Abstract
Purpose
In chicks, plus defocus retards eye growth, thickens the choroid, and activates glucagonergic amacrine cells, probably releasing glucagon. Glucagon receptor antagonists (expected to inhibit compensation to plus defocus) and agonists (expected to block myopia induction by form deprivation) were administered to eyes of chicks, to test the hypothesis that glucagon mediates the induction of changes in eye growth by plus defocus.
Methods
Seven-day-old (P7) chick eyes were injected intravitreally with peptides at concentrations of ~10−9 to 10−5 M in 20 μL (injection volume). The glucagon-receptor antagonists [des-His1,des- Phe6,Glu9]-glucagon-NH2 (des- Phe6-antagonist) and [des-His1,Glu9]-glucagon-NH2 (Phe6-antagonist) were administered daily for 4 to 5 days to plus-defocused eyes. Agonists (porcine glucagon-[1–29] and [Lys17,18,Glu21]-glucagon-NH2) were monocularly administered daily for 5 days to form-deprived eyes. The contralateral eye remained open and received saline. After treatment, eyes were refracted, measured, and examined for histologic changes.
Results
The Phe6-antagonist at 10−5 M (in the syringe) inhibited changes in both refractive error and axial length compensation induced by +7-D lens wear; however, des-Phe6-antagonist (10−5 M) had weak, inconsistent effects and did not antagonize the action of exogenous glucagon. Glucagon prevented ocular elongation and myopia and induced choroidal thickening in form-deprived eyes. [Lys17,18,Glu21]-glucagon-NH2 had little effect at 1037 M, but at 10−6 to 10−5 M altered rod structure and inhibited eye growth.
Conclusions
Exogenous glucagon inhibited the growth of form-deprived eyes, whereas Phe6-antagonist inhibited compensation to plus defocus, as might be expected if glucagon is an endogenous mediator of emmetropization. The reason for the failure of des-Phe6-antagonist to counteract the effects of exogenous glucagon requires further investigation.
It has been suggested that retinal neurotransmitters or neuromodulators, such as dopamine,1 acetylcholine,2 basic fibroblast growth factor,3 vasoactive intestinal polypeptide,4 and glutamate,5 play important roles in visual regulation of eye growth. Recent studies in chicks suggest that glucagon also plays such a role.6,7 Glucagon, a 29-amino-acid peptide synthesized in the pancreas, intestines, and brain, is one of several products formed by enzymatic cleavage of the polypeptide precursor, proglucagon.8 Proglucagon belongs to the secretin-glucagon superfamily of peptides, which act through a matching family of G-protein-coupled receptors coupled to stimulation of adenylyl cyclase, phospholipase C, or other effector mechanisms.9 Glucagon-like immunoreactivity is found in a single class of amacrine cells in the avian retina.7,10 In the chick, the glucagon-containing cells are stimulated by conditions that suppress ocular elongation (plus-lens treatment, recovery from form-deprivation [FD] myopia), but not by conditions that permit or induce ocular elongation (form deprivation, minus-lens treatment).7,11 The release of glucagon during plus defocus may be inferred from the supposition that induction of immediate early genes, such as Egr-1 and fos, is due to depolarization and calcium entry, and the finding that proglucagon mRNA levels in the retina increase with plus lens wear.6 Conversely, the absence of immediate early gene production during form deprivation or negative lens wear implies minimal release of peptides from glucagon amacrine cells and suggests that the lack of glucagon is responsible for the development of experimental myopia.7 In support of this hypothesis, administration of the synthetic glucagon receptor agonist Lys17,18,Glu21-glucagon-NH2 has been found to inhibit the axial elongation of the eye that would otherwise result from negative lens wear12 or form deprivation.13 These studies implicate the release of retinal glucagon and activation of a glucagon receptor in mediating an ocular “stop”-growth signal.
In the studies reported herein, the hypothesis that glucagon is released from the chick retina during plus defocus, thereby mediating an effect of plus defocus on ocular growth and refraction, was tested further. This hypothesis predicts that the administration of exogenous glucagon or the glucagon receptor agonist, Lys17,18,Glu21-glucagon-NH2, should inhibit the development of FD myopia. In addition, if glucagon is released maximally during plus defocus, then glucagon receptor antagonists should block the action of endogenous glucagon and prevent compensatory changes in eyes treated with plus lenses or recovering from FD myopia. Finally, if the glucagon receptor mediates a plus-defocus signal, pretreatment with a glucagon receptor antagonist should inhibit the effects of exogenous glucagon administration on the development of FD myopia. Some of these results have been reported previously in abstract13,14 and thesis13 form.
Methods
Experiment 1: The Effect of Glucagon-Related Agents on Refractive Development and Ocular Growth
Animals
Male White Leghorn chickens (Gallus gallus domesticus) were obtained from Lilydale Hatcheries (Calgary, Alberta, Canada) within 24 hours of hatching (post-hatching day [P]0) and kept on a 12-hour light–dark cycle (lights on at 7:00 AM) at ~25°C. On post-hatching day 7 (P7), groups of six chicks were transferred into clear plastic cages under eight 4-ft 34-W fluorescent lamps, 4 to 8 ft overhead, and beside two 34-W fluorescent lights running from the floor to the top of the wall, resulting in an illuminance of 250 lux at the base of each cage. Chick starter feed (Purina, Richmond, IN) and water were provided ad libitum. The care and use of animals were in accordance with the guidelines of the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were reviewed and approved by the Animal Care Committee of the University of Calgary, Faculty of Medicine.
Visual Manipulations and Injection Protocols
Four different manipulations of the visual environment were used to test the effects of glucagon-related agents on refractive error development and ocular growth.
Glucagon Receptor Antagonist and Plus-Lens–Induced Hyperopia
Myopic defocus was imposed in the left eye of P7 chicks with a +7-D plastic contact lens mounted on a ring of pharmaceutical blister-wrap (plus-lens wear). The lens mount was attached to a circular piece of Velcro, the complementary half of which was glued to the periorbital feathers of the chick. This allowed the lens to be applied and removed easily. The lens was cleaned of dust three times a day during the light phase to ensure that the vision of the chick was not degraded. The right (control) eye was allowed unrestricted vision (no goggle). Chicks received daily intravitreal injection (described later) of the glucagon receptor antagonist [des-His1,Glu9]-glucagon-NH2 (Phe6-antagonist) or saline into the treated (left) eye and saline only into the control (right) eye for 4 days (P7–P10) before ocular measurements were taken on P11 (Table 1).
Table 1.
Experiments to Test the Effects of Glucagon-Related Agents on Refractive Error and Ocular Growth
| Drug | Action | Drug Concentration (M) | n | Treatment |
|---|---|---|---|---|
| Glucagon (glucagon) | Agonist | 10−9, 10−8, 10−7, 10−6, 10−5 or saline | 6 | Myopia induction |
| P7: FD | ||||
| P7–P11: Daily injection (5 total) | ||||
| P12: Measurements | ||||
| [Lys17,18Glu21]-glucagon | Agonist | 10−9, 10−8, 10−7, 10−6, 10−5 or saline | 4–5 | Myopia induction |
| P7: FD | ||||
| P7–P11: Daily injection (5 total) | ||||
| P12: Measurements | ||||
| [des-His1, des-Phe6, Glu9]-glucagon (des-Phe6-antagonist) | Antagonist | 10−5 or saline | 6 | Recovery (myopic defocus) |
| P7: FD | ||||
| P14: Remove goggle (recovery) | ||||
| P14–P17: Daily injection (4 total) | ||||
| P18: Measurements | ||||
| [des-His1, Glu9]-glucagon (Phe6-antagonist) | Antagonist | 10−5 or saline | 4 | Plus-lens wear (myopic defocus) |
| P7: 37-D lens | ||||
| P7–P10: Daily injection (4 total) | ||||
| P11: Measurements |
The first column identifies the peptide used, and gives in parentheses the shortened name used in the text. The second column notes whether the drug acts as an agonist or antagonist at glucagon receptors. The third column gives the concentration of drug delivered in 20 μL in the syringe. The fourth column gives the number of animals (n) in each experiment, and the fifth column describes the treatment protocol where P7 is posthatching day 7. FD, form deprivation; measurements, refraction, wet weight, and axial length measured by electronic calipers.
Glucagon Receptor Antagonist and Recovery from Experimentally Induced Myopia
FD myopia was induced in P7 chicks for 7 days by attaching a translucent diffuser goggle (made from roughened pharmaceutical blister wrap) to the feathers around the treated eye, using contact cement (Prestite; LePage, Brampton, Ontario, Canada). On P14, the goggle was removed, allowing the chicks unrestricted but myopically defocused (initially greater than −10 D) vision (“recovery”). The control eye was allowed unrestricted vision. Chicks received daily intravitreal injections of the glucagon receptor antagonist [des-His1,des-Phe6,Glu9]-glucagon-NH2 (des-Phe6-antagonist), or a mouse monoclonal antibody that is an antagonist to the human glucagon receptor, hGR-2 F6,15 or saline into both the treated eye and control eyes, for 4 days (P14–P17), before ocular measurements were taken on P18 (Table 1).
Glucagon Receptor Agonists and Experimentally Induced Myopia
FD myopia was induced in the treated eye of P7 chicks as just described. The control eye was allowed unrestricted vision. Chicks received daily intravitreal injections of a glucagon receptor agonist—glucagon or [Lys17,18-Glu21]-glucagon-NH2—or saline into the treated eye and saline into the control eye, for 5 days (P7–P11), before ocular measurements were taken on P12 (Table 1).
Glucagon Receptor Agonist or Antagonist Treatment and Unrestricted Vision
Both the treated and control eyes of chicks remained uncovered during the duration of the experiment. Chicks received daily intravitreal injection of a glucagon receptor agonist—glucagon or [Lys17,18,Glu21]-glucagon-NH2—or the des-Phe6-antagonist, or saline into the treated eye and saline into the control eye for 5 days (P7–P11), before ocular measurements were taken on P12.
Intraocular Injection
Before injection of glucagon-related agents into the vitreous, chicks were anesthetized with 2.0% halothane in 50% O2/50% NO2. The vitreous of the FD eye was injected through the posterodorsal side of the eye under aseptic conditions with 20 μL of either peptide or saline (treated eye, n = 4 to 6 at each dose), whereas the open contralateral eye was injected with saline (control eye) via a 25-μL syringe (26 gauge needle; Hamilton, Reno, NV). The doses stated in the Results section and Figures represent the drug concentration in 20 μL in the syringe. The total vitreous volume in 7-to 14-day-old chicks is ~300 to 350 μL, of which a constant 150 to 175 μL is gel (Rushforth DA, Stell WK, unpublished data, 2003). Since diffusion, uptake, destruction, or binding of the injected peptide could greatly affect its effective concentration in the vitreous and retina, for convenience, the concentrations of substances in the vitreous were assumed to be approximately 20/200× (or 1/10×) those in the injected solutions. However, in the Results section and Figures, doses are given as the drug concentration in 20 μL in the syringe, so that the reader can make an independent estimate of the concentration presented to membrane receptors in the tissues that line the vitreous cavity.
Agents Injected
The glucagon receptor agonists tested in experiment 1 were natural porcine glucagon, hereafter called glucagon(1–29) or simply glucagon (70%–80% glucagon, from porcine pancreas extract, cat. no. G3157; Sigma-Aldrich, Oakville, Ontario, Canada), and the higher affinity, peptidase-protected agonist analogue [Lys17,18,Glu21]-glucagon-NH2 (custom-synthesized by Jung-Mo Ahn in the laboratory of VJH). The glucagon receptor agonists were delivered in saline over the concentration range 10−9 to 10−5 M in 20 μL in the syringe. The glucagon receptor antagonists tested were the des-Phe6-antagonist (custom-synthesized by Jung-Mo Ahn and Dev Trivedi in the laboratory of VJH) and the Phe6-antagonist; 97% pure synthetic peptide, cat. no. G1651; Sigma-Aldrich), delivered in saline at 10−5 M stock in 20 μL in the syringe. A mouse monoclonal antibody to the human glucagon receptor, hGR-2 F6, which is known to act as a competitive glucagon receptor antagonist in human and rat,15 was also tested at 2.7 × 10−7, 2.7 × 10−5, and 1.35 × 10−4 M in the syringe. This antibody, generously donated by Robert Bywater (Novo Nordisk A/S, Bagsvaerd, Denmark), was supplied at a concentration of 4.07 mg/mL (~27 μM) in distilled water and was injected either without dilution or after dilution at 1:10 to 1:100 in sterile 0.9% saline. For one experiment, the antibody was concentrated 5×, to ~20 mg/mL (1.35 × 10−4 M), by centrifugal filtration (Microcon YM50 filter; Millipore Corp., Bedford, MA) at ~10g overnight at 4°C and then liberated by reverse centrifugation; recovery was 95%.
Ocular Measurements
Refractive error was measured by streak retinoscopy without cycloplegia to ±0.5 D. Streak retinoscopy was consistently performed at a distance of 30 cm, and no correction was made for working distance or the small-eye artifact.16 The animals were killed by intraperitoneal injection of pentobarbital (Euthanyl; Biomeda, Foster City, CA). The eyes were removed and cleaned of extraneous orbital tissue, wet weight (±10 mg) was measured by an electronic balance, and axial length (±0.2 mm) was measured by digital calipers.
Histology and Immunocytochemistry
Impairment of eye growth and a consequent hyperopic shift in refraction can result from toxic insults to photoreceptors and/or pigment epithelium (RPE).21 The extreme inhibition of growth observed in most eyes treated with the highest doses of the agonist analogue [Lys17,18,Glu21]-glucagon-NH2, but not glucagon or the antagonists, suggested the possibility of toxicity. To check for toxic effects, both form-deprived and open eyes were given daily injections for 5 days of either [Lys17,18,Glu21]-glucagon-NH2 (10−5 M in the syringe) or saline, as described earlier. Treated eyes were enucleated and hemisected, the vitreous gel removed, and the posterior halves immersed in 4% paraformaldehyde (pH 7.4) in 0.1 M phosphate buffer (PB) for 1 hour. Tissues were washed in PB, cryoprotected in 30% sucrose in PB, sectioned on a cryostat, and immunohistochemically labeled as previously described.22 Cryosections were stained with toluidine blue or labeled with a mouse monoclonal rhodopsin antibody, Rho4D2 (1:50; gift of Robert Molday, University of British Columbia, Vancouver, BC, Canada), followed by Alexa Fluor 488 goat anti-mouse IgG (H+L) conjugate (1:1000; Molecular Probes, Eugene, OR). Sections were viewed in bright-field or fluorescence mode, respectively, and images were captured with a monochrome digital camera (Spot; Diagnostic Instruments, Sterling Heights, MI) and transferred to image-analysis software for formatting (Photoshop; Adobe Systems, Mountain View, CA). The effect on rods was quantified by counting abnormal rod cells per field (~380 μm retinal length) in all fields (typically 17) in an entire section.
Experiment 2: The Effect of Glucagon-Related Agents on Choroidal Thickness
Animals
Male White Leghorn chickens (Gallus gallus domesticus) were purchased and maintained as for Experiment 1.
Visual Manipulations and Injection Protocols
Three manipulations of the visual environment were used to test the effects of glucagon-related agents (10−5 M in 20 μL in the syringe) on choroidal thickness. These were (1) the effects of the Phe6-antagonist on plus-lens–induced hyperopia, (2) the effects of the des-Phe6-antagonist on recovery from experimentally induced myopia, and (3) the effects of glucagon on experimentally induced myopia. The methods were essentially the same as those used in experiment 1, except that in this case the effects of [Lys17,18,Glu21]-glucagon-NH2 and the monoclonal antibody hGR-2 F6 were not retested. Also, in this experiment, the effects of the des-Phe6-antagonist on choroidal thickness during recovery from experimentally induced myopia were determined after five injections, rather than four injections as in experiment 2.
Ocular Measurements
Changes in choroidal thickness were determined under halothane anesthesia by high-resolution A-scan ultrasonography, as described in detail elsewhere,17 allowing estimates of choroidal thickness to be obtained with a resolution of ±21 μm.18 The A-scan ultrasonograph was not obtained until after experiment 1 was completed, and it was therefore not possible to determine axial dimensions of the eyes in experiment 1. However, A-scan ultrasonography data for the effects of glucagon-related agents on axial ocular dimensions in FDM will be reported in the accompanying article (Vessey KA, et al. 19).
Experiment 3: The Effects of Pretreatment with a Glucagon Receptor Antagonist on the Actions of Exogenous Glucagon on Refractive Error, Ocular Growth, and Choroidal Thickness
Animals
The Lilydale chicks ceased to be available after the completion of experiments 1 and 2. Thereafter, male White Leghorn chickens (Gallus gallus domesticus) were obtained from Clark Hy-Line Hatcheries (Brandon, Manitoba, Canada) within 48 hours of hatching (P2). In this new strain of chicks from Clark Hy-Line, preliminary experiments showed that daily intraocular injection of saline alone significantly inhibited the increase in axial elongation in form-deprived eyes compared with eyes of form-deprived animals that were not injected. Therefore, it was necessary to increase the interval between injections to 2 days, at which interval injection per se had a minimal effect.
Visual Manipulations and Injection Protocols
FD myopia was induced in the left (treated) eye of P7 chicks, and the right (control) eye was allowed unrestricted vision. Chicks were separated into one of four groups, and on P7, P9, P11, P13, and P15, they received a series of two intraocular injections (20 μL, injection volume) separated by an interval of 20 minutes. The interval between intraocular injections was instituted so that in the groups in which the antagonist was administered, the antagonist could reach and bind the glucagon receptor before administration of the agonist.20 Saline-treated chicks (group 1) received an intravitreal injection of saline into both the left (treated) eye and the right (control) eye, 20 minutes before a second injection of saline into each eye. Glucagon-treated chicks (group 2) received an intravitreal injection of saline into the treated and control eyes 20 minutes before administration of 20 μL glucagon (10−6 M stock in the syringe) to the treated eye and saline to the control eye. Glucagon receptor antagonist-treated chicks (group 3) received an intravitreal injection of des-Phe6-antagonist (10−4 M in the syringe) into the treated eye and saline into the control eye, 20 minutes before administration of saline to both the treated and control eyes. Glucagon receptor antagonist/glucagon-treated chicks (group 4) received an intravitreal injection of des-Phe6-antagonist (10−4 M in the syringe) to the treated eye and saline to the control eye 20 minutes before administration of glucagon (10−6M in the syringe) to the treated eye and saline to the control eye.
Ocular Measurements
Refractive error was measured by streak retinoscopy without cycloplegia to ±0.5 D. Changes in vitreous chamber depth and choroidal thickness were determined by high-resolution A-scan ultrasonography, and the final wet weight of the eyes was measured with an electronic balance.
Statistics
All experimental groups (agent and dose) comprised four to seven chicks, (i.e., 4–7 pairs of experimental and control eyes). Differences between values for the experimental and control eyes were compared statistically on computer (Prism 4.0; GraphPad Software Inc., San Diego, CA). Screening of the raw data for treated and control eyes separately using a multiple analysis of variance (ANOVA) with a Newman-Keuls posttest showed that the treatments in the experimental (left) eye had no significant effect on the contralateral control (right) eye, regardless of whether the left eye was treated with saline or a glucagon-related agent—that is, within any experiment, the data for control eyes in different treatment groups were statistically indistinguishable from one another. Therefore, for simplicity and clarity, results are expressed as the mean of the differences between data from the experimental (treated) and the contralateral (control) eyes, or the treated minus the control result in each animal. For each treatment, the treated – control (T – C) data were averaged and are expressed in the text, tables, and graphs as the mean ± SD.
For statistical analysis of the T – C data, when experimental groups of five or greater were used, parametric statistical analyses were completed after an initial test to confirm that the data were distributed in a Gaussian fashion. An ANOVA was used to compare three or more treatment groups for a given response parameter, and a Newman-Keuls posttest was used to identify which pairs of treatment-group data were significantly different. Where only two conditions were compared, differences were assessed with an unpaired two-tailed Student’s t-test. In the two experimental groups in which n = 4, the data could not be assumed to be normally distributed, and a nonparametric analysis (Mann-Whitney test) was used to compare the effects of the two different treatments. A difference between the mean/median of separate experimental conditions was considered to be significant at P < 0.05.
Results
Experiments 1 and 2: The Effect of Glucagon-Related Agents on Refractive Development, Ocular Growth, and Choroidal Thickness
The Effect of Glucagon Receptor Antagonists on Compensation to Plus-Lens–Induced Defocus
Exogenous glucagon receptor antagonists were expected to inhibit the development of plus-lens-induced hyperopia, by blocking the axial elongation-inhibiting actions of endogenous glucagon, which should be maximally secreted under this condition. Phe6-antagonist (10−5 M in the syringe) prevented the growth-inhibition caused by plus-lens wear (Table 2). The induction of hyperopic refractive error by +7-D lens wear in saline-treated eyes (T – C, +2.5 ± 0.6 D) was not only inhibited by treatment with Phe6-antagonist, but actually was supplanted by the development of mild myopia (T – C, +2.3 ± 1.0 D; n = 4; Mann-Whitney test, P = 0.03). Treatment with this antagonist also retarded the defocus-induced decreases in axial length as measured by electronic calipers (saline, −0.36 ± 0.2 mm; Phe6-antagonist, +0.8 ± 0.15 mm; n = 4; Mann-Whitney test, P = 0.03) and, although not significantly, wet weight (saline, −17 ± 15 mg; Phe6-antagonist +2 ± 9 mg; n = 4; Mann-Whitney test, P = 0.11). However, Phe6-antagonist had no effect on the increase in choroidal thickness otherwise observed in saline-treated eyes wearing +7-D lenses (261 ± 24 vs. 247 ± 35 μm, Phe6-antagonist vs. saline; n = 4; Mann-Whitney test, P = 0.67; Table 3).
Table 2.
The Effect of Glucagon Receptor Antagonists (10−5 M in the Syringe, Injected Intravitreally) or Saline on the Measurements of Chick Eyes Experiencing Myopic Defocus
|
Refraction (D) |
Wet Weight (mg) |
Axial Length (mm) |
|||||
|---|---|---|---|---|---|---|---|
| Drug (10−5M) | Treatment | Saline | Drug | Saline | Drug | Saline | Drug |
| des-Phe6 | Recovery (n =6) | −1.2 ± 1.6 | −6.2 ± 2.2 *P < 0.01 | 96 ± 28 | 67 ± 38 P = 0.16 | 0.80 ± 0.32 | 0.39 ± 0.24 *P = 0.03 |
| Phe6 | 37-D lens (n =4) | 2.5 ± 0.6 | −2.3 ± 1.0 *P = 0.03 | −17 ± 15 | 2 ± 9 P = 0.11 | −0.36 ± 0.2 | 0.08 ± 0.15 *P = 0.03 |
Data are presented as the mean interocular difference (treated – control eye) ± SD (standard deviation of the mean).
Significant difference between saline- and drug-treated animals: Student’s t-test for des-Phe6-antagonist (des-Phe6) treatment groups (n = 6), and a Mann-Whitney non-parametric test for Phe6-antagonist (Phe6) treatment groups (n = 4).
Table 3.
The Effects of Glucagon-Related Agents (10−5 M in the Syringe, Injected Intravitreally) or Saline on Choroidal Thickness in Chick Eyes
|
Choroidal Thickness (μm) (Treated – Control eye) |
||||
|---|---|---|---|---|
| Drug (10−5M) | Treatment | Saline | Drug | PValue |
| Glucagon | FD myopia (n = 6) | 62 ± 13 | 266 ± 34 | P < 0.0001* |
| des-Phe6 | Recovery (n = 6) | 361 ± 87 | 412 ± 90 | P = 0.34 |
| Phe6 | 3 7-D lens (n = 4) | 247 ± 35 | 261 ± 24 | P = 0.67 |
Data are presented as the mean interocular difference ± SD (standard deviation of the mean).
indicates a significant difference between saline- and drug-treated animals using a Student’s t-test for glucagon and des-Phe6-antagonist (des-Phe6) treatment groups, n = 6, and a Mann-Whitney nonparametric test for Phe6-antagonist (Phe6) treatment groups, n = 4.
The Effect of Glucagon Receptor Antagonists on Recovery from FD Myopia
Exogenous glucagon receptor antagonists were expected similarly to inhibit recovery from FD myopia by blocking the axial elongation-inhibiting actions of endogenous glucagon, which should be maximally secreted under this condition. The results of experiments using the antagonist des-Phe6-antagonist and the monoclonal antibody hGR2 provided only weak and inconsistent support for this hypothesis.
In eyes previously made myopic by form deprivation, daily intravitreal injection of des-Phe6-antagonist (10−5 M in the syringe), for 4 days after removing the goggle inhibited the recovery of emmetropic refraction (Table 2). In saline-treated eyes, refraction recovered from −9 D to emmetropia, but in eyes treated with des-Phe6-antagonist, refraction recovered only to −6 D (n = 6, Student’s t-test, P < 0.01). The basis of this effect was unclear, because both saline and des-Phe6-antagonist-treated eyes retained the same amount of excess weight (n = 6; Student’s t-test, P = 0.15), and the axial lengths of saline-treated recovering eyes (0.80 ± 0.32 mm), as measured by electronic calipers, were actually significantly longer than eyes treated with the antagonist (0.39 ± 0.24 mm; n = 6; Student’s t-test, P = 0.03). One possible explanation for the lack of correlation between refractive error and axial length is that this drug instead affected choroidal thickness, altering the depth of the vitreous chamber and thus refractive error. However, des-Phe6-antagonist (10−5 M in the syringe), administered for 5 days to a separate batch of animals, did not result in any reduction in the increased choroidal thickness otherwise recorded in saline-treated eyes (412 ± 90 vs. 361 ± 87 μm, des-Phe6-antagonist versus saline; n = 6; Student’s t-test, P = 0.34; Table 3).
Eyes recovering from FD myopia were also treated with the monoclonal antibody hGR2, which is an antagonist at the human and rat glucagon receptor, at 2.7 × 10−7, 2.7 × 10−5, and 1.35 × 10−4 M in the syringe. Even at the highest dose, this agent had no significant effect on the changes in refraction (n = 6; Student’s t-test, P = 0.66), wet weight (n = 6; Student’s t-test, P = 0.71), and axial length (n = 6; Student’s t-test, P = 0.86) that occurred during recovery (data not shown).
The Effect of Glucagon Receptor Agonists on FD Myopia
Glucagon receptor agonists were hypothesized to inhibit the development of FD myopia and were indeed found to have this effect. In our chicks, at this age, the normal rate of vitreal elongation in open control eyes, as determined by high-resolution A-scan ultrasonography, was ~60 μm/d. After 1 day, form deprivation increased the rate of vitreal elongation by ~80 μm/d, to a total of ~140 μm/d in form-deprived eyes (Ayotte AL, Stell WK, unpublished results, 2005).
Daily injection of glucagon (10−5 M in the syringe) for 5 days significantly inhibited the development of deprivation-induced myopic refraction of approximately −9 D observed in saline-treated FD eyes (Fig. 1a; n = 6; ANOVA with a Newman-Keuls posttest, e.g., saline versus glucagon 10−5M; P < 0.001). Glucagon also inhibited the excessive gain in wet weight (T – C) of approximately 50 mg (Fig. 1b; n = 6; ANOVA with a Newman-Keuls posttest, e.g., saline versus glucagon 10−5M; P < 0.05) and the excessive increase in total axial length of approximately 0.4 mm (as measured by electronic calipers) that are normally induced in saline-treated FD eyes, although not significantly (Fig. 1c; n = 6; ANOVA; P = 0.11). These effects of glucagon were concentration dependent (Fig. 1). Glucagon (10−5 M in the syringe, one dose only) was also found to induce choroidal thickening in FD eyes (266 ± 34 μm) compared with saline-treated FD eyes (62 ± 13 μm; n = 6; Student’s t-test, P < 0.0001; Table 3).
Figure 1.
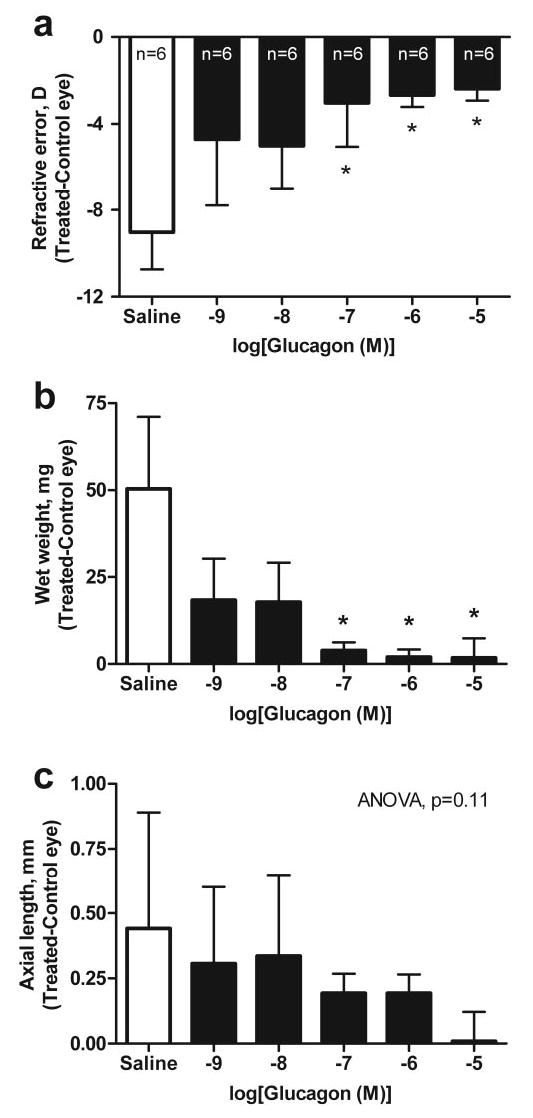
The effect of glucagon or saline on refractive error and ocular growth in the form-deprived chick eye. The effects of saline and increasing doses of glucagon (concentration is expressed as log(M) in 20 μL in the syringe) on (a) refractive error, (b) wet weight, and (c) axial length determined by caliper measurements in form-deprived eyes. Bars and error bars, mean interocular difference (T – C eye) ± SD, for n animals. *Effects of glucagon were significantly different from those of saline (ANOVA, Newman-Keuls posttest, P < 0.05).
The agonist [Lys17,18,Glu21]-glucagon-NH2, which is five to seven times as effective as glucagon at inducing cAMP in rat hepatocytes,23 had no effect on FD myopia in the chick at 10−9 to 10−7 M in the syringe (Fig. 2). At 10−7 and 10−6 M, however, [Lys17,18,Glu21]-glucagon-NH2 abruptly reversed the deprivation-induced ocular weight gain (Fig. 2b; n = 5, ANOVA with a Newman-Keuls posttest, e.g., saline versus glucagon 10−5M; P < 0.01) and myopic shift in refraction (Fig. 2a; n = 5; ANOVA with the Newman-Keuls posttest, P < 0.001), so that the treated form-deprived eyes were smaller and more hyperopic than the contralateral open eyes. Of interest, [Lys17,18,Glu21]-glucagon-NH2 did not significantly diminish the increase in axial length observed in saline-treated FD eyes (Fig. 2c; n = 5; ANOVA; P = 0.22). Given the discontinuity of the concentration–response function, it was not possible to determine the EC50 for this agent, but the transition from ineffective to fully effective concentrations for refractive error and wet weight occurred in the range of 10−7 to 10−6 M (in the syringe).
Figure 2.
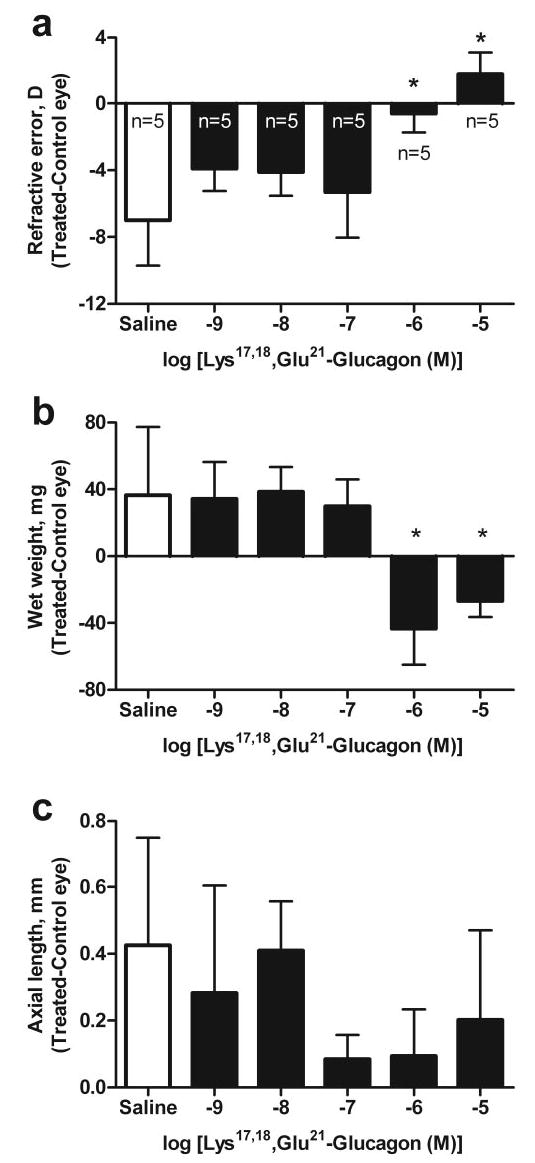
The effect of Lys17,18,Glu21-glucagon (SA) or saline on refractive error and ocular growth in the form-deprived chick eye. The effects of saline and increasing doses of Lys17,18,Glu21-glucagon- NH2 (concentration is expressed as log(M) in 20 μL in the syringe) on (a) refractive error, (b) wet weight, and (c) axial length determined by caliper measurements in form-deprived eyes. Bars and error bars represent the mean interocular difference (T – C eye) ± SD of the mean, for n animals. *Effects of Lys17,18,Glu21-glucagon- NH2 were significantly different from those of saline (ANOVA, Newman-Keuls post test, P < 0.05).
Effect of Glucagon-Related Agents on Growth of Eyes with Unrestricted Vision
The glucagon receptor antagonist des-Phe6-antagonist had no effect on refractive error (0.2 ± 0.4 D; n = 6; Student’s t-test, P = 0.62; Fig. 3), wet weight (1.1 ± 10.2 mg; n = 6; Student’s t-test, P = 0.88), or axial length (0.02 ± 0.18 mm; n = 6; Student’s t-test, P = 0.51) of eyes with unrestricted vision compared with saline-treated open eyes. Similarly, in eyes with unrestricted vision, glucagon (10−5 M in the syringe) caused only a small and nonsignificant hyperopic shift in refraction, from an interocular difference of +0.13 ± 0.2 D in saline-treated open eyes to +0.4 ± 0.9 D in glucagon-treated eyes (n = 6 each; Student’s t-test, P = 0.39; Fig. 3). Glucagon also did not significantly affect the wet weight (saline 3.9 ± 3.0 mg versus glucagon 1.8 ± 14 mg; Student’s t-test, P = 0.99) or axial length (saline 0.01 ± 0.13 mm versus glucagon −0.02 ± 0.16 mm; Student’s t-test, P = 0.71) of eyes with unrestricted vision.
Figure 3.
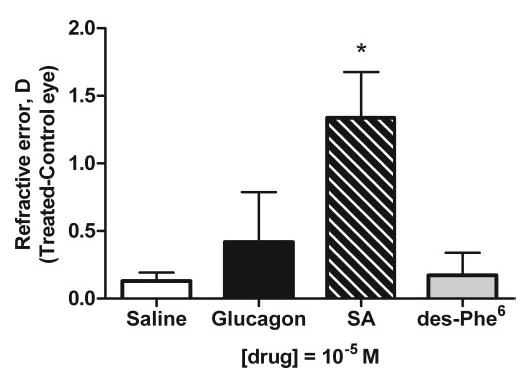
The effect of glucagon-related agents on refractive error of eyes with unrestricted vision. The effects of daily injections for five consecutive days of saline, glucagon, Lys17,18,Glu21-glucagon-NH2 (SA), and des-Phe6-antagonist (des-Phe6) at a concentration of 10−5 M in 20 μL in the syringe on refractive error. Bars and error bars represent the mean interocular difference (T – C) ± SD, for n = 6 animals. *Effects of Lys17,18,Glu21-glucagon-NH2 (SA) on refractive error were significantly different from those in the saline group (Student’s t-test, P < 0.05).
In eyes with unrestricted vision, as in FD eyes, the agonist, [Lys17,18,Glu21]-glucagon-NH2 (10−5 M in the syringe) caused a small but significant hyperopic shift of +1.3 ± 0.8 D (n = 6; Student’s t-test, P = 0.01; Fig. 3). However, [Lys17,18,Glu21]-glucagon-NH2 had no significant effect on wet weight (−14.5 ± 20.2 mg; n = 6, Student’s t-test, P = 0.08) or axial length (0.33 ± 0.25 mm; n = 6, Student’s t-test, P = 0.13) compared with saline-treated open eyes.
Alterations to Rod Photoreceptors in Retinas Treated with [Lys17,18,Glu21]-Glucagon-NH2
The discontinuous dose–response function and the induction of hyperopia in most eyes treated with the highest doses of the agonist analogue [Lys17,18,Glu2]-glucagon-NH2, but not glucagon or the antagonist, suggested the possibility of toxicity. Histologic study revealed changes in the photoreceptors, suggesting a nonpharmacological basis for restriction of growth by [Lys17,18,Glu21]-glucagon-NH2 in both form-deprived eyes and eyes with unrestricted vision (Fig. 4). No alteration in structure was apparent in the toluidine-blue-stained sections of [Lys17,18,Glu21]-glucagon-NH2-treated retinas (Figs. 4b1, 4d1). Immunocytochemical labeling for rhodopsin, however, showed a consistent and highly significant association between treatment with this peptide and abnormalities in rods, signified by whole-cell rhodopsin labeling of rods in treated retinas (Figs. 4b2, 4d2). Treatment was often associated with swelling, shortening, and disruption of rod outer segments as well. Sometimes the retinal pigment epithelium also appeared ragged or disrupted in treated eyes (Fig. 4b1). The incidence of rod abnormalities was substantial in all retinas from treated eyes, whether form deprived (mean = 21.2 abnormal rods/microscope field) or open (mean = 13.5 abnormal rods/field). In contrast, virtually no altered rods were present in saline-treated retinas (Figs. 4a2, 4c2), whether form deprived or open. Whole-cell rod labeling was observed only rarely in eight saline-treated open eyes (mean = 0.1 abnormal rods/field) and none at all in three saline-treated FD eyes.
Figure 4.
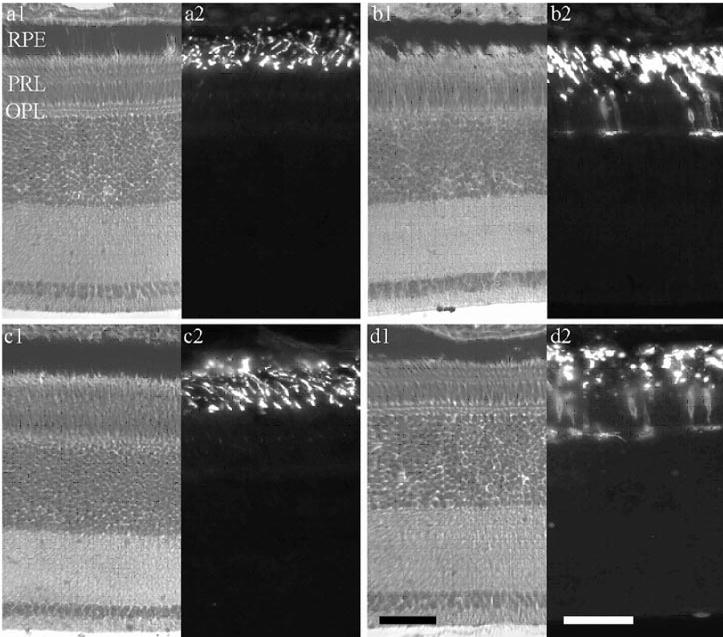
High concentrations of [Lys17,18,Glu21]-glucagon-NH2 alter rods in the chick retina. Micrographs of retinas from eyes, open or form deprived, treated with saline or a dose of [Lys17,18,Glu21]-glucagon-NH2 that significantly reduced ocular growth and caused a hyperopic shift in refraction (10−5 M in the syringe, five daily injections). Each pair shows (left) histologic stain with toluidine blue and (right) darkfield immunofluorescence for rhodopsin. (a) Open, saline; (b) open [Lys17,18,Glu21]-glucagon-NH2; (c) form-deprived, saline; and (d) form-deprived, [Lys17,18,Glu21]-glucagon-NH2. RPE, retinal pigment epithelium; PRL, photoreceptor layer. Scale bar, 50 μm (d1 holds also for a1, b1, c1; d2 holds for a2, b2, c2).
Experiment 3: The Effects of Pretreatment with a Glucagon Receptor Antagonist on the Actions of Exogenous Glucagon on Refractive Error, Ocular Growth, and Choroidal Thickness
To further elucidate the actions of glucagon and to determine whether the glucagon receptor mediates a “stop” signal, the glucagon receptor antagonist des-Phe6-antagonist was tested for its ability to inhibit the effects of exogenous glucagon administration on the development of FD myopia. As previously shown, injection of glucagon (10−6 M in the syringe) into form-deprived eyes every second day suppressed the development of myopic refractive error (−5.1 ± 2.5 vs. −17.2 ± 2.1 D, saline [SAL]/glucagon [GLUC] versus SAL/SAL; n = 7; ANOVA with the Newman-Keuls posttest, P < 0.001; Fig. 5a) and excessive eye weight (71 ± 35 vs. 140 ± 51 mg, SAL/GLUC versus SAL/SAL; n = 7; ANOVA with a Newman-Keuls posttest, P < 0.001; Fig. 5b) seen in saline-treated FD eyes. Glucagon induced significant choroidal thickening (n = 7; ANOVA with a Newman-Keuls posttest, P < 0.01; Fig. 5c) and also a decrease in vitreous elongation (n = 7; ANOVA with the Newman-Keuls posttest, P < 0.001; Fig. 5d) compared with saline-injected FD controls; effects on anterior chamber depth and the thickness of lens and retina were not statistically significant (data not shown). Des-Phe6-antagonist (10−4 M in the syringe, 100× the concentration of glucagon), by itself, significantly lessened the deprivation-induced increase in ocular wet weight, although the effects of this drug on all other parameters were not statistically significant. Injection of des-Phe6-antagonist 20 minutes before glucagon, to achieve optimal antagonism, had no significant inhibitory effect on the actions of glucagon on refractive error or vitreous chamber depth (Figs. 5a, 5c; n = 7; ANOVA with the Newman-Keuls posttest, comparing ANT/SAL versus ANT/GLUC, P < 0.05 for refractive error and vitreous chamber depth). Only the action of the antagonist on the choroidal-thickening induced by glucagon was not decisive. Glucagon induced choroidal thickening in FD eyes compared with saline-treated FD eyes, and administration of the antagonist induced no change in choroidal thickness compared with that in saline-treated FD eyes; however, preadministration of des-Phe6-antagonist followed by glucagon did not significantly affect choroidal thickness compared with treatment with glucagon alone or des-Phe6-antagonist alone (n = 7; ANOVA with the Newman-Keuls posttest, P > 0.05 for both SAL/GLUC versus ANT/GLUC and ANT/SAL versus ANT/GLUC).
Figure 5.
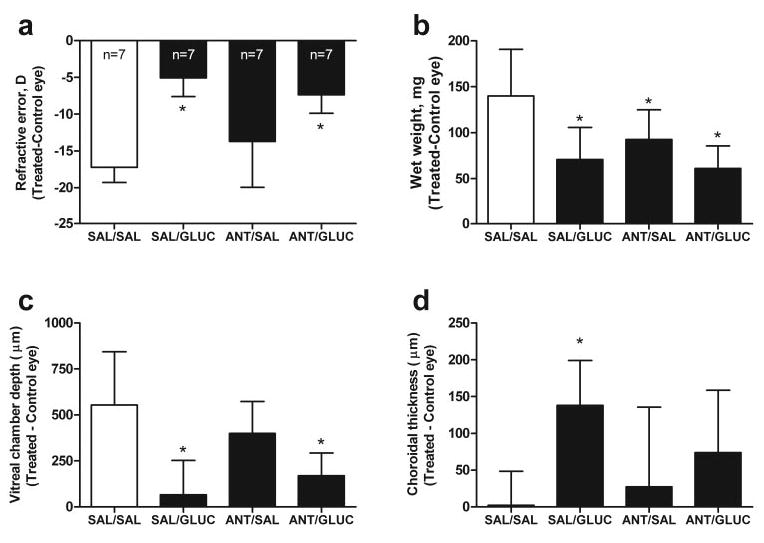
The effects of pretreatment with a glucagon receptor antagonist on the actions of exogenous glucagon on refractive error, ocular growth, and choroidal thickness. Form-deprived experimental eyes were injected with (1) saline both injections (SAL/SAL); (2) saline followed by glucagon (10−6 M in the syringe (SAL/GLUC); (3) des-Phe6-antagonist (10−4 M in the syringe) followed by saline (ANT/SAL); and (4) des-Phe6-antagonist, followed by glucagon (ANT/GLUC). Bars and error bars, mean interocular difference (T – C eye) ± SD of the mean for (a) refractive error, (b) ocular wet weight, (c) vitreous chamber depth, and (d) choroidal thickness for (n) animals. *Effects of a treatment were significantly different from the SAL/SAL group (ANOVA, Newman-Keuls post test, P < 0.05).
Discussion
We found that exogenous glucagon acted as predicted, restraining ocular growth and inducing choroidal thickening in form-deprived eyes. The glucagon receptor antagonist Phe6-antagonist inhibited changes in both refractive error and axial length compensation induced by +7-D lens wear, but had no effect on the choroidal thickening induced by plus defocus. These results are consistent with a role for an increase in endogenous glucagon release in inhibiting excessive eye growth and myopia in the chick, so that, conversely, when the actions of glucagon are inhibited, myopia is induced. The other antagonist, des-Phe6-antagonist (10−5 M), however, had weak and inconsistent effects and did not antagonize the action of exogenous glucagon. The lack of effect of des-Phe6-antagonist may be due to its 32-fold lesser affinity for the glucagon receptor than for glucagon itself, as determined in the mammalian system24; however, further experiments are needed to confirm this lesser affinity in the chick.
Effects of Glucagon Receptor Antagonists on Compensation for Plus-Defocus–Induced Changes in Refractive Error and Ocular Growth
If glucagon is synthesized in and released from glucagon amacrine cells in response to plus defocus, then it should act on targets in the interior of the eye to compensate for plus defocus. Thus, when the rate of release of endogenous glucagon is high, as it is likely to be under plus defocus, antagonists should block the growth-inhibiting and choroid-thickening actions of endogenous glucagon.
The glucagon receptor antagonist Phe6-antagonist (10−5 M in the syringe) inhibited changes in both refractive error and axial length compensation induced by +7-D lens wear. This effect might be expected if glucagon is released during plus defocus and is consistent with previous findings that the same antagonist inhibits refractive error compensation to plus lens wear.12 The previous results were obtained using doses of the antagonist, 0.01 to 0.25 nanomoles injected, that were similar to the 0.2 nanomoles injected in the present study.12 In another study, the Phe6-antagonist (1.4 × 10−5 M in 20 μL) was also found to reduce the inhibition of ocular elongation observed during +7-D lens wear, although refractive error was not measured (Zhu X, et al. IOVS 2001;42:ARVO Abstract 318). In that study and in the present study, the Phe6-antagonist did not affect choroidal thickness, which was otherwise enlarged by plus defocus. In general, the actions of Phe6-antagonist on refraction and ocular growth, with the exception of choroidal thickness, were consistent with the hypothesis that endogenous glucagon mediates a plus defocus signal.
The effects of the other antagonists, des-Phe6-antagonist and the monoclonal antibody hGR2, on plus defocus experienced during recovery from FD myopia were less supportive of a role for endogenous glucagon in compensation to plus defocus. Furthermore, even in 100-fold molar excess, the des-Phe6-antagonist was unable to block the effects of exogenous glucagon on FD myopia. Further experiments are necessary to explain definitively why these antagonist effects were so small and inconsistent, whereas the agonist effects were both dramatic and robust. There are several possible explanations: (1) Both the antagonist des-Phe6-antagonist and the antibody hGR2 may act weakly at chicken glucagon receptors. Indeed, des-Phe6-antagonist has 13-fold lesser affinity for the mammalian glucagon receptor than does Phe6-antagonist and 32-fold lesser affinity for the receptor than does glucagon itself.24 Furthermore, unlike mammalian glucagon, the glucagon receptor antagonists used in this study have not been tested previously on chicken receptors for glucagon and related peptides, and cross-species activity cannot be assumed. (2) Antagonist action may have been too brief to counteract the defocus–compensation mechanism(s) effectively. Whereas the bioavailability of these peptides has not been determined, it is likely to be cut short by, for example, binding, internalization, degradation by enzymes, and diffusion away from the retina within minutes to hours after injection, as occurs with the dopamine receptor antagonist, spiperone.25 (3) Glucagon may activate a receptor other than the glucagon receptor, and the glucagon antagonists may be unable to block its action. Glucagon is derived from the peptide proglucagon, which can be cleaved to produce many other bioactive peptides, each with a specific receptor.9 Glucagon may be activating a related receptor from the same family of proglucagon-derived peptide receptors, although with much lower affinity than the endogenous ligand. This explanation is rendered unlikely, however, by the inability of glucagon/secretin-related peptides to prevent FD myopia, as reported in the accompanying paper (Vessey KA, et al.19). (4) Alternatively, glucagon could activate plus defocus compensation through receptors coupled to transduction cascades not involving adenylate cyclase/cAMP signaling.26 This seems to be a plausible explanation, because the antagonists tested so far appear to be selective for the adenylylcyclase–coupled receptors and may even be agonists at receptors coupled to other transduction pathways.23,27 Further experiments, beyond the scope of the present study, are necessary to test these hypotheses.
Effect of Glucagon on the Development of FD Myopia
Previous studies have shown that glucagon-immunoreactive amacrine cells in the chick retina respond to plus defocus and unrestricted vision by increasing the expression of the immediate-early gene product, ZENK (Zif268, Egr-1).7,11 This suggests that the activation of glucagonergic amacrine cells by these optical conditions could result in an increase in neurotransmitter release, thereby mediating the compensatory changes in ocular growth and refraction that defocus is known to induce. The results of this study are consistent with this hypothesis, showing that administration of glucagon inhibits the excessive ocular axial elongation, wet weight gain, and myopia in form-deprived eyes and stimulates choroidal thickening, thus mimicking the actions of plus defocus on ocular growth and refraction. The significant differences in wet weight between saline- and glucagon-treated form-deprived eyes, in the absence of statistically significant axial length differences (in experiment 1) may be due in part to the use of different measurement techniques. The wet weight results, obtained using an electronic balance, were much less variable than the axial length results, obtained using electronic calipers, although a trend toward a decrease in axial length with glucagon concentration was also observed (ANOVA, P = 0.11). Furthermore, in experiment 3, glucagon was found to inhibit vitreal and axial elongation as measured using A-scan ultrasonography, in agreement with the results of a previous study showing that glucagon inhibits axial elongation in chick eyes reared with negative lenses.12 Finally, recent experiments in our laboratory (Beloukhina N, et al. IOVS 2005;46:ARVO E-Abstract 3337) have shown that glucagon is a potent inhibitor of equatorial expansion in the chick eye. This action of glucagon, which was not assessed in the present study, may explain instances in which glucagon inhibits weight gain more than axial elongation. Thus, the findings of this study, that glucagon inhibits the development of FD myopia, are consistent with the hypothesis that endogenous glucagon may be a signal for the inhibition of ocular growth during plus defocus.
In open eyes (normal, unrestricted vision), the retina receives in-focus and defocused images, and glucagon amacrine cells are partially activated.7 In this condition, the effects of the exogenous glucagon would be superimposed on the effects of endogenous glucagon and therefore might be expected to be variable and small, and this is what we observed. Furthermore, many retinal pathways and messenger systems besides those via glucagonergic amacrine cells are likely to be activated during unrestricted viewing, some of which could inhibit the plus-defocus–induced release or action of the glucagon-related messenger(s). Such activation would be desirable, for example, to maintain emmetropia by preventing the plus-defocus messengers from further inhibiting eye growth and thus making the eye hyperopic.
Anomalous Action of the Glucagon Receptor Agonist [Lys17,18,Glu21]-Glucagon-NH2 on the Development of FD Myopia and the Cellular Integrity of the Chick Retina
The glucagon receptor agonist [Lys17,18,Glu21]-glucagon-NH2 produced effects on the development of FD myopia similar to those reported previously in chicks wearing +7-D lenses.12 In the present study, [Lys17,18,Glu21]-glucagon-NH2 at concentrations of 10−9 to 10−7 M in 20 μL in the syringe had no effect on refractive error, axial length, or wet weight of treated eyes. However at 10−6 and 10−5 M, [Lys17,18,Glu21]-glucagon-NH2 reduced the growth rate of treated eyes below that in control eyes, causing a hyperopic shift in refraction and a hyperinhibition of axial elongation of the eye. This induction of hyperopia was also seen in open eyes (unrestricted vision) that were treated with [Lys17,18,Glu21]-glucagon-NH2 (10−5 M). At the highest dose (10−5 M) alterations to the rod photoreceptors and retinal pigmented epithelium (RPE) were observed. Immunolabeling for rhodopsin, a protein that is usually expressed only in the outer segments of the rod photoreceptor, was found throughout the cell bodies of many rods in [Lys17,18,Glu21]-glucagon-NH2-treated retinas. This aberrant labeling of rhodopsin protein throughout the photoreceptor has been shown to occur during retinal disease.28 Furthermore, the rod outer segments of the tissues treated with [Lys17,18,Glu21]-glucagon-NH2 displayed swelling and shortening, which can be indicative of disease. Because damage to the photoreceptors and RPE is known to cause growth-inhibition and hyperopia,21 the effects of [Lys17,18,Glu21]-glucagon-NH2 could be due to a toxic rather than a physiologic mechanism, even though toxicity of this peptide has not been reported previously and it is not obvious why it should have this effect.
Possible Relevance to Plus-Defocus Compensation in Mammals
Plus-defocus compensation is known to occur, not only in chicks, but also in mammalian experimental models in macaque monkey29 and marmoset,30 among others.31 Visual modulation of eye growth has also been reported recently in the mouse (Schaeffel F, et al. IOVS 2004;45:ARVO E-Abstract 182). Could the same mechanisms be responsible for defocus compensation in mammals as in chicks?
In principle, the conservation of mechanisms for defocus compensation is likely. In any animal that is dependent on high-quality visual information for survival, vitreous chamber depth must be matched precisely to focal length. One possible implication of the results reported herein, therefore, would be that glucagon amacrine cells also mediate plus-defocus compensation in mammals. In support of this notion, the same early-response transcription factor (Egr-1) that is induced by plus defocus in glucagon amacrine cells in the chick7 has been reported to be induced also by defocus in macaque32 and tree shrew (Stell WK, et al. IOVS 2004;45:ARVO E-Abstract 1159). The cells in which Egr-1 is induced in mammalian retinas, however, are not known to produce glucagon. Except in one study that lacked appropriate immunocytochemical controls,33 mammalian retinas have been reported to contain little or no glucagon.34 Furthermore, whereas high-affinity glucagon binding sites have been detected in rat retina,35 glucagon has not been found to be effective at stimulating cAMP production in rabbit retina.36 In contrast, vasoactive intestinal peptide (VIP) has been identified in mammalian retinas by immunoassay and immunocytochemistry37 and stimulates cAMP synthesis with a submicromolar EC50 in rabbit retinas.36 Perhaps VIP and its receptor, which are related to (but distinct from) glucagon and its receptor, mediate plus defocus compensation in mammalian retinas, as some have proposed.38
Summary and Conclusions
We have shown in the chick that glucagon significantly inhibits the development of FD myopia and causes the choroid of treated eyes to thicken. Furthermore, Phe6-antagonist inhibited the development of hyperopia in eyes treated with plus lenses. This suggests that exogenous and endogenous glucagon may contribute to visual regulation of the refractive state, reducing axial length by restraining scleral growth and stimulating choroidal thickening. The failure of the other receptor antagonists to counteract consistently the effects of either plus defocus or exogenous glucagon, however, requires further research.
Footnotes
Disclosure: K.A. Vessey, None; K.A. Lencses, None; D.A. Rush-forth, None; V.J. Hruby, None; W.K. Stell, None
Supported by National Eye Institute Grant R01EY13187 and the Canadian Institutes of Health Research (WKS).
References
- 1.Stone RA, Lin T, Laties AM, Iuvone PM. Retinal dopamine and form-deprivation myopia. Proc Natl Acad Sci USA. 1989;86:704–706. doi: 10.1073/pnas.86.2.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stone RA, Lin T, Laties AM. Muscarinic antagonist effects on experimental chick myopia. Exp Eye Res. 1991;52:755–758. doi: 10.1016/0014-4835(91)90027-c. [DOI] [PubMed] [Google Scholar]
- 3.Rohrer B, Stell WK. Basic fibroblast growth factor (bFGF) and transforming growth factor beta (TGF-beta) act as stop and go signals to modulate postnatal ocular growth in the chick. Exp Eye Res. 1994;58:553–561. doi: 10.1006/exer.1994.1049. [DOI] [PubMed] [Google Scholar]
- 4.Seltner RL, Stell WK. The effect of vasoactive intestinal peptide on development of form deprivation myopia in the chick: a pharmacological and immunocytochemical study. Vision Res. 1995;35:1265–1270. doi: 10.1016/0042-6989(94)00244-g. [DOI] [PubMed] [Google Scholar]
- 5.Fujikado T, Hosohata J, Omoto T. ERG of form deprivation myopia and drug induced ametropia in chicks. Curr Eye Res. 1995:79–86. doi: 10.3109/02713689609017614. [DOI] [PubMed] [Google Scholar]
- 6.Buck C, Schaeffel F, Simon P, Feldkaemper M. Effects of positive and negative lens treatment on retinal and choroidal glucagon and glucagon receptor mRNA levels in the chicken. Invest Ophthalmol Vis Sci. 2004;45:402–409. doi: 10.1167/iovs.03-0789. [DOI] [PubMed] [Google Scholar]
- 7.Fischer AJ, McGuire JJ, Schaeffel F, Stell WK. Light- and focus-dependent expression of the transcription factor ZENK in the chick retina. Nat Neurosci. 1999;2:706–712. doi: 10.1038/11167. [DOI] [PubMed] [Google Scholar]
- 8.Drucker DJ, Asa S. Glucagon gene expression in vertebrate brain. J Biol Chem. 1988;263:13475–13478. [PubMed] [Google Scholar]
- 9.Mayo KE, Miller LJ, Bataille D, et al. International union of pharmacology. XXXV. The glucagon receptor family. Pharmacol Rev. 2003;55:167–194. doi: 10.1124/pr.55.1.6. [DOI] [PubMed] [Google Scholar]
- 10.Eldred WD, Ammermuller J, Schechner J, Behrens UD, Weiler R. Quantitative anatomy, synaptic connectivity and physiology of amacrine cells with glucagon-like immunoreactivity in the turtle retina. J Neurocytol. 1996;25:347–364. doi: 10.1007/BF02284807. [DOI] [PubMed] [Google Scholar]
- 11.Bitzer M, Schaeffel F. Defocus-induced changes in ZENK expression in the chicken retina. Invest Ophthalmol Vis Sci. 2002;43:246–252. [PubMed] [Google Scholar]
- 12.Feldkaemper MP, Schaeffel F. Evidence for a potential role of glucagon during eye growth regulation in chicks. Vis Neurosci. 2002;19:755–766. doi: 10.1017/s0952523802196064. [DOI] [PubMed] [Google Scholar]
- 13.Lencses KA. Glucagon Amacrine Cells Regulate Ocular Growth and Refraction in Chick. University of Calgary, 2002. MSc thesis.
- 14.Stell WK, Lencses KA, Ahn J-M, Hruby VJ. Glucagon-synthesizing amacrine cells mediate choroidal compensation for plus-defocus in the chick eye (abstract) Eur J Neurosci. 2000;12 (suppl 11):487. [Google Scholar]
- 15.Skovgaard RN. Monoclonal Antibody to Human Glucagon Receptor: a Glucagon-Receptor Antagonist Copenhagen; Novo Nordisk; 1996. PhD Thesis.
- 16.Glickstein M, Millodot M. Retinoscopy and eye size. Science. 1970;168:605–606. doi: 10.1126/science.168.3931.605. [DOI] [PubMed] [Google Scholar]
- 17.Luft WA, Ming Y, Stell WK. Variable effects of previously untested muscarinic receptor antagonists on experimental myopia. Invest Ophthalmol Vis Sci. 2003;44:1330–1338. doi: 10.1167/iovs.02-0796. [DOI] [PubMed] [Google Scholar]
- 18.Nickla DL, Wildsoet C, Wallman J. Visual influences on diurnal rhythms in ocular length and choroidal thickness in chick eyes. Exp Eye Res. 1998;66:163–181. doi: 10.1006/exer.1997.0420. [DOI] [PubMed] [Google Scholar]
- 19.Vessey KA, Rushforth DA, Stell WK. Glucagon- and secretin-related peptides differentially alter ocular growth and the development of form-deprivation myopia in chicks. Invest Ophthalmol Vis Sci. 2005;46:3932–3942. doi: 10.1167/iovs.04-1027. [DOI] [PubMed] [Google Scholar]
- 20.Chan WY, Rockway TW, Hruby VJ. Long-acting oxytocin antagonists: effects of 2-D-stereoisomers substitution on antagonist potency and duration of action. Proc Soc Exp Biol Med. 1987;185:187–192. doi: 10.3181/00379727-185-42533. [DOI] [PubMed] [Google Scholar]
- 21.Westbrook AM, Crewther SG, Liang H, et al. Formoguanamine-induced inhibition of deprivation myopia in chick is accompanied by choroidal thinning while retinal function is retained. Vision Res. 1995;35:2075–2088. doi: 10.1016/0042-6989(94)00282-q. [DOI] [PubMed] [Google Scholar]
- 22.Fischer AJ, Seltner RL, Poon J, Stell WK. Immunocytochemical characterization of quisqualic acid- and N-methyl-D-aspartate-induced excitotoxicity in the retina of chicks. J Comp Neurol. 1998;393:1–15. [PubMed] [Google Scholar]
- 23.Azizeh BY, Van Tine BA, Trivedi D, Hruby VJ. Pure glucagon antagonists: biological activities and cAMP accumulation using phosphodiesterase inhibitors. Peptides. 1997;18:633–641. doi: 10.1016/s0196-9781(97)00131-9. [DOI] [PubMed] [Google Scholar]
- 24.Van Tine BA, Azizeh BY, Trivedi D, et al. Low level cyclic adenosine 3′,5′-monophosphate accumulation analysis of [des-His1, des-Phe6, Glu9] glucagon-NH2 identifies glucagon antagonists from weak partial agonists/antagonist. Endocrinology. 1996;137:3316–3322. doi: 10.1210/endo.137.8.8754757. [DOI] [PubMed] [Google Scholar]
- 25.Rohrer B, Spira AW, Stell WK. Apomorphine blocks form-deprivation myopia in chickens by a dopamine D2-receptor mechanism acting in retina or pigmented epithelium. Vis Neurosci. 1993;10:447–453. doi: 10.1017/s0952523800004673. [DOI] [PubMed] [Google Scholar]
- 26.Wakelam MJ, Murphy GJ, Hruby VJ, Houslay MD. Activation of two signal-transduction systems in hepatocytes by glucagon. Nature. 1986;323:68–71. doi: 10.1038/323068a0. [DOI] [PubMed] [Google Scholar]
- 27.Unson CG, Gurzenda EM, Merrifield RB. Biological activities of des-His1[Glu9]glucagon amide, a glucagon antagonist. Peptides. 1989;10:1171–1177. doi: 10.1016/0196-9781(89)90010-7. [DOI] [PubMed] [Google Scholar]
- 28.Sethi CS, Lewis GP, Fisher SK, et al. Glial remodeling and neural plasticity in human retinal detachment with proliferative vitreo-retinopathy. Invest Ophthalmol Vis Sci. 2005;46:329–335. doi: 10.1167/iovs.03-0518. [DOI] [PubMed] [Google Scholar]
- 29.Hung LF, Crawford ML, Smith EL. Spectacle lenses alter eye growth and the refractive status of young monkeys. Nat Med. 1995;1:761–765. doi: 10.1038/nm0895-761. [DOI] [PubMed] [Google Scholar]
- 30.Troilo D, Judge SJ. Ocular development and visual deprivation myopia in the common marmoset (Callithrix jacchus) Vision Res. 1993;33:1311–1324. doi: 10.1016/0042-6989(93)90039-y. [DOI] [PubMed] [Google Scholar]
- 31.Edwards MH. Animal models of myopia: a review. Acta Ophthalmol Scand. 1996;74:213–219. doi: 10.1111/j.1600-0420.1996.tb00078.x. [DOI] [PubMed] [Google Scholar]
- 32.Zhong XW, Ge J, Smith EL, III, Stell WK. Image defocus modulates activity of bipolar and amacrine cells in macaque retina. Invest Ophthalmol Vis Sci. 2004;45:2065–2074. doi: 10.1167/iovs.03-1046. [DOI] [PubMed] [Google Scholar]
- 33.Das A, Pansky B, Budd GC. Glucagon-like immunoreactivity in mouse and rat retina. Neurosci Lett. 1985;60:215–218. doi: 10.1016/0304-3940(85)90246-0. [DOI] [PubMed] [Google Scholar]
- 34.Tornqvist K, Ehinger B. Glucagon immunoreactive neurons in the retina of different species. Graefes Arch Clin Exp Ophthalmol. 1983;220:1–5. doi: 10.1007/BF02307008. [DOI] [PubMed] [Google Scholar]
- 35.Fernandez-Durango R, Sanchez D, Fernandez-Cruz A. Identification of glucagon receptors in rat retina. J Neurochem. 1990;54:1233–1237. doi: 10.1111/j.1471-4159.1990.tb01953.x. [DOI] [PubMed] [Google Scholar]
- 36.Schorderet M, Hof P, Magistretti PJ. The effects of VIP on cyclic AMP and glycogen levels in vertebrate retina. Peptides. 1984;5:295–298. doi: 10.1016/0196-9781(84)90222-5. [DOI] [PubMed] [Google Scholar]
- 37.Ekman R, Tornqvist K. Glucagon and VIP in the retina. Invest Ophthalmol Vis Sci. 1985;26:1405–1409. [PubMed] [Google Scholar]
- 38.Stone RA, Laties AM, Raviola E, Wiesel TN. Increase in retinal vasoactive intestinal polypeptide after eyelid fusion in primates. Proc Natl Acad Sci USA. 1988;85:257–260. doi: 10.1073/pnas.85.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]


