Abstract
Purpose: To accurately assess the full spectrum of end stage renal disease (ESRD) in Wilms tumor survivors by combining the unique resources of the National Wilms Tumor Study Group (NWTSG) and the U.S. Renal Data System (USRDS), and to confirm preliminary reports of an increased incidence of ESRD in those with the Wilms tumor-aniridia (WAGR) syndrome.
Material and Methods: ESRD was ascertained for 5,910 patients enrolled on NWTSG studies during 1969-1994 both by record linkage to USRDS and by direct follow-up. Cumulative ESRD incidence was estimated accounting for inter-current mortality.
Results: Ten of 115 cases of ESRD (9%) were ascertained by NWTSG alone, 13 (11%) by USRDS alone and 92 (80%) by both. Cumulative incidence of ESRD at 20 years from diagnosis of unilateral Wilms tumor (WT) was 74% for 17 patients with Deny-Drash syndrome (DDS), 36% for 37 patients with WAGR syndrome, 7% for 125 male patients with hypospadias or cryptorchism (GU anomalies) and 0.6% for 5,347 patients with none of these conditions. The incidence for bilateral Wilms tumor was 50% for DDS (n=6), 90% for WAGR (n=10), 25% for GU anomaly (n=25) and 12% for other patients (n=409). ESRD for patients with WAGR syndrome or GU anomalies tended to occur relatively late, often during or after adolescence.
Conclusions: The risk of ESRD is remarkably low for the majority of WT patients. Those with WAGR syndrome or associated GU anomalies, however, are at higher risk and should be screened indefinitely to facilitate prospective management of impaired renal function.
Keywords: Denys-Drash syndrome, genitourinary anomalies, record linkage, renal failure, WAGR syndrome, WT1 mutation
The childhood kidney cancer known as Wilms tumor (WT) is a major success story for cancer treatment, with modern rates of cure approaching 90%.1 Interest is increasingly focused on the lifelong health prospects for the growing number of survivors, who may be at risk of second malignancies and congestive heart failure. Development of end stage renal disease (ESRD) is of particular concern due to the possibility of progression of bilateral WT, irradiation of the opposite kidney in those with unilateral disease or hyperfiltration of the remaining nephrons following loss of a major portion of renal tissue.2,3
The clinical trials of the National Wilms Tumor Study Group (NWTSG) are a valuable resource for study of long term effects of childhood cancer treatment.4,5 They enrolled a large fraction of North American patients with WT and emphasized early on the importance of long term follow-up. The first NWTSG study of renal failure (RF) identified progressive disease in both kidneys, Denys-Drash syndrome (DDS) and radiation nephritis as its most important causes.6 Alerted by case reports of ESRD associated with the Wilms tumor-aniridia (WAGR) syndrome 7, subsequent studies demonstrated that WAGR patients were indeed at high risk.8,9 They excluded cases of ESRD following bilateral nephrectomy for progressive disease, however, and some uncertainty remained regarding completeness of ascertainment.
An opportunity to confirm the preliminary results was presented by Medicare, which began in 1975 to fund dialysis and kidney transplants for the entire U.S. This was shortly after the NWTSG began operations and assured that virtually all NWTSG patients who developed ESRD would be covered. Since 1994, reporting of ESRD has been mandatory even for non-Medicare patients. The U.S. Renal Data System (USRDS) was established in 1988 to facilitate research using these records. 10 By combining the unique resources of the NWTSG and the USRDS, the present study improves on previous work by having a longer follow-up period and more complete ascertainment of ESRD, including that due to bilateral nephrectomy for progressive WT. Separate results are given for patients with unilateral and bilateral WT, and improved statistical methods are used to estimate cumulative incidence.
METHODS
Patients
Between October, 1969 and September, 1994, 6,216 patients from U.S. institutions were enrolled on one of the first four NWTSG studies and had complete baseline data submitted. Consent for participation of all patients was obtained by investigators at the initial treating institution. Those with rhabdoid tumor of kidney (n=105) or clear cell sarcoma of kidney (n=201), none of whom developed ESRD, were excluded on grounds that they did not have WT per se. 11 The remaining 5,910 patients were divided into two cohorts. Cohort I consisted of 5,526 patients with unilateral disease at the time of WT diagnosis. Cohort II consisted of 384 patients with synchronous bilateral WT or (n=5) WT in a solitary kidney at diagnosis, plus 66 patients from Cohort I who later developed metachronous WT in the contralateral kidney and were moved to Cohort II at that time. Each cohort was subdivided into four groups on the basis of information on congenital malformation syndromes and anomalies abstracted from registration cards, flow sheets, surgery and pathology records or family questionnaires. The four groups were patients with DDS, WAGR syndrome, male genitourinary (GU) anomalies (hypospadias and/or cryptorchism) or none of the above.
Record linkage
Identifying data for all patients including, where available, social security number (SSN), name, gender and date of birth (DOB) were submitted to the USRDS in October, 2002. Following approval by U.S. government officials, data for patients whose records matched those in the 2002 USRDS database were released to the NWTSG in June, 2003. Visual inspection of available records was used to confirm matches identified by computerized criteria. The USRDS data items used here were the match criteria and the date of the first ESRD service. June 30, 2001 was chosen as the closing date to allow time for reporting of ESRD to the USRDS. The protocol for this record linkage study was approved by the Institutional Review Board of the Fred Hutchinson Cancer Research Center.
Definition of Endpoint
Treatment with chronic dialysis or kidney transplant was the USRDS criterion for ESRD and this criterion was therefore also applied to NWTSG patients. Three patients who died from ESRD after the family refused such treatment also were included as cases of ESRD at the time of death. Where laboratory results were available to NWTSG, patients identified with ESRD invariably had repeated serum creatinine levels well above 3.0 mg/dl.
Statistical Methods
Patients were ‘on study’ from the date of diagnosis of unilateral or bilateral WT until the earliest report to NWTSG or USRDS of treatment for ESRD (n=112), death (n=877, including the 3 counted as having ESRD at that time) or the closing date June 30, 2001 (n=4921). The cumulative incidence of ESRD was estimated treating death as a competing risk.12 This represents the proportion of patients who developed ESRD by each specified time point, taking into account that some did not due to prior death. Ninety-five percent confidence intervals (CI) were estimated similarly. Graphs of cumulative incidence in some small subgroups were truncated at the time that follow-up for all patients in that subgroup ceased. Overall survival was estimated actuarially using NWTSG records alone.
RESULTS
Match criteria
In all 115 cases of ESRD were identified, 55 in Cohort I and 60 in Cohort II. Ninety-two cases (80%) were ascertained by both NWTSG and USRDS. Thirteen (11%) were ascertained only by USRDS, 8 because of loss to follow-up by NWTSG before the onset of ESRD. No records suggesting the presence of ESRD were submitted to NWTSG for the remaining five patients. Ten ESRD cases (9%) were ascertained by NWTSG only, 3 because they never received dialysis or transplant (see below) and one who died in 1973 after starting dialysis. The remaining six apparently either failed to match the USRDS database (none had a SSN on record) or were never reported to it. The match criteria for the 105 patients identified by USRDS included: SSN, name and DOB for 41; SSN and DOB for 11; full name, DOB and gender for 45; SSN alone for 5; partial name, DOB and sex for 2; and partial SSN, DOB and sex for 1.
ESRD identified by NWTSG
Forty-one of 53 ESRD cases (77%) in Cohort II ascertained by NWTSG had either bilateral nephrectomy, or nephrectomy and partial nephrectomy with less than 25% of renal tissue remaining. None of 48 ESRD cases identified by NWTSG in Cohort I developed ESRD from surgical treatment of WT. Three WAGR patients who died of uremia after their families declined treatment for renal failure were classified as having ESRD at the time of death. Two patients in Cohort II were treated medically for advanced renal failure but died prior to initiation of dialysis or transplant and therefore were not classified as having ESRD in the analysis of cumulative incidence. One was a DDS patient who expired with tumor present four days after serum creatinine reached 13mg/dl. The other was a GU anomaly patient who died of acute myelogenous leukemia shortly after serum creatinine reached 8.7mg/dl.
Cumulative incidence of ESRD and survival in groups defined by malformation syndromes Table 1 and Figures 1 and 2 show the main results. Nearly half (27/55) the ESRD cases in Cohort I occurred in the small fraction of patients with DDS, WAGR syndrome or a GU anomaly. For two DDS patients the ESRD preceded the diagnosis of WT and for the remainder it occurred within 6.5 years of diagnosis. For the WAGR and all but one of the GU anomaly patients, by contrast, ESRD did not occur until at least 6 years from diagnosis and 12 years of age. The cumulative incidence of ESRD for 5,347 patients with no such congenital condition was 0.6% at 20 years from diagnosis. At this time 1016 (19%) of Cohort I patients were still actively followed by NWTSG.
Table 1.
Cumulative incidence of ESRD and overall survival at 20 years from diagnosis of Wilms tumor
| Congenital malformation group | No. of patients | Mean FU time (years) | No. of renal failures observed | Incidence of ESRD at 20 years from diagnosis (95% CI) | No. of deaths observed | Survival at 20 years from diagnosis (95% CI) |
|---|---|---|---|---|---|---|
| Cohort I: Unilateral Wilms tumor | ||||||
| DDS | 17 | 10.6 | 12 | 74% (45-89%) | 4 | 68% (31-88%) |
| WAGR | 37 | 14.0 | 11 | 36% (18-55%) | 8 | 78% (55-90%) |
| GU anomalies | 125 | 14.2 | 4 | 6.7% (1.9-16%) | 10 | 93% (86-96%) |
| None | 5,347 | 12.7 | 28 | 0.6% (0.4-1.0%) | 730 | 85% (84-86%) |
| Total | 5,526 | 12.7 | 55 | 1.3% (0.9%-1.7%) | 752 | 85% (84-86%) |
| Cohort II:Bilateral Wilms tumor | ||||||
| DDS | 6 | 13.4 | 3 | 50% (11-80%) | 1 | 83% (27-98%) |
| WAGR | 10 | 11.1 | 5 | 90% (47-99%) | 3 | 56% (19-85%) |
| GU anomalies | 25 | 15.2 | 8 | 25% (8-47%) | 6 | 79% (57-91%) |
| None | 409 | 11.3 | 44 | 11.5% (8-15%) | 115 | 70% (64-74%) |
| Total | 450 | 11.5 | 60 | 15% (11-19%) | 125 | 70% (65-75%) |
Abbreviations: CI = confidence interval; DDS = Denys-Drash syndrome; FU = follow-up; WAGR = Wilms tumor-aniridia syndrome
Figure 1.
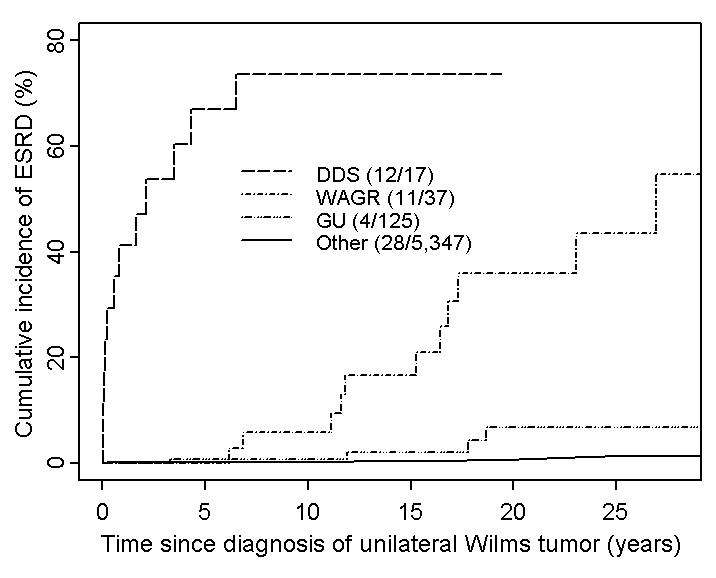
Cumulative incidence of ESRD in patients with unilateral Wilms tumor, by congenital malformation group. Abbreviations: DDS=Denys-Drash syndrome; WAGR=Wilms tumor-aniridia syndrome; GU=hypospadias/cryptorchism. The DDS curve stops at the maximum follow-up time observed for patients in this subgroup.
Figure 2.
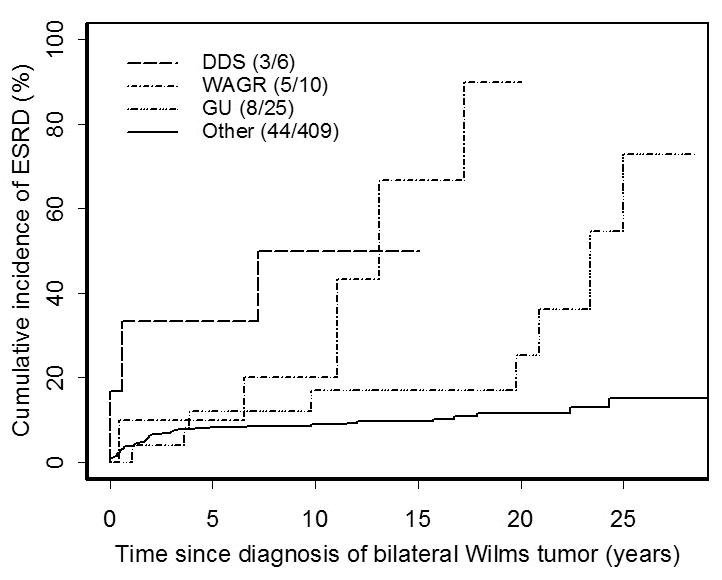
Cumulative incidence of ESRD in patients with bilateral Wilms tumor, by congenital malformation group. Abbreviations: DDS=Denys-Drash syndrome; WAGR=Wilms tumor-aniridia syndrome; GU=hypospadias/cryptorchism. The curves for DDS, WAGR and GU stop at the maximum follow-up time observed for patients in each subgroup.
The cumulative incidence of ESRD for Cohort II patients with no GU anomaly or syndrome was 11.5% at 20 years. All but 10 of the 44 ESRD events occurred within 5 years of WT diagnosis. For those ascertained within 5 years by NWTSG, 30/32=94% were due to bilateral nephrectomy, whereas only 4/8=50% of ESRD cases ascertained after 5 years had this etiology. Two of 3 cases of ESRD in DDS patients occurred either before or within one year of WT diagnosis, as did the renal failure in the DDS patient who died from progressive WT (see above). Only WAGR patients and those with GU anomalies had substantial risk of ESRD beyond 10 years from WT diagnosis.
DISCUSSION
This investigation strongly reinforces the preliminary NWTSG results. The 20 year cumulative incidence of ESRD for former patients with unilateral WT was found to be quite low, less than 1%; it was less than 12% for those with bilateral disease. While some possibility of under-ascertainment of ESRD remains, this seems minimal given the national coverage of USRDS and the high degree of overlap between cases identified by both systems. These results should greatly reassure the families of patients who are cured of their initial WT.
The excess of ESRD in Cohort II over Cohort I was due primarily to surgical removal of the remaining kidney for progressive WT, especially during the first five years following diagnosis. Otherwise, the patterns of ESRD in the two cohorts were similar (Figures 1 and 2).
Patients at highest risk of ESRD had one of the rare congenital malformation syndromes associated with a constitutional mutation or deletion of the tumor suppressor gene WT1. DDS was likely under-ascertained during the early years of the NWTSG, but its prevalence has since stabilized at approximately 4-5 cases per 1,000 WT patients (unpublished NWTSG data). DDS patients usually have a missense point mutation in the 8th or 9th exons of WT1, although nonsense mutations expected to result in a truncated WT1 protein have also been observed.13 Mice engineered to express mutated forms of WT1 develop glomerulosclerosis, possibly through alteration of the glomerular capillaries.14,15 These laboratory studies support the idea that the severe DDS phenotype, with mesangial sclerosis of the glomeruli leading to early renal failure, is the result of a mutated WT1 gene product acting in a dominant negative fashion.13,16
The proportion of NWTSG patients with WAGR syndrome has remained constant at approximately 7-8 per 1,000.9 This syndrome invariably occurs in patients with an interstitial deletion at chromosome 11p13 that involves WT1 and PAX6.17 A recent study of Wt1 knockout mice expressing one or two copies of the WT1 transgene suggests that reduced WT1 expression causes down regulation of genes involved in podocyte function leading to mesangial sclerosis of the glomeruli and renal failure.18 Hence WT1 deletion and consequent reduction in the gene product might well contribute to the ESRD found in WAGR patients.
The third group of WT patients with high risk of ESRD, especially in Cohort II, consists of males with hypospadias or cryptorchism but without the nephropathy of DDS or the aniridia of WAGR. Diller and colleagues19 found that 7 of 8 WT patients in whom constitutional WT1 mutations were detected had cryptorchism and 3 also had bilateral WT. DDS and WAGR patients were excluded. Their data suggest that as many as one-fourth of cryptorchid WT patients may harbor germline WT1 mutations, perhaps even a greater fraction if the WT is bilateral. Hence WT1 alteration seems a plausible explanation also for the increased rate of ESRD observed among patients with these characteristic GU anomalies. A retrospective case-control study comparing the frequency of constitutional WT1 mutations in WT patients who developed ESRD and in matched controls would be of interest.
CONCLUSIONS
Renal failure leading to ESRD was an uncommon development among surviving WT patients who had none of the phenotypic manifestations of WT1 mutation or deletion. Only a few patients had congenital malformations associated with WT1 alterations and confidence limits on their estimated risks were correspondingly wide. Nonetheless, the risks were clearly elevated. Most patients with WAGR syndrome or WT with GU anomalies are expected to survive childhood and should be monitored for microalbuminuria, proteinuria, hypertension and level of renal function. Prospective study of renal pathology by histology or ultrasound in those who develop renal failure could help determine the extent to which the decline in renal function was related to therapy induced tubulointerstitial damage, to hyperfiltration injury or to the mesangial sclerosis associated with DDS.16 Children with impaired renal function may benefit from renoprotective therapies such as angiotensin converting enzyme inhibitors and angiotensin receptor blockers and control of hypertension based on the National Kidney Foundation clinic practice guidelines for chronic kidney disease.20 Such treatment may slow progression of renal failure and plans for timely initiation of dialysis or transplant can be managed prospectively.
ACKNOWLEDGEMENT
We thank investigators of the Pediatric Oncology Group, the Children”s Cancer Group and the health professionals who managed the care of children entered on the National Wilms Tumor Group Studies.
Footnotes
Portions of the reported data were supplied by the United States Renal Data System. This study was performed as a deliverable under Contract No. N01-DK-9-2343 (National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Maryland). Support was also provided by United States Public Health Service grants no. CA 42326 and CA 54498.
REFERENCES
- 1.Grundy PE, Green DM, Coppes MJ, et al. Renal tumors. In: Pizzo PA, Poplack DG, editors. Principles and Practice of Pediatric Oncology. Fourth Edition. Lippincott, Williams & Wilkins; Philadelphia: 2002. p. 865. chapt. 30. [Google Scholar]
- 2.Mitus A, Tefft M, Fellers FX. Long-term follow-up of renal function of 108 children who underwent nephrectomy for malignant disease. Pediatrics. 1969;44:912. [PubMed] [Google Scholar]
- 3.Anderson S, Meyer TW, Brenner BM. The Role of Hemodynamic Factors in the Initiation and Progression of Renal-Disease. Journal of Urology. 1985;133:363. doi: 10.1016/s0022-5347(17)48980-4. [DOI] [PubMed] [Google Scholar]
- 4.D′Angio GJ, Breslow N, Beckwith B, et al. Treatment of Wilms′ tumor, results of the third National Wilms′ Tumor Study. Cancer. 1989;64:349. doi: 10.1002/1097-0142(19890715)64:2<349::aid-cncr2820640202>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 5.Green DM, Breslow NE, Beckwith JB, et al. Comparison between single-dose and divided-dose administration of dactinomycin and doxorubicin for patients with Wilms′ tumor: A report from the National Wilms′ Tumor Study Group. J Clin Oncol. 1998;16:237. doi: 10.1200/JCO.1998.16.1.237. [DOI] [PubMed] [Google Scholar]
- 6.Ritchey ML, Green DM, Thomas PRM, et al. Renal failure in Wilms′ tumor patients: a report from the National Wilms′ Tumor Study Group. Med Pediatr Oncol. 1996;26:75. doi: 10.1002/(SICI)1096-911X(199602)26:2<75::AID-MPO1>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 7.Wilms H, Back E, Kirste G. Aniridia-Wilms-syndrome in end stage renal-failure. Klinische Wochenschrift. 1986;64:800. doi: 10.1007/BF01732191. [DOI] [PubMed] [Google Scholar]
- 8.Breslow NE, Takashima J, Ritchey ML, et al. Renal failure in the Denys-Drash and Wilms tumor-aniridia syndromes. Cancer Res. 2000;60:4030. [PubMed] [Google Scholar]
- 9.Breslow NE, Norris R, Norkool PA, et al. Characteristics and outcomes of children with the Wilms Tumor-Aniridia syndrome: A report from the National Wilms Tumor Study Group. J Clin Oncol. 2003;21:4579. doi: 10.1200/JCO.2003.06.096. [DOI] [PubMed] [Google Scholar]
- 10.US Renal Data System: USRDS 2003 Annual Data Report. Am J Kidney Dis. 2004;42:S1. [Google Scholar]
- 11.Beckwith JB, Palmer NF. Histopathology and prognosis of Wilms tumor. Cancer. 1978;41:1937. doi: 10.1002/1097-0142(197805)41:5<1937::aid-cncr2820410538>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 12.Gooley TA, Leisenring W, Crowley J, et al. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999:695. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 13.Huff V. Genotype/phenotype correlation in Wilms′ Tumor. Med Pediatr Oncol. 1996;27:408. doi: 10.1002/(SICI)1096-911X(199611)27:5<408::AID-MPO4>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 14.Patek CE, Little MH, Fleming S, et al. A zinc finger truncation of murine WT1 results in the characteristic urogenital abnormalities of Denys-Drash syndrome. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:2931. doi: 10.1073/pnas.96.6.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Natoli TA, Liu J, Eremina V, et al. A mutant form of the Wilms′ tumor suppressor gene WT1 observed in Denys-Drash syndrome interferes with glomerular capillary development. Journal of the American Society of Nephrology. 2002;13:2058. doi: 10.1097/01.asn.0000022420.48110.4b. [DOI] [PubMed] [Google Scholar]
- 16.Habib R, Loirat C, Gubler MC, et al. The nephropathy associated with male pseudohermaphroditism and Wilms′ tumor (Drash syndrome): A distinctive glomerular lesion - report of 10 cases. Clinical Nephrology. 1985;24:269. [PubMed] [Google Scholar]
- 17.Ton CCT, Hirvonen H, Miwa H, et al. Positional cloning and characterization of a paired box-containing and homeobox-containing gene from the aniridia region. Cell. 1991;67:1059. doi: 10.1016/0092-8674(91)90284-6. [DOI] [PubMed] [Google Scholar]
- 18.Guo JK, Menke AL, Gubler MC, et al. WT1 is a key regulator of podocyte function: reduced expression levels cause crescentic glomerulonephritis and mesangial sclerosis. Human Molecular Genetics. 2002;11:651. doi: 10.1093/hmg/11.6.651. [DOI] [PubMed] [Google Scholar]
- 19.Diller L, Ghahremani M, Morgan J, et al. Constitutional WT1 mutations in Wilms′ tumor patients. J Clin Oncol. 1998;16:3634. doi: 10.1200/JCO.1998.16.11.3634. [DOI] [PubMed] [Google Scholar]
- 20.National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. American Journal of Kidney Disease. 2002;39:S17. [PubMed] [Google Scholar]


