Abstract
We report the identification, cDNA cloning, and molecular characterization of a novel, constitutive nucleolar protein. The cDNA-deduced amino acid sequence of the human protein defines a polypeptide of a calculated mass of 61.5 kDa and an isoelectric point of 9.9. Inspection of the primary sequence disclosed that the protein is a member of the family of “DEAD-box” proteins, representing a subgroup of putative ATP-dependent RNA helicases. ATPase activity of the recombinant protein is evident and stimulated by a variety of polynucleotides tested. Immunolocalization studies revealed that protein NOH61 (nucleolar helicase of 61 kDa) is highly conserved during evolution and shows a strong accumulation in nucleoli. Biochemical experiments have shown that protein NOH61 synthesized in vitro sediments with ∼11.5 S, i.e., apparently as homo-oligomeric structures. By contrast, sucrose gradient centrifugation analysis of cellular extracts obtained with buffers of elevated ionic strength (600 mM NaCl) revealed that the solubilized native protein sediments with ∼4 S, suggestive of the monomeric form. Interestingly, protein NOH61 has also been identified as a specific constituent of free nucleoplasmic 65S preribosomal particles but is absent from cytoplasmic ribosomes. Treatment of cultured cells with 1) the transcription inhibitor actinomycin D and 2) RNase A results in a complete dissociation of NOH61 from nucleolar structures. The specific intracellular localization and its striking sequence homology to other known RNA helicases lead to the hypothesis that protein NOH61 might be involved in ribosome synthesis, most likely during the assembly process of the large (60S) ribosomal subunit.
INTRODUCTION
Nucleoli, the most conspicuous intranuclear structures in eukaryotic cells, are known to be the main site of ribosome biosynthesis (Hadjiolov, 1985; Scheer and Weisenberger, 1994; Scheer and Hock, 1999). More recently, however, it has also become clear that the nucleolus has additional functions and may be involved in the transport or assembly of various kinds of ribonucleoprotein particles (reviewed by Pederson, 1998).
The production of preribosomal particles is a complex process, involving the transcription of the rRNA genes, the processing of the primary transcripts, and the addition of proteins to the nascent preribosomes as well as the incorporation of the 5S rRNA, which is synthesized outside of the nucleolus. Besides the rRNA gene clusters and flanking sequences, nucleoli contain a large number of proteins and RNA components that are not part of mature cytoplasmic ribosomes but are involved in the transcriptional (Reeder, 1990; Paule, 1993) and post-transcriptional regulation of ribosome synthesis (Olson, 1990). Among these are small nucleolar RNAs (snoRNAs), which play a major role in the endo- and exonucleolytic processing of the large preribosomal precursor as well as in the specific modification of rRNA molecules by remodeling certain uridine residues into pseudouridines and by tagging ribose moieties with methyl groups (Tollervey, 1996; Tollervey and Kiss, 1997; Smith and Steitz, 1997; Weinstein and Steitz, 1999). A number of nonribosomal proteins that may be components of nucleolar structural elements, proteins that are transiently associated with preribosomes, and nucleocytoplasmic shuttle proteins have been identified, but only some of them were characterized in detail (reviewed by Melese and Xue, 1995; Shaw and Jordan, 1995; Busch, 1997).
Although ribosome biogenesis has been extensively studied in higher eukaryotes (Eichler and Craig, 1994), mainly biochemical strategies and genetic approaches performed in the yeast Saccharomyces cerevisiae contributed to the identification of various cis-elements and trans-acting factors participating in this process (Venema and Tollervey, 1995; Tollervey, 1996). In addition to snoRNAs, enzymes for rRNA modification, and endo- and exonucleases, the importance of another class of trans-acting factors predicted to function enzymatically in ribosome biogenesis became evident, i.e., the ATP-dependent RNA helicases.
RNA helicases, which can be classified into subfamilies (Gorbalenya and Koonin, 1993), have been identified in most organisms ranging from bacteria to human. They have been implicated in cellular processes as diverse as pre-mRNA splicing, rRNA processing, ribosome assembly, translation, nucleocytoplasmic transport of mRNA, RNA degradation, mRNA stability, germ line development, and embryogenesis (reviewed by Schmid and Linder, 1992; Fuller-Pace, 1994; de la Cruz et al., 1999). To date, a number of putative RNA helicases, mainly identified in the yeast S. cerevisiae, have been described to play a functional role in ribosome biogenesis, and for some of them a nucleolar localization could be demonstrated. Nevertheless, only a few mammalian nucleolar RNA helicases have been identified and characterized in more detail.
In the course of our studies aimed at the identification of novel nonribosomal nucleolar proteins to get a clearer picture of the protein composition within the nucleolus, we have cloned the cDNA of a previously unknown 61-kDa protein (NOH61 [nucleolar helicase of 61 kDa]) from HeLa cells. Analysis of the primary sequence deduced from this cDNA clone has revealed that the encoded protein belongs to the DEAD-(Asp-Glu-Ala-Asp) box protein family representing a subclass of putative RNA helicases. Localization studies have disclosed that protein NOH61 is a constitutive component of actively transcribing nucleoli and thus represents a novel nucleolar helicase-type protein. In biochemical studies we further show that the protein is specifically enriched in fractions containing the precursors of the large ribosomal subunit (65S preribosomal particle) but is absent from mature ribosomes. From the latter observation, together with its exclusive nucleolar localization and the remarkably high evolutionary conservation, we conclude that we have identified a protein that may have an essential function during ribosome biogenesis.
MATERIALS AND METHODS
Biological Material
Clawed toads (Xenopus laevis) were purchased from the South African Snake Farm (Krysna, Republic of South Africa).
Cell culture lines used in this study were maintained under standard conditions and included cervical adenocarcinoma, line HeLa, SV40-transformed fibroblasts, line SV80, vulvar squamous cell carcinoma, line A-431, spontaneously transformed keratinocytes, line HaCaT, breast adenocarcinoma, line MCF-7, astrocytoma-derived glioma cell line U333 CG/343 MG, primary liver carcinoma line PLC (all human origin), bovine mammary gland–derived line BMGE+H, rat vascular smooth muscle–derived line RV, embryonic mouse line 3T3-L1, rat kangaroo PtK2, and X. laevis kidney epithelium XLKE, line A6. For sources of all cell lines see American Type Culture Collection (Manassas, VA) and previous reports from this laboratory (Franke et al., 1979; Schmid et al., 1983; Cordes et al., 1996).
Isolation and Characterization of cDNA Clones Coding for Protein NOH61
A human keratinocyte cDNA expression library (HL11106; Clontech, Heidelberg, Germany) was screened with monoclonal antibody (mAb) 240-14-10. This mAb, which showed a strong nuclear staining on human cultured cells when analyzed by immunofluorescence microscopy, was selected during the screening procedure of hybridoma cell lines resulting from mice immunized with a synthetic peptide deduced from the primary sequence of the human Tpr protein (SERQAPRAPQSPRRPPHPLPPR, aa 2049–2070; cf. Cordes et al., 1997). The isolation and the characterization of this antibody will be described in detail elsewhere.
One of the cDNA clones obtained was selected, plaque purified, and subcloned into the Bluescript vector (pBTSK; Stratagene, Heidelberg, Germany) for subsequent sequencing. This partial cDNA clone (pBT-240) of 1794 nucleotides in length encodes a protein of 425 aa with a high sequence homology to putative ATP-dependent RNA helicases. To isolate the corresponding full-length cDNA clone, a HeLa cDNA expression library was rescreened with the random-primed, 32P-labeled fragment derived from pBT-240 after digestion with NcoI (nucleotides 29–1588). After three rounds of screening, 12 reactive cDNA clones were isolated and characterized by restriction mapping and sequencing. All of them could be assigned to a sequence contig comprising an open reading frame (ORF) of 547 aa that is preceded by an in-frame-stop codon at position −33 relative to the start methionine, which meets the “−3 = A” requirement of Kozak (1989). One cDNA clone, termed pBT-NOH61, contains the entire ORF and 3′-noncoding regions as well as part of the 5′-noncoding region and was used for the experiments described in this study.
Sequence analyses were performed using the Heidelberg Unix Sequence Analysis Resources software package.
RNA Isolation, Northern Blot Hybridization, and Coupled In Vitro Transcription–Translation
Total RNA from HeLa and HaCaT cells was prepared as described by Chirgwin et al. (1979). Poly(A)+ RNA was obtained using an mRNA purification kit (Pharmacia, Freiburg, Germany). RNAs (5 μg) were separated on 1% agarose gels containing 0.6% formaldehyde, transferred to Biodyne A filters (Pall, Dreieich, Germany), hybridized with a 32P-labeled full-length DNA fragment derived from clone pBT-NOH61 by EcoRI–XhoI digestion, washed, and processed by autoradiography essentially as described by Heid et al. (1994).
For the in vitro synthesis of [35S]methionine-labeled protein, we used the TNT coupled reticulocyte lysate (Promega, Heidelberg, Germany) programmed by the construct pBT-NOH61.
Generation of Peptide-specific Antibodies against the Human NOH61 Protein
Guinea pig antibodies specific for protein NOH61 were obtained by immunization with synthetic peptides (Schnölzer et al., 1992) representing various parts of the amino acid sequence deduced from the cDNA sequence (indicated in Figure 1B). In the experiments reported here, antibodies NOH61-2.1 and NOH61-5.1 directed against the peptide sequences GPKGDKASDPEAGV (aa 332–345) and EKLKTYFEDNPRDLQ (aa 458–472), respectively, were routinely used after affinity purification on iodoacetyl-immobilized peptide (Mertens et al., 1996). Antibody NOH61-4.2 (aa 23–36; TDLGWSRPTLIQEK) showed the same specificities. All three antibodies varied in their cross-reactivities throughout different species.
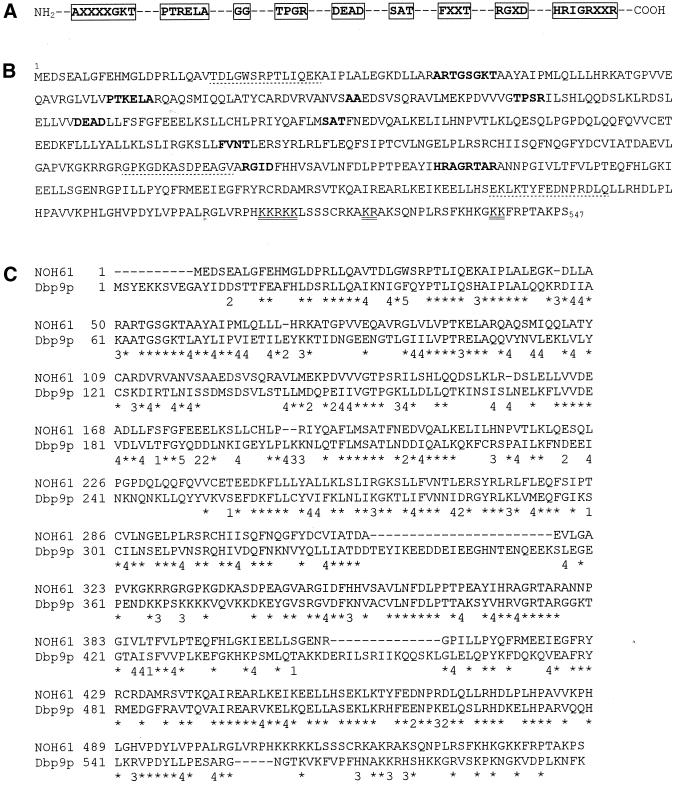
Figure 1.
Amino acid sequence of the human helicase-type protein NOH61 and comparison with its putative yeast homologue Dbp9p. (A) Schematic representation of highly conserved amino acid regions characteristic of putative ATP-dependent RNA helicases of the DEAD-box protein family (Schmid and Linder, 1992; Pause and Sonenberg, 1993). The nine conserved motifs are boxed (X indicates any amino acid). (B) Amino acid sequence of the protein NOH61 deduced from the cDNA insert of pBT-NOH61 (EMBL accession number AJ131712, 1926 bp). The helicase core region extends from aa 51 to 378. The nine conserved motifs characteristic of the family of DEAD-box proteins are all present in the primary sequence of NOH61 (indicated by bold letters). Putative nuclear localization signals are double underlined, and sequences used for generating antibodies are marked by dotted lines. (C) Sequence comparison of human protein NOH61 with the yeast protein Dbp9p (EMBL accession number Q06218). Horizontal bars are omissions introduced to optimize the alignment. The symbols below the primary sequences are defined as follows: *, identical amino acids; 1–5, conservative exchanges (1, aliphatic, polar; 2, acidic; 3, basic; 4, aliphatic, nonpolar; 5, aromatic).
Isolation and Fractionation of Xenopus Oocyte Nuclei and Egg Extract
Nuclei of mature (stages IV–VI; Dumont, 1972) X. laevis oocytes were obtained by mass isolation, a large-scale procedure described by Scalenghe et al. (1978) and modified by Kleinschmidt and Franke (1982). Subsequent fractionation of nuclear contents by differential centrifugation was performed as described in detail by Hügle et al. (1985), resulting in fractions termed “low-speed pellet” (LSP), “high-speed pellet” (HSP), and “high-speed supernatant” (HSS). The LSP fractions were cleared from yolk proteins by Freon extraction (Evans and Kay, 1991). The preparation of egg extracts was as described by Cordes et al. (1993).
Preparation of Total Cellular Lysates and Cell Extracts and Isolation of Nuclei and Nucleoli from HeLa Cells
Total cellular lysates of cultured cells were obtained as described by Schmidt-Zachmann et al. (1998).
Cellular extract of HeLa cells used for sucrose gradient centrifugation was prepared by the following method. Confluently grown cells were washed three times with PBS and lysed directly on the culture dish with 1 ml HeLa lysis buffer (50 mM Tris-HCl, pH 7.4, 0.5% Triton-X100, 1 mM β-mercaptoethanol, 600 mM NaCl, and, for inhibition of proteases, 100 μM Pefablock, 20 μM pepstatin, and 20 μM leupeptin) per 10-cm dish for 2 min at room temperature. The lysates were transferred to a 1.5-ml tube and further incubated for 20 min on ice. The lysed cells were cleared from cellular debris by centrifugation (15 min, 14,000 × g, 4°C), and the resulting supernatant was directly used for biochemical studies.
The preparation of nuclei and nucleoli from HeLa cells was done according to the NP-40 method described by Chan and Chan (1995) with some minor modifications. All steps were performed at 4°C with freshly prepared, precooled solutions, and critical steps were microscopically controlled. Adherently grown HeLa cells (2 × 108) were trypsinized and washed twice with 20 ml of PBS and once with 20 ml of buffer A (10 mM Tris-HCl, pH 7.4, 8.3 mM KCl, 1.3 mM NaCl, and 1.5 mM MgSO4). After each step, the cells were sedimented (10 min, 660 × g) and finally resuspended in 20 ml of buffer A. Cells were swollen for 30 min on ice, sedimented as described above, and resuspended in 20 ml of buffer B (buffer A complemented with 0.5% NP-40 and 1 mM PMSF). Nuclei and cytosol were separated by passing the suspension eight times through a 23-gauge needle followed by 20 dounces in a glass–glass homogenizer with a tight-fitting pestle. Crude nuclei were sedimented (10 min, 1000 × g), resuspended in 10 ml of buffer C (buffer A containing 1 mM PMSF), sedimented again (10 min, 1000 × g), and resuspended in 10 ml of buffer S1 (0.25 M sucrose, 1.5 mM MgSO4, 0.5 mM spermidine, 0.2 mM spermin, and 1 mM PMSF). The suspension was underlaid with 15 ml of buffer S2 (0.88 M sucrose and 0.05 mM MgSO4) and centrifuged (15 min, 2500 × g). The sediment containing the purified nuclei was resuspended in 1 ml of buffer S3 (0.34 M sucrose, 0.05 mM MgSO4, 0.5 mM spermidine, 0.2 mM spermin, and 1 mM PMSF). A 100-μl aliquot of the nuclear suspension was removed and stored at −80°C or processed for SDS-PAGE (see below). The residual 900 μl were diluted to a final volume of 10 ml with buffer S3 and sonicated with a Branson (Danbury, CT) Sonifier (six to eight 125-W bursts, 10 s each, followed by 20 s of cooling on ice) until no intact nuclei were visible. The suspension was underlaid with 15 ml of buffer S2 and centrifuged (15 min, 2500 × g). The sediment containing the purified nucleoli was resuspended in 1 ml of buffer S3 and stored at −80°C or processed for SDS-PAGE (see below).
Nuclear and nucleolar fractions were prepared for SDS-PAGE by addition of 1 vol of 2× SDS-PAGE sample buffer and 10 μl of Benzonase (Merck, Darmstadt, Germany) and incubation for 15 min on ice. Immediately before loading onto SDS-PAGE gels, the fractions were heated for 10 min at 95°C and centrifuged (2 min, 14,000 × g; room temperature).
Sucrose Gradient Density Centrifugation and Gel Filtration
Four hundred microliters of cell lysate obtained from HeLa cells were separated by centrifugation in a 5–30% linear sucrose gradient (in HeLa lysis buffer without detergent). Upon centrifugation (SW40 rotor, Beckman Instruments, Palo Alto, CA; 17 h, 36,000 rpm, 4°C), fractions of 0.4 ml were collected from the top (light) to the bottom (heavy) of the gradient, and proteins were precipitated with 20% trichloroacetic acid and separated by SDS-PAGE. BSA, catalase, and thyroglobulin (all from Pharmacia) were used as S-value reference proteins in parallel gradients.
HSP fractions of Xenopus oocyte nuclei were resuspended in Tris-buffered saline (TBS; 10 mM Tris-HCl, pH 7.4, 100 mM NaCl, 1 mM MgCl2, and 1 mM β-mercaptoethanol) and fractionated in 10–40% linear sucrose gradients made up in TBS. The isolation of preribosomal particles as well as the preparation of ribosomes and ribosomal subunits have been described in detail before (Hügle et al., 1985).
For gel filtration, 75 μl of reticulocyte lysate containing protein NOH61 synthesized in vitro were mixed with 125 μl of elution buffer (10 mM Tris-HCl, pH 7.4, 83 mM KCl, 17 mM NaCl, 2 mM MgCl2, and 2 mM β-mercaptoethanol) and loaded onto a Superdex 200 HR10/30 column (Pharmacia) at 4°C. For calibration, dextran blue and reference proteins (ferritin, catalase, aldolase, BSA, and ovalbumin; all from Pharmacia) were fractionated in parallel. Proteins were eluted in the buffer described above, and fractions of 200 μl were collected and analyzed by SDS-PAGE and autoradiography.
Gel Electrophoresis and Immunoblotting
Protein fractions were separated by SDS-PAGE according to the method of Thomas and Kornberg (1975). The polypeptides were transferred to nitrocellulose and visualized by Ponceau S staining. The nitrocellulose membranes were blocked in TBS containing 0.05% Tween 20 (TBST) and 5% nonfat dry milk for 1 h and then incubated at room temperature with affinity-purified antibodies (diluted 1:400) for 1 h in TBST and 5% nonfat dry milk. Bound antibodies were detected by chemiluminescence using the ECL system (Amersham, Braunschweig, Germany) after incubation with horseradish peroxidase–coupled secondary antibodies (Dianova, Hamburg, Germany), diluted 1:10,000 in TBST and 5% nonfat dry milk for 1 h.
DNA Transfection
For transient expression in PLC cells, the NOH61 cDNA was subcloned as a blunt-ended NcoI–XhoI fragment into the mammalian expression vector pEGFP-C1 (Clontech), previously cut with BglII and blunt ended. The resulting fusion construct consists of the full-length NOH61 protein fused to the carboxyl terminus of the green fluorescent protein (GFP). The correct in-frame fusion of the cDNA was controlled by sequencing. The primary structure of the ∼87-kDa GFP-NOH61 fusion protein reads GFP(aa 1–238)-SGLRS-NOH61(aa1–547). Transfections were carried out using FuGene (Roche Biochemicals, Mannheim, Germany) as the transfection reagent according to the manufacturer's protocol. Immunofluorescence microscopy analysis was performed 24 h after transfection.
Expression and Purification of His-tagged Protein NOH61
To express an amino-terminal His6-tagged version of protein NOH61, the blunt-ended NcoI–XhoI fragment derived from clone pBT-NOH61 was subcloned into the vector pQE-30 (Qiagen, Hilden, Germany), previously cut with BamHI and blunt ended. The resulting pQE-30-His6-NOH61 plasmid was transformed into Escherichia coli M15[pREP4] cells. Transformed cells were grown and induced with 1 mM isopropyl-1-thio-β-d-galactopyranoside for 5 h. Finally, the cells were harvested and stored at −80°C. The bacterial pellet was resuspended in lysis buffer containing 50 mM NaH2PO4, pH 8.0, 500 mM NaCl, 14 mM β-mercaptoethanol, and 10 mM imidazole, supplemented with protease inhibitor mixture tablets (Roche). The lysate was obtained by sonication, treated for 10 min with Benzonase (Merck), and cleared by centrifugation at 10,000 rpm at 4°C for 60 min in a Beckman Instruments (Palo Alto, CA) JA 20 rotor. Ni-nitrilotriacetic acid superflow beads (Qiagen) were added to the bacterial lysate, and the mixture was incubated at 4°C for 1.5 h. Beads were poured into a column, the flow through fraction was collected, and the column was washed extensively with lysis buffer followed by several washes with lysis buffer supplemented with 20 mM imidazole. Proteins bound to Ni-nitrilotriacetic acid beads were eluted with lysis buffer containing 250 mM imidazole. The eluted proteins were dialyzed against PBS and either used directly for the ATPase assays or stored at −80°C until use. We usually obtained ∼2 mg of protein His6-NOH61 from 400 ml of culture. The protein has the tendency to precipitate; where necessary, precipitated protein was removed by centrifugation.
ATPase Hydrolysis Assays
ATPase assays were carried out in 50 mM Tris-HCl, pH 7.2, 5 mM MgCl2, 1 mM DTT, and 1 μCi [γ-32P]ATP (New England Nuclear Life Science, Köln, Germany) in a final concentration of 0.1 mM ATP. Reactions contained 200 ng of His6-NOH61 and, unless otherwise stated, 10 μg of polynucleotides (total HeLa RNA, synthetic homopolymers of RNAs [Sigma, München, Germany], double-stranded DNA [dsDNA], and single-stranded DNA [ssDNA] in a final volume of 20 μl). The reaction mixtures were incubated for 1 h at 37°C, stopped by adding 3 μl of 0.5 M EDTA, and placed on ice. Two microliters of each reaction were spotted onto polyethyleneimine-cellulose thin-layer chromatography plates (Merck). The chromatography was performed in 1 M formic acid and 0.5 M LiCl and analyzed by autoradiography. The relative amounts of free radioactive phosphate in the reaction mixture were evaluated from the dried plates using a Fujifilm BAS 1500 phosphorimager (Raytest, Straubenhardt, Germany).
Immunofluorescence Microscopy
For immunofluorescence microscopy studies on cultured cells, the cells grown on coverslips were routinely fixed with methanol (7 min, −20°C) and acetone (30 s, −20°C). Alternatively, cells were fixed with 3% paraformaldehyde and 2% sucrose in PBS (7 min, room temperature) and permeabilized with 0.5% Triton X-100 (5 min on ice).
Nuclease digestion was performed according to the method of Spector et al. (1991). For actinomycin D (AMD) experiments, the cultured cells were incubated for 4 h in fresh medium containing a 5 μg/ml concentration of the drug.
After fixation, cells were washed twice in PBS and incubated with purified guinea pig antibodies (1:100 in PBS) for 20 min at room temperature. After several washes in PBS, cells were incubated for 20 min with Texas Red–labeled goat anti-guinea pig secondary antibodies (Dianova), diluted 1:200 in PBS. The cells were then washed again with PBS, dehydrated in ethanol, air dried, and mounted with Fluoromount (Biozol, Eching, Germany). In some experiments, cells were stained with DAPI (0.1 μg/ml; Serva, Heidelberg, Germany) for 5 min during incubation with the secondary antibodies.
Immunoelectron Microscopy
HeLa cells grown on coverslips were briefly washed with PBS, fixed for 5 min in −20°C methanol, and permeabilized in −20°C acetone for 2 min. Incubation with the primary antibody (affinity-purified serum NOH61-2.1, diluted 1:50) was performed in a wet chamber for 5 h at room temperature. After three washes with PBS for 5 min each, bound antibodies were reacted overnight at room temperature with anti-guinea pig immunoglobulin G (IgG)-conjugated nanogold (Nanoprobes, Stony Brook, NY), diluted 1:50. After several washes with PBS for 5 min each, cells were fixed with 2.5% glutaraldehyde in 0.05 M cacodylate buffer for 15 min at 4°C. Silver enhancement was carried out as described by Uchida et al. (1996). Subsequently, cells were postfixed with 0.01% osmium tetroxid solution and processed for flat embedding in Epon as described (Franke et al., 1978).
RESULTS
Isolation and Analysis of a cDNA Clone Encoding a Helicase-Type 61-kDa Protein from HeLa Cells
In the course of functional and structural analysis of the Tpr protein, a constitutive component of the nuclear pore complex–attached intranuclear filaments (Cordes et al., 1997), mAbs were raised against a synthetic peptide (hTpr-Pep2) deduced from the cDNA sequence coding for the human Tpr protein (European Molecular Biology Laboratory [EMBL] accession number U69668; Cordes et al., 1997). Among others, we obtained one mAb (240-14-10), which on immunofluorescence microscopy did not show the expected punctate staining in the nuclear periphery, as is characteristic for Tpr, but stained the nuclear interior in a more uniform, finely dotted manner. The antibody reacted with a ∼100-kDa protein in immunoblots of SDS-PAGE–separated proteins of whole cellular lysates from different human cultured cells, such as epithelial cells of lines HeLa and HaCaT, respectively (V.C. Cordes and M.S. Schmidt-Zachmann, unpublished results).
This antibody was used to screen a human keratinocyte cDNA expression library. One of the positive clones, termed pBT-240, was isolated, purified to homogeneity, and sequenced. The cDNA insert of clone pBT-240 consisted of 1794 base pairs (bp) and contained an ORF encoding a protein of 425 aa. The analysis of this sequence by BLAST search revealed the presence of several sequence motifs characteristic for ATP-dependent RNA helicases. Because the 3′ end of the cDNA was missing, a Uni-Zap HeLa cDNA expression library was screened with the random prime–labeled cDNA fragment of clone pBT-240. Several positive recombinants, all containing cDNA inserts of ∼2 kb, were obtained, and sequencing revealed that they overlapped extensively. One clone, denoted pBT-NOH61, was further analyzed in detail (see EMBL database, accession number AJ131712).
Clone pBT-NOH61 (1926 bp) contains an initiation codon at position 68, an ORF of 1644 bp, and a 3′ untranslated region of 215 bp with a consensus AATAAA polyadenylation signal 21 bp upstream of the poly(A) tail of 18 bp. The ORF encodes a polypeptide of 547 aa, with a calculated molecular mass of 61.5 kDa and an isoelectric point of 9.9 (Figure 1B). Comparison of the inferred amino acid sequence with proteins in the current databases revealed that the 61.5-kDa protein shares extensive identity with a family of proteins thought to function as ATP-dependent RNA helicases.
One of the five classes of helicases based on sequence homology (Gorbalenya and Koonin, 1993), are the so-called “DEAD-box” proteins. A key feature of all DEAD-box RNA helicases is the presence of nine characteristic motifs spaced from each other by conserved distances (Schmid and Linder, 1992; Pause and Sonenberg, 1993), which are schematically represented in Figure 1A. Despite some minor differences, these motifs are all present in the 61-kDa protein (Figure 1B). Therefore, we consider this “helicase-type” 61-kDa protein as a member of the DEAD-box protein subfamily of putative ATP-dependent RNA helicases.
We have also noted two putative nuclear localization signals at the very carboxyl terminus of the molecule, one of the monopartite type (aa 509–513; KKRKK) and the other of the bipartite type [aa 522–539; KR(x)14KK; Figure 1B, underlined; cf. Dingwall and Laskey, 1991]. Potential phosphorylation sites are found for cAMP-dependent kinase, protein kinase C, and casein kinase II, although the biological significance of these sites has to be investigated. Another interesting feature of the primary sequence is the presence of an almost ideal leucine zipper in the amino-terminal third of the molecule (aa 146–173), a sequence motif known to be involved in the maintenance of protein–protein interactions (e.g., see Alber, 1992).
In searches of current databases we noted besides the general sequence homology to members of the DEAD-box protein family a striking homology of the 61-kDa protein with a hypothetical so far uncharacterized RNA helicase termed Dbp9p (DEAD-box-protein 9) from S. cerevisiae (EMBL accession number Q06218). The overall amino acid sequence identity between the human 61-kDa protein and the putative homologue in yeast is 40%; their similarity, including conservative exchanges, is even 47%. Interestingly, the sequence identity over the carboxyl-terminal 140 aa of both proteins, i.e., downstream of the conserved motifs, is even higher (47% identity and 56% similarity, respectively; cf. Figure 1C). The high conservation suggests that these proteins represent homologous molecules that most likely participate in a fundamental cellular process.
Molecular Characterization of the cDNA Clone Encoding Protein NOH61
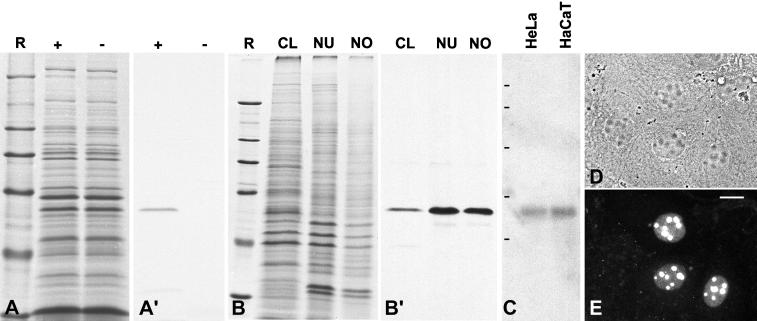
In vitro transcription and translation of cDNA clone pBT-NOH61 using the reticulocyte lysate system yielded a product of ∼61 kDa, consistent with the calculated molecular mass of the encoded protein (Figure 2, A and A′). A polypeptide of similar size was detected on immunoblots probed with antibodies raised against synthetic peptides deduced from the cDNA sequence of pBT-NOH61 (see Figure 1B). The 61-kDa protein was detectable in total cell lysates from HeLa cells and was significantly enriched in the nuclear and nucleolar fractions, respectively (Figure 2, B and B′).
Figure 2.
Molecular characterization of the cDNA clone encoding the helicase-type protein NOH61. (A) Coomassie blue staining of SDS-PAGE–separated rabbit reticulocyte lysates after in vitro transcription and translation in the presence (+) or absence (−) of the pBT-NOH61 template. R, reference proteins: 205, 116, 97.4, 66, 45, and 29 kDa (from top to bottom). (A′) Corresponding autoradiograph of translation products. (B) Coomassie blue staining of various cellular fractions of HeLa cells separated by SDS-PAGE (CL, total cell lysate; NU, fraction enriched in nuclei; NO, fraction enriched in nucleoli). Reference proteins (R) are the same as in A. (B′) Corresponding immunoblot probed with antibody NOH61-5.1, which reacts specifically with a 61-kDa protein. This protein is highly enriched in the nuclear and nucleolar fractions. (C) Identification of specific mRNAs by Northern blot analysis. Poly(A)+ RNA from HeLa and HaCaT cells, respectively, separated by agarose gel electrophoresis was hybridized with a random prime–labeled NOH61-specific probe. Note the reaction of a single band corresponding to an ∼2-kb RNA. RNA size markers of 9.5, 7.4, 4.4, 2.4, and 1.4 kb are indicated on the left (from top to bottom). (D) Phase-contrast microscopy of methanol-fixed human hepatocellular carcinoma (PLC) cells after transfection with a chimeric cDNA encoding protein NOH61 fused to GFP. (E) Corresponding immunofluorescence micrograph. The expressed GFP-NOH61 fusion protein localizes almost exclusively in nucleoli. Some cells show in addition a weak staining of the nucleoplasm. Bar, 15 μm.
Poly(A)+ RNA from HeLa and HaCaT cells was probed in Northern blot experiments, using the random prime–labeled cDNA fragment derived from pBT-NOH61. A specific signal corresponding to an mRNA of ∼2 kb was detected (Figure 2C), indicating that the isolated cDNA clone was of full length. To investigate the expression profile of protein NOH61, the level of mRNA expression in six human tissues was examined (heart, brain, liver, pancreas, placenta, and lung) by Northern blot analysis. Again, a single transcript of ∼2 kb was detected in all tissues analyzed. Consistently, the intensity of the radioactive signal was slightly reduced in liver and pancreas, although the significance of this observation is still unclear (our unpublished results).
To analyze the intracellular localization of the 61-kDa protein, a chimeric cDNA construct encoding the full-length protein fused to the carboxyl terminus of GFP was transfected into human liver carcinoma cells (PLC), and the distribution of the ectopically expressed fusion protein was subsequently examined by immunofluorescence microscopy. The GFP-NOH61 fusion protein was almost exclusively enriched in nucleoli. In addition to the strong nucleolar staining, weak labeling of the nucleoplasm was observed (Figure 2, D and E). From this result we conclude that we have cloned a novel nucleolar, helicase-type protein, termed NOH61.
Given the differences in molecular mass and intracellular localization of the protein encoded by the pBT-NOH61 cDNA and the nuclear antigen detected by the mAb 240-14-10 used for the original screening procedure, we assume that the mAb probably recognizes identical or overlapping epitopes present in both proteins. On the other hand, we cannot exclude that the cDNA clone was isolated by serendipity.
Biochemical Characterization of Protein NOH61
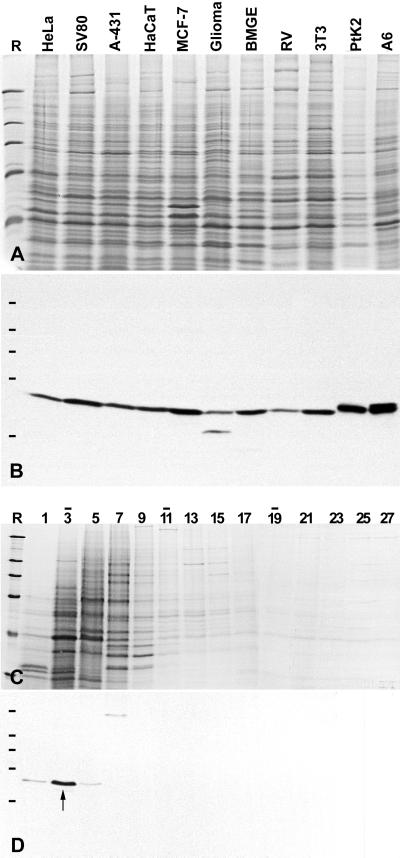
In immunoblots of total proteins isolated from cultured cells of different origin (human, bovine, rat, mouse, rat kangaroo, and Xenopus), all three antibodies raised against peptides representing the pBT-NOH61 sequence (see Figure 1B) reacted exclusively with the 61-kDa polypeptide (Figure 3, A and B). They varied in their cross-species reactivities but showed the same specificities. Because antibodies NOH61-5.1 gave the most intense signal in Western blots, they were routinely used in the majority of the experiments. The broad cross-reactivity indicates that the NOH61 protein has been highly conserved during evolution.
Figure 3.
Identification of protein NOH61 in different cultured cell lines and analysis of its native state by sucrose gradient centrifugation. (A) Coomassie blue–stained total cellular proteins of cultured cells of different origin (see MATERIALS AND METHODS). (B) Corresponding autoradiogram showing ECL detection of the antigenic polypeptide using antibody NOH61-5.1. (C) Cellular extracts of HeLa cells were fractionated after sucrose gradient centrifugation, separated by SDS-PAGE, and stained with Coomassie blue. Fraction numbers are indicated on top of the lanes (the top of the gradient is on the left). Bars indicate the peak positions of the reference proteins BSA (4.3S; fraction 3), catalase (11.3S; lane 11), and thyroglobulin (16.5S; fraction 19). (D) Corresponding immunoblot using antibody NOH61-2.1. The bulk of protein NOH61 is recovered in fraction 3 with a sedimentation coefficient of ∼4.3 S (marked by an arrow). Reference proteins (R) are the same as in Figure 2.
On cell extractions with detergent and intermediate salt concentrations, followed by cell fractionation, protein NOH61 remained structure associated. The protein became partially solubilized in buffers containing 500–600 mM NaCl and was completely released upon additional treatment with Benzonase for the removal of nucleic acids (our unpublished results). The native state of protein NOH61 solubilized in buffers of high ionic strength (600 mM NaCl) was analyzed by density gradient centrifugation (Figure 3, C and D). Immunoblotting of the resulting fractions revealed that the protein sedimented with ∼4.3 S (Figure 3D, fraction 3), indicating a monomeric state. By contrast, when the in vitro translation product was analyzed by sucrose gradient centrifugation and gel filtration experiments under near physiological ionic conditions (100 mM NaCl), protein NOH61 sedimented with ∼11.3 S and eluted in a distinct peak corresponding to an apparent mass of ∼300,000 (our unpublished results). These results suggest that protein NOH61 is able to form distinct homo-oligomeric complexes.
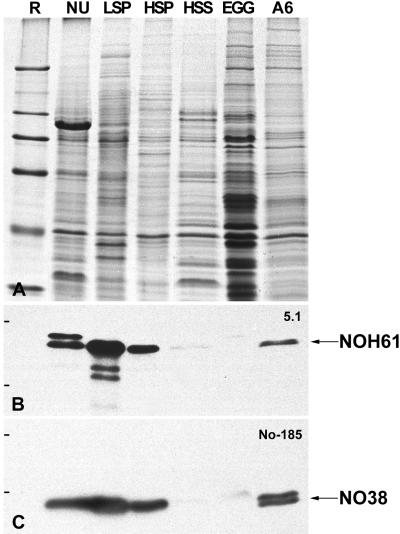
Our transfection experiments had already suggested that protein NOH61 was a nucleolar protein capable of redirecting a reporter protein first to the nucleus and then to the nucleolus. Because fractionation of isolated Xenopus oocyte nuclei by differential centrifugation leads to well-defined protein fractions characterized by specific nuclear components (cf. Hügle et al., 1985; Köhler et al., 1997; Schmidt-Zachmann et al., 1998), the distribution of protein NOH61 in such fractions from oocyte nuclei, egg extracts, and somatic cells was analyzed by immunoblotting with the NOH61-specific antibody (Figure 4). Antibody NOH61-5.1, which shows the strongest cross-reactivity with the Xenopus homologue, recognized its antigen in total oocyte nuclei as well as in the LSP and HSP fractions, indicating that protein NOH61 is a nuclear protein bound to relatively large structures, i.e., the nucleoli (LSP) and extranucleolar nucleoplasmic particles (HSP), respectively. The protein was only present in trace amounts in the HSS fraction containing soluble nuclear proteins, not detectable in the egg extract, but was present again in somatic cells (Figure 4B). The identity and integrity of the fractions were ascertained by reprobing the nitrocellulose filter with mAb No-185 directed against nucleolar protein NO38 (Schmidt-Zachmann et al., 1987), known to be enriched in the LSP and HSP but absent from the HSS fraction (Figure 4C; cf. Schmidt-Zachmann et al., 1998).
Figure 4.
Identification of NOH61 in different nuclear fractions from Xenopus oocytes. (A) Coomassie blue staining of various nuclear fractions of Xenopus oocytes and somatic cells, respectively, separated by SDS-PAGE. Total mass-isolated oocyte nuclei (NU), proteins of the LSP, HSP, and HSS fractions of fractionated oocyte nuclei, Xenopus egg extract (Egg), and total cellular proteins of Xenopus XLKE-A6 cells (A6) are shown. (B) Corresponding immunoblot probed with antibody NOH61-5.1, which shows a strong cross-reactivity with the putative Xenopus homologue in Western blots. Protein NOH61 is detectable in all fractions analyzed, except the egg extract. The identity of the slightly larger polypeptide band recognized by the antibody in the lane containing total oocyte nuclei is unknown. (C) To ascertain the fractionation procedure, the immunoblot shown in B was reprobed with mAb No-185 directed against the well-characterized nucleolar protein NO38. Reference (R) proteins are the same as in Figure 2.
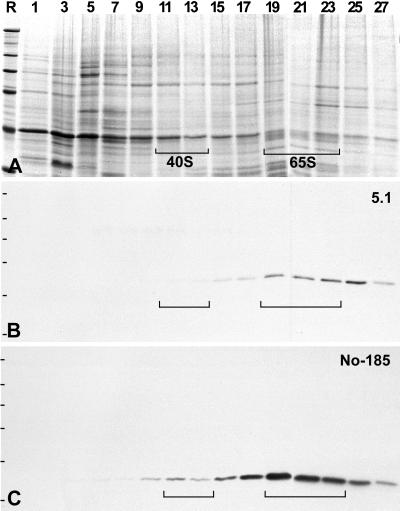
The detection of protein NOH61 in the HSP fraction was of special interest, because it has been shown that this fraction is highly enriched in free nucleoplasmic preribosomes (Hügle et al., 1985). To examine whether protein NOH61 might be complexed with preribosomal particles, the HSP fraction was further separated by centrifugation in 10–40% sucrose gradients, and the resulting fractions were analyzed by SDS-PAGE and immunoblotting (Figure 5). Indeed, protein NOH61 was specifically enriched in fractions containing the precursors for the large ribosomal subunit, recognized as 65S particles (Figure 5B). Even after longer exposure, protein NOH61 could not be detected in fractions of precursors for the 40S small ribosomal subunit. In contrast, nucleolar proteins NO38 (Figure 5C) and NO29 could be identified as components of both preribosomal particles as previously described (Schmidt-Zachmann et al., 1987; Zirwes et al., 1997). It should be noted that protein NOH61 was not detectable in mature cytoplasmic ribosomes, as determined by immunoblotting experiments on 40S and 60S ribosomal subunits and by immunocytochemistry (our unpublished results).
Figure 5.
Identification of NOH61 in free nucleoplasmic preribosomal particles separated by sucrose gradient centrifugation of HSP fractions from Xenopus oocyte nuclei. (A) Coomassie blue staining of the resulting gradient fractions. The fraction numbers are indicated at the top of the lanes. Brackets mark the positions of the preribosomal presursor molecules. (B) Corresponding immunoblot after incubation with antibody NOH61-5.1. Protein NOH61 can be detected in the 65S preribosomal particle but not in the 40S precursor for the small ribosomal subunit. (C) In comparison, reprobing of the identical immunoblot with mAb No-185 against nucleolar protein NO38 discloses the association of the latter protein with both 40S and 65S preribosomal particles. Reference (R) proteins are the same as in Figure 2.
Taken together, the results from the various immunoblot and gradient sedimentation experiments suggest that protein NOH61 is a highly conserved, nucleolar component that specifically associates with precursor particles for the large ribosomal subunit.
ATPase Activity of Protein NOH61
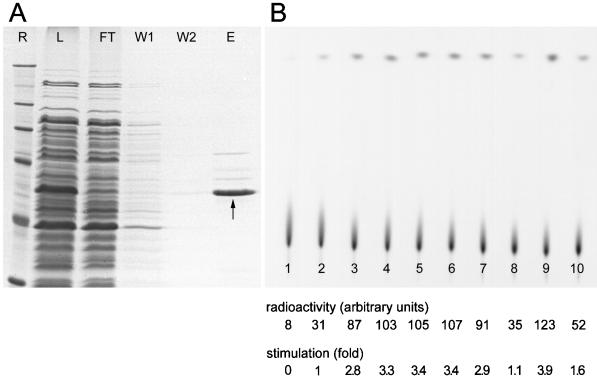
The presence of the DEAD-box motif in protein NOH61 leads to the prediction that this protein might exhibit ATPase activity. To facilitate the biochemical characterization of the protein, we overexpressed it in E. coli as a fusion protein with a six-histidine tag inserted at its amino terminus. Although a small portion was insoluble, most of the protein was found in the soluble fraction of the bacterial cell extract. The recombinant protein yielded upon purification on a nickel-agarose column was ∼95% pure, as estimated from a Coomassie blue-stained gel (Figure 6A). The identity of the protein was verified by Western blot analysis using anti-polyhistidine and NOH61-specific antibodies, respectively (our unpublished results). The faint bands coeluting with NOH61 are most likely E. coli-contaminating proteins, because they also appeared in eluates of cells transformed with the empty vector pQE-30 (our unpublished results).
Figure 6.
Expression and purification of His6-NOH61 exhibiting ATPase activity. (A) His6-NOH61 was purified as described in MATERIALS AND METHODS. The resulting protein fractions, analyzed by SDS-PAGE, are as follows: R, reference proteins are the same as in Figure 2; L, total cell lysate (soluble fraction) from bacteria after induction with isopropyl-1-thio-β-d-galactopyranoside; FT, flow-through fraction of the Ni-agarose column; W1, proteins washed from the column with 10 mM imidazole; W2, proteins washed from the column with 20 mM imidazole; E, proteins eluted from the column in the presence of 250 mM imidazole. The position of the recombinant protein is indicated by an arrow. (B) ATPase activity of purified recombinant protein His6-NOH61. An autoradiograph of a polyethyleneimine-cellulose thin-layer chromatography plate shows the separation of radioactive phosphate released from [γ32P]ATP in ATP hydrolysis assays. Lane 1 shows the ATP input; in lane 2 no polynucleotide was added; in lanes 3–10, ATP hydrolysis was stimulated in the presence of the polynucleotides poly(A), poly(C), poly(G), poly(U), poly(I), HeLa total RNA, dsDNA, and ssDNA. Reactions shown in lanes 2–10 contain 200 ng His6-NOH61. The signal intensity and fold-stimulation of ATPase activity are indicated. Each value represents the average of four independent experiments. A representative experiment is shown. Quantitations were performed on a phosphorimager.
As presented in Figure 6B, the recombinant protein NOH61 was found to be an active ATPase in the presence of various polynucleotides in vitro. However, multiple recombinant protein preparations exhibited significant ATPase activity in the absence of RNA or DNA. This activity was not reduced after additional treatment of the recombinant protein while still bound to the Ni column with DNaseI or RNaseA to release any bacterial nucleic acid bound during purification (our unpublished results). To test for a possible specificity of the ATPase reaction toward the nucleic acid substrate, we compared the stimulatory effect of a variety of RNAs and DNAs. Overall, the ATPase activity of recombinant protein NOH61 was stimulated most efficiently by poly(A), poly(C), poly(G), poly(U), poly(I), and dsDNA (2.8- to 3.9-fold on initial rates in the absence of polynucleotides), whereas total HeLa RNA and ssDNA were less efficient (1.1- to 1.6-fold). These results indicate that protein NOH61 has an intrinsic ATPase activity, which can be increased in the presence of a variety of polynucleotides.
Immunolocalization Studies at the Light Microscopic Level
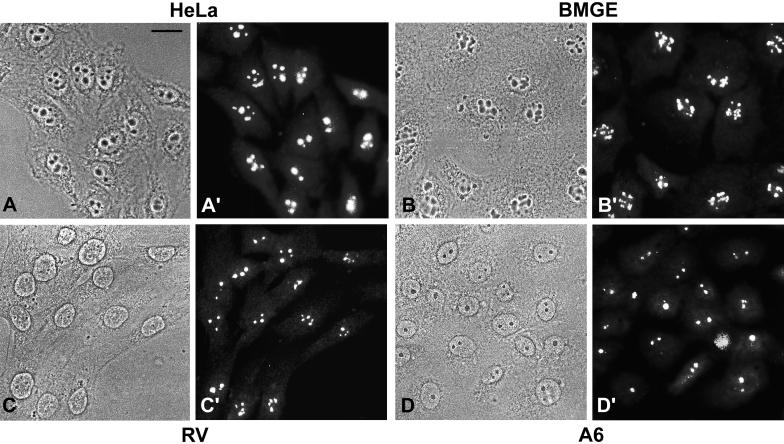
To examine the intracellular location of native protein NOH61, we performed immunocytochemistry on monolayer cell cultures of different vertebrate species, including human (Figure 7, A and A′), bovine (Figure 7, B and B′), rat (Figure 7, C and C′), and amphibian (Figure 7, D and D′). In all cases, antibody NOH61-5.1 brightly stained the nucleoli and, to a lesser extent and only in some cells, the nucleoplasm in a finely dispersed manner. The specific nucleolar localization of protein NOH61 was also confirmed by immunolocalization studies on frozen sections through tissues of various species (our unpublished results).
Figure 7.
Immunolocalization studies in cultured cells from different species. Phase-contrast micrographs are shown in A–D, and the corresponding immunofluorescence micrographs are shown in A′–D′. (A and A′) Human cervical adenocarcinoma cells of line HeLa. (B and B′) Bovine mammary gland–derived cells, line BMGE+H. (C and C′) Rat vascular smooth muscle–derived cells, line RV. (D and D′) Xenopus kidney epithelial cells, line A6. Antibody NOH61-5.1 shows a strong and specific labeling of nucleoli in all cell lines analyzed. Sometimes, a few cells show additional weak labeling of the nucleoplasm and the cytoplasm. Bar, 20 μm
Interestingly, the intracellular distribution of protein NOH61 changed drastically during mitosis. In metaphase and anaphase, i.e., upon desintegration of nucleolar structures, immunofluorescence was noted on the surfaces of the chromosomes, in addition to a diffuse staining of the perichromosomal cytoplasm (our unpublished results). This pattern of distribution during mitosis is similar to the mitotic behavior described for proteins NO38 and NO29 (Schmidt-Zachmann et al., 1987; Zirwes et al., 1997) and is characteristic for a subset of nucleolar proteins, primarily those located in the granular component of the nucleolus (Olson, 1990).
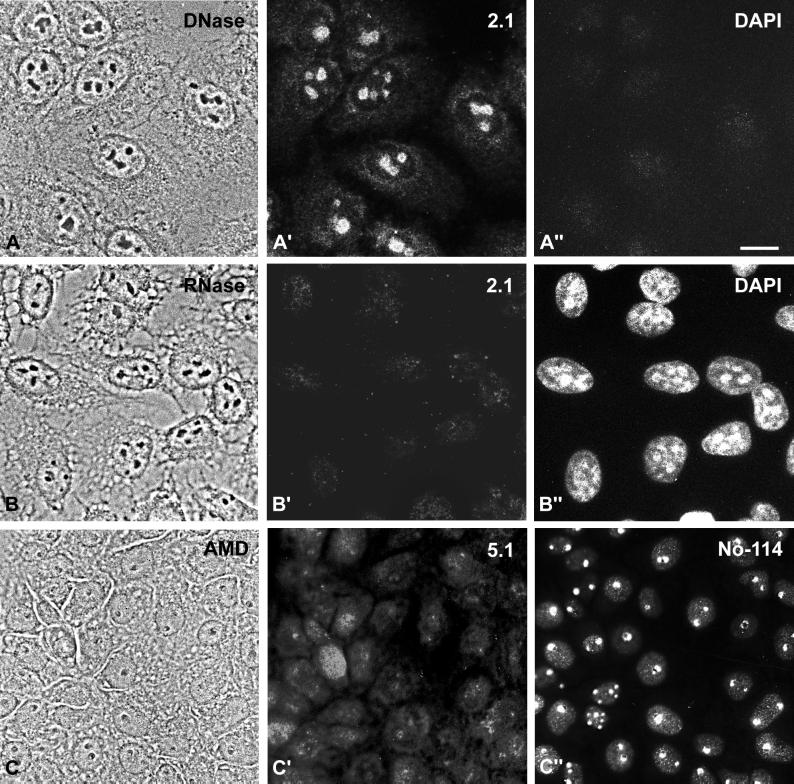
Because protein NOH61 belongs to the family of putative ATP-dependent RNA helicases, we have analyzed its intracellular distribution after treatment with nucleases and the transcription inhibitor AMD (Figure 8). After incubation of cultured cells with DNase, protein NOH61 has still been seen in nucleoli, although the staining has been weaker and more diffuse compared with untreated cells (Figures 7, A and A′, and 8, A and A′). Moreover, small amounts of the protein have been detected in the cytoplasm, seemingly concentrated at the nuclear envelope (Figure 8, A–A"). The distribution of protein NOH61 is particularly affected by treatment with RNase (Figure 8, B–B"), resulting in complete release from nucleolar structures, suggesting that protein NOH61 binds to RNA transcripts and probably is a component of larger ribonucleoprotein particles. Upon treatment of Xenopus A6 cells with AMD (Figure 8C), protein NOH61 was released from the nucleoli and translocated in part to the nucleoplasm and also to the cytoplasm (Figure 8C′). A very similar translocation has been reported for protein NO38/B23 after treatment with AMD and other cytotoxic drugs (Yung et al., 1985; Chan, 1992). When the same cells were counterstained with mAb No-114 directed against the 180-kDa nucleolar protein from X. laevis, also termed xNopp180 (Schmidt-Zachmann et al., 1984; Cairns and McStay, 1995), a specific labeling of the dense fibrillar component of segregated nucleoli was seen (Figure 8C"). These experiments indicate that protein NOH61 binds to RNA and that its nucleolar accumulation depends on ongoing transcription.
Figure 8.
Intracellular distribution of NOH61 in cells treated with nucleases and transcription inhibitors, respectively. (A–A") HeLa cells (phase-contrast image shown in A) were treated with DNase and subsequently incubated with antibody NOH61-2.1 (A′). The protein was still associated with nucleoli, although some weak staining in the cytoplasm was visible. (A") Corresponding DAPI staining. (B–B") HeLa cells (phase-contrast image shown in B) were incubated with RNase A and analyzed by indirect immunofluorescence microscopy using antibody NOH61-2.1 (B′) and DAPI (B"), respectively. NOH61 was no longer detectable in nucleoli of the treated cells. (C–C") Xenopus kidney epithelial cells, line A6, were treated with AMD (phase-contrast image shown in C) and subsequently stained with antibody NOH61-5.1 (C′) and mAb No-114 (C"). While the 180-kDa nucleolar protein recognized by mAb No-114 (cf. Schmidt-Zachmann et al., 1984) was specifically retained in the dense fibrillar component of the segregated nucleoli, protein NOH61 was released and distributed throughout the cells. Bar, 15 μm.
Subnucleolar Localization of Protein NOH61
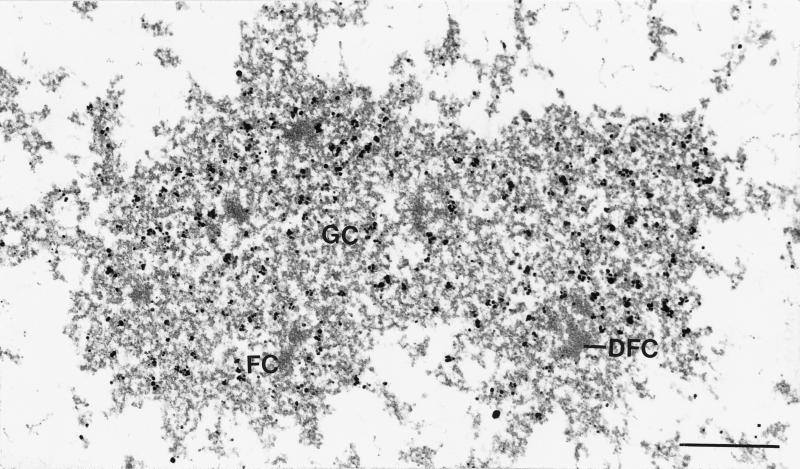
To investigate the intranucleolar distribution of this highly conserved nucleolar protein in greater detail, immunoelectron microscopy has been performed on HeLa cells. Here as in most other cells, interphase nucleoli are usually characterized by the presence of three major elements, which can be distinguished under the electron microscope: the fibrillar center, the dense fibrillar component, and the granular component. Protein NOH61 was significantly enriched in the granular component, whereas the fibrillar centers and the dense fibrillar component were practically devoid of gold particles (Figure 9). Again, this localization suggests that NOH61 may have a role in later stages of the processing of the preribosomal particles.
Figure 9.
Immunoelectron microscopy of protein NOH61 within the nucleolus of HeLa cells. Antibody NOH61-2.1 was detected by secondary antibodies coupled to nanogold particles. The granular component (GC) is specifically labeled, whereas fibrillar centers (FC) and the dense fibrillar component (DFC) are practically free of gold particles. Bar, 0.5 μm.
DISCUSSION
In the present study we have identified and characterized a novel 61-kDa nucleolar protein, designated NOH61, which is present in various cell types from human to X. laevis. The high evolutionary conservation of the amino acid sequence between the human protein and its putative homologue in yeast, the wide cross-species reactivity of antibodies, and the consistent localization in the nucleolar compartment suggest that this protein participates in a fundamental cellular process, probably in later stages of pre-rRNA processing and preribosome assembly.
Protein NOH61 is closely related to ATP-dependent RNA helicases, i.e., enzymes that unwind nucleic acid duplexes. Five classes of helicases can be grouped on the basis of sequence homology (Gorbalenya and Koonin, 1993), of which the “superfamily” II comprises the members of the so-called DEAD-box proteins. This family is characterized by a core region of ∼300–350 aa showing strong homology to translation initiation factor eIF-4A, the prototype of the DEAD-box protein family (Linder et al., 1989). Several highly conserved sequence elements are found within the core region, and for some of them their functions have been determined by studying the effects of mutations on ATP and RNA binding, ATP hydrolysis, and unwinding activity (Pause and Sonenberg, 1993). Our demonstration that NOH61 exhibits the capacity to hydrolyze ATP is not surprising, because many proteins that possess the superfamily II helicase domain have been shown previously to be ATPases. However, NOH61 ATPase activity displayed only a modest stimulation (1.6- to 3.9-fold) in the presence of polynucleotides, in apparent contrast to other DEAD-box proteins (for example, see proteins Dbp5p [Tseng et al., 1998] and Ded1p [Iost et al., 1999]). Nevertheless, it should be noted that there are several viral proteins whose ATPase activity is stimulated only weakly by nucleic acids (Kadare and Haenni, 1997). To investigate this apparent difference, comparative quantitative data regarding the ATPase activity of these proteins would be required. Moreover, the observed nucleic acid–independent ATPase activity is not unique to protein NOH61. The Drosophila Vasa protein (Liang et al., 1994) and the yeast PRP22 protein (Wagner et al., 1998) have been described as exhibiting RNA-independent ATPase activities.
To date, we have not identified NOH61 RNA specificity as a cofactor for ATP hydrolysis. One possibility would be that specific RNA substrates exist that remain to be discovered. For example, it has been described that the ATPase activity of protein Slt22p, which has a functional role in pre-mRNA splicing, is stimulated preferentially by annealed U2/U6 small nuclear RNAs (Xu et al., 1996). Among the DEAD-box proteins that have been studied biochemically, only DbpA from E. coli shows a strong RNA substrate specificity (Fuller-Pace et al., 1993). Most of the DEAD-box proteins identified so far do not show any substrate specificity in the ATPase assay or show a specificity that seems unrelated to their assumed functions.
The assembly of ribosomes is a highly elaborated process, including the transcription of pre-rRNAs, the processing of the primary transcripts, and the coordinated assembly with ribosomal as well as with nonribosomal proteins to form the two ribosomal subunits (reviewed by Hadjiolov, 1985; Warner, 1990). It is widely believed that this process requires the assembly of a large processing complex for pre-rRNA maturation, similar to the spliceosome where splicing reactions of the pre-mRNAs take place (reviewed by Moore et al., 1993; Krämer, 1996). The crucial steps in the biogenesis of ribosomes have been extensively examined in diverse eukaryotic organisms (reviewed by Söllner-Webb et al., 1991; Paule, 1993; Grummt, 1999), but most of the results unraveling this process at the molecular level came from biochemical and genetic studies in the yeast S. cerevisiae (e.g., Planta et al., 1995; van Nues et al., 1995; Venema and Tollervey, 1995).
Because it is known that ribosome assembly requires ATP, it is a special challenge to identify the proteins involved in nucleolar ATP metabolism. The ATP-dependent RNA helicases are a major class of factors predicted to function in ribosome biogenesis and have attracted special interest in recent years. To date, 10 different putative RNA helicases have been shown to be implicated in ribosome biogenesis in yeast. Although all these proteins share high sequence homology, they appear to serve distinct functions and cannot functionally substitute for each other. This specificity may also be reflected by sequence differences mainly found in the amino- and carboxyl-terminal regions. These sequence elements vary in length and composition and probably confer substrate specificity, e.g., by modulating the binding to RNA and/or accessory proteins. Moreover, they may be involved in regulation of the intracellular distribution of the respective helicase (for a recent review, see de la Cruz et al., 1999). Interestingly, protein NOH61 and its putative yeast homologue (Dbp9p) display an extraordinarily high sequence conservation in the carboxyl-terminal region. We are currently performing a detailed mutational analysis of protein NOH61 to identify sequence elements required for nuclear uptake and nucleolar accumulation. In addition, this approach will allow us to identify and further characterize the functional domains present in this molecule. Surprisingly, in higher eukaryotics only a few nucleolar RNA helicases have been described and their precise functions are largely unknown: Xenopus An3 protein (Gururajan et al., 1994; Gururajan and Weeks, 1997), protein Dbp45A from Drosophila (Lavoie et al., 1993), and the human proteins p68 (Iggo et al., 1991), Ski2w (Qu et al., 1998), and the Gu/RH-II helicase (Flores-Rozas and Hurwitz, 1993; Valdez et al., 1996, 1997). Protein NOH61 represents a new member of widespread occurrence in this fast growing family of proteins.
Protein NOH61 does not contain known RNA binding motifs such as the ribonucleoprotein motif or the Arg/Gly-rich domains found in various other DEAD-box proteins, which are characteristic for single-stranded nucleic acid–binding proteins (Serin et al., 1997, and references therein). On the other hand, the protein is released from nucleolar structures upon treatment with RNase and translocated from the residual nucleoli upon inhibition of transcription by AMD. Whether protein NOH61 can bind directly to RNA remains to be examined.
In general, helicases may catalyze different events during ribosome synthesis: they may 1) disrupt secondary structures that otherwise block the activity of exonucleases, 2) control the association and dissociation of snoRNAs by regulating the intramolecular unwinding of rRNA secondary structures, and 3) result in structural rRNA influencing the binding capacity of ribosomal and nonribosomal proteins during the assembly process. In the current state of our studies, we cannot predict in which of these reactions protein NOH61 might be involved. On the other hand, our finding that the protein, which exhibits ATPase activity in vitro, is specifically complexed with the nucleoplasmic precursor molecules for the large ribosomal subunit strongly suggests that NOH61 plays an important role in the assembly of the 60S ribosomal subunit. A similar functional participation has recently been postulated by de la Cruz et al. (1999) for the yeast homologue, although the latter protein has not been studied yet. As the next step, the identification of molecules interacting with NOH61 should allow decoding of its nucleolar function more precisely.
ACKNOWLEDGMENTS
We gratefully acknowledge Dieter Werner and Frances Fuller-Pace for advice on the ATPase assay. We thank Christine Grund, Astrid Hofmann, and Nadja Pfetzer for expert technical assistance, Ingrid Kempter and Ralf Zimbelmann for performing the antibody screen, Hans-Richard Rackwitz for preparing and KLH coupling of synthetic peptides, Andreas Hunziker for competent sequencing work, Jutta Osterholt for preparing the photographs, and Eva Ouis for arranging the typescript. Specifically, we acknowledge Werner W. Franke for stimulating discussions and critical reading of the manuscript. This study was supported by Deutsche Forschungsgemeinschaft grant Schm 862/3-1 (to M.S.S.-Z.).
Abbreviations used:
- AMD
actinomycin D
- bp
base pairs
- dsDNA
double-stranded DNA
- EMBL
European Molecular Biology Laboratory
- GFP
green fluorescent protein
- HSP
high-speed pellet
- HSS
high-speed supernatant
- LSP
low-speed pellet
- mAb
monoclonal antibody
- ORF
open reading frame
- snoRNA
small nucleolar RNA
- ssDNA
single-stranded DNA
- TBS
Tris-buffered saline
- TBST
TBS containing 0.05% Tween 20
REFERENCES
- Alber T. Structure of the leucine zipper. Curr Opin Genet Dev. 1992;2:205–210. doi: 10.1016/s0959-437x(05)80275-8. [DOI] [PubMed] [Google Scholar]
- Busch H. Nucleolar and nucleolonemal proteins of cancer cells. J Tumor Marker Oncol. 1997;12:4–68. [Google Scholar]
- Cairns C, McStay B. Identification and cDNA cloning of a Xenopus nucleolar phosphoprotein, xNopp180, that is the homolog of the rat nucleolar protein Nopp140. J Cell Sci. 1995;108:3339–3347. doi: 10.1242/jcs.108.10.3339. [DOI] [PubMed] [Google Scholar]
- Chan PK. Characterization and cellular localization of nucleophosmin/B23 in HeLa cells treated with selected cytotoxic agents (studies of B23-translocation mechanism) Exp Cell Res. 1992;203:174–181. doi: 10.1016/0014-4827(92)90053-b. [DOI] [PubMed] [Google Scholar]
- Chan PK, Chan FY. Nucleophosmin/B23 (NPM) oligomer is a major and stable entity in HeLa cells. Biochim Biophys Acta. 1995;1262:37–42. doi: 10.1016/0167-4781(95)00044-h. [DOI] [PubMed] [Google Scholar]
- Chirgwin JM, Przybyla AE, MacDonald RJ, Rutter WJ. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979;18:5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cordes VC, Reidenbach S, Franke WW. Cytoplasmic annulate lamellae in cultured cells: composition, distribution, and mitotic behavior. Cell Tissue Res. 1996;284:177–191. doi: 10.1007/s004410050578. [DOI] [PubMed] [Google Scholar]
- Cordes VC, Reidenbach S, Köhler A, Stuurman N, van Driel R, Franke WW. Intranuclear filaments containing a nuclear pore complex protein. J Cell Biol. 1993;123:1333–1344. doi: 10.1083/jcb.123.6.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes VC, Reidenbach S, Rackwitz H-R, Franke WW. Identification of protein p270/Tpr as a constitutive component of the nuclear pore complex-attached intranuclear filaments. J Cell Biol. 1997;136:515–529. doi: 10.1083/jcb.136.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Cruz J, Kressler D, Linder P. Unwinding RNA in Saccharomyces cerevisiae: DEAD-box proteins and related families. Trends Biochem Sci. 1999;24:192–198. doi: 10.1016/s0968-0004(99)01376-6. [DOI] [PubMed] [Google Scholar]
- Dingwall C, Laskey RA. Nuclear targeting sequences—a consensus? Trends Biochem Sci. 1991;16:478–481. doi: 10.1016/0968-0004(91)90184-w. [DOI] [PubMed] [Google Scholar]
- Dumont JN. Oogenesis in Xenopus laevis (Daudin). I. Stages of oocyte development in laboratory-maintained animals. J Morphol. 1972;136:153–164. doi: 10.1002/jmor.1051360203. [DOI] [PubMed] [Google Scholar]
- Eichler DC, Craig N. Processing of eukaryotic ribosomal RNA. Prog Nucleic Acid Res Mol Biol. 1994;49:197–239. doi: 10.1016/s0079-6603(08)60051-3. [DOI] [PubMed] [Google Scholar]
- Evans JP, Kay BK. Biochemical fractionation of oocytes. Methods Cell Biol. 1991;36:133–148. doi: 10.1016/s0091-679x(08)60275-7. [DOI] [PubMed] [Google Scholar]
- Flores-Rozas H, Hurwitz J. Characterization of a new RNA helicase from nuclear extracts of HeLa cells which translocates in the 5′ to 3′ direction. J Biol Chem. 1993;268:21372–21383. [PubMed] [Google Scholar]
- Franke WW, Grund C, Osborn M, Weber K. The intermediate-sized filaments in rat kangaroo PtK2 cells. I. Morphology in situ. Cytobiologie. 1978;17:365–391. [PubMed] [Google Scholar]
- Franke WW, Schmid E, Winter S, Osborn M, Weber K. Widespread occurrence of intermediate-sized filaments of the vimentin-type in cultured cells from diverse vertebrates. Exp Cell Res. 1979;123:25–46. doi: 10.1016/0014-4827(79)90418-x. [DOI] [PubMed] [Google Scholar]
- Fuller-Pace FV. RNA helicases: modulators of RNA structure. Trends Cell Biol. 1994;4:271–274. doi: 10.1016/0962-8924(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Fuller-Pace FV, Nicol SM, Reid AD, Lane DP. DbpA: a DEAD box protein specifically activated by 23S rRNA. EMBO J. 1993;12:3619–3626. doi: 10.1002/j.1460-2075.1993.tb06035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya AE, Koonin EV. Helicases: amino acid sequence comparisons and structure-function relationships. Curr Opin Struct Biol. 1993;3:419–429. [Google Scholar]
- Grummt I. Regulation of mammalian ribosomal gene transcription by RNA polymerase I. Prog Nucleic Acid Res Mol Biol. 1999;62:109–154. doi: 10.1016/s0079-6603(08)60506-1. [DOI] [PubMed] [Google Scholar]
- Gururajan R, Mathews L, Longo FJ, Weeks DL. An3 mRNA encodes an RNA helicase that colocalizes with nucleoli in Xenopus oocytes in a stage-specific manner. Proc Natl Acad Sci USA. 1994;91:2056–2060. doi: 10.1073/pnas.91.6.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gururajan R, Weeks DL. An3 protein encoded by a localized maternal mRNA in Xenopus laevis is an ATPase with substrate-specific RNA helicase activity. Biochim Biophys Acta. 1997;1350:169–182. doi: 10.1016/s0167-4781(96)00155-8. [DOI] [PubMed] [Google Scholar]
- Hadjiolov AA. Cell Biology Monographs. Vol. 12 1985. , The Nucleolus and Ribosome Biogenesis, Vienna: Springer-Verlag. [Google Scholar]
- Heid HW, Schmidt A, Zimbelmann R, Schäfer S, Winter-Simanowski S, Stumpp S, Keith M, Figge U, Schnölzer M, Franke WW. Cell type-specific desmosomal plaque proteins of the plakoglobin family: plakophilin 1 (band 6 protein) Differentiation. 1994;58:113–131. doi: 10.1046/j.1432-0436.1995.5820113.x. [DOI] [PubMed] [Google Scholar]
- Hügle B, Scheer U, Franke WW. Ribocharin: a nuclear Mr 40,000 protein specific to precursor particles of the large ribosomal subunit. Cell. 1985;41:615–627. doi: 10.1016/s0092-8674(85)80034-9. [DOI] [PubMed] [Google Scholar]
- Iggo RD, Jamieson DJ, MacNeill SA, Southgate J, McPheat J, Lane DP. p68 RNA helicase: identification of a nucleolar form and cloning of related genes containing a conserved intron in yeasts. Mol Cell Biol. 1991;11:1326–1333. doi: 10.1128/mcb.11.3.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iost I, Dreyfuss M, Linder P. Ded1p, a DEAD-box protein required for translation initiation in Saccharomyces cerevisiae, is an RNA helicase. J Biol Chem. 1999;274:17677–17683. doi: 10.1074/jbc.274.25.17677. [DOI] [PubMed] [Google Scholar]
- Kadare G, Haenni A-L. Virus-encoded RNA helicases. J Virol. 1997;71:2583–2590. doi: 10.1128/jvi.71.4.2583-2590.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschmidt JA, Franke WW. Soluble acidic complexes containing histones H3 and H4 in nuclei of Xenopus laevis oocytes. Cell. 1982;29:799–809. doi: 10.1016/0092-8674(82)90442-1. [DOI] [PubMed] [Google Scholar]
- Köhler A, Schmidt-Zachmann MS, Franke WW. AND-1, a natural chimeric DNA-binding protein, combines an HMG-box with regulatory WD-repeats. J Cell Sci. 1997;110:1051–1062. doi: 10.1242/jcs.110.9.1051. [DOI] [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989;108:229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krämer A. The structure and function of proteins involved in mammalian pre-mRNA splicing. Annu Rev Biochem. 1996;65:367–409. doi: 10.1146/annurev.bi.65.070196.002055. [DOI] [PubMed] [Google Scholar]
- Lavoie CA, Harvey M, Lasko PF. Dbp45A encodes a Drosophila DEAD box protein with similarity to a putative yeast helicase involved in ribosome assembly. Biochim Biophys Acta. 1993;1216:140–144. doi: 10.1016/0167-4781(93)90052-f. [DOI] [PubMed] [Google Scholar]
- Liang L, Diehl-Jones W, Lasko P. Localization of vasa protein to the Drosophila pole plasm is independent of its RNA-binding and helicase activities. Development. 1994;120:1201–1211. doi: 10.1242/dev.120.5.1201. [DOI] [PubMed] [Google Scholar]
- Linder P, Lasko PF, Ashburner M, Leroy P, Nielsen PJ, Nishi K, Schnier J, Slonimski PP. Birth of the D-E-A-D box. Nature. 1989;337:121–122. doi: 10.1038/337121a0. [DOI] [PubMed] [Google Scholar]
- Melese T, Xue Z. The nucleolus: an organelle formed by the act of building a ribosome. Curr Opin Cell Biol. 1995;7:319–324. doi: 10.1016/0955-0674(95)80085-9. [DOI] [PubMed] [Google Scholar]
- Mertens C, Kuhn C, Franke WW. Plakophilins 2a and 2b: constitutive proteins of dual location in the karyoplasm and the desmosomal plaque. J Cell Biol. 1996;135:1009–1025. doi: 10.1083/jcb.135.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore MJ, Query CC, Sharp PA. Splicing of precursors to messenger RNAs by the spliceosome. In: Gesteland RF, Atkins JF, editors. The RNA World. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1993. pp. 303–357. [Google Scholar]
- Olson MOJ. The role of proteins in nucleolar structure and function. In: Strauss PR, Wilson SH, editors. The Eukaryotic Nucleus. Vol. 2. Caldwell, NY: Telford; 1990. pp. 519–559. [Google Scholar]
- Paule MR. Polymerase I transcription, termination and processing. Gene Expr. 1993;3:1–9. [PMC free article] [PubMed] [Google Scholar]
- Pause A, Sonenberg N. Helicases and RNA unwinding in translation. Curr Opin Struct Biol. 1993;3:953–959. [Google Scholar]
- Pederson T. The plurifunctional nucleolus. Nucleic Acids Res. 1998;26:3871–3876. doi: 10.1093/nar/26.17.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planta RJ, Goncalves PM, Mager WH. Global regulators of ribosome biosynthesis in yeast. Biochem Cell Biol. 1995;73:825–834. doi: 10.1139/o95-090. [DOI] [PubMed] [Google Scholar]
- Qu X, Yang Z, Zhang S, Shen L, Dangel AW, Hughes JH, Redman KL, Wu LC, Yu CY. The human DEVH-box protein Ski2w from the HLA is localized in nucleoli and ribosomes. Nucleic Acids Res. 1998;26:4068–4077. doi: 10.1093/nar/26.17.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeder RH. rRNA synthesis in the nucleolus. Trends Genet. 1990;6:390–395. doi: 10.1016/0168-9525(90)90298-k. [DOI] [PubMed] [Google Scholar]
- Scalenghe F, Buscaglia M, Steinheil C, Crippa M. Large scale isolation of nuclei and nucleoli from vitellogenic oocytes in Xenopus laevis. Chromosoma. 1978;66:299–308. doi: 10.1007/BF00328531. [DOI] [PubMed] [Google Scholar]
- Scheer U, Hock R. Structure and function of the nucleolus. Curr Opin Cell Biol. 1999;11:385–390. doi: 10.1016/S0955-0674(99)80054-4. [DOI] [PubMed] [Google Scholar]
- Scheer U, Weisenberger D. The nucleolus. Curr Opin Cell Biol. 1994;6:354–359. doi: 10.1016/0955-0674(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Schmid E, Schiller DL, Grund C, Stadler J, Franke WW. Tissue type-specific expression of intermediate filament proteins in a cultured epithelial cell line from bovine mammary gland. J Cell Biol. 1983;96:37–50. doi: 10.1083/jcb.96.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid SR, Linder P. D-E-A-D protein family of putative RNA helicases. Mol Microbiol. 1992;6:283–291. doi: 10.1111/j.1365-2958.1992.tb01470.x. [DOI] [PubMed] [Google Scholar]
- Schmidt-Zachmann MS, Hügle B, Scheer U, Franke WW. Identification and localization of a novel nucleolar protein of high molecular weight by a monoclonal antibody. Exp Cell Res. 1984;153:327–346. doi: 10.1016/0014-4827(84)90604-9. [DOI] [PubMed] [Google Scholar]
- Schmidt-Zachmann MS, Hügle-Dörr B, Franke WW. A constitutive nucleolar protein identified as a member of the nucleoplasmin family. EMBO J. 1987;6:1881–1890. doi: 10.1002/j.1460-2075.1987.tb02447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Zachmann MS, Knecht S, Krämer A. Molecular characterization of a novel, widespread nuclear protein that colocalizes with spliceosome components. Mol Biol Cell. 1998;9:143–160. doi: 10.1091/mbc.9.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnölzer M, Alewood P, Jones A, Alewood D, Kent SB. In situ neutralization in Boc-chemistry solid phase peptide synthesis. Rapid, high yield assembly of difficult sequences. Int J Pept Protein Res. 1992;40:180–193. doi: 10.1111/j.1399-3011.1992.tb00291.x. [DOI] [PubMed] [Google Scholar]
- Serin G, Joseph G, Ghisolfi L, Bauzan M, Erard M, Amalric F, Bouvet P. Two RNA-binding domains determine the RNA-binding specificity of nucleolin. J Biol Chem. 1997;272:13109–13116. doi: 10.1074/jbc.272.20.13109. [DOI] [PubMed] [Google Scholar]
- Shaw PJ, Jordan EG. The nucleolus. Annu Rev Cell Dev Biol. 1995;11:93–121. doi: 10.1146/annurev.cb.11.110195.000521. [DOI] [PubMed] [Google Scholar]
- Smith CM, Steitz JA. Sno storm in the nucleolus: new roles for myriad small RNPs. Cell. 1997;89:669–672. doi: 10.1016/s0092-8674(00)80247-0. [DOI] [PubMed] [Google Scholar]
- Söllner-Webb B, Pape L, Ryan K, Mougey EB, Poretta R, Nikolov E, Paalman MH, Lazdins I, Martin C. Expression of mouse and frog rRNA genes: transcription and processing. Mol Cell Biochem. 1991;104:149–154. doi: 10.1007/BF00229814. [DOI] [PubMed] [Google Scholar]
- Spector DL, Fu X-D, Maniatis T. Associations between distinct pre-mRNA splicing components and the cell nucleus. EMBO J. 1991;10:3467–3481. doi: 10.1002/j.1460-2075.1991.tb04911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JO, Kornberg RD. An octamer of histones in chromatin and free in solution. Proc Natl Acad Sci USA. 1975;72:2626–2630. doi: 10.1073/pnas.72.7.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollervey D. Small nucleolar RNAs guide ribosomal RNA methylation. Science. 1996;273:1056–1057. doi: 10.1126/science.273.5278.1056. [DOI] [PubMed] [Google Scholar]
- Tollervey D, Kiss T. Function and synthesis of small nucleolar RNAs. Curr Opin Cell Biol. 1997;9:337–342. doi: 10.1016/s0955-0674(97)80005-1. [DOI] [PubMed] [Google Scholar]
- Tseng SS-I, Weaver PL, Liu Y, Hitomi M, Tartakoff AM, Chang T-H. Dbp5p, a cytosolic RNA helicase, is required for poly(A)+ RNA export. EMBO J. 1998;17:2651–2662. doi: 10.1093/emboj/17.9.2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida N, Honjo Y, Johnson KR, Wheelock MJ, Takeichi M. The catenin/cadherin adhesion system is localized in synaptic junctions bordering transmitter release zones. J Cell Biol. 1996;135:767–779. doi: 10.1083/jcb.135.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez BC, Henning D, Busch RK, Woods K, Flores-Rozas H, Hurwitz J, Perlaky L, Busch H. A nucleolar RNA helicase recognized by autoimmune antibodies from a patient with watermelon stomach disease. Nucleic Acids Res. 1996;24:1220–1224. doi: 10.1093/nar/24.7.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez BC, Henning D, Perlaky L, Busch RK, Busch H. Cloning and characterization of Gu/RH-II binding protein. Biochem Biophys Res Commun. 1997;234:335–340. doi: 10.1006/bbrc.1997.6642. [DOI] [PubMed] [Google Scholar]
- van Nues RW, Venema J, Rientjes JM, Dirks-Mulder A, Raue HA. Processing of eukaryotic pre-rRNA: the role of the transcribed spacers. Biochem Cell Biol. 1995;73:789–801. doi: 10.1139/o95-087. [DOI] [PubMed] [Google Scholar]
- Venema J, Tollervey D. Processing of pre-ribosomal RNA in Saccharomyces cerevisiae. Yeast. 1995;11:1629–1650. doi: 10.1002/yea.320111607. [DOI] [PubMed] [Google Scholar]
- Wagner JDO, Jankowsky E, Company M, Pyle AM, Abelson JN. The DEAH-box protein PRP22 is an ATPase that mediates ATP-dependent mRNA release from the spliceosome and unwinds RNA duplexes. EMBO J. 1998;17:2926–2937. doi: 10.1093/emboj/17.10.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner JR. The nucleolus and ribosome formation. Curr Opin Cell Biol. 1990;2:521–527. doi: 10.1016/0955-0674(90)90137-4. [DOI] [PubMed] [Google Scholar]
- Weinstein LB, Steitz JA. Guided tours: from precursor snoRNA to functional snoRNP. Curr Opin Cell Biol. 1999;11:378–384. doi: 10.1016/S0955-0674(99)80053-2. [DOI] [PubMed] [Google Scholar]
- Xu D, Nouraini S, Field D, Tang S-J, Friesen JD. An RNA-dependent ATPase associated with U2/U6 snRNAs in pre-mRNA splicing. Nature. 1996;381:709–713. doi: 10.1038/381709a0. [DOI] [PubMed] [Google Scholar]
- Yung BY, Busch RK, Busch H, Mauger AB, Chan PK. Effects of actinomycin D analogs on nucleolar phosphoprotein B23 (37,000 daltons/pI 5.1) Biochem Pharmacol. 1985;34:4059–4063. doi: 10.1016/0006-2952(85)90387-9. [DOI] [PubMed] [Google Scholar]
- Zirwes RF, Schmidt-Zachmann MS, Franke WW. Identification of a small, very acidic constitutive nucleolar protein (NO29) as a member of the nucleoplasmin family. Proc Natl Acad Sci USA. 1997;94:11387–11392. doi: 10.1073/pnas.94.21.11387. [DOI] [PMC free article] [PubMed] [Google Scholar]