Abstract
Sucralose is a non-nutritive halogenated sucrose derivative that has been described by humans as tasting predominately sweet with little or no aftertaste. In this study we examined the preference for sucralose in adult male Sprague Dawley rats. A standard 24 hr two-bottle test was used to compare a wide range of sucralose concentrations (0.0003–10g/L; 0.8 μM–25 mM) with water. The rats did not prefer sucralose to water at low concentrations (0.0003–0.3 g/L) and avoided sucralose at high concentrations (1–10g/L). Although there are many similarities in the taste preference of humans, mice, and rats, these results suggest that male rats do not prefer sucralose and avoid it at high concentrations. An awareness of the potential species differences in preference testing for novel sweeteners is critical for the taste and nutritional research communities.
Keywords: taste, preference test, species differences, rat, Splenda
1. Introduction
In 1998, the FDA approved sucralose for use in United States as a “no calorie” artificial sweetener. Unlike other artificial sweeteners, this compound is derived from and structurally similar to sucrose with a taste profile as described by humans as remarkably similar to sucrose [1]. The important structural differences between the two compounds are three chlorine atoms that replace the hydroxyl groups on the sucrose molecule to form sucralose (1′,6′-dichloro-1′,6′-dideoxy β-d-fructofuranosyl-4′-chloro-4′-deoxy-α-d-galactopyranoside). The chlorination stabilizes the sucralose molecule, which prevents it from being degraded or metabolized [2, 3] and makes it intensely (~600 times) sweeter than sucrose on a per weight basis [1]. Likewise, it has been shown to be a safe sugar alternative for diabetics [4, 5] and has been manufactured for general consumption as the primary sweetening agent in Splenda ®. Sucralose also has been shown to activate cells transfected with the rat sweet taste receptor [6]. Despite these qualities, published studies of the preference for sucralose have been mostly limited to humans subjects [1, 7, 8] pigs [9] and mice [10,11].
In a study that investigated the preference for sweet compounds compared with water in two inbred strains of mice Bachmanov and colleagues [10] demonstrated four typical patterns of preference, which consisted of either indifference–preference, indifference–preference–avoidance, indifference–avoidance, or only indifference. The purpose of this study was to determine the pattern of preference for sucralose in male rats. This was done by using a series of two-bottle tests that compared a wide range of sucralose concentrations, 0.0003 to 10 g/L, with water. Adult male Sprague Dawley rats were chosen as an animal model, since they are one of the most common experimental subjects used by taste and nutritional sciences laboratories. Recently and independent of this study, Sclafani and Clare (2004) investigated the preference for sucralose to water in female Sprague Dawley rats using a range of sucralose concentrations from 0.25 to 4 g/L [12].
2. Materials and Methods
2.1 Animals
Thirteen naive male Sprague Dawley rats (Charles River, Wilmington, MA), with an initial weight of 475–525 g were individually housed and placed on a 12/12 h light-dark schedule (lights on at 0700h). Rats received ad libitum standard laboratory chow (Global Diet-2018, Harlan Teklad, Madison, WI) and a bottle of filtered (5 μm filtration) tap water at the front of their cages, unless otherwise noted. Animal protocols were approved by the Institutional Animal Care and Use Committee of the Pennsylvania State University, College of Medicine, and were in accordance with NIH guidelines.
2.2 Experimental design
All rats received one bottle containing water and the other bottle containing one concentration of sucralose (MW 397.64; McNeil Specialty, New Brunswick, NJ). The range of concentration tested was 0.0003–10 g/L (0.0008–25 mM) with each concentration increasing by half-log steps. Each sucralose concentration was prepared with filtered tap water and testing for 48 hr. The bottles were switched every 24hr to control for possible side preference. [10,11].
2.3 Statistical analysis
Intakes in ml were averaged for both days expressed as mean ± SEM per 24 hr. Percent preference was expressed by taking the volume of sucralose divided by the total volume of fluid (sucralose and water) multiplied by 100. The intakes of sucralose and water were analyzed by dependent t-test. In that case, significance was set at α = 0.005, since we applied Bonferroni correction (0.05/10 t-test). Percent preference was analyzed by one-way ANOVA with repeated measures and post-hoc Neuman-Keuls. All statistical analyses were performed with Statisica 6.0 software (StatSoft Inc., Tulsa, OK) and significance was set at α = 0.05.
3. Results
The rats consumed more water than sucralose at all concentrations, this effect was not significant at the lower concentrations, but did reach significance at the higher concentrations. The highest amount of sucralose, 18.38 ± 5.72 ml, was consumed at the 0.01 g/L, but in comparison 22.49 ± 3.84 ml of water was also consumed at this concentration. The least amount of sucralose, 1.04 ± 0.39 ml, was consumed at 10 g/L, in comparison 32.62 ± 1.92 ml of water was consumed at this concentration. Multiple dependent t-test revealed that the intakes for sucralose were significant less than water at 1, 5 and 10 g/L (p< 0.0001, for each concentration), see Figure 1.
Fig. 1. Two bottle test for sucralose, intakes expressed as mean ± SEM.
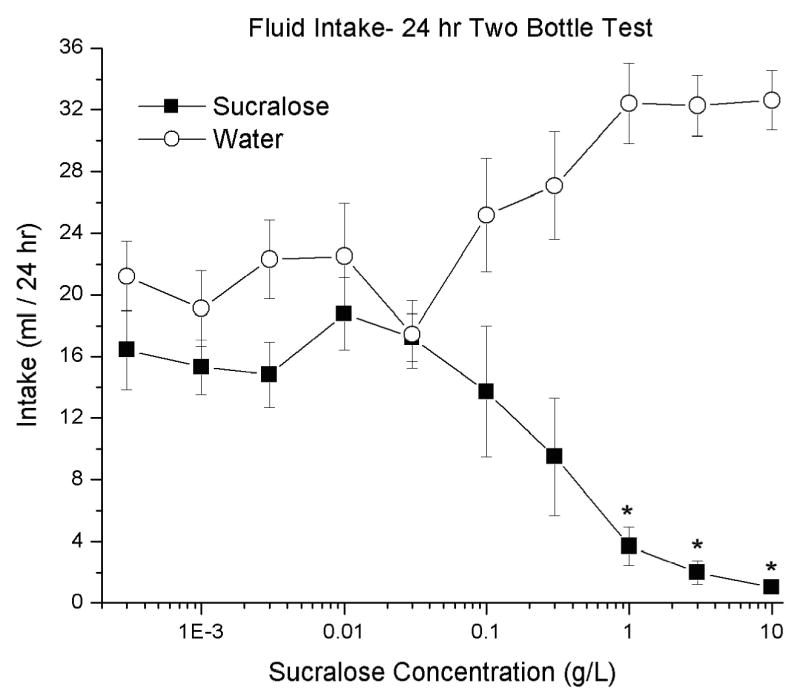
Multiple 24 hr two bottle test comparing a single sucralose concentration (0.0003–10 g/L) with water; * (p< 0.0001).
A one–way ANOVA with repeated measures revealed that as sucralose concentration increased percent preferences decreased significantly (F (9,108) = 10.92, P<0.00001), see Figure 2a. Post-hoc tests determined that the percent preferences for 1–10g/L were different from the percent preference of the 0.0003–0.1 g/L concentrations (p< 0.01; for all concentrations). A difference was also determined between 0.3 g/L and the 0.01, 0.03 and 10 g/L concentrations (p < 0.05; for all concentrations, labeled as “a” in Figure 2a). An examination of the individual preferences of all thirteen rats revealed that two rats had a 79–94% preference for 0.1 and 0.3 g/L sucralose, whereas another rat had a 72–84% preference for 0.01 and 0.03 g/L sucralose, see Figure 2b.
Fig. 2. Preference for sucralose concentrations in 24 hr two bottle test.
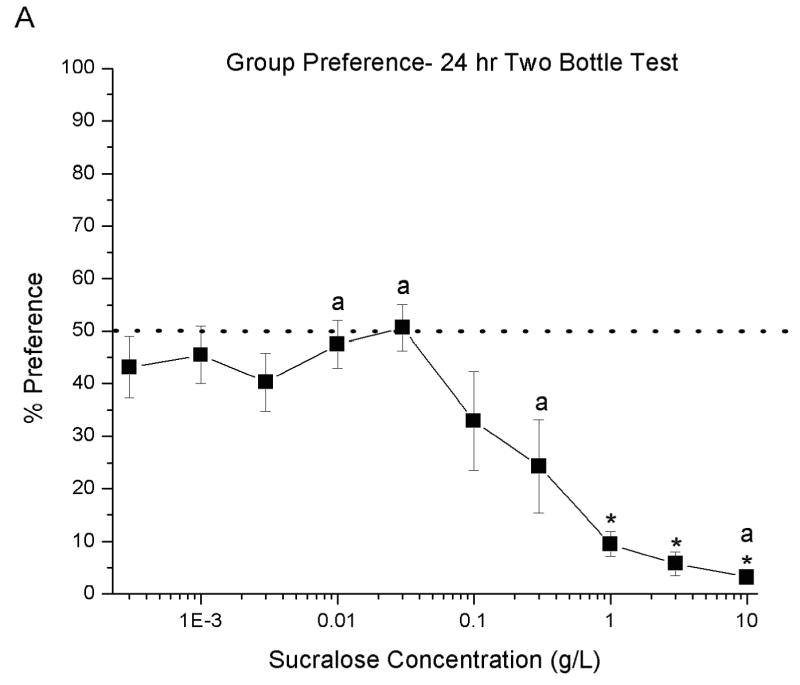
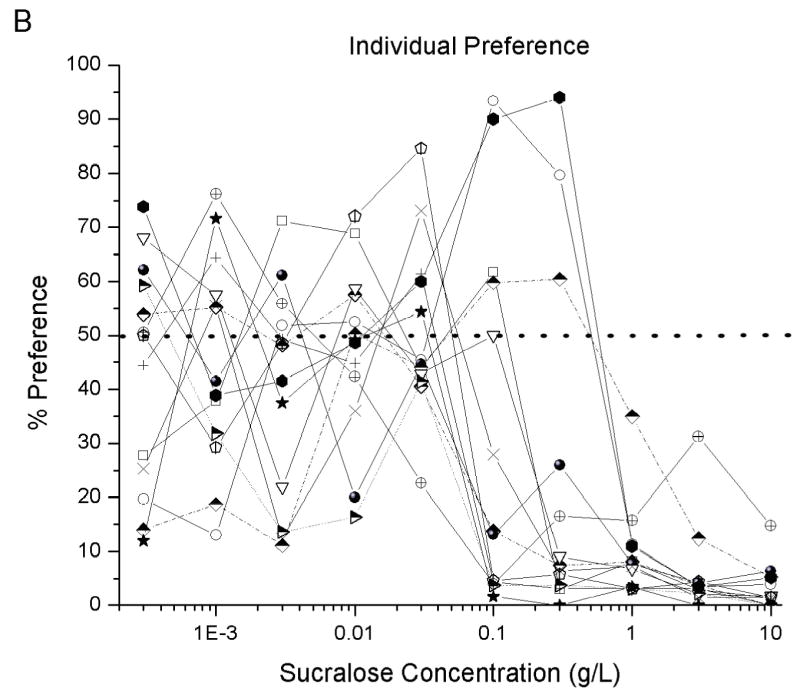
Percent preference expressed as Sucralose concentration intake/(Water intake + Sucralose concentration) multiplied by 100. Therefore, total avoidance was 0%, indifference 50%, and a strong preference 100%. In graph A, post- hoc tests revealed difference between * (p < 0.01) from 0.0003–0.03 g/L and preferences labeled with an “a” are significant difference (p < 0.05) from each other. Graph B shows individual rat preference for sucralose.
4. Discussion
The overall purpose of this experiment was to examine whether adult male Sprague Dawley rats prefer the non-caloric sweetener sucralose to water. These results indicated that male rats exhibited a characteristic indifference-avoidance pattern of preference for sucralose in that they do not prefer sucralose to water at low concentrations and avoid it at higher concentrations. The avoidance threshold for a solution is defined as the lowest concentration of a solution that is consumed in significantly smaller amount than that of water [10], from this experiment it can be determined that the avoidance threshold for sucralose in adult male Sprague Dawley rats to be approximately 1 g/L.
In a recent study by Sclafani and Clare [12] that examined the preference for sucralose in female Sprague Dawley rats, it was determined in a two-bottle test with water that half (n = 6) of the rats had a weak preference (78%) for sucralose, whereas the other half (n = 6) avoided (11%) sucralose. Also, they found that the sucralose preferring rats had the highest preference for the 0.5 g/L concentration. In the present study, only three rats had a relatively strong preference (72–94%) for sucralose over water. Rather than preferring a single concentration, each of the three sucralose preferring rats in our study had the highest individual preference for three different sucralose concentrations (0.03, 0.1 and 0.3 g/L, respectively). Sclafani and Clare also reported the results of a preliminary study that suggested that male rats avoid sucralose at 0.5 g/L. Although only a single concentration was tested, the Sclafani and Clare results are consistent with our present results and overlap with the avoidance observed in female rats at 0.2 and 1.0g/L concentrations. The discrepancy between the individual preferences, however, in this study and that of Sclafani and Clare may be related to sex of the rats used in the studies, since females, compared with males, tend to have a stronger preference for sweet solutions [13]. Sclafani and Clare also compared the preference for sucralose with that of saccharin or a mixture of sucrose + sucralose versus sucrose. In all cases, saccharin and sucrose were strongly preferred to sucralose or a mixture containing sucralose. In a separate group of experiments that used naive adult male Sprague Dawley rats (n=18), our laboratory previously reported similar findings [14]. Male rats in that study had a strong preference ( > 90%) for either 0.3M sucrose or 3mM saccharin solution versus sucralose (0.18–0.35g/L). Also, when sucralose (0.05 g/L) was added to either sweetener (0.3M sucrose or 3mM saccharin) it did not enhance the preference of the sweetener and rats tended to avoid the sucrose + sucralose solution. Taken together, this suggests that Sprague Dawley rats do not prefer and will even avoid sucralose or solutions containing sucralose. These results are quite surprising, particularly because it has been determine that humans characterize 0.18 and 0.35 g/L sucralose as tasting similar to 8.05 and 12% sucrose, respectively, with very little or no aftertaste [1,8].
Dissimilarity between human sweet preference and ingestive behaviors exhibited by rats has been indicated with other artificial sweeteners [16, 17]. In particular, it has been demonstrated that female Sprague Dawley rats have a weak preference (68–75%) for the dipeptide sweetener, aspartame (L-aspartyl-L-phenylalanine methyl ester), to water in a two-bottle test. This weak preference was only demonstrated at relatively high aspartame concentrations (0.5% and 1.0%) and in rats that were previously trained to drink 4% and 32% sucrose [17]. These results are consistent with in vitro experiments that demonstrated HEK cells transfected with the rat sweet taste receptor, T1R2/T1R3, are not responsive to 2.5 mM (0.07%) aspartame [6]. Surprisingly, these same cells were responsive to 1mM (0.4 g/L) sucralose [6].
The preference or avoidance for sucralose appears to be considerably different between rodent species (i.e., mice versus rats) and between mice strains [10,11]. At concentrations that our rats trended toward avoidance, C57BL/6 ByJ mice have been shown to prefer sucralose to water (0.1–10 g/L) [10] and (0.08–4.8g/L)[11]. In comparison to C57BL/6, 129P3/J mice, which are less responsive to a variety of sweeteners, have a relatively weaker preference for sucralose and are more limited in the concentrations they prefer (1–10 g/L) [10] and (0.8–4.8 g/L)[11]. In a follow-up study using whole chorda tympani nerve recordings, it was demonstrated that C57BL/6 ByJ mice had a threshold response to sucralose at 1 g/L. Interestingly in the strain that had a weaker preference for sucralose, the 129P3/J mice, a threshold neural response was not reached at the concentrations tested (0.1–1 g/L) [15], suggesting that the peripheral taste sensory components may be responsible for a difference in preference for sucralose. Along these lines, Damak and colleagues (2003) performed a two-bottle preference test (tastant vs. water) in T1R3 knockout mice to examine the role of the sweet taste receptor, in vivo. In that study and similar to our present findings, they reported T1R3 knockouts had an indifference to low concentrations of sucralose (0.004–0.8g/L, 0.01–2 mM) and an avoidance to high concentration of sucralose (4–4.8g/L, 2–12 mM)[11]. This suggests that avoidance at high concentration of sucralose is not mediated by the T1R3 sweet taste receptor. In addition it has been shown that polymorphisms in the Tas1R3 gene (which encodes the T1R3 component of the sweet taste receptor) in thirty mouse strains is correlated with a difference in saccharin preference between mice strains [18]. It is tempting to propose the notion that polymorphisms in Tas1R3 may be responsible for the indifference response to low concentrations of sucralose exhibited by male Sprague Dawley rats, while inbred mice [10], pigs [9] and humans [1, 7, 8] prefer sucralose solutions. This, however, does not explain why male rats avoid sucralose at high concentrations.
The present findings caution the research community against generalization of taste preference data obtained in different species in general, and in case of artificial sweeteners in particular. These differences also call for further investigation of underlying factors that may shed light on basic receptor and signal processing mechanisms in the gustatory system.
Acknowledgments
This research was supported by National Institutes Health grants DC04751, DK065709, and NS046872. The authors thank McNeil Nutritionals for their gift of sucralose, Mr. K. N. Patel for his technical assistance, and Dr. A. Sclafani for his helpful comments on these experiments. Part of this data was presented at the meeting of the Society for the Study of Ingestive Behavior, 2004, Cincinnati, Ohio, USA.
References
- 1.Wiet SG, Miller GA. Does chemical modification of tastants merely enhance their intrinsic taste qualities? Food Chemistry. 1997;58:305–311. [Google Scholar]
- 2.Roberts A, Renwick AG, Sims J, Snodin DJ. Sucralose metabolism and pharmacokinetics in man. Food Chem Toxicol. 2000;38 (Suppl 2):S31–S41. doi: 10.1016/s0278-6915(00)00026-0. [DOI] [PubMed] [Google Scholar]
- 3.Sims J, Roberts A, Daniel JW, Renwick AG. The metabolic fate of sucralose in rats. Food Chem Toxicol. 2000;38 (Suppl 2):S115–S121. doi: 10.1016/s0278-6915(00)00034-x. [DOI] [PubMed] [Google Scholar]
- 4.Mezitis NH, Maggio CA, Koch P, Quddoos A, Allison DB, Pi-Sunyer FX. Glycemic effect of a single high oral dose of the novel sweetener sucralose in patients with diabetes. Diabetes Care. 1996;19:1004–1005. doi: 10.2337/diacare.19.9.1004. [DOI] [PubMed] [Google Scholar]
- 5.Grotz VL, Henry RR, McGill JB, Prince MJ, Shamoon H, Trout JR, Pi-Sunyer FX. Lack of effect of sucralose on glucose homeostasis in subjects with type 2 diabetes. J Am Diet Assoc. 2003;103:1607–1612. doi: 10.1016/j.jada.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 6.Li X, Staszewski L, Xu H, Durick K, Zoller M, Adler E. Human receptors for sweet and umami taste . Proc Natl Acad Sci USA. 2002;99:4692–4696. doi: 10.1073/pnas.072090199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schiffman SS, Booth BJ, Losee ML, Pecore SD, Warwick ZS. Bitterness of sweeteners as a function of concentration . Brain Res Bull. 1995;36:505–513. doi: 10.1016/0361-9230(94)00225-p. [DOI] [PubMed] [Google Scholar]
- 8.Dubois GE, Walters DE, Schiffman SS, Warwick ZS, Booth BJ, et al., Concentration-response relationships of sweeteners. In: Walter DE, Orthoefer FT, Dubois GE (eds). Sweeteners: Discovery, Molecular Design, and Chemoreception. Washington, DC: American Chemical Society, 1991. pp. 261–276.
- 9.Glaser D, Wanner M, Tinti JM, Nofre C. Gustatory responses of pigs to various natural and artificial compounds known to be sweet in man. Food Chemistry. 2000;68:375–385. [Google Scholar]
- 10.Bachmanov AA, Tordoff MG, Beauchamp GK. Sweetener preference of C57BL/6ByJ and 129P3/J mice. Chem Senses. 2001;26:905–913. doi: 10.1093/chemse/26.7.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Varadarajan V, Zou S, Jiang P, Ninomiya Y, Margolskee RF. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science. 2003;301(5634):850–3. doi: 10.1126/science.1087155. [DOI] [PubMed] [Google Scholar]
- 12.Sclafani A, Clare RA. Female rats show a bimodal preference response to the artificial sweetener sucralose. Chem Senses. 2004;29:523–528. doi: 10.1093/chemse/bjh055. [DOI] [PubMed] [Google Scholar]
- 13.Valenstein ES, Kakolewski JW, Cox VC. Sex differences in taste preference for glucose and saccharin solutions. Science. 1967;156:942–943. doi: 10.1126/science.156.3777.942. [DOI] [PubMed] [Google Scholar]
- 14.Bello NT, Brockley MR, Hajnal A. Male rats lack a preference for sucralose solutions. Appetite. 2004;42:340. [Google Scholar]
- 15.Inoue M, McCaughey SA, Bachmanov AA, Beauchamp GK. Whole nerve chorda tympani responses to sweeteners in C57BL/6ByJ and 129P3/J mice . Chem Senses. 2001;26:915–923. doi: 10.1093/chemse/26.7.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fisher GL, Pfaffmann C, Brown E. Dulcin and saccharin taste in squirrel monkeys, rats, and men. Science. 1965;150:506–507. doi: 10.1126/science.150.3695.506. [DOI] [PubMed] [Google Scholar]
- 17.Sclafani A, Abrams M. Rats show only a weak preference for the artificial sweetener aspartame. Physiol Behav. 1986;37:253–256. doi: 10.1016/0031-9384(86)90228-3. [DOI] [PubMed] [Google Scholar]
- 18.Reed DR, Li S, Li X, Huang L, Tordoff MG, Startling-Roney R, Taniguchi K, West DB, Ohmen JD, Beauchamp GK, Bachmanov AA. Polymorphisms in the taste receptor gene (Tas1r3) region are associated with saccharin preference in 30 mouse strains. J Neurosci. 2004;24(4):938–946. doi: 10.1523/JNEUROSCI.1374-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]


