Abstract
Biological selectivity is shown to vary with medium osmotic strength and temperature. Selectivity reversals occur at 4°C and at an external osmolality of 0.800 indicating that intracellular hydration and endosolvent (intracellular water) structure are important determinants in selectivity. Magnetic resonance measurements of line width by steady-state nuclear magnetic resonance (NMR) indicate a difference in the intracellular water signal of 16 Hz between the K form and Na form of Escherichia coli, providing additional evidence that changes in the ionic composition of cells are accompanied by changes in endosolvent structure. The changes were found to be consistent with the thermodynamic and magnetic resonance properties of aqueous electrolyte solutions. Calculation of the dependence of ion-pairing forces on medium dielectric reinforces the role of endosolvent structure in determining ion exchange selectivity.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cope F. W. Nuclear magnetic resonance evidence using D2O for structured water in muscle and brain. Biophys J. 1969 Mar;9(3):303–319. doi: 10.1016/S0006-3495(69)86388-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
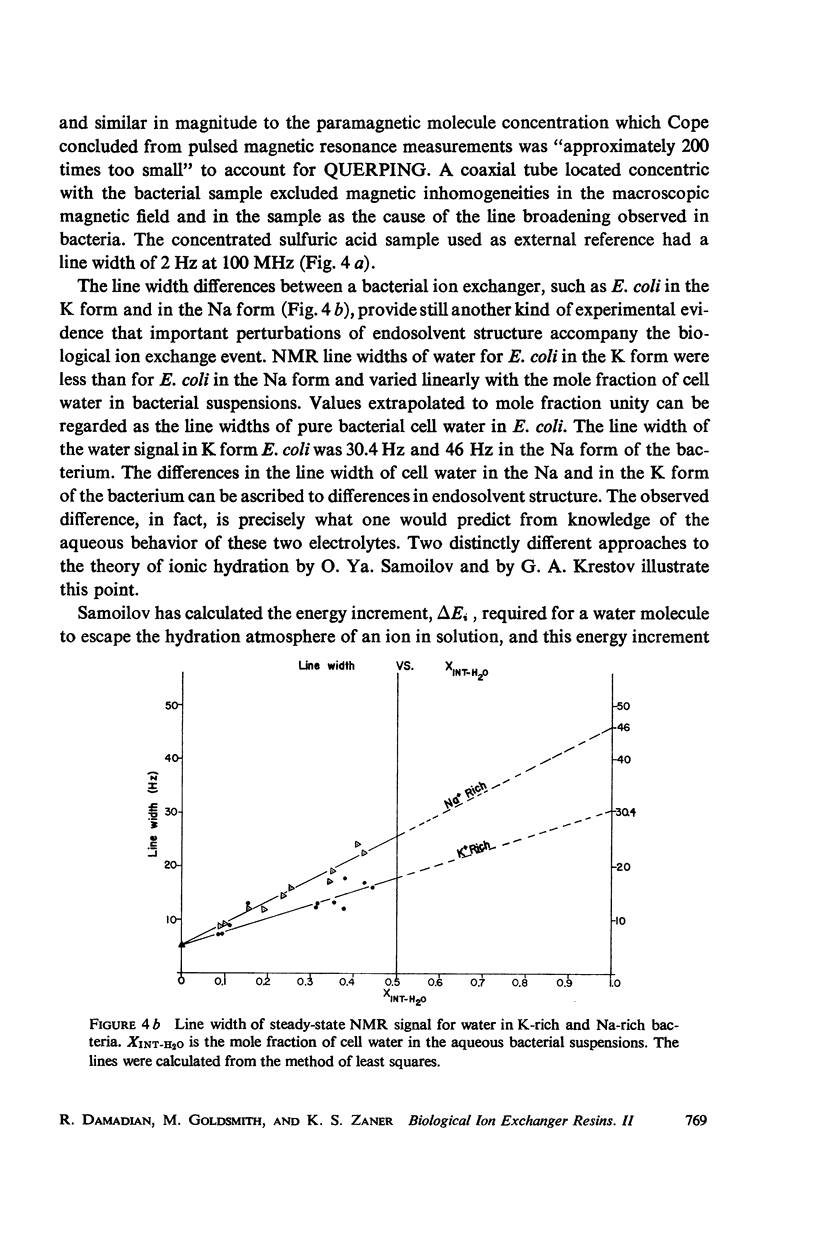
- Damadian R. Biological ion exchanger resins. I. Quantitative electrostatic correspondence of fixed charge and mobile counter ion. Biophys J. 1971 Sep;11(9):739–760. doi: 10.1016/S0006-3495(71)86251-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damadian R. Ion metabolism in a potassium accumulation mutant of Escherichia coli B. I. Potassium metabolism. J Bacteriol. 1968 Jan;95(1):113–122. doi: 10.1128/jb.95.1.113-122.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foter M. J., Rahn O. Growth and Fermentation of Bacteria Near Their Minimum Temperature. J Bacteriol. 1936 Nov;32(5):485–497. doi: 10.1128/jb.32.5.485-497.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREGOR H. P., FREDERICK M. Thermodynamic properties of ion exchange resins; free energy of swelling as related to ion selectivities. Ann N Y Acad Sci. 1953 Nov 11;57(3):87–104. doi: 10.1111/j.1749-6632.1953.tb36389.x. [DOI] [PubMed] [Google Scholar]
- Hazlewood C. F., Nichols B. L., Chamberlain N. F. Evidence for the existence of a minimum of two phases of ordered water in skeletal muscle. Nature. 1969 May 24;222(5195):747–750. doi: 10.1038/222747a0. [DOI] [PubMed] [Google Scholar]
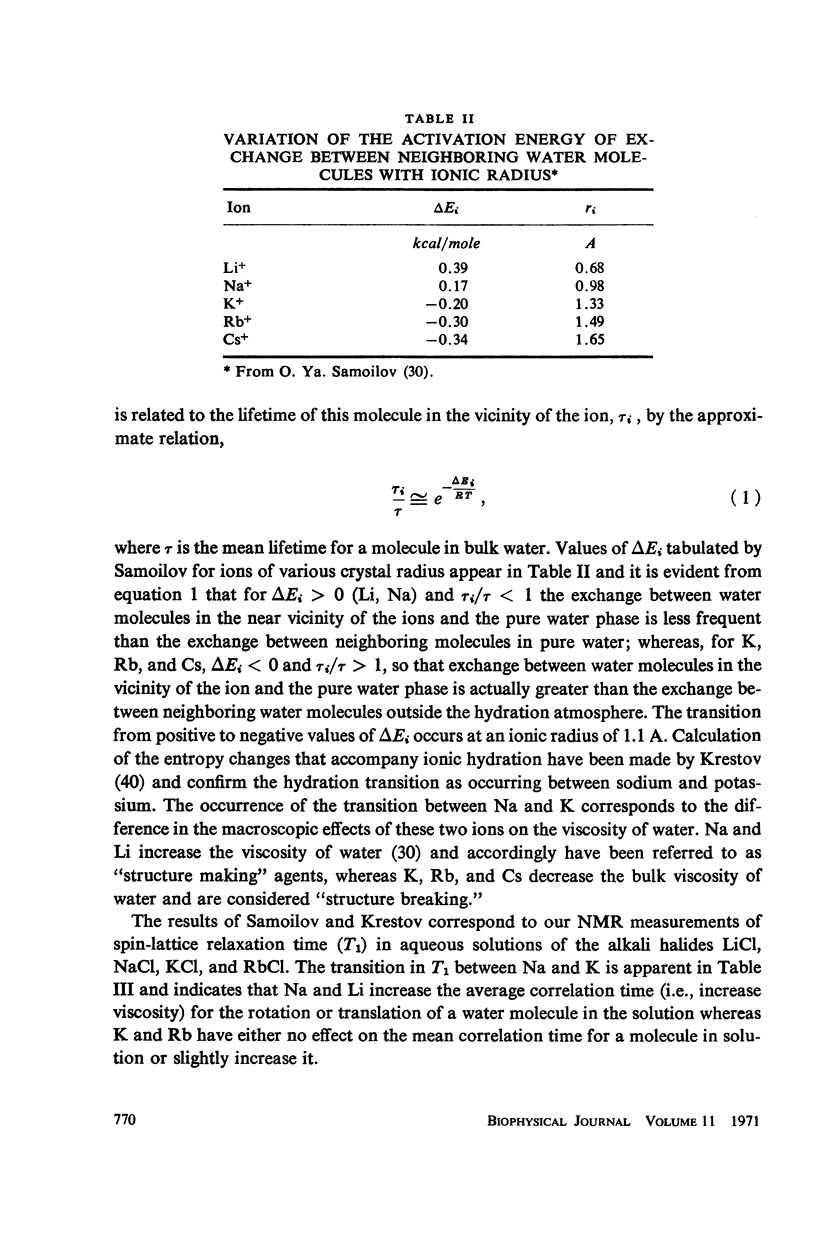
- OPPENHEIMER C. H., DROST-HANSEN W. A relationship between multiple temperature optima for biological systems and the properties of water. J Bacteriol. 1960 Jul;80:21–24. doi: 10.1128/jb.80.1.21-24.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]



