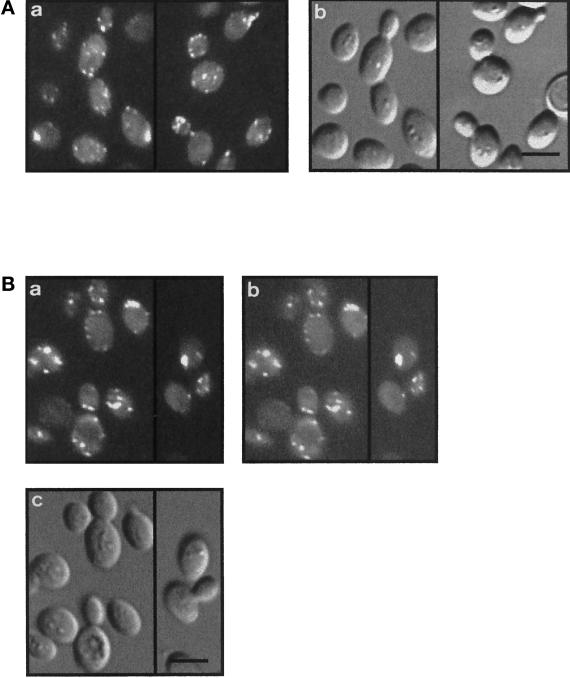
Both Arp2 (Moreau et al., 1996) and Arp3 (Winter et al., 1997) have been localized to actin patches. Because Arc35p belongs to the same complex and exhibits an actin defect, we determined the localization of Arc35p. Because our α-Arc35p antibodies failed to recognize the protein in fixed cells (our unpublished results), we integrated an Arc35-GFP chimera at the ARC35 locus. The chimeric protein was fully functional based on cell morphology, F-actin stainings (Figure 3A), and endocytosis assays (our unpublished results). Arc35-GFP localized predominantly to sites of active growth in a Wt background (Figure 3A) with some cortical staining visible in the mother cell. Arc35-GFP fluorescence was not observed on cable-like structures. To test for colocalization of actin and Arc35p, an Arc35-GFP–expressing strain was stained to visualize F-actin (Figure 3B). Although integrity of the actin cytoskeleton was affected by the use of sorbitol during fixation to preserve Arc35-GFP, the signal from Arc35-GFP was found to overlap nicely with the staining of F-actin for all visible actin patches. Because the cables were lost when using this special fixation protocol, it was impossible to determine localization of Arc35-GFP to those structures.
Figure 3.
Arc35-GFP colocalizes with filamentous actin. (A) A Wt strain expressing Arc35-GFP (RH4161) was visualized with epifluorescence (a) or Nomarski optics (b). Bar, 5 μm. (B) A Wt strain expressing Arc35-GFP (RH4161) was grown at 24°C, stained for F-actin with rhodamine–phalloidin, and visualized either with Nomarski optics (c) or with epifluorescence filters suitable to distinguish between GFP and rhodamine signals. The F-actin staining is shown in a, and the signal of Arc35-GFP is shown in b. Bar, 5 μm.
Overexpression of Calmodulin Restores Growth of arc35–1 at 37°C
Because the arc35–1 mutant exhibited defects in actin organization, in endocytosis, and in nuclear division (Schaerer-Brodbeck and Riezman, 2000), analogous to cmd1 mutants, we wanted to test for a potential genetic interaction between Arc35p and Cmd1p. We found that overexpression of Cmd1p rescued the growth defect of arc35–1 at 37°C (Figure 4B, sector B). Growth of the Wt control strain was not affected by overexpression of Cmd1p at any temperature (Figure 4A). No suppression of the ts growth of various cmd1 mutants was observed upon overexpression of Arc35p (our unpublished results).
Intragenic complementation studies of ts cmd1 mutants have revealed at least four distinct, essential cellular functions for calmodulin (Ohya and Botstein, 1994). For a summary of the ts cmd1 mutant phenotypes, see Table 3. Class A mutants (e.g., cmd1-226) are defective in the organization of the actin cytoskeleton. Class B mutants (cmd1-228) mislocalize calmodulin. Class C mutants (cmd1-239) are defective for establishment/elongation of the nuclear spindle, and class D mutants (cmd1-231) do not form a bud. In addition, calmodulin performs at least two different functions in endocytosis (Geli et al., 1998). One function, affected by the cmd1-247 mutation and referred to as the Cmd1-247 pathway, acts through the type I myosins. The other function, impaired by the cmd1-228 mutation and referred to as the Cmd1-228 pathway, operates via an unknown target. The cmd1-226 mutation affects both endocytic functions of calmodulin. To examine genetically which of the functions of Cmd1p Arc35p interacts with, Wt and arc35-1 mutant cells were transformed with a series of high-copy plasmids expressing the ts cmd1 alleles described above. All of the resulting strains were tested for growth at 37°C. Overexpression of the mutant alleles did not affect growth of the Wt control strain at either 24 or 37°C (Figure 4A). The ts growth of arc35-1 (Figure 4B, sector A) could be suppressed by overexpression of Cmd1-226p (panel C), Cmd1-231p (panel F), or Cmd1-247p (panel G). Overexpression of neither Cmd1-228p (panel D) nor Cmd1-239p (panel E) could restore growth at 37°C to arc35–1. The lack of suppression by the B and C class calmodulin mutants (for a summary of the data, see Table 4) indicates that Arc35p may share two functions with calmodulin, calmodulin localization (Cmd1-228 pathway) and nuclear division (Cmd1-239 pathway). At least one of the functions of Arc35p may be calcium-dependent because overexpression of Cmd1-3p, which is defective for calcium binding in vitro (Geiser et al., 1991), did not compensate for loss of Arc35p activity (Figure 4B, panel H). Moreover, a genetic interaction between ARC35 and CMD1 was confirmed in crosses. An arc35-1 mutant was crossed to cmd1-3, cmd1-226, cmd1-228, cmd1-239, cmd1-231, or cmd1-247 strains. The resulting diploids were sporulated, and tetrads were dissected. No double mutants between arc35-1 and cmd1-3, cmd1-228, or cmd1-239 were obtained, indicating a synthetic lethal interaction (our unpublished results). This correlates well with the suppression of ts growth data.
Table 3.
Summary of the calmodulin mutant phenotypes
| Mutant allele | Class | Phenotype at 37°C | Endocytosis at 37°C |
|---|---|---|---|
| cmd1-226 | A | Delocalized actin | − |
| cmd1-228 | B | Delocalized calmodulin | − |
| cmd1-239 | C | Defective mitotic spindle formation | + |
| cmd1-231 | D | No bud emergence | + |
| cmd1-247 | − | ||
| cmd1-3 | No Ca2+ binding | + |
The phenotypes for one representative allele of each calmodulin mutant class are shown. These are based on results of Geiser et al. (1991), Ohya and Botstein (1994), and Geli et al. (1998).
Table 4.
Summary of the suppression of the defects observed in arc31-1 by different ts calmodulin mutant alleles
| Growth | Actin | Endocytosis | Interacts with Arc35p | |
|---|---|---|---|---|
| arc35-1, 24° | + | − | + | |
| arc35-1, 37° | − | − | − | |
| arc35-1 + pASZ12, 37° | − | − | − | |
| arc35-1 + Cmd1p, 37° | + | + | + | + |
| arc35-1 + Cmd1-226p, 37° | + | − | − | − |
| arc35-1 + Cmd1-228p, 37° | − | − | − | − |
| arc35-1 + Cmd1-239p, 37° | − | + | + | − |
| arc35-1 + Cmd1-231p, 37° | + | + | + | + |
| arc35-1 + Cmd1-247p, 37° | + | + | + | + |
| arc35-1 + Cmd1-3p, 37° | − | +/− | +/− | − |
Interestingly, the ts growth of an arp2-1 strain (Figure 4C) or an arp3-1 strain (our unpublished results) was not suppressed by overexpression of calmodulin or selected mutant calmodulin proteins. This suggests that the genetic interaction observed between the Arp2/3 complex and Cmd1p could be unique to Arc35p.
Overexpression of Cmd1p and Distinct Cmd1 Mutant Proteins Restores Endocytosis in arc35-1
We wanted to determine whether overexpression of Cmd1p suppresses the endocytic defect of arc35-1. A Wt and arc35-1 strain expressing Cmd1p were assayed for receptor-mediated endocytosis after a preincubation of 20 min at 37°C (Figure 1A). Overexpression of Cmd1p reduced the internalization kinetics slightly in a Wt background. In the arc35-1 background, the endocytic rate was increased to ∼80% of Wt levels upon overexpression of Cmd1p if compared with the mutant strain without plasmid.
The collection of ts cmd1 alleles was assayed for their ability to restore endocytosis in arc35-1 at 37°C upon overexpression (Figure 1B). None of the mutant alleles was found to decrease the internalization rate more than Cmd1p upon overexpression in a Wt background. This allowed a direct comparison of the internalization rates observed for the different arc35-1 strains. Overexpression of Cmd1-239p (nuclear defect), Cmd1-231p (bud emergence), or Cmd1-247p (myosin endocytic function) restored endocytosis in arc35-1 to almost Wt level as observed for overexpression of Cmd1p. Overexpression of Cmd1-226p or Cmd1-228p did not improve the internalization kinetics. Overexpression of Cmd1-3p restored endocytosis to 50% of the Wt level. The data (for a summary, see Table 4) suggest that Arc35p shares specifically one endocytic function with Cmd1p, namely the Cmd1-228 but not the Cmd1-247 pathway.
An involvement of other subunits of the Arp2/3 complex in endocytosis has been demonstrated previously (Moreau et al., 1997). We found that the ts arp2-1 strain, which fails to internalize uracil permease (Moreau et al., 1997), showed a reduced rate of α-factor internalization already at the permissive temperature (Figure 1B, arp2-1, 24°C). The internalization rate decreased further upon a 20 min preincubation at 37°C (arp2-1, 37°C). Overexpression of calmodulin did not improve the internalization kinetics. This points to different defects affecting endocytosis in mutants of the Arp2/3 complex and is in good correlation with the findings for the actin defects of the different mutants.
The Same Calmodulin Mutant Proteins that Restore Endocytosis Suppress the Actin Defect of arc35-1
It has been shown that defects in the organization of the actin cytoskeleton result frequently in increased or decreased sensitivity to the actin depolymerizing drug latA (Ayscough et al., 1997). We found that the arc35-1 mutation leads to almost twofold sensitivity to latA at 24°C if compared with a Wt control (Figure 5B; Wt+pASZ12, arc35-1+pASZ12). Overexpression of Cmd1p in arc35-1 resulted in a restoration of Wt sensitivity to latA (Figure 5B). In support of the idea that latA sensitivity is a measure of integrity of the actin cytoskeleton, the actin cytoskeleton of an arc35-1 strain expressing Cmd1p was found to be properly organized at 37°C (Figure 5A, panel c; compare with arc35-1 at the same temperature in panel a).
Figure 5.
Sensitivity of arc35-1 expressing various calmodulin mutant proteins to latrunculin A. (A) An arc35-1 strain (RH3429) without plasmid (a, b) or overexpressing Cmd1p from pCMD1 (c, d) was preincubated for 3 h, stained with rhodamine–phalloidin, and visualized with epifluorescence. Bar, 5 μm. (B) Wt (RH2877) and arc35-1 (RH3429) strains bearing the empty control plasmid (pASZ12) or overexpressing the indicated calmodulin mutant protein from pASZ12 were used for determination of the apparent relative sensitivity to latrunculin A.
In contrast to overexpression of Wt Cmd1p (Figure 5A, panel d), overexpression of most other ts cmd1 alleles in arc35-1 did not restore Wt morphology (Schaerer-Brodbeck and Riezman, 2000). We have shown previously that overexpression of Cmd1-228p did not restore proper actin localization in arc35-1 cells, whereas high doses of Cmd1-239p did (Schaerer-Brodbeck and Riezman, 2000); however, it may be misleading to draw any conclusions concerning restoration of the actin integrity in mutant cells with an abnormal morphology. Therefore, we determined sensitivity to latA of arc35-1 strains overexpressing the collection of ts cmd1 alleles as a measure for integrity of the actin cytoskeleton. Overexpression of any of the ts alleles, except for Cmd1-3p, did not alter the sensitivity of a Wt strain to latA (Figure 5B). Overexpression of Cmd1-3p increased the sensitivity of the Wt strain to latA 1.3-fold. Overexpression of Cmd1-239p, Cmd1-231p, or Cmd1-247p in arc35-1 restored latA sensitivity to Wt values (Figure 5B). Overexpression of Cmd1-228p did not decrease the sensitivity of arc35-1 to latA, whereas overexpression of Cmd1-226p increased the sensitivity of arc35-1 to latA. Overexpression of Cmd1-3p (calcium binding) resulted in some reduction of sensitivity of arc35-1 to latA, although the suppression was not complete. It is striking that calmodulin mutant proteins that cannot suppress the endocytic defect of arc35-1 cannot suppress the increased sensitivity to latA either.
Arc35p Is Required to Localize Calmodulin to Sites of Active Growth
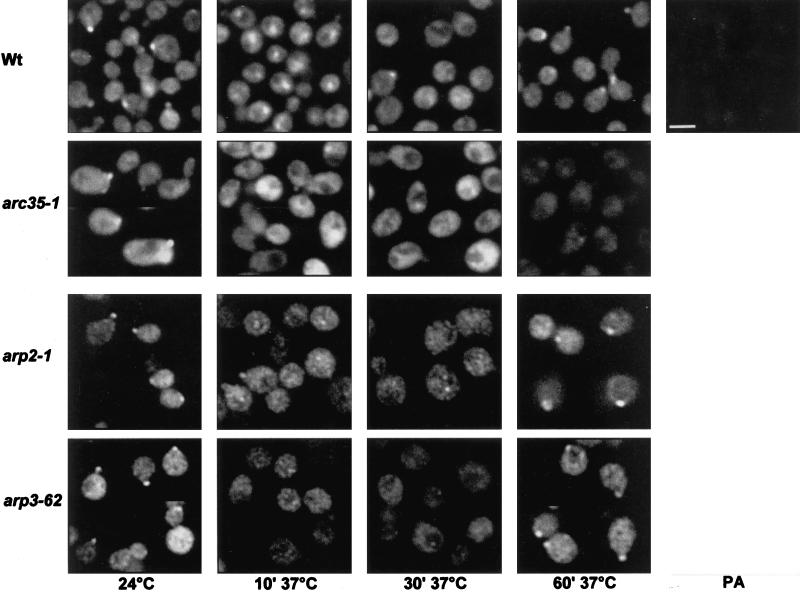
The pathway affected by cmd1-228 is required to localize calmodulin to the sites of active growth. Because the endocytic defect in an arc35-1 mutant is most likely linked to the Cmd1-228 function, we determined the intracellular localization of Cmd1p in an arc35-1 mutant at the restrictive temperature. Wt and arc35-1 strains were processed for immunofluorescence with the α-Cmd1p antibody after various time intervals at 37°C (Figure 6). The Wt strain showed localization of calmodulin to the sites of active growth at 24°C as described previously (Ayscough et al., 1997). After the shift to 37°C, calmodulin underwent a transient relocalization to the cytoplasm. Recovery of cortical calmodulin localization began 30 min after the shift and was completed after 1 h at 37°C. To confirm the specificity of the observed signal, the Wt strain was stained with calmodulin antibody that had been preabsorbed with recombinant calmodulin (PA). The fluorescent images revealed that the signal observed was due to calmodulin.
Figure 6.
Arc35p is required to localize calmodulin to the cortical sites. Wt (RH2877), arc35-1 (RH3429), arp2-1 (RH4164), and arp3-1 (Y90) strains were grown at 24°C and then shifted to 37°C for 10, 30, or 60 min before fixation and staining for calmodulin by indirect immunofluorescence. The staining obtained using preabsorbed α-calmodulin antibody is also shown (PA). Bar, 5 μm.
In arc35-1 mutant cells, Cmd1p was mainly localized to the sites of active growth, although increased cytoplasmic staining was observed (Figure 6, arc35-1, 24°C). Staining of calmodulin at sites of active growth disappeared completely upon shift for 10 min to 37°C as in Wt cells. In contrast to Wt cells, a relocalization of calmodulin to sites of active growth was not seen in the arc35-1 mutant at 37°C. In contrast, no mislocalization of Arc35-GFP in any of the ts cmd1 mutants was observed (our unpublished results), indicating that Arc35p localization does not depend on Cmd1p.
It should be considered that the observed mislocalization of Cmd1p in arc35-1 cells on shift to 37°C could be caused by misorganization of the actin cytoskeleton. To address this question, Cmd1p localization was analyzed in arp2 and arp3 mutants, which fail to organize the actin cytoskeleton properly at 37°C (Moreau et al., 1996; our own observation). At 24°C, both mutants localized Cmd1p to the sites of active growth (Figure 6). A short shift to 37°C caused mislocalization of Cmd1p to the cytoplasm as observed for Wt and arc35-1 cells. In contrast to the arc35-1 mutant, recovery of cortical calmodulin was observed in arp2 and arp3 mutants within 1 h at 37°C. Although the kinetics of recovery was slightly decreased when compared with Wt, disruption of the actin cytoskeleton by the arp2-1 and arp3-1 mutations did not affect the long-term localization of Cmd1p. It is therefore unlikely that the mislocalization of Cmd1p observed in arc35-1 cells is caused by the failure to organize the actin cytoskeleton properly. These data suggest that Arc35p is required to localize calmodulin to the sites of active growth.
Arc35p Interacts Specifically with Cmd1p
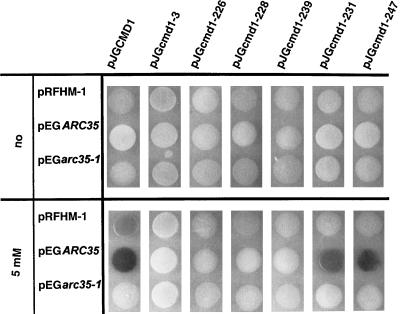
The data presented suggest a role for Arc35p as a regulator of calmodulin localization and probably function. We therefore investigated a possible physical interaction between Arc35p and Cmd1p with the two-hybrid system and coimmunoprecipitations. For the analysis with the yeast two-hybrid system, ARC35 and arc35-1 were used as baits (pEG series), and CMD1 and the collection of ts cmd1 mutant were used as prey (pJG series). β-Galactosidase activity indicative of interaction was monitored at 37°C on X-Gal–enriched plates containing no calcium or 5 mM calcium. Strong β-galactosidase activity was detected in the strain bearing the ARC35 bait and CMD1 prey on plates with 5 mM calcium (Figure 7).
Figure 7.
Two-hybrid interaction between Arc35p and Cmd1p. ARC35 and arc35-1 were cloned in frame to the lexA DNA binding domain of pEG202. CMD1 and the collection of ts cmd1 alleles were cloned in frame behind the B42 transactivation domain of pJG4-5. β-Galactosidase activity indicative of interaction was monitored on X-Gal plates at 37°C supplemented with no calcium or 5 mM calcium.
The observation that only some ts cmd1 alleles are capable of suppressing the ts growth and/or the endocytic defect of the arc35-1 strain prompted us to determine the interaction profile of Arc35p with the same calmodulin mutants (Figure 7). Strong β-galactosidase activity was detected in the strains bearing the ARC35 bait and either the cmd1-231 or cmd1-247 prey. Strains bearing the ARC35 bait and the cmd1-226, cmd1-228, or cmd1-239 preys did not show detectable β-galactosidase activity. These data (for a summary, see Table 4) support the hypothesis that Arc35p exerts its functions via the pathways affected by cmd1-226/228 and cmd1-239 alleles. No interaction was monitored in a strain expressing the ARC35 bait and the cmd1-3 prey, indicating Ca2+-dependent binding. β-galactosidase activity was not observed with the cmd1 preys when the ARC35 bait was replaced with arc35-1 as a bait, showing that functional Arc35p is required for this interaction. The strain bearing the Drosophila bicoid protein as a control for the specificity of the assay did not show any significant interaction.
To confirm the interaction seen in the two-hybrid interaction trap, an extract of Wt cells was used for immunoprecipitations with the α-calmodulin antiserum in the presence or absence of 5 mM calcium. The immunoprecipitates (P) and supernatants (S) were assayed for Arc35p and Cmd1p content by immunoblotting. Arc35p was recovered significantly in the immunoprecipitates using Cmd1p antibody (α-Cmd1p) for immunoprecipitation in the presence of 5 mM calcium (Figure 8). No recovery was detected without calcium. At 5 mM calcium, no Arc35p was recovered in the immunoprecipitates when protein A-Sepharose (prot. A) or preimmune serum (preimm.) was used for immunoprecipitation, confirming the specificity of the interaction. Because the interaction between Arc35p and Cmd1p could be mediated by filamentous actin, the immunoprecipitates and supernatants were assayed for the actin content as well (Figure 8). Actin was found to precipitate nonspecifically in all control reactions where no Arc35p could be recovered in the immunopellets. It is therefore unlikely that filamentous actin mediates the observed interaction. Unlike experiments with the two-hybrid system, a strain expressing Cmd1-3p could not be used for an independent confirmation of the calcium dependence of the observed interaction because Cmd1-3p is not recognized by our antibody (our unpublished results).
DISCUSSION
The Arp2/3 complex is involved in the organization and integrity of the cortical actin cytoskeleton (Zigmond, 1998; for review, see Machesky and Gould, 1999). Analysis of an arc35-1 (ts) mutant, defective in a relatively uncharacterized component of the complex, also revealed defects in the organization of the actin cytoskeleton. The mutant displayed exaggerated actin cables along the mother–daughter cell axis, and F-actin aggregates localized predominantly in the bud neck. Although accumulation of F-actin aggregates has been demonstrated for arp2 (Moreau et al., 1996) and arp3 (Winter et al., 1997) mutants, an increase in actin cable staining has not been seen in arp2/3 mutants. The phenotype of arc35-1 suggests a role for the Arp2/3 complex, not only in actin polymerization (Welch et al., 1998) but also in dynamics of actin cables.
Suppression of arc35-1 by Calmodulin
We have shown that overexpression of calmodulin can suppress the ts growth, the endocytic defect, and the actin defect of the arc35-1 mutant. This suppression was specific to arc35-1 and was not observed for arp2-1 or arp3-1. The unusual actin defect of arc35-1 compared with other mutants in the Arp2/3 complex and the unique genetic interaction observed between Arc35p and Cmd1p suggest that Arc35p may regulate some aspects of F-actin dynamics via calmodulin and that its function may be different from other subunits of the complex; however, we cannot rule out that the differences we see between mutants in the Arp2/3 complex are due to the specific alleles rather than individual functions of the subunits.
We observed that overexpression of cmd1 alleles that do not affect endocytosis restored normal actin staining and endocytosis in the arc35-1 mutant. Overexpression of Cmd1-3p, which does not bind calcium (Geiser et al., 1991), could only partially suppress the endocytic and actin defect of arc35-1, although a ts cmd1–3 strain internalizes α-factor with Wt kinetics (Kübler et al., 1994). On the other hand, overexpression of two cmd1 alleles that affect endocytosis, cmd1-226 and cmd1-228, could not restore the actin and endocytic defects, whereas overexpression of another allele, cmd1-247, could. For a summary of the suppression data, see Table 4. These data are consistent with the previous hypothesis (Geli et al., 1998) that calmodulin plays at least two roles in endocytosis. One role is to regulate the type I myosin, and this function is affected by the cmd1-247 and cmd1-226 mutations. The target of the second function, which is affected by the cmd1-226 and cmd1-228 mutations, is still unknown. Our data would suggest that Arc35p is required for actin organization and endocytosis and that it is associated with the Cmd1-228 function of calmodulin. Thus, it is likely that the endocytic function defined by the arc35-1 mutation is independent of the type I myosins.
One striking observation is that both the cmd1-228 and arc35-1 mutants mislocalize cortical calmodulin after shift to the restrictive temperature. For other mutants in the Arp2/3 complex, recovery of cortical calmodulin after a shift to the restrictive temperature was observed, albeit with slower kinetics than in the Wt control strain. Because both arc35-1 and cmd1-228 mislocalize calmodulin and are defective for receptor-mediated endocytosis as well, it is tempting to speculate that the endocytic function of Arc35p is to localize cortical calmodulin. The endocytic defect could be a direct consequence of the mislocalization of calmodulin in the arc35-1 strain. One line of evidence argues against this simple model. In Wt cells, after short times of temperature shift to 37°C (up to 30 min), calmodulin is transiently localized to the cytoplasm. At the same time, endocytosis occurs with normal kinetics. We therefore postulate that Arc35p is not only required for localizing calmodulin cortically, but could be required for interaction of calmodulin with the target of the Cmd1-228 function. Prolonged incubation at 37°C results in localization of Cmd1p back to the cortical sites in Wt cells. It is possible that during this time at 37°C in which wild-type cells recover calmodulin localization, the target of the Cmd1-228 function remains in place; however, in arc35-1 cells, calmodulin is never relocalized cortically, perhaps because of lack of interaction with Arc35-1p. As a consequence, the target of the Cmd1-228 function may become mislocalized or inactivated, which might be the cause of the endocytic defect in both the cmd1-228 and arc35-1 mutants. This idea is supported by the observation that the endocytic defect of an arc35-1 strain is not immediate after a shift to restrictive temperature but requires 15–20 min preincubation.
Overexpression of some calmodulin mutant proteins suppressed the ts growth phenotype of arc35-1 cells, whereas others, such as Cmd1-228p and Cmd1-239p, could not. In addition, overexpression of Cmd1-226p in arc35-1 restored growth at 37°C without restoring endocytosis or actin organization. This indicates that the actin/endocytic defect of arc35-1 cells is not the only determinant of ts growth and suggests that Arc35p regulates another essential function through calmodulin. We have evidence that Arc35p is involved in spindle elongation during mitosis via a function affected by the cmd1-239 allele (Schaerer-Brodbeck and Riezman, 2000).
Arc35p Interacts with Calmodulin in a Calcium-dependent Manner
We have characterized Arc35p as a regulator of Cmd1p localization and activity. Two-hybrid data showed that Arc35p interacts optimally with Cmd1p under conditions of 5 mM extracellular calcium. Consistent with the calcium stimulation is the fact that Arc35p–Cmd1-3p interaction was not detectable even with 5 mM extracellular calcium. This suggests that the calcium-binding domains of calmodulin loaded with calcium strengthen the interaction with Arc35p.
Results using calmodulin mutant proteins in the two-hybrid assays showed that the interaction we detected is specific and most likely significant for function. For a summary of the interaction data, see Table 4. Calmodulin mutant proteins that could suppress the ts growth defect of arc35-1 (Cmd1-231p, Cmd1-247p) bound Arc35p strongly in the assay, whereas ones that could not suppress the ts growth defect or the endocytic defect (Cmd1-226p, Cmd1-228p, Cmd1-239p) did not. Coimmunoprecipitation assays were in entire agreement with the two-hybrid studies and indicate that Arc35p and Cmd1p can be found together in a protein complex. In addition, our data suggest that the interaction observed may not be mediated through filamentous actin. This is the first suggestion of complex formation between these two important players in regulation of the dynamics of the actin cytoskeleton. We cannot determine from these experiments whether the interaction of the two proteins is direct or through other proteins.
The internalization step of endocytosis in yeast has been shown to require calmodulin, but apparently the calcium binding capacity of calmodulin is not essential for this calmodulin function because cmd1-3 mutant cells do not show a strong endocytic defect (Kübler et al., 1994). On the other hand, we show here that calcium is needed to detect an interaction between Cmd1p and Arc35p, two proteins required for endocytosis. One possibility to explain this is if the interaction between the two proteins would be dynamic and calcium ions would favor the state in which the two proteins interact. This would allow detection of a stable complex in vitro. In vivo a transient interaction without calcium could be sufficient for endocytic function.
In summary, the data presented show that Arc35p is required for endocytosis and provide evidence that its endocytic function is mediated through actin and calmodulin. This major point is supported by multiple genetic interactions as well as a physical association between Arc35p and Cmd1p. Calmodulin has two functions in endocytosis. One of these is associated with the type I myosin and is not affected in the arc35-1 mutant. The other calmodulin function, characterized by the cmd1-228 allele, is affected in the arc35-1 mutant. For the moment, it seems that this novel calmodulin-dependent function of the Arp2/3 complex is mediated by the Arc35p subunit; however, our studies do not address the question of whether this function of Arc35p requires association with other subunits of the complex.
ACKNOWLEDGMENTS
We thank Kathleen d'Hondt, Agustin Alconada, Antje Heese-Peck, and Ruben Lombardi for critical reading of the manuscript, and members of the Riezman laboratory for discussions. We are grateful to D. Botstein for providing the cmd1 strains, to R. Brent for sending the two-hybrid strains and plasmids, and to B. Winsor for sending strains and sharing unpublished data. We acknowledge the technical assistance of N. Stern, T. Eberle, and T. Aust. This work was funded by the Canton Basel-Stadt and by grants from Roche Research Foundation and the Swiss National Science Foundation to H.R.
REFERENCES
- Adams AEM, Pringle JR. Relationship of actin and tubulin distribution to bud growth in wild-type and morphogenetic-mutant Saccharomyces cerevisiae. J Cell Biol. 1984;98:934–945. doi: 10.1083/jcb.98.3.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayscough KR, Stryker J, Pokala N, Sanders M, Crews P, Drubin D. High rates of actin filament turnover in budding yeast and roles for actin in establishment and maintenance of cell polarity revealed using the actin inhibitor Latrunculin-A. J Cell Biol. 1997;137:399–416. doi: 10.1083/jcb.137.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bénédetti H, Raths S, Crausaz F, Riezman H. The END3 gene encodes a protein that is required for the internalization step of endocytosis and for actin cytoskeleton organization in yeast. Mol Biol Cell. 1994;5:1023–1037. doi: 10.1091/mbc.5.9.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clague MJ. Molecular aspects of the endocytic pathway. Biochem J. 1998;336:271–282. doi: 10.1042/bj3360271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis TN, Urdea MS, Masiarz FR, Thorner J. Isolation of the yeast calmodulin gene: calmodulin is an essential protein. Cell. 1986;47:423–431. doi: 10.1016/0092-8674(86)90599-4. [DOI] [PubMed] [Google Scholar]
- Della Rocca GJ, Mukhin YV, Garnovskaya MN, Daaka Y, Clark GJ, Luttrell LM, Lefkowitz RJ, Raymond JR. Serotonin 5-HT1A receptor-mediated Erk activation requires calcium/calmodulin-dependent receptor activation. J Biol Chem. 1999;274:4749–4753. doi: 10.1074/jbc.274.8.4749. [DOI] [PubMed] [Google Scholar]
- Dower WJ, Miller JF, Ragsdale CW. High efficiency transformation of Escherichia coli by high voltage electroporation. Nucleic Acids Res. 1988;16:6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulic V, Egerton M, Elguindi I, Raths S, Singer B, Riezman H. Yeast endocytosis assays. Methods Enzymol. 1991;194:697–710. doi: 10.1016/0076-6879(91)94051-d. [DOI] [PubMed] [Google Scholar]
- Durrbach A, Louvard D, Coudrier E. Actin filaments facilitate two steps of endocytosis. J Cell Sci. 1996;109:457–465. doi: 10.1242/jcs.109.2.457. [DOI] [PubMed] [Google Scholar]
- Enrich C, Apodaca G, Mostov KE. Calmodulin regulates the intracellular trafficking in epithelial cells. Z Gastroenterol. 1996;34:83–85. [PubMed] [Google Scholar]
- Estojak J, Brent R, Golemis EA. Correlation of two-hybrid affinity data with in vitro measurements. Mol Cell Biol. 1995;15:5820–5829. doi: 10.1128/mcb.15.10.5820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman SD, Katz MH, Parker EM, Gelrud A. Endocytosis at the apical plasma membrane of pancreatic acinar cells is regulated by tyrosine kinases. Am J Physiol. 1999;276:C306–311. doi: 10.1152/ajpcell.1999.276.2.C306. [DOI] [PubMed] [Google Scholar]
- Geiser JR, van Tuinen D, Brockerhoff SE, Neff MM, Davis TN. Can calmodulin function without binding calcium? Cell. 1991;65:949–959. doi: 10.1016/0092-8674(91)90547-c. [DOI] [PubMed] [Google Scholar]
- Geli MI, Wesp A, Riezman H. Distinct functions of calmodulin are required for the uptake step of receptor-mediated endocytosis in yeast: the type I myosin Myo5p is one of the calmodulin targets. EMBO J. 1998;17:635–647. doi: 10.1093/emboj/17.3.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz D, St. Jean A, Woods RA, Schiestl RH. Improved method for high-efficiency transformation of intact yeast cells. Nucleic Acids Res. 1992;20:1425. doi: 10.1093/nar/20.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz RD, Sugino A. New yeast-E. coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pairs restriction sites. Gene. 1988;74:527–534. doi: 10.1016/0378-1119(88)90185-0. [DOI] [PubMed] [Google Scholar]
- Gottlieb TA, Ivanov IE, Adesnik M, Sabatini DD. Actin microfilaments play a critical role in endocytosis at the apical but not the basolateral surface of polarized epithelial cells. J Cell Biol. 1993;120:695–710. doi: 10.1083/jcb.120.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyuris J, Golemis EA, Chertkov H, Brent R. Cdi1, a human G1 and S phase protein phosphatase that associates with Cdk2. Cell. 1993;75:791–803. doi: 10.1016/0092-8674(93)90498-f. [DOI] [PubMed] [Google Scholar]
- Hicke L, Zanolari B, Pypaert M, Rohrer J, Riezman H. Transport through the yeast endocytic pathway occurs through morphologically distinct compartments and requires an active secretory pathway and Sec18/N-ethylmaleimide-sensitive fusion protein. Mol Biol Cell. 1997;8:13–31. doi: 10.1091/mbc.8.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hishinuma S, Naiki A, Tsuga H, Young JM. Ca2+/calmodulin-mediated regulation of agonist-induced sequestration of Gq protein-coupled histamine H1 receptors in human U373 MG astrocytoma cells. J Neurochem. 1998;71:2626–2633. doi: 10.1046/j.1471-4159.1998.71062626.x. [DOI] [PubMed] [Google Scholar]
- Horvath A, Riezman H. Rapid protein extraction from yeast. Yeast. 1994;10:1305–1310. doi: 10.1002/yea.320101007. [DOI] [PubMed] [Google Scholar]
- Jackman MR, Shurety W, Ellis JA, Luzio JP. Inhibition of apical but not basolateral endocytosis of ricin and folate in Caco-2 cells by cytochalasin D. J Cell Sci. 1994;107:2547–2556. doi: 10.1242/jcs.107.9.2547. [DOI] [PubMed] [Google Scholar]
- Kelleher JF, Atkinson SJ, Pollard TD. Sequences, structural models, and cellular localization of the actin-related proteins Arp2 and Arp3 from Acanthamoeba. J Cell Biol. 1995;131:385–397. doi: 10.1083/jcb.131.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kübler E, Riezman H. Actin and fimbrin are required for the internalization step of endocytosis in yeast. EMBO J. 1993;12:2855–2862. doi: 10.1002/j.1460-2075.1993.tb05947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kübler E, Schimmöller F, Riezman H. Calcium-independent calmodulin requirement for endocytosis in yeast. EMBO J. 1994;13:5539–5546. doi: 10.1002/j.1460-2075.1994.tb06891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamaze C, Fujimoto LM, Yin HL, Schmid SL. The actin cytoskeleton is required for receptor-mediated endocytosis in mammalian cells. J Biol Chem. 1997;272:20332–20335. doi: 10.1074/jbc.272.33.20332. [DOI] [PubMed] [Google Scholar]
- Llorente A, Garred O, Kaae Holm P, Eker P, Jacobsen J, van Deurs B, Sandvig K. Effect of calmodulin antagonists on endocytosis and intracellular transport of ricin in polarized MDCK cells. Exp Cell Res. 1996;227:298–308. doi: 10.1006/excr.1996.0279. [DOI] [PubMed] [Google Scholar]
- Machesky LM. Cell motility: complex dynamics at the leading edge. Curr Biol. 1997;7:R164–R167. doi: 10.1016/s0960-9822(97)70079-4. [DOI] [PubMed] [Google Scholar]
- Machesky LM, Atkinson SJ, Ampe C, Vandekerckhove J, Pollard TD. Purification of a cortical complex containing two unconventional actins from Acanthamoeba by affinity chromatography on profilin-agarose. J Cell Biol. 1994;127:107–115. doi: 10.1083/jcb.127.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machesky LM, Gould KL. The Arp2/3 complex: a multifunctional actin organizer. Curr Opin Cell Biol. 1999;11:117–121. doi: 10.1016/s0955-0674(99)80014-3. [DOI] [PubMed] [Google Scholar]
- Machesky LM, Reeves E, Wientjes F, Mattheyse FJ, Grogan A, Totty NF, Burlingame AL, Hsuan JJ, Segal AW. Mammalian actin-related protein 2/3 complex localizes to regions of lamellipodial protrusion and is composed of evolutionary conserved proteins. Biochem J. 1997;328:105–112. doi: 10.1042/bj3280105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCollum D, Feoktistova A, Morphew M, Balasubramanian M, Gould KL. The Schizosaccharomyces pombe actin-related protein, Arp3, is a component of the cortical actin cytoskeleton and interacts with profilin. EMBO J. 1996;15:6438–6446. [PMC free article] [PubMed] [Google Scholar]
- Moreau V, Galan J-M, Devilliers G, Haguenauer-Tsapis R, Winsor B. The yeast actin-related protein Arp2 is required for the internalization step of endocytosis. Mol Biol Cell. 1997;8:1361–1375. doi: 10.1091/mbc.8.7.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau V, Madania A, Martin RP, Winsor B. The Saccharomyces cerevisiae actin-related protein Arp2 is involved in the actin cytoskeleton. J Cell Biol. 1996;134:117–132. doi: 10.1083/jcb.134.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, Ghosh RN, Maxfield FR. Endocytosis. Physiol Rev. 1997;77:759–803. doi: 10.1152/physrev.1997.77.3.759. [DOI] [PubMed] [Google Scholar]
- Mullins RD, Heuser JA, Pollard TD. The interaction of Arp2/3 complex with actin: nucleation, high affinity pointed end capping, and formation of branching networks of filaments. Proc Natl Acad Sci USA. 1998;95:6181–6186. doi: 10.1073/pnas.95.11.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins RD, Stafford WF, Pollard TD. Structure, subunit topology, and actin-binding activity of the Arp2/3 complex from Acanthamoeba. J Cell Biol. 1997;136:331–343. doi: 10.1083/jcb.136.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn AL, Riezman H. Endocytosis is required for growth of vacuolar H+-ATPase-defective yeast: identification of six new END genes. J Cell Biol. 1994;127:373–386. doi: 10.1083/jcb.127.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohya Y, Botstein D. Diverse essential functions revealed by complementing yeast calmodulin mutants. Science. 1994;263:963–966. doi: 10.1126/science.8310294. [DOI] [PubMed] [Google Scholar]
- Pley UM, Alibert C, Brodsky FM, Parham P. The interaction of calmodulin with clathrin-coated vesicles, triskelions, and light-chains. J Biol Chem. 1995;270:2395–2402. doi: 10.1074/jbc.270.5.2395. [DOI] [PubMed] [Google Scholar]
- Reneke JE, Blumer KJ, Courchesne WE, Thorner J. The carboxy-terminal segment of the yeast α-factor receptor is a regulatory domain. Cell. 1988;55:221–234. doi: 10.1016/0092-8674(88)90045-1. [DOI] [PubMed] [Google Scholar]
- Riezman H, Munn AL, Geli MI, Hicke L. Actin-, myosin-, and ubiquitin-dependent endocytosis. Experientia. 1996;52:1033–1041. doi: 10.1007/BF01952099. [DOI] [PubMed] [Google Scholar]
- Ruden DM, Ma J, Li Y, Wood K, Ptashne M. Generating yeast transcriptional activators containing no yeast protein sequence. Nature. 1991;350:250–252. doi: 10.1038/350250a0. [DOI] [PubMed] [Google Scholar]
- Salisbury JL, Condeelis JS, Satir P. Role of coated vesicles, microfilaments, and calmodulin in receptor-mediated endocytosis by cultured B lymphoblastoid cells. J Cell Biol. 1980;87:132–141. doi: 10.1083/jcb.87.1.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- Schaerer-Brodbeck C, Riezman H. Saccharomyces cerevisiae Arc35p works through two genetically separable calmodulin functions to regulate the actin and tubulin cytoskeletons. J Cell Sci. 2000;113:521–532. doi: 10.1242/jcs.113.3.521. [DOI] [PubMed] [Google Scholar]
- Sherman S, Fink G, Lawrence C. Methods in Yeast Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1974. [Google Scholar]
- Shurety W, Bright NA, Luzio JP. The effects of cytochalasin D and phorbol myristate acetate on the apical endocytosis of ricin in polarized Caco-2 cells. J Cell Sci. 1996;109:2927–2935. doi: 10.1242/jcs.109.12.2927. [DOI] [PubMed] [Google Scholar]
- Singer-Krüger B, Ferro-Novick S. Use of a synthetic lethal screen to identify yeast mutants impaired in endocytosis, vacuolar protein sorting and the organization of the cytoskeleton. Eur J Cell Biol. 1997;74:365–375. [PubMed] [Google Scholar]
- Singer-Krüger B, Frank R, Crausaz F, Riezman H. Partial purification and characterization of early and late endosomes from yeast. J Biol Chem. 1993;268:14376–14386. [PubMed] [Google Scholar]
- Sohrmann M, Fankhauser C, Brodbeck C, Simanis V. The dmf1/mid1 gene is essential for correct positioning of the division septum in fission yeast. Genes Dev. 1996;10:2707–2719. doi: 10.1101/gad.10.21.2707. [DOI] [PubMed] [Google Scholar]
- Stotz A, Linder P. The ADE2 gene from Saccharomyces cerevisiae: sequence and new vectors. Gene. 1990;95:91–98. doi: 10.1016/0378-1119(90)90418-q. [DOI] [PubMed] [Google Scholar]
- Valentijn KM, Gumkowski FD, Jamieson JD. The subapical actin cytoskeleton regulates secretion and membrane retrieval in pancreatic acinar cells. J Cell Sci. 1999;112:81–96. doi: 10.1242/jcs.112.1.81. [DOI] [PubMed] [Google Scholar]
- Wach A, Brachat A, Alberti-Segui C, Rebischung C, Philippsen P. Heterologous HIS3 marker and GFP reporter modules for PCR-targeting in Saccharomyces cerevisiae. Yeast. 1997;13:1065–1075. doi: 10.1002/(SICI)1097-0061(19970915)13:11<1065::AID-YEA159>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Welch MD, DePace AH, Verma S, Iwamatsu A, Mitchison TJ. The human Arp2/3 complex is composed of evolutionary conserved subunits and is localized to cellular regions of dynamic actin filament assembly. J Cell Biol. 1997a;138:375–384. doi: 10.1083/jcb.138.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch MD, Iwamatsu A, Mitchison TJ. Actin polymerization is induced by Arp2/3 protein complex at the surface of Listeria monocytogenes. Nature. 1997b;385:265–268. doi: 10.1038/385265a0. [DOI] [PubMed] [Google Scholar]
- Welch MD, Rosenblatt J, Skoble J, Portnoy D, Mitchison TJ. Interaction of human Arp2/3 complex and the Listeria monocytogenes ActA protein in actin filament nucleation. Science. 1998;281:105–108. doi: 10.1126/science.281.5373.105. [DOI] [PubMed] [Google Scholar]
- Wendland B, Emr SD, Riezman H. Protein traffic in the yeast endocytic and vacuolar protein sorting pathways. Curr Opin Cell Biol. 1998;10:513–522. doi: 10.1016/s0955-0674(98)80067-7. [DOI] [PubMed] [Google Scholar]
- Wendland B, McCaffery JM, Xiao Q, Emr SD. A novel fluorescence-activated cell sorter-based screen for yeast endocytosis mutants identifies a yeast homologue of mammalian eps15. J Cell Biol. 1996;135:1485–1500. doi: 10.1083/jcb.135.6.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter D, Podtelejnikov AV, Mann M, Li R. The complex containing actin-related proteins Arp2 and Arp3 is required for the motility and integrity of yeast actin patches. Curr Biol. 1997;7:519–529. doi: 10.1016/s0960-9822(06)00223-5. [DOI] [PubMed] [Google Scholar]
- Winter DC, Choe EY, Li R. Genetic dissection of the budding yeast Arp2/3 complex: a comparison of the in vivo and structural roles of individual subunits. Proc Natl Acad Sci USA. 1999;96:7288–7293. doi: 10.1073/pnas.96.13.7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond SH. Actin cytoskeleton: the Arp2/3 complex gets to the point. Curr Biol. 1998;8:R654–R657. doi: 10.1016/s0960-9822(07)00415-0. [DOI] [PubMed] [Google Scholar]