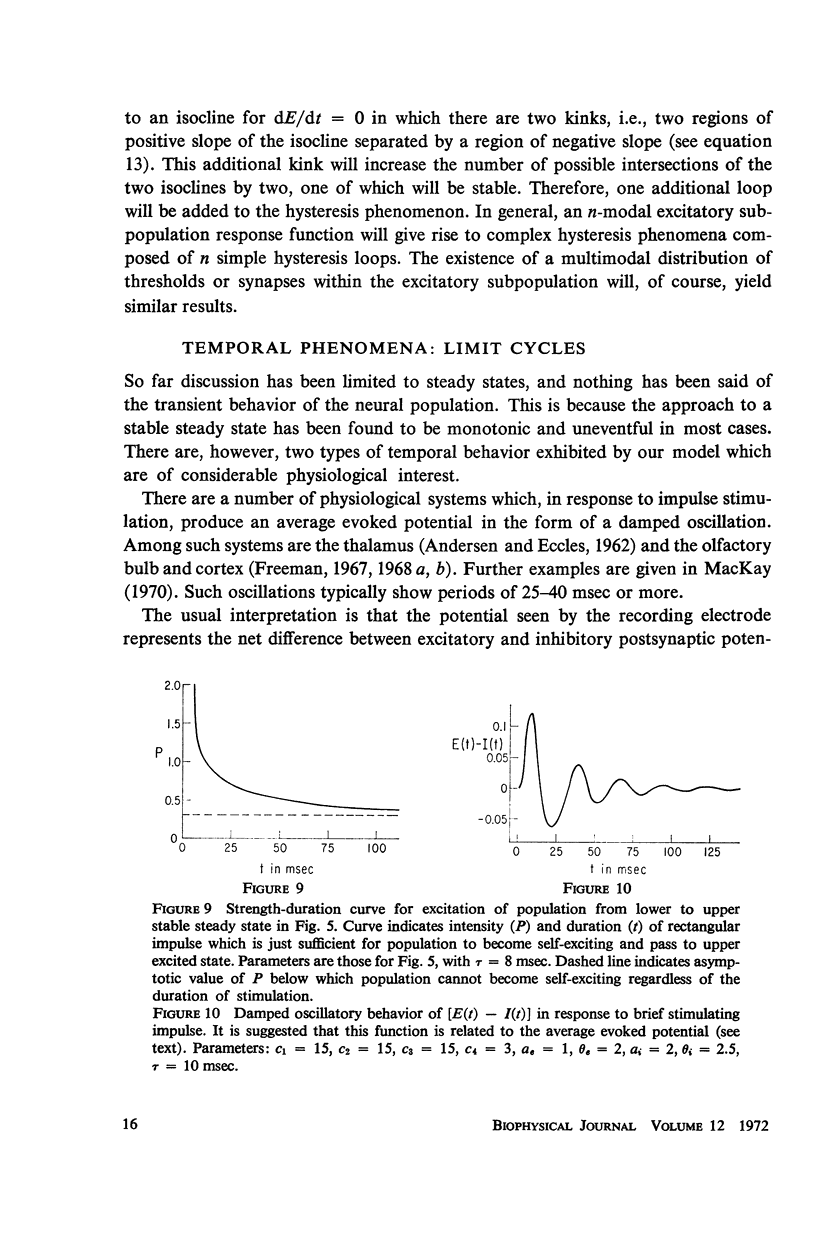
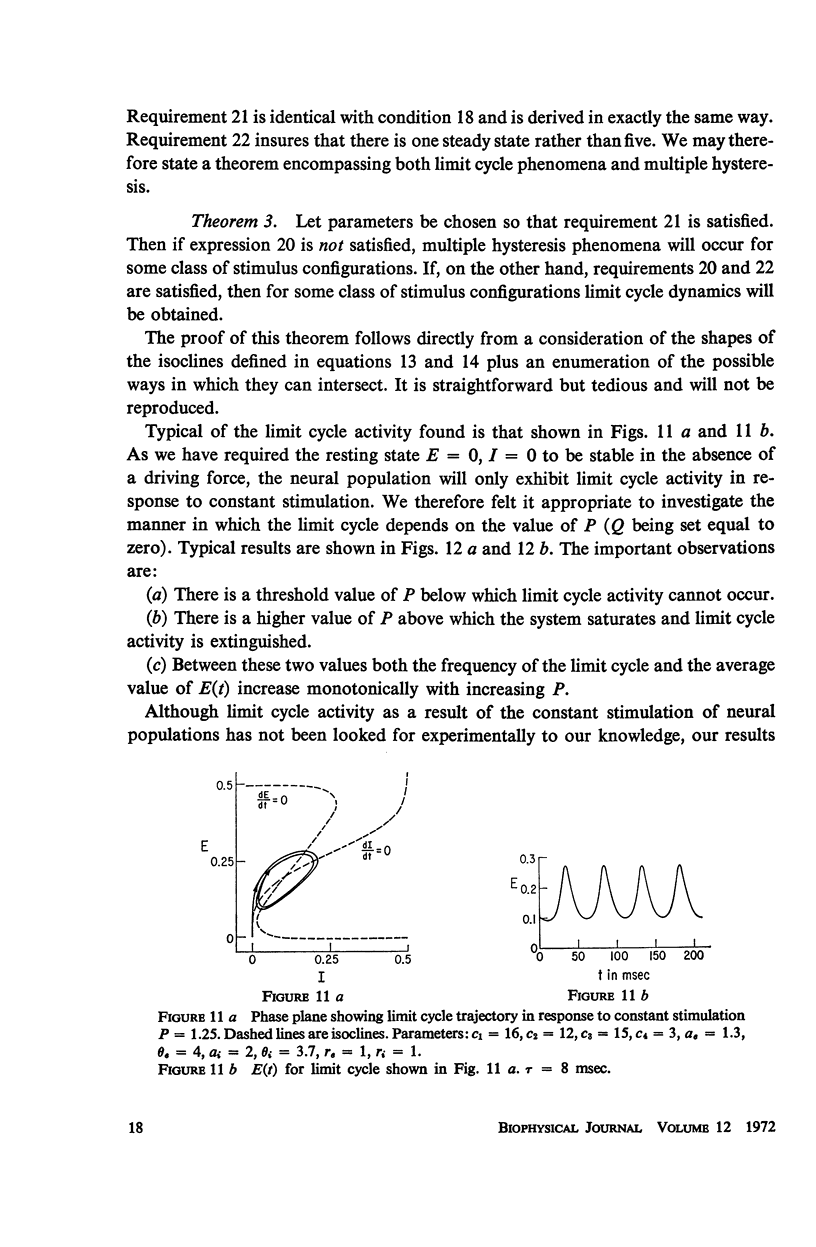
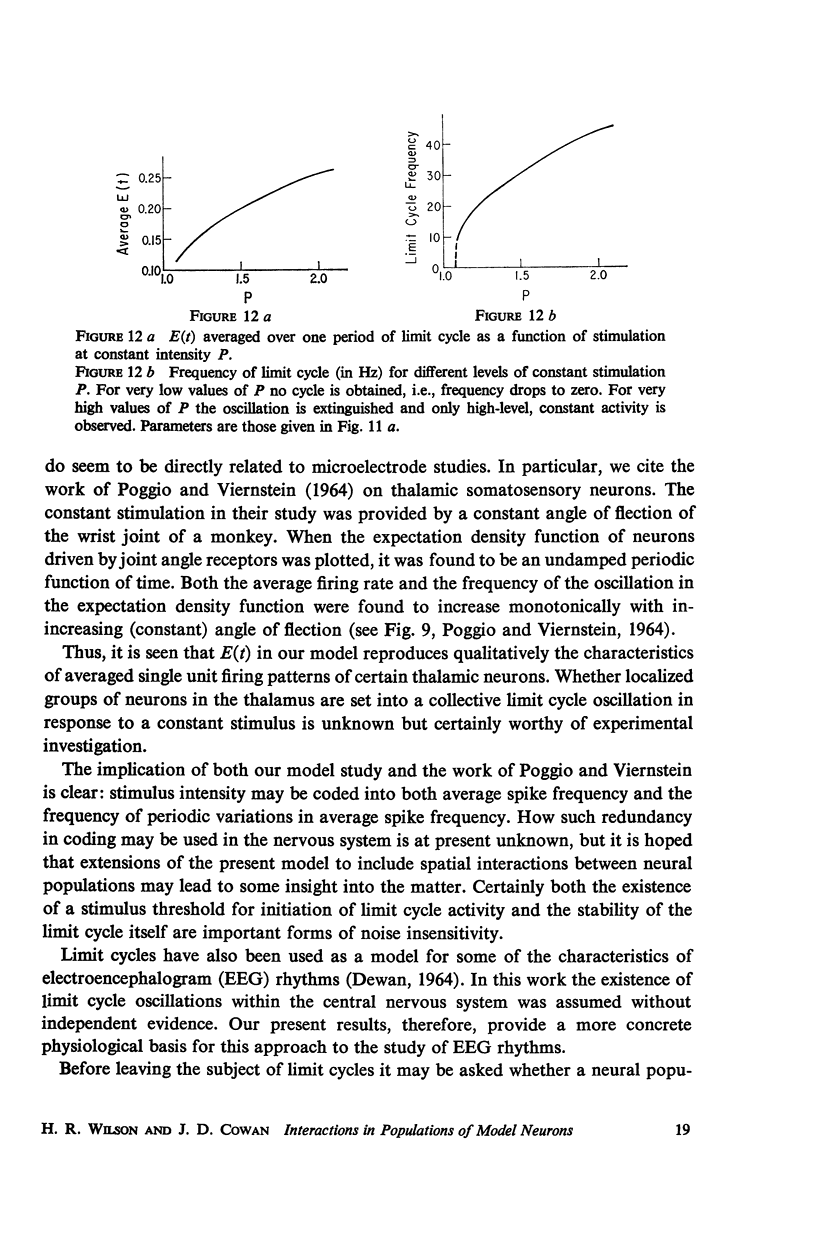
Abstract
Coupled nonlinear differential equations are derived for the dynamics of spatially localized populations containing both excitatory and inhibitory model neurons. Phase plane methods and numerical solutions are then used to investigate population responses to various types of stimuli. The results obtained show simple and multiple hysteresis phenomena and limit cycle activity. The latter is particularly interesting since the frequency of the limit cycle oscillation is found to be a monotonic function of stimulus intensity. Finally, it is proved that the existence of limit cycle dynamics in response to one class of stimuli implies the existence of multiple stable states and hysteresis in response to a different class of stimuli. The relation between these findings and a number of experiments is discussed.
Full text
PDF
















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN P., ECCLES J. Inhibitory phasing of neuronal discharge. Nature. 1962 Nov 17;196:645–647. doi: 10.1038/196645a0. [DOI] [PubMed] [Google Scholar]
- Anninos P. A., Beek B., Csermely T. J., Harth E. M., Pertile G. Dynamics of neural structures. J Theor Biol. 1970 Jan;26(1):121–148. doi: 10.1016/s0022-5193(70)80036-4. [DOI] [PubMed] [Google Scholar]
- CRAGG B. G., TEMPERLEY H. N. Memory: the analogy with ferromagnetic hysteresis. Brain. 1955;78(2):304–316. doi: 10.1093/brain/78.2.304. [DOI] [PubMed] [Google Scholar]
- Dewan E. M. Nonlinear oscillations and electroencephalography. J Theor Biol. 1964 Jul;7(1):141–159. doi: 10.1016/0022-5193(64)90047-5. [DOI] [PubMed] [Google Scholar]
- FUORTES M. G., MANTEGAZZINI F. Interpretation of the repetitive firing of nerve cells. J Gen Physiol. 1962 Jul;45:1163–1179. doi: 10.1085/jgp.45.6.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fender D., Julesz B. Extension of Panum's fusional area in binocularly stabilized vision. J Opt Soc Am. 1967 Jun;57(6):819–830. doi: 10.1364/josa.57.000819. [DOI] [PubMed] [Google Scholar]
- Freeman W. J. Relations between unit activity and evoked potentials in prepyriform cortex of cats. J Neurophysiol. 1968 May;31(3):337–348. doi: 10.1152/jn.1968.31.3.337. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. RECEPTIVE FIELDS OF CELLS IN STRIATE CORTEX OF VERY YOUNG, VISUALLY INEXPERIENCED KITTENS. J Neurophysiol. 1963 Nov;26:994–1002. doi: 10.1152/jn.1963.26.6.994. [DOI] [PubMed] [Google Scholar]
- Harth E. M., Csermely T. J., Beek B., Lindsay R. D. Brain functions and neural dynamics. J Theor Biol. 1970 Jan;26(1):93–120. doi: 10.1016/s0022-5193(70)80035-2. [DOI] [PubMed] [Google Scholar]
- RALL W. A statistical theory of monosynaptic input-output relations. J Cell Physiol. 1955 Dec;46(3):373–411. doi: 10.1002/jcp.1030460302. [DOI] [PubMed] [Google Scholar]
- RALL W. Experimental monosynaptic input-output relations in the mammalian spinal cord. J Cell Physiol. 1955 Dec;46(3):413–437. doi: 10.1002/jcp.1030460303. [DOI] [PubMed] [Google Scholar]
- RALL W., HUNT C. C. Analysis of reflex variability in terms of partially correlated excitability fluctuation in a population of motoneurons. J Gen Physiol. 1956 Jan 20;39(3):397–422. doi: 10.1085/jgp.39.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]



