Abstract
In response to oxidative stress, eukaryotic cells induce transcription of genes required for detoxification of oxidants. Here we present evidence that oxidative stress stimuli are transmitted by a multistep phosphorelay system to the Spc1/Sty1 stress-activated protein kinase in the fission yeast Schizosaccharomyces pombe. The fission yeast mpr1+ gene encodes a novel protein with a histidine-containing phosphotransfer domain homologous to the budding yeast Ypd1. Spc1 activation upon oxidative stress is severely impaired in the Δmpr1 mutant as well as in the mpr1HQ strain, in which the putative phosphorylation site Mpr1-His221 is substituted with glutamine. In response to oxidative stress, Mpr1 binds to the Mcs4 response regulator that functions upstream of the Spc1 cascade, suggesting that Mcs4 is a cognate response regulator for Mpr1. Unexpectedly, when exposed to hydrogen peroxide, Δmpr1 cells can induce the catalase gene ctt1+, one of the transcriptional targets of the Spc1 pathway, and survive oxidative stress in the absence of significant Spc1 activation. We have found that Pap1, a bZIP transcription factor homologous to human c-Jun, can mediate induction of ctt1+ expression upon oxidative stress independently of the Spc1 stress-activated protein kinase. These studies show that oxidative stress stimuli are transmitted by multiple pathways to induce specific gene expression.
INTRODUCTION
Stress-activated protein kinases (SAPKs) form an evolutionarily conserved subfamily of the MAPKs and are responsive to diverse environmental stress stimuli rather than growth factors and other mitogenic stimuli (Waskiewicz and Cooper, 1995; Kyriakis and Avruch, 1996; Ip and Davis, 1998). The prototype of SAPKs, Hog1, was first identified in the budding yeast Saccharomyces cerevisiae (Brewster et al., 1993). In response to high-osmolarity stress, Hog1 is activated by a MAPK kinase (MEK) homologue, Pbs2, which is in turn activated by three redundant MEK kinase (MEKK) homologues, Ssk2, Ssk22, and Ste11 (Maeda et al., 1995; Posas and Saito, 1997). This Hog1 MAPK cascade is known to be regulated by a variation of the two-component system, multistep phosphorelay, which is composed of the Sln1, Ypd1, and Ssk1 proteins (Posas et al., 1998). Under low-osmolarity conditions, the “sensor kinase” Sln1 autophosphorylates a histidine residue within its kinase domain, and this phosphoryl group is subsequently transferred to an aspartate residue in the “receiver domain” of Sln1 (Maeda et al., 1994). Ypd1 acts as a phosphotransferase and transfers the phosphoryl group from Sln1 to an aspartate residue in the receiver domain of Ssk1, with a high-energy phosphohistidine intermediate at Ypd1-His64 (Posas et al., 1996). Phosphorylation inhibits the Ssk1 “response regulator” from activating the Ssk2 MEKK. Once cells are exposed to a high-osmolarity environment, the Sln1 kinase is inactivated, and binding of unphosphorylated Ssk1 to the N terminus of Ssk2 induces the activation of Ssk2 (Posas and Saito, 1998). Thus, the four-step His-Asp-His-Asp phosphorelay in the Sln1-Ypd1-Ssk1 pathway negatively regulates the Hog1 MAPK cascade. The two-component system is widespread among prokaryotic signaling pathways to sense and respond to a variety of environmental conditions, but it is less common among eukaryotic species (Loomis et al., 1997). Thus, regulation of the Hog1 MAPK cascade by a two-component system is an intriguing connection between eukaryotic and prokaryotic signaling modules.
In contrast to Hog1, which is responsive only to osmolarity stress (Schüller et al., 1994), SAPKs in mammals and the fission yeast Schizosaccharomyces pombe are activated by diverse forms of stress (reviewed by Banuett, 1998). In S. pombe, a SAPK called Spc1 (also known as Sty1 or Phh1) is activated by high osmolarity, oxidative stress, and heat shock, and Δspc1 mutant cells rapidly lose viability under these stress conditions (Millar et al., 1995; Shiozaki and Russell, 1995; Kato et al., 1996). Spc1 is activated through phosphorylation of Thr-171 and Tyr-173, which is carried out by a MEK homologue, Wis1. Wis1 is strongly activated in response to osmostress and oxidative stress, whereas heat shock induces relatively weak and transient Wis1 activation (Nguyen and Shiozaki, 1999). However, heat shock inhibits the Pyp1 and Pyp2 tyrosine phosphatases, negative regulators of Spc1, which results in strong activation of Spc1 (Nguyen and Shiozaki, 1999). Thus, there are at least two independent pathways that transmit different stress stimuli to Spc1: osmostress and oxidative stress are transmitted by Wis1 activation, and heat shock is mediated by inhibition of Pyp1/Pyp2.
It remains largely unknown how osmostress and oxidative stress are sensed and transmitted to the Wis1 MEK. Activation of Wis1 requires phosphorylation by a MEKK, and mutations of the MEKK phosphorylation sites in Wis1 abolish Wis1 activation in response to stress (Shiozaki et al., 1998; Nguyen and Shiozaki, 1999), implying that stress signals are transmitted to Wis1 by MEKKs. Two MEKKs activating Wis1 have been identified, Wis4 (also known as Wik1 and Wak1) (Samejima et al., 1997; Shieh et al., 1997; Shiozaki et al., 1997) and Win1 (Samejima et al., 1998). Proceeding farther upstream, Mcs4, a homologue of the Ssk1 response regulator, is believed to regulate the Wis4 MEKK (Cottarel, 1997; Shieh et al., 1997; Shiozaki et al., 1997). Stress-induced activation of Spc1 is partially impaired in strains lacking functional Mcs4, suggesting that Mcs4 positively regulates the Spc1 MAPK cascade. High sequence homology between Mcs4 and budding yeast Ssk1 implies that fission yeast also has a two-component signaling system upstream of the SAPK pathway.
The outcome of Spc1 activation in response to stress is induction of various stress-response genes, such as gpd1+ and ctt1+ (Degols et al., 1996; Wilkinson et al., 1996; Degols and Russell, 1997). gpd1+ encodes glycerol-3-phosphate dehydrogenase (Pidoux et al., 1990), a key enzyme in glycerol synthesis that is important to increase intracellular osmolarity in a high-osmolarity environment (Ohmiya et al., 1995). Cytoplasmic catalase encoded by ctt1+ decomposes hydrogen peroxide (H2O2) and protects cells from oxidative stress (Nakagawa et al., 1995). Transcription of these stress-response genes is under the regulation of a bZIP transcription factor, Atf1 (also known as Gad7) (Takeda et al., 1995; Kanoh et al., 1996). Both Δspc1 and Δatf1 mutants are defective in stress-induced expression of the same set of genes and are sensitive to various stress treatments (Shiozaki and Russell, 1996; Wilkinson et al., 1996). Moreover, Atf1 is phosphorylated by Spc1 both in vivo and in vitro (Shiozaki and Russell, 1996). These results strongly suggest that Atf1 is a downstream target of Spc1. Atf1 is most homologous to ATF-2, a key substrate of the human SAPKs, p38 and c-Jun N-terminal kinases (Gupta et al., 1995; Livingstone et al., 1995; van Dam et al., 1995; Raingeaud et al., 1996), underscoring the high conservation between the S. pombe and human SAPK pathways. Recently, Toone et al. (1998) proposed that the Pap1 transcription factor, which has significant homology and similar DNA-binding specificity to mammalian c-Jun (Toda et al., 1991, 1992), is a target transcription factor for the Spc1 MAPK under oxidative stress conditions (reviewed by Wilkinson and Millar, 1998). Pap1 is required for induction of ctt1+ and other genes in response to oxidative stress. In addition, oxidative stress brings about nuclear accumulation of Pap1 in a spc1+-dependent manner (Toone et al., 1998). It should be noted, however, that Pap1 is not a substrate of the Spc1 MAPK (Wilkinson et al., 1996), and accumulation of Pap1 in the nucleus is much slower than rapid induction of gene expression after oxidative stress (Toone et al., 1998). Thus, the physiological meaning of Pap1 regulation by Spc1 is not well understood.
In this report, we have characterized the fission yeast Mpr1, which is highly homologous to the Ypd1 response regulator phosphotransferase in budding yeast. Δmpr1 cells are defective in Spc1 activation by oxidative stress but not other forms of stress. In addition, the conserved phosphorylation site, His-221, is essential for Mpr1 function, and Mpr1 binds to the Mcs4 response regulator in response to oxidative stress. These results strongly suggest that Mpr1 and Mcs4 are part of a multistep phosphorelay that transmits oxidative stress signals to the Spc1 MAPK cascade. Unexpectedly, Δmpr1 cells are not sensitive to oxidative stress or defective in induced expression of ctt1+ after oxidative stress. Our data strongly suggest that the Pap1 transcription factor can mediate oxidative stress–induced ctt1+ expression independently of Spc1 activation. We propose that multiple independent pathways mediate oxidative stress signals to ctt1+ expression, which may be important to sensing and responding to different forms of oxidative stress.
MATERIALS AND METHODS
Yeast Strains and General Techniques
S. pombe strains used in this study are listed in Table 1. They are derivatives of 972 h− and 975 h+ (Mitchison, 1970). Growth media as well as basic genetic and biochemical techniques for S. pombe have been described (Moreno et al., 1991; Alfa et al., 1993). S. pombe cells were grown in yeast extract medium YES and synthetic minimal medium EMM2.
Table 1.
S. pombe strains used in this study
| Strain | Genotype | Source or reference |
|---|---|---|
| PR109 | h− | Laboratory stock |
| CHP429 | h− his7-366 ade6-M216 | Laboratory stock |
| JM544 | h− wis1∷ura4+ | Laboratory stock |
| TP108-3c | h− pap1∷ura4+ | Toda et al. (1991) |
| KS1366 | h− spc1∷ura4+ | Laboratory stock |
| KS1376 | h− spc1∶HA6H(ura4+) | Shiozaki and Russell (1995) |
| KS1497 | h− atf1∷ura4+ | Shiozaki and Russell (1996) |
| KS1533 | h− spc1∷ura4+ atf1∷ura4+ | Shiozaki and Russell (1996) |
| KS1575 | h+ his7-366 ade6-M210 spc1∷ura4+ | Shiozaki and Russell (1996) |
| KS2081 | h− wis1DD∶12myc(ura4+) | Shiozaki et al. (1998) |
| KS2088 | h− spc1∶HA6H(ura4+) wis1DD∶12myc(ura4+) | Shiozaki et al. (1998) |
| KS2096 | h− spc1∶HA6H(ura4+) wis1∶12myc(ura4+) | Shiozaki et al. (1998) |
| CA220 | h− mcs4∷ura4+ spc1∶HA6H(ura4+) | This study |
| CA279 | h− his7-366 mpr1∷his7+ spc1∶HA6H(ura4+) | This study |
| CA288 | h+ his7-366 mpr1∷his7+ | This study |
| CA290 | h− mcs4∶12myc(ura4+) spc1∶HA6H(ura4+) | This study |
| CA334 | h− atf1∷ura4+ pap1∷ura4+ | This study |
| CA337 | h− his7-366 mcs4∶12myc(ura4+) mpr1∷his7+ | This study |
| CA356 | h− pap1∷ura4+ wis1DD∶12myc(ura4+) | This study |
| CA385 | h− mpr1∶HA6H(ura4+) spc1∶HA6H(ura4+) | This study |
| CA403 | h− mpr1HQ∶HA6H(ura4+) spc1∶HA6H(ura4+) | This study |
| CA420 | h− his7-366 mcs4∷ura4+ mpr1∷his7+ spc1∶HA6H(ura4+) | This study |
| CA423 | h+ his7-366 mcs4∷ura4+ prr1∷his7+ spc1∶HA6H(ura4+) | This study |
| CA425 | h− his7-366 prr1∷his7+ spc1∶HA6H(ura4+) | This study |
All strains are leu1-32 ura4-D18.
Gene Disruption of mpr1+
Using S. pombe genomic DNA as a template, we amplified a 1.4-kilobase (kb) DNA fragment that contains the entire mpr1+ ORF by PCR with a pair of primers, EcNd-mpr1 (5′-CC GGA ATT CAT ATG AGT GTA TAT CGT GAT AAC ATG-3′; EcoRI restriction site is underlined) and mpr1-3Kp (5′-CCG GGG TAC CAT GCC ACG ACC GTA AGA ACG-3′; KpnI restriction site is underlined). After digesting with EcoRI and KpnI, this fragment was cloned into pBluescript (Stratagene, La Jolla, CA). The resultant plasmid (pBS-mpr1+) was digested with HindIII and XhoI, to which a 1.9-kb XbaI–SmaI fragment of his7+ (Apolinario et al., 1993) was ligated. The mpr1::his7+ construct was released by restriction digestion with EcoRI and KpnI and used to transform a diploid strain constructed by mating CHP429 and KS1575. Stable His+ transformants were selected, and disruption of one of the mpr1+ loci in the diploid was confirmed by Southern hybridization. Sporulation and tetrad dissection generated viable haploid segregants of mpr1::his7+ spc1::ura4+ and mpr1::his7+ spc1+, which indicated that mpr1+ is not essential for cellular viability.
Construction of the mpr1:HA6H and mpr1HQ:HA6H Alleles
The mpr1HQ mutant gene, which has the His-221→Gln substitution, was created by the overlap extension method with the use of PCR (Higuchi et al., 1988; Ho et al., 1989). Two separate amplification reactions were performed with pBS-mpr1+ as the template with the use of a first pair of primers, EcNd-mpr1 and MPR3HQ (5′-CC TTT AAG GAA TTG CCC CAA CG-3′; the codon corresponding to the substitution from His to Gln is in boldface), and a second pair of primers, MPR5HQ (5′-CG TTG GGG CAA TTC CTT AAA GG-3′) and mpr1-3Kp. These PCR products were purified by agarose gel electrophoresis, mixed at a 1:1 ratio, and then subjected to another PCR with the use of the primers PS288-MPR (5′-T GCA CTG CAG GCT GCT AAT GAG ACG GCT GGC GC-3′; PstI restriction site is underlined) and MPR-NT2 (5′-GT TTA GCG GCC GCC TGT AGA AGA ATT TTT TTC ATA AAA GTC AAG-3′; NotI restriction site is underlined). The same pair of primers was also used to amplify the wild-type mpr1+ sequence with the use of the pBS-mpr1+ plasmid as a template. The 0.6-kb PstI–NotI fragments of these PCR products were cloned in pUra4-HA6H to add a C-terminal tag of two copies of hemagglutinin (HA) epitope and six consecutive histidine residues (Shiozaki and Russell, 1997). The resultant pUra4-mpr1-HA6H and pUra4-mpr1HQ-HA6H plasmids were used to transform wild-type strain PR109 after being linearized at the BclI site in the mpr1 ORF. Stable Ura+ transformants were selected, and integration of the plasmids at the mpr1+ locus was confirmed by Southern hybridization. Furthermore, the mpr1 sequence was amplified by PCR with the use of the genomic DNA from the transformants, and the introduced mutation was confirmed by DNA sequencing. In these strains, the mpr1:HA6H and mpr1HQ:HA6H constructs are expressed from the chromosomal mpr1+ promoter.
Purification and Detection of the Spc1, Mpr1, and Mpr1HQ Proteins with the HA6H Tag
The HA6H sequence encoding two copies of the HA epitope and six consecutive histidine residues (Shiozaki and Russell, 1995, 1997) was used to tag the chromosomal spc1+, mpr1+, and mpr1HQ genes. Cells were grown to midlog phase in liquid YES medium at 30°C and subjected to stress treatments with 0.6 M KCl, 0.3 mM H2O2, or heat shock at 48°C, as described previously (Shiozaki et al., 1997). Cells were harvested by rapid filtration (Shiozaki and Russell, 1997), and HA6H-tagged proteins were purified on Ni-nitrilotriacetic acid (NTA)–agarose beads under denaturing conditions, followed by immunoblotting with anti-HA (12CA5; Boehringer Mannheim, Indianapolis, IN) and anti-phospho-p38 MAPK (New England Biolabs, Beverly, MA) antibodies (Shiozaki and Russell, 1997). HRP-conjugated secondary antibodies (Promega, Madison, WI) and ECL Plus Reagent (Amersham Pharmacia, Arlington Heights, IL) were used for detection and quantification with the Storm System (Molecular Dynamics, Sunnyvale, CA).
H2O2 Sensitivity Assay and RNA Analysis
S. pombe cells were grown in YES medium to midlog phase at 30°C. Aliquots of the culture were incubated for 1 h in the presence of 0, 1, 2, 4, and 8 mM H2O2. Cells were washed, diluted, and plated on YES agar (Degols et al., 1996). Percent viability was determined after 3 d of incubation at 30°C. Survival curves in Figures 6 and 8 represent the mean values of three independent experiments. Northern hybridization analyses of ctt1+ and leu1 have been described by Degols and Russell (1997).
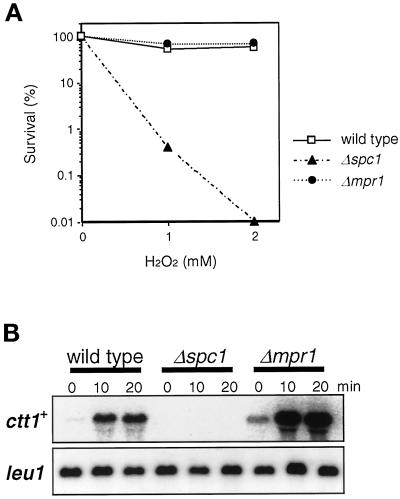
Figure 6.
Δmpr1 cells are not sensitive to oxidative stress. (A) Wild-type (PR109), Δspc1 (KS1366), and Δmpr1 (CA288) cells were grown to midlog phase in YES medium at 30°C, and H2O2 was added at concentrations of 0, 1, and 2 mM. After 1 h of incubation at 30°C, cells were washed, diluted, and plated on YES agar medium. The survival of each strain was evaluated in terms of its colony-forming ability after 3 d of incubation at 30°C. Results from an average of three independent experiments are shown. (B) Oxidative stress–induced expression of the catalase gene, ctt1+, in Δmpr1 cells. The strains used in A were treated with 0.3 mM H2O2 in YES medium, and aliquots of cells were harvested at the indicated times for Northern hybridization analysis of the ctt1+ mRNA. The leu1+ probe served as a control. ctt1+ expression was strongly induced in Δmpr1 cells but was not detectable in Δspc1 cells.
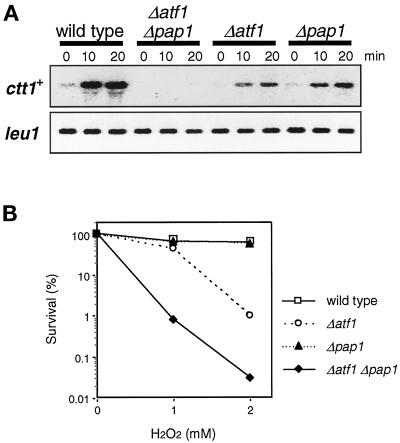
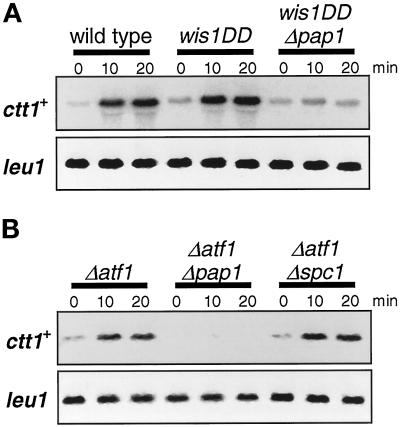
Figure 8.
Two transcription factors, Atf1 and Pap1, regulate expression of ctt1+ in response to oxidative stress. (A) Wild-type (PR109), Δatf1 (KS1497), Δpap1 (TP108-3c), and Δatf1 Δpap1 (CA334) strains were grown to midlog phase in YES medium at 30°C and treated with 0.3 mM H2O2. Aliquots of cells were harvested at the indicated times for Northern hybridization analysis with the ctt1+ and leu1+ probes. Compared with wild-type cells, ctt1+ expression after oxidative stress was significantly impaired in Δatf1 and Δpap1 mutants and was not detectable in Δatf1 Δpap1 double mutants. (B) The strains used in A were grown to midlog phase in YES medium at 30°C, and H2O2 was added at concentrations of 0, 1, and 2 mM. After 1 h of incubation at 30°C, cells were washed, diluted, and plated on YES agar medium. The survival of each strain was evaluated in terms of its colony-forming ability after 3 d of incubation at 30°C. Results from an average of three independent experiments are shown. The Δatf1 mutant showed higher sensitivity to H2O2 than the wild-type cells, and this sensitivity was further accentuated in the Δpap1 background.
Detection of Mpr1-Mcs4 Association In Vivo
The pREP1-KZ-mpr1 and pREP1-KZ-mpr1HQ plasmids were constructed by cloning of the wild-type mpr1+ and mutant mpr1HQ genes in pREP1-KZ vector (Shiozaki and Russell, 1997); they express GST-Mpr1 or GST-Mpr1HQ fusion proteins, respectively, under the regulation of the thiamine-repressible nmt1 promoter (Maundrell, 1990). The pREP1-KZ-mpr1 plasmid can complement the Δmpr1 defect in Spc1 activation upon oxidative stress, indicating that the GST tag does not disturb the Mpr1 function. CA337 (Δmpr1 mcs4:myc) cells were transformed with the plasmids pREP1-KZ-mpr1 and pREP1-KZ-mpr1HQ, and transformants were grown at 30°C for 23 h in EMM2 medium supplemented with 0.03 μM thiamine to induce expression of the GST fusion proteins at a low level. After stress treatments, cells were harvested by rapid filtration and disrupted in lysis buffer (50 mM Tris-HCl, pH 8.0, 5 mM EDTA, 100 mM NaCl, 1 mM 2-mercaptoethanol, 10% glycerol, 0.1 mM Na3VO4). The protein concentration of each lysate was normalized with the use of a Bio-Rad (Richmond, CA) protein assay before immunoblot analysis of Mcs4 (see Figure 4A, bottom panel) and incubation with 10 μl (bed volume) of glutathione (GSH)-Sepharose beads (Amersham Pharmacia). After extensive washes with lysis buffer containing 0.7% Triton X-100, proteins bound to the beads were analyzed by immunoblotting with anti-GST and anti-myc (BabCO, Richmond, CA) antibodies (Shiozaki and Russell, 1997).
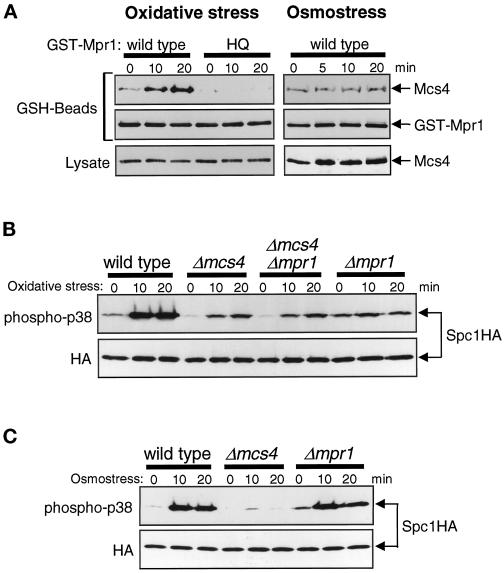
Figure 4.
Mpr1 functions upstream of the Mcs4 response regulator. (A) Oxidative stress induces physical association between Mpr1 and Mcs4. Strain CA337 has chromosomal mcs4+ tagged with the sequence encoding the myc epitope. This strain was transformed with pREP1-KZ-mpr1 and pREP1-KZ-mpr1HQ plasmids, which express GST fusion proteins of wild-type and His-221→Gln mutant Mpr1, respectively, under the regulation of the thiamine-repressible nmt1 promoter. The transformants were grown in EMM2 medium with 0.03 μM thiamine to induce expression of the GST fusion proteins at a low level and treated with either oxidative stress induced by 0.3 mM H2O2 (left panels) or high-osmolarity stress induced by 0.6 M KCl (right panels) for the indicated times. Cell lysates were absorbed to GSH-Sepharose beads, and after extensive washes, proteins bound to the beads (GSH-Beads) were analyzed by immunoblotting with anti-myc and anti-GST antibodies. The amount of Mcs4 detected in the crude cell lysates (Lysate) did not change significantly after the stress treatments. (B) Wild-type (KS1376), Δmcs4 (CA220), Δmpr1 (CA279), and Δmcs4 Δmpr1 (CA420) strains carrying the spc1:HA6H allele were grown to midlog phase at 30°C in YES medium and treated with oxidative stress induced by 0.3 mM H2O2. Aliquots of cells were harvested at the indicated times, and Spc1 was purified by Ni-NTA chromatography, followed by immunoblotting with anti-phospho-p38 and anti-HA antibodies. The pattern of Spc1 activation in the Δmcs4 Δmpr1 double mutant is identical to that in the Δmcs4 mutant before and after oxidative stress. (C) Wild-type (KS1376), Δmcs4 (CA220), and Δmpr1 (CA279) strains were treated with high-osmolarity stress induced by 0.6 M KCl, and Spc1 activation was examined as described for B.
RESULTS
Mpr1 Is Homologous to Ypd1, a Response Regulator Phosphotransferase in Budding Yeast
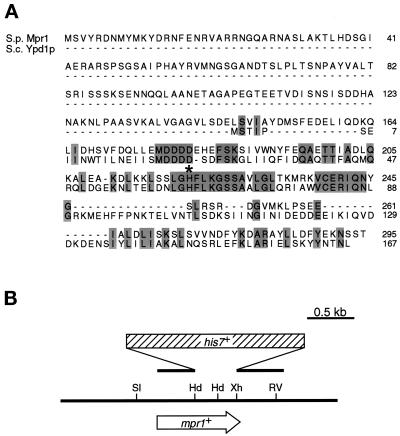
Previous studies identified Mcs4 as a regulator of the Spc1 MAPK cascade. Mcs4 is most homologous to the Ssk1 response regulator in budding yeast (Shieh et al., 1997; Shiozaki et al., 1997). To examine whether the Spc1 cascade is regulated by a multistep phosphorelay similar to the budding yeast Sln1-Ypd1-Ssk1 pathway, we performed a computer search for Ypd1 homologues in the S. pombe genome sequences submitted to GenBank by the Sanger Center. An 888-base pair uninterrupted ORF (SPBC725.02) in chromosome II was found to encode a protein of 295 amino acid residues with a significant homology to Ypd1 (Figure 1A). This putative 32.5-kDa protein is significantly larger than Ypd1, at 165 amino acid residues; however, its C-terminal region of 130 residues is 43% identical to the Ypd1 sequence. Notably, residues 62–71 of Ypd1, containing the histidine phosphorylation site, are completely conserved in this fission yeast gene, which was therefore named mpr1+ (multistep phosphorelay). On the other hand, no apparent homology to any known proteins was found in the N-terminal 160 residues of Mpr1.
Figure 1.
Mpr1 is a fission yeast homologue of the budding yeast Ypd1 protein, a response regulator phosphotransferase. (A) Alignment of the amino acid sequence of Mpr1 with that of Ypd1. Identical residues are shown by shaded boxes. An asterisk marks the phosphorylation site His-64 of Ypd1, which is also conserved in Mpr1. The nucleotide sequence of mpr1+ is available in the GenBank database under accession number AL034352 (ORF, SPBC725.02). (B) Restriction map of the mpr1+ locus and the mpr1::his7+ construct for gene disruption. The HindIII–XhoI fragment encoding residues 136–281 was replaced with the his7+ marker gene (Apolinario et al., 1993) to disrupt the mpr1+ gene. Restriction enzyme sites: Hd, HindIII; RV, EcoRV; Sl, SalI; Xh, XhoI.
Mpr1 Is Important for Oxidative Stress Signaling to SAPK
To study the cellular function of Mpr1, gene disruption of mpr1+ was performed. In. S. cerevisiae, mutational inactivation of YPD1 brings about lethal hyperactivation of the Hog1 MAPK, which can be suppressed by the hog1Δ mutation (Posas et al., 1996). Therefore, a spc1::ura4+/spc1+ heterozygous diploid strain, which has one of the spc1 loci disrupted with the ura4+ gene, was transformed with the mpr1::his7+ plasmid construct (Figure 1B). Integration of this plasmid to one of the mpr1+ loci replaced the Mpr1 sequence homologous to Ypd1 with the his7+ marker gene, which was confirmed by Southern hybridization. The resultant diploid mpr1::his7+/mpr1+ spc1::ura4+/spc1+ strain was sporulated and subjected to tetrad analysis. All of the dissected tetrads gave rise to four viable spores with 2:2 segregation of His+ and His− phenotypes regardless of the uracil auxotrophy, indicating that mpr1+ is not essential for viability in both spc1+ and spc1− backgrounds. A haploid strain with the disrupted mpr1+ gene (Δmpr1), however, showed ∼12% longer doubling time than that of wild-type cells under the standard growth condition (in YES medium at 30°C). Δmpr1 cells also grew and formed colonies on YES agar plates containing 1 M KCl (our unpublished results), suggesting that mpr1+ is not essential for cellular survival of high-osmolarity conditions.
We next examined whether, like Ypd1, Mpr1 is involved in stress signaling to the SAPK cascade in fission yeast. Activation of Spc1 in response to stress treatments was compared between wild-type and Δmpr1 cells with the use of strains in which chromosomal spc1+ is tagged with the HA6H sequence encoding the HA epitope and six consecutive histidine residues (Shiozaki and Russell, 1995). Anti-phospho-p38 antibodies were used in immunoblotting to detect the active form of Spc1 phosphorylated on both Thr-171 and Tyr-173 (Shiozaki and Russell, 1997; Nguyen and Shiozaki, 1999). Under normal growth conditions, the level of active Spc1 in Δmpr1 cells is 60–70% higher than that in wild-type cells (Figure 2, lanes 0), whereas no apparent difference was observed in the amount of the Spc1 protein detected by anti-HA antibodies. High-osmolarity stress induced by 0.6 M KCl (Figure 2A) and oxidative stress induced by 0.3 mM H2O2 (Figure 2B) increased active Spc1 four- to fivefold in wild-type cells. On the other hand, oxidative stress–induced activation of Spc1 in Δmpr1 cells was only 60% of that in wild-type cells (Figure 2B), although no apparent difference was detected between the two strains in the level of Spc1 activation after osmostress (Figure 2A). We also observed that heat shock–induced activation of Spc1 in the Δmpr1 strain was comparable to that in the wild-type strain (our unpublished results). Thus, Spc1 activation in response to oxidative stress, but not other forms of stress, is defective in the Δmpr1 mutant. These results implicate Mpr1 in oxidative stress signaling to the Spc1 SAPK cascade, which contrasts with the function of Ypd1 in osmostress signaling of budding yeast (Posas et al., 1996).
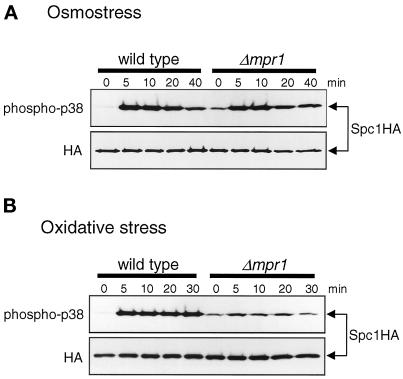
Figure 2.
Mpr1 is important for activation of Spc1 in response to oxidative stress. Wild-type (KS1376) and Δmpr1 (CA279) strains carrying the spc1:HA6H allele were grown to midlog phase at 30°C in YES medium and treated with either high-osmolarity stress induced by 0.6 M KCl (A) or oxidative stress induced by 0.3 mM H2O2 (B). Aliquots of cells were harvested at the indicated times, and Spc1 was purified by Ni-NTA chromatography, followed by immunoblotting with anti-phospho-p38 and anti-HA antibodies. Δmpr1 mutant cells showed a significant defect in Spc1 activation upon oxidative stress, whereas osmostress induced strong activation of Spc1 in Δmpr1 mutants and wild-type cells.
The Putative Histidine Phosphorylation Site of Mpr1 Is Crucial for Oxidative Stress Signaling
Sequence conservation of the histidine phosphorylation site between Mpr1 and Ypd1 (Figure 1A) prompted us to test whether phosphorylation of His-221 in Mpr1 is required for oxidative stress signaling. Site-directed mutagenesis was used to construct the mpr1HQ mutant gene, in which His-221 was substituted with an unphosphorylatable residue, glutamine. The chromosomal mpr1+ locus was replaced with the mpr1HQ gene, which was tagged with the HA6H sequence for purification by Ni-NTA chromatography and detection by anti-HA antibodies. Phenotypes of the control strain expressing wild-type Mpr1 tagged with the HA6H sequence were indistinguishable from those of cells expressing untagged Mpr1; strong activation of Spc1 was observed in response to osmostress and oxidative stress (Figure 3, mpr1:HA). In contrast, oxidative stress–induced Spc1 activation was significantly reduced in the mpr1HQ mutant strain, whereas high osmolarity activated Spc1 in mpr1HQ cells to a level comparable to that in the control strain (Figure 3). Thus, like Δmpr1 cells, the mpr1HQ mutant is defective in oxidative stress–induced activation of Spc1, although anti-HA immunoblotting detected both the Mpr1 and Mpr1HQ proteins at similar levels (Figure 3, lower panel). These results indicate that the putative phosphorylation site, His-221, is required for the Mpr1 function in oxidative stress signaling to Spc1.
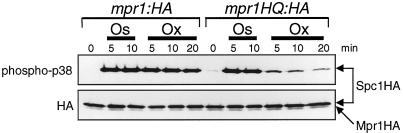
Figure 3.
The putative histidine phosphorylation site, His-221, is required for Mpr1 function in oxidative stress signaling to Spc1. The mpr1HQ mutant gene with the His-221→Gln substitution was created by site-directed mutagenesis and fused to the HA6H tag, which was used to replace the mpr1+ locus of an spc1:HA6H strain. The resultant strain (CA403) and the control strain, which has the wild-type mpr1+ locus tagged with HA6H (CA385), were grown to midlog phase at 30°C in YES medium and then treated with osmostress induced by 0.6 M KCl (Os) and oxidative stress induced by 0.3 mM H2O2 (Ox) for the indicated times. Spc1 as well as wild-type and mutant Mpr1 were purified on Ni-NTA beads and analyzed by immunoblotting with anti-phospho-p38 and anti-HA antibodies. The Mpr1 and Mpr1HQ proteins were detected as weak bands below the Spc1 bands in anti-HA immunoblotting. Stress-induced Spc1 activation in the mpr1:HA strain was indistinguishable from that in strains expressing untagged Mpr1, indicating that the HA6H tag does not disturb the Mpr1 function.
Mpr1 Interacts with the Mcs4 Response Regulator
The results described above suggest that, like Ypd1, Mpr1 may mediate stress signals by transferring a phosphoryl group from its histidine residue to a response regulator. Because a response regulator homologue, Mcs4, regulates the Spc1 MAPK cascade (Cottarel, 1997; Shieh et al., 1997; Shiozaki et al., 1997), it is likely that Mpr1 functions as a phosphotransferase for Mcs4. To explore this possibility, physical interaction between Mpr1 and Mcs4 in vivo was examined. We constructed a strain in which chromosomal mcs4+ was tagged with the sequence encoding the myc epitope for detection. Phenotypes of this mcs4:myc strain were indistinguishable from those of strains expressing untagged Mcs4 (our unpublished results), indicating that the Mcs4 function was not disturbed by the myc epitope. The mcs4:myc strain was transformed with pREP1-KZ-mpr1 and pREP1-KZ-mpr1HQ, which express wild-type Mpr1 and the His-221→Gln mutant (Mpr1HQ), respectively, as fusion with GST. Expression of GST-Mpr1 and GST-Mpr1HQ was induced at a relatively low level from the thiamine-repressible nmt1 promoter of the pREP1 vector (Maundrell, 1990) in the growth medium containing 0.03 μM thiamine. Isolation of the GST-Mpr1 protein on GSH-Sepharose beads resulted in copurification of Mcs4, whereas little Mcs4 was recovered with the mutant GST-Mpr1HQ protein (Figure 4A). Moreover, the amount of Mcs4 coprecipitated with GST-Mpr1 increased significantly during the experiment after cells were exposed to oxidative stress induced by H2O2. In contrast, osmostress did not affect the interaction between GST-Mpr1 and Mcs4 (Figure 4A, right panels), indicating that the induced interaction between Mpr1 and Mcs4 is specific to oxidative stress.
We next examined the phenotype of the Δmcs4 Δmpr1 double mutant in Spc1 activation upon oxidative stress. Compared with wild-type cells, exposure to H2O2 brought about a reduced level of Spc1 activation in Δmcs4 cells (Figure 4B), indicating that Mcs4 positively regulates Spc1 activation (Shieh et al., 1997; Shiozaki et al., 1997). In Δmpr1 cells, the level of active Spc1 before stress was somewhat higher than in wild-type cells, but only slight activation of Spc1 was induced by oxidative stress. We found that the pattern of Spc1 activation before and after oxidative stress in the Δmcs4 Δmpr1 double mutant was indistinguishable from that in the Δmcs4 strain (Figure 4B); therefore, Δmcs4 appears to be epistatic to mpr1+. This observation is consistent with the idea that Mpr1 transmits oxidative stress signals to the Spc1 cascade through the Mcs4 response regulator and that Mpr1 cannot affect Spc1 activity in the absence of Mcs4. However, Δmpr1 and Δmcs4 cells showed significantly different phenotypes under osmostress conditions (Figure 4C); only weak activation of Spc1 was observed in Δmcs4 cells exposed to high osmolarity, whereas Spc1 activation in Δmpr1 cells was comparable to that in wild-type cells under the same conditions.
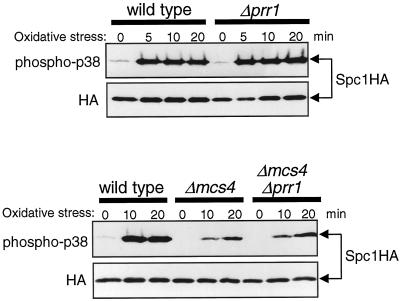
In the Δmcs4 and Δmcs4 Δmpr1 strains, weak activation of Spc1 was induced in response to oxidative stress (Figure 4B), indicating that oxidative stress stimuli can be transmitted to Spc1 independently of Mpr1 and Mcs4. Recently, the prr1+ gene encoding a novel response regulator was reported, which is important for cellular resistance against oxidative stress in S. pombe (Ohmiya et al., 1999). To test whether the Prr1 response regulator is involved in oxidative stress signaling to Spc1, H2O2-induced activation of Spc1 in prr1 null mutant (Δprr1) cells was examined. The Δprr1 mutation showed no apparent defect in Spc1 activation before or after oxidative stress in either the mcs4+ (Figure 5, top) or the Δmcs4 (Figure 5, bottom) background, suggesting that Prr1 is not important for oxidative stress signaling to Spc1.
Figure 5.
The Prr1 response regulator is not important for Spc1 activation after oxidative stress. Wild-type (KS1376), Δprr1 (CA425), Δmcs4 (CA220), and Δmcs4 Δprr1 (CA423) strains carrying the spc1:HA6H allele were grown to midlog phase at 30°C in YES medium and treated with oxidative stress induced by 0.3 mM H2O2. Aliquots of cells were harvested at the indicated times, and Spc1 was purified by Ni-NTA chromatography, followed by immunoblotting with anti-phospho-p38 and anti-HA antibodies.
Mpr1 Is Not Essential for Cellular Resistance against Oxidative Stress
Because spc1 mutants are supersensitive to H2O2 (Degols et al., 1996; Toone et al., 1998), we also examined the H2O2 sensitivity of the Δmpr1 mutant, which is defective in Spc1 activation upon oxidative stress. Wild-type, Δspc1, and Δmpr1 cells were incubated for 1 h with 1 and 2 mM H2O2 in the growth medium, and the survival of each strain was evaluated in terms of their colony-forming ability after plating. As shown in Figure 6A, Δmpr1 cells showed H2O2 resistance similar to that of wild-type cells, whereas significantly higher loss of viability was observed with Δspc1 cells under the same conditions.
In response to oxidative stress, Spc1 activation brings about induced expression of the ctt1+ gene encoding cytosolic catalase (Wilkinson et al., 1996; Degols and Russell, 1997), which decomposes H2O2 to protect cells from oxidative damage (Nakagawa et al., 1995). Northern blot experiments demonstrated that, in Δmpr1 cells, ctt1+ expression was higher than in wild-type cells in the absence of stress, and ctt1+ was further induced upon exposure to H2O2 (Figure 6B). On the other hand, ctt1+ mRNA was hardly detected in Δspc1 cells before or after oxidative stress. Although Mpr1 plays an important role in the activation of Spc1 in response to oxidative stress, these data indicate that Mpr1 is not essential for the induction of ctt1+ expression and other cellular responses to protect cells from oxidative stress.
Oxidative Stress Can Induce ctt1+ Expression Independently of Spc1 Activation
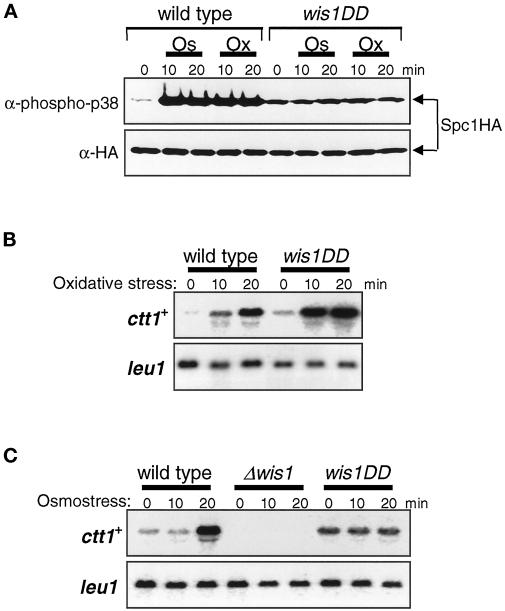
The results described above indicate that in Δmpr1 cells, expression of ctt1+ is strongly induced in the absence of a significant increase in cellular Spc1 activity. However, Δspc1 mutants are totally defective in ctt1+ expression upon oxidative stress (Degols and Russell, 1997) (Figure 6B), suggesting that Spc1 activity is essential for induction of ctt1+ expression. One hypothesis to explain both of these observations is that ctt1+ expression has an absolute requirement for a certain level of Spc1 activity, but oxidative stress stimuli can trigger induction of ctt1+ in the absence of any increase in Spc1 activity. To test this hypothesis, we examined ctt1+ expression in the wis1DD mutant strain, which expresses a mutant form of the Wis1 MEK that is constitutively active because of aspartic acid substitutions at the MEKK phosphorylation sites (Shiozaki et al., 1998; Nguyen and Shiozaki, 1999). In contrast to wild-type cells, the activation level of Spc1 in wis1DD cells was not affected by osmostress and oxidative stress (Figure 7A). As shown in Figure 7B, ctt1+ expression was strongly induced by oxidative stress in wis1DD cells, which suggests that induction of ctt1+ upon oxidative stress does not require an increase in Spc1 activity. In contrast, ctt1+ induction by high-osmolarity stress appeared to be mediated by Spc1 activation (Figure 7C). The level of ctt1+ mRNA was constant both before and after osmostress in wis1DD cells, whereas strong induction of ctt1+ was observed in wild-type cells. As in the Δspc1 cells, ctt1+ expression was not detectable in the Δwis1 cells. Thus, Spc1 activity is necessary for ctt1+ expression, but oxidative stress can induce ctt1+ expression independently of stress-induced activation of Spc1. In contrast, induction of ctt1+ upon osmostress is dependent on an increase in Spc1 activity.
Figure 7.
Oxidative stress but not osmostress can induce ctt1+ expression independently of Spc1 activation. (A) Wild-type (KS2096) and wis1DD (KS2088) strains carrying the spc1:HA6H allele were grown to midlog phase at 30°C in YES medium and treated with either high-osmolarity stress induced by 0.6 M KCl (Os) or oxidative stress induced by 0.3 mM H2O2 (Ox). Aliquots of cells were harvested at the indicated times, and Spc1 was purified by Ni-NTA chromatography, followed by immunoblotting with anti-phospho-p38 and anti-HA antibodies. The level of active Spc1 does not change in wis1DD cells even after osmostress and oxidative stress. (B and C) Wild-type (PR109), wis1DD (KS2081), and Δwis1 (JM544) cells were grown to midlog phase in YES medium and treated with oxidative stress induced by 0.3 mM H2O2 (B) and high-osmolarity stress induced by 0.6 M KCl (C). Aliquots of cells were harvested at the indicated times for Northern hybridization analysis with the ctt1+ and leu1+ probes. In wis1DD cells, ctt1+ expression was increased significantly in response to oxidative stress but not osmostress.
Both the Atf1 and Pap1 Transcription Factors Regulate ctt1+ Expression in Response to Oxidative Stress
Two transcription factors, Atf1 and Pap1, are implicated in expression of the ctt1+ gene (Wilkinson et al., 1996; Degols and Russell, 1997; Toone et al., 1998). Toone et al. (1998) recently proposed that ctt1+ expression in response to high osmolarity is induced by the Spc1-Atf1 pathway, but oxidative stress–induced expression of ctt1+ is wholly dependent on the Spc1-Pap1 pathway. In contrast to this model, we found that Atf1 is also important in induced expression of ctt1+ after oxidative stress. Expression of ctt1+ in Δatf1, Δpap1, and Δatf1 Δpap1 mutants was examined by Northern hybridization (Figure 8A). Compared with wild-type cells, expression of ctt1+ upon H2O2 treatments was dramatically reduced in both Δatf1 and Δpap1 strains, and ctt1+ mRNA was undetectable in Δatf1 Δpap1 double mutant cells. These data clearly indicate that both Atf1 and Pap1 are required for the full expression of ctt1+ upon oxidative stress. In addition, the defective phenotypes of Δatf1 and Δpap1 in ctt1+ expression appear to be additive, suggesting that Atf1 and Pap1 can independently regulate transcription of ctt1+.
Consistent with these observations, we found that Δatf1 mutant cells are more sensitive to H2O2 than wild-type cells (Figure 8B), although it was previously reported that Δatf1 cells did not show increased sensitivity to oxidative stress (Toone et al., 1998). Only a slight difference was observed between wild-type cells and Δatf1 cells when treated with 1 mM H2O2; however, only 1% of Δatf1 cells survived exposure to 2 mM H2O2, compared with 60% survival of the same treatment with the wild-type strain. In addition, the Δatf1 Δpap1 double mutant showed more severe sensitivity to H2O2 than the Δatf1 single mutant (Figure 8B), indicating that both Atf1 and Pap1 are involved in the cellular response to oxidative stress. With a higher concentration of H2O2, the Δpap1 single mutant also showed increased sensitivity; when exposed to 4 mM H2O2, the viability of Δpap1 and Δatf1 cells decreased to 1 and 0.01% of that of wild-type cells, respectively.
Spc1-independent Induction of ctt1+ upon Oxidative Stress Is Mediated by Pap1
Having established that both Atf1 and Pap1 are involved in the expression of ctt1+ upon oxidative stress, we next examined which factor, Atf1 or Pap1, is responsible for oxidative stress–induced ctt1+ expression in the presence of constitutive Spc1 activity (Figure 7). Even in the absence of stress, activation of Spc1 by Wis1 overexpression induces the expression of various stress-response genes that are regulated by the Spc1 pathway (Degols et al., 1996). We found that ctt1+ expression was also induced by Wis1 overexpression in wild-type and Δpap1 cells but not in Δatf1 cells (our unpublished results), which indicates that activated Spc1 induces ctt1+ expression through Atf1 rather than Pap1. Therefore, we tested whether Pap1 is involved in the Spc1-independent induction of ctt1+ in wis1DD cells upon oxidative stress. As shown in Figure 9A, the Δpap1 mutation largely impaired the induction of ctt1+ after oxidative stress in wis1DD cells, and the level of ctt1+ mRNA in the wis1DD Δpap1 strain showed little change before and after H2O2 treatments. Thus, Pap1 has an essential role in inducing ctt1+ expression upon oxidative stress when cells have constitutive Spc1 activity. In other words, in the Δpap1 background, induction of ctt1+ expression is mediated by oxidative stress–induced activation of Spc1, presumably through Atf1.
Figure 9.
Pap1 can regulate ctt1+ expression upon oxidative stress independently of the Spc1 pathway. (A) Wild-type (PR109), wis1DD (KS2081), and wis1DD Δpap1 (CA356) strains were grown to midlog phase in YES medium at 30°C and treated with 0.3 mM H2O2. Aliquots of cells were harvested at the indicated times for Northern hybridization analysis with the ctt1+ and leu1+ probes. In wis1DD cells, which have constitutive Spc1 activity, ctt1+ induction upon oxidative stress was dependent on Pap1. (B) Northern analysis similar to that described for A was performed with Δatf1 (KS1497), Δatf1 Δpap1 (CA334), and Δatf1 Δspc1 (KS1533). In the Δatf1 background, the induction of ctt1+ upon oxidative stress was dependent on Pap1 but not Spc1.
A simple model to explain the data described above is that two independent inputs regulate ctt1+ expression in response to oxidative stress. One input is the Spc1-Atf1 pathway in which stress-activated Spc1 induces ctt1+ transcription through Atf1. The other input is mediated by the Pap1 transcription factor that can induce ctt1+ expression upon oxidative stress independently of Spc1 activation. A prediction from this model is that in the Δatf1 mutant, induction of ctt1+ by oxidative stress is dependent on Pap1 but not Spc1. As expected, the experiments shown in Figure 9B demonstrated that after oxidative stress, the pattern of ctt1+ expression in Δatf1 Δspc1 double mutant cells was indistinguishable from that in Δatf1 cells, whereas ctt1+ mRNA was not detectable in Δatf1 Δpap1 cells. Collectively, these results strongly suggest that Pap1 can regulate ctt1+ transcription in response to oxidative stress independently of the Spc1 MAPK.
DISCUSSION
Reactive oxygen species (ROS) generated as by-products of aerobic metabolism or by environmental chemicals cause damage to DNA, proteins, and cellular structures. Yeast has been used as a model system to study cellular oxidative stress responses in eukaryotes, and a set of genes required for detoxification of ROS has been identified (Moradas-Ferreira et al., 1996). However, little is known about the mechanism whereby eukaryotic cells detect ROS and modulate expression of the antioxidant defense genes. The data presented in this paper strongly suggest that, in fission yeast, proteins homologous to members of two-component systems are involved in oxidative stress signaling to a MAPK cascade that regulates transcription of stress-response genes.
Mpr1 is most homologous to the budding yeast Ypd1 protein, which transfers a phosphoryl group from the receiver domain of Sln1 to the Ssk1 response regulator in osmostress signaling to the Hog1 MAPK cascade. Phosphorylation of Ypd1-His64 is an intermediate in the Sln1-Ypd1-Ssk1 phosphorelay, and substitution of His-64 with glutamine abolishes the Ypd1 function (Posas et al., 1996). We found that the sequence encompassing His-64 of Ypd1 is completely conserved in Mpr1 and that the mutant Mpr1 protein with glutamine substitution of the putative phosphorylation site, His-221, is not functional. Furthermore, physical interaction between Mpr1 and the Mcs4 response regulator was observed. These data suggest that, like Ypd1, Mpr1 also functions as a response regulator phosphotransferase.
Considering the highly conserved architecture of the Mpr1-Mcs4 pathway and the budding yeast Ypd1-Ssk1 pathway, it is surprising that Mpr1 and Mcs4 are involved in transmitting oxidative stress rather than osmostress signals to a SAPK cascade. Under low-osmolarity conditions, phosphotransfer from Ypd1 to the Ssk1 response regulator inhibits Ssk1 from activating the Ssk2 MEKK in budding yeast, and the ypd1 null mutation causes hyperactivation of Hog1 (Posas et al., 1996). Δmpr1 cells have a higher basal level of Spc1 activity, which is suppressed in the Δmcs4 Δmpr1 double mutant, implying that, like Ypd1, Mpr1 also suppresses the Mcs4 response regulator from activating the Spc1 MAPK cascade under normal growth conditions. However, when cells are exposed to oxidative stress, the Mpr1 function is required for induction of Spc1 activation; Spc1 activation in response to oxidative stress is significantly impaired in mpr1 mutants. Thus, under oxidative stress conditions, Mpr1 may positively regulate Mcs4 to activate the downstream MEKKs. Importantly, significant activation of Spc1 was observed upon oxidative stress even in the Δmcs4 and Δmcs4 Δmpr1 mutants, indicating that oxidative stress stimuli can be transmitted to the Spc1 cascade independently of the Mpr1-Mcs4 pathway (Figure 10). Therefore, it is also possible that the Mpr1 function may be required for coordinated action of the two pathways transmitting oxidative stress signals to the MEKKs. In mpr1 mutants, deregulated Mcs4 might hamper the other pathway in activation of the MEKKs.
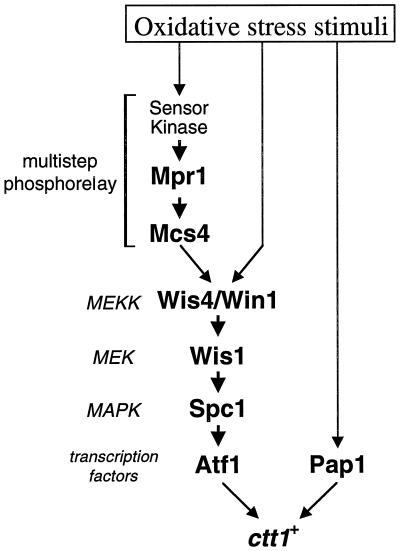
Figure 10.
Oxidative stress signaling pathways that lead to transcription of the ctt1+ gene. A multistep phosphorelay composed of an unknown sensor kinase, Mpr1, and the Mcs4 response regulator transmits oxidative stress signals to the Spc1 MAPK cascade. Oxidative stress induces partial activation of Spc1 even in the Δmcs4 Δmpr1 strain, suggesting that oxidative stress can be transmitted to the Spc1 cascade by additional pathways. Activated Spc1 induces transcription of ctt1+ and other stress-response genes through the Atf1 transcription factor. The Pap1 transcription factor can also mediate ctt1+ expression in response to oxidative stress independently of the Spc1 pathway.
It is very likely that Mpr1-Mcs4 forms part of a multistep phosphorelay similar to the Sln1-Ypd1-Ssk1 in budding yeast. The S. pombe genome-sequencing project has identified two ORFs (SPAC27E2.09 and SPCC74.06) encoding “sensor kinases” similar to Sln1. Both of these proteins have C-terminal receiver domains as well as histidine kinase domains and, therefore, can form His-Asp-His-Asp phosphorelays with Mpr1 and Mcs4. Interestingly, the sensor kinase encoded by SPCC74.06 is a transmembrane protein with a PAS domain that is common among the sensor proteins in bacterial two-component systems responsive to redox or related stimuli (reviewed by Zhulin et al., 1997). Therefore, SPCC74.06 may be a good candidate as an oxidative stress sensor.
Interestingly, oxidative stress induces association between Mpr1 and the Mcs4 response regulator. We are not aware of any known example of stimuli-induced complex formation between phosphotransferase and response regulator proteins in two-component systems. The His-221→Gln mutation in Mpr1 abolishes interaction with Mcs4, implying that phosphorylation of Mpr1 modulates interaction with Mcs4. It is conceivable that signaling to the Spc1 MAPK cascade may involve not only phosphotransfer from Mpr1 to Mcs4 but also complex formation between these proteins, which is currently under investigation.
When Δmpr1 cells are exposed to oxidative stress, only a minor increase of Spc1 activity is detectable; however, the catalase gene ctt1+ is strongly induced and Δmpr1 cells show H2O2 resistance comparable to that of wild-type cells. This is an unexpected result, because ctt1+ expression is known to be under the regulation of the Spc1 pathway; ctt1+ expression is undetectable in Δspc1 and Δwis1 cells, and these mutants are hypersensitive to oxidative stress (Degols et al., 1996; Wilkinson et al., 1996; Degols and Russell, 1997). We found that oxidative stress, but not osmostress, can induce ctt1+ expression in wis1DD cells, which have constitutive Spc1 activity, suggesting that ctt1+ expression requires a certain level of Spc1 activity, but oxidative stress can induce ctt1+ independently of an increase in Spc1 activity.
Spc1 regulates a number of stress-response genes through the Atf1 transcription factor (Shiozaki and Russell, 1996; Wilkinson et al., 1996), and induction of ctt1+ upon osmostress (Wilkinson et al., 1996) and UV irradiation (Degols and Russell, 1997) is impaired in Δatf1 mutants. Recently, Toone et al. (1998) reported that the Δpap1 mutant, but not the Δatf1 mutant, is defective in H2O2-induced expression of ctt1+ and is sensitive to oxidative stress, phenotypes similar to those of Δspc1 mutants. These observations led to the model that Spc1 regulates Pap1 under oxidative stress but regulates Atf1 in response to other forms of stress (Toone et al., 1998; reviewed by Wilkinson and Millar, 1998). However, this model raises perplexing questions. How does activated Spc1 choose either Atf1 or Pap1, depending on the stress that activates Spc1? Why is Spc1 unable to regulate Atf1 only when activated by oxidative stress? The data presented in this paper draw a rather different picture. First, oxidative stress–induced expression of ctt1+ is partially impaired in both Δatf1 and Δpap1, and ctt1+ mRNA is undetectable in the Δatf1 Δpap1 double mutant, indicating that both Atf1 and Pap1 are involved in ctt1+ expression upon oxidative stress. Consistently, Δatf1 cells lose viability rapidly in liquid medium with H2O2, although the H2O2 sensitivity of Δatf1 cells was not apparent when grown on agar medium containing H2O2 in the previous study (Toone et al., 1998). Second, induced ctt1+ expression upon oxidative stress is dependent on Pap1 in the wis1DD strain, which has constitutive Spc1 activity, suggesting that Pap1 can mediate ctt1+ expression independently of stress-induced activation of Spc1. Third, in Δatf1 cells, ctt1+ induction in response to oxidative stress is dependent on Pap1 but not Spc1. It was previously reported that in Δspc1 cells, unphosphorylated Atf1 represses ctt1+ expression both before and after stress (Degols and Russell, 1997). However, in the absence of Atf1, induction of ctt1+ by oxidative stress is detectable and appears to be mediated by Pap1 independently of Spc1. A simple model to explain these data is that ctt1+ expression upon oxidative stress is regulated by two independent pathways, the Spc1-Atf1 pathway and Pap1 (Figure 10). Regardless of the form of stress, activated Spc1 phosphorylates Atf1 to induce ctt1+ expression. Under oxidative stress conditions, Pap1 can also induce ctt1+ expression independently of Spc1. Pap1 has a NES sequence sensitive to oxidative stress; the Pap1 NES contains cysteine residues essential for its function, and the thiol group of these residues might serve as a redox sensor (Kudo et al., 1999). However, our data do not exclude the possibility that Pap1 is also subjected to auxiliary regulation by oxidative stress–activated Spc1. It should be noted that ctt1+ is inducible by oxidative stress in the Δatf1 Δspc1 double mutant but not in the Δspc1 single mutant, suggesting that Pap1 cannot induce ctt1+ under transcriptional repression by unphosphorylated Atf1.
In summary, the data presented here suggest that fission yeast has at least three pathways sensing oxidative stress stimuli to induce the catalase gene ctt1+ (Figure 10). In addition to the phosphorelay system with Mpr1-Mcs4, an Mcs4-independent pathway transmits oxidative stress signals to the Spc1 MAPK cascade, which regulates ctt1+ through Atf1. Oxidative stress can also induce ctt1+ through the Pap1 transcription factor independently of the Spc1 pathway. Studies in budding yeast also suggest that cellular defense against oxidative stress is complex and may involve multiple signaling pathways (Moradas-Ferreira et al., 1996). It is possible that different sensory mechanisms with partly overlapping specificities contribute to the detection of various ROS and prooxidants to minimize damage to the cell.
ACKNOWLEDGMENTS
We thank Mitsue Shiozaki for technical assistance, Paul Russell and Valley Stewart for helpful discussion, and Jonathan Millar and Takashi Toda for strains. A.N.N. was supported by the National Institutes of Health Molecular and Cellular Biology Training Program at the University of California-Davis (T32 GM07377). This research was supported by National Institutes of Health grant GM59788 and American Cancer Society grant IRG-95-125-04 awarded to K.S.
REFERENCES
- Alfa C, Fantes P, Hyams J, McLeod M, Warbrick E. Experiments with Fission Yeast: A Laboratory Course Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1993. [Google Scholar]
- Apolinario E, Nocero M, Jin M, Hoffman CS. Cloning and manipulation of the Schizosaccharomyces pombe his7+ gene as a new selectable marker for molecular genetic studies. Curr Genet. 1993;24:491–495. doi: 10.1007/BF00351711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banuett F. Signaling in the yeasts: an informational cascade with links to the filamentous fungi. Microbiol Mol Biol Rev. 1998;62:249–274. doi: 10.1128/mmbr.62.2.249-274.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewster JL, de Valoir T, Dwyer ND, Winter E, Gustin MC. An osmosensing signal transduction pathway in yeast. Science. 1993;259:1760–1763. doi: 10.1126/science.7681220. [DOI] [PubMed] [Google Scholar]
- Cottarel G. Mcs4, a two-component system response regulator homologue, regulates the Schizosaccharomyces pombe cell cycle control. Genetics. 1997;147:1043–1051. doi: 10.1093/genetics/147.3.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degols G, Russell P. Discrete roles of the Spc1 kinase and the Atf1 transcription factor in the UV response of Schizosaccharomyces pombe. Mol Cell Biol. 1997;17:3356–3363. doi: 10.1128/mcb.17.6.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degols G, Shiozaki K, Russell P. Activation and regulation of the Spc1 stress-activated protein kinase in Schizosaccharomyces pombe. Mol Cell Biol. 1996;16:2870–2877. doi: 10.1128/mcb.16.6.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Campbell D, Dérijard B, Davis RJ. Transcription factor ATF2 regulation by the JNK signal transduction pathway. Science. 1995;267:389–393. doi: 10.1126/science.7824938. [DOI] [PubMed] [Google Scholar]
- Higuchi R, Krummel B, Saiki RK. A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res. 1988;16:7351–7367. doi: 10.1093/nar/16.15.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- Ip YT, Davis RJ. Signal transduction by the c-Jun N-terminal kinase (JNK): from inflammation to development. Curr Opin Cell Biol. 1998;10:205–219. doi: 10.1016/s0955-0674(98)80143-9. [DOI] [PubMed] [Google Scholar]
- Kanoh J, Watanabe Y, Ohsugi M, Iino Y, Yamamoto M. Schizosaccharomyces pombe gad7+ encodes a phosphoprotein with a bZIP domain, which is required for proper G1 arrest and gene expression under nitrogen starvation. Genes Cells. 1996;1:391–408. doi: 10.1046/j.1365-2443.1996.d01-247.x. [DOI] [PubMed] [Google Scholar]
- Kato TJ, Okazaki K, Murakami H, Stettler S, Fantes PA, Okayama H. Stress signal, mediated by a Hog1-like MAP kinase, controls sexual development in fission yeast. FEBS Lett. 1996;378:207–212. doi: 10.1016/0014-5793(95)01442-x. [DOI] [PubMed] [Google Scholar]
- Kudo N, Taoka H, Toda T, Yoshida M, Horinouchi S. A novel nuclear export signal sensitive to oxidative stress in the fission yeast transcription factor Pap1. J Biol Chem. 1999;274:15151–15158. doi: 10.1074/jbc.274.21.15151. [DOI] [PubMed] [Google Scholar]
- Kyriakis JM, Avruch J. Sounding the alarm: protein kinase cascades activated by stress and inflammation. J Biol Chem. 1996;271:24313–24316. doi: 10.1074/jbc.271.40.24313. [DOI] [PubMed] [Google Scholar]
- Livingstone C, Patel G, Jones N. ATF-2 contains a phosphorylation-dependent transcriptional activation domain. EMBO J. 1995;14:1785–1797. doi: 10.1002/j.1460-2075.1995.tb07167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis WF, Shaulsky G, Wang N. Histidine kinases in signal transduction pathways of eukaryotes. J Cell Sci. 1997;110:1141–1145. doi: 10.1242/jcs.110.10.1141. [DOI] [PubMed] [Google Scholar]
- Maeda T, Takekawa M, Saito H. Activation of yeast PBS2 MAPKK by MAPKKKs or by binding of an SH3-containing osmosensor. Science. 1995;269:554–558. doi: 10.1126/science.7624781. [DOI] [PubMed] [Google Scholar]
- Maeda T, Wurgler-Murphy SM, Saito H. A two-component system that regulates an osmosensing MAP kinase cascade in yeast. Nature. 1994;369:242–245. doi: 10.1038/369242a0. [DOI] [PubMed] [Google Scholar]
- Maundrell K. nmt1 of fission yeast. J Biol Chem. 1990;265:10857–10864. [PubMed] [Google Scholar]
- Millar JBA, Buck V, Wilkinson MG. Pyp1 and Pyp2 PTPases dephosphorylate an osmosensing MAP kinase controlling cell size at division in fission yeast. Genes Dev. 1995;9:2117–2130. doi: 10.1101/gad.9.17.2117. [DOI] [PubMed] [Google Scholar]
- Mitchison JM. Physiological and cytological methods for Schizosaccharomyces pombe. Methods Cell Physiol. 1970;4:131–146. [Google Scholar]
- Moradas-Ferreira P, Costa V, Piper P, Mager W. The molecular defenses against reactive oxygen species in yeast. Mol Microbiol. 1996;19:651–658. doi: 10.1046/j.1365-2958.1996.403940.x. [DOI] [PubMed] [Google Scholar]
- Moreno S, Klar A, Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Nakagawa CW, Mutoh N, Hayashi Y. Transcriptional regulation of catalase gene in the fission yeast Schizosaccharomyces pombe: molecular cloning of the catalase gene and Northern blot analyses of the transcript. J Biochem. 1995;118:109–116. doi: 10.1093/oxfordjournals.jbchem.a124864. [DOI] [PubMed] [Google Scholar]
- Nguyen AN, Shiozaki K. Heat shock-induced activation of stress MAP kinase is regulated by threonine- and tyrosine-specific phosphatases. Genes Dev. 1999;13:1653–1663. doi: 10.1101/gad.13.13.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmiya R, Kato C, Yamada H, Aiba H, Mizuno T. A fission yeast gene (prr1+) that encodes a response regulator implicated in oxidative stress response. J Biochem. 1999;125:1061–1066. doi: 10.1093/oxfordjournals.jbchem.a022387. [DOI] [PubMed] [Google Scholar]
- Ohmiya R, Yamada H, Nakashima K, Aiba H, Mizuno T. Osmoregulation of fission yeast: cloning of two distinct genes encoding glycerol-3-phosphate dehydrogenase, one of which is responsible for osmotolerance for growth. Mol Microbiol. 1995;18:963–973. doi: 10.1111/j.1365-2958.1995.18050963.x. [DOI] [PubMed] [Google Scholar]
- Pidoux AL, Fawell EH, Armstrong J. Glyerol-3-phosphate dehydrogenase homologue from Schizosaccharomyces pombe. Nucleic Acids Res. 1990;18:7145. doi: 10.1093/nar/18.23.7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posas F, Saito H. Osmotic activation of the HOG MAPK pathway via Ste11p MAPKKK: scaffold role of Pbs2p MAPKK. Science. 1997;276:1702–1705. doi: 10.1126/science.276.5319.1702. [DOI] [PubMed] [Google Scholar]
- Posas F, Saito H. Activation of the yeast SSK2 MAP kinase kinase kinase by the SSK1 two-component response regulator. EMBO J. 1998;17:1385–1394. doi: 10.1093/emboj/17.5.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posas F, Takekawa M, Saito H. Signal transduction by MAP kinase cascades in budding yeast. Curr Opin Microbiol. 1998;1:175–182. doi: 10.1016/s1369-5274(98)80008-8. [DOI] [PubMed] [Google Scholar]
- Posas F, Wurgler-Murphy SM, Maeda T, Witten EA, Thai TC, Saito H. Yeast HOG1 MAP kinase cascade is regulated by a multiple phosphorelay mechanism in the SLN1-YPD1-SSK1 ‘two-component’ osmosensor. Cell. 1996;86:865–875. doi: 10.1016/s0092-8674(00)80162-2. [DOI] [PubMed] [Google Scholar]
- Raingeaud J, Whitmarsh AJ, Barrett T, Dérijard B, Davis RJ. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol Cell Biol. 1996;16:1247–1255. doi: 10.1128/mcb.16.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samejima I, Mackie S, Fantes PA. Multiple modes of activation of the stress-responsive MAP kinase pathway in fission yeast. EMBO J. 1997;16:6162–6170. doi: 10.1093/emboj/16.20.6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samejima I, Mackie S, Warbrick E, Weisman R, Fantes PA. The fission yeast mitotic regulator win1+ encodes a MAP kinase kinase kinase that phosphorylates and activates Wis1 MAP kinase kinase in response to high osmolarity. Mol Biol Cell. 1998;9:2325–2335. doi: 10.1091/mbc.9.8.2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüller C, Brewster JL, Alexander MR, Gustin MC, Ruis H. The HOG pathway controls osmotic regulation of transcription via the stress response element (STRE) of the Saccharomyces cerevisiae CTT1 gene. EMBO J. 1994;13:4382–4389. doi: 10.1002/j.1460-2075.1994.tb06758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh J-C, Wilkinson MG, Buck V, Morgan BA, Makino K, Millar JBA. The Mcs4 response regulator coordinately controls the stress-activated Wak1-Wis1-Sty1 MAP kinase pathway and fission yeast cell cycle. Genes Dev. 1997;11:1008–1022. doi: 10.1101/gad.11.8.1008. [DOI] [PubMed] [Google Scholar]
- Shiozaki K, Russell P. Cell-cycle control linked to the extracellular environment by MAP kinase pathway in fission yeast. Nature. 1995;378:739–743. doi: 10.1038/378739a0. [DOI] [PubMed] [Google Scholar]
- Shiozaki K, Russell P. Conjugation, meiosis and the osmotic stress response are regulated by Spc1 kinase through Atf1 transcription factor in fission yeast. Genes Dev. 1996;10:2276–2288. doi: 10.1101/gad.10.18.2276. [DOI] [PubMed] [Google Scholar]
- Shiozaki K, Russell P. Stress-activated protein kinase pathway in cell cycle control of fission yeast. Methods Enzymol. 1997;283:506–520. doi: 10.1016/s0076-6879(97)83040-6. [DOI] [PubMed] [Google Scholar]
- Shiozaki K, Shiozaki M, Russell P. Mcs4 mitotic catastrophe suppressor regulates the fission yeast cell cycle through the Wik1-Wis1-Spc1 kinase cascade. Mol Biol Cell. 1997;8:409–419. doi: 10.1091/mbc.8.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiozaki K, Shiozaki M, Russell P. Heat stress activates fission yeast Spc1/Sty1 MAPK by a MEKK-independent mechanism. Mol Biol Cell. 1998;9:1339–1349. doi: 10.1091/mbc.9.6.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda T, Toda T, Kominami K, Kohnosu A, Yanagida M, Jones N. Schizosaccharomyces pombe atf1+ encodes a transcription factor required for sexual development and entry into stationary phase. EMBO J. 1995;14:6193–6208. doi: 10.1002/j.1460-2075.1995.tb00310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda T, Shimanuki M, Saka Y, Yamano H, Adachi Y, Shirakawa M, Kyogoku Y, Yanagida M. Fission yeast pap1-dependent transcription is negatively regulated by an essential nuclear protein, crm1. Mol Cell Biol. 1992;12:5474–5484. doi: 10.1128/mcb.12.12.5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda T, Shimanuki M, Yanagida M. Fission yeast genes that confer resistance to staurosporine encode an AP-1-like transcription factor and a protein kinase related to the mammalian ERK1/MAP2 and budding yeast FUS3 and KSS1 kinases. Genes Dev. 1991;5:60–73. doi: 10.1101/gad.5.1.60. [DOI] [PubMed] [Google Scholar]
- Toone WM, Kuge S, Samuels M, Morgan BA, Toda T, Jones N. Regulation of the fission yeast transcription factor Pap1 by oxidative stress: requirement for the nuclear export factor Crm1 (Exportin) and the stress-activated MAP kinase Sty1/Spc1. Genes Dev. 1998;12:1453–1463. doi: 10.1101/gad.12.10.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dam H, Wilhelm D, Herr I, Steffen A, Herrlich P, Angel P. ATF-2 is preferentially activated by stress-activated protein kinases to mediate c-jun induction in response to genotoxic agents. EMBO J. 1995;14:1798–1811. doi: 10.1002/j.1460-2075.1995.tb07168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waskiewicz AJ, Cooper JA. Mitogen and stress response pathways: MAP kinase cascades and phosphatase regulation in mammals and yeast. Curr Opin Cell Biol. 1995;7:798–805. doi: 10.1016/0955-0674(95)80063-8. [DOI] [PubMed] [Google Scholar]
- Wilkinson MG, Millar JBA. SAPKs and transcription factors do the nucleocytoplasmic tango. Genes Dev. 1998;12:1391–1397. doi: 10.1101/gad.12.10.1391. [DOI] [PubMed] [Google Scholar]
- Wilkinson MG, Samuels M, Takeda T, Toone WM, Shieh J-C, Toda T, Millar JBA, Jones N. The Atf1 transcription factor is a target for the Sty1 stress-activated MAP kinase pathway in fission yeast. Genes Dev. 1996;10:2289–2301. doi: 10.1101/gad.10.18.2289. [DOI] [PubMed] [Google Scholar]
- Zhulin IB, Taylor BL, Dixon R. PAS domain S-boxes in archaea, bacteria and sensors for oxygen and redox. Trends Biochem Sci. 1997;22:331–333. doi: 10.1016/s0968-0004(97)01110-9. [DOI] [PubMed] [Google Scholar]