Abstract
GPI-linked hemagglutinin (GPI-HA) of influenza virus was thought to induce hemifusion without pore formation. Cells expressing either HA or GPI-HA were bound to red blood cells, and their fusion was compared by patch-clamp capacitance measurements and fluorescence microscopy. It is now shown that under more optimal fusion conditions than have been used previously, GPI-HA is also able to induce fusion pore formation before lipid dye spread, although with fewer pores formed than those induced by HA. The GPI-HA pores did not enlarge substantially, as determined by the inability of a small aqueous dye to pass through them. The presence of 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate or octadecylrhodamine B in red blood cells significantly increased the probability of pore formation by GPI-HA; the dyes affected pore formation to a much lesser degree for HA. This greater sensitivity of pore formation to lipid composition suggests that lipids are a more abundant component of a GPI-HA fusion pore than of an HA pore. The finding that GPI-HA can induce pores indicates that the ectodomain of HA is responsible for all steps up to the initial membrane merger and that the transmembrane domain, although not absolutely required, ensures reliable pore formation and is essential for pore growth. GPI-HA is the minimal unit identified to date that supports fusion to the point of pore formation.
INTRODUCTION
The fusion protein of influenza virus, hemagglutinin (HA), shares many common structural features with other viral fusion proteins (Bullough et al., 1994; Chan et al., 1997; Weissenhorn et al., 1998) and has served as a prototypic fusion protein. GPI-HA is a protein in which the ectodomain of HA is coupled to a membrane via a GPI linkage (Kemble et al., 1993). The transmembrane (TM) domains and cytoplasmic tails are absent for GPI-HA. The ectodomains of GPI-HA (when produced in the presence of the mannosidase inhibitor deoxymannojirmycin to prevent processing of terminal oligosaccharides) and HA are essentially the same, and when fusion is triggered at low pH, they behave similarly (Kemble et al., 1993).
The hypothesis is widely held that hemifusion is a key intermediate stage of membrane fusion (Palade, 1975). Hemifusion is defined as a membrane configuration in which contacting, outer lipid monolayers have merged and inner leaflets are apposed into a single bilayer, the hemifusion diaphragm, which has become the only barrier separating aqueous compartments. Thus, in hemifusion, lipid continuity has been established but aqueous continuity has not. The hemifusion hypothesis received considerable support when it was shown that GPI-HA was able to induce lipid dye spread without aqueous contents mixing (Kemble et al., 1994; Melikyan et al., 1995). This hemifusion did not proceed to full fusion. Several examples of HA-mediated hemifusion, in addition to that of GPI-HA, have since been observed. Under less than optimal conditions for fusion, HA itself can also lead predominantly to end-state hemifusion without pore formation (Melikyan et al., 1997; Chernomordik et al., 1998). An HA with the NH2-terminal residue of the fusion peptide mutated (from glycine to serine) yields, under optimal fusion conditions, the same result (Qiao et al., 1999). That is, small changes in HA or conditions less drastic than the elimination of the TM domain can also lead to hemifusion. At the other extreme, the elimination of a major portion of HA (∼75% of the protein), a much more severe truncation than that of GPI-HA, yields a peptide that may have led to hemifusion between phospholipid vesicles (Kim et al., 1998; Epand et al., 1999). End-state hemifusion has been observed in several other viral fusion systems (Cleverley and Lenard, 1998; Munoz-Barroso et al., 1998). When HA induces lipid dye spread before pore opening, pores do not form subsequently (Chernomordik et al., 1998), so it is possible that, in general, hemifusion is an aberrant side reaction, not a part of fusion, that occurs only when fusion pore formation is prevented. It is not known whether the pathways that lead to hemifusion and fusion deviate from each other at an early step after fusion is triggered or deviate late in the reaction, perhaps just before the point that a fusion pore forms. If the former is the case, hemifusion would be of more limited interest. Because fusion has not ensued whenever hemifusion has been observed, the relationship between hemifusion and full fusion is still uncertain.
It has come to be appreciated that when GPI-HA cells are hemifused to red blood cells (RBCs), aqueous contents are observed to mix for a fraction of hemifused cell pairs, the precise fraction varying from inconsequential to substantial, depending on the conditions (Melikyan et al., 1995; Nüssler et al., 1997). But it has not been clear whether these aqueous connections were due to bona fide fusion and whether they bore any relation to HA-mediated fusion pores. For example, hemifusion may be the natural end state mediated by GPI-HA, with the aqueous continuities caused by “leaks” or some other local instabilities in the end-state hemifusion diaphragm rather than by a true fusion process (Nüssler et al., 1997). In this article, we focus on an examination of the aqueous continuities that occur between GPI-HA cells and RBCs. Using optimal fusion conditions and sensitive electrophysiological techniques, we have determined that the GPI-HA–induced aqueous pathway is initiated as a stepwise increase in conductance, and using simultaneous fluorescence measurements, we found that the aqueous pathways occur before lipid dye spread. In other words, when GPI-HA generates aqueous continuity, it does so via the formation of true fusion pores. But pore formation occurs to a lesser extent, and end-state hemifusion occurs to a greater extent, for GPI-HA than for HA. Also, pores induced by GPI-HA do not enlarge sufficiently for aqueous dyes to pass through them consistently and reliably.
GPI-HA pore formation necessitates a reevaluation of concepts that were based on the assumption that GPI-HA induces only end-state hemifusion. The observation of the GPI-HA pore immediately demonstrates that the ectodomain of HA anchored to a membrane can induce fusion pores, even though it does so with less efficiency than full-length HA. Whereas it had been thought that the TM domain was essential to create pores, it is now clear that, instead, the TM domain is important to induce pores efficiently and is essential for the full pore enlargement that is necessary to release the viral nucleocapsid.
MATERIALS AND METHODS
Reagents
Carboxyfluorescein (CF), octadecylrhodamine B (R18), 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI), and rhodamine-tagged dextran (RD; molecular mass = 40,000 D) were purchased from Molecular Probes (Eugene, OR). Neuraminidase (type V from Clostridium perfringens), N-tosyl-l-phenylalanine chloromethyl ketone–treated trypsin, PKH-26 cell tracer, methyl-β-cyclodextrin, and chlorpromazine were obtained from Sigma Chemical (St. Louis, MO). Lissamine rhodamine sulfonyl dioleoylphosphatidylethanolamine (RhoPE) was purchased from Avanti Polar Lipids (Alabaster, AL).
Labeling of RBCs
Human RBCs were isolated and labeled with membrane dye essentially as described (Morris et al., 1989; Melikyan et al., 1995) on the same day the blood was drawn. Lipophilic dyes were introduced by injection from 1 mg/ml stock solutions in ethanol. Five milliliters of a 1% suspension of RBCs in PBS was labeled with either 2.5 or 5 μg of R18, resulting in R18 occupying ∼1 or 2%, respectively, of the area of the RBC membrane. Labeling with DiI was carried out similarly, except that 10 or 20 μg of DiI was injected into the RBC suspension. This resulted in ∼4 or 8% dye in the RBC membrane, respectively. These determinations of membrane concentrations of lipophilic dyes were made by solubilizing the labeled RBCs with detergent so that the dye was diluted to the point that any self-quenching was relieved. The fluorescence of the solubilized dye was compared against standard curves, allowing the amount of dye incorporated into the RBCs to be determined. Because both dyes freely translocate from one monolayer of a membrane to the other, referred to as “flip-flop” (Melikyan et al., 1996), the values assume that the incorporated R18 and DiI are distributed equally in both inner and outer monolayers. We identify the labeled RBCs by the approximate percentage of dye in the monolayers (i.e., 1%R18-RBCs and 2%R18-RBCs, 4%DiI-RBCs and 8%DiI-RBCs). The calculated percentages provide estimates of total area of the RBC membrane occupied by the lipid dyes. Because the protein occupies a significant fraction of the RBC area, the lipid dyes are, on a mole basis, a larger percentage of total lipid within the labeled RBC membrane. Injecting 15 μg of RhoPE to 5 ml of a 1% RBC suspension yielded ∼5% RhoPE in the outer monolayer only—RhoPE does not flip-flop (Melikyan et al., 1996). These labeled RBCs are denoted as 5%RhoPE-RBCs. PKH-26 labeling was performed according to the manufacturer's instructions, except that “diluent C” was not used. Five microliters of dye was injected per 2 ml of a 5% RBC suspension in PBS (the final concentration of PKH-26 was 2.5 μM). The membrane concentration of PKH-26 was not determined. These cells are denoted as PKH-RBCs. As required, 2.5 mM of the aqueous dye CF was loaded into unlabeled or lipid probe–labeled RBCs by mild hypotonic lysis (Melikyan et al., 1995).
Cell Growth, Treatment, and Fluorescence Microscopy Measurements
CHO cells constitutively expressing the HA of the X-31 strain (HA300a; Kemble et al., 1993), referred to as HA cells, and those expressing GPI-HA, referred to as GPI-HA cells, were obtained from Dr. J. White (University of Virginia, Charlottesville, VA) and maintained in glutamate-deficient medium supplemented with 250 μM 1-deoxymannojirmycin (Calbiochem-Novabiochem, San Diego, CA), as described previously (Kemble et al., 1994; Melikyan et al., 1995). To obtain cells for fusion experiments, cells were lifted from a culture dish by a brief treatment with 0.5 mg/ml trypsin and 0.5 mM EDTA, reseeded on 1.5-mm coverslips in complete growth medium, and placed in a CO2 incubator for 1 h. Cells were then washed with PBS and treated with 0.1 mg/ml neuraminidase and 0.01 mg/ml N-tosyl-l-phenylalanine chloromethyl ketone–treated trypsin for 10 min at room temperature. Trypsin was quenched by adding an excess of growth medium; cells were washed and incubated with a suspension of labeled RBCs for 10 min. Unbound RBCs were removed by washing the cells twice with PBS. Cells with an RBC adhered to them were stored on ice and used for experiments within 4–5 h.
The extent of fusion between RBCs and HA-expressing cells was determined by fluorescence video microscopy as described (Melikyan et al., 1997). Several culture dishes were used for each experiment. Fusion was triggered by exposing cells to a 20 mM succinate-buffered solution adjusted to pH 4.8 (unless specified otherwise) at 37°C for 2 min, and the solution was then reneutralized to pH 7.4. Ten minutes after the cells were brought back to neutral pH, the extent of fusion was determined by microscopically observing the fractions of HA- and GPI-HA–expressing cells that were stained with membrane and/or aqueous dye. In some cases, after pH was brought back to neutral, 0.5 mM chlorpromazine was added for 1 min to determine whether this membrane-permeable, cationic agent could promote aqueous dye transfer for cells in a state of hemifusion (Melikyan et al., 1997).
Simultaneous Electrophysiological and Video Microscopy Measurements
Fusion pore formation between RBCs and HA-expressing cells was monitored in the whole-cell patch-clamp configuration by time-resolved admittance measurements, and pore conductance was calculated exactly as described previously (Markosyan et al., 1999; Melikyan et al., 1999; Qiao et al., 1999): a phase shift of the output current with respect to the command sine wave voltage was introduced by the entire system, and the phase angle was corrected by capacitance dithering (Neher and Marty, 1982). Cells were bathed in a solution of 150 mM N-methylglucamine aspartate, 5 mM MgCl2, 2 mM Cs-HEPES, pH 7.2. Patch pipettes were filled with 155 mM Cs-glutamate, 5 mM MgCl2, 5 mM bis-(o-aminophenoxy)-N,N,N′,N′-tetraacetic acid, 10 mM Cs-HEPES, pH 7.4. Fusion was triggered by ejecting an acidic solution of the same composition as the bathing solution (but buffered with 20 mM Cs-succinate) under low pressure through another pipette positioned ∼50–60 μm from the cell; the resulting low pH was maintained for 2–2.5 min. For all experiments except those shown in Figure 4, GPI-HA or HA cells with only one bound RBC were chosen for study. For the experiments shown in Figure 4, more than one RBC ghost was sometimes bound when measuring aqueous dye spread. For these cases, the fraction of RBC ghosts that fused was electrically determined by counting the number of capacitative discharge spikes that resulted when two cells with different resting potentials fused (Spruce et al., 1989). It has been shown that this procedure reliably measures the number of fusion events when several RBCs are bound (Melikyan et al., 1999). The redistribution of fluorescent dyes from a RBC into HA- and GPI-HA–expressing cells was monitored with a 40×, 0.6 numerical aperture objective (Nikon, Garden City, NY) and an intensified CCD camera (XR GenIII+, Stanford Photonics, Stanford, CA) and recorded on videotape (S-VHS recorder SVO-9500MD, Sony, Park Ridge, NJ). Electrical and fluorescence recordings were synchronized, and data were analyzed off line. The onset of fluorescent dye transfer was routinely determined visually by playing the tape recorder in frame-by-frame mode. For a few individual experiments, the moments for the onset of dye spread were determined with the use of computer analysis, as described previously (Qiao et al., 1999). Briefly, an area of a GPI-HA cell adjacent to the bound RBC was selected, and the brightness of this region was measured over time. The average fluorescence within the region of interest before and after dye spread was curve fitted to straight lines (SigmaPlot, Jandel Scientific, San Rafael, CA). The intercept of these two lines was taken as the time that dye began to spread (Figure 1A). These times obtained by digitizing images were similar to the times that lipid dye was visually determined to begin spreading.
Figure 4.
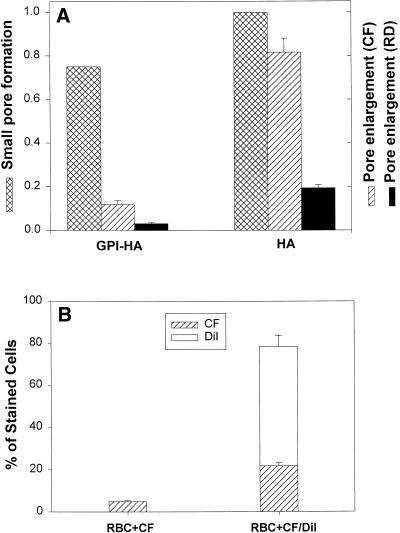
Enlargement of fusion pores induced by GPI-HA and HA. (A) Fusion was triggered by locally applying pH 4.8 at 37°C for 2 min or until a pore formed. The fraction of bound RBC ghosts coloaded with CF and RD that fused was determined electrically (cross-hatched bars) by counting capacitative discharges resulting from fusion. In separate measurements of aqueous dye spread, the same batch of RBC ghosts was used and fusion was triggered at 37°C by reducing pH to 4.8 for 2 min followed by reneutralization at room temperature. Dye spread was monitored 10 min after acidification. Although, as determined electrically (cross-hatched bars), pores formed for almost the same percentage of GPI-HA cells with a bound RBC as for HA cells, CF (striped bars) did not pass as readily through GPI-HA pores. For HA cells, pores enlarged sufficiently to permit CF to transfer into 80% of the cells and to permit RD (solid bars) to transfer into ∼20% of the cells. (B) Transfer of CF through GPI-HA pores (striped bars) is greater when the RBC ghosts are labeled with DiI (second bar). But only ∼25% of the GPI-HA cells that became stained with DiI (open bar) also received CF. Different batches of cells were used in A and B; consequently, their extents of CF transfer are somewhat different.
Figure 1.
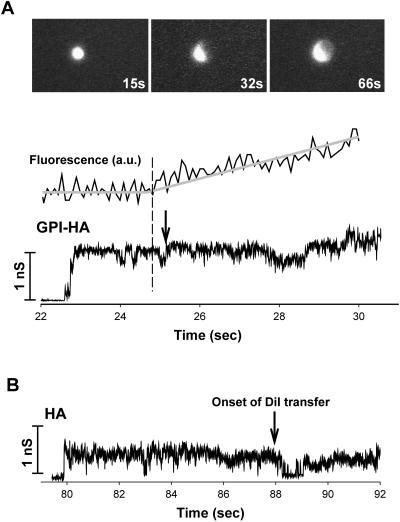
GPI-HA can induce fusion pores before lipid dye spread. (A) Fluorescence images illustrating the spread of lipid dye are shown in the top panel for GPI-HA cells. The 4%DiI-RBC is bright. The average brightness of a region of the GPI-HA cell adjacent to the RBC was measured (fluorescence, in arbitrary units [a.u.]) over time. The brightness before and after dye spread was fitted to straight lines. The intercept of the two lines (marked by the dashed vertical line) was similar to the time for onset of dye spread determined visually (arrow). The traces for pore conductance and fluorescence were synchronized in time; the times after acidification are shown on the pore conductance trace. (B) A typical conductance trace of a fusion pore for HA cells. For the HA cell, the moment of dye spread, indicated by the arrow, was determined visually. Fusion pore conductances were calculated every 5 ms from changes in the in-phase and out-of-phase components of cell admittance. Fusion was triggered in these experiments at pH 4.8 and 30°C.
RESULTS
GPI-HA Induces Fusion Pores before Lipid Dye Spread
RBCs were labeled with a lipid dye and bound to GPI-HA cells or to HA cells. We used electrical admittance measurements to follow the formation and evolution of fusion pores and simultaneously monitored membrane dye redistribution. Fusion was triggered by applying a low-pH solution from a second pipette placed near the cell-RBC pair. For all GPI-HA cells with a bound fluorescently labeled RBC, one of two outcomes was observed: either lipid dye spread without subsequent formation of a pore or fusion pores formed before the onset of membrane dye redistribution.1 We operationally refer to an outcome as “hemifusion” if lipid dye spread without pore formation and as “fusion” if a pore formed before dye spread. Which outcome predominated depended on experimental conditions. A region of the GPI-HA cell adjacent to a dye-labeled RBC was marked, and its mean brightness of fluorescence intensity (Figure 1A, images) was measured over time (Figure 1A, fluorescence trace). For 4%DiI-RBCs (4% refers to the percentage, on a mole basis, of lipid dye incorporated into the RBC membrane; see MATERIALS AND METHODS) bound to a GPI-HA cell, the dye was observed to spread a few seconds after a pore formed (Figure 1A). Based on sensitive electrical measurements, GPI-HA is clearly able to induce fusion pores.
In a comparison of pores formed by GPI-HA and those formed by HA (Figure 1B), several significant differences were observed. First, GPI-HA–mediated pores did not flicker open and closed (as judged by pore conductance transiently returning to baseline; Figure 1A), whereas pores formed by HA (Figure 1B) did flicker and did so extensively. (However, the GPI-HA pores could fluctuate between different levels.) Second, the initial conductances of GPI-HA pores were consistently higher than those of HA pores. Also, the GPI-HA pore allowed DiI to pass readily; this movement was more restricted for HA pores, as inferred from the greater delays between the formation of an HA pore and subsequent lipid dye movement than the delays observed for GPI-HA pores (see Figure 1 for typical examples; see Figure 2 for the distribution of times of dye spread). In the case of GPI-HA, DiI was observed to transfer from the RBCs within a few seconds after pore formation. In contrast, with HA cells, the DiI spread at significantly longer times after pore formation. This could have occurred because HA pores were smaller than GPI-HA pores, because the TM domain of HA pores hindered lipid dye movement, or by a combination of the two causes. The more facile movement of lipid dye through GPI-HA pores was not limited to DiI: R18 also transferred more readily through GPI-HA pores (our unpublished results). Finally, as we now show, the GPI-HA and HA pores exhibited different patterns of growth.
Figure 2.
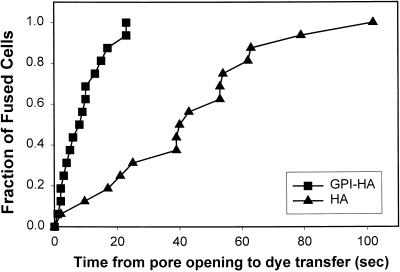
The times between pore formation and observation of DiI spread were rank ordered into cumulative distributions for GPI-HA (▪) and HA (▴). These times were significantly longer for HA cells. 4%DiI-RBCs were used. Fusion was triggered at pH 4.8, and cells were maintained at 30°C.
GPI-HA Pores Did Not Enlarge Sufficiently to Pass Aqueous Dye
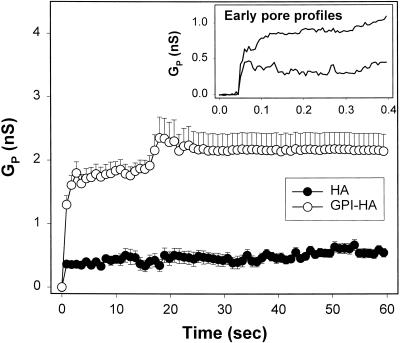
We quantitatively established the behavior of pores formed with GPI-HA and HA cells by plotting the average pore conductance as a function of time from all experiments for each cell type under a given condition (Figure 3). Although individual features, such as flickering, are obliterated in these plots, they do allow a determination of whether the ensemble behavior of pores formed by GPI-HA and HA are the same. With 4%DiI-RBCs as target, pores induced by GPI-HA had an average initial conductance of ∼0.5 nS (Figure 3, inset, upper curve), from which they grew to ∼1 nS within 0.5 s. By ∼10 s, their conductance levels reached ∼2–2.5 nS, and they did not enlarge further (Figure 3, ○). (Of course, any individual pore usually showed behaviors that deviated from the average.) Pores induced for HA cells were initially smaller (Figure 3, inset, lower curve), and their average conductance did not grow beyond ∼0.5 nS, even after 1 min (Figure 3, ●). Electrical measurements are not well suited to observing HA-mediated pores for times longer than ∼1 min because of increases in membrane conductance caused by activated HA (Qiao et al., 1999). Therefore, to assess pore enlargement at longer times, we used fluorescence microscopy to compare the ability of aqueous dyes of different sizes to pass through GPI-HA and HA pores.
Figure 3.
The increase in conductance of GPI-HA and HA pores for the first minute after formation. Initially (inset), the average conductance was significantly larger for pores induced by GPI-HA (upper trace) than for pores generated by HA (lower trace). GPI-HA pores (○) eventually reached an average conductance on the order of 2 nS but did not enlarge further. HA pores (●) remained small. Average conductances of fusion pores were determined every 5 ms. Points were decimated for visual clarity. Bars indicate the SEM. The average pore conductance was calculated from 10 GPI-HA pores and 8 HA pores at pH 4.8, 30°C, with 4%DiI-RBCs.
RBC ghosts were coloaded with both the small dye CF (molecular mass ∼ 400 D) and the large RD (molecular mass ∼ 40,000 D), and their transfer was measured (Melikyan et al., 1997). Because some portion of the GPI-HA cells will hemifuse rather than fuse, we needed to eliminate this hemifusing fraction. In other words, the fraction of GPI-HA cells that became stained by aqueous dyes had to be compared with the fraction of cells that actually formed pores. Therefore, in separate experiments on the same batch of GPI-HA cells, HA cells, and RBC ghosts, pore formation was measured electrically to definitively establish what fraction of bound RBCs fused (Figure 4A, cross-hatched bars). This fraction was then compared with the fraction of GPI-HA (and HA) cells that acquired each of the two aqueous dyes. Fusion was triggered at 37°C by reducing pH to 4.8, and pore formation was electrically detected for almost 80% of the RBC ghosts bound to GPI-HA cells (Figure 4A, GPI-HA, cross-hatched bar) and for virtually every RBC bound to an HA cell (HA, cross-hatched bar). CF did not pass well through the GPI-HA pores (striped bar), and RD transferred through an even smaller fraction (solid bar). Thus, the GPI-HA pores did not enlarge. (The finding that CF did not permeate GPI-HA pores despite the relatively large total electrical conductance [2–2.5 nS; Figure 3] may indicate that several small pores formed, rather than a single pore that enlarged somewhat.) In contrast, CF readily moved through the pores of HA cells (Figure 4A, HA, striped bar), and RD passed more readily (solid bar) than for GPI-HA cells. The ability of a GPI-HA–induced pore to enlarge depended on whether lipid dye was incorporated into the RBC membrane. Loading CF into RBC ghosts and labeling the membrane with DiI led to greater transfer of CF than if the ghosts were loaded with only CF (Figure 4B). But the extents were still significantly less than occurred for HA pores (even in the absence of DiI). Thus, the presence of the TM domain is critical for significant pore enlargement.
GPI-HA Pore Formation Depends on pH and Temperature
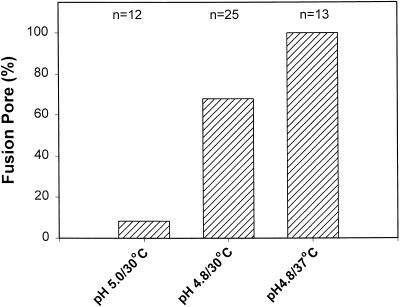
GPI-HA cells with exactly one bound 4%DiI-RBC were selected. At 30°C, a much larger fraction of cells fused at pH 4.8 (Figure 5, second bar) than at pH 5.0 (first bar), as measured electrically. Also at pH 4.8, pores were always observed (13 of 13) at 37°C. Thus, conditions can be optimized to the point that GPI-HA induces fusion pores virtually without failure, although the conditions that induce efficient pore formation are pointedly more limited for GPI-HA than for HA. Importantly, fusion pore formation by GPI-HA was promoted by both higher temperature and lower pH (Figure 5). This pH and temperature profile of GPI-HA pore formation is similar to that of HA-mediated fusion.
Figure 5.
pH and temperature dependence of the formation of GPI-HA pores. At pH 5.0 and 30°C (first bar), fusion pores were registered electrically in only 1 of 12 experiments. At pH 4.8 and 30°C (second bar), pores were observed in 17 of 25 experiments. Increasing temperature to 37°C (third bar) resulted in highly efficient fusion (13 of 13 experiments). 4%DiI-RBCs were used.
GPI-HA–induced Fusion Depends on the Lipid Composition of the RBC Membrane
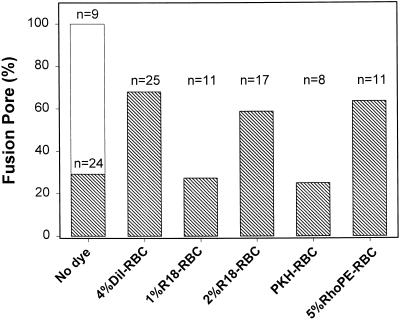
Because the TM domain of HA is absent from GPI-HA, one would expect lipid to be part of a GPI-HA–induced fusion pore. If so, the lipid composition of the target RBC membrane should strongly affect pore formation. (As we have shown, lipid dye affected pore enlargement [Figure 4B].) By including different lipid dyes at various concentrations in the RBCs, we were not only able to alter composition but could monitor both hemifusion and fusion as well. For these experiments, we reduced pH to 4.8 at 30°C so that fusion was not maximally stimulated (Figure 5). We could thus quantitatively determine if the incorporation of a lipid dye into RBCs gave more fusion or less fusion than in the absence of dye. Compared with unlabeled RBCs, pore formation was greater for 4%DiI-RBCs, 2%R18-RBCs, and 5%RhoPE-RBCs (Figure 6); 2 mol % R18 had the same effect in facilitating the production of GPI-HA pores as 4 mol % DiI. PKH-RBCs or 1%R18-RBCs did not promote pore formation.
Figure 6.
The extent to which GPI-HA pore formation depends on the presence of lipid dye. RBCs, either unlabeled or labeled with the indicated membrane dye, were bound to GPI-HA–expressing cells, and a GPI-HA cell (with one bound RBC) was patch clamped in the whole-cell mode. Pore formation was detected by electrical admittance measurements, and the redistribution of a fluorescent dye was monitored simultaneously by fluorescence microscopy. Fusion was triggered at 30°C by applying a pH 4.8 solution. Either a pore formed or a pore did not form, but lipid dye always spread in virtually every experiment (i.e., every GPI-HA cell fused or hemifused). In the case of unlabeled RBCs, if a fusion pore did not form by 5 min after exposure to low pH, the experiment was terminated. In contrast to GPI-HA cells, pores always formed between HA cells and unlabeled RBCs (open bar) under these conditions. A higher percentage of fusion was generally observed with RBC ghosts (Figure 4, cross-hatched bars) than for intact RBCs (this figure). All experiments of this study, except for those shown in Figure 4, used intact RBCs. The numbers of experiments (n) carried out for each condition are given above the bars.
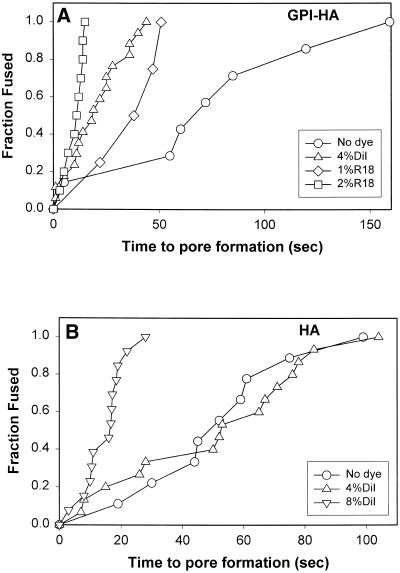
Incorporating either R18 (Figure 7A, ⋄ and □) or DiI (▵) into RBC membranes not only increased the extent of pore formation for GPI-HA cells (Figure 6) but also accelerated the rate of pore formation above that of unlabeled RBCs (Figure 7A, ○) in a dye concentration–dependent manner. Kinetics became faster as the R18 (Figure 7A, ⋄ vs. □) or DiI concentration (our unpublished results) increased.
Figure 7.
Kinetics of fusion pore formation for GPI-HA cells (A) and HA cells (B). RBCs were either unlabeled (○) or labeled with DiI (▵ and ▿) or R18 (⋄ and □) at the indicated mole fraction ratios. (A) Cumulative distributions of waiting times from acidification until pore formation for experiments in which a GPI-HA pore formed before lipid dye transfer. Because the probability of fusion pore formation was low with unlabeled RBCs and those labeled with a low concentration of R18 (i.e., hemifusion usually occurred), in these cases the sample sizes are small. Each point represents the waiting time from acidification until pore formation for an individual experiment. (B) HA invariably induced pore formation. Including 4 mol % DiI (▵) did not speed up pore formation compared with unlabeled RBCs (○), but including 8 mol % DiI did (▿). Any fusion pores that formed for PKH-RBCs did so at long times after acidification and are not shown.
Whereas less than one-third of the GPI-HA cells exhibited fusion pores in the absence of membrane dye at 30°C, pores almost always formed between HA cells and RBCs (Figure 6, open bar), illustrating that the TM domain of HA helps ensure pore formation and that a wider latitude of conditions reliably promotes fusion for HA cells than for GPI-HA cells. Despite the higher extent of fusion for HA cells, their kinetics of pore formation (Figure 7B, ○) were comparable to those of GPI-HA cells (Figure 7A, ○). Fusion pore formation was less sensitive to the presence of lipid dye for HA than for GPI-HA: kinetics were the same for unlabeled RBCs and for 4%DiI-RBCs (Figure 7B, ▵) but were faster for 8%DiI-RBCs (▿).
Not every fluorescent probe speeds the rate of fusion and hemifusion. GPI-HA cells hemifused to PKH-RBCs substantially more slowly than to R18-RBCs and DiI-RBCs; with PKH-26 as probe, it took half of the GPI-HA cells >210 s after pH was decreased to become fluorescently labeled. But PKH-26 does not appear to greatly affect the extent of pore formation (Figure 6). Because the chemical identity of PKH-26 is proprietary information that has not been released, we did not characterize its quantitative effects on the extent and kinetics of pore formation.
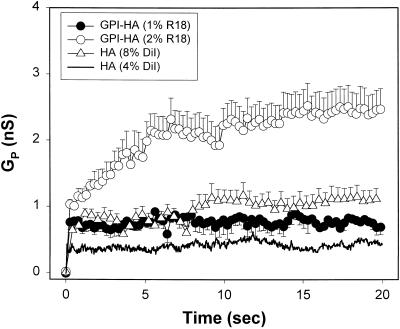
Electrical measurements show that the presence of lipid dyes alters the growth of GPI-HA pores immediately after formation. Increasing the concentration of R18 in the RBC membrane from 1% (Figure 8, ●) to 2% (○) led to a more rapid increase in conductance. As shown above (Figure 4B), the presence of DiI in the RBC membrane facilitated enlargement of some pores to the point that they could pass CF. The greater CF transfer observed may be due at least partially to the ability of DiI itself to promote pore formation (Figure 6). Increased concentrations of lipid probe also caused HA pores to become larger: inclusion of a high concentration of DiI in the RBCs (8%DiI-RBCs; Figure 8, ▵) led to a larger HA pore at, or soon after, pore formation than did a lower concentration (4%DiI-RBCs; solid curve).
Figure 8.
Lipid dye effects on GPI-HA and HA pores. The mean conductance of GPI-HA pores increased for 2% R18-RBCs (○, n = 6) to more than twice the conductance of 1% R18-RBCs (●, n = 3). Including 8 mol % DiI in the RBCs (▵, n = 9) resulted in somewhat larger HA pore conductances than including 4 mol % DiI in the RBCs (solid curve, redrawn from Figure 3).
DISCUSSION
In this study, we have shown that GPI-HA is capable of inducing not only hemifusion, as was appreciated previously (Kemble et al., 1994; Melikyan et al., 1995), but small fusion pores as well. The occurrence of pores was highly sensitive to pH and depended on temperature, as would be expected of an HA-mediated process. We also found that the occurrence of pore formation was quite sensitive to the presence of lipid dye. Because HA and GPI-HA can cause either hemifusion or pore formation, it is natural to consider the relationship of these two outcomes.
The State of Hemifusion May Be Either Transitional or End State
The term “hemifusion” is classically defined as continuity of outer lipid monolayers without merger of inner monolayers and without pore formation. Operationally, the observation of lipid dye spread is evidence of outer monolayer continuity, and the absence of aqueous continuity is evidence that a pore has not formed and, therefore, that hemifusion has occurred. Electrical detection of pores is sufficiently sensitive that if a pore does form, it will be unambiguously identified. Lipid dye spread assays are comparatively much less sensitive than electrical assays (Cohen and Melikyan, 1998), and lipid dye may not be observed to spread even after the formation of a small fusion pore (Tse et al., 1993; Zimmerberg et al., 1994). In general, when hemifusion has been observed, pores do not form subsequently (Chernomordik et al., 1998; Qiao et al., 1999); thus, the only unambiguously observable hemifusion has been hemifusion as an end state. Therefore, we consider it useful to distinguish between observable “end-state” hemifusion and what we will refer to as “transitional” hemifusion, which is hemifusion that proceeds to full fusion. Until it can be unambiguously shown to occur, transitional hemifusion must be considered a conjectured state that is hypothesized to be an intermediate of full fusion.
Why Was It Thought That GPI-HA Did Not Induce Fusion Pores?
It was originally shown that at pH 5.2 and 37°C, GPI-HA induces lipid dye, but not aqueous dye (lucifer yellow), transfer from RBCs. It was thus proposed that GPI-HA induces only end-state hemifusion and that the TM domain of HA was absolutely essential for pore formation (Kemble et al., 1994; Melikyan et al., 1995). Aqueous dye mixing and continuity of inner membrane leaflets were observed, with the amounts depending on conditions (Melikyan et al., 1995; Nüssler et al., 1997). Each of these continuities would signify fusion. But the transfers were usually assayed at relatively long times after acidification, and they increased over the course of about 1 h. Also, lipid dye incorporated into inner leaflets of RBC ghosts could spread without transfer of aqueous dye. It was thus interpreted that the diaphragm of end-state hemifusion was prone to instability, leading to leaks in the end-state diaphragm rather than the occurrence of a bona fide fusion event (Nüssler et al., 1997). This was in accord with the finding that the hemifusion diaphragm that forms between GPI-HA and planar phospholipid bilayer membranes often developed electrical leaks (which might have obscured the electrical signature of any fusion pores that did form) (Melikyan et al., 1995). With the understanding of GPI-HA–generated pores gained from the present study, we can appreciate why the experimental results of previous studies with RBCs as target were obtained.
Very few GPI-HA pores enlarged. In the absence of membrane dye (Kemble et al., 1994), the relatively small aqueous dye CF transferred from RBC ghosts into only a small percentage of the GPI-HA cells that fused, as determined electrically (Figure 4). For larger aqueous dyes (e.g., lucifer yellow), even less transfer would be expected (Kemble et al., 1994). When RBCs were labeled with lipid dye, more aqueous continuity was observed, with transfer greater at 37°C than at 23°C (Melikyan et al., 1995; Nüssler et al., 1997). Also, more fusion occurred at pH 4.8 than at pH 5.0. It is now appreciated that as fusion conditions are made less optimal, the amount of fusion is reduced and the extent of end-state hemifusion is increased (Melikyan et al., 1997; Chernomordik et al., 1998). The temperature and pH dependence of GPI-HA–mediated aqueous pathways previously observed are typical of HA-induced fusion, and we would now expect it of GPI-HA–mediated pore formation. Therefore, the data from previous studies were accurate and the interpretations were logical, but it was not appreciated, until the present study, that GPI-HA can either induce pore formation upstream of end-state hemifusion or induce end-state hemifusion, with the outcome strongly dependent on temperature, pH, and the presence of lipid dye. It was also not appreciated that GPI-HA pores do not enlarge sufficiently to permit significant transfer of aqueous dye.
For GPI-HA, the Occurrence of Fusion Depends on the Lipid Probe
The amount of fluorescent lipid dye needed to be placed in membranes for dye spread to be detected is not insignificant: it is usually a few percentage points, on a mole basis, of total lipid (Cohen and Melikyan, 1998). Thus, in addition to serving as a probe, the dye itself becomes a membrane constituent that can affect fusion. We have found that the formation and enlargement of a GPI-HA pore is very sensitive to lipid composition, much more so than the formation and enlargement of a pore by HA trimers. (However, high concentrations of lipid dye did affect the formation and early conductance of HA pores [Figures 7 and 8].) If the structure of the GPI-HA pore is essentially lipidic, this would explain GPI-HA pore sensitivity to lipid. Similarly, if the wall of an HA pore contains the TM domain, pore sensitivity to lipid changes would be relatively less but would still exist.
It is known that spontaneous monolayer curvature (i.e., the natural tendency of monolayers to bend in one direction or another) is an important property of lipids that affects the formation of fusion pores in an understood manner (Chernomordik et al., 1995). When lipid composition is varied, however, many parameters other than spontaneous curvature are altered, each of which may affect fusion in ways not yet understood. How lipid probes affect pore formation for GPI-HA is not known, nor is it known whether they do so through a common property. R18, DiI, and RhoPE all promoted pore formation, but what feature they may have in common that would cause this is not obvious: R18 and DiI are cationic, confer a more negative spontaneous monolayer curvature, and flip-flop across monolayers of membranes; RhoPE is anionic, confers positive spontaneous monolayer curvature, and does not flip-flop (Melikyan et al., 1996; Razinkov et al., 1998). R18 affected pore formation and enlargement at lower concentrations than did DiI: 2% R18 and 4% DiI speeded kinetics (Figure 7A), increased the percentage of fusion (Figure 6), and promoted greater pore conductance (Figures 3 and 8) to about the same degrees. The lipid dye PKH-26 (whose structure is not known) slowed fusion of GPI-HA without altering its extent. The fact that these probes affect fusion in ways that cannot be predicted demonstrates the practical importance of using lipid dyes at minimal concentrations.
GPI-HA Is the Smallest Identified Unit That Promotes Pore Formation
Previous studies have been done to determine if isolated portions of the ectodomain of HA in solution can promote hemifusion or fusion. Adding almost the entire ectodomain of HA (commonly known as BHA; Brand and Skehel, 1972) to a solution bathing cells and then decreasing pH yields neither aqueous nor membrane continuities (White et al., 1982; Wharton et al., 1986). This demonstrates that the ectodomain in isolation is not functional. (When a much smaller portion of the ectodomain—already in its low-pH conformation—was added to solutions bathing liposomes, lipid dye spread, but with significant leakage of aqueous contents; surprisingly, dye spread occurred only after pH was decreased [Epand et al., 1999].) GPI-HA represents the minimal portion of HA identified to date that can unambiguously support hemifusion and/or pore formation. It would appear that the ectodomain of HA must be anchored to a membrane, either through a lipid or a TM domain, to induce fusion.
The Role of the TM Domain in Pore Formation and Pore Growth
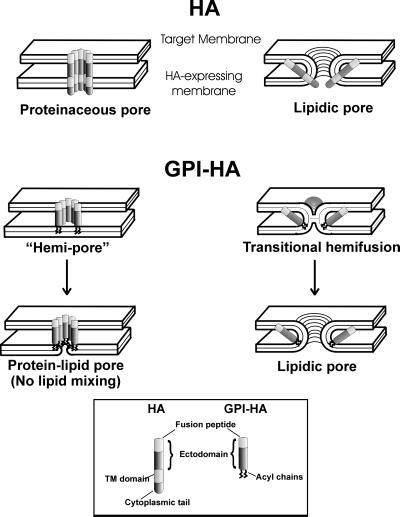
It has been proposed that the initial pore of HA is composed solely of protein (Figure 9, HA, proteinaceous pore), in which case the TM domains would form the structure of the pore within the HA-expressing membrane and the fusion peptides would line the lumen of the pore within the target membrane (Lindau and Almers, 1995). The previous findings that GPI-HA only caused hemifusion would be consistent with this model: the lipid anchor of a “hemi-pore” cannot line the lumen of the pore of the GPI-HA–expressing membrane (Figure 9). However, our finding that GPI-HA can induce pore formation before observation of lipid dye spread is contrary to this model. The formation of GPI-HA pores strongly suggests that the initial GPI-HA pore must be essentially a lipidic structure. The observed effects of lipid composition on the kinetics and extent of formation of GPI-HA pores, as well as on the early growth of the pore, also directly support the lipidic nature of the pore. Although one could conceive that a hemi-pore converts to a “protein-lipid pore” (Figure 9), this would not account for the observed facile mixing of lipid. To affect lipid continuity at the moment of pore formation, it is almost imperative that hemifusion occurs before the formation of the GPI-HA pore. Because GPI-HA pores do not result from the experimentally observed end-state hemifusion, these lipidic pores should arise directly out of transitional hemifusion (Figure 9). The same or a similar intermediate state of fusion just before lipid dye spread and pore formation (captured by decreasing pH to an optimal value but maintaining cells at 4°C) occurs for the fusion of HA and GPI-HA cells to RBCs; this intermediate state is likely to be at or immediately before the point of transitional hemifusion (Chernomordik et al., 1998). Because HA and GPI-HA induce the same intermediate state, we envision that transitional hemifusion is crucial to HA-induced fusion, rather than viewing hemifusion as solely an end-state condition that occurs as an aberrant side reaction. The pathways for pore formation and end-state hemifusion probably diverge at transitional hemifusion (Chernomordik et al., 1998).
Figure 9.
Transitional hemifusion as an intermediate of HA- and GPI-HA–mediated membrane fusion. GPI-HA pores (lipidic pores) can naturally result from the state of transitional hemifusion by reconfiguration of a few lipids within the initial hemifusion diaphragm. (The same would be the case for HA pores.) If hemifusion never occurred, and instead GPI-HA generated a “hemi-pore” as an intermediate of a “protein-lipid pore,” lipid dye would not be able to spread, nor would the rearrangement of lipids be likely to proceed in the GPI-HA–expressing membrane. All experimental evidence is consistent with HA inducing pores by means essentially the same as those used by GPI-HA. We thus propose that HA-mediated fusion proceeds through transitional hemifusion as well.
Fusion pores still form when TM domains of proteins unrelated to fusion are substituted for those of the fusion proteins (Wilk et al., 1996; Odell et al., 1997; Schroth-Diez et al., 1998; Melikyan et al., 1999). However, particular residues may be critical for fusion, because point mutations within TM domains can drastically reduce mixing of aqueous contents (Cleverley and Lenard, 1998; Taylor and Sanders, 1999) and even prevent lipid dye transfer (Melikyan et al., 1999). The extremely limited CF and RD transfer through GPI-HA pores compared with HA pores (Figure 4) shows that the TM domain ensures not only pore formation but pore growth as well. The present study suggests that in exocytosis (and intracellular trafficking) the TM domains of SNARE proteins within a coiled-coil complex (Sutton et al., 1998) are not only important for the formation of a fusion pore but also may be crucial for enlarging the pore to a size that allows passage of small molecules such as neurotransmitters and hormones.
In conclusion, the ectodomain of HA anchored to a membrane is sufficient to promote fusion pore formation. The TM domains, although not essential to pore generation, facilitate the creation of the fusion pore when they are present and are critical for appreciable pore enlargement. We envision that pores are created out of transitional hemifusion and that the TM domains insert into, and become structural elements of, the otherwise lipidic pore walls. TM domains thereby affect the pore's initial conductance, growth, and lipid dye movement.
ACKNOWLEDGMENTS
We thank Judith White for providing cells and Sofya Brener for steady technical support. Drs. Yuri Chizmadzhev, Judith White, and Joshua Zimmerberg provided critical readings of previous versions of the manuscript. This work was supported by National Institutes of Health grants GM-27367 and GM-54787.
Abbreviations used:
- CF
6-carboxyfluorescein
- DiI
1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate
- HA
hemagglutinin
- R18
octadecylrhodamine B
- RD
rhodamine-tagged dextran
- RhoPE
lissamine rhodamine sulfonyl dioleoylphosphatidylethanolamine
- TM
transmembrane
Footnotes
When hemifusion (lipid mixing before pore formation) occurred, a fusion pore was subsequently detected in only 3 of 32 experiments. The times between observed dye spread and pore formation were long: 17, 26, and 116 s. This is similar to the finding that if lipid dye is observed to spread between a RBC and a cell expressing HA before fusion pore formation, pore formation does not ensue (Chernomordik et al., 1998). It has been found that for hemifusion of GPI-HA cells to RBCs, a flickering pore was occasionally observed electrically after lipid dye spread (Frolov et al., 1997).
REFERENCES
- Brand CM, Skehel JJ. Crystalline antigen from the influenza virus envelope. Nat New Biol. 1972;238:145–147. doi: 10.1038/newbio238145a0. [DOI] [PubMed] [Google Scholar]
- Bullough PA, Hughson FM, Skehel J, Wiley DC. Structure of influenza hemagglutinin at the pH of membrane fusion. Nature. 1994;371:37–43. doi: 10.1038/371037a0. [DOI] [PubMed] [Google Scholar]
- Chan DC, Fass D, Berger JM, Kim PS. Core structure of gp41 from the HIV envelope glycoprotein. Cell. 1997;89:263–273. doi: 10.1016/s0092-8674(00)80205-6. [DOI] [PubMed] [Google Scholar]
- Chernomordik L, Kozlov MM, Zimmerberg J. Lipids in biological membrane fusion. J Membr Biol. 1995;146:1–14. doi: 10.1007/BF00232676. [DOI] [PubMed] [Google Scholar]
- Chernomordik LV, Frolov VA, Leikina E, Bronk P, Zimmerberg J. The pathway of membrane fusion catalyzed by influenza hemagglutinin: restriction of lipids, hemifusion, and lipidic fusion pore formation. J Cell Biol. 1998;140:1369–1382. doi: 10.1083/jcb.140.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleverley DZ, Lenard J. The transmembrane domain in viral fusion: essential role for a conserved glycine residue in vesicular stomatitis virus G protein. Proc Natl Acad Sci USA. 1998;95:3425–3430. doi: 10.1073/pnas.95.7.3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen FS, Melikyan GB. Methodologies in the study of cell-cell fusion. Methods. 1998;16:215–226. doi: 10.1006/meth.1998.0670. [DOI] [PubMed] [Google Scholar]
- Epand RF, Macosko JC, Russell CJ, Shin YK, Epand RM. The ectodomain of HA2 of influenza virus promotes rapid pH dependent membrane fusion. J Mol Biol. 1999;286:489–503. doi: 10.1006/jmbi.1998.2500. [DOI] [PubMed] [Google Scholar]
- Frolov VA, Leikina E, Bronk P, Chernomordik L, Zimmerberg J. Wild-type HA induces hemifusion between cell membranes. Biophys J. 1997;72:A14. [Google Scholar]
- Kemble GW, Danieli T, White JM. Lipid-anchored influenza hemagglutinin promotes hemifusion, not complete fusion. Cell. 1994;76:383–391. doi: 10.1016/0092-8674(94)90344-1. [DOI] [PubMed] [Google Scholar]
- Kemble GW, Henis YI, White JM. GPI- and transmembrane-anchored influenza hemagglutinin differ in structure and receptor binding activity. J Cell Biol. 1993;122:1253–1265. doi: 10.1083/jcb.122.6.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CH, Macosko JC, Shin YK. The mechanism for low-pH-induced clustering of phospholipid vesicles carrying the HA2 ectodomain of influenza hemagglutinin. Biochemistry. 1998;37:137–144. doi: 10.1021/bi971982w. [DOI] [PubMed] [Google Scholar]
- Lindau M, Almers W. Structure and function of fusion pores in exocytosis and ectoplasmic membrane fusion. Curr Opin Cell Biol. 1995;7:509–517. doi: 10.1016/0955-0674(95)80007-7. [DOI] [PubMed] [Google Scholar]
- Markosyan RM, Melikyan GB, Cohen FS. Tension of membranes expressing the hemagglutinin of influenza virus inhibits fusion. Biophys J. 1999;77:943–952. doi: 10.1016/S0006-3495(99)76945-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melikyan GB, Brener SA, Ok DC, Cohen FS. Inner but not outer membrane leaflets control the transition from glycosylphosphatidylinositol-anchored influenza hemagglutinin-induced hemifusion to full fusion. J Cell Biol. 1997;136:995–1005. doi: 10.1083/jcb.136.5.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melikyan GB, Deriy BN, Ok DC, Cohen FS. Voltage-dependent translocation of R18 and DiI across lipid bilayers leads to fluorescence changes. Biophys J. 1996;71:2680–2691. doi: 10.1016/S0006-3495(96)79459-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melikyan GB, Lin S, Roth MG, Cohen FS. Amino acid sequence requirements of the transmembrane and cytoplasmic domains of influenza virus hemagglutinin for viable membrane fusion. Mol Biol Cell. 1999;10:1821–1836. doi: 10.1091/mbc.10.6.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melikyan GB, White JM, Cohen FS. GPI-anchored influenza hemagglutinin induces hemifusion to both red blood cell and planar bilayer membranes. J Cell Biol. 1995;131:679–691. doi: 10.1083/jcb.131.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SJ, Sarkar DP, White JM, Blumenthal R. Kinetics of pH-dependent fusion between 3T3 fibroblasts expressing influenza hemagglutinin and red blood cells. J Biol Chem. 1989;264:3972–3978. [PubMed] [Google Scholar]
- Munoz-Barroso I, Durell S, Sakaguchi K, Appella E, Blumenthal R. Dilation of the human immunodeficiency virus-1 envelope glycoprotein fusion pore revealed by the inhibitory action of a synthetic peptide from gp41. J Cell Biol. 1998;140:315–323. doi: 10.1083/jcb.140.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E, Marty A. Discrete changes of cell membrane capacitance observed under conditions of enhanced secretion in bovine adrenal chromaffin cells. Proc Natl Acad Sci USA. 1982;79:6712–6716. doi: 10.1073/pnas.79.21.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nüssler F, Clague MJ, Herrmann A. Meta-stability of the hemifusion intermediate induced by glycosylphosphatidylinositol-anchored influenza hemagglutinin. Biophys J. 1997;73:2280–2291. doi: 10.1016/S0006-3495(97)78260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odell D, Wanas E, Yan J, Ghosh HP. Influence of membrane anchoring and cytoplasmic domains on the fusogenic activity of vesicular stomatitis virus glycoprotein G. J Virol. 1997;71:7996–8000. doi: 10.1128/jvi.71.10.7996-8000.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975;189:347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Qiao H, Armstrong RT, Melikyan GB, Cohen FS, White JM. A specific point mutant at position 1 of the influenza hemagglutinin fusion peptide displays a hemifusion phenotype. Mol Biol Cell. 1999;10:2759–2769. doi: 10.1091/mbc.10.8.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razinkov VI, Melikyan GB, Epand RM, Epand RF, Cohen FS. Effects of spontaneous bilayer curvature on influenza virus-mediated fusion pores. J Gen Physiol. 1998;112:409–422. doi: 10.1085/jgp.112.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroth-Diez B, Ponimaskin E, Reverey H, Schmidt MF, Herrmann A. Fusion activity of transmembrane and cytoplasmic domain chimeras of the influenza virus glycoprotein hemagglutinin. J Virol. 1998;72:133–141. doi: 10.1128/jvi.72.1.133-141.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruce AE, Iwata A, White JM, Almers W. Patch clamp studies of single cell-fusion events mediated by a viral fusion protein. Nature. 1989;342:555–558. doi: 10.1038/342555a0. [DOI] [PubMed] [Google Scholar]
- Sutton RB, Fasshauer D, Jahn R, Brunger AT. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 Å resolution. Nature. 1998;395:347–353. doi: 10.1038/26412. [DOI] [PubMed] [Google Scholar]
- Taylor GM, Sanders DA. The role of the membrane-spanning domain sequence in glycoprotein-mediated membrane fusion. Mol Biol Cell. 1999;10:2803–2815. doi: 10.1091/mbc.10.9.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse FW, Iwata A, Almers W. Membrane flux through the pore formed by a fusogenic viral envelope protein during cell fusion. J Cell Biol. 1993;121:543–552. doi: 10.1083/jcb.121.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenhorn W, Carfi A, Lee KH, Skehel JJ, Wiley DC. Crystal structure of the Ebola virus membrane fusion subunit, GP2, from the envelope glycoprotein ectodomain. Mol Cell. 1998;2:605–616. doi: 10.1016/s1097-2765(00)80159-8. [DOI] [PubMed] [Google Scholar]
- Wharton SA, Skehel JJ, Wiley DC. Studies of influenza hemagglutinin-mediated membrane fusion. Virology. 1986;149:27–35. doi: 10.1016/0042-6822(86)90083-8. [DOI] [PubMed] [Google Scholar]
- White J, Helenius A, Gething MJ. Hemagglutinin of influenza virus expressed from a cloned gene promotes membrane fusion. Nature. 1982;300:658–659. doi: 10.1038/300658a0. [DOI] [PubMed] [Google Scholar]
- Wilk T, Pfeiffer T, Bukovsky A, Moldenhauer G, Bosch V. Glycoprotein incorporation and HIV-1 infectivity despite exchange of the gp160 membrane-spanning domain. Virology. 1996;218:269–274. doi: 10.1006/viro.1996.0190. [DOI] [PubMed] [Google Scholar]
- Zimmerberg J, Blumenthal R, Sarkar DP, Curran M, Morris SJ. Restricted movement of lipid and aqueous dyes through pores formed by influenza hemagglutinin during cell fusion. J Cell Biol. 1994;127:1885–1894. doi: 10.1083/jcb.127.6.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]